
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Allergy , 30 March 2022
Sec. Drug, Venom & Anaphylaxis
Volume 3 - 2022 | https://doi.org/10.3389/falgy.2022.874772
This article is part of the Research Topic Drug Allergy Diagnosis and Testing View all 5 articles
 Masao Yamaguchi1*
Masao Yamaguchi1* Akiko Komiya2
Akiko Komiya2 Maho Suzukawa3
Maho Suzukawa3 Rikiya Koketsu4
Rikiya Koketsu4 Risa Shiragami1
Risa Shiragami1 Motoyasu Iikura5
Motoyasu Iikura5 Hiroyuki Nagase6
Hiroyuki Nagase6Drug hypersensitivity can be an important problem during pharmacological management of various diseases. Patients diagnosed as having a drug allergy usually need to avoid the offending drug, either temporarily or for life. Another way of overcoming a drug allergy is to establish desensitization using the allergen drug itself. We previously investigated in vitro desensitization of human basophils using a subthreshold dose of an IgE-crosslinking reagent. We found that basophil desensitization occurred in a dose-dependent manner over a period of one to several hours. We think that inducible basophil desensitization occurring without histamine release may explain, at least in part, the clinical features of drug desensitization in type 1 drug allergy.
Most, if not all, clinicians have encountered adverse events during administration of drugs for diseases. Anaphylaxis, an immediate–type systemic allergic reaction, accounts for a significant portion of such adverse events (1). An IgE-mediated (type I) allergic reaction is the typical mechanism. Mast cells and basophils have numerous high–affinity IgE receptors (FcεRI) on their surface. In sensitized subjects, cross-linkage of IgE by the eliciting drug results in activation of mast cells and basophils, followed by release of their preformed mediators such as histamine, in addition to newly–synthesized mediators, including lipid mediators.
Drug-induced anaphylactic reactions have been extensively investigated from both the clinical and basic viewpoints. However, we have a poor understanding of how sensitive subjects react to exposure to very low allergen doses, i.e., below threshold. One outcome may be desensitization, but another may be worsening of the allergic reaction; the relationship between these two outcomes has been unclear.
We previously investigated basophil desensitization in vitro by using a simple method: human basophils were preincubated with low–dose anti-IgE antibody for various lengths of time (hours or days), and the cells were then stimulated with high–dose anti-IgE or the sensitizing allergen (2). The histamine released into the supernatant was then measured. When basophils were preincubated for 24 h with subthreshold or lower concentrations of anti-IgE antibody, the cells' shape and surface FcεRI density did not undergo significant changes, but the histamine releasability of the cells was suppressed. That suppression, i.e., desensitization, induced by anti-IgE was dose- and time-dependent. IgE-dependent histamine releasability was completely suppressed when basophils were preincubated with a near-threshold concentration of anti-IgE for 4 h. The time–course of basophil desensitization in the presence of subthreshold anti-IgE varied greatly among the tested subjects, with some being completely desensitized within only 1 h (2). Using basophils from mite-sensitive subjects, preincubation with a subthreshold allergen (Der f 2) dose induced complete unresponsiveness to later stimulation with the same allergen, and nearly complete unresponsiveness to later stimulation with high–dose anti-IgE antibody. Those findings suggest that both specific and non-specific desensitization of basophils took place (3). However, we did not perform more detailed studies to try to distinguish between specific and non-specific desensitization.
Through these analyses, we learned that in vitro basophil desensitization can be induced not only in the presence of high–dose allergen but also subthreshold or low–dose allergen, and that the time required for basophil desensitization by subthreshold allergen exposure varies widely from ≤ 1 h to 4 h among subjects (2, 4). Based on these results, we think the schema may reflect the features of untreated basophils (Figure 1A) and during the desensitization procedure applied to drug-sensitive patients (Figure 1B). In clinical settings, desensitization protocols usually use two-fold increments at 15-min intervals. We feel that those protocols may be optimal for safe, effective and efficient induction of basophil (and probably also mast cell) desensitization, culminating in elimination of reactivity to the drug.
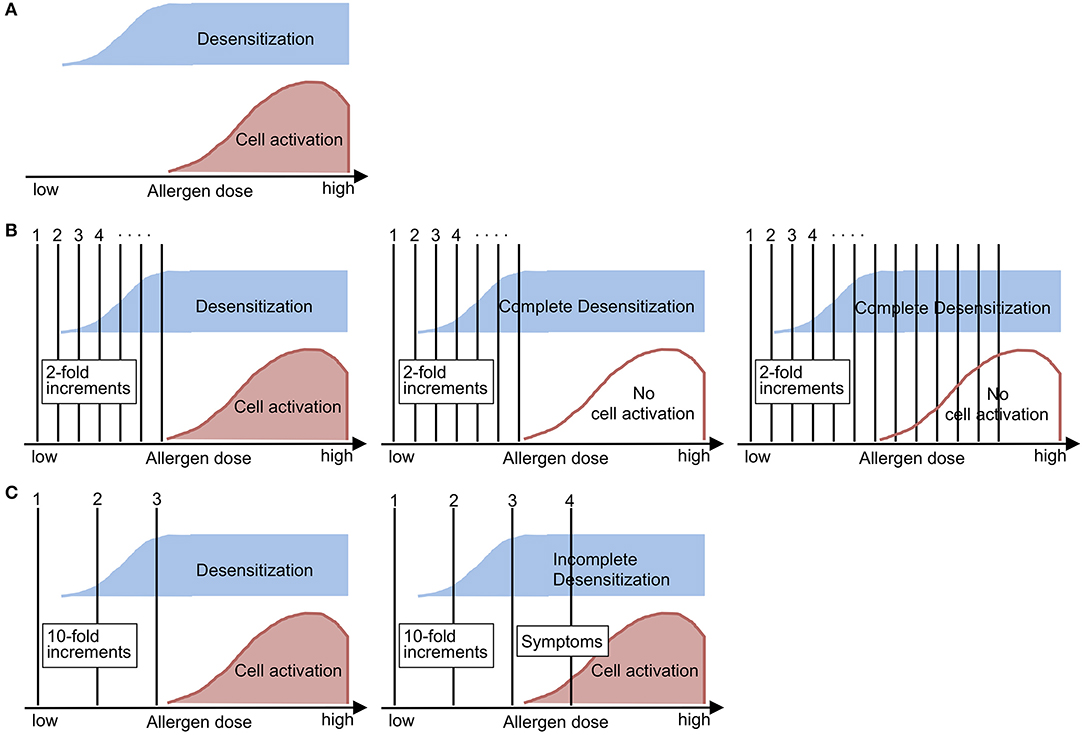
Figure 1. Schematic demonstrations of (A) the nature of allergen-induced outcomes, (B) desensitization and (C) the challenge test in sensitized subjects, as suggested by the findings of in vitro analysis of basophil desensitization. (A) Allergen-induced desensitization can occur below the lowest allergen dose that can induce cell activation (i.e., threshold dose). (B) During the desensitization procedure, the initial doses will induce dose-dependent desensitization (left). When complete desensitization is induced (middle), then the subject can accept incremental doses of allergen without manifesting allergic symptoms (right). (C) During the challenge test, the initial doses will not be able to induce complete desensitization (left), so the subject will manifest allergic symptoms when the incremental dose reaches the range of cell activation (right). This figure was translated and modified from the original figure that we published in a Japanese journal (4).
On the other hand, challenge tests utilize 10-fold or higher increments in typical settings, although the reason of those protocols has not been established. Based on the features of basophil desensitization (Figure 1A), the challenge test procedure may need to be performed rapidly enough to avoid complete desensitization during dose escalation; thus, cellular activation and either a systemic or local allergic reaction occurs when exposed to an above-threshold dose (Figure 1C). Although drug desensitization and challenge tests are performed in different situations, challenge tests can theoretically be followed by a desensitization procedure, since administration of the sensitizing drug at an above-threshold dose will accelerate desensitization and will lead to tolerance to the subsequent administration of a threshold or higher dose of the drug.
This article includes speculation and has several limitations. First, we did not perform detailed in vivo analyses of basophil functions during drug desensitization procedures in the clinical setting. Second, we did not test mast cell functions, but we note that precise characterization of ex vivo mast cell desensitization is a very difficult task. In addition, our analyses did not elucidate activation markers such as CD63 or CD203c, or the intracellular signals that induce basophil desensitization, although other researchers are accumulating findings through active scrutiny of human basophils (3, 5–8). Moreover, we do not know the extent to which desensitization induced by exposure to subthreshold allergen is similar or related to that induced by high–dose allergen. The difference between specific and non-specific desensitization is also important and needs to be investigated. Although these limitations exist, based on our daily experience we think that the relationships between desensitization and cell activation during drug dose escalation shown in Figure 1 may reflect the clinical situation.
In separate studies, we found that basophils desensitized by preincubation with subthreshold anti-IgE or anti-FcεRI antibody showed not only a suppressed response to IgE-mediated stimulation, but also enhanced responses to non-IgE-mediated stimulation, i.e., histamine release induced by Ca ionophore A23187 and chemokines, chemotaxis toward eotaxin, and expression of the CD69 surface activation marker (2, 9–11). The latter phenomenon may be out of the scope of this manuscript, and the mechanism has not yet been clarified. However, enhanced responses to non-IgE-mediated stimulation may partly explain the clinical impact of trace–level allergen exposure in patients with chronic allergic diseases, and the nature and clinical implication need to be investigated.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Teikyo University Ethical Review Board. The patients/participants provided their written informed consent to participate in this study.
MY, AK, MS, RK, and MI performed the in vitro basophil studies. MY, AK, MS, RK, MI, and HN performed the tests in clinical settings. MY and RS wrote the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Drs. Koichi Hirai and Ken Ohta for directing the budding projects, Ms. Miki Mori for her excellent technical assistance, and Ms. Yasuko Asada for her secretarial help.
1. Broyles AD, Banerji A, Castells M. Practical guidance for the evaluation and management of drug hypersensitivity: general concepts. J Allergy Clin Immunol Pract. (2020) 8:S3–15. doi: 10.1016/j.jaip.2020.08.002
2. Komiya A, Hirai K, Iikura M, Nagase H, Yamada H, Miyamasu M, et al. Induction of basophil desensitization in physiological medium: enhancement after IgE-dependent upregulation of surface IgE binding on basophils. Int Arch Allergy Immunol. (2003) 130:40–50. doi: 10.1159/000068374
3. Mendoza GR, Minagawa K. Subthreshold and suboptimal desensitization of human basophils. II. Nonspecificity and irreversibility of desensitization. Int Arch Allergy Appl Immunol. (1982) 69:282–4. doi: 10.1159/000233185
4. Yamaguchi M. Management of drug allergy – an update. Arerugi. (2021) 70:75–9. doi: 10.15036/arerugi.70.75
5. Dispenza MC, Bochner BS, MacGlashan DW Jr. Targeting the FcεRI pathway as a potential strategy to prevent food-induced anaphylaxis. Front Immunol. (2020). 11:614402. doi: 10.3389/fimmu.2020.614402
6. MacGlashan D Jr. Subthreshold desensitization of human basophils re-capitulates the loss of Syk and FcεRI expression characterized by other methods of desensitization. Clin Exp Allergy. (2012). 42:1060–70. doi: 10.1111/j.1365-2222.2012.04013.x
7. MacGlashan D Jr, Undem BJ. Inducing an anergic state in mast cells and basophils without secretion. J Allergy Clin Immunol. (2008) 121:1500–6. doi: 10.1016/j.jaci.2008.04.019
8. Oka T, Rios EJ, Tsai M, Kalesnikoff J, Galli SJ. Rapid desensitization induces internalization of antigen-specific IgE on mouse mast cells. J Allergy Clin Immunol. (2013) 132:922–32. doi: 10.1016/j.jaci.2013.05.004
9. Suzukawa M, Hirai K, Iikura M, Nagase H, Komiya A, Yoshimura-Uchiyama C, et al. IgE- and FcεRI-mediated migration of human basophils. Int Immunol. (2005) 17:1249–55. doi: 10.1093/intimm/dxh301
10. Suzukawa M, Komiya A, Yoshimura-Uchiyama C, Kawakami A, Koketsu R, Nagase H, et al. IgE- and FcεRI-mediated enhancement of surface CD69 expression in basophils: role of low-level stimulation. Int Arch Allergy Immunol. (2007) 143(Suppl 1):56–9. doi: 10.1159/000101406
Keywords: challenge test, basophil, anaphylaxis, IgE, desensitization, cell activation
Citation: Yamaguchi M, Komiya A, Suzukawa M, Koketsu R, Shiragami R, Iikura M and Nagase H (2022) Findings of in vitro Analyses of Basophil Functions May Help Us Better Understand Drug Desensitization. Front. Allergy 3:874772. doi: 10.3389/falgy.2022.874772
Received: 13 February 2022; Accepted: 14 March 2022;
Published: 30 March 2022.
Edited by:
Renan Rangel Bonamigo, Federal University of Rio Grande do Sul, BrazilReviewed by:
Scott P. Commins, University of North Carolina at Chapel Hill, United StatesCopyright © 2022 Yamaguchi, Komiya, Suzukawa, Koketsu, Shiragami, Iikura and Nagase. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masao Yamaguchi, bXlhbWFAbWVkLnRlaWt5by11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.