
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Allergy , 05 December 2022
Sec. Drug, Venom & Anaphylaxis
Volume 3 - 2022 | https://doi.org/10.3389/falgy.2022.1050048
This article is part of the Research Topic Highlights in Drug, Venom & Anaphylaxis View all 5 articles
 Nikolaos Kitsos1*
Nikolaos Kitsos1* Dimitrios Cassimos2
Dimitrios Cassimos2 Grigorios Trypsianis2
Grigorios Trypsianis2 Ioannis Xinias1
Ioannis Xinias1 Emmanouil Roilides1
Emmanouil Roilides1 Ioanna Grivea3
Ioanna Grivea3 Elpis Mantadakis2
Elpis Mantadakis2 Antigoni Mavroudi1
Antigoni Mavroudi1
Background: Adverse antibiotic reactions caused by an immunological mechanism are known as allergic reactions. The percentage of reported antibiotic allergies is likely to differ from the one validated after a drug provocation test (DPT) with the culprit antibiotic. This study aimed to compare the percentage of children who were thought to be allergic to a certain antibiotic with those who have a true allergy, as confirmed by DPTs. We also validated Skin Prick Tests (SPTs) and Intradermal Tests (IDTs) by assessing their sensitivity and specificity, in diagnosing antibiotic allergies using DPT as the gold standard. Furthermore, we investigated epidemiological risk factors such as personal and family history of atopic disease and eosinophilia.
Methods: Children with a history of possible allergic reaction to an antibiotic underwent a diagnostic procedure that included: (1) Eosinophil blood count, (2) SPTs, (3) IDTs and (4) DPTs. The parameters were compared with Pearson's Chi-Square and Fisher's Exact Test. Several risk factors that were found significant in univariate analysis, such as personal and family history of atopic disease, and positive SPTs and IDTs were examined with multiple logistic regression analysis to see if they were related to a higher risk for a positive DPT.
Results: Semi-synthetic penicillin was the most common group of antibiotics thought to cause allergic reactions in this study. Overall, 123 children with a personal history of an adverse reaction to a certain antibiotic, were evaluated. In 87.8% of the cases, the symptoms had occurred several hours after administration of the culprit antibiotic. Both SPTs and IDTs had low sensitivity but high specificity. Moreover, they had a high positive predictive value (PPV). In contrast, eosinophilia was not recognized as a risk factor. Seventeen patients (13.8%) had a true antibiotic allergy, as confirmed by a positive DPT. A positive IDT was a strong predictor of a positive DPT, along with a positive personal and family history of atopy.
Conclusion: SPTs and IDTs are very reliable in confirming antibiotic allergy when found positive. A negative result of a SPT highly predicts a negative DPT. A positive IDT and a positive personal and family history of atopy were recognized as significant risk factors for antibiotic allergy.
Beta-lactam antibiotics are the most common cause of drug-induced hypersensitivity reactions in children. However, non-beta-lactam (NBL) antibiotics can also cause hypersensitivity responses, estimated to be between 1% and 3% of the general population (1). Most articles on antibiotic allergy have focused on beta-lactam hypersensitivity, whereas reactions to NBLs have been presented mainly as case reports (2). The management of antibiotic allergy begins with identifying the culprit antibiotic based on a comprehensive medical history. A detailed medical history for allergies and a meticulous physical examination are critical for accurately diagnosing drug-induced reactions (3).
To identify immediate allergic reactions to antibiotics, SPTs and IDTs are available. IDTs are used for immediate as well as late reactions and are evaluated immediately and after 24 and 72 h. For late reactions, an infiltrating erythematous wheal is characterized as a positive reaction. Immediate positive reactions consist of a wheal with surrounding erythema. However, only the wheal is measured. A SPT is considered positive if the wheal has a minimum diameter of 3 mm (4, 5). However, these are mainly standardized for beta-lactam antibiotics and not for other antibiotic groups. Skin and/or provocation tests may be used to confirm reactions (6–9).
Even though the percentage of children with reported allergies vs. those confirmed by DPTs has been determined previously, in some populations, the validity of SPTs and IDTs has not been adequately defined in the current literature. Therefore, this study aimed to provide additional evidence regarding the diagnostic value of SPTs and IDTs in diagnosing antibiotic allergies. Furthermore, several risk factors, such as peripheral eosinophilia and personal and family history of atopic disease were investigated for an association with the risk of true antibiotic allergy.
Our study took place at the University Pediatric Allergy Department of the Hippokrateion Hospital of Thessaloniki. We recruited children aged 1 to 15 years old, who were referred with a clinical diagnosis of antibiotic allergy (Figure 1). The bioethics committee of the Aristotle University of Thessaloniki approved the research design and protocol (number of approval 424, 20/01/22). The initial selection of the children was carried out using the standardized European Academy of Allergy and Clinical Immunology (EAACI) questionnaire of the European Network of Drug Allergy (ENDA) (10). The questionnaire included a detailed medical and allergy history (both personal and family history), the initial symptoms of the allergic reaction, the culprit antibiotic, the cause of antibiotic treatment, the treatment of the hypersensitivity reaction, the organ systems affected by the reaction, and finally the route and dose of the administered drug.
Drug hypersensitivity reactions that occurred 1–6 h after the drug administration were considered immediate reactions, whereas those that occurred after 6 h or more were considered late reactions.
The exclusion criteria were: (1) Age: infants < 1-year-old and teenagers > 15-years-old, (2) Previous anaphylaxis to the culprit antibiotic, (3) Severe skin and mucosal reactions (e.g., drug reaction with eosinophilia and systemic symptoms, Stevens-Johnson syndrome, toxic epidermal necrolysis, acute generalized exanthematous pustulosis) to the culprit antibiotic (Figure 1).
We performed SPTs and IDTs on all subjects. Both SPTs, IDTs, and DPTs were performed by medical and nursing personnel trained to identify and treat anaphylactic reactions. The concentration of the drugs used for the skin tests had been proven to be nonirritating in previous studies, shown in Table 1 (11, 12).
We performed DPTs on all subjects. The initial dose of the examined antibiotic administered to the subjects was 1/4 of the maximum single unit dose, followed within 30 min intervals by administration of half of the maximum single unit dose and finally, the maximum single unit dose. The latter was calculated according to the patient's age and weight. The culprit antibiotic was subsequently administered at home for four more consecutive days (13).
Statistical analysis was performed using IBM Statistical Package for the Social Sciences (SPSS), version 19.0 (IBM Corp., Armonk, NY, United States). Quantitative variables are expressed as mean ± standard deviation (SD) and qualitative variables are expressed as absolute and relative (%) frequencies. The association between qualitative variables was assessed using the chi-square test and Fisher's exact test. For the evaluation of the independent effect of children's characteristics on the risk of being diagnosed with an antibiotic allergy, multiple stepwise logistic regression analyses were used and adjusted for all children's characteristics of interest, i.e., sex, age, history of atopic disease, eosinophilia, positive SPT, and IDT. Odds ratios (OR) with their 95% confidence intervals (CI) were estimated as the measure of the above associations. All tests were two-tailed and statistical significance was set at p values <0.05.
Nine hundred and ninety-one (991) children visited the University Pediatric Allergy Department between December 2017 and February 2020. Overall, 123 children reported hypersensitivity reactions to antibiotics, 73 boys and 50 girls. The mean age was 7.5 years (SD ± 4.5). Thirty-nine children (31.7%) had an atopic background (eczema, food allergy, allergic rhinitis or allergic asthma). Demographics of the study population are shown in Table 2. The most common diagnosis was a respiratory tract infection (95.1%).
The analysis showed that most reported reactions were due to the penicillin group (58.6%). In 30.1% (n = 37) of the cases, the suspected drug was amoxicillin, while in 28.5% (n = 35) of the cases, the suspected drug was the combination of amoxicillin with clavulanic acid. Cephalosporines and macrolides represented 27.6% (n = 34) and 13.8% (n = 17) of suspected antibiotic allergy, respectively.
In our research, 87.8% (n = 108) of the patients had symptoms that occurred several hours after the antibiotic administration, and in 12.2% (n = 15) of the cases, symptoms appeared in the first hour after the antibiotic administration. We also found that most children (92.7%, n = 114) had symptoms from the skin, such as urticaria, angioedema, flushing, or maculopapular rash. A percentage of 6.5% had symptoms from the gastrointestinal tract, such as vomiting, nausea, and abdominal pain.
Seventeen out of 123 patients (13.8%) had a positive DPT. One out of seventeen patients (5.8%) with a positive DPT developed a severe systemic reaction that fulfilled the diagnostic criteria of anaphylaxis. This single patient was a 12-year-old girl with a suspected clarithromycin allergy. She developed a fine papular exanthema 2 h after the intake of the third dose of clarithromycin. During DPT, 30 min after the intake of the last dose, she developed a maculopapular rash over large areas of her body, along with abdominal pain, severe vomiting, diarrhea, and malaise. She received symptomatic therapy with administration of adrenaline, antihistamines, and intravenous fluids. Most of the patients, i.e., 94.2% of them (16 out of 17), presented a delayed positive DPT with mild self-limited symptoms. Fifteen out of 16 patients presented the symptoms at home during the second day of the DPT (Supplementary Table S1). These patients had symptoms from the skin, corresponding to a grade 1 systemic allergic reaction according to the World Allergy Organization grading system (14).
It was then investigated whether the SPTs and the IDTs are valid for allergy diagnosis. For this purpose, we calculated sensitivity, specificity, PPV, and negative predictive value (NPV) compared to the DPTs outcome, which is considered the “gold standard” for the diagnosis of drug allergy for all antibiotic groups, as well as for beta-lactams and macrolides, respectively. SPTs were found to have a sensitivity of 11.8% (2/17 = 0.118), a specificity of 100% (106/106 = 1), a PPV of 100% (2/2 = 1), a NPV of 87.6% (106/121 = 0.876) and accuracy 87.8% (80.7–93.0), respectively. IDTs had a sensitivity of 23.5% (4/17 = 0.235), a specificity of 99.1% (105/106 = 0.991), a PPV of 80% (4/5 = 0.8), a NPV of 89% (105/118 = 0.890), and accuracy of 88.6% (81.6–93.6), respectively. These values (sensitivity, specificity, PPV, and NPV) were also calculated for penicillins, cephalosporins, and macrolides separately, as shown in Table 3.
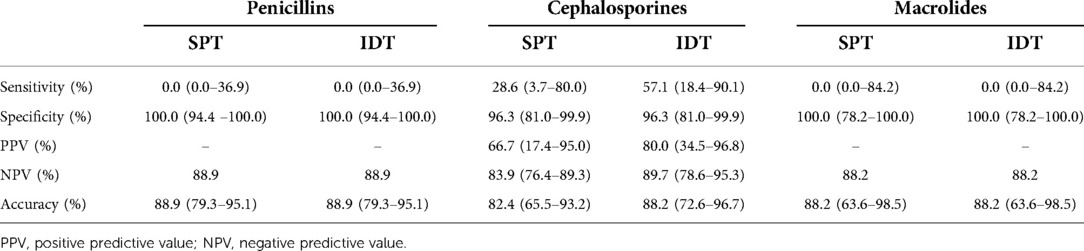
Table 3. Sensitivity, specificity and predictive values of penicillins, cephalosporins and macrolides.
Univariate statistical analysis revealed that a positive DPT was more frequent in children with a family history of atopic disease (p = 0.027) and even more in children with a co-existing personal history of atopic disease (p = 0.013).
A correlation was also observed between positive SPTs and positive DPTs (p < 0.0001), and positive IDTs and positive DPTs (p < 0.0001).
A tendency towards a high frequency of positive DPTs was observed in male subjects (p = 0.125).
No statistically significant correlation was found between eosinophilia and positive DPTs (Table 1).
Multiple logistic regression analysis showed that the simultaneous presence of a personal and family history of atopy (p = 0.002) and positive intradermal tests (p = 0.002) were the two independent predictors for a positive challenge test in children with possible drug allergies. Specifically, children with positive intradermal tests were 66 times more likely to have a positive challenge (Adjusted Odds Ratio – aOR: 66.14, 95% CI: 4.56–960.18), and children with a personal and family history of atopy were 11 times more likely to have a positive challenge (aOR: 11.21, 95% CI: 2.45–51.27).
In this study, 123 children with a clinical diagnosis of antibiotic allergy were evaluated for true drug allergy by a process that included history, physical examination, skin tests (SPTs and IDTs), and finally, DPTs. True antibiotic allergy was confirmed in 13.8% of the cases. In most cases, a semi-synthetic penicillin was the culprit antibiotic, followed by cephalosporines and macrolides.
Self-reported drug allergy has a prevalence of 8% in the general population (15). Beta-lactam allergy has been estimated to occur in up to 15% of hospitalized patients (16). While drug allergy is relatively uncommon, many children are labeled as “allergic” to various medications, particularly antibiotics. This study evaluated a group of 123 children with reported antibiotic allergy for true antibiotic allergy, which was confirmed in 17 patients (13.8%). Therefore, false antibiotic allergy had a high reported prevalence of more than 86%. Antibiotic allergy overdiagnosis is a significant healthcare issue. Thus, due to the overuse of the term “allergy”, e.g., in the presence of skin rashes most commonly caused by viral infections, many children are falsely labeled as allergic to an antibiotic. This can lead to the extensive use of alternative antibiotics (17). Many children who carry the label of being allergic to an antibiotic are treated with alternatives that may be less effective, leading to difficult to treat infections and contributing to the development of bacterial resistance (15, 18, 19).
SPTs and IDTs demonstrated relatively low sensitivity but high specificity. Positive skin tests, both SPTs and IDTs, strongly predicted a positive DPT to the culprit antibiotic. A positive personal and family history of allergy was recognized as a risk factor for true antibiotic allergy.
Delayed-type hypersensitivity reactions occurred in 94.2% of the patients with a positive DPT. Only one child (0.8%) developed true anaphylaxis out of the 123 patients. This finding is consistent with previous studies showing a low probability of potentially life-threatening reactions during DPT (20). Of interest, this female patient had no history of a severe reaction during her previous exposure to the antibiotic that had been implicated. Therefore, patients who report mild symptomatology after taking an antibiotic to which they experienced an allergic reaction may have more severe systemic symptomatology when a diagnostic DPT is performed.
We sought to assess the accuracy of SPTs and IDTs, in predicting antibiotic allergy and, in comparison to DPT, which is considered the “gold standard” for a definite diagnosis. Sensitivity was 11.8% and 23.5% for SPTs and IDTs, respectively, while specificity was 100% and 99.1%, respectively. Moreover, the PPV and NPV were high for SPTs and IDTs (PPV: 100% and 80%, and NPV 87.6% and 89% for SPTs and IDTs, respectively). Similarly, a systematic review and a prospective study assessing the diagnostic accuracy of SPTs in penicillin-allergic patients reported a high specificity but a low sensitivity of less than 50% in predicting hypersensitivity reactions. Overall, the results of that review suggested that, at least in patients reporting a delayed mild reaction, skin tests may have a high specificity (97.4%) and NPV, but a low PPV and sensitivity (19.3%) in identifying patients who will develop a hypersensitivity reaction when exposed to penicillin (21, 22).
Another study by Yoon et al. reported that the IDT for cephalosporins had a sensitivity of 0%, a specificity of 97.5%, a NPV of 99.7%, and a PPV of 0% when challenged with the same drugs that were positive in the skin test (23). The present study showed that IDTs had high specificity in predicting a positive DPT.
Our study also revealed that the simultaneous presence of personal and family history of atopic disease and a positive IDT were the two independent factors related to a positive DPT, and consequently true drug allergy. As reported in 1993 by Gadde J et al., previous findings showed no association between penicillin allergy and atopy (24), although patients with asthma were more prone to severe reactions (25). However, there are several studies suggesting that drugs causing delayed hypersensitivity adverse reactions in the presence of HLA alleles include allopurinol, antiretrovirals (namely abacavir and nevirapine), aromatic amine anticonvulsants (in particular carbamazepine and phenytoin), and sulfonamides (25–27). An immunological response to certain drug antigens may be triggered in patients carrying specific HLA alleles, leading to T-cell activation and clonal expansion (28). Moreover, recent studies show an association between the HLA-DRB1∗10:01 allele and hypersensitivity reactions to penicillin (29).
Α limitation of the current study is that the statistical analysis concerning the validity of the tests, SPTs and IDTs, specifically the sensitivity, specificity, PPV, and NPV were performed on a relatively small patient sample, in whom the diagnosis of antibiotic allergy was made after the positive challenge test, and therefore their statistical power is low. Re-evaluation in larger patient samples is required. In addition, the inclusion of these patients in future meta-analyses will lead to more reliable results.
In summary, in the current study, true antibiotic allergy was confirmed in a small number of patients, 13.8% of those with a clinical diagnosis of antibiotic allergy. Semisynthetic penicillins were the most frequently implicated antibiotics, followed by cephalosporins and macrolides. Most allergic reactions to antibiotics are of delayed type, with the skin being the most frequently affected organ, followed by the gastrointestinal tract. SPTs and IDTs had high specificity and positive and negative predictive values. A positive IDT was a strong predictor of a positive DPT. Regarding IDTs, there is no published data showing a statistical association between skin tests and DPTs outcomes. Interestingly enough, the simultaneous presence of a positive personal and family history of atopy was also associated with an increased risk for a positive DPT, and subsequently the diagnosis of true antibiotic allergy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Bioethics commitee, Aristotle University of Thessaloniki. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
NK was responsible for the sample collection, the conduct of skin tests and provocation test and the writing of the manuscript. NK, DC and AM contributed to conception and design of the study, GT performed the statistical analysis. ER and IG contributed to the review, editing and preparation of the manuscript. EM contributed to the formal analysis, supervision, validation, and writing, review and editing of the manuscript. AM had the senior authorship. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/falgy.2022.1050048/full#supplementary-material.
1. Merk HF, Bickers DR. Hypersensitivity to non-β-lactam antibiotics. Allergol Select. (2022) 6:11–7. doi: 10.5414/ALX02311E
2. Guvenir H, Dibek Misirlioglu E, Capanoglu M, Vezir E, Toyran M, Kocabas CN. Proven non-β-lactam antibiotic allergy in children. Int Arch Allergy Immunol. (2016) 169(1):45–50. doi: 10.1159/000443830
3. Thong BY. Update on the management of antibiotic allergy. Allergy Asthma Immunol Res. (2010) 2(2):77–86. doi: 10.4168/aair.2010.2.2.77
4. Host A, Andrea S, Charkin S, Diaz-Vasquez C, Dreborg S, Eigenmann PA, et al. Allergy testing in children: why, who, when and how? Allergy. (2003) 58(7):559–69. doi: 10.1034/j.1398-9995.2003.00238.x
5. Duce K, Gouldstone A. A practical guide to carrying out skin-prick allergy testing. Nurs Times. (2006) 102(48):28–9.17193775
6. Arnold A, Sommerfield A, Ramgolam A, Rueter K, Muthusamy S, Noble V, et al. The role of skin testing and extended antibiotic courses in assessment of children with penicillin allergy: an Australian experience. J Paediatr Child Health. (2019) 55(4):428–32. doi: 10.1111/jpc.14220
7. Phillips EJ, Bigliardi P, Bircher AJ, Broyles A, Chang YS, Chung WH, et al. Controversies in drug allergy: testing for delayed reactions. J Allergy Clin Immunol. (2019) 143(1):66–73. doi: 10.1016/j.jaci.2018.10.030
8. Marrs T, Fox AT, Lack G, du Toit G. The diagnosis and management of antibiotic allergy in children: systematic review to inform a contemporary approach. Arch. Dis. Child. (2015) 100:583–8. doi: 10.1136/archdischild-2014-306280
9. Mill C, Primeau M-N, Medoff E, Lejtenyi C, O'Keefe A, Netchiporouk E, et al. Assessing the diagnostic proper-ties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. (2016) 170:e160033. doi: 10.1001/jamapediatrics.2016.0033
10. Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International consensus on drug allergy. Allergy. (2014) 69(4):420–37. doi: 10.1111/all.12350
11. Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, et al. Skin test concentrations for systemically administered drugs – an ENDA/EAACI drug allergy interest group position paper. Allergy. (2013) 68(6):702–12. doi: 10.1111/all.12142
12. Barni S, Butti D, Mori F, Pucci N, Rossi ME, Cianferoni A, et al. Azithromycin is more allergenic than clarithromycin in children with suspected hypersensitivity reaction to macrolides. J Investig Allergol Clin Immunol. (2015) 25(2):128–32.25997306
13. Chiriac AM, Rerkpattanapipat T, Bousquet PJ, Molinari N, Demoly P. Optimal step doses for drug provocation tests to prove beta-lactam hypersensitivity. Allergy. (2017) 72(4):552–61. doi: 10.1111/all.13037
14. Cox LS, Sanchez-Borges M, Lockey RF. World allergy organization systemic allergic reaction grading system: is a modification needed? J Allergy Clin Immunol Pract. (2017) 5(1):58–62.e5. doi: 10.1016/j.jaip.2016.11.009
15. Ponvert C, Perrin Y, Bados-Albiero A, Le Bourgeois M, Karila C, Delacourt C, et al. Allergy to beta-lactam antibiotics in children: results of a 20-year study based on clinical history, skin and challenge tests. Pediatr. Allergy Immunol. (2011) 22:411–8. doi: 10.1111/j.1399-3038.2011.01169.x
16. van Dijk SM, Gardarsdottir H, Wassenberg MW, Oosterheert JJ, de Groot MC, Rockmann H. The high impact of penicillin allergy registration in hospitalized patients. J Allergy Clin Immunol Pract. (2016) 4:926–31. doi: 10.1016/j.jaip.2016.03.009
17. Macy E, Romano A, Khan D. Practical management of a biotic hypersensitivity in 2017. J Allergy Clin Immunol Pract. (2017) 5(3):577–86. doi: 10.1016/j.jaip.2017.02.014
18. Mittmann N, Knowles SR, Gomez M, Fish JS, Cartotto R, Shear NH. Evaluation of the extent of under-reporting of serious adverse drug reactions: the case of toxic epidermal necrolysis. Drug Saf. (2004) 27:477–87. doi: 10.2165/00002018-200427070-00004
19. Messaad D, Sahla H, Benahmed S, Godard P, Bousquet J, Demoly P. Drug provocation tests in patients with a history suggesting an immediate drug hypersensitivity reaction. Ann Intern Med. (2004) 140:1001–6. doi: 10.7326/0003-4819-140-12-200406150-00009
20. Cardoso-Fernandes A, Blumenthal KG, Chiriac AM, Tarrio I, Afonso-João D, Delgado L, et al. Frequency of severe reactions following penicillin drug provocation tests: a Bayesian meta-analysis. Clin Transl Allergy. (2021) 11(4):e12008. doi: 10.1002/clt2.12008
21. Sousa-Pinto B, Tarrio I, Blumenthal KG, Araújo L, Azevedo LF, Delgado L, et al. Accuracy of penicillin allergy diagnostic tests: a systematic review and meta-analysis. J Allergy Clin Immunol. (2021) 147(1):296–308. doi: 10.1016/j.jaci.2020.04.058
22. Ibáñez MD, Rodríguez Del Río P, Lasa EM, Joral A, Ruiz-Hornillos J, Muñoz C, et al. Penicillin allergy in children (APENIN) task force. Pediatric allergy committee, spanish society of allergy and clinical immunology (SEAIC). prospective assessment of diagnostic tests for pediatric penicillin allergy: from clinical history to challenge tests. Ann Allergy Asthma Immunol. (2018) 121(2):235–244.e3. doi: 10.1016/j.anai.2018.05.013
23. Yoon SY, Park SY, Kim S, Lee T, Lee YS, Kwon HS, et al. Validation of the cephalosporin intradermal skin test for predicting immediate hypersensitivity: a prospective study with drug challenge. Allergy. (2013) 68(7):938–44. doi: 10.1111/all.12182
24. Gadde J, Spence M, Wheeler B, Adkinson NF Jr. Clinical experience with penicillin skin testing in a large inner-city STD clinic. JAMA. (1993) 270(20):2456–63. doi: 10.1001/jama.1993.03510200062033
25. Bock SA, Muñoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. (2001) 107(1):191–3. doi: 10.1067/mai.2001.112031
26. Borchers AT, Lee JL, Naguwa SM, Cheema GS, Gershwin ME. Stevens–Johnson syndrome and toxic epidermal necrolysis. Autoimmun Rev. (2008) 7:598–605. doi: 10.1016/j.autrev.2008.06.004
27. Phillips EJ, Mallal SA. Pharmacogenetics of drug hypersensitivity. Pharmacogenomics. (2010) 11:973–87. doi: 10.2217/pgs.10.77
28. Yang MS, Kang MG, Jung JW, Song WJ, Kang HR, Cho SH, et al. Clinical features and prognostic factors in severe cutaneous drug reactions. Int Arch Allergy Immunol. (2013) 162:346–54. doi: 10.1159/000354918
Keywords: drug allergy, skin tests, drug provocation test, children, graded challenge
Citation: Kitsos N, Cassimos D, Trypsianis G, Xinias I, Roilides E, Grivea I, Mantadakis E and Mavroudi A (2022) Drug allergy evaluation in children with suspected mild antibiotic allergy. Front. Allergy 3:1050048. doi: 10.3389/falgy.2022.1050048
Received: 21 September 2022; Accepted: 24 October 2022;
Published: 5 December 2022.
Edited by:
Simon Blank, Technical University of Munich and Helmholtz Center Munich, GermanyReviewed by:
Jarmila Celakovska, Charles University, Czechia© 2022 Kitsos, Cassimos, Trypsianis, Xinias, Roilides, Grivea, Mantadakis and Mavroudi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nikolaos Kitsos bmtpdHNvc0BnbWFpbC5jb20=
Specialty Section: This article was submitted to Drug, Venom & Anaphylaxis, a section of the journal Frontiers in Allergy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.