- 1Comparative Medicine, The Interuniversity Messerli Research Institute, University of Veterinary Medicine Vienna, Medical University Vienna, University of Vienna, Vienna, Austria
- 2Institute of Pathophysiology and Allergy Research, Center of Pathophysiology, Infectiology and Immunology, Medical University of Vienna, Vienna, Austria
Although iron is one of the most abundant elements on earth, about a third of the world's population are affected by iron deficiency. Main drivers of iron deficiency are beside the chronic lack of dietary iron, a hampered uptake machinery as a result of immune activation. Macrophages are the principal cells distributing iron in the human body with their iron restriction skewing these cells to a more pro-inflammatory state. Consequently, iron deficiency has a pronounced impact on immune cells, favoring Th2-cell survival, immunoglobulin class switching and primes mast cells for degranulation. Iron deficiency during pregnancy increases the risk of atopic diseases in children, while both children and adults with allergy are more likely to have anemia. In contrast, an improved iron status seems to protect against allergy development. Here, the most important interconnections between iron metabolism and allergies, the effect of iron deprivation on distinct immune cell types, as well as the pathophysiology in atopic diseases are summarized. Although the main focus will be humans, we also compare them with innate defense and iron sequestration strategies of microbes, given, particularly, attention to catechol-siderophores. Similarly, the defense and nutritional strategies in plants with their inducible systemic acquired resistance by salicylic acid, which further leads to synthesis of flavonoids as well as pathogenesis-related proteins, will be elaborated as both are very important for understanding the etiology of allergic diseases. Many allergens, such as lipocalins and the pathogenesis-related proteins, are able to bind iron and either deprive or supply iron to immune cells. Thus, a locally induced iron deficiency will result in immune activation and allergic sensitization. However, the same proteins such as the whey protein beta-lactoglobulin can also transport this precious micronutrient to the host immune cells (holoBLG) and hinder their activation, promoting tolerance and protecting against allergy. Since 2019, several clinical trials have also been conducted in allergic subjects using holoBLG as a food for special medical purposes, leading to a reduction in the allergic symptom burden. Supplementation with nutrient-carrying lipocalin proteins can circumvent the mucosal block and nourish selectively immune cells, therefore representing a new dietary and causative approach to compensate for functional iron deficiency in allergy sufferers.
Introduction
The ability of iron to act as an electron receptor or donor forms the fundamental basis for its essential role in supporting basic cellular processes, of which oxygen transport via iron-containing heme in hemoglobin is the most well-known (1). As such, iron is not only essential for humans but extends to almost all organisms that we consume (e.g., plants, animals), symbiotically live with as commensal microbes or are pathogenic and infect us.
Although iron is one of the most common elements on earth, about a third of the world's population are affected by iron deficiency, with, predominantly, infants, preschool children, young menstruating women, and women in the second/third trimester of pregnancy and postpartum being affected (2, 3). In western countries, female gender and persons with a vegetarian or vegan diet, blood donors but also elite endurance athletes due to inflammation-induced functional iron deficiency are at greater risk (4).
Besides blood loss, there are two main drivers for iron deficiency, chronic lack of dietary iron, and/or a hampered uptake machinery usually as a result of immune activation. Iron is closely linked with our immune system as the major contributor for systematic iron recycling; shuttling and distribution are the macrophages, which are also key cells in innate immunity, with their iron status determining activation or suppression of the immune machinery.
Many respiratory allergens, such as pathogenesis-related proteins and lipocalins, are able to deprive antigen-presenting cells from iron, thereby initiating presentation and immune activation. Iron deficiency also favors survival of Th2-cells, facilitates antibody class switching, and is also an essential contributor in the effector phase as a lack of iron primes mast cells for degranulation.
In this review, we highlight the most important interconnections between iron metabolism and allergies, the effect of iron deprivation on distinct immune cell types, as well as the pathophysiology in atopic diseases. Although the main focus will be humans, we also compare them with innate defense and iron sequestration strategies of microbes and plants important for the etiology of allergic diseases and give epidemiology, preclinical and clinical evidence for exploiting the iron-immune regulatory axis to combat the atopic march.
Basic Iron Features
Iron is present in our body mainly in the ferrous (Fe2+, acting as an electron donor) or ferric form (Fe3+, an electron acceptor). Under anaerobic conditions, the ferrous form, which preferentially binds to nitrogen and sulfur ligands (5), is favored, whereas, in oxygen-rich environments, ferric iron is the most dominant form. Due to its incredible high affinity to oxygen, “free iron” is biochemically dangerous as it can damage tissue by catalyzing the formation of oxygen radicals that attack cellular membranes, proteins, and DNA (1) (Haber-Weiss reaction). Hence, under healthy conditions, no appreciable concentration of “free iron” is present as iron is virtually always present in a complexed form (e.g., as heme) and/or protein-bound form (e.g., bound to transferrin, lactoferrin, etc.) (6). Moreover, iron uptake is highly regulated with a sophisticated iron-uptake machinery existing not only in humans (7) but also in bacteria (8), fungi, and plants (9), emphasizing that iron acquisition is always an active, regulated process.
Non-Transferrin Bound Iron and the Labile Iron Pool
The non-transferrin bound iron pool (NTBI) represents the presence of iron, not bound by transferrin in the circulation. As such, it comprises the ferric iron-binding proteins lactoferrin and ceruloplasmin, a copper-containing ferroxidase that is essential to export iron out from the tissue to the circulation. It includes members of the lipocalin family, such as LCN1 and LCN2 (10–12), binding to a plethora of iron-siderophore complexes but also to heme as the lipocalin alpha1-microglobulin (13–16). Moreover, heme-binding proteins, such as hemopexin and peroxynitrite isomerase THAP4 (17), as well as haptoglobulin binding to heme-containing hemoglobin and a large number of poorly defined low molecular weight, belong to the NTBI. Known low-molecular weight compounds of the NTBI are ferric iron-binding citric acid, being the major representative here (18) but extending to amino acids, such as glycine and asparagine (19), ATP/AMP, and catecholamines [dopamine (20), norepinephrine (21), and epinephrine (22)]. Dietary-derived catechol flavonoids have also been suggested to be part of the NTBI that partake in iron homeostasis (23).
Intracellularly, iron concentration is about 1 μM but may range from 0.5 to 10 μM (24, 25) and is part of the so-called labile iron pool, LIP, for further incorporation into iron-dependent enzymes and electron transfer proteins, with glutathione acting presumably as a cellular buffer (26). The ferritin H subunit (FTH) oxidizes ferrous to ferric iron for storage within ferritin. Although the ferrous form seems to be intracellular prevalent, endogenous ferric-binding siderophore such as 2,5-dihydroxybenzoic acid (26) also partakes in iron transport and homeostasis (26), with a deficiency here causing intracellular iron accumulation.
Iron Status in the Steady State
The human body contains about 4-to-5-g iron with men having, on average, 50 mg/kg and women about 38 mg/kg. Roughly, two thirds of the total body iron is contained in heme within hemoglobins in red blood cells (27), with the next biggest store being the liver (≈1 g) and the mononuclear phagocyte system (≈0.6 g), in which iron is stored in ferritin (28) as ferrihydrates and in hemosiderin, which is a poorly defined iron-storage complex, presumably composed of ferritin, denatured ferritin, and other materials (29). About 0.3 g of iron in heme is present in the myoglobins of the muscles (30, 31). All other cellular iron-containing proteins and enzymes are estimated to bind a total of about 8 mg of iron.
Dietary Iron Uptake
The daily uptake of iron through food is about 1–2 mg, just as high as the daily loss of iron through desquamation of the enterocytes lining the gut or of the skin and due to smaller bleedings. Iron may leave the body also through urine, bile or sweat, although in considerable smaller and usually neglectable amounts (32–34).
About 10–20 mg iron is consumed daily via the normal diet representing the major iron source in humans, of which a tenth is absorbed. Within the digestive tract, iron is present in two forms: as heme iron (meat, fish) and non-heme iron (cocoa, legumes, cereals, fruits) of which heme-iron uptake is about five times more efficiently absorbed than non-heme iron. Its bioavailability is further determined by the individual iron status and physiological condition and is reflected by the production of hepcidin (35).
The chief area of iron absorption is the duodenum and the proximal jejunum (36), which is more acidic, with a pH ranging from 4 to 5 than the rest of small intestines, with a pH range between 7 and 9. It is also the site where pancreatic juices and bile enter the small intestines.
Heme iron is transported as heme (from meat) into the enterocytes via the known transporter for folate being the high-affinity folate transporter PCP/HCP1 (SLC46A1) (37–39), and also the duodenal cytochrome b; Dcytb is able to bind on the lumen and on the cytoplasmic side to heme molecules (40–44).
For non-heme iron, which is typically ferric iron chelated by low molecular weight compounds (e.g., plants, meat), reduction by ascorbic acid and/or duodenal ferric reductases, such as cytochrome b, Dcytb, STEAP2, and FRRS1 (41, 42), has to precede before uptake via the divalent metal-ion transporter 1, DMT1, and ZIP14 is initiated (44, 45). Iron-carrying proteins, such as lactoferrin (46), transferrin (47), or ferritin from food, are efficiently absorbed without depending on reduction or heme transporter via receptor-mediated, clathrin-dependent endocytosis: ferritin via SCARA5 (48), lactoferrin via ITLN1 (49). Moreover, glycine and asparagine, but not other amino acids (19), promote iron absorption (50) (Figure 1).
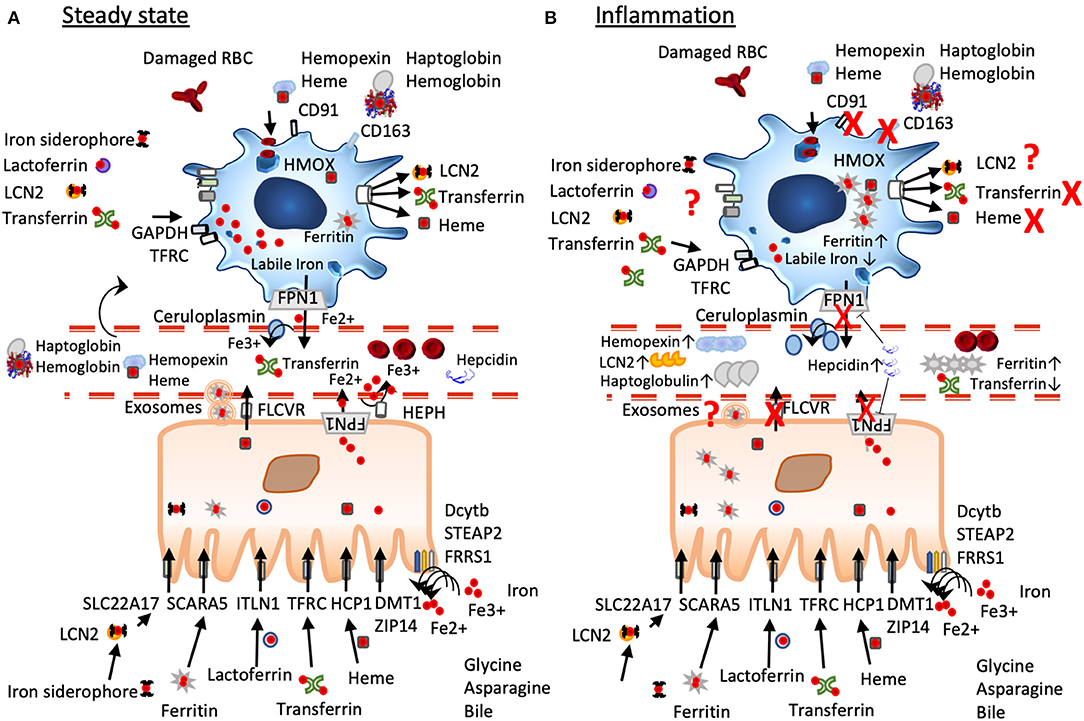
Figure 1. A simplified scheme of iron homeostasis under steady-state and inflammatory conditions. (A) Under non-inflamed steady-state conditions, iron is reduced by ferric reductases (Dcytb, STEAP2, FRRS1) in the intestinal lumen to ferrous iron before import via DMT1 and ZIP14, heme iron is transported via the folate receptor HCP1, lactoferrin via ITLN1, dietary ferritin uptake occurs via SCARA 5, and chelated iron can be captured by LCN2 and transported by the enterocytes via SLC22A17. Cellular iron export occurs via ferroportin often aided by hephaestin and/or ceruloplasmin, ferritin seems to be exported via exosomal pathways, heme is exported via FLCVR. Macrophages under steady state have an anti-inflammatory phenotype characterized by a large labile iron pool, low ferritin-levels, and expression of iron importers such as CD163. They constantly take up but also export iron that derives from damaged red blood cells, from heme-hemopexin, haptoglobin-hemoglobin, LCN2, transferrin, and lactoferrin. (B) Under inflammation, iron mobilization is blocked due to increased expression of hepcidin that leads to FPN degradation and trapping iron inside the cells. Macrophages change to an inflammatory phenotype inhibiting iron import and export, their ferritin-levels are increased, while their labile iron pool is decreased. In the circulation levels of ferritin, hemopexin, haptoglobulin, and lipocalin 2 are elevated, while serum iron and transferrin are decreased.
Iron can also be transported via the lymphatic system, with bile itself contributing to iron absorption (51–53). Newer dietary iron-supplementation formulation encapsules iron [ferrous iron (54)] with a phospholipid bilayer generating a liposomal iron or surround ferric iron in sucrosomes (starchlike vesicles) (55), which leads to uptake of iron via the lymphatic system and circumvent hepcidin-mediated blockage of iron absorption (56).
Once in the cell, iron is exported via the iron exporter ferroportin 1 (IREG1, MTP1, SLC40A1, FPN1, HFE4) (57), often with the help of Hephaestin HEPH or ceruloplasmin CP and is released into the circulation. Ferroportin-mediated iron efflux is calcium activated and functions as an iron/calcium antiporter (58).
Heme iron export occurs via the Feline leukaemic virus receptor (FLVCR) (59, 60), which is also highly expressed in enterocytes, and is dependent on hemopexin (61, 62). Ferritin seems to be exported via exosomes (63) (Figure 1). In general, iron excretion is suppressed by inflammation and enhanced during erythropoiesis and hypoxia (44).
Dietary phytates, representing inositol polyphosphates typically contained in nuts, seeds, and grains, form insoluble precipitates with iron (64) and thus inhibit dietary uptake (65). Similarly, fruit- and plant-derived polyphenolic compounds are known to reduce the bioavailability for non-heme iron as many of these bind with high affinity to iron (66). Upon consumption, flavonoid concentrations in plasma can reach 1–10 μM (67) and thus may considerably influence iron homeostasis (68, 69). Consequently, consumption of large quantities of purified polyphenols has been reported to decrease the volunteers' iron status (70–73). However, when these polyphenols are already in complex with iron, dietary administration of polyphenol-iron complexes had been demonstrated to contribute to an improved iron and redox status in vivo (74, 75).
Iron Regulation
In 2001, hepcidin, which is highly conserved between species and only 25-amino acids long, was discovered as the key regulator for systemic iron homeostasis (76). It is mainly secreted by the liver in response to iron overload or inflammation (77), but, also, parietal cells of the stomach (78) and macrophages synthesize and secrete hepcidin. Under steady state, hepcidin is found in the plasma in a protein-bound and free-circulating form (79), with only the latter being excreted into the urine (80). Reported hepcidin concentration in the circulation is about 7.8 nM in men, 4.1 nM in pre-, and 8.5 nM in post-menopausal women (81). Radiolabeled hepcidin accumulated in the ferroportin-rich organs, liver, spleen, and proximal duodenum (82).
Hepcidin decreases plasma iron levels by blocking iron absorption in the duodenum and iron release from macrophages, thus targeting the two entrance gates for iron into the circulation. Molecularly, it binds to ferroportin (FPN), inducing its internalization, ubiquitinylation, and consecutive degradation of FPN in the lysoproteasome (77), while iron is retained within the cells (81, 83). Hepcidin is also negatively regulated by folic acid, cobalamin, or vitamin D (84).
Under iron-replete conditions, increasing body iron levels cause an increased hepcidin expression, hampering further iron accumulation and acquisition in macrophage and liver cells, and decreased dietary iron absorption; the result is a reduction in serum iron (85). In contrast, when more iron is needed, hepcidin decreases, permitting macrophages to release iron and allowing an enhance uptake of dietary iron via the gut.
As hepcidin is also an acute phase reactant, it is upregulated during inflammation to remove iron from the circulation along with iron-binding proteins, such as lactoferrin, haptoglobulin, hemopexin, lipocalin 2, and ferritin (81, 86). Due to its dual role in iron regulation and inflammation, hepcidin levels in the circulation reflect on the one hand ongoing inflammation as well as the need of iron; consequently, in conditions of severe anemia and inflammation, low hepcidin levels will prevail despite the presence of inflammation (87).
Iron in the Circulation
Iron is then delivered to most tissues via circulating transferrin, which carries roughly 2 mg of this metal in the steady state (88). Hemopexin also seems to partake in distributing dietary heme iron, which accounts for two-thirds of absorbed body iron, as a lack of hemopexin leads to heme accumulation in the enterocyte and impedes heme distribution (89). In healthy men, plasma iron turnover ranges from 25 to 35 mg (90) per day, of which only 5 to 10% is provided by absorption of dietary iron in the gut, the rest being predominantly iron recycled from monocytes and macrophages of the liver, adipose tissue, bone marrow, spleen, and lymph nodes (91). Regarding serum levels, most iron-associated proteins dedicated to distributing and mobilizing iron are increased in situations of greater iron demand such as transferrin, hemopexin, soluble transferrin receptor, and ceruloplasmin (92, 93), while serum iron is low. In contrast, reduced levels of the same proteins in the serum/plasma at steady-state condition usually describe the consequence of an effective iron delivery to the target tissues (e.g., transferrin-iron binding to transferrin receptor 1 CD71, heme-hemopexin complex binding to CD91 expressed on hepatocytes, monocytes, and macrophages in the spleen and liver, haptoglobulin-hemoglobin binding on CD163 expressed on M2-macrophages) and indicate an improved iron status.
In contrast to the widely disturbed transferrin receptor 1 TFRC responsible for iron import via iron-sated transferrin, transferrin receptor 2 (373) (mainly expressed by hepatocytes, erythroid cells, but also by basophils and eosinophils) bind to erythropoietin (94, 372), exert a regulatory function (95) and do not participate in increasing tissue iron. Ablation or mutation of this receptor leads to iron overload (95, 96) in the respected tissue.
Iron Deficiency in Humans
As iron homeostasis is quite complex, there is still no international consensus that clearly defines iron deficiency (97) with the World Health Organization (WHO) defining anemia as circulating hemoglobin (Hb) levels <12. g/dL in non-pregnant women and <13. g/dL in men (98, 99). However, normal Hb distribution varies not only with sex but also with ethnicity and physiological status; thus, recommended adjustment factors are given by the WHO according to, e.g., smoking habits and people living above 1,000-m altitude (100). Ferritin is a good indicator for iron stores, but also, here, adjustments are done (101) and recommended as ferritin is elevated upon infection or inflammation (102). Thus, the assessment of the iron status is not precise, since the available biomarkers reflect the iron status of different compartments in the body: serum ferritin assesses stored iron, while serum iron and the percentage of transferrin saturation reflect the iron supply to tissues. Serum transferrin receptor, erythrocyte ferritin, and red cell zinc protoporphyrin are indicators for the iron supply to the bone marrow, whereas the percentage of hypochromic red blood cells, mean corpuscular volume, and reticulocyte hemoglobin reflect the use of iron by the bone marrow. As these biomarkers are affected by age, sex, disease (infections, inflammation), life style (e.g., blood donations, smoking, drugs, physical fitness), there is currently no single standardized test that can diagnose iron deficiency without anemia, and even the use of multiple tests can only partially overcome the limitations of individual tests, especially because many iron markers are elevated during inflammatory responses or mild immune activation (103).
According to the Global Burden of Disease Study 2016, estimated 1.24 billion individuals are affected by iron deficiency anemia, with the figures for the global prevalence of iron deficiency without anemia being estimated at least double.
Immune activation and iron balance are intertwined, with a change in the iron status always modulating the immunological reactivity. This is reflected in the two main entities of iron deficiency being anemia and “functional iron deficiency.” However, various shades and mixed forms between these two are possible. During functional iron deficiency, iron is not “mobilized,” leading to functional impairments of cells and tissues. Only in severe cases, this results in anemia, which represents the most extreme example of iron deficiency. In mild to moderate cases of iron deficiency, anemia is not present, although the function of tissues and cells is already compromised.
Virtually, every immune activation results in functional iron deficiency (4, 104–108), where, despite sufficient iron stores in the liver and mononuclear phagocyte system (macrophages), iron mobilization is inhibited and dietary iron absorption is decreased by hepcidin, the master regulator of iron uptake. As such, even in healthy adults, iron deficiency is a driver of low-grade chronic inflammation (109).
Persons with functional iron deficiencies usually suffer from underlying chronic or metabolic diseases such as autoimmune (110, 111) and atopic diseases (108, 112–115), chronic kidney diseases (56, 116, 117), congestive heart failure (118–120), chronic pulmonary diseases (121–123), and obesity (124, 125), in which iron deficiency is associated with a worsened prognosis and outcome (103, 104, 126–133). Interestingly, iron deficiency is also associated with an increased risk for thrombosis (134, 135).
Iron Recycling by Macrophages—the Direct Link to our Immune System
As duodenal dietary iron uptake only accounts for 1–2 mg of the daily acquirements, iron is recycled largely through the erythrocyte hemoglobin cycle as the novo synthesis of hemoglobin consumes about 25 mg iron per day. Iron is recycled from senescent red blood cells by macrophages. Recycling occurs predominantly in the spleen by the for this purpose specialised red pulp macrophages and to a lesser degree also Kupfer cells in the liver can recycle iron from red blood cells. Both macrophage-types in the splenic red pulp as well as in the liver have by default an anti-inflammatory phenotype and are critical for maintaining systemic iron concentration (130).
Macrophages are the principal cells responsible for handling iron in mammals, and, thus, any change in the iron status has a direct impact on the innate and, indirectly, on the adaptive immune system.
Macrophages are present in all tissues and classically appreciated for their surveillance role in pathogen recognition. They have crucial homeostatic function, including cell repair, phagocytic clearance of apoptotic and senescent cells, and even cell death. Moreover, in the last decade, their function to support and restore the tissue homeostatic balance, by acting, on the one hand, as sensors for the local iron demands and, on the other hand, providing the local environment with the essential trace element iron, became apparent (130).
Macrophages are sentinels, who are highly plastic, and whole spectra of macrophage subtypes and activation status exist, ranging from an M1-like proinflammatory to an M2-like tissue repair phenotype. Importantly, they markedly differ in their iron handling (136). Indeed, M2 macrophages usually express highly CD163, the hemoglobin/haptoglobin receptor, have low ferritin levels, while having a large labile iron pool LIP, and the iron-export protein, ferroportin FPN, is highly expressed (Figure 2). In contrast, M1 macrophages do not partake in iron sequestration, although they favor an iron storage phenotype having a low LIP, increased ferritin-levels and decreased FPN expression (Figure 2) (126, 137, 138).
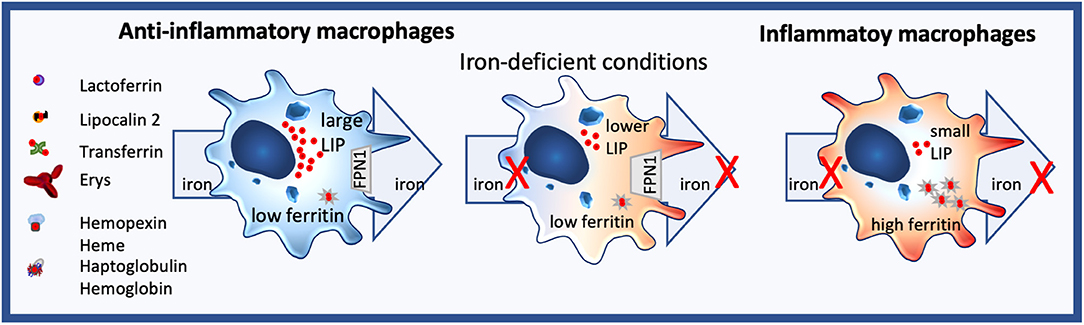
Figure 2. Iron homeostasis in macrophages. Anti-inflammatory macrophages constantly take up but also export iron and are characterized by a large labile iron pool (LIP) and low ferritin levels. In contrast, inflammatory macrophages neither import nor efflux iron, their LIP is small, while ferritin expression is high. Under iron-deficient conditions, no iron can be distributed by anti-inflammatory macrophages, changing their phenotype towards a more inflammatory state.
Of note, in the healthy steady-state conditions, the increased iron uptake by phagocytosis of senescent red blood cells, uptake of hemoglobin (139, 140), hemoglobin-haptoglobin complexes (141, 142), heme-hemopexin (143–145), iron-siderophore laden lipocalin 2 (LCN2) (146–150), iron-laden ferritin (138, 151–155) does not induce inflammation, but, rather, contrarily promotes an anti-inflammatory macrophage phenotype and thus contributes to immune suppression, regulation, and restoration of the tissue homeostatic function as, simultaneously, they serve as iron-rich nurse cells supporting other cells and tissues with iron (148).
In line, macrophage-derived transferrin has been shown to contain already iron and supports lymphocyte proliferation (156).
Atopic Diseases and Micronutrients
The tendency to develop allergies, also called atopy, affects almost one third of the Western population and is partly inherited. Especially in our affluent society, the development of allergy is paradoxically characterized by a lack of contacts and the absence of micronutrients.
On the one hand, the lack of contact with people, animals, and germs leaves the immune system untrained, and, thus, several deficiencies of innate proteins, such as LCN2 (157), lactoferrin (158), uteroglobin (SCGB1A1) (159), Cathelicidin antimicrobial peptide (160), have been described in atopic individuals compared to non-allergic ones, which further underline the lack of microbial contact but also the lack of nutritional support by commensal microbes in atopic individuals.
On the other hand, a lack of micronutrients signals danger to the immune cells and often leads—through this heightened alertness—to an exaggerated immune response, which is such a typical characteristic in individuals with allergy (161, 162). Due to the heightened immune response, patients with atopic diseases also have an increased risk to develop autoimmune diseases (113).
In contrast, studies reveal that the earlier children have contact with other children, as well as animals, the less likely they are suffering from allergies (163). The probability of developing an allergy decreases with the number of siblings and the ownership of pets (164), for example, dogs, and it is proven that regular stays in the immediate vicinity of farms protect against the development of asthma and hay fever (165).
Micronutritional Deficiencies in Atopic Individuals
Especially in the perinatal period, an adequate nutrition is pivotal to avoid an atopic predisposition (166, 167). A plethora of studies affirm that atopics suffer from numerous micronutrient deficiencies (114, 115, 168–180), such as vitamins A (181), E, (182, 183), and D, as well as folic acid and iron (112, 162). Although usually widely overlooked, these micronutrients have a profound impact on our genes and our immune system, resulting in many epigenetic changes affecting immune-associated genes (167, 184), but, most importantly, being also associated with enhanced inflammatory responses.
In respect to epigenetic changes, iron deficiency is known to alter key metabolic and epigenetic pathways, particularly of neural cells, including the phosphorylation of proteins involved in iron sequestration, glutamate metabolism, and histone methylation (185–187); also, liver hepcidin expression, as well as the liver BMP-SMAD signaling pathway, is suppressed by microRNA (188, 189); however, no significant differences in circulating microRNAs between iron-deficient and -replete persons have been observed (190), although some seem to participate in iron homeostatic events (191).
Vitamin A/D and iron homeostasis are very closely linked, making it difficult to distinguish the individual contributions of each micronutrient. For example, vitamin A promotes regulatory T cells (192) but also impacts macrophages and is a known contributor for iron mobilization (193) and—uptake (194), whereas deficiencies of both iron and vitamin A are associated with inflammation (195, 196).
Similarly, iron is also essential for vitamin D synthesis (197), so that people with iron deficiency usually have vitamin D deficiency too (198, 199), which likewise is linked to inflammation (200).
Preventive Diets
Regardless of the inadequate exposure of atopic individuals with people, animals, and microbes, the “right diet” can also prevent or alleviate allergic disease. The 2021 GINA (371) guideline recommends micronutrient intake in the form of fruits and vegetables not only to prevent asthma but also to improve asthma control and reduce the risk of exacerbation (Evidence A) (201). Among foods, milk and, here, in particular, the whey protein content appears to reduce the risk of atopy (atopic dermatitis, rhinitis, asthma) (202–204), and this association has been shown, especially for drinking unprocessed raw milk. Indeed, even allergic children could tolerate raw milk better than pasteurized shop milk, showing less allergic symptoms upon drinking raw milk in a human pilot study (205). The atopy preventive effect of milk correlates with the amount of whey proteins present in the milk (206, 207) and is lost by thermal treatment (204, 208).
The whey protein content in the milk is highest in summer when the animals are kept on pastures and is lower in winter (209, 210). Grazing also strongly affects the iron as well as polyphenol content in milk, which has, indeed, higher antioxidant properties than vitamin C or E (211). The polyphenol content in milk depends on the forage composition and ranges from 3.7 to 35.8 g per-liter milk (212, 213), whereas reported iron concentrations vary from 57 μg (214) to 1,500 μg per liter (215), which correspond to roughly 1–26 μM iron per-liter milk.
Due to the loss of the heat-sensitive protective factors in whey, the ultra-high temperature UHT milk usually offered today does not prevent atopy. In this regard, it is remarkable that the main component of the whey is the heat-sensitive beta-lactoglobulin (BLG) (216) with constitutes 50–60% of all whey proteins, from which we show that it has a tolerogenic effect when loaded with micronutrients.
BLG is a known binder of many polyphenols [catechins (217, 218)], quercetin (219, 220), luteolin (221), rutin (220), etc., which increases the anti-oxidant activity of BLG (218, 222, 223) and leads to enhanced intestinal uptake of these polyphenols (224). Concurrently, depletion of BLG reduces the antioxidant activities of milk by 50%, and, also, heating (that destroys BLG) reduces the antioxidant activity (225, 226), while purified BLG is only considered a mild antioxidant (225).
Similarly, there are numerous reports showing the iron-binding abilities of BLG (222, 224, 227, 228) as the major component in whey (229) improve iron absorption (230–233).
Milk processing such as pasteurization has been shown to cause aggregation of whey proteins (216) to impair the ligand-binding capacity of BLG—shown with ligands such as retinol and palmitic acid (234), while, at the same time, its antigenicity increases (234). Milk processing has also been described to decrease copper and iron content (235) in milk.
Epidemiology and Clinical Evidence of Iron Deficiency in Atopic Diseases
With regard to iron deficiency and atopic diseases, large epidemiology consistently demonstrated that children with allergies have an up to eight-fold greater risk of developing iron deficiency anemia than children without allergies (112, 114). The greater anemic risk in allergic children is clinically relevant as iron deficiency during the years of growth not only causes fatigue and anemia but also affects the small intestinal function and cognitive development (attention, sensory perception, emotions, intelligence). Physicians caring for children with atopic diseases should clarify in their current practice whether fatigue is due to sleep loss caused by atopic dermatitis or asthma or whether an undiagnosed anemia is present.
Iron deficiency can be “inherited” as the nutritional state of the mother is passed to the child. As such, the iron status of pregnant women already predetermines the later allergy risk of children. Several studies demonstrated that a good iron status of the expectant mothers lowered the risk of children of developing atopic dermatitis or asthma (172, 176, 236, 237). Low maternal hemoglobin levels are also associated with increased IgE antibody levels and lower lung volume in the child. Higher maternal transferrin concentrations during pregnancy, reflecting a lower iron status, were associated with an increased risk of a child's physician-diagnosed inhalant allergy (238). In an Italian study, supplementing mothers with iron and folic acid during their pregnancy compared to women without nutrient supplementation reduced the risk of their children developing atopic dermatitis by the age of 6 years by 80% (176). An inverse association was also illustrated between cord blood iron levels (173) right after delivery and the development of atopic urticaria, infantile eosinophilia, and wheeze at 4 years of age (172, 173).
Even in adults, the anemia risk is pertained in allergic individuals. A Korean study analyzing health insurance records from the health care system revealed that men with allergies had a 3.5-fold higher risk of being anemic than non-allergic men, while, in women, this difference was only about half as large (115). A possible explanation for this gender discrepancy could be the natural fluctuations in women's iron status, which often change due to menstrual cycles, pregnancies, and contraceptive methods (copper IUD), as well as due to the general greater tendency for iron deficiency in women to be left untreated, even in the absence of allergies.
By the same token, patients with anemic diseases are also more likely to develop atopic diseases and asthma. Elevated IgE is a common phenomenon observed in anemic patients, which is not related to parasitic infestations (239). Patients with chronic, even life-threatening anemia as with beta-thalassemia major (Cooley's anemia)—having impaired hemoglobin synthesis, which is often accompanied by enlarged spleens, livers and hearts—are more likely to have atopic diseases (240, 241) and suffer from asthma (241–244). Similarly, also subjects with atopic dermatitis have a greater risk to suffer from coronary heart disease, angina, peripheral artery disease, and anemia (245).
Summing up, the studies provide evidence that, indeed, atopy and iron deficiency are interconnected, making anemia more common in allergic people than in non-allergic individuals.
Immune Cells Under Iron-Deficient Conditions
Neutrophils, Natural Killer Cells, and Macrophages—Lower ROS Formation, Despite Increased Activity
Neutrophils, monocytes/macrophages (246, 247) and NK cells (248) use iron to combat pathogens. During intracellular infection, they release iron-loaded lactoferrin into their phagocytic vacuoles where ferrous iron functions as a catalyst of the Haber-Weiss reaction, generating reactive oxygen species (ROS) (249). Hence, under iron-deficient conditions, ROS formation and microbicidal killing are impaired.
As macrophages also are the principal cells for iron distribution, iron-deficient conditions hamper their iron-distribution capability, shifting the macrophage toward a more pro-inflammatory phenotype. Consequently, nutritional iron deficiency has been implicated in low-grad inflammation (250) and shifting of monocytes to a more inflammatory state in children (251) and infants (252) (Figure 2).
Lymphocytes–Survival Advantage for Th2 Cells
An important aspect of iron deficiency is that the decrease in red blood cells is often accompanied by an increase of the white blood cell population, in which particularly the lymphocytic population is significantly increased (253). Within the lymphocytes, however, particularly CD4+ cells and the CD4/CD8 ratio is reduced (253, 254).
Iron chelation inhibits T cell proliferation, as T cell activation leads to expression of TfR1 for iron uptake. As such, iron chelation partake in apoptosis induction of proliferating, activated T-lymphocytes, but not of resting peripheral blood lymphocytes or granulocytes (255). Besides iron-uptake via transferrin, also, active uptake of oligomeric ferric citrate has been reported for T cells (256, 257). T lymphocytes also actively modulate the NTBI pool by uptake and export, with T cell deficiency associated with iron accumulation in the liver and pancreas (258).
The acidity of lysosomes also seems to partake in iron homeostasis and cell proliferation. Under lysosomal pH augmentation, cellular iron via TfR1 is impaired, decreasing cellular viability and proliferation, whereas iron supplementation by augmenting the NTBI pool bypasses the need for functional and acidic lysosomes and rescues cellular viability and proliferation in T cells (259).
In regard, to T cell subtypes, particularly, inflammation-associated Th1 cells are sensitive to iron-deficient conditions (260) as iron regulates the IFN-gamma/STAT1 signaling pathway (261).
Iron import into T cells seems also to affect T cell polarization, as import of iron via iron-siderophore-laden LCN2 has been demonstrated to suppress TH17 polarization in a vasculitis model (262).
In contrast, patients with iron overload have relative lower numbers of CD3 + T cells, while their percentage of regulatory T (Treg) cells and the ratio of CD4/CD8 seemed increased (263).
Th2 clones exhibit larger chelatable iron pools than Th1 clones and are less affected by deferoxamine treatment or TfR1 blocking (264), resulting in a survival advantage of Th2 cells under iron-deficient conditions (260, 265, 266) (Figure 3). Consequently, iron deficiency prones the system toward Th2 (267), induces splenomegaly in mice (268), and induces increased IL-4 secretion in the supernatants of anti–CD3-treated splenocytes compared to controls (268).
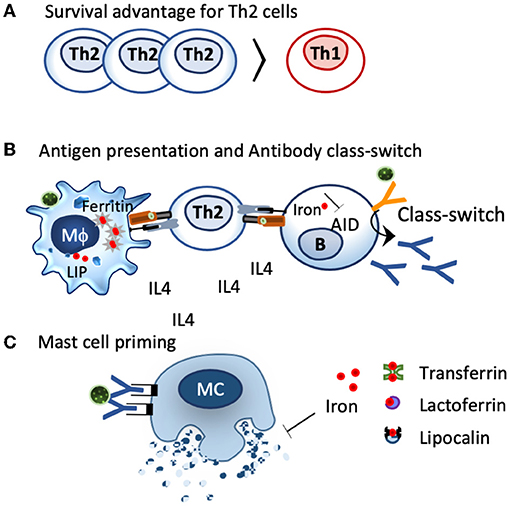
Figure 3. Impact of iron deficiency on immune cells. (A). Th2 cells characterized by IL4 secretion have a greater chelatable iron pool compared to Th1 cells and have a survival advantage under iron-deficient conditions. (B). Iron-deficient conditions modulate iron handling in macrophages and shift them towards a more activated, inflammatory status, which facilates antigen presentation. The activation-induced cytidine deaminase (AID), an enzyme responsible for class switch and affinity maturation, is repressed by iron. Iron-deficient conditions favor AID activation and class switch. (C) Local iron deprivation induces mast cell degranulation, whereas iron repletion by transferrin, lactoferrin, and lipocalins suppresses their activation.
Similarly, also in humans, iron deficiency per se generates a Th2 environment. In the seminal African study, which examined the immune status of children with or without iron deficiency, a marked elevation of the Th2 mediator interleukin 4 was also seen in children with iron deficiency, but not in iron-repleted children (269).
As such, under iron-deficient conditions, a Th2 environment is evidently created, which is the basic prerequisite for allergic sensitization (Figure 3).
B Cells—Promotion of Antibody Class Switch and Affinity Maturation
Iron deficiency also affects antibody-producing B cells, as the enzyme responsible for antibody class switching and affinity maturation, the activation-induced cytidine deaminase, AID, is activated under iron-deficient conditions, while ferrous iron specifically inhibits this enzyme (270). In line, a lack of iron impairs in B cells adequate transfer of ferrous iron to the protoporphyrin IX in the mitochondria, thereby hampering heme synthesis and maintaining Bach2 activation (271), an essential transcription factor not only for class switching and affinity maturation but also an important regulator for T reg differentiation and the macrophage function (272).
In line, iron fortification of Vietnamese school children, but not deworming strategies, significantly improved hemoglobin, serum ferritin, and led to a significant decrease in the measured IgE-levels (239), with another study also reporting a decline in antibodies upon iron fortification in women (273). In contrast, decreased hemoglobin levels due to autoimmune hemolytic anemia, in which antibodies attack red blood cells (274), or because of infections (275) such as plasmodium falciparum malaria, digesting hemoglobin of the red blood cells (leading to anemia), are correlated with increased IgE-levels and severity (276).
The corollary of iron deficiency is, therefore, an antibody class switch toward IgE as iron deficiency simultaneously promotes a Th2 environment (Figure 3).
Mast Cells—Ready to Burst
Mast cells, the main contributor for immediate allergic reactions, are particularly sensitive to iron deprivation. In these cells, intradermal application of the iron binder desferrioxamine, an iron chelator used in the clinics against iron overload, depletes the tissue and the resident mast cells of iron, resulting in histamine release and wheal formation (277). The iron binder is so effective that there have been endeavors to use the iron binder desferrioxamine instead of histamine as a positive control in skin tests. Reversely, iron delivery through transferrin, lactoferrin, or even iron-loaded beta-lactoglobulin (holoBLG) inhibits mast cell activation (12, 278–281) (Figure 3).
Interestingly, mast cells may also be involved in Th2-associated alopecia with an iron-restricted diet, resulting in hair loss in a murine model using IL10-deficient mice (282).
All in all, the degree of iron under- or oversupply seems to contribute directly to the reactivity of mast cells and, therefore, also on the symptom burden of allergic sufferers.
Sequestration Strategies and Defense Mechanisms in Microbes and Plants
Common Concepts in Bacteria and Fungi and Plants
Most bacteria and fungi require iron for their growth. In contrast to humans, in which iron is stored and transported predominantly within proteins, a very large pool of iron is present in bacteria (283) and fungi (284) in chelated form by low molecular compounds, with iron stored mainly in vacuoles and not within ferritin. Also, plants store iron in vacuoles and ferritin, although the distribution here varies with the type and development stage of the plant.
Bacterial and Fungal Iron Acquisition Strategy
Bacteria and fungi such as Alternaria alternata thus usually have two types of siderophores: internal siderophores, such as fungal ferricrocin (285), and siderophores that are excreted such as coprogen for acquisition of environmental iron. Intracellular siderophores have been described to serve for iron storage and being involved in sporulation. In contrast, bacteria and fungi use exogenous siderophores, but also xenosiderophores, synthesized by other microorganisms, to acquire environmental iron as some microorganisms do not produce siderophores (286). The feeding with xenosiderophores is a widely used approach in bioassays in order to demonstrate their growth-promoting activity, and cross feeding is a widely observed feature of the microbial world (287) but also seems to extend to the host. Commensal bacteria such as Bacteroides fragilis have been reported to contribute to iron homeostasis of macrophage and be capable to modulate the immune response of macrophage (288). Siderophores may contribute thus in the nutritional provision of iron; in some cases, also binding to other metals such as copper, manganese, and zinc has been described, not only to support the microbial community, but that of the host too.
Indication for that exists in murine models in which the use of broad-spectrum antibiotics resulted in anemia and an altered immune homeostasis with diminished granulocytes and B cells (289), with fecal microbiota transfer partly reverting the hematopoietic changes (290). Antibiotic treatment also aggravated atopic dermatitis in a murine model (291, 292). In line, it is well established that individuals with atopic diseases (rhinitis, asthma, dermatitis, food allergy) have a reduced microbial (fungal and bacterial) diversity (108, 293–303), which may result in a diminished nutritional support by the commensal microbiota. The microbiota strongly manipulates the immune system. The composition and localization of the commensal microbiota in allergics may thus directly impact the homeostatic iron status of the host, but more studies here need to be done.
Bacteria use numerous iron uptake pathways, which include iron uptake from transferrin, ferritin, lactoferrin, siderophores, or heme. All of these uptake pathways require an active transport, although not all bacteria have all systems; e.g., Listeria monocytogenes, a facultative intracellular pathogen, can acquire iron through transferrin, lactoferrin, ferritin, and hemoglobin, but does not secrete any siderophores. Rather, it can use several hydroxamate (ferrichrome, ferrichrome A and ferrioxamine B) and catecholate (enterobactin and corynebactin) siderophores from other organisms, and it can use additional iron-binding compounds, such as hosts' catecholamines (304), gram-negative bacteria Neisseria spp., can acquire ferric iron directly from lactoferrin and serum transferrin via the TbpA/TbpB receptor (305, 306), and many bacteria exploit heme iron as a nutritional source (307) by secreting extracellular heme-binding proteins such as HasA (gram negative) and NEAT (gram positive) hemophores that either recognize heme and/or the host hemoproteins, such as hemoglobin, hemoglobin–haptoglobin and heme-hemopexin via HxuA hemophores (306, 308) to sequester and translocate iron into their cytoplasm (309).
Iron Chelators: Siderophores and Flavonoids
Animals and humans provide a particularly low-iron habitat for bacteria and fungi. Consequently, siderophore production and access do play crucial roles in determining the course of an infection.
Siderophores are ferric iron–chelating molecules with very high ferric-ion association constants (1020-1049 M−1), which effectively remove iron from the host's iron–protein complexes. They are usually classified by their chemical moieties used to chelate the ferric iron, which are catechol-, hydroxamate or α-hydroxycarboxylate- moieties (Figure 4), but also mixed forms exist (162). Dependent on the moiety and the rest of the structure as well as salt type, ionic strength and temperature, there exist optimal pH-ranges for the respected siderophore types, with ferric iron usually complexed in an octahedral hexadental arrangement. Although dependent on the specific conditions, tris- and bis-catechol -ferric complexes possess some of the highest known stability constants of metal-ligand chelates, with the pH required to establish these bis- and tris-complexes being typically reported to be above pH 7 (310). In contrast, hydroxamates (311) usually have a wide roptimal pH range from 4 to 9, and described optimal chelation conditions for alpha-hydroxycarboxylates usually lie within the pH of 5–7 (66).
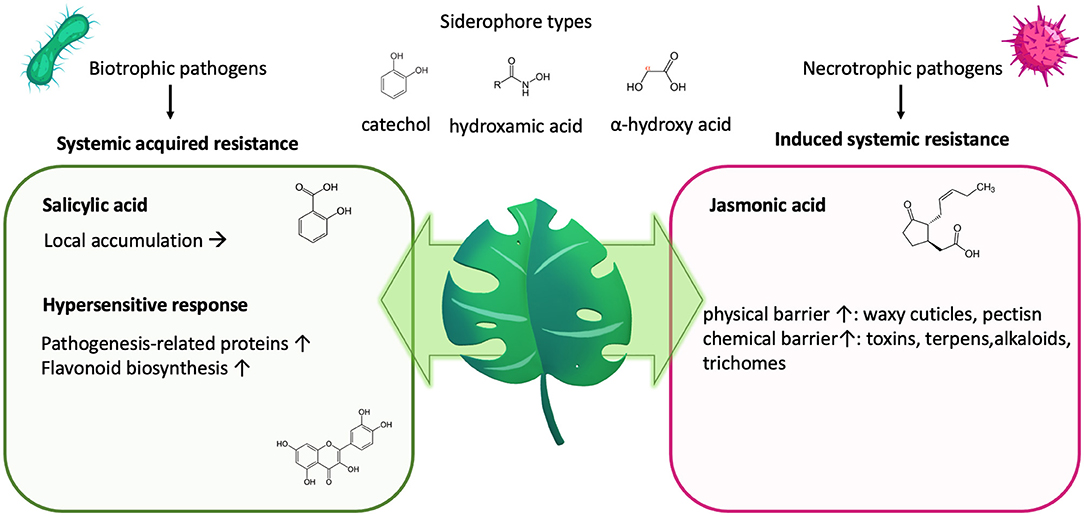
Figure 4. Plant defense and nutrition. Plants will impede biotrophic pathogens, releasing siderophores to sequester iron by initiating a local “hypersensitive response” as part of their “systemic acquired resistance.” This activates the salicylic acid, leading to its accumulation on site and the synthesis of pathogenesis-related proteins and polyphenols/flavonoids. Both can impede nutrient deprivation by the invading pathogen. In contrast, induced systemic resistance counter regulates the systemic-acquired resistance but leads to fortification of the physical and chemical barrier.
Generally, siderophore production is downregulated at low pH and upregulated with high pH (312).
Siderophores anti-oxidative and anti-inflammatory properties are widely acknowledged (313) as they can impede ROS formation.
As the biosynthesis of siderophores needs energy in form of carbon sources and ATP, it determines with the microbial growth rate, which kind of population will colonize a low-iron habitat. Microorganisms that continuously produce siderophores are unknown in nature. Similarly, siderophore production in fungi starts just after germination from conidiospores and are contained in the spore wall material, which is released during germination (314).
As secondary metabolites siderophores are generally defined for not being directly involved in the growth, development, and reproduction of the organisms, but mediate ecological interactions, which may produce a selective advantage for the microbes or plants. As such, microbial siderophores usually belong to the class of nonribosomal peptides (315) and/or polyketides (316), from which a number of very powerful medicinal products are known for, ranging from antibiotics (e.g., vancomycin) to immunosuppressive drugs, such as ciclosporin.
Similarly, many fruits and plants synthesize phenolics/polyphenols/flavonoids with described anti-oxidative and anti-inflammatory attributes, that—as their microbial counterpart—are categorized as secondary metabolites and have a very high affinity to iron due to the presence of catechol structures. For flavonoids, the reported complex stability constants for catechol are 43.7; for quercetin 44.2; and for catechine 47.4 (67) and thus comparable to the iron affinity of microbial siderophores, with the strongest known catechol-siderophore enterobactin having a complex stability constant of 49 at physiological pH (317).
Of note, many flavonoids-binding iron such as luteolin (318), apigenin, quercetin (319), catechin, rutin, naringenin, fisetin (320), and epicatechin have been attributed an anti-allergic activity in vitro and in in vivo models (321, 322). With a double-blind, placebo-controlled study using topical cream containing vitamin E, epigallocatechin gallate and grape seed procyanidins improving atopic dermatitis (323), and O-methylated catechins reducing symptoms of Japanese cedar pollinosis (324).
Plant Defense and Iron Availability
Iron availability is dictated by the soil redox potential and pH. In soils that are aerobic or of higher pH, iron is readily oxidized, and is predominately in the form of insoluble ferric oxides. At lower pH, the ferric iron is freed from the oxide and becomes more available for uptake by roots. Because 30% of the world's cropland is too alkaline for optimal plant growth (e.g., calcareous soils in which the addition of lime increases the pH), graminaceous plants (grasses, cereals, and rice) secrete phytosiderophores (e.g., mugeneic acid), but also chemical compounds with catechol moieties have been described such as fraxetin (325), which are released into the soil to sequester iron (326).
Importantly, similarly than in the mammalian system, iron deficiency alone has been demonstrated to be enough to prime the plant immune response (327) and activate flavonoid (328, 329) and phytosiderophore synthesis (330).
Plants will impede pathogens by increasing their resistance via “induced systemic resistance” (Figure 4), which involves the synthesis of jasmonic acid and ethylene and leads to an increase of the physical or chemical barrier of the host plant (331). Simultaneously, upon infection, also, “systemic acquired resistance “is initiated, which is analogous to our innate immune system and mediated by synthesis of salicylic acid, leading to its accumulation, but also to the transcription of a wide range of “pathogenesis-related” proteins (332–334) as well as the synthesis of flavonoids (328, 335, 336) (Figure 4). Both pathways counter regulate each other, with salicylic acid inhibiting jasmonic acid signaling (336).
In response to pathogens, the salicylic acid pathway elicits a rapid local reaction or “hypersensitive response” to limit the area of infection for biotrophic pathogens, which require living tissue to gain nutrients. In the case of necrotrophic pathogens, hypersensitive response might even be beneficial to the pathogen, as they require dead plant cells to obtain nutrients.
Strikingly, many major allergens are derived from these pathogenesis-related protein families that are induced by the plants to prevent nutritional deprivation (337, 338).
Also, beneficial root-associated mutualistic microbes living in the rhizosphere, like bacteria and fungi, besides impacting on plant nutrition and growth, can further boost plant defenses, rendering the entire plant more resistant to pathogens (339). These beneficial microbes secrete siderophores to facilitate plant iron acquisition with ectorhizosphere and rhizoplane bacteria described to release predominantly hydroxamate-type siderophores, whereas endophytic bacteria rather producing catechol-type siderophores (340) for plant uptake. Interestingly, several different bacterial genera, especially in plant-growth-promoting rhizobacteria, synthesize salicylic acid, the key compound of the systemic acquired resistance in plants, to ultimately incorporate them into catechol-based siderophores (341).
Importantly, although a mutualistic relationship between hosts and microbial siderophores exists, at the same time, not only a competition between excreted siderophores for the metal but also for capturing these iron-siderophore complexes is always prevalent.
Allergens or Tolerogens: the Role of Proteins Carrying Micronutrients
Only a few protein families are capable to become allergens under physiological conditions; thus, virtually, all major allergens of animal origin belong to the lipocalin family, specifically in the lipocalin subfamily of “retinoic acid-binding proteins” (11, 342) and a considerable part of the major respiratory allergens of plant origin belongs to the pathogenesis-related-10 (PR-10) protein family10 or originates from the prolamin (2S albumin, lipid-binding proteins, LTPs) and cupin (7S, 11S) superfamilies (216, 343).
Apart from belonging either to animal or plant allergen families, they do have several features in common with the most essential one, that these proteins belong to the innate defense system in the respected animals/plants. They, therefore, possess an inherent affinity to our immune system, and their uptake occurs mostly receptor mediated and via the lymphatic system. The described allergen families have “pockets” in which they can very effectively bind and transport micronutrients, such as iron complexes, fatty acids (344), flavonoids (217–221) or vitamins (10, 281, 345–348). In this way, they can deprive pathogens of nutrients or, conversely, provide nutrients to the immune cells.
As such, many major allergens are capable to bind to flavonoids with known iron-binding capacity, making them nutrient binders. Consequently, the natural ligand of the pathogenesis-related PR-10 proteins major birch pollen allergen Bet v 1 has been identified as quercetin-3-O-sophoroside (349); for the major hazelnut allergen Cor a 1, being quercetin-3-O-(2″-O-β-D-glucopyranosyl)-β-D-galactopyranoside (350), and also Fra a 1 and Fra a 3 have been crystalized with catechin ligands (351). Also, other major allergens from peanuts have been well investigated with Ara h 2 and Ara h 6, belonging to the 2S family, binding to the flavonoid epigallocatechin-3-gallate (352), Ara h8 binding to quercetin, (353) as well as epicatechin (354) and Ara h 1 from the 7S family, forming large complexes by binding to proanthocyanidins, which are oligomers, consisting of catechin and epicatechin and their gallic acid esters (355).
Mammalian lipocalin allergens closely resemble endogenous human lipocalin proteins, such as Lipocalin-2, LCN2 (11, 157), a natural acute phase defense proteins that binds environmental iron and can deliver this iron directly and a receptor-mediated to immune cells (157, 162). They are usually excreted and thus are found in the dander, urine, fur, and saliva of animals (356). LCN2 is involved in numerous iron-dependent processes of the innate immune arm and is also critical to renal development. Iron transport by lipocalins requires the presence of a siderophore, since lipocalins usually have no measurable affinity for iron alone (357). Consequently, LCN2 binds only to iron chelated by siderophores, thereby being also microbicidal. Simultaneously, it acts as an immune regulator as the iron-containing form of LCN2 (holoLCN2) increases the intracellular iron content of macrophages, while the iron-free form decreases the intracellular iron content (358). Thus, raising of the labile iron pool content by iron-loaded LCN2 form promotes the development of anti-inflammatory cells (359–361), while the lowering of their intracellular iron content causes their activation. Importantly, LCN2 is able to activate or suppress the immune cells—dependent on the nutritional supply it provides.
Due to its resemblance to lipocalin 2, mammalian lipocalins, such as the bovine beta-lactoglobulin BLG, are similarly taken up via the lymphatic system (216, 362); in a receptor-mediated fashion and via this route, their ligands will predominantly transport to the residing immune cells. It can even reach the lactal system of nursing mothers and serves as a marker for maternal dietary proteins in breast milk as it is not naturally present in human milk (363). In a series of studies exploiting the lymphatic pathway for targeted micronutritional supply of iron (10, 12, 281), zinc (281), and vitamins (346) by BLG, we provided evidence that micronutrients were transported to immune cells, and that this nutritional supply was accompanied with the establishment of immune resilience in an allergen-independent fashion (12, 348) in a prophylactic setting, as well as in already sensitized mice, this leads to a significant reduction of the symptom burden upon allergen challenge (281).
Our studies, but also these of others (364, 365), have demonstrated that, in the absence of micronutrients, particularly of iron, proteins of the innate defense arm in mammals and plants in their apo-(empty) form are able to elicit a Th2 response in vitro and in vivo (10, 12, 346, 347) as an encounter of these proteins in an “empty” form with our immune system enables them to locally deplete these cells from iron or vitamins, thereby triggering a danger signal and evoking an immune response. In contrast, when these proteins carry micronutrients and are present as holo-(loaded) proteins, they contribute to the nutritional balance of the immune cell and actively contribute to tolerance development (10, 12, 162, 281, 345–348).
Thus, upon contact with the holo-proteins, the immune nutritional balance is not disturbed, enabling the establishment of immune resilience (12), which protects against atopy.
In situations of infections or inflammation, which requires an increased micronutritional supply, or when nutritional deficiencies are already prevalent, apo-proteins can bind to micronutrients, further aggravating the micronutritional deficiency present in these cells, which not only activates these immune cells but also results that exogenous innate defense proteins are recognized as a threat and turn into allergens.
Clinical Studies: Balancing Micronutrient Requirements as a Strategy to Ameliorate Allergic Diseases
Based on the preclinical studies, we sought clinical translation of our research efforts and combined the whey protein BLG with catechines, iron, zinc, and vitamin A into a lozenge (holoBLG lozenge) to be used as a food for special medical purposes (FSMP). The ultimate objective was to investigate in clinical studies whether, indeed, the targeted transport of micronutrients to immune cells by holoBLG was effective and could have an influence on immune cell reactivity and the allergic symptom load in allergic individuals.
Of note, the amount of iron included in the lozenge is with <1 mg/lozenge rather low, and, therefore, the lozenge cannot be considered as an iron supplement per se, but it does contain iron in a form that enables transport by BLG via the lymph and is roughly equivalent to the estimated daily iron requirement of human leukocytes.
In the 2019 and 2020 conducted double-blind, placebo-controlled clinical trial with women allergic to birch and/or grass pollen allergy, 6-month supplementation with holo-BLG lozenge resulted in a total nasal symptom score (TNSS) improvement after nasal provocation by 42% after, compared with 13% in the placebo group. The combined symptom-medication score, considered the gold standard of allergen immunotherapy, (366) was in the group, taking the holoBLG lozenges 45% lower in the birch pollen peak season and 40% lower in the grass pollen season compared to the placebo-supplemented study arm. Additionally, blood values improved, and peripheral blood monocytic cells had, compared to the monocytes of the placebo arm, a significant higher labile iron pool (12, 347, 367, 368).
Another clinical study with house dust mite allergic patients was also conducted in 2020, in which the symptoms were objectively assessed and recorded in an allergen exposure chamber before and after 3 months of holoBLG supplementation. Here, holoBLG supplementation resulted in a 60% reduction of the TNSS (369). Moreover, a long-lasting effect was apparent, as even 7 to 8 months later these patients had lower total symptom score and a perceived higher well-being on re-exposure in the allergen exposure chamber, indicating a long-lasting nature of the induced immune resilience (370).
It has to be emphasized that in both atopic cohorts, dietary application of the holoBLG lozenge containing micronutrients, that are dedicated for the immune cell compartments, ameliorated allergic symptoms in a completely allergen-independent manner.
Further studies are currently being conducted with cat allergic patients to investigate in other atopic cohorts, whether compensating micronutritional deficiencies in the immune cell compartments is a further causal strategy to support immune resilience in an allergen-independent manner.
Discussion
Iron is a trace element essential for nearly every organism and needed for oxygen transport, cellular respiration, but also contributing in immune regulation. Its access is tightly controlled due to its high affinity for oxygen, requiring that iron always has to be present in a complexed and/or protein-bound form; otherwise, reactive oxygen species are generated with detrimental effects.
Here, we collected evidences that functional iron deficiency not only promotes allergy development but also increases the clinical symptom burden in allergic patients.
Atopic individuals lack—besides Vitamin A and D—iron, which profoundly affects our immune system as deficiencies here render our cells hyper-sensitive.
The dual role of macrophages as the central hub for iron handling but also as a major contributor in immunity has the consequence that iron deficiency directly impacts these cells and shifts them under iron poor conditions to a more inflammatory phenotype.
Iron deficiency is sufficient to create a Th2-milieu to favor affinity maturation and antibody class switching and to prime mast cells for degranulation. Consequently, iron deficiency sets the whole body on alert.
Although this a very desired response to infections, it also turns, otherwise, harmless proteins to allergens.
Indeed, comparing the defense system in the plant with ours is particularly revealing as, here, it becomes apparent how intricate nutrition and defense are intertwined and that stealing and sharing often go hand in hand. On the one hand, the biotrophic pathogen needs its nutrients from the host and secretes anti-inflammatory siderophores, and its attack is being counteracted by pathogenesis-related proteins, hindering nutritional retrieval. On the other hand, microbes synthesize their siderophores from salicylic acid and share the nutrients bound by siderophores with their host, thereby promoting the growth and health of the plant. Similarly, interactions can be assumed in humans with uptake of flavonoids being well-documented, but also the commensal microbial communities will participate in the nutritional provision of the human host, with the secondary metabolites of some commensal bacteria already known to be capable to modulate iron handling in human macrophages.
Exactly, these ecological interactions seem lacking in individuals with atopy, with the microbial communities either not able or not sharing their precious micronutrients with the host but also the individuals with atopy secreting less lipocalin and other innate proteins capable to capture this precious siderophore-complexed iron. Due to the precarious nutritional status, the antigen-presenting cells of atopic persons are also much more sensitive to potential “nutrient” thieves in the form of allergens. In contrast, encountering these allergens with micronutrients seems to turn them into friends and tolerogens.
Once functional iron deficiency is established, dietary iron absorption is hindered by hepcidin, resulting that those persons with functional-iron deficiency (and inflammation) are in the vicious cycle, in which they need more iron but have to exploit different nutritional approaches to compensate their iron requirements, as, otherwise, their immune systems remain hyperactive. Here, evidence is given that one dietary approach is by the lymphatic route using the whey protein beta-lactoglobulin as a carrier for micronutrients.
Our preclinical as well as clinical studies demonstrated that iron can be selectively transported to the myeloid cells through holoBLG, thereby reestablishing immune resilience. Indeed, supplementation with holoBLG could simulate “the protective farm effect” as, also here, protection against allergies could be achieved in a completely allergen-independent manner.
To date, specific allergen immunotherapy is considered the only causative treatment option for ameliorating atopic diseases. However, providing immune cells with micronutrients shows a strikingly similar efficacy, in a completely allergen-independent manner. It emphasizes that micronutritional provision is another causative cure against allergies that should be included in the current practice.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
This study was supportd by the Danube Allergy Research Cluster (DARC) project #08 to Erika Jensen-Jarolim, Karl Landsteiner University Krems, Austria.
Conflict of Interest
The author declares inventorship of EP2894478 (Roth-Walter F et al. Method and means for diagnosing and treating allergy.) (applicant Biomedical International R+D GmbH, Vienna, Austria), the basis for the holoBLG lozenge. FR-W received research funding from Biomedical International R+D GmbH, Vienna, Austria, Bencard Allergie GmbH, Munich, Germany and Vienna, Austria, and Allergy Therapeutics, Worthing, UK. Moreover, she received lecture honoraria by FOMF, VAEM, Bencard Allergie GmbH, Munich, Germany and Vienna, Austria, and Allergy Therapeutics, Worthing, UK.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Venkataramani V. Iron homeostasis and metabolism: two sides of a coin. Adv Exp Med Biol. (2021) 1301:25–40. doi: 10.1007/978-3-030-62026-4_3
3. Kinyoki D, Osgood-Zimmerman AE, Bhattacharjee NV, Local Burden of Disease Anaemia C, Kassebaum NJ, Hay SI. Anemia prevalence in women of reproductive age in low- and middle-income countries between 2000 and 2018. Nat Med. (2021) 27:1761–82. doi: 10.1038/s41591-021-01498-0
4. Camaschella C, Girelli D. The changing landscape of iron deficiency. Mol Aspects Med. (2020) 75:100861. doi: 10.1016/j.mam.2020.100861
5. Chipperfield JR, Ratledge C. Salicylic acid is not a bacterial siderophore: a theoretical study. Biometals. (2000) 13:165–8. doi: 10.1023/A:1009227206890
6. Anderson GJ, Frazer DM. Current understanding of iron homeostasis. Am J Clin Nutr. (2017) 106:1559S−66S. doi: 10.3945/ajcn.117.155804
7. Bogdan AR, Miyazawa M, Hashimoto K, Tsuji Y. Regulators of Iron Homeostasis: New Players in Metabolism, Cell Death, and Disease. Trends Biochem Sci. (2016) 41:274–86. doi: 10.1016/j.tibs.2015.11.012
8. Wandersman C, Delepelaire P. Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol. (2004) 58:611–47. doi: 10.1146/annurev.micro.58.030603.123811
9. Hanikenne M, Esteves SM, Fanara S, Rouached H. Coordinated homeostasis of essential mineral nutrients: a focus on iron. J Exp Bot. (2021) 72:2136–53. doi: 10.1093/jxb/eraa483
10. Roth-Walter F, Pacios LF, Gomez-Casado C, Hofstetter G, Roth GA, Singer J, et al. The major cow milk allergen Bos d 5 manipulates T-helper cells depending on its load with siderophore-bound iron. PLoS ONE. (2014) 9:e104803. doi: 10.1371/journal.pone.0104803
11. Jensen-Jarolim E, Pacios LF, Bianchini R, Hofstetter G, Roth-Walter F. Structural similarities of human and mammalian lipocalins, and their function in innate immunity and allergy. Allergy. (2016) 71:286–94. doi: 10.1111/all.12797
12. Roth-Walter F, Afify SM, Pacios LF, Blokhuis BR, Redegeld F, Regner A, et al. Cow's milk protein beta-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells. J Allergy Clin Immunol. (2021) 147:321–34 e324. doi: 10.1016/j.jaci.2020.05.023
13. Larsson J, Allhorn M, Kerstrom B. The lipocalin alpha(1)-microglobulin binds heme in different species. Arch Biochem Biophys. (2004) 432:196–204. doi: 10.1016/j.abb.2004.09.021
14. Nalepa AI, Taing JJ, Savitsky A, Knipp M. Preparation of cysteine-34-nitroxide spin labeled human alpha(1)-microglobulin. Protein Expr Purif. (2013) 88:33–40. doi: 10.1016/j.pep.2012.11.004
15. Matz JM, Drepper B, Blum TB, Van Genderen E, Burrell A, Martin P, et al. A lipocalin mediates unidirectional heme biomineralization in malaria parasites. Proc Natl Acad Sci USA. (2020) 117:16546–56. doi: 10.1073/pnas.2001153117
16. Bergwik J, Kristiansson A, Allhorn M, Gram M, Akerstrom B. Structure, Functions, and Physiological Roles of the Lipocalin alpha1-Microglobulin (A1M). Front Physiol. (2021) 12:645650. doi: 10.3389/fphys.2021.645650
17. De Simone G, Ascenzi P, Polticelli F. Nitrobindin: An Ubiquitous Family of All beta-Barrel Heme-proteins. IUBMB Life. (2016) 68:423–8. doi: 10.1002/iub.1500
18. Adam FI, Bounds PL, Kissner R, Koppenol WH. Redox properties and activity of iron-citrate complexes: evidence for redox cycling. Chem Res Toxicol. (2015) 28:604–14. doi: 10.1021/tx500377b
19. Christensen JM, Ghannam M, Ayres JW. Effects of divalent amino acids on iron absorption. J Pharm Sci. (1984) 73:1245–8. doi: 10.1002/jps.2600730913
20. Dichtl S, Haschka D, Nairz M, Seifert M, Volani C, Lutz O, et al. Dopamine promotes cellular iron accumulation and oxidative stress responses in macrophages. Biochem Pharmacol. (2018) 148:193–201. doi: 10.1016/j.bcp.2017.12.001
21. Miethke M, Skerra A. Neutrophil gelatinase-associated lipocalin expresses antimicrobial activity by interfering with L-norepinephrine-mediated bacterial iron acquisition. Antimicrob Agents Chemother. (2010) 54:1580–9. doi: 10.1128/AAC.01158-09
22. Sneader W. The discovery and synthesis of epinephrine. Drug News Perspect. (2001) 14:491–4. doi: 10.1358/dnp.2001.14.8.858417
23. Baccan MM, Chiarelli-Neto O, Pereira RM, Esposito BP. Quercetin as a shuttle for labile iron. J Inorg Biochem. (2012) 107:34–9. doi: 10.1016/j.jinorgbio.2011.11.014
24. Meister A. Glutathione metabolism and its selective modification. J Biol Chem. (1988) 263:17205–8. doi: 10.1016/S0021-9258(19)77815-6
25. Roth-Walter F, Starkl P, Zuberbier T, Hummel K, Nobauer K, Razzazi-Fazeli E, et al. Glutathione exposes sequential IgE-epitopes in ovomucoid relevant in persistent egg allergy. Mol Nutr Food Res. (2013) 57:536–44. doi: 10.1002/mnfr.201200612
26. Hider RC, Kong XL. Glutathione: a key component of the cytoplasmic labile iron pool. Biometals. (2011) 24:1179–87. doi: 10.1007/s10534-011-9476-8
27. Pishchany G, Skaar EP. Taste for blood: hemoglobin as a nutrient source for pathogens. PLoS Pathog. (2012) 8:e1002535. doi: 10.1371/journal.ppat.1002535
28. Michel FM, Hosein HA, Hausner DB, Debnath S, Parise JB, Strongin DR. Reactivity of ferritin and the structure of ferritin-derived ferrihydrite. Biochim Biophys Acta. (2010) 1800:871–85. doi: 10.1016/j.bbagen.2010.05.007
29. Saito H. Storage Iron Turnover from a New Perspective. Acta Haematol. (2019) 141:201–8. doi: 10.1159/000496324
30. Zhang AS, Enns CA. Iron homeostasis: recently identified proteins provide insight into novel control mechanisms. J Biol Chem. (2009) 284:711–5. doi: 10.1074/jbc.R800017200
31. Winter WE, Bazydlo LA, Harris NS. The molecular biology of human iron metabolism. Lab Med. (2014) 45:92–102. doi: 10.1309/LMF28S2GIMXNWHMM
32. Aktories K, Hofmann F, Förstermann U, Starke K, Wollenberg P. Eisen- Pharmakologie des Eisenmangels. In: Allgemeine und spezielle Pharmakologie und Toxikologie. Elsevier- Urban and Fischer.
33. Munoz M, Garcia-Erce JA, Remacha AF. Disorders of iron metabolism. Part II: iron deficiency and iron overload. J Clin Pathol. (2011) 64:287–96. doi: 10.1136/jcp.2010.086991
35. Demeyer D, De Smet S, Ulens M. The near equivalence of haem and non-haem iron bioavailability and the need for reconsidering dietary iron recommendations. Eur J Clin Nutr. (2014) 68:750–1. doi: 10.1038/ejcn.2014.58
36. Huebers H, Huebers E, Forth W, Rummel W. Binding of iron to a non-ferritin protein in the mucosal cells of normal and iron-deficient rats during absorption. Life Sci I. (1971) 10:1141–8. doi: 10.1016/0024-3205(71)90274-8
37. Latunde-Dada GO, Takeuchi K, Simpson RJ, Mckie AT. Haem carrier protein 1 (HCP1): Expression and functional studies in cultured cells. FEBS Lett. (2006) 580:6865–70. doi: 10.1016/j.febslet.2006.11.048
38. Nakai Y, Inoue K, Abe N, Hatakeyama M, Ohta KY, Otagiri M, et al. Functional characterization of human proton-coupled folate transporter/heme carrier protein 1 heterologously expressed in mammalian cells as a folate transporter. J Pharmacol Exp Ther. (2007) 322:469–76. doi: 10.1124/jpet.107.122606
39. Le Blanc S, Garrick MD, Arredondo M. Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism. Am J Physiol Cell Physiol. (2012) 302:C1780–5. doi: 10.1152/ajpcell.00080.2012
40. Mckie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, Mudaly E, et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science. (2001) 291:1755–9. doi: 10.1126/science.1057206
41. Ludwiczek S, Rosell FI, Ludwiczek ML, Mauk AG. Recombinant expression and initial characterization of the putative human enteric ferric reductase Dcytb. Biochemistry. (2008) 47:753–61. doi: 10.1021/bi701793a
42. Oakhill JS, Marritt SJ, Gareta EG, Cammack R, Mckie AT. Functional characterization of human duodenal cytochrome b (Cybrd1): Redox properties in relation to iron and ascorbate metabolism. Biochim Biophys Acta. (2008) 1777:260–8. doi: 10.1016/j.bbabio.2007.12.001
43. Da Silva GF, Shinkarev VP, Kamensky YA, Palmer G. Spectroscopic evidence of the role of an axial ligand histidinate in the mechanism of adrenal cytochrome b561. Biochemistry. (2012) 51:8730–42. doi: 10.1021/bi301127k
44. Lane DJ, Bae DH, Merlot AM, Sahni S, Richardson DR. Duodenal cytochrome b (DCYTB) in iron metabolism: an update on function and regulation. Nutrients. (2015) 7:2274–96. doi: 10.3390/nu7042274
45. Hansen SL, Trakooljul N, Liu HC, Moeser AJ, Spears JW. Iron transporters are differentially regulated by dietary iron, and modifications are associated with changes in manganese metabolism in young pigs. J Nutr. (2009) 139:1474–9. doi: 10.3945/jn.109.105866
46. Chierici R, Sawatzki G, Tamisari L, Volpato S, Vigi V. Supplementation of an adapted formula with bovine lactoferrin. 2. Effects on serum iron ferritin and zinc levels. Acta Paediatr. (1992) 81:475–9. doi: 10.1111/j.1651-2227.1992.tb12277.x
47. Huebers HA, Huebers E, Csiba E, Rummel W, Finch CA. The significance of transferrin for intestinal iron absorption. Blood. (1983) 61:283–90. doi: 10.1182/blood.V61.2.283.283
48. Li JY, Paragas N, Ned RM, Qiu A, Viltard M, Leete T, et al. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev Cell. (2009) 16:35–46. doi: 10.1016/j.devcel.2008.12.002
49. Theil EC, Chen H, Miranda C, Janser H, Elsenhans B, Nunez MT, et al. Absorption of iron from ferritin is independent of heme iron and ferrous salts in women and rat intestinal segments. J Nutr. (2012) 142:478–83. doi: 10.3945/jn.111.145854
50. Layrisse M, Garcia-Casal MN, Solano L, Baron MA, Arguello F, Llovera D, et al. Iron bioavailability in humans from breakfasts enriched with iron bis-glycine chelate, phytates and polyphenols. J Nutr. (2000) 130:2195–9. doi: 10.1093/jn/130.9.2195
51. Sanyal AJ, Shiffmann ML, Hirsch JI, Moore EW. Premicellar taurocholate enhances ferrous iron uptake from all regions of rat small intestine. Gastroenterology. (1991) 101:382–9. doi: 10.1016/0016-5085(91)90015-D
52. Sanyal AJ, Hirsch JI, Moore EW. Evidence that bile salts are important for iron absorption. Am J Physiol. (1994) 266:G318–323. doi: 10.1152/ajpgi.1994.266.2.G318
53. Fini A, Feroci G, Fazio G, Zuman P. Interaction of iron(II) with bile salts. J Inorg Biochem. (1997) 68:251–6. doi: 10.1016/S0162-0134(97)00093-7
54. Russo G, Guardabasso V, Romano F, Corti P, Samperi P, Condorelli A, et al. Monitoring oral iron therapy in children with iron deficiency anemia: an observational, prospective, multicenter study of AIEOP patients (Associazione Italiana Emato-Oncologia Pediatrica). Ann Hematol. (2020) 99:413–20. doi: 10.1007/s00277-020-03906-w
55. Gomez-Ramirez S, Brilli E, Tarantino G, Munoz M. Sucrosomial((R)) Iron: A new generation iron for improving oral supplementation. Pharmaceuticals (Basel). (2018) 11:97. doi: 10.3390/ph11040097
56. Batchelor EK, Kapitsinou P, Pergola PE, Kovesdy CP, Jalal DI. Iron deficiency in chronic kidney disease: updates on pathophysiology, diagnosis, and treatment. J Am Soc Nephrol. (2020) 31:456–68. doi: 10.1681/ASN.2019020213
57. Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. (2000) 403:776–81. doi: 10.1038/35001596
58. Deshpande CN, Ruwe TA, Shawki A, Xin V, Vieth KR, Valore EV, et al. Calcium is an essential cofactor for metal efflux by the ferroportin transporter family. Nat Commun. (2018) 9:3075. doi: 10.1038/s41467-018-05446-4
59. Quigley JG, Yang Z, Worthington MT, Phillips JD, Sabo KM, Sabath DE, et al. Identification of a human heme exporter that is essential for erythropoiesis. Cell. (2004) 118:757–66. doi: 10.1016/j.cell.2004.08.014
60. Latunde-Dada GO, Simpson RJ, Mckie AT. Recent advances in mammalian haem transport. Trends Biochem Sci. (2006) 31:182–8. doi: 10.1016/j.tibs.2006.01.005
61. Thul PJ, Akesson L, Wiking M, Mahdessian D, Geladaki A, Ait Blal H, et al. A subcellular map of the human proteome. Science. (2017) 356:eaal3321. doi: 10.1126/science.aal3321
62. 0.Proteinatlas.Org/Ensg00000162769-Flvcr1 FLVCR1 [Online]. proteinatlas.org. Available: https://www.proteinatlas.org/ENSG00000162769-FLVCR1 (accessed November 29, 2021).
63. Truman-Rosentsvit M, Berenbaum D, Spektor L, Cohen LA, Belizowsky-Moshe S, Lifshitz L, et al. Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood. (2018) 131:342–52. doi: 10.1182/blood-2017-02-768580
64. Clemens S. Zn and Fe biofortification: the right chemical environment for human bioavailability. Plant Sci. (2014) 225:52–7. doi: 10.1016/j.plantsci.2014.05.014
65. Hanson LN, Engelman HM, Alekel DL, Schalinske KL, Kohut ML, Reddy MB. Effects of soy isoflavones and phytate on homocysteine, C-reactive protein, and iron status in postmenopausal women. Am J Clin Nutr. (2006) 84:774–80. doi: 10.1093/ajcn/84.4.774
66. Dell'mour M, Schenkeveld W, Oburger E, Fischer L, Kraemer S, Puschenreiter M, et al. Analysis of iron-phytosiderophore complexes in soil related samples: LC-ESI-MS/MS versus CE-MS. Electrophoresis. (2012) 33:726–33. doi: 10.1002/elps.201100466
67. Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. (2009) 53:75–100. doi: 10.1007/s12013-009-9043-x
68. Hunt JR, Roughead ZK. Nonheme-iron absorption, fecal ferritin excretion, and blood indexes of iron status in women consuming controlled lactoovovegetarian diets for 8 wk. Am J Clin Nutr. (1999) 69:944–52.
69. Suliburska J, Bogdanski P, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res. (2012) 149:315–22. doi: 10.1007/s12011-012-9448-z
70. Ullmann U, Haller J, Bakker GC, Brink EJ, Weber P. Epigallocatechin gallate (EGCG) (TEAVIGO) does not impair nonhaem-iron absorption in man. Phytomedicine. (2005) 12:410–5. doi: 10.1016/j.phymed.2004.07.001
71. Tako E, Reed SM, Budiman J, Hart JJ, Glahn RP. Higher iron pearl millet (Pennisetum glaucum L.) provides more absorbable iron that is limited by increased polyphenolic content. Nutr J. (2015) 14:11. doi: 10.1186/1475-2891-14-11
72. Ahmad Fuzi SF, Koller D, Bruggraber S, Pereira DI, Dainty JR, Mushtaq S. A 1-h time interval between a meal containing iron and consumption of tea attenuates the inhibitory effects on iron absorption: a controlled trial in a cohort of healthy UK women using a stable iron isotope. Am J Clin Nutr. (2017) 106:1413–21. doi: 10.3945/ajcn.117.161364
73. Sajadi Hezaveh Z, Azarkeivan A, Janani L, Hosseini S, Shidfar F. The effect of quercetin on iron overload and inflammation in beta-thalassemia major patients: a double-blind randomized clinical trial. Complement Ther Med. (2019) 46:24–8. doi: 10.1016/j.ctim.2019.02.017
74. Imessaoudene A, Merzouk H, Berroukeche F, Mokhtari N, Bensenane B, Cherrak S, et al. Beneficial effects of quercetin-iron complexes on serum and tissue lipids and redox status in obese rats. J Nutr Biochem. (2016) 29:107–15. doi: 10.1016/j.jnutbio.2015.11.011
75. Mazhar M, Kabir N, Simjee SU. Quercetin modulates iron homeostasis and iNOS expression of splenic macrophages in a rat model of iron deficiency anemia. Chin J Nat Med. (2018) 16:580–9. doi: 10.1016/S1875-5364(18)30095-5
76. Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA. (2001) 98:8780–5. doi: 10.1073/pnas.151179498
77. Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. (2004) 113:1271–6. doi: 10.1172/JCI200420945
78. Schwarz P, Kubler JA, Strnad P, Muller K, Barth TF, Gerloff A, et al. Hepcidin is localised in gastric parietal cells, regulates acid secretion and is induced by Helicobacter pylori infection. Gut. (2012) 61:193–201. doi: 10.1136/gut.2011.241208
79. Van Swelm RP, Wetzels JF, Verweij VG, Laarakkers CM, Pertijs JC, Van Der Wijst J, et al. Renal Handling of Circulating and Renal-Synthesized Hepcidin and Its Protective Effects against Hemoglobin-Mediated Kidney Injury. J Am Soc Nephrol. (2016) 27:2720–32. doi: 10.1681/ASN.2015040461
80. Huang ML, Austin CJ, Sari MA, Rahmanto YS, Ponka P, Vyoral D, et al. Hepcidin bound to alpha2-macroglobulin reduces ferroportin-1 expression and enhances its activity at reducing serum iron levels. J Biol Chem. (2013) 288:25450–65. doi: 10.1074/jbc.M113.471573
81. Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, Klaver SM, Kroot JJ, Van Tienoven D, et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood. (2011) 117:e218–225. doi: 10.1182/blood-2011-02-337907
82. Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. (2005) 106:2196–9. doi: 10.1182/blood-2005-04-1766
83. Ramey G, Deschemin JC, Durel B, Canonne-Hergaux F, Nicolas G, Vaulont S. Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica. (2010) 95:501–4. doi: 10.3324/haematol.2009.014399
84. Bacchetta J, Zaritsky JJ, Sea JL, Chun RF, Lisse TS, Zavala K, et al. Suppression of iron-regulatory hepcidin by vitamin D. J Am Soc Nephrol. (2014) 25:564–72. doi: 10.1681/ASN.2013040355
85. Nakanishi T, Hasuike Y, Nanami M, Yahiro M, Kuragano T. Novel iron-containing phosphate binders and anemia treatment in CKD: oral iron intake revisited. Nephrol Dial Transplant. (2015). doi: 10.1093/ndt/gfv268
86. Nita E, Bairaktari E, Kolios G, Migkos MP, Somarakis GP, Markatseli T, et al. Role of hepcidin in anemia of chronic disease in rheumatoid arthritis. J Lab Physicians. (2021) 13:317–22. doi: 10.1055/s-0041-1732827
87. Abuga KM, Muriuki JM, Uyoga SM, Mwai K, Makale J, Mogire RM, et al. Hepcidin regulation in Kenyan children with severe malaria and non-typhoidal Salmonella bacteremia. Haematologica. (2021). doi: 10.3324/haematol.2021.279316
88. Ganz T. Macrophages and systemic iron homeostasis. J Innate Immun. (2012) 4:446–53. doi: 10.1159/000336423
89. Fiorito V, Geninatti Crich S, Silengo L, Aime S, Altruda F, Tolosano E. Lack of plasma protein hemopexin results in increased duodenal iron uptake. PLoS ONE. (2013) 8:e68146. doi: 10.1371/journal.pone.0068146
91. Pantopoulos K, Porwal SK, Tartakoff A, Devireddy L. Mechanisms of mammalian iron homeostasis. Biochemistry. (2012) 51:5705–24. doi: 10.1021/bi300752r
92. Srigiridhar K, Nair KM. Iron-deficient intestine is more susceptible to peroxidative damage during iron supplementation in rats. Free Radic Biol Med. (1998) 25:660–5. doi: 10.1016/S0891-5849(98)00086-0
93. Richardson DR. Role of ceruloplasmin and ascorbate in cellular iron release. J Lab Clin Med. (1999) 134:454–65. doi: 10.1016/S0022-2143(99)90166-X
94. Roetto A, Mezzanotte M, Pellegrino RM. The functional versatility of transferrin receptor 2 and its therapeutic value. Pharmaceuticals (Basel). (2018) 11. doi: 10.3390/ph11040115
95. Wortham AM, Goldman DC, Chen J, Fleming WH, Zhang AS, Enns CA. Extrahepatic deficiency of transferrin receptor 2 is associated with increased erythropoiesis independent of iron overload. J Biol Chem. (2020) 295:3906–17. doi: 10.1074/jbc.RA119.010535
96. Ali MK, Kim RY, Brown AC, Donovan C, Vanka KS, Mayall JR, et al. Critical role for iron accumulation in the pathogenesis of fibrotic lung disease. J Pathol. (2020) 251:49–62. doi: 10.1002/path.5401
97. Cappellini MD, Comin-Colet J, De Francisco A, Dignass A, Doehner W, Lam CS, et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am J Hematol. (2017) 92:1068–78. doi: 10.1002/ajh.24820
98. UNICEF/UNU/WHO. Iron Deficiency Anaemia: Assessment, Prevention, and Control. (2001). Available online at: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf
99. World Health Organization C.F.D.C.a.P. Assessing the iron status of populations. Second edition, including Literature Reviews (2007). Available online at: https://www.who.int/publications/i/item/9789241596107
100. WHO (2011). Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization.
101. Mei Z, Addo OY, Jefferds ME, Sharma AJ, Flores-Ayala RC, Brittenham GM. Physiologically based serum ferritin thresholds for iron deficiency in children and non-pregnant women: a US National Health and Nutrition Examination Surveys (NHANES) serial cross-sectional study. Lancet Haematol. (2021) 8:e572–82. doi: 10.1016/S2352-3026(21)00168-X
102. WHO (2020). WHO Guidelin on Use of Ferritin Concentration to Assess in Individuals and Populations. Geneva: World Health Organization.
103. Ross AC. Impact of chronic and acute inflammation on extra- and intracellular iron homeostasis. Am J Clin Nutr. (2017) 106:1581S−7S. doi: 10.3945/ajcn.117.155838
104. Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. (2019) 133:40–50. doi: 10.1182/blood-2018-06-856500
105. Yambire KF, Rostosky C, Watanabe T, Pacheu-Grau D, Torres-Odio S, Sanchez-Guerrero A, et al. Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. Elife. (2019) 8:e51031. doi: 10.7554/eLife.51031
106. Fertrin KY. Diagnosis and management of iron deficiency in chronic inflammatory conditions (CIC): is too little iron making your patient sick? Hematology. Am Soc Hematol Educ Program. (2020) 2020:478–86. doi: 10.1182/hematology.2020000132
107. Tahir E, Ayotte P, Little M, Belanger RE, Lucas M, Mergler D, et al. Anemia, iron status, and associated protective and risk factors among children and adolescents aged 3 to 19 years old from four First Nations communities in Quebec. Can J Public Health. (2020) 111:682–93. doi: 10.17269/s41997-020-00304-7
108. Petje LM, Jensen SA, Szikora S, Sulzbacher M, Bartosik T, Pjevac P, et al. Functional iron-deficiency in women with allergic rhinitis is associated with symptoms after nasal provocation and lack of iron-sequestering microbes. Allergy. (2021) 76:2882–6. doi: 10.1111/all.14960
109. Wieczorek M, Schwarz F, Sadlon A, Abderhalden LA, De Godoi Rezende Costa Molino C, Spahn DR, et al. Iron deficiency and biomarkers of inflammation: a 3-year prospective analysis of the DO-HEALTH trial. Aging Clin Exp Res. (2021) 34:515–25. doi: 10.1007/s40520-021-01955-3
110. Chang R, Chu KA, Lin MC, Chu YH, Hung YM, Wei JC. Newly diagnosed iron deficiency anemia and subsequent autoimmune disease: a matched cohort study in Taiwan. Curr Med Res Opin. (2020) 36:985–92. doi: 10.1080/03007995.2020.1748585
111. Luo J, Wang X, Yuan L, Guo L. Iron deficiency, a risk factor of thyroid disorders in reproductive-age and pregnant women: a systematic review and meta-analysis. Front Endocrinol (Lausanne). (2021) 12:629831. doi: 10.3389/fendo.2021.629831
112. Drury KE, Schaeffer M, Silverberg JI. Association between atopic disease and anemia in US children. JAMA Pediatr. (2016) 170:29–34. doi: 10.1001/jamapediatrics.2015.3065
113. Krishna MT, Subramanian A, Adderley NJ, Zemedikun DT, Gkoutos GV, Nirantharakumar K. Allergic diseases and long-term risk of autoimmune disorders: longitudinal cohort study and cluster analysis. Eur Respir J. (2019) 54:1900476. doi: 10.1183/13993003.00476-2019
114. Rhew K, Oh JM. Association between atopic disease and anemia in pediatrics: a cross-sectional study. BMC Pediatr. (2019) 19:455. doi: 10.1186/s12887-019-1836-5
115. Rhew K, Brown JD, Oh JM. Atopic disease and anemia in korean patients: cross-sectional study with propensity score analysis. Int J Environ Res Public Health. (2020) 17:1978. doi: 10.3390/ijerph17061978
116. Albaramki J, Hodson EM, Craig JC, Webster AC. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. (2012) 1:CD007857. doi: 10.1002/14651858.CD007857.pub2
117. Susantitaphong P, Alqahtani F, Jaber BL. Efficacy and safety of intravenous iron therapy for functional iron deficiency anemia in hemodialysis patients: a meta-analysis. Am J Nephrol. (2014) 39:130–41. doi: 10.1159/000358336
118. Zhang J, Hu S, Jiang Y, Zhou Y. Efficacy and safety of iron therapy in patients with chronic heart failure and iron deficiency: a systematic review and meta-analysis based on 15 randomised controlled trials. Postgrad Med J. (2020) 96:766–76. doi: 10.1136/postgradmedj-2019-137342
119. Osman M, Syed M, Balla S, Kheiri B, Faisaluddin M, Bianco C. A Meta-analysis of intravenous iron therapy for patients with iron deficiency and heart failure. Am J Cardiol. (2021) 141:152–3. doi: 10.1016/j.amjcard.2020.11.025
120. Reinhold J, Papadopoulou C, Baral R, Vassiliou VS. Iron deficiency for prognosis in acute coronary syndrome - A systematic review and meta-analysis. Int J Cardiol. (2021) 328:46–54. doi: 10.1016/j.ijcard.2020.12.021
121. Nickol AH, Frise MC, Cheng HY, Mcgahey A, Mcfadyen BM, Harris-Wright T, et al. A cross-sectional study of the prevalence and associations of iron deficiency in a cohort of patients with chronic obstructive pulmonary disease. BMJ Open. (2015) 5:e007911. doi: 10.1136/bmjopen-2015-007911
122. Cloonan SM, Mumby S, Adcock IM, Choi AMK, Chung KF, Quinlan GJ. The “Iron”-y of Iron Overload and Iron Deficiency in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. (2017) 196:1103–12. doi: 10.1164/rccm.201702-0311PP
123. Pizzini A, Aichner M, Sonnweber T, Tancevski I, Weiss G, Loffler-Ragg J. The Significance of iron deficiency and anemia in a real-life COPD cohort. Int J Med Sci. (2020) 17:2232–9. doi: 10.7150/ijms.46163
124. Zhao L, Zhang X, Shen Y, Fang X, Wang Y, Wang F. Obesity and iron deficiency: a quantitative meta-analysis. Obes Rev. (2015) 16:1081–93. doi: 10.1111/obr.12323
125. Teng IC, Tseng SH, Aulia B, Shih CK, Bai CH, Chang JS. Can diet-induced weight loss improve iron homoeostasis in patients with obesity: a systematic review and meta-analysis. Obes Rev. (2020) 21:e13080. doi: 10.1111/obr.13080
126. Corna G, Campana L, Pignatti E, Castiglioni A, Tagliafico E, Bosurgi L, et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. (2010) 95:1814–22. doi: 10.3324/haematol.2010.023879
127. Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. (2013) 165:575–82 e573. doi: 10.1016/j.ahj.2013.01.017
128. Ruiter G, Lanser IJ, De Man FS, Van Der Laarse WJ, Wharton J, Wilkins MR, et al. Iron deficiency in systemic sclerosis patients with and without pulmonary hypertension. Rheumatology (Oxford). (2014) 53:285–92. doi: 10.1093/rheumatology/ket331
129. Lewis GD, Malhotra R, Hernandez AF, Mcnulty SE, Smith A, Felker GM, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA. (2017) 317:1958–66. doi: 10.1001/jama.2017.5427
130. Winn NC, Volk KM, Hasty AH. Regulation of tissue iron homeostasis: the macrophage “ferrostat”. JCI Insight. (2020) 5. doi: 10.1172/jci.insight.132964
131. Guedes M, Muenz D, Zee J, Lopes MB, Waechter S, Stengel B, et al. Serum biomarkers of iron stores are associated with worse physical health-related quality of life in nondialysis-dependent chronic kidney disease patients with or without anemia. Nephrol Dial Transplant. (2021) 36:1694–703. doi: 10.1093/ndt/gfab050
132. Lanser L, Burkert FR, Bellmann-Weiler R, Schroll A, Wildner S, Fritsche G, et al. Dynamics in anemia development and dysregulation of iron homeostasis in hospitalized patients with COVID-19. Metabolites. (2021) 11:653. doi: 10.3390/metabo11100653
133. Roth-Walter F. Compensating functional iron-deficiency in patients with allergies with targeted micronutrition. Allergo J Int. (2021) 30:130–4. doi: 10.1007/s40629-021-00171-9
134. Livesey JA, Manning RA, Meek JH, Jackson JE, Kulinskaya E, Laffan MA, et al. Low serum iron levels are associated with elevated plasma levels of coagulation factor VIII and pulmonary emboli/deep venous thromboses in replicate cohorts of patients with hereditary haemorrhagic telangiectasia. Thorax. (2012) 67:328–33. doi: 10.1136/thoraxjnl-2011-201076
135. Potaczek DP, Jankowska EA, Wypasek E, Undas A. Iron deficiency: a novel risk factor of recurrence in patients after unprovoked venous thromboembolism. Pol Arch Med Wewn. (2016) 126:159–65. doi: 10.20452/pamw.3311
136. De Sousa M, Smithyman A, Tan C. Suggested models of ecotaxopathy in lymphoreticular malignancy. A role for iron-binding proteins in the control of lymphoid cell migration. Am J Pathol. (1978) 90:497–520.
137. Momotani E, Whipple DL, Thiermann AB. The distribution of ferritin, lactoferrin and transferrin in granulomatous lymphadenitis of bovine paratuberculosis. J Comp Pathol. (1988) 99:205–14. doi: 10.1016/0021-9975(88)90072-2
138. Recalcati S, Invernizzi P, Arosio P, Cairo G. New functions for an iron storage protein: the role of ferritin in immunity and autoimmunity. J Autoimmun. (2008) 30:84–9. doi: 10.1016/j.jaut.2007.11.003
139. Kragsnaes MS, Fredberg U, Stribolt K, Kjaer SG, Bendix K, Ellingsen T. Stereological quantification of immune-competent cells in baseline biopsy specimens from achilles tendons: results from patients with chronic tendinopathy followed for more than 4 years. Am J Sports Med. (2014) 42:2435–45. doi: 10.1177/0363546514542329
140. Rubio-Navarro A, Amaro Villalobos JM, Lindholt JS, Buendia I, Egido J, Blanco-Colio LM, et al. Hemoglobin induces monocyte recruitment and CD163-macrophage polarization in abdominal aortic aneurysm. Int J Cardiol. (2015) 201:66–78. doi: 10.1016/j.ijcard.2015.08.053
141. Schaer DJ, Boretti FS, Schoedon G, Schaffner A. Induction of the CD163-dependent haemoglobin uptake by macrophages as a novel anti-inflammatory action of glucocorticoids. Br J Haematol. (2002) 119:239–43. doi: 10.1046/j.1365-2141.2002.03790.x
142. Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. (2004) 94:119–26. doi: 10.1161/01.RES.0000109414.78907.F9
143. Liang X, Lin T, Sun G, Beasley-Topliffe L, Cavaillon JM, Warren HS. Hemopexin down-regulates LPS-induced proinflammatory cytokines from macrophages. J Leukoc Biol. (2009) 86:229–35. doi: 10.1189/jlb.1208742
144. Lin T, Sammy F, Yang H, Thundivalappil S, Hellman J, Tracey KJ, et al. Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. J Immunol. (2012) 189:2017–22. doi: 10.4049/jimmunol.1103623
145. Vinchi F, Costa Da Silva M, Ingoglia G, Petrillo S, Brinkman N, Zuercher A, et al. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood. (2016) 127:473–86. doi: 10.1182/blood-2015-08-663245
146. Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. (2004) 432:917–21. doi: 10.1038/nature03104
147. Mertens C, Kuchler L, Sola A, Guiteras R, Grein S, Brune B, et al. Macrophage-derived iron-bound lipocalin-2 correlates with renal recovery markers following sepsis-induced kidney damage. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21207527
148. Urbschat A, Thiemens AK, Mertens C, Rehwald C, Meier JK, Baer PC, et al. Macrophage-secreted lipocalin-2 promotes regeneration of injured primary murine renal tubular epithelial cells. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21062038
149. Mertens C, Schnetz M, Rehwald C, Grein S, Elwakeel E, Weigert A, et al. Iron-bound lipocalin-2 from tumor-associated macrophages drives breast cancer progression independent of ferroportin. Metabolites. (2021) 11. doi: 10.3390/metabo11030180
150. Watzenboeck ML, Drobits B, Zahalka S, Gorki AD, Farhat A, Quattrone F, et al. Lipocalin 2 modulates dendritic cell activity and shapes immunity to influenza in a microbiome dependent manner. PLoS Pathog. (2021) 17:e1009487. doi: 10.1371/journal.ppat.1009487
151. Persson HL, Vainikka LK, Eriksson HB, Wennerstrom U. Lane-Hamilton syndrome: ferritin protects lung macrophages against iron and oxidation. Chest. (2011) 139:361–7. doi: 10.1378/chest.10-0818
152. Nybakken G, Gratzinger D. Myelodysplastic syndrome macrophages have aberrant iron storage and heme oxygenase-1 expression. Leuk Lymphoma. (2016) 57:1893–902. doi: 10.3109/10428194.2015.1121259
153. Sottile R, Federico G, Garofalo C, Tallerico R, Faniello MC, Quaresima B, et al. Iron and Ferritin Modulate MHC Class I Expression and NK Cell Recognition. Front Immunol. (2019) 10:224. doi: 10.3389/fimmu.2019.00224
154. Mesquita G, Silva T, Gomes AC, Oliveira PF, Alves MG, Fernandes R, et al. H-Ferritin is essential for macrophages' capacity to store or detoxify exogenously added iron. Sci Rep. (2020) 10:3061. doi: 10.1038/s41598-020-59898-0
155. Hu ZW, Chen L, Ma RQ, Wei FQ, Wen YH, Zeng XL, et al. Comprehensive analysis of ferritin subunits expression and positive correlations with tumor-associated macrophages and T regulatory cells infiltration in most solid tumors. Aging (Albany NY). (2021) 13:11491–506. doi: 10.18632/aging.202841
156. Djeha A, Perez-Arellano JL, Hayes SL, Brock JH. Transferrin synthesis by macrophages: up-regulation by gamma-interferon and effect on lymphocyte proliferation. FEMS Microbiol Immunol. (1992) 5:279–82. doi: 10.1111/j.1574-6968.1992.tb05912.x
157. Roth-Walter F, Schmutz R, Mothes-Luksch N, Lemell P, Zieglmayer P, Zieglmayer R, et al. Clinical efficacy of sublingual immunotherapy is associated with restoration of steady-state serum lipocalin 2 after SLIT: a pilot study. World Allergy Organ J. (2018) 11:21. doi: 10.1186/s40413-018-0201-8
158. Choi GS, Shin SY, Kim JH, Lee HY, Palikhe NS, Ye YM, et al. Serum lactoferrin level as a serologic biomarker for allergic rhinitis. Clin Exp Allergy. (2010) 40:403–10. doi: 10.1111/j.1365-2222.2009.03414.x
159. Johansson S, Keen C, Stahl A, Wennergren G, Benson M. Low levels of CC16 in nasal fluid of children with birch pollen-induced rhinitis. Allergy. (2005) 60:638–42. doi: 10.1111/j.1398-9995.2005.00775.x
160. Dilek F, Gultepe B, Ozkaya E, Yazici M, Gedik AH, Cakir E. Beyond anti-microbial properties: the role of cathelicidin in allergic rhinitis. Allergol Immunopathol (Madr). (2016) 44:297–302. doi: 10.1016/j.aller.2015.07.006
161. Tulic MK, Hodder M, Forsberg A, Mccarthy S, Richman T, D'vaz N, et al. Differences in innate immune function between allergic and nonallergic children: new insights into immune ontogeny. J Allergy Clin Immunol. (2011) 127:470–8 e471. doi: 10.1016/j.jaci.2010.09.020
162. Roth-Walter F, Pacios LF, Bianchini R, Jensen-Jarolim E. Linking iron-deficiency with allergy: role of molecular allergens and the microbiome. Metallomics. (2017) 9:1676–92. doi: 10.1039/C7MT00241F
163. Karvonen AM, Lampi J, Keski-Nisula L, Auvinen J, Toppila-Salmi S, Jarvelin M, et al. Farm environment during pregnancy and childhood and polysensitization at the age of 31: prospective birth cohort study in Finland. J Investig Allergol Clin Immunol. (2021) 31:44–51. doi: 10.18176/jiaci.0455
164. Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. (2002) 288:963–72. doi: 10.1001/jama.288.8.963
165. Riedler J, Braun-Fahrlander C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. (2001) 358:1129–33. doi: 10.1016/S0140-6736(01)06252-3
166. Van Esch B, Porbahaie M, Abbring S, Garssen J, Potaczek DP, Savelkoul HFJ, et al. The impact of milk and its components on epigenetic programming of immune function in early life and beyond: implications for allergy and asthma. Front Immunol. (2020) 11:2141. doi: 10.3389/fimmu.2020.02141
167. Acevedo N, Alashkar Alhamwe B, Caraballo L, Ding M, Ferrante A, Garn H, et al. Perinatal and early-life nutrition, epigenetics, and allergy. Nutrients. (2021) 13. doi: 10.3390/nu13030724
168. Magdelijns FJ, Mommers M, Penders J, Smits L, Thijs C. Folic acid use in pregnancy and the development of atopy, asthma, and lung function in childhood. Pediatrics. (2011) 128:e135–144. doi: 10.1542/peds.2010-1690
169. Triche EW, Lundsberg LS, Wickner PG, Belanger K, Leaderer BP, Bracken MB. Association of maternal anemia with increased wheeze and asthma in children. Ann Allergy Asthma Immunol. (2011) 106:131–9 e131. doi: 10.1016/j.anai.2010.11.007
170. Rosenlund H, Magnusson J, Kull I, Hakansson N, Wolk A, Pershagen G, et al. Antioxidant intake and allergic disease in children. Clin Exp Allergy. (2012) 42:1491–500. doi: 10.1111/j.1365-2222.2012.04053.x
171. Toyran M, Kaymak M, Vezir E, Harmanci K, Kaya A, Ginis T, et al. Trace element levels in children with atopic dermatitis. J Investig Allergol Clin Immunol. (2012) 22:341–4.
172. Nwaru BI, Hayes H, Gambling L, Craig LC, Allan K, Prabhu N, et al. An exploratory study of the associations between maternal iron status in pregnancy and childhood wheeze and atopy. Br J Nutr. (2014) 112:2018–27. doi: 10.1017/S0007114514003122
173. Weigert R, Dosch NC, Bacsik-Campbell ME, Guilbert TW, Coe CL, Kling PJ. Maternal pregnancy weight gain and cord blood iron status are associated with eosinophilia in infancy. J Perinatol. (2015) 35:621–6. doi: 10.1038/jp.2015.21
174. Yang AR, Kim YN, Lee BH. Dietary intakes and lifestyle patterns of Korean children and adolescents with atopic dermatitis: Using the fourth and fifth Korean National Health and Nutrition Examination Survey (KNHANES IV,V), 2007-11. Ecol Food Nutr. (2016) 55:50–64. doi: 10.1080/03670244.2015.1072813
175. Pereira De Jesus S, Den Dekker HT, De Jongste JC, Reiss IK, Steegers EA, Jaddoe VWV, et al. Maternal hemoglobin and hematocrit levels during pregnancy and childhood lung function and asthma. The Generation R Study Pediatr Pulmonol. (2018) 53:130–7. doi: 10.1002/ppul.23733
176. Fortes C, Mastroeni S, Mannooranparampil TJ, Di Lallo D. Pre-natal folic acid and iron supplementation and atopic dermatitis in the first 6 years of life. Arch Dermatol Res. (2019) 311:361–7. doi: 10.1007/s00403-019-01911-2
177. Lara-Corrales I, Huang CM, Parkin PC, Rubio-Gomez GA, Posso-De Los Rios CJ, Maguire J, et al. Vitamin D level and supplementation in pediatric atopic dermatitis: a randomized controlled trial. J Cutan Med Surg. (2019) 23:44–9. doi: 10.1177/1203475418805744
178. Liu X, Yang G, Luo M, Lan Q, Shi X, Deng H, et al. Serum vitamin E levels and chronic inflammatory skin diseases: A systematic review and meta-analysis. PLoS ONE. (2021) 16:e0261259. doi: 10.1371/journal.pone.0261259
179. Nowak S, Wang H, Schmidt B, Jarvinen KM. Vitamin D and iron status in children with food allergy. Ann Allergy Asthma Immunol. (2021) 127:57–63. doi: 10.1016/j.anai.2021.02.027
180. Petriashvili M. (2021). Impact of maternal vitamin d status on the formation of atopic dermatitis in young children. Glob Pediatr Health 8, 2333794X211022916. doi: 10.1177/2333794X211022916
181. Riccioni G, Bucciarelli T, Mancini B, Di Ilio C, Della Vecchia R, D'orazio N. Plasma lycopene and antioxidant vitamins in asthma: the PLAVA study. J Asthma. (2007) 44:429–32. doi: 10.1080/02770900701421880
182. Mills K, Lay J, Wu W, Robinette C, Kesic MJ, Dreskin SC, et al. Vitamin E, gamma-tocopherol, diminishes ex vivo basophil response to dust mite allergen. Allergy. (2014) 69:541–4. doi: 10.1111/all.12371
183. Yang H, Chen JS, Zou WJ, Tan Q, Xiao YZ, Luo XY, et al. Vitamin A deficiency exacerbates extrinsic atopic dermatitis development by potentiating type 2 helper T cell-type inflammation and mast cell activation. Clin Exp Allergy. (2020) 50:942–53. doi: 10.1111/cea.13687
184. Potaczek DP, Harb H, Michel S, Alhamwe BA, Renz H, Tost J. Epigenetics and allergy: from basic mechanisms to clinical applications. Epigenomics. (2017) 9:539–71. doi: 10.2217/epi-2016-0162
185. Lien YC, Condon DE, Georgieff MK, Simmons RA, Tran PV. Dysregulation of neuronal genes by fetal-neonatal iron deficiency anemia is associated with altered dna methylation in the rat hippocampus. Nutrients. (2019) 11. doi: 10.3390/nu11051191
186. Barks AK, Liu SX, Georgieff MK, Hallstrom TC, Tran PV. Early-life iron deficiency anemia programs the hippocampal epigenomic landscape. Nutrients. (2021) 13:3857. doi: 10.3390/nu13113857
187. Erber LN, Luo A, Gong Y, Beeson M, Tu M, Tran P, et al. Iron Deficiency Reprograms Phosphorylation Signaling and Reduces O-GlcNAc Pathways in Neuronal Cells. Nutrients. (2021) 13:179. doi: 10.3390/nu13010179
188. Zumbrennen-Bullough KB, Wu Q, Core AB, Canali S, Chen W, Theurl I, et al. MicroRNA-130a is up-regulated in mouse liver by iron deficiency and targets the bone morphogenetic protein (BMP) receptor ALK2 to attenuate BMP signaling and hepcidin transcription. J Biol Chem. (2014) 289:23796–808. doi: 10.1074/jbc.M114.577387
189. Huang Y, Zhang H, Wang C, Zhou J, Li Y, Hu C. DNA methylation suppresses liver Hamp expression in response to iron deficiency after bariatric surgery. Surg Obes Relat Dis. (2020) 16:109–18. doi: 10.1016/j.soard.2019.10.005
190. Gunnarsdottir MG, Jonsson T, Halldorsdottir AM. Circulating plasma microRNAs as biomarkers for iron status in blood donors. Transfus Med. (2019) 29:52–8. doi: 10.1111/tme.12554
191. Ozdemir ZC, Duzenli Kar Y, Bor O. Whole Blood miR-210, miR-122, miR-223 Expression levels and their relationship with iron status parameters and hypercoagulability indices in children with iron deficiency anemia. J Pediatr Hematol Oncol. (2021) 43:e328–35. doi: 10.1097/MPH.0000000000002127
192. Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. (2007) 204:1775–85. doi: 10.1084/jem.20070602
193. Jang JT, Green JB, Beard JL, Green MH. Kinetic analysis shows that iron deficiency decreases liver vitamin A mobilization in rats. J Nutr. (2000) 130:1291–6. doi: 10.1093/jn/130.5.1291
194. Suharno D, West CE, Muhilal, Karyadi D, Hautvast JG. Supplementation with vitamin A and iron for nutritional anaemia in pregnant women in West Java, Indonesia. Lancet. (1993) 342:1325–8. doi: 10.1016/0140-6736(93)92246-P
195. Campbell RK, Shaikh S, Schulze K, Arguello M, Ali H, Wu L, et al. Micronutrient and inflammation status following one year of complementary food supplementation in 18-month-old rural bangladeshi children: a randomized controlled trial. Nutrients. (2020) 12:1452. doi: 10.3390/nu12051452
196. Defnet AE, Shah SD, Huang W, Shapiro P, Deshpande DA, Kane MA. Dysregulated retinoic acid signaling in airway smooth muscle cells in asthma. FASEB J. (2021) 35:e22016. doi: 10.1096/fj.202100835R
197. Malczewska-Lenczowska J, Sitkowski D, Surala O, Orysiak J, Szczepanska B, Witek K. The association between iron and vitamin d status in female elite athletes. Nutrients. (2018) 10:167. doi: 10.3390/nu10020167
198. Blanco-Rojo R, Perez-Granados AM, Toxqui L, Zazo P, De La Piedra C, Vaquero MP. Relationship between vitamin D deficiency, bone remodelling and iron status in iron-deficient young women consuming an iron-fortified food. Eur J Nutr. (2013) 52:695–703. doi: 10.1007/s00394-012-0375-8
199. Lee JA, Hwang JS, Hwang IT, Kim DH, Seo JH, Lim JS. Low vitamin D levels are associated with both iron deficiency and anemia in children and adolescents. Pediatr Hematol Oncol. (2015) 32:99–108. doi: 10.3109/08880018.2014.983623
200. De La Cruz-Gongora V, Salinas-Rodriguez A, Flores-Aldana M, Villalpando S. Etiology of anemia in older mexican adults: the role of hepcidin, vitamin A and vitamin D. Nutrients. (2021) 13:3814. doi: 10.3390/nu13113814
201. Wood LG, Garg ML, Smart JM, Scott HA, Barker D, Gibson PG. Manipulating antioxidant intake in asthma: a randomized controlled trial. Am J Clin Nutr. (2012) 96:534–43. doi: 10.3945/ajcn.111.032623
202. Lothian JB, Grey V, Lands LC. Effect of whey protein to modulate immune response in children with atopic asthma. Int J Food Sci Nutr. (2006) 57:204–11. doi: 10.1080/09637480600738294
203. Suarez-Varela MM, Alvarez LG, Kogan MD, Ferreira JC, Martinez Gimeno A, Aguinaga Ontoso I, et al. Diet and prevalence of atopic eczema in 6 to 7-year-old schoolchildren in Spain: ISAAC phase III. J Investig Allergol Clin Immunol. (2010) 20:469–75.
204. Loss G, Apprich S, Waser M, Kneifel W, Genuneit J, Buchele G, et al. The protective effect of farm milk consumption on childhood asthma and atopy: the GABRIELA study. J Allergy Clin Immunol. (2011) 128:766–73 e764. doi: 10.1016/j.jaci.2011.07.048
205. Abbring S, Kusche D, Roos TC, Diks MaP, Hols G, Garssen J, et al. Milk processing increases the allergenicity of cow's milk-Preclinical evidence supported by a human proof-of-concept provocation pilot. Clin Exp Allergy. (2019) 49:1013–25. doi: 10.1111/cea.13399
206. Brick T, Schober Y, Bocking C, Pekkanen J, Genuneit J, Loss G, et al. omega-3 fatty acids contribute to the asthma-protective effect of unprocessed cow's milk. J Allergy Clin Immunol. (2016) 137:1699–706 e1613. doi: 10.1016/j.jaci.2015.10.042
207. Abbring S, Hols G, Garssen J, Van Esch B. Raw cow's milk consumption and allergic diseases—The potential role of bioactive whey proteins. Eur J Pharmacol. (2019) 843:55–65. doi: 10.1016/j.ejphar.2018.11.013
208. Abbring S, Ryan JT, Diks MaP, Hols G, Garssen J, Van Esch B. Suppression of food allergic symptoms by raw cow's milk in mice is retained after skimming but abolished after heating the milk-a promising contribution of alkaline phosphatase. Nutrients. (2019) 11:1499. doi: 10.3390/nu11071499
209. Kuczynska B, Puppel K, Golebiewski M, Metera E, Sakowski T, Sloniewski K. Differences in whey protein content between cow's milk collected in late pasture and early indoor feeding season from conventional and organic farms in Poland. J Sci Food Agric. (2012) 92:2899–904. doi: 10.1002/jsfa.5663
210. Stergiadis S, Leifert C, Seal CJ, Eyre MD, Nielsen JH, Larsen MK, et al. Effect of feeding intensity and milking system on nutritionally relevant milk components in dairy farming systems in the North East of England. J Agric Food Chem. (2012) 60:7270–81. doi: 10.1021/jf301053b
211. Fardet A, Rock E. In vitro and in vivo antioxidant potential of milks, yoghurts, fermented milks and cheeses: a narrative review of evidence. Nutr Res Rev. (2018) 31:52–70. doi: 10.1017/S0954422417000191
212. Besle JM, Viala D, Martin B, Pradel P, Meunier B, Berdague JL, et al. Ultraviolet-absorbing compounds in milk are related to forage polyphenols. J Dairy Sci. (2010) 93:2846–56. doi: 10.3168/jds.2009-2939
213. Kuhnen S, Moacyr JR, Mayer JK, Navarro BB, Trevisan R, Honorato LA, et al. Phenolic content and ferric reducing-antioxidant power of cow's milk produced in different pasture-based production systems in southern Brazil. J Sci Food Agric. (2014) 94:3110–7. doi: 10.1002/jsfa.6654
214. Sola-Larrañaga C, Cristina Sola-Larrañaga I. Chemometric analysis of minerals and trace elements in raw cow milk from the community of Navarra, Spain. Food Chem. (2009) 112:189–96. doi: 10.1016/j.foodchem.2008.05.062
215. Gulati A, Galvin N, Lewis E, Hennessy D, O'donovan M, Mcmanus JJ, et al. Outdoor grazing of dairy cows on pasture versus indoor feeding on total mixed ration: Effects on gross composition and mineral content of milk during lactation. J Dairy Sci. (2018) 101:2710–23. doi: 10.3168/jds.2017-13338
216. Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer's patches. Allergy. (2008) 63:882–90. doi: 10.1111/j.1398-9995.2008.01673.x
217. Chen W, Wang W, Ma X, Lv R, Balaso Watharkar R, Ding T, et al. Effect of pH-shifting treatment on structural and functional properties of whey protein isolate and its interaction with (-)-epigallocatechin-3-gallate. Food Chem. (2019) 274:234–41. doi: 10.1016/j.foodchem.2018.08.106
218. Tao F, Xiao C, Chen W, Zhang Y, Pan J, Jia Z. Covalent modification of beta-lactoglobulin by (-)-epigallocatechin-3-gallate results in a novel antioxidant molecule. Int J Biol Macromol. (2019) 126:1186–91. doi: 10.1016/j.ijbiomac.2019.01.017
219. Salvi A, Carrupt P, Tillement J, Testa B. Structural damage to proteins caused by free radicals: asessment, protection by antioxidants, and influence of protein binding. Biochem Pharmacol. (2001) 61:1237–42. doi: 10.1016/S0006-2952(01)00607-4
220. Mirpoor SF, Hosseini SMH, Nekoei AR. Efficient delivery of quercetin after binding to beta-lactoglobulin followed by formation soft-condensed core-shell nanostructures. Food Chem. (2017) 233:282–9. doi: 10.1016/j.foodchem.2017.04.126
221. Li X, Lu Y, Deng R, Zheng T, Lv L. Chemical components from the haulm of Artemisia selengensis and the inhibitory effect on glycation of beta-lactoglobulin. Food Funct. (2015) 6:1841–6. doi: 10.1039/C5FO00117J
222. Zommara M, Toubo H, Sakono M, Imaizumi K. Prevention of peroxidative stress in rats fed on a low vitamin E-containing diet by supplementing with a fermented bovine milk whey preparation: effect of lactic acid and beta-lactoglobulin on the antiperoxidative action. Biosci Biotechnol Biochem. (1998) 62:710–7. doi: 10.1271/bbb.62.710
223. Bartfay WJ, Davis MT, Medves JM, Lugowski S. Milk whey protein decreases oxygen free radical production in a murine model of chronic iron-overload cardiomyopathy. Can J Cardiol. (2003) 19:1163–8.
224. Wang X, Ai T, Meng XL, Zhou J, Mao XY. In vitro iron absorption of alpha-lactalbumin hydrolysate-iron and beta-lactoglobulin hydrolysate-iron complexes. J Dairy Sci. (2014) 97:2559–66. doi: 10.3168/jds.2013-7461
225. Liu HC, Chen WL, Mao SJ. Antioxidant nature of bovine milk beta-lactoglobulin. J Dairy Sci. (2007) 90:547–55. doi: 10.3168/jds.S0022-0302(07)71538-2
226. Kim YE, Kim JW, Cheon S, Nam MS, Kim KK. Alpha-Casein and Beta-Lactoglobulin from Cow Milk Exhibit Antioxidant Activity: A Plausible Link to Antiaging Effects. J Food Sci. (2019) 84:3083–90. doi: 10.1111/1750-3841.14812
227. Guzzi R, Rizzuti B, Labate C, Zappone B, De Santo MP. Ferric Ions Inhibit the Amyloid Fibrillation of beta-Lactoglobulin at High Temperature. Biomacromolecules. (2015) 16:1794–801. doi: 10.1021/acs.biomac.5b00371
228. Cruz-Huerta E, Martinez Maqueda D, De La Hoz L, Da Silva VS, Pacheco MT, Amigo L, et al. Short communication: Identification of iron-binding peptides from whey protein hydrolysates using iron (III)-immobilized metal ion affinity chromatography and reversed phase-HPLC-tandem mass spectrometry. J Dairy Sci. (2016) 99:77–82. doi: 10.3168/jds.2015-9839
229. Banjare IS, Gandhi K, Sao K, Sharma R. Spray-dried whey protein concentrate-iron complex: preparation and physicochemical characterization. Food Technol Biotechnol. (2019) 57:331–40. doi: 10.17113/ftb.57.03.19.6228
230. Miglioranza LH, Matsuo T, Caballero-Cordoba GM, Dichi JB, Cyrino ES, Oliveira IB, et al. Effect of long-term fortification of whey drink with ferrous bisglycinate on anemia prevalence in children and adolescents from deprived areas in Londrina, Parana, Brazil. Nutrition. (2003) 19:419–21. doi: 10.1016/S0899-9007(02)00933-4
231. Kim J, Paik HD, Yoon YC, Park E. Whey protein inhibits iron overload-induced oxidative stress in rats. J Nutr Sci Vitaminol (Tokyo). (2013) 59:198–205. doi: 10.3177/jnsv.59.198
232. Wang J, Radics G, Whelehan M, O'driscoll A, Healy AM, Gilmer JF, et al. Novel iron-whey protein microspheres protect gut epithelial cells from iron-related oxidative stress and damage and improve iron absorption in fasting adults. Acta Haematol. (2017) 138:223–32. doi: 10.1159/000480632
233. Banjare IS, Gandhi K, Sao K, Arora S, Pandey V. Physicochemical properties and oxidative stability of milk fortified with spray-dried whey protein concentrate-iron complex and in vitro bioaccessibility of the added iron. Food Technol Biotechnol. (2019) 57:48–58. doi: 10.17113/ftb.57.01.19.5945
234. Song CY, Chen WL, Yang MC, Huang JP, Mao SJ. Epitope mapping of a monoclonal antibody specific to bovine dry milk: involvement of residues 66-76 of strand D in thermal denatured beta-lactoglobulin. J Biol Chem. (2005) 280:3574–82. doi: 10.1074/jbc.M407031200
235. Zurera-Cosano G, Moreno-Rojas R, Amaro-Lopez M. Effect of processing on contents and relationships of mineral elements of milk. Food Chem. (1994) 51:75–8. doi: 10.1016/0308-8146(94)90050-7
236. Shaheen SO, Macdonald-Wallis C, Lawlor DA, Henderson AJ. Haemoglobin concentrations in pregnancy and respiratory and allergic outcomes in childhood: Birth cohort study. Clin Exp Allergy. (2017) 47:1615–24. doi: 10.1111/cea.13034
237. Bedard A, Lewis SJ, Burgess S, Henderson AJ, Shaheen SO. Maternal iron status during pregnancy and respiratory and atopic outcomes in the offspring: a Mendelian randomisation study. BMJ Open Respir Res. (2018) 5:e000275. doi: 10.1136/bmjresp-2018-000275
238. Quezada-Pinedo HG, Mensink-Bout SM, Reiss IK, Jaddoe VWV, Vermeulen MJ, Duijts L. Maternal iron status during early pregnancy and school-age, lung function, asthma, and allergy: The Generation R Study. Pediatr Pulmonol. (2021) 56:1771–8. doi: 10.1002/ppul.25324
239. Le Huong T, Brouwer ID, Nguyen KC, Burema J, Kok FJ. The effect of iron fortification and de-worming on anaemia and iron status of Vietnamese schoolchildren. Br J Nutr. (2007) 97:955–62. doi: 10.1017/S0007114507659029
240. Vierucci A, De Martino M, Di Palma A, Novembre E, Rossi ME, Resti M, et al. The multitransfused beta-thalassemic child: a model for the study of IgE response. Ann Allergy. (1986) 56:158–61.
241. Patel AP, Krupani S, Stark JM, Mosquera RA, Waller DK, Gonzales T, et al. Validation of the breathmobile case identification survey for asthma screening in children with sickle cell disease. J Asthma. (2021) 58:782–90. doi: 10.1080/02770903.2020.1729381
242. Pardalos G, Kanakoudi-Tsakalidis F, Malaka-Zafiriu M, Tsantali H, Athanasiou-Metaxa M, Kallinikos G, et al. Iron-related disturbances of cell-mediated immunity in multitransfused children with thalassemia major. Clin Exp Immunol. (1987) 68:138–45.
243. De A, Agrawal S, Morrone K, Zhang J, Bjorklund NL, Manwani D, et al. Airway inflammation and lung function in sickle cell disease. Pediatr Allergy Immunol Pulmonol. (2019) 32:92–102. doi: 10.1089/ped.2019.1014
244. Hsieh HY, Huang LC, Yu HR, Kuo KC, Chen WH, Su CH, et al. Pediatric thalassemic patients have higher incidence of asthma: a nationwide population-based retrospective cohort study. PLoS ONE. (2021) 16:e0258727. doi: 10.1371/journal.pone.0258727
245. Pandher K, Ghamrawi RI, Heron CE, Feldman SR. Controversial cardiovascular and hematologic comorbidities in atopic dermatitis. Arch Dermatol Res. (2021). doi: 10.1007/s00403-021-02240-z
246. Hallquist NA, Mcneil LK, Lockwood JF, Sherman AR. Maternal-iron-deficiency effects on peritoneal macrophage and peritoneal natural-killer-cell cytotoxicity in rat pups. Am J Clin Nutr. (1992) 55:741–6. doi: 10.1093/ajcn/55.3.741
247. Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. (2001) 131:568S−79S; discussion 580S. doi: 10.1093/jn/131.2.568S
248. Littwitz-Salomon E, Moreira D, Frost JN, Choi C, Liou KT, Ahern DK, et al. Metabolic requirements of NK cells during the acute response against retroviral infection. Nat Commun. (2021) 12:5376. doi: 10.1038/s41467-021-25715-z
249. Khan A, Singh P, Srivastava A. Synthesis, nature and utility of universal iron chelator—Siderophore: a review. Microbiol Res. (2018) 212–213:103–11. doi: 10.1016/j.micres.2017.10.012
250. Baum P, Toyka KV, Bluher M, Kosacka J, Nowicki M. Inflammatory mechanisms in the pathophysiology of diabetic peripheral neuropathy (DN)-new aspects. Int J Mol Sci. (2021) 22:10835. doi: 10.3390/ijms221910835
251. Dhankar N, Gupta R, Jain SL, Mandal S, Sarkar B. Perturbation of monocyte subsets in iron-deficient children - a shift to a pro-inflammatory state? Allergol Immunopathol (Madr). (2021) 49:42–7. doi: 10.15586/aei.v49i6.91
252. Munoz C, Olivares M, Schlesinger L, Lopez M, Letelier A. Increased in vitro tumour necrosis factor-alpha production in iron deficiency anemia. Eur Cytokine Netw. (1994) 5:401–4.
253. Aly SS, Fayed HM, Ismail AM, Abdel Hakeem GL. Assessment of peripheral blood lymphocyte subsets in children with iron deficiency anemia. BMC Pediatr. (2018) 18:49. doi: 10.1186/s12887-018-0990-5
254. Das I, Saha K, Mukhopadhyay D, Roy S, Raychaudhuri G, Chatterjee M, et al. Impact of iron deficiency anemia on cell-mediated and humoral immunity in children: a case control study. J Nat Sci Biol Med. (2014) 5:158–63. doi: 10.4103/0976-9668.127317
255. Hileti D, Panayiotidis P, Hoffbrand AV. Iron chelators induce apoptosis in proliferating cells. Br J Haematol. (1995) 89:181–7. doi: 10.1111/j.1365-2141.1995.tb08927.x
256. Arezes J, Costa M, Vieira I, Dias V, Kong XL, Fernandes R, et al. Non-transferrin-bound iron (NTBI) uptake by T lymphocytes: evidence for the selective acquisition of oligomeric ferric citrate species. PLoS ONE. (2013) 8:e79870. doi: 10.1371/journal.pone.0079870
257. Jabara HH, Boyden SE, Chou J, Ramesh N, Massaad MJ, Benson H, et al. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat Genet. (2016) 48:74–8. doi: 10.1038/ng.3465
258. Pinto JP, Arezes J, Dias V, Oliveira S, Vieira I, Costa M, et al. Physiological implications of NTBI uptake by T lymphocytes. Front Pharmacol. (2014) 5:24. doi: 10.3389/fphar.2014.00024
259. Weber RA, Yen FS, Nicholson SPV, Alwaseem H, Bayraktar EC, Alam M, et al. Maintaining iron homeostasis is the key role of lysosomal acidity for cell proliferation. Mol Cell. (2020) 77:645–55 e647. doi: 10.1016/j.molcel.2020.01.003
260. Leung S, Holbrook A, King B, Lu HT, Evans V, Miyamoto N, et al. Differential inhibition of inducible T cell cytokine secretion by potent iron chelators. J Biomol Screen. (2005) 10:157–67. doi: 10.1177/1087057104272394
261. Regis G, Bosticardo M, Conti L, De Angelis S, Boselli D, Tomaino B, et al. Iron regulates T-lymphocyte sensitivity to the IFN-gamma/STAT1 signaling pathway in vitro and in vivo. Blood. (2005) 105:3214–21. doi: 10.1182/blood-2004-07-2686
262. Schreiber A, Rousselle A, Klocke J, Bachmann S, Popovic S, Bontscho J, et al. Neutrophil Gelatinase-Associated Lipocalin Protects from ANCA-Induced GN by Inhibiting TH17 Immunity. J Am Soc Nephrol. (2020) 31:1569–84. doi: 10.1681/ASN.2019090879
263. Chen J, Lu WY, Zhao MF, Cao XL, Jiang YY, Jin X, et al. Reactive oxygen species mediated T lymphocyte abnormalities in an iron-overloaded mouse model and iron-overloaded patients with myelodysplastic syndromes. Ann Hematol. (2017) 96:1085–95. doi: 10.1007/s00277-017-2985-y
264. Thorson JA, Smith KM, Gomez F, Naumann PW, Kemp JD. Role of iron in T cell activation: TH1 clones differ from TH2 clones in their sensitivity to inhibition of DNA synthesis caused by IgG Mabs against the transferrin receptor and the iron chelator deferoxamine. Cell Immunol. (1991) 134:126–37. doi: 10.1016/0008-8749(91)90336-A
265. Erb KJ, Ruger B, Von Brevern M, Ryffel B, Schimpl A, Rivett K. Constitutive expression of interleukin (IL)-4 in vivo causes autoimmune-type disorders in mice. J Exp Med. (1997) 185:329–39. doi: 10.1084/jem.185.2.329
266. Weiss G, Bogdan C, Hentze MW. Pathways for the regulation of macrophage iron metabolism by the anti-inflammatory cytokines IL-4 and IL-13. J Immunol. (1997) 158:420–5.
267. Naderi N, Etaati Z, Rezvani Joibari M, Sobhani SA, Hosseni Tashnizi S. Immune deviation in recurrent vulvovaginal candidiasis: correlation with iron deficiency anemia. Iran J Immunol. (2013) 10:118–26.
268. Kuvibidila SR, Velez M, Gardner R, Penugonda K, Chandra LC, Yu L. Iron deficiency reduces serum and in vitro secretion of interleukin-4 in mice independent of altered spleen cell proliferation. Nutr Res. (2012) 32:107–15. doi: 10.1016/j.nutres.2011.12.005
269. Nyakeriga AM, Williams TN, Marsh K, Wambua S, Perlmann H, Perlmann P, et al. Cytokine mRNA expression and iron status in children living in a malaria endemic area. Scand J Immunol. (2005) 61:370–5. doi: 10.1111/j.1365-3083.2005.01573.x
270. Li G, Pone EJ, Tran DC, Patel PJ, Dao L, Xu Z, et al. Iron inhibits activation-induced cytidine deaminase enzymatic activity and modulates immunoglobulin class switch DNA recombination. J Biol Chem. (2012) 287:21520–9. doi: 10.1074/jbc.M112.366732
271. Jang KJ, Mano H, Aoki K, Hayashi T, Muto A, Nambu Y, et al. Mitochondrial function provides instructive signals for activation-induced B-cell fates. Nat Commun. (2015) 6:6750. doi: 10.1038/ncomms7750
272. Afzali B, Gronholm J, Vandrovcova J, O'brien C, Sun HW, Vanderleyden I, et al. BACH2 immunodeficiency illustrates an association between super-enhancers and haploinsufficiency. Nat Immunol. (2017) 18:813–23. doi: 10.1038/ni.3753
273. Rizwan Ahmad AM, Ahmed W, Iqbal S, Mushtaq MH, Anis RA. Iron and prebiotic fortified flour improves the immune function of iron deficient women of childbearing age. Pak J Pharm Sci. (2020) 33:253–61.
274. Duan N, Zhao M, Wang Y, Qu Y, Liu H, Wang H, et al. Expression of BTK/p-BTK is different between CD5(+) and CD5(-) B lymphocytes from autoimmune hemolytic anemia/evans syndromes. Hematology. (2019) 24:588–95. doi: 10.1080/16078454.2019.1652005
275. Noureldin MS, Shaltout AA. Anti-schistosomal IgE and its relation to gastrointestinal allergy in breast-fed infants of Schistosoma mansoni infected mothers. J Egypt Soc Parasitol. (1998) 28:539–50.
276. Seka-Seka J, Brouh Y, Yapo-Crezoit AC, Atseye NH. The role of serum immunoglobulin E in the pathogenesis of Plasmodium falciparum malaria in Ivorian children. Scand J Immunol. (2004) 59:228–30. doi: 10.1111/j.0300-9475.2004.01337.x
277. Magro AM, Brai M. Evidence for lipoxygenase activity in induction of histamine release from rat peritoneal mast cells by chelated iron. Immunology. (1983) 49:1–8.
278. Mecheri S, Peltre G, Lapeyre J, David B. Biological effect of transferrin on mast cell mediator release during the passive cutaneous anaphylaxis reaction: a possible inhibition mechanism involving iron. Ann Inst Pasteur Immunol. (1987) 138:213–21. doi: 10.1016/S0769-2625(87)80072-7
279. Theobald K, Gross-Weege W, Keymling J, Konig W. Purification of serum proteins with inhibitory activity on the histamine release in vitro and/or in vivo. Int Arch Allergy Appl Immunol. (1987) 82:295–7. doi: 10.1159/000234211
280. Nakashima K, Takeuchi T, Shirakawa T. Differentiation, distribution, and chemical state of intracellular trace elements in LAD2 mast cell line. Biol Trace Elem Res. (2005) 108:105–14. doi: 10.1385/BTER:108:1-3:105
281. Afify SM, Regner A, Pacios LF, Blokhuis BR, Jensen SA, Redegeld FA, et al. Micronutritional supplementation with a holoBLG-based FSMP (food for special medical purposes)-lozenge alleviates allergic symptoms in BALB/c mice: Imitating the protective farm effect. Clin Exp Allergy. (2022) 52:426–41. doi: 10.1111/cea.14050
282. Vanderford DA, Greer PK, Sharp JM, Chichlowski M, Rouse DC, Selim MA, et al. Alopecia in IL-10-deficient mouse pups is c-kit-dependent and can be triggered by iron deficiency. Exp Dermatol. (2010) 19:518–26. doi: 10.1111/j.1600-0625.2009.01032.x
283. Miethke M. Molecular strategies of microbial iron assimilation: from high-affinity complexes to cofactor assembly systems. Metallomics. (2013) 5:15–28. doi: 10.1039/C2MT20193C
284. Winkelmann G. Ecology of siderophores with special reference to the fungi. Biometals. (2007) 20:379–92. doi: 10.1007/s10534-006-9076-1
285. Voss B, Kirschhofer F, Brenner-Weiss G, Fischer R. Alternaria alternata uses two siderophore systems for iron acquisition. Sci Rep. (2020) 10:3587. doi: 10.1038/s41598-020-60468-7
286. Saha M, Sarkar S, Sarkar B, Sharma BK, Bhattacharjee S, Tribedi P. Microbial siderophores and their potential applications: a review. Environ Sci Pollut Res Int. (2016) 23:3984–99. doi: 10.1007/s11356-015-4294-0
287. Fritts RK, Mccully AL, Mckinlay JB. Extracellular Metabolism Sets the Table for Microbial Cross-Feeding. Microbiol Mol Biol Rev. (2021) 85. doi: 10.1128/MMBR.00135-20
288. Verma S, Prescott R, Cherayil BJ. The commensal bacterium Bacteroides fragilis down-regulates ferroportin expression and alters iron homeostasis in macrophages. J Leukoc Biol. (2019) 106:1079–88. doi: 10.1002/JLB.2A1018-408RR
289. Hider RC, Kong X. Chemistry and biology of siderophores. Nat Prod Rep. (2010) 27:637–57. doi: 10.1039/b906679a
290. Josefsdottir KS, Baldridge MT, Kadmon CS, King KY. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood. (2017) 129:729–39. doi: 10.1182/blood-2016-03-708594
291. Lee MJ, Kang MJ, Lee SY, Lee E, Kim K, Won S, et al. Perturbations of gut microbiome genes in infants with atopic dermatitis according to feeding type. J Allergy Clin Immunol. (2018) 141:1310–9. doi: 10.1016/j.jaci.2017.11.045
292. Kim HJ, Lee SH, Hong SJ. Antibiotics-Induced Dysbiosis of Intestinal Microbiota Aggravates Atopic Dermatitis in Mice by Altered Short-Chain Fatty Acids. Allergy Asthma Immunol Res. (2020) 12:137–48. doi: 10.4168/aair.2020.12.1.137
293. Kalliomaki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol. (2001) 107:129–34. doi: 10.1067/mai.2001.111237
294. Candela M, Rampelli S, Turroni S, Severgnini M, Consolandi C, De Bellis G, et al. Unbalance of intestinal microbiota in atopic children. BMC Microbiol. (2012) 12:95. doi: 10.1186/1471-2180-12-95
295. Ling Z, Li Z, Liu X, Cheng Y, Luo Y, Tong X, et al. Altered fecal microbiota composition associated with food allergy in infants. Appl Environ Microbiol. (2014) 80:2546–54. doi: 10.1128/AEM.00003-14
296. Berni Canani R, Sangwan N, Stefka AT, Nocerino R, Paparo L, Aitoro R, et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. (2016) 10:742–50. doi: 10.1038/ismej.2015.151
297. Chen CC, Chen KJ, Kong MS, Chang HJ, Huang JL. Alterations in the gut microbiotas of children with food sensitization in early life. Pediatr Allergy Immunol. (2016) 27:254–62. doi: 10.1111/pai.12522
298. Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. (2016). doi: 10.1038/nm.4176
299. Chua HH, Chou HC, Tung YL, Chiang BL, Liao CC, Liu HH, et al. intestinal dysbiosis featuring abundance of ruminococcus gnavus associates with allergic diseases in infants. Gastroenterology. (2018) 154:154–67. doi: 10.1053/j.gastro.2017.09.006
300. Boutin RCT, Sbihi H, Mclaughlin RJ, Hahn AS, Konwar KM, Loo RS, et al. Composition and associations of the infant gut fungal microbiota with environmental factors and childhood allergic outcomes. MBio. (2021) 12:e0339620. doi: 10.1128/mBio.03396-20
301. Hyytiainen H, Kirjavainen PV, Taubel M, Tuoresmaki P, Casas L, Heinrich J, et al. Microbial diversity in homes and the risk of allergic rhinitis and inhalant atopy in two European birth cohorts. Environ Res. (2021) 196:110835. doi: 10.1016/j.envres.2021.110835
302. Petersen C, Dai DLY, Boutin RCT, Sbihi H, Sears MR, Moraes TJ, et al. A rich meconium metabolome in human infants is associated with early-life gut microbiota composition and reduced allergic sensitization. Cell Rep Med. (2021) 2:100260. doi: 10.1016/j.xcrm.2021.100260
303. Joseph CL, Sitarik AR, Kim H, Huffnagle G, Fujimura K, Yong GJM, et al. Infant gut bacterial community composition and food-related manifestation of atopy in early childhood. Pediatr Allergy Immunol. (2022) 33:e13704. doi: 10.1111/pai.13704
304. Caza M, Kronstad J. Shared and distinct mechanisms of iron acquisition by bacterial and fungal pathogens of humans. Front Cell Infect Microbiol. (2013) 3:80. doi: 10.3389/fcimb.2013.00080
305. Ekins A, Khan AG, Shouldice SR, Schryvers AB. Lactoferrin receptors in gram-negative bacteria: insights into the iron acquisition process. Biometals. (2004) 17:235–43. doi: 10.1023/B:BIOM.0000027698.43322.60
306. Zambolin S, Clantin B, Chami M, Hoos S, Haouz A, Villeret V, et al. Structural basis for haem piracy from host haemopexin by Haemophilus influenzae. Nat Commun. (2016) 7:11590. doi: 10.1038/ncomms11590
307. Tong Y, Guo M. Bacterial heme-transport proteins and their heme-coordination modes. Arch Biochem Biophys. (2009) 481:1–15. doi: 10.1016/j.abb.2008.10.013
308. Porcheron G, Garenaux A, Proulx J, Sabri M, Dozois CM. Iron, copper, zinc, and manganese transport and regulation in pathogenic Enterobacteria: correlations between strains, site of infection and the relative importance of the different metal transport systems for virulence. Front Cell Infect Microbiol. (2013) 3:90. doi: 10.3389/fcimb.2013.00090
309. Page MGP. The Role of Iron and Siderophores in Infection, and the Development of Siderophore Antibiotics. Clin Infect Dis. (2019) 69:S529–37. doi: 10.1093/cid/ciz825
310. Holten-Andersen N, Harrington MJ, Birkedal H, Lee BP, Messersmith PB, Lee KY, et al. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc Natl Acad Sci USA. (2011) 108:2651–5. doi: 10.1073/pnas.1015862108
311. Neilands JB. Hydroxamic acids in nature. Science. (1967) 156:1443–7. doi: 10.1126/science.156.3781.1443
312. Eisendle M, Oberegger H, Buttinger R, Illmer P, Haas H. Biosynthesis and uptake of siderophores is controlled by the PacC-mediated ambient-pH Regulatory system in Aspergillus nidulans. Eukaryot Cell. (2004) 3:561–3. doi: 10.1128/EC.3.2.561-563.2004
313. Paauw A, Leverstein-Van Hall MA, Van Kessel KP, Verhoef J, Fluit AC. Yersiniabactin reduces the respiratory oxidative stress response of innate immune cells. PLoS ONE. (2009) 4:e8240. doi: 10.1371/journal.pone.0008240
314. Li Y, Wang Z, Liu X, Song Z, Li R, Shao C, et al. Siderophore biosynthesis but not reductive iron assimilation is essential for the dimorphic fungus nomuraea rileyi conidiation, dimorphism transition, resistance to oxidative stress, pigmented microsclerotium formation, and virulence. Front Microbiol. (2016) 7:931. doi: 10.3389/fmicb.2016.00931
315. Barry SM, Challis GL. Recent advances in siderophore biosynthesis. Curr Opin Chem Biol. (2009) 13:205–15. doi: 10.1016/j.cbpa.2009.03.008
316. Ahmadi MK, Fawaz S, Jones CH, Zhang G, Pfeifer BA. Total biosynthesis and diverse applications of the nonribosomal peptide-polyketide siderophore yersiniabactin. Appl Environ Microbiol. (2015) 81:5290–8. doi: 10.1128/AEM.01373-15
317. Butler A, Theisen RM. Iron(III)-siderophore coordination chemistry: reactivity of marine siderophores. Coord Chem Rev. (2010) 254:288–96. doi: 10.1016/j.ccr.2009.09.010
318. Kritas SK, Saggini A, Varvara G, Murmura G, Caraffa A, Antinolfi P, et al. Luteolin inhibits mast cell-mediated allergic inflammation. J Biol Regul Homeost Agents. (2013) 27:955–9.
319. Jafarinia M, Sadat Hosseini M, Kasiri N, Fazel N, Fathi F, Ganjalikhani Hakemi M, et al. Quercetin with the potential effect on allergic diseases. Allergy Asthma Clin Immunol. (2020) 16:36. doi: 10.1186/s13223-020-00434-0
320. Higa S, Hirano T, Kotani M, Matsumoto M, Fujita A, Suemura M, et al. Fisetin, a flavonol, inhibits TH2-type cytokine production by activated human basophils. J Allergy Clin Immunol. (2003) 111:1299–306. doi: 10.1067/mai.2003.1456
321. Finn DF, Walsh JJ. Twenty-first century mast cell stabilizers. Br J Pharmacol. (2013) 170:23–37. doi: 10.1111/bph.12138
322. Singh A, Demont A, Actis-Goretta L, Holvoet S, Leveques A, Lepage M, et al. Identification of epicatechin as one of the key bioactive constituents of polyphenol-enriched extracts that demonstrate an anti-allergic effect in a murine model of food allergy. Br J Nutr. (2014) 112:358–68. doi: 10.1017/S0007114514000877
323. Patrizi A, Raone B, Neri I, Gurioli C, Carbonara M, Cassano N, et al. Randomized, controlled, double-blind clinical study evaluating the safety and efficacy of MD2011001 cream in mild-to-moderate atopic dermatitis of the face and neck in children, adolescents and adults. J Dermatolog Treat. (2016) 27:346–50. doi: 10.3109/09546634.2015.1115814
324. Masuda S, Maeda-Yamamoto M, Usui S, Fujisawa T. 'Benifuuki' green tea containing o-methylated catechin reduces symptoms of Japanese cedar pollinosis: a randomized, double-blind, placebo-controlled trial. Allergol Int. (2014) 63:211–7. doi: 10.2332/allergolint.13-OA-0620
325. Siso-Terraza P, Luis-Villarroya A, Fourcroy P, Briat JF, Abadia A, Gaymard F, et al. Accumulation and secretion of coumarinolignans and other coumarins in arabidopsis thaliana roots in response to iron deficiency at high pH. Front Plant Sci. (2016) 7:1711. doi: 10.3389/fpls.2016.01711
326. Connorton JM, Balk J, Rodriguez-Celma J. Iron homeostasis in plants—a brief overview. Metallomics. (2017) 9:813–23. doi: 10.1039/C7MT00136C
327. Ceballos-Laita L, Gutierrez-Carbonell E, Lattanzio G, Vazquez S, Contreras-Moreira B, Abadia A, et al. Protein profile of Beta vulgaris leaf apoplastic fluid and changes induced by Fe deficiency and Fe resupply. Front Plant Sci. (2015) 6:145. doi: 10.3389/fpls.2015.00145
328. Gondor OK, Janda T, Soos V, Pal M, Majlath I, Adak MK, et al. Salicylic acid induction of flavonoid biosynthesis pathways in wheat varies by treatment. Front Plant Sci. (2016) 7:1447. doi: 10.3389/fpls.2016.01447
329. Wasli H, Jelali N, Saada M, Ksouri R, Cardoso SM. Insights on the adaptation of foeniculum vulgare mill to iron deficiency. Applied Sciences (Switzerland). (2021) 11. doi: 10.3390/app11157072
330. Bocchini M, Bartucca ML, Ciancaleoni S, Mimmo T, Cesco S, Pii Y, et al. Iron deficiency in barley plants: phytosiderophore release, iron translocation, and DNA methylation. Front Plant Sci. (2015) 6:514. doi: 10.3389/fpls.2015.00514
331. Trapet PL, Verbon EH, Bosma RR, Voordendag K, Van Pelt JA, Pieterse CMJ. Mechanisms underlying iron deficiency-induced resistance against pathogens with different lifestyles. J Exp Bot. (2021) 72:2231–41. doi: 10.1093/jxb/eraa535
332. Hwang H-J, Kim H, Yu H-J, Oh M-H, Lee I, Kim S-G. Gene encoding pathogenesis-related 10 protein of Lithospermum erythrorhizon is responsive to exogenous stimuli related to the plant defense system. Plant Science. (2003) 165:1297–302. doi: 10.1016/S0168-9452(03)00341-8
333. Liu J-J, Ekramoddoullah AKM. The family 10 of plant pathogenesis-related proteins: Their structure, regulation, and function in response to biotic and abiotic stresses. Physiol Mol Plant Pathol. (2006) 68:3–13. doi: 10.1016/j.pmpp.2006.06.004
334. Ali S, Ganai BA, Kamili AN, Bhat AA, Mir ZA, Bhat JA, et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol Res 212-213. (2018) 29–37. doi: 10.1016/j.micres.2018.04.008
335. Tajik S, Zarinkamar F, Soltani BM, Nazari M. Induction of phenolic and flavonoid compounds in leaves of saffron (Crocus sativus L.) by salicylic acid. Scientia Horticulturae. (2019) 257:108751. doi: 10.1016/j.scienta.2019.108751
336. Yamamoto R, Ma G, Zhang L, Hirai M, Yahata M, Yamawaki K, et al. Effects of salicylic acid and methyl jasmonate treatments on flavonoid and carotenoid accumulation in the juice sacs of satsuma mandarin in vitro. Applied Sciences (Switzerland). (2020) 10:1–13. doi: 10.3390/app10248916
337. Sinha M, Singh RP, Kushwaha GS, Iqbal N, Singh A, Kaushik S, et al. Current overview of allergens of plant pathogenesis related protein families. ScientificWorldJournal. (2014) 2014:543195. doi: 10.1155/2014/543195
338. Aglas L, Soh WT, Kraiem A, Wenger M, Brandstetter H, Ferreira F. Ligand Binding of PR-10 Proteins with a Particular Focus on the Bet v 1 Allergen Family. Curr Allergy Asthma Rep. (2020) 20:25. doi: 10.1007/s11882-020-00918-4
339. Romera FJ, García MJ, Lucena C, Martínez-Medina A, Aparicio MA, Ramos J, et al. Induced systemic resistance (ISR) and Fe deficiency responses in dicot plants. Front Plant Sci. (2019) 10:287. doi: 10.3389/fpls.2019.00287
340. Grobelak A, Hiller J. Bacterial siderophores promote plant growth: Screening of catechol and hydroxamate siderophores. Int J Phytoremediation. (2017) 19:825–33. doi: 10.1080/15226514.2017.1290581
341. Mishra AK, Baek KH. Salicylic Acid Biosynthesis and Metabolism: A Divergent Pathway for Plants and Bacteria. Biomolecules. (2021) 11. doi: 10.3390/biom11050705
342. Hesselink RW, Findlay JB. Expression, characterization and ligand specificity of lipocalin-1 interacting membrane receptor (LIMR). Mol Membr Biol. (2013) 30:327–37. doi: 10.3109/09687688.2013.823018
343. Stewart GA, Thompson PJ. The biochemistry of common aeroallergens. Clin Exp Allergy. (1996) 26:1020–44. doi: 10.1046/j.1365-2222.1996.d01-405.x
344. Kushibiki S, Hodate K, Kurisaki J, Shingu H, Ueda Y, Watanabe A, et al. Effect of beta-lactoglobulin on plasma retinol and triglyceride concentrations, and fatty acid composition in calves. J Dairy Res. (2001) 68:579–86. doi: 10.1017/S0022029901005040
345. Roth-Walter F, Gomez-Casado C, Pacios LF, Mothes-Luksch N, Roth GA, Singer J, et al. Bet v 1 from Birch Pollen is a Lipocalin-like Protein acting as Allergen only when devoid of Iron by promoting Th2 lymphocytes. J Biol Chem. (2014). doi: 10.1074/jbc.A114.567875
346. Hufnagl K, Ghosh D, Wagner S, Fiocchi A, Dahdah L, Bianchini R, et al. Retinoic acid prevents immunogenicity of milk lipocalin Bos d 5 through binding to its immunodominant T-cell epitope. Sci Rep. (2018) 8:1598. doi: 10.1038/s41598-018-19883-0
347. Hufnagl K, Afify SM, Braun N, Wagner S, Wallner M, Hauser M, et al. Retinoic acid-loading of the major birch pollen allergen Bet v 1 may improve specific allergen immunotherapy: In silico, in vitro and in vivo data in BALB/c mice. Allergy. (2020) 75:2073–7. doi: 10.1111/all.14259
348. Afify SM, Pali-Scholl I, Hufnagl K, Hofstetter G, El-Bassuoni Ma-R, Roth-Walter F, et al. Bovine Holo-Beta-Lactoglobulin Cross-Protects Against Pollen Allergies in an Innate Manner in BALB/c Mice: Potential Model for the Farm Effect. Front Immunol. (2021) 12:176. doi: 10.3389/fimmu.2021.611474
349. Seutter Von Loetzen C, Hoffmann T, Hartl MJ, Schweimer K, Schwab W, Rosch P, et al. Secret of the major birch pollen allergen Bet v 1: identification of the physiological ligand. Biochem J. (2014) 457:379–90. doi: 10.1042/BJ20130413
350. Jacob T, Von Loetzen CS, Reuter A, Lacher U, Schiller D, Schobert R, et al. Identification of a natural ligand of the hazel allergen Cor a 1. Sci Rep. (2019) 9:8714. doi: 10.1038/s41598-019-44999-2
351. Casanal A, Zander U, Dupeux F, Valpuesta V, Marquez JA. Purification, crystallization and preliminary X-ray analysis of the strawberry allergens Fra a 1E and Fra a 3 in the presence of catechin. Acta Crystallogr Sect F Struct Biol Cryst Commun. (2013) 69:510–4. doi: 10.1107/S1744309113006945
352. Vesic J, Stambolic I, Apostolovic D, Milcic M, Stanic-Vucinic D, Cirkovic Velickovic T. Complexes of green tea polyphenol, epigalocatechin-3-gallate, and 2S albumins of peanut. Food Chem. (2015) 185:309–17. doi: 10.1016/j.foodchem.2015.04.001
353. Offermann LR, Yarbrough J, Mcbride J, Hurlburt BK, Maleki SJ, Pote SS, et al. Structure of PR 10 Allergen Ara h 8.01 with Quercetin. (2022). Available at: https://www.rcsb.org/structure/6B1DRCSB PDB
354. Hurlburt BK, Offermann LR, Mcbride JK, Majorek KA, Maleki SJ, Chruszcz M. Structure and function of the peanut panallergen Ara h 8. J Biol Chem. (2013) 288:36890–901. doi: 10.1074/jbc.M113.517797
355. Van Boxtel EL, Van Den Broek LA, Koppelman SJ, Vincken JP, Gruppen H. Peanut allergen Ara h 1 interacts with proanthocyanidins into higher molecular weight complexes. J Agric Food Chem. (2007) 55:8772–8. doi: 10.1021/jf071585k
356. Schafer T, Merkl J, Klemm E, Wichmann HE, Ring J. We and our pets: allergic together? Acta Vet Hung. (2008) 56:153–61. doi: 10.1556/avet.56.2008.2.2
357. Gomez-Casado C, Roth-Walter F, Jensen-Jarolim E, Diaz-Perales A, Pacios LF. Modeling iron-catecholates binding to NGAL protein. J Mol Graph Model. (2013) 45:111–21. doi: 10.1016/j.jmgm.2013.08.013
358. Devireddy LR, Gazin C, Zhu X, Green MR. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. (2005) 123:1293–305. doi: 10.1016/j.cell.2005.10.027
359. Guo H, Jin D, Chen X. Lipocalin 2 is a regulator of macrophage polarization and NF-kappaB/STAT3 pathway activation. Mol Endocrinol. (2014) 28:1616–28. doi: 10.1210/me.2014-1092
360. Meier JK, Schnetz M, Beck S, Schmid T, Dominguez M, Kalinovic S, et al. Iron-Bound Lipocalin-2 Protects Renal Cell Carcinoma from Ferroptosis. Metabolites. (2021) 11. doi: 10.3390/metabo11050329
361. 0.Proteinatlas.Org/Ensg00000148346-Lcn2 LCN2 [Online]. proteinatlas.org. Available: https://www.proteinatlas.org/ENSG00000148346-LCN2 (accessed January 13, 2022).
362. Li X, Wei L, Jia L, Li M, Zhu L, Liu L, et al. Identification and characterization of cow's milk proteins from the rat intestinal lymph using a proteomic strategy. Proteomics. (2013) 13:2649–56. doi: 10.1002/pmic.201300097
363. Meyer R, Chebar Lozinsky A, Fleischer DM, Vieira MC, Du Toit G, Vandenplas Y, et al. Diagnosis and management of Non-IgE gastrointestinal allergies in breastfed infants-An EAACI Position Paper. Allergy. (2020) 75:14–32. doi: 10.1111/all.13947
364. Chodaczek G, Saavedra-Molina A, Bacsi A, Kruzel ML, Sur S, Boldogh I. Iron-mediated dismutation of superoxide anion augments antigen-induced allergic inflammation: effect of lactoferrin. Postepy Hig Med Dosw (Online). (2007) 61:268–76.
365. Tong P, Gao L, Gao J, Li X, Wu Z, Yang A, et al. Iron-induced chelation alleviates the potential allergenicity of ovotransferrin in a BALB/c mouse model. Nutr Res. (2017) 47:81–9. doi: 10.1016/j.nutres.2017.09.009
366. Pfaar O, Demoly P, Gerth Van Wijk R, Bonini S, Bousquet J, Canonica GW, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. (2014) 69:854–67. doi: 10.1111/all.12383
367. Bartosik T, Jensen SA, Afify S, Bianchini R, Hufnagl K, Hofstetter G, et al. Ameliorating allergic symptoms by supplementing micronutritional deficiencies in immune cells with a holoBLG-based FSMP (food for specific medical purposes)-lozenge in a double-blind placebo-controlled trial. In: Annual Congress of the European Academy of Allergy and Clinical Immunology EAACI 2021. Allergy (2021).
368. Bartosik T, Jensen SA, Afify SM, Bianchini R, Hufnagl K, Hofstetter G, et al. Ameliorating Atopy by Compensating Micronutritional Deficiencies in Immune cells: a Double-Blind Placebo-Controlled Pilot Study. J Allergy Clin Immunol Pract. (2022). doi: 10.1016/j.jaip.2022.02.028. [Epub ahead of print].
369. Bergmann KC, Graessel A, Raab J, Banghard W, Krause L, Becker S, et al. Targeted micronutrition via holo-BLG based on the farm effect in house dust mite allergic rhinoconjunctivitis patients—first evaluation in a standardized allergen exposure chamber. Allergo J Int. (2021). doi: 10.1007/s40629-021-00163-9
370. Bergmann K-C, Raab J, Krause L, Becker S, Kugler S, Zuberbier T, et al. Long-term benefits of targeted micronutrition with the holoBLG lozenge in house dust mite allergic patients. Allergo J Int. (2022). doi: 10.1007/s40629-021-00197-z
371. G.I.F. Asthma. GINA. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. (2021). Available from: www.ginasthma.org
372. Artuso I, Lidonnici MR, Altamura S, et al. Transferrin receptor 2 is a potential novel therapeutic target for beta-thalassemia: evidence from a murine model. Blood. (2018) 132:2286–97. doi: 10.1182/blood.2019001583
373. https://Www.Proteinatlas.Org/Ensg00000106327-Tfr2TFR2 [Online]. proteinatlas.org. Available: https://www.proteinatlas.org/ENSG00000106327-TFR2 (accessed November 29, 2021).
Keywords: iron-deficiency, atopic diseases, pathogenesis-related proteins, siderophores, polyphenols, lipocalin, holoBLG, immunonutrition
Citation: Roth-Walter F (2022) Iron-Deficiency in Atopic Diseases: Innate Immune Priming by Allergens and Siderophores. Front. Allergy 3:859922. doi: 10.3389/falgy.2022.859922
Received: 22 January 2022; Accepted: 03 March 2022;
Published: 10 May 2022.
Edited by:
Ines Swoboda, University of Applied Sciences Wien, AustriaReviewed by:
Josefina Zakzuk, University of Cartagena, ColombiaDaniel P. Potaczek, University of Marburg, Germany
Copyright © 2022 Roth-Walter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Franziska Roth-Walter, ZnJhbnppc2thLnJvdGgtd2FsdGVyQG1lZHVuaXdpZW4uYWMuYXQ=; ZnJhbnppc2thLnJvdGgtd2FsdGVyQHZldG1lZHVuaS5hYy5hdA==
 Franziska Roth-Walter
Franziska Roth-Walter