- 1Division of Allergy and Clinical Immunology, Department of Medicine, McGill University Health Centre (MUHC), McGill University, Montreal, QC, Canada
- 2Division of Allergy and Clinical Immunology Department of Paediatrics, Central Second Health Cluster, Ministry of Health, Riyadh, Saudi Arabia
- 3Department of Internal Medicine, Security Forces Hospital Program, Riyadh, Saudi Arabia
- 4Faculty of Medicine, McGill University, Montreal, QC, Canada
- 5The Research Institute of the McGill University Health Centre, McGill University, McGill University Health Centre (MUHC), Montreal, QC, Canada
- 6Allergy Clinic, Copenhagen University Hospital, Gentofte, Denmark
- 7Department of Clinical Medicine, Copenhagen University, Copenhagen, Denmark
- 8Pharmacy Department, Montreal General Hospital, Montreal, QC, Canada
- 9Division of Allergy, Immunology and Dermatology, Montreal Children’s Hospital, McGill University Health Centre (MUHC), McGill University, Montreal, QC, Canada
- 10Centre for Antibiotic Allergy and Research, Department of Infectious Diseases, Austin Health, Heidelberg, VIC, Australia
Introduction
Although rare, anaphylaxis to the COVID-19 vaccine is a public concern. The rate of vaccine-related anaphylaxis in Canada is estimated to be 1.08 per 100,000 doses for the Pfizer-BioTech® vaccine and 0.77 per 100,000 doses for the Moderna® Spikevax COVID-19 vaccine (1). Recent data showed a variation in the incidence of vaccine-related anaphylaxis, depending on the definitions used for this acute reaction (2). Multiple mechanisms have been suggested to explain the underlying causes for the reported immediate reactions to the COVID-19 vaccines (3, 4). Studies that demonstrated tolerance to the second dose of the COVID-19 vaccine in patients with a history of anaphylaxis to the first dose (5–7) support a non-IgE-mediated mechanism. In November 2021, we shared our results of a successful desensitization protocol for the mRNA COVID-19 vaccine for six patients who had reported anaphylaxis to their first dose (8). With the evolving and reassuring data about the safety of subsequent doses in patients with a previous history of anaphylaxis, we re-evaluated the tolerance to the COVID-19 vaccine by performing a booster dose challenge.
Methods
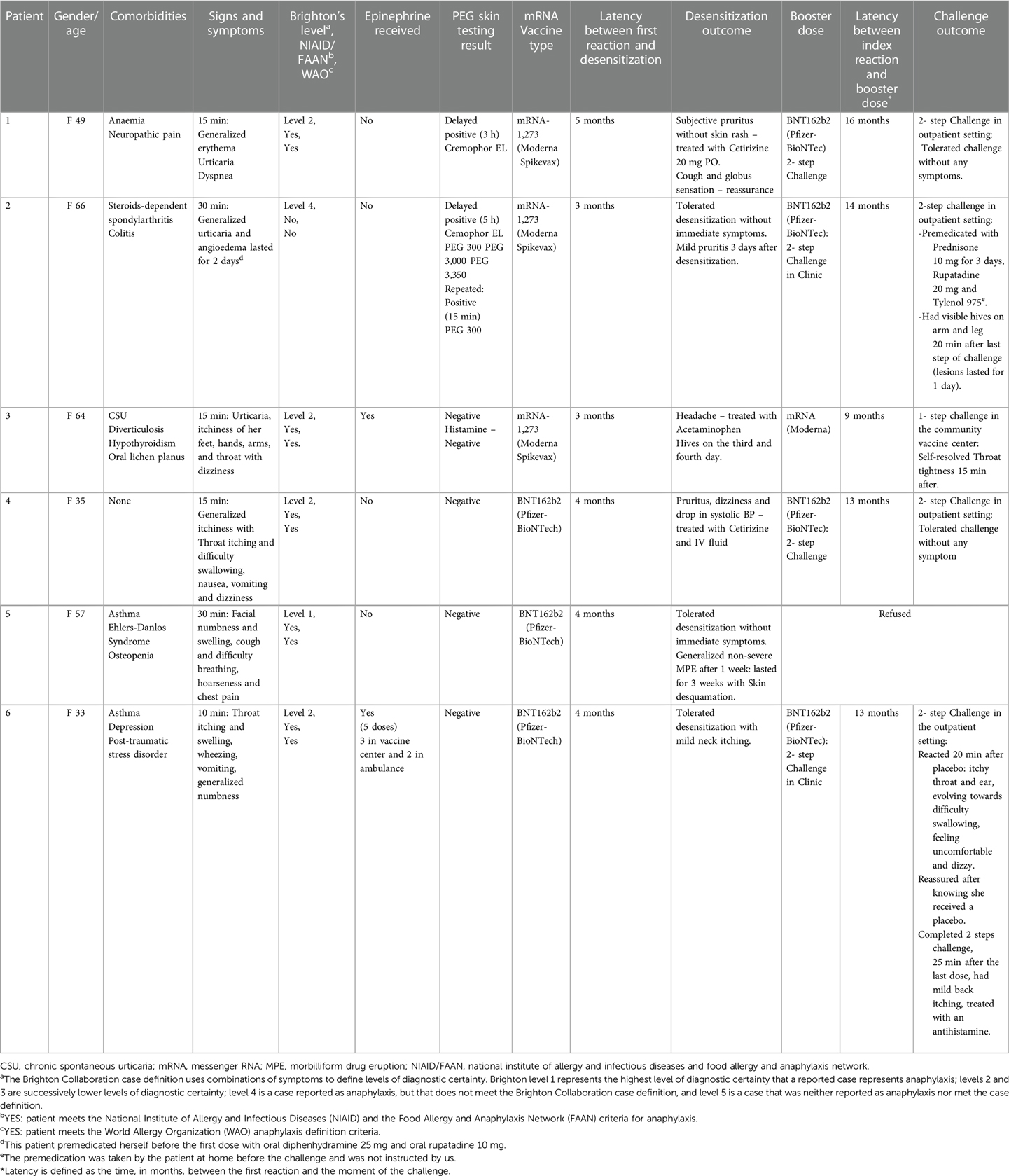
Patients were recruited as part of a large prospective 12-month COVID-19 vaccine study (ARCOV) (9). Individuals considered at risk for anaphylaxis to the COVID-19 vaccine were prospectively recruited. Six patients were selected based on a reported history of anaphylaxis to the first dose of the COVID-19 vaccine. The Brighton Collaboration case definition was used to define the levels of diagnostic certainty based on the reported symptoms (10). Brighton level 1 determines the highest level of diagnostic certainty that a reported case represents anaphylaxis; levels 2 and 3 are successively lower levels of diagnostic certainty; level 4 defines cases reported as anaphylaxis that do not meet the Brighton Collaboration case definition; and level 5 refers to cases that are neither reported as anaphylaxis nor met the case definition. Among our six patients who reported a history of anaphylaxis, four met level 2 Brighton's criteria, and two met level 3 and 4 criteria. As per our previously published protocol, PEG skin prick testing was performed during the initial assessment for all the patients with lower molecular weight (MW) PEGs: polyoxyl 35 hydrogenated castor oil (Cremophor EL) (527 mg/ml), PEG 300 (100% wt./vol), PEG 3,000 (50% wt./vol), PEG 3,350 (50% wt./vol), polysorbate 80 (20% wt./vol), and high MW PEG 20,000 (0.01%, 0.1%, 1%, and 10% wt./vol) (8). All six patients had safely received the second dose of the culprit COVID-19 vaccine using a desensitization protocol consisting of a graded dose administration followed by a 60-min observation period. Three patients received the Moderna® mRNA-1,273 and three the Pfizer-BioNTech® BNT162b2 vaccine. We offered a booster dose of the Pfizer-BioNTech® COVID-19 vaccine using a two-step blinded placebo-controlled challenge with a 1-hour observation period in a monitored setting. We defined tolerance to a subsequent dose as either (1) no immediate symptoms after the COVID-19 vaccine dose administration or (2) symptoms that were mild, self-limited, and resolved with oral antihistamines alone.
Results
All six patients were females aged 33–66 years. Five patients had a past medical history of drug or vaccine allergy. PEG SPT was initially performed and resulted in delayed positive in 2 patients. The first patient had a delayed positive (3 h) to Cremophor EL and the second patient had a delayed positive (5 h) to Cremophor EL, PEG 300, PEG 3,000, and PEG 3,350. Skin testing was repeated for the second patient, resulting in an immediate positive for PEG 300.
Of the six patients administered the booster vaccine doses, one received a one-step challenge in the community and reported no adverse reactions, and one refused the 3rd vaccine dose (Table 1). The remaining four patients completed the two-step blinded placebo-controlled challenge in a controlled outpatient setting. One patient taking regular doses of daily prednisone 5 mg was premedicated with prednisone 10 mg for three days, rupatadine 20 mg and acetaminophen 975 mg on the day of the challenge. She completed the challenge without any severe systemic reaction. However, she developed hives on her left arm and right leg 20 min after completing the challenge. The urticarial skin eruption persisted, and she required prolongation of the prednisone 10 mg and rupatadine 20 mg for one more day. A second patient reported symptoms 20 min after receiving 0.1 ml of saline (placebo). She complained of itchy throat and ears, difficulty breathing, swelling inside her ears and feeling very uncomfortable. The patient was informed that she had been given a placebo in this context. After reassurance, she was able to complete the challenge safely. Twenty-five minutes after the last dose, she reported mild back itching and received 20 mg of cetirizine which resolved her symptoms. The remaining two patients completed the challenge uneventfully.
Discussion
Rechallenging patients with a history of anaphylaxis to the mRNA CoV-2 vaccine is still discouraged because of the unknown safety of the procedure and the lack of understanding of the possible mechanisms involved (Figure 1) (4, 11). We previously demonstrated the safety of administrating the second vaccine dose using desensitization or a graded dose protocol. This cautious approach aimed at ensuring that patients could safely complete their vaccinations (8). In this study, we safely dispensed the vaccine booster in our small cohort by administering a 3rd vaccine dose in a 2-step challenge protocol. Our findings suggest that non-IgE-mediated mechanisms, including potentially direct mast cell activation, could explain the initial reactions (Figure 1) (4). Similar results were described by Krantz et al. when they challenged eight patients with a prior history of anaphylaxis (6). Their data revealed that serum tryptase levels at the reaction time were normal when collected. Unfortunately, we did not obtain serum tryptase in our patients.
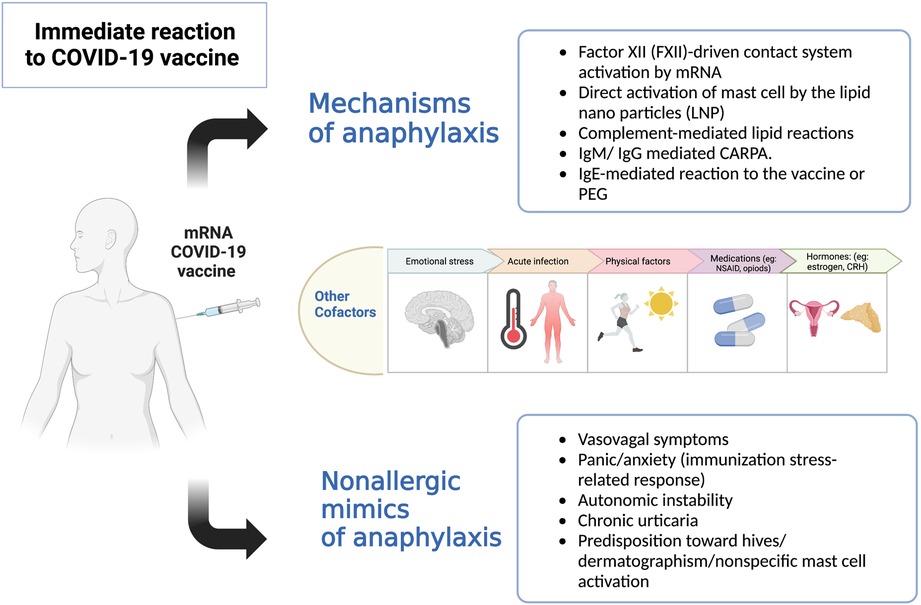
Figure 1. Potential mechanisms of immediate reactions to the COVID-19 vaccines (4).Legend: Multiple suggested mechanisms of anaphylaxis to the COVID-19 vaccine: (1) Exogenous nucleic acids activate factor XII leading to contact activation and production of bradykinin, causing increased vascular permeability, angioedema, hypotension and bronchoconstriction. (2) Direct activation of mast cells by lipid nanoparticles (LNP) via various receptors, e.g., opioids receptor, mast cell related G protein-coupled receptors X2 (MRGPRX2). (3) Lipid nanoparticles (LNP) in mRNA vaccine include neutral lipids, which may activate anaphylatoxins complement component 3a (C3a) and complement component 5a (C5a), which leads to the release of histamine, leukotrienes, prostaglandins that can lead to flushing, hives, hypoxia, vasodilatation, and hypotension. (4) Forming previous antibodies (IgM, IgG) against PEG or LNP can bind to complement and cross-link with the Fc receptor on mast cells leading to degranulation. (5) IgE against PEG on vaccine can cause anaphylaxis in patients with true PEG allergy. Host cofactors (genetic and environmental) can modify mast cell activation and increase predisposition to an immediate reaction. Other nonallergic reactions mimicking anaphylaxis should be considered in assessing patients with immediate reactions.
Administering a new vaccine to patients with a previous history of anaphylaxis, including vaccines and drugs, is challenging as it requires prompt action to identify and treat possible symptoms of anaphylaxis. Interestingly, in our study, 2 out of 6 patients required epinephrine to manage their initial reaction, and one received five doses of epinephrine before arrival at the hospital. This patient had a history of anxiety and post-traumatic stress disorder (PTSD) and reacted to the placebo during the challenge. This case demonstrates the often-encountered dilemma of distinguishing patient anxiety and psychosomatic symptoms mimicking “allergic reactions” from the true anaphylaxis (12).
The European Academy of Allergy & Clinical Immunology has recommended skin testing with PEG for patients with an allergic reaction to the COVID-19 vaccine (13). However, several studies found that patients with positive skin tests tolerated the vaccination and some patients with negative skin tests developed a reaction (14). The accuracy of PEG skin testing in the context of a reported mRNA vaccine reaction is yet to be established (3). PEG-allergic patients can tolerate the mRNA vaccine (15). However, this tolerance of mRNA vaccines does not rule out PEG allergy, and patients who tolerate the mRNA vaccines may nevertheless experience severe reactions to PEG (3). Performing this testing on our patients did not assist us in determining the tolerance of the second dose of the RNA COVID-19 vaccination (14, 16). In our view, a delayed positive skin test is not a sign of PEG hypersensitivity, and the utility and validity of testing remain unknown.
We revisited the initial reactions and used different diagnostic criteria for anaphylaxis (Table 1). All patients met Brighton's criteria with different diagnostic certainty. However, one patient did not meet the NIAID or WAO Criteria (2020) (16, 17). The anaphylaxis definition varies depending on the diagnostic criteria used. Furthermore, Brighton's criteria overestimate the anaphylaxis prevalence (2). We believe that genuine anaphylactic reactions to the COVID-19 vaccination are infrequent. A case-by-case evaluation should be performed to confirm or refute the initial anaphylactic diagnosis and thus offer the opportunity for a vaccine challenge allowing the completion of the scheduled immunization program.
Conclusion
Patients with a history of possible anaphylaxis should be assessed in an allergy unit to validate the initial reaction. A history of suspected anaphylaxis to the COVID-19 vaccine may not be a contraindication for receiving subsequent vaccine doses in an allergist-supervised setting. Large-scale studies are required to understand better the underlying mechanisms for the immediate reactions reported to the COVID-19 vaccine.
Data availability statement
The authors confirm that the results supporting the findings of this study are available within the article and/or its supplementary materials. Other data supporting the findings of this study are available from the corresponding author on request.
Ethics statement
The study was approved by the McGill University Health Center Research Ethics Board (REB# ARCOV / 2021-7510). All participants included provided a written informed consent.
Author contributions
IA: performed the literature review and wrote the manuscript with supervision from AC. FA, MF, GI, and AC: recruited patients and performed the initial desensitization protocol. IA and AC: carried out and supervised the challenges. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Government of Canada. Canadian COVID-19 vaccination safety report (2022). Available at: https://health-infobase.canada.ca/covid-19/vaccine-safety/ (Accessed).
2. Hourihane JO, Byrne AM, Blümchen K, Turner PJ, Greenhawt M. Ascertainment bias in anaphylaxis safety data of COVID-19 vaccines. J Allergy Clin Immunol Pract. (2021) 9(7):2562–6. doi: 10.1016/j.jaip.2021.04.025
3. Copaescu AM, Rosa Duque JS, Phillips EJ. What have we learned about the allergenicity and adverse reactions associated with the severe acute respiratory syndrome coronavirus 2 vaccines: one year later. Ann Allergy Asthma Immunol. (2022) 129(1):40–51. doi: 10.1016/j.anai.2022.03.030
4. Risma KA, Edwards KM, Hummell DS, Little FF, Norton AE, Stallings A, et al. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. (2021) 147(6):2075–82.e2. doi: 10.1016/j.jaci.2021.04.002
5. Krantz MS, Kwah JH, Stone CA Jr, Phillips EJ, Ortega G, Banerji A, et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. (2021) 181(11):1530–3. doi: 10.1001/jamainternmed.2021.3779
6. Krantz MS, Bruusgaard-Mouritsen MA, Koo G, Phillips EJ, Stone CA Jr, Garvey LH. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: don’t give up on the second dose!. Allergy. (2021) 76(9):2916–20. doi: 10.1111/all.14958
7. Chu DK, Abrams EM, Golden DBK, Blumenthal KG, Wolfson AR, Stone CA Jr, et al. Risk of second allergic reaction to SARS-CoV-2 vaccines: a systematic review and meta-analysis. JAMA Intern Med. (2022) 182(4):376–85. doi: 10.1001/jamainternmed.2021.8515
8. AlMuhizi F, Ton-Leclerc S, Fein M, Tsoukas C, Garvey LH, Lee D, et al. Successful desensitization to mRNA COVID-19 vaccine in a case series of patients with a history of anaphylaxis to the first vaccine dose. Front Allergy. (2022) 3:825164. doi: 10.3389/falgy.2022.825164
9. ALMuhizi F, Fein M, Gabrielli S, Gilbert L, Tsoukas C, Ben-Shoshan M, et al. Allergic reactions to the coronavirus disease 2019 vaccine (ARCOV) study: the McGill university health centre experience. Ann Allergy Asthma Immunol. (2022) 129(2):182–8.e1. doi: 10.1016/j.anai.2022.05.014
10. Kohl KS, Gidudu J, Bonhoeffer J, Braun MM, Buettcher M, Chen RT, et al. The development of standardized case definitions and guidelines for adverse events following immunization. Vaccine. (2007) 25(31):5671–4. doi: 10.1016/j.vaccine.2007.02.063
11. CDC. Interim clinical considerations for use of mRNA COVID-19 vaccines (2022). Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html (Accessed).
12. Hause AM, Gee J, Johnson T, Jazwa A, Marquez P, Miller E, et al. Anxiety-Related adverse event clusters after janssen COVID-19 vaccination - five U.S. Mass vaccination sites, April 2021. MMWR Morb Mortal Wkly Rep. (2021) 70(18):685–8. doi: 10.15585/mmwr.mm7018e3
13. Wolfson AR, Robinson LB, Li L, McMahon AE, Cogan AS, Fu X, et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. (2021) 9(9):3308–20.e3. doi: 10.1016/j.jaip.2021.06.010
14. Picard M, Drolet JP, Masse MS, Filion CA, ALMuhizi F, Fein M, et al. Safety of COVID-19 vaccination in patients with polyethylene glycol allergy: a case series. J Allergy Clin Immunol Pract. (2022) 10(2):620–5.e1. doi: 10.1016/j.jaip.2021.11.021
15. Brockow K, Mathes S, Fischer J, Volc S, Darsow U, Eberlein B, et al. Experience with polyethylene glycol allergy-guided risk management for COVID-19 vaccine anaphylaxis. Allergy. (2022) 77(7):2200–10. doi: 10.1111/all.15183
16. Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report–second national institute of allergy and infectious disease/food allergy and anaphylaxis network symposium. J Allergy Clin Immunol. (2006) 117(2):391–7. doi: 10.1016/j.jaci.2005.12.1303
Keywords: COVID-19, vaccine, mRNA, anaphylaxis, allergic reaction, desensitization, challenge, allergy
Citation: AlOtaibi I, Almuhizi F, Ton-Leclerc S, Fein M, Tsoukas C, Garvey LH, Lee D, Ben-Shoshan M, Isabwe GAC and Copaescu AM (2023) Anaphylaxis induced by mRNA COVID-19 vaccines: follow-up and booster dose after previous desensitization. Front. Allergy 4:1056619. doi: 10.3389/falgy.2023.1056619
Received: 29 September 2022; Accepted: 12 April 2023;
Published: 3 May 2023.
Edited by:
Simon Blank, Technical University of Munich and Helmholtz Center Munich, GermanyReviewed by:
Marina Labella, Regional University Hospital of Malaga, SpainGianfranco Calogiuri, Ospedale Vito Fazzi, Italy
© 2023 AlOtaibi, Almuhizi, Ton-Leclerc, Fein, Tsoukas, Garvey, Lee, Ben-Shoshan, Isabwe and Copaescu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana M. Copaescu YW5hLmNvcGFlc2N1QGdtYWlsLmNvbQ==
 Ibtihal AlOtaibi
Ibtihal AlOtaibi Faisal Almuhizi3
Faisal Almuhizi3 Shaonie Ton-Leclerc
Shaonie Ton-Leclerc Christos Tsoukas
Christos Tsoukas Lene Heise Garvey
Lene Heise Garvey Derek Lee
Derek Lee Ghislaine A. C. Isabwe
Ghislaine A. C. Isabwe Ana M. Copaescu
Ana M. Copaescu