- 1Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
- 2Division of Sport, Health and Exercise Sciences, College of Health, Medicine and Life Sciences, Brunel University London, Uxbridge, United Kingdom
Exercise induced bronchoconstriction (EIB) describes the transient narrowing of the airways that follows vigorous exercise. It commonly occurs in children and adults who have asthma and in elite athletes. The primary stimulus is proposed to be loss of water, by evaporation, from the airway surface due to conditioning inspired air. The mechanism, whereby this evaporative loss of water provokes contraction of the bronchial smooth muscle, is thought to be an increase in osmolarity of the airway surface liquid. The increase in osmolarity causes mast cells to release histamines, prostaglandins, and leukotrienes. It is these mediators that contract smooth muscle causing the airways to narrow.
Exercise-induced bronchoconstriction (EIB) describes a transient narrowing of the airways that follows vigorous exercise. It most commonly occurs in children and adults with asthma and in elite athletes (1, 2). From early controversies about the primary stimulus of EIB (i.e., loss of water vs. loss of heat from the airways as a result of conditioning of large volumes of inhaled air during exercise-hyperpnea) (3) to the establishment of the mechanism of EIB (i.e., bronchial smooth muscle contraction in response to an osmotic-driven inflammation in the lower airways) (4) and subsequent identification of specific mediators involved (i.e., histamines, prostaglandins, and leukotrienes) (5–10), significant progress has been made in the field over the last 50 years. Herein, we summarize (using select illustrations) our current understanding of the stimuli, mechanisms, and mediators released in EIB.
Exercise-induced bronchoconstriction (EIB) is usually identified by measuring peak expiratory flow (PEF) or preferably forced expiratory volume in 1 s (FEV1). These lung function measurements are made immediately before exercise and for at least 10–20 min after exercise. Surrogates for exercise, such as dry air hyperpnea and osmotic agents (e.g., hypertonic saline or mannitol), may be used to identify the potential for EIB (11). When using exercise to identify EIB, it is important to consider exercise modality, as exercise by running is more potent than exercise by walking, swimming, or cycling (12). The intensity and duration of the exercise are also important (Figures 1A,B) (13). The exercise intensity needs to be sufficient to increase the participant's heart rate within a couple of minutes to 85% or more of their predicted maximum heart rate for exercise (14). This intensity should be sustained for at least 6 min in adults and 4–5 min in children. A nose clip is used to ensure that, during exercise, the subject is breathing by mouth. To reduce the risk of a false negative test, the inspired air needs to be “dry” with a water content of less than 10 mg H2O per liter. This value is achieved when the inspired air temperature is 23°C or less and its relative humidity is 50% or less (15). Medical air inhaled from cylinders usually meets these conditions. EIB is identified by documenting a reduction from the pre-exercise PEF or FEV1 of 13% in children and 10%–15% in adults (16, 17). These values are based on observations made in healthy non-asthmatics performing exercise under the same conditions. For those currently receiving treatment for asthma, appropriate times since they last took a medication must be documented, together with details of dose and duration of treatment (18).
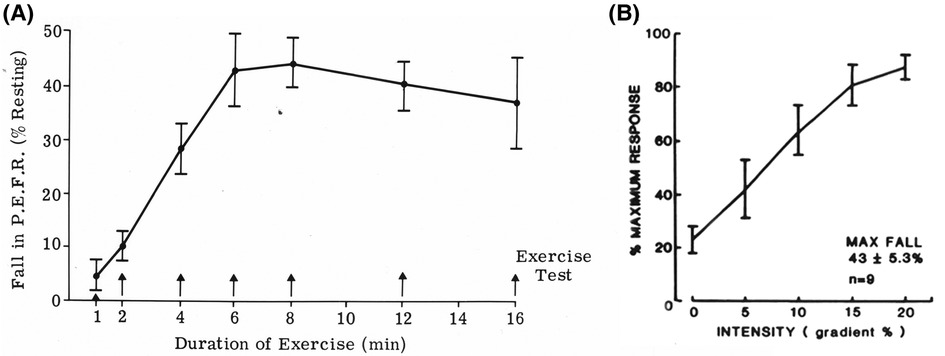
Figure 1. (A) Effect of the duration of exercise on the reduction in peak expiratory flow rate induced by treadmill running at constant speed and slope. Each point represents the mean of tests in 10 participants with asthma who performed each duration on a separate occasion. Modified from Silverman and Anderson (13). (B) Effect of gradient (workload) on the reduction in peak expiratory flow rate induced by treadmill running at constant speed for 6 min. Each point represents the mean of tests in nine participants who performed each gradient on a separate occasion. Modified from Silverman and Anderson (13).
Under most climatic conditions, when air is inspired, it needs to be heated to body temperature and fully humidified before entering the lower airways. At resting levels of ventilation, the conditioning of the inspired air to 37°C and full saturation of 44 mg H2O per liter can be achieved with nasal breathing. As ventilation increases during exercise, there is a need to reduce airway resistance, which is achieved by switching from nose breathing to mouth breathing. As a result of this switch, and due to the larger volume of air entering the respiratory tract, the lower airways are needed to fully condition the inspired air (19).
Mathematical models of the human airways suggest that the cumulative volume of airway surface liquid in the first 6–10 generations of lower airways is likely less than 1 ml. This value is based on the surface area of these generations and the depth of the airway surface liquid or periciliary fluid (19, 20). This fluid contains sodium and chloride ions and has an osmolarity of about 300 milliosmoles (similar to blood osmolarity). It has been proposed that, during exercise, the rate of water lost by evaporation from these generations due to conditioning the inspired air exceeds the rate of water return to the airway surface (Figure 2) (21). Under such conditions, a transient increase in osmolarity of the airway surface liquid is predicted to occur. It is the increase in osmolarity that has been proposed as the stimulus causing the airways to narrow in response to exercise (3). This proposal has been supported by (i) in vitro studies demonstrating that human lung mast cells release bronchoconstrictor substances when osmolarity increases (22–24) and (ii) in vivo studies showing a similar reduction in lung function after inhaling agents with high osmolarity [e.g., an aerosol of a saline solution (25) or a dry powder of mannitol (26, 27)] and after an exercise or a dry air hyperpnea test.
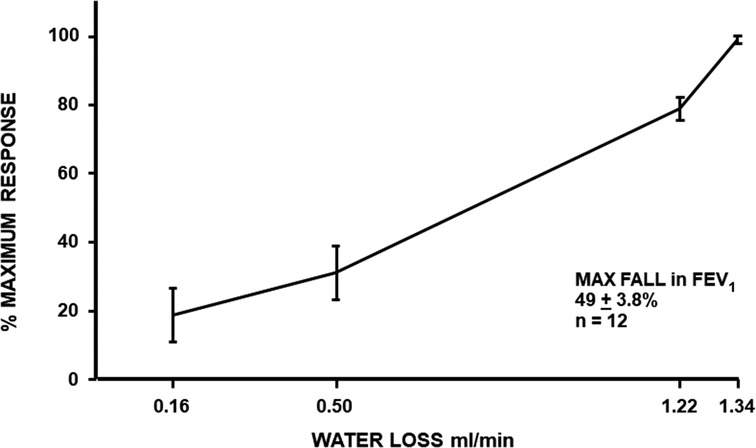
Figure 2. Maximum percentage fall in FEV1 in relation to the rate of loss of water from the airways during four exercise tests, each performed while inspiring air of a different condition (hot wet, warm humid, ambient, and medical air) in 12 participants with exercise-induced bronchoconstriction. Based on the data reported by Anderson et al. (21).
The mechanism whereby an increase in osmolarity can act as a stimulus for EIB is via the release of inflammatory mediators. The principal source of these mediators, or biomarkers, is most likely mast cells situated on or near the airway surface (28) (Figure 3). Further, it has been reported that the density of mast cells per volume is greater in those with EIB relative to normal controls and asthmatics without EIB (29).
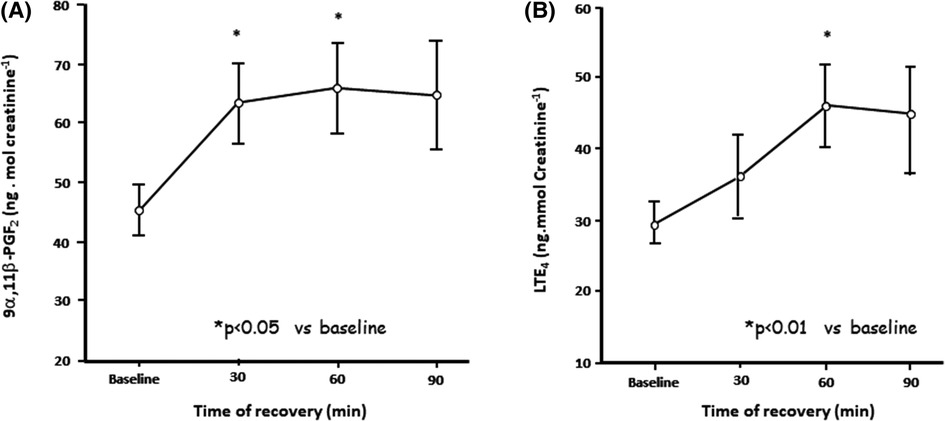
Figure 3. Change in urinary excretion of levels of (A) prostaglandin D2 metabolite 9 alpha 11 beta prostaglandin F2 and (B) leukotriene E4 levels over 90 min following 6 min of eucapnic voluntary hyperpnea in eight untrained asthmatics. Reproduced from Anderson and Kippelen (18).
Mast cell mediators include histamines, leukotrienes E4, and prostaglandins D2 (5, 6, 30–32). All these mediators would appear to contribute to the bronchoconstriction induced by hyperpnea of dry air (33). Histamine is however less potent in causing bronchoconstriction than the leukotrienes (34). Originally, mast cell mediators were identified using pharmacological antagonists, which either prevent the release or the action of these mediators (7–9). These mediators are thought to act on bronchial smooth muscle cells to cause contraction and narrowing of the airways, ultimately manifesting as EIB.
To assess the effects of pharmacological agents, both the % fall in FEV1 or PEF and the area under the curve over time have been used (7). Histamines are preformed mediators and, as such, are immediately available for release to provoke bronchoconstriction. This is likely why histamine is an important contributor to the maximum % fall in PEF or FEV1 after exercise. Because histamines are rapidly metabolized, these are not likely to be important in prolonging the bronchoconstriction due to exercise. Antihistamines do not prevent EIB and are not recommended for treatment; however, the histamine antagonist, loratadine, has been shown to reduce the severity of EIB when taken for 3 days before exercise (8) (Figure 4).
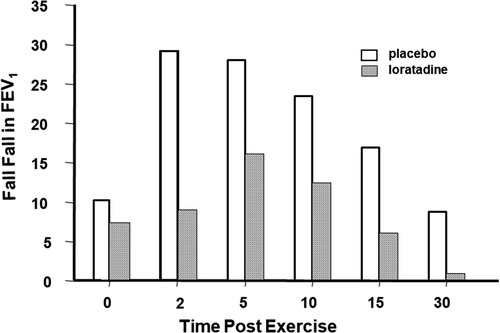
Figure 4. Maximum percentage fall in FEV1 at various times following an exercise test in 11 children who had received 10 mg of the antihistamine loratadine or its placebo once daily for 3 days. Drawn from Baki and Orhan (8).
In contrast to histamines, the mast cell mediators, prostaglandin D2 and Leukotriene E4, are not preformed. For this reason, they are likely to be released from the mast cell later than histamines. Further, unlike histamines, PGD2 and LTE4 are not rapidly metabolized. Studies using pharmacological antagonists indicate that PGD2 and LTE4 contribute significantly to the area under the response curve over time. For example the leukotriene antagonist montelukast reduces the severity of EIB, although it does not completely inhibit EIB, as other mediators are also involved. The combination of montelukast and loratadine reduced the concentration of mediators in sputum following exercise, and this was associated with EIB inhibition (28). Sodium cromoglycate (SCG), a mast cell stabilizer, has been reported to inhibit the release of a metabolite of prostaglandin D2 in response to hyperpnea with dry air (Figure 5) (10). Another study reported the inhibitory effects of the beta2 adrenoceptor agonist terbutaline, given by inhalation in a dose of 500 μg, on mast cell-derived mediator release in EIB (Figure 6) (35). As it had previously been shown that 200 μg of inhaled salbutamol was more effective in preventing EIB than 4 mg of salbutamol taken orally (36), it is likely that a dual action (i.e., relaxation of the airway smooth muscle plus stabilization of mast cells) is responsible for the prophylactic effect of inhaled beta2 adrenoceptor agonists on EIB.
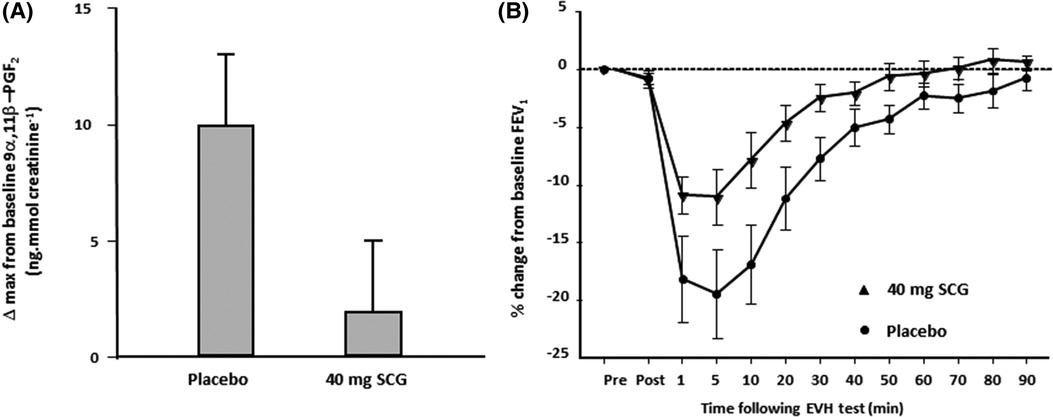
Figure 5. (A) Change in the maximum value for 9 alpha, 11 beta prostaglandin F2, a metabolite of prostaglandin D2, in 11 athletes following 8 min of eucapnic voluntary hyperpnea with dry air performed 10 min after inhaling 40 mg of SCG and after placebo. Reproduced from Anderson and Kippelen (18). (B) Percentage change in FEV1 from baseline over 90 min in 11 athletes following 8 min of eucapnic voluntary hyperpnea with dry air performed 10 min after inhaling 40 mg of SCG (triangles) and after placebo (closed circles). Reproduced from Anderson and Kippelen (18).

Figure 6. (A) Individual values for the maximum fall in FEV1 in 27 athletes in response to 8 min of eucapnic voluntary hyperpnea with dry air performed 15 min after inhaling 500 μg of terbutaline sulfate and its placebo. The broken line identifies the threshold for a positive EIB test (i.e., a fall in FEV1 of at least 10%). Reproduced from Simpson et al. (35). (B) Median values (and interquartile range) for the urinary concentration of 11 β-PGF2α at baseline and 30 and 60 min post-eucapnic voluntary hyperpnea with dry air following inhalation of 500 μg of terbutaline sulfate and its placebo in 18 athletes with EIB. Reproduced from Simpson et al. (35).
In conclusion, inflammatory mediators are released in response to exercise and, in those individuals with asthma, are likely to account for the airway narrowing following exercise. As such, preventative methods for EIB should focus on reducing the stimulus (i.e., hyperosmolarity in the lower airways), inhibiting mediators release, or counteracting the mediators’ effects.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor AS declared a past collaboration with the authors.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Anderson SD. Exercise-induced asthma: stimulus, mechanism, and management. In: Barnes PJRI, Thomson NC, editors. Asthma: basic mechanisms and clinical management. London: Academic Press (1988). p. 503–22.
2. Fitch KD, Sue-Chu M, Anderson SD, Boulet LP, Hancox RJ, McKenzie DC, et al. Asthma and the elite athlete: summary of the International Olympic Committee’s consensus conference, Lausanne, Switzerland, January 22-24, 2008. J Allergy Clin Immunol. (2008) 122(2):254–60.e7. doi: 10.1016/j.jaci.2008.07.003
3. Anderson SD. Is there a unifying hypothesis for exercise-induced asthma? J Allergy Clin Immunol. (1984) 73(5 Pt 2):660–5. doi: 10.1016/0091-6749(84)90301-4
4. Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is. J Allergy Clin Immunol. (2000) 106(3):453–9. doi: 10.1067/mai.2000.109822
5. Anderson SD, Bye PT, Schoeffel RE, Seale JP, Taylor KM, Ferris L. Arterial plasma histamine levels at rest, and during and after exercise in patients with asthma: effects of terbutaline aerosol. Thorax. (1981) 36(4):259–67. doi: 10.1136/thx.36.4.259
6. O’Sullivan S, Roquet A, Dahlén B, Larsen F, Eklund A, Kumlin M, et al. Evidence for mast cell activation during exercise-induced bronchoconstriction. Eur Respir J. (1998) 12(2):345–50. doi: 10.1183/09031936.98.12020345
7. Kemp JP, Dockhorn RJ, Shapiro GG, Nguyen HH, Reiss TF, Seidenberg BC, et al. Montelukast once daily inhibits exercise-induced bronchoconstriction in 6- to 14-year-old children with asthma. J Pediatr. (1998) 133(3):424–8. doi: 10.1016/S0022-3476(98)70281-1
8. Baki A, Orhan F. The effect of loratadine in exercise-induced asthma. Arch Dis Child. (2002) 86(1):38–9. doi: 10.1136/adc.86.1.38
9. Edelman JM, Turpin JA, Bronsky EA, Grossman J, Kemp JP, Ghannam AF, et al. Oral montelukast compared with inhaled salmeterol to prevent exercise-induced bronchoconstriction. A randomized, double-blind trial. Exercise study group. Ann Intern Med. (2000) 132(2):97–104. doi: 10.7326/0003-4819-132-2-200001180-00002
10. Kippelen P, Larsson J, Anderson SD, Brannan JD, Dahlén B, Dahlén SE. Effect of sodium cromoglycate on mast cell mediators during hyperpnea in athletes. Med Sci Sports Exerc. (2010) 42(10):1853–60. doi: 10.1249/MSS.0b013e3181da4f7d
11. Anderson SD, Kippelen P. Assessment of EIB: what you need to know to optimize test results. Immunol Allergy Clin North Am. (2013) 33(3):363–80. doi: 10.1016/j.iac.2013.02.006
12. Anderson SD, Connolly NM, Godfrey S. Comparison of bronchoconstriction induced by cycling and running. Thorax. (1971) 26(4):396–401. doi: 10.1136/thx.26.4.396
13. Silverman M, Anderson SD. Standardization of exercise tests in asthmatic children. Arch Dis Child. (1972) 47(256):882–9. doi: 10.1136/adc.47.256.882
14. Carlsen KH, Engh G, Mørk M. Exercise-induced bronchoconstriction depends on exercise load. Respir Med. (2000) 94(8):750–5. doi: 10.1053/rmed.2000.0809
15. Haby MM, Anderson SD, Peat JK, Mellis CM, Toelle BG, Woolcock AJ. An exercise challenge protocol for epidemiological studies of asthma in children: comparison with histamine challenge. Eur Respir J. (1994) 7(1):43–9. doi: 10.1183/09031936.94.07010043
16. Godfrey S, Springer C, Bar-Yishay E, Avital A. Cut-off points defining normal and asthmatic bronchial reactivity to exercise and inhalation challenges in children and young adults. Eur Respir J. (1999) 14(3):659–68. doi: 10.1034/j.1399-3003.1999.14c28.x
17. Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, et al. An official American thoracic society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med. (2013) 187(9):1016–27. doi: 10.1164/rccm.201303-0437ST
18. Anderson SD, Kippelen P. Stimulus and mechanism of exercise-induced bronchoconstriction. Breathe. (2010) 7(1):25–33. doi: 10.1183/18106838.0701.025
19. Daviskas E, Gonda I, Anderson SD. Local airway heat and water vapour losses. Respir Physiol. (1991) 84(1):115–32. doi: 10.1016/0034-5687(91)90023-C
20. Daviskas E, Gonda I, Anderson SD. Mathematical modeling of heat and water transport in human respiratory tract. J Appl Physiol (1985). (1990) 69(1):362–72. doi: 10.1152/jappl.1990.69.1.362
21. Anderson SD, Schoeffel RE, Follet R, Perry CP, Daviskas E, Kendall M. Sensitivity to heat and water loss at rest and during exercise in asthmatic patients. Eur J Respir Dis. (1982) 63(5):459–71.7140877
22. Eggleston PA, Kagey-Sobotka A, Lichtenstein LM. A comparison of the osmotic activation of basophils and human lung mast cells. Am Rev Respir Dis. (1987) 135(5):1043–8. doi: 10.1164/arrd.1987.135.5.1043
23. Säfholm J, Manson ML, Bood J, Al-Ameri M, Orre AC, Raud J, et al. Mannitol triggers mast cell-dependent contractions of human small bronchi and prostacyclin bronchoprotection. J Allergy Clin Immunol. (2019) 144(4):984–92. doi: 10.1016/j.jaci.2019.04.031
24. Gulliksson M, Palmberg L, Nilsson G, Ahlstedt S, Kumlin M. Release of prostaglandin D2 and leukotriene C4 in response to hyperosmolar stimulation of mast cells. Allergy. (2006) 61(12):1473–9. doi: 10.1111/j.1398-9995.2006.01213.x
25. Smith CM, Anderson SD. Inhalational challenge using hypertonic saline in asthmatic subjects: a comparison with responses to hyperpnoea, methacholine and water. Eur Respir J. (1990) 3(2):144–51. doi: 10.1183/09031936.93.03020144
26. Kersten ET, Driessen JM, van der Berg JD, Thio BJ. Mannitol and exercise challenge tests in asthmatic children. Pediatr Pulmonol. (2009) 44(7):655–61. doi: 10.1002/ppul.21034
27. Brannan JD, Koskela H, Anderson SD, Chew N. Responsiveness to mannitol in asthmatic subjects with exercise- and hyperventilation-induced asthma. Am J Respir Crit Care Med. (1998) 158(4):1120–6. doi: 10.1164/ajrccm.158.4.9802087
28. Hallstrand TS, Moody MW, Wurfel MM, Schwartz LB, Henderson WR, Aitken ML. Inflammatory basis of exercise-induced bronchoconstriction. Am J Respir Crit Care Med. (2005) 172(6):679–86. doi: 10.1164/rccm.200412-1667OC
29. Lai Y, Altemeier WA, Vandree J, Piliponsky AM, Johnson B, Appel CL, et al. Increased density of intraepithelial mast cells in patients with exercise-induced bronchoconstriction regulated through epithelially derived thymic stromal lymphopoietin and IL-33. J Allergy Clin Immunol. (2014) 133(5):1448–55. doi: 10.1016/j.jaci.2013.08.052
30. Manning PJ, Rokach J, Malo JL, Ethier D, Cartier A, Girard Y, et al. Urinary leukotriene E4 levels during early and late asthmatic responses. J Allergy Clin Immunol. (1990) 86(2):211–20. doi: 10.1016/S0091-6749(05)80068-5
31. Wenzel SE. Arachidonic acid metabolites: mediators of inflammation in asthma. Pharmacotherapy. (1997) 17(1 Pt 2):3S–12S.9017783
32. Komi EAD, Bjermer L. Mast cell-mediated orchestration of the immune responses in human allergic asthma: current insights. Clin Rev Allergy Immunol. (2019) 56(2):234–47. doi: 10.1007/s12016-018-8720-1
33. Bood JR, Sundblad BM, Delin I, Sjödin M, Larsson K, Anderson SD, et al. Urinary excretion of lipid mediators in response to repeated eucapnic voluntary hyperpnea in asthmatic subjects. J Appl Physiol (1985). (2015) 119(3):272–9. doi: 10.1152/japplphysiol.00301.2015
34. O'Hickey SP, Arm JP, Rees PJ, Spur BW, Lee TH. The relative responsiveness to inhaled leukotriene E4, methacholine and histamine in normal and asthmatic subjects. Eur Respir J. (1988) 1(10):913–7. doi: 10.1183/09031936.93.01100913
35. Simpson AJ, Bood JR, Anderson SD, Romer LM, Dahlén B, Dahlén SE, et al. A standard, single dose of inhaled terbutaline attenuates hyperpnea-induced bronchoconstriction and mast cell activation in athletes. J Appl Physiol (1985). (2016) 120(9):1011–7. doi: 10.1152/japplphysiol.00700.2015
Keywords: exercise, asthma, bronchoconstriction, mast cells, inflammatory mediators
Citation: Anderson SD and Kippelen P (2023) A proposal to account for the stimulus, the mechanism, and the mediators released in exercise-induced bronchoconstriction. Front. Allergy 4:1004170. doi: 10.3389/falgy.2023.1004170
Received: 27 July 2022; Accepted: 25 September 2023;
Published: 6 November 2023.
Edited by:
Andrew Simpson, University of Hull, United KingdomReviewed by:
Hans Haverkamp, Washington State University Health Sciences Spokane, United StatesNeil Williams, Nottingham Trent University, United Kingdom
© 2023 Anderson and Kippelen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandra D. Anderson c2FuZHJhLmFuZGVyc29uQHN5ZG5leS5lZHUuYXU=
 Sandra D. Anderson
Sandra D. Anderson Pascale Kippelen
Pascale Kippelen