
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Allergy , 14 July 2021
Sec. Drug, Venom & Anaphylaxis
Volume 2 - 2021 | https://doi.org/10.3389/falgy.2021.690837
This article is part of the Research Topic Adverse Vaccine Reactions View all 6 articles
 Valerie Chiang1†
Valerie Chiang1† Agnes S. Y. Leung2†
Agnes S. Y. Leung2† Elaine Y. L. Au1
Elaine Y. L. Au1 Marco H. K. Ho3
Marco H. K. Ho3 Tak Hong Lee4
Tak Hong Lee4 Adrian Y. Y. Wu5
Adrian Y. Y. Wu5 Gary W. K. Wong2†
Gary W. K. Wong2† Philip H. Li6*†
Philip H. Li6*†Background: Mass coronavirus disease 2019 (COVID-19) vaccination to achieve herd immunity is an effective means to mitigate the current COVID-19 pandemic. Reports of COVID-19 vaccine-associated allergic reactions and lack of clear local guidance are contributing factors leading to a low vaccine acceptance rate in the community. A task force of experts from the Hong Kong Institute of Allergy (HKIA) has been formed to address current needs.
Objective: To formulate a set of consensus statements (CS) on COVID-19 vaccine allergy safety (VAS) in Hong Kong.
Methods: A nominated task force of experts managing patients with drug and vaccine allergies in Hong Kong formulated the CS by the Delphi method. An agreement was a priori defined as ≥80% consensus.
Results: A total of 11 statements met the criteria for consensus with good overall agreement among task force members, including seven statements on pre-vaccination recommendations and four statements on vaccination and post-vaccination guidance. Individuals with a history of suspected allergic reaction to prior COVID-19 vaccination should not receive further COVID-19 vaccination, and other groups at risk of COVID-19 vaccine-associated allergic reactions have been identified. The importance of pre-vaccination and post-vaccination assessment by frontline healthcare workers and evaluation by allergists are highlighted.
Conclusion: The CS provides pragmatic and timely guidance for local frontline healthcare providers on decisions regarding COVID-19 VAS.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease 2019 (COVID-19) has infected more than 100 million people globally, taken away the lives of more than 2 million individuals, and caused irretrievable damage to society (1). Vaccination is the single most effective way to reduce deaths and severe illness caused by SARS-CoV-2, and mass vaccination of 60–70% of the total population to achieve herd immunity appears to be the most effective public health intervention to control the pandemic (2).
However, even before the commencement of the COVID-19 vaccination program in Hong Kong, the overall vaccine acceptance rate was lower than 40% (3). This low acceptance rate correlated with perceived harm of COVID-19 vaccination and trust (or lack of) in the healthcare system. Likewise, since the start of the COVID-19 vaccination program, there has been much anxiety and fear regarding the possibility of COVID-19 vaccine-associated allergic reactions. This has greatly impacted the confidence of the general public and thus the low rate of vaccine uptake.
Many countries have released their guidelines regarding COVID-19 vaccine allergy safety (VAS), including how to screen people with a higher risk of COVID-19 vaccine-associated allergies and how to approach patients with suspected reactions (4–9). Despite having experienced cases of possible vaccine-associated allergic reactions already, no clear guidance has been made available in Hong Kong regarding COVID-19 VAS so far. Even among healthcare professionals, there have been many uncertainties on how to evaluate and approach this important population-wide dilemma.
In view of this, the Hong Kong Institute of Allergy (HKIA) formed a local task force of experts in the field of Immunology and Allergy to formulate a set of consensus statements (CS) on the pressing issue of COVID-19 VAS in Hong Kong.
Consensus statements were formulated by the Delphi method, soliciting the opinions of experts managing patients with drug and vaccine allergies in Hong Kong (10). All members of this task force were nominated representatives of the HKIA and consisted of two academic representatives [PHL and ASYL], two public sector representatives working in the VAS Clinic of the Hospital Authority Hong Kong West Cluster [VC and EYLA], two private sector representatives [TL and AYYW], and two professional body representatives [MHKH and GWKW]. PHL also acted as the facilitator. No ethics approval was required as no human or animal subjects were involved.
In the first Delphi round, a structured questionnaire containing a set of proposed CS encompassing important issues pertaining to COVID-19 VAS in Hong Kong was formulated by the task force. The statements were formed based on various aspects regarding VAS from the pre-vaccination phase (e.g., screening and management of patients at higher risk of COVID-19 vaccine-associated allergies, requirements of medical regulations, and infrastructure), vaccination process (e.g., monitoring requirements, management of potential allergic reactions), and post-vaccination events (e.g., referral of suspected COVID-19 vaccine-associated allergies). In the second round of Delphi, all members of the task force completed the above-described questionnaire via an online anonymized system. They were not required to answer all questions and could select “no opinion” to any of the statements. In the third and final round of Delphi, the task force reviewed the aggregated responses of the questionnaires. If further clarification or elaboration on any statements was required, the questionnaire was adapted and sent back to participants with feedback. The term “drugs” was defined to include both non-vaccine and vaccine medications.
Responses were graded as “Strongly Agree,” “Tend to Agree,” “Neither Agree nor Disagree,” “Tend to Disagree,” and “Strongly Disagree” for each respective statement, scoring +1, +0.5, 0, −0.5, and −1, respectively. The consensus was a priori defined as 80% or more responses to “Strongly Agree” or “Tend to Agree.” Scores are reported as the mean and SD (scale from +1 to −1). More extreme scores and lower SD, therefore, indicated stronger consensus.
A total of 11 statements reached consensus after multiple rounds of Delphi. All members of the task force were invited to further elaborate on the details and suggest refinements to certain statements that reached consensus as deemed necessary. Results after revision following the final round of Delphi were summarized in Table 1, Figure 1, and were as follows:
Pre-vaccination:
CS #1: Some people may be at higher risk of COVID-19 vaccine-associated allergic reactions, including those with:
- Suspected allergic reaction(s) to prior COVID-19 vaccination.
- History of anaphylaxis or at risk of anaphylaxis.
- History of severe immediate-type allergic reactions to multiple foods or more than one class of drugs.
There was 100% agreement (Score: +0.63 ± 0.23) with CS #1. The definitions of anaphylaxis and severe reactions were based on the National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network (NIAID/FAAN) criteria (Table 2) and modified Ring and Messmer grading (Table 3), respectively (11, 12). Immediate-type reactions were defined as reactions with symptom onset within 1 h following allergen exposure.
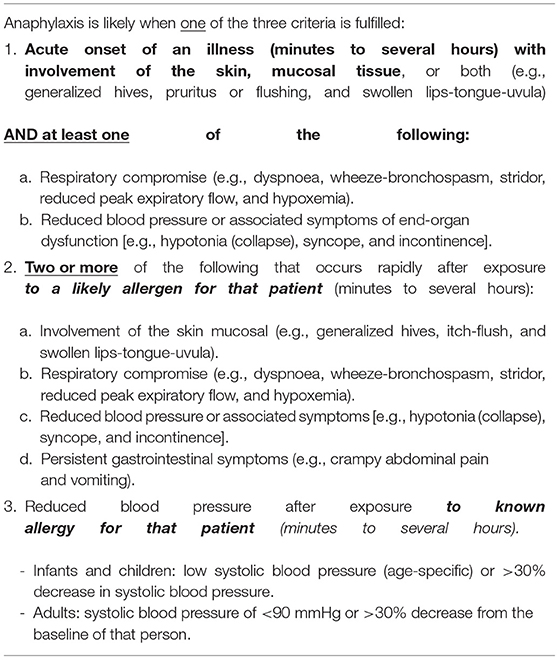
Table 2. Anaphylaxis according to National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network (11) (NIAID/FAAN).
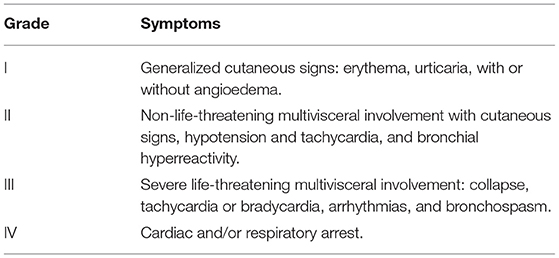
Table 3. Modified ring and messmer grading (12).
CS #2: People with a history of suspected allergic reaction to prior COVID-19 vaccination should not receive further COVID-19 vaccination until allergist evaluation.
There was 100% agreement (Score: +1.00 ± 0.00) with CS #2.
CS #3: People with a history of suspected anaphylaxis or severe allergic reactions may be referred for allergist evaluation prior to COVID-19 vaccination.
There was 88% agreement (Score: +0.75 ± 0.53) with CS #3.
CS #4: People with a history of drug allergies to more than one class of drugs may be referred for allergist review prior to COVID-19 vaccination.
There was 88% agreement (Score: +0.63 ± 0.52) with CS #4.
CS #5: Full excipient lists should be mandated and made available in all product inserts of registered drugs to facilitate the evaluation of COVID-19 vaccine-associated allergic reactions.
There was 88% agreement (Score: +0.81 ± 0.38) with CS #5.
CS #6: Pre-vaccination vaccine or excipient allergy testing should not be routinely performed, especially for people not at higher risk of COVID-19 vaccine-associated allergic reactions.
There was 100% agreement (Score: +0.94 ± 0.18) with CS #6.
CS #7: Prior to vaccination, people should be screened for factors associated with a higher risk of COVID-19 vaccine-associated allergic reactions.
There was 88% agreement (Score: +0.69 ± 0.70) with CS #7.
Vaccination:
CS #8: Healthcare providers should be sufficiently prepared to recognize and treat allergic reactions properly, with adrenaline autoinjectors and antihistamines available.
There was 88% agreement (Score: +0.81 ± 0.37) with CS #8.
CS #9: When an immediate-type allergic reaction following COVID-19 vaccination is suspected, blood for serum tryptase should be saved from 30 min to 4 h (preferably within 2 h) of symptom onset.
There was 100% agreement (Score: +0.69 ± 0.37) with CS #9.
CS #10: People should be routinely observed for at least 15 min after the COVID-19 vaccination. Those at higher risk of COVID-19 vaccine-associated allergic reactions should be observed for at least 30 min after vaccination.
There was 100% agreement (Score: +1.00 ± 0.00) with CS #10.
Post-vaccination:
CS #11: People with suspected allergic reactions following COVID-19 vaccination should be referred for allergist evaluation.
There was 100% agreement (Score: +0.94 ± 0.18) with CS #11.
Patient fear regarding the risk associated with a new vaccine may lead to vaccine hesitancy, the major hurdle in attaining herd immunity. To provide reassurance and support toward the success of the global vaccination effort, we present the first set of CS of Hong Kong on the approach to COVID-19 VAS. Given the novel and emerging nature of COVID-19 vaccination and associated allergic reactions, there is still a paucity of evidence or published literature on such events. Despite this, many authorities in various other countries have also published recommendations mostly based on expert opinion (Table 4). Thus, it is not possible to create an evidence-based treatment guideline, instead, the consensus described in this report comprises the collective expertise of members of the HKIA task force. This is in-line with various recommendations published in other countries (Table 4). It is likely that as more experience becomes available for COVID-19 vaccination, clinical practices will evolve so recommendations will be updated from a background of accumulated evidence. Nonetheless, in the interim, given the pressing and urgent need for such recommendations to facilitate the decisions of healthcare providers about various aspects of COVID-19 VAS in Hong Kong, it was felt essential that a series of CS are produced without delay.
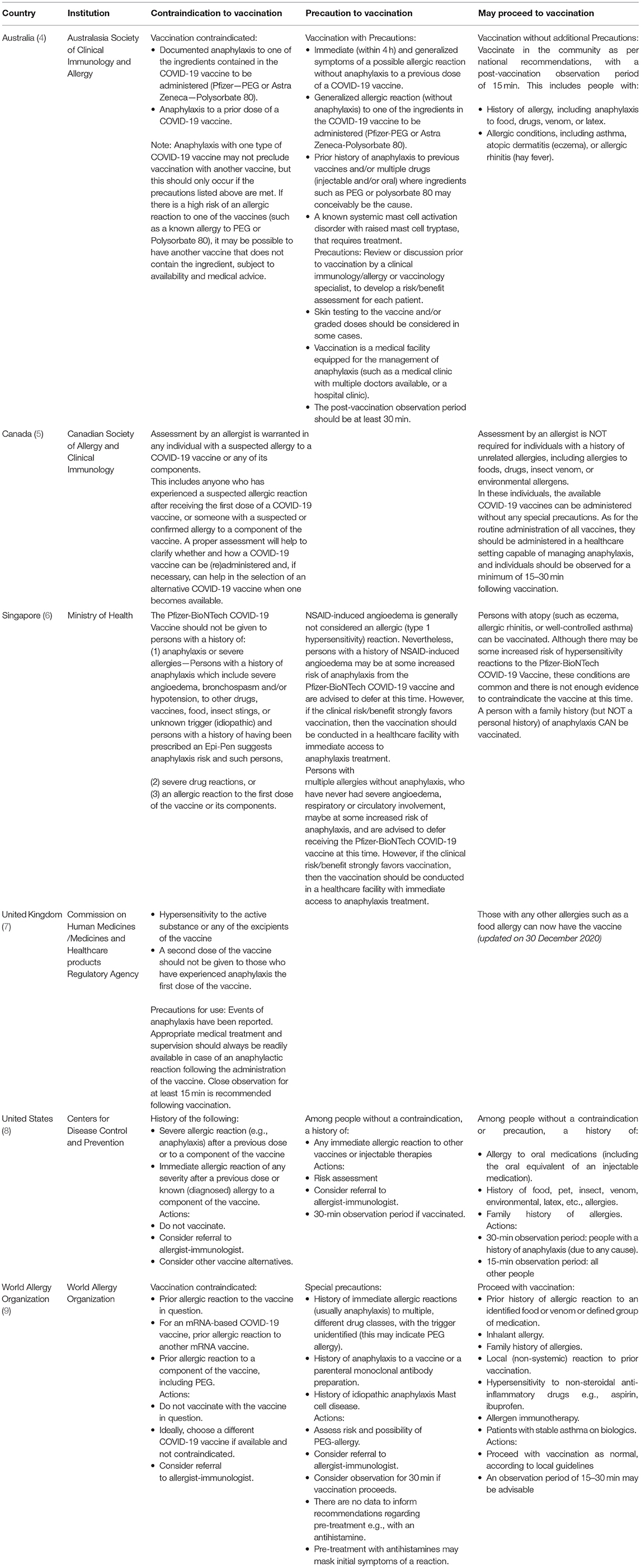
Table 4. Summary of recommendations regarding COVID-19 vaccine allergy safety (VAS) in other countries.
CS #1–4 pertains to defining those people at higher risk of potential COVID-19 vaccine-associated allergies. The groups defined in CS #1 are in concordance with the broader guidance published by the Department of Health (DH), which suggests that referral to specialists in Immunology and Allergy for assessment should be considered before vaccination for those with a “history of reactions to multiple classes of drugs or anaphylaxis; history of anaphylaxis or at risk of anaphylaxis; severe reactions to other allergens, e.g., drugs/ foods/ insects (especially injectables) and severe reactions to other vaccines or prior COVID-19 vaccine” (13). In addition to the recommendations of DH, we offer definitions for “anaphylaxis” and “severe reactions” by drawing references to the well-established NIAID/FAAN criteria and Ring and Messmer grading. We also suggest focusing current definitions to describe immediate-type reactions only, given that current recommendations do not pertain to delayed-type vaccine-associated reactions (14).
CS #2–4 provide details on the recommendations on the three different groups described in CS #1. However, it is important to note that except for the history of previous reactions to prior COVID-19 vaccination, the other higher-risk groups were proposed based on pragmatic (rather than purely medical or evidence-based) reasons. Although patients with a history of anaphylaxis have been cautioned against, or even excluded from, COVID-19 vaccination in some countries, we were unaware of any definite evidence that these factors were associated with an increased risk of subsequent COVID-19 vaccine-elicited allergic reactions. Based on current literature, around 31% of patients experiencing anaphylaxis after messenger RNA (mRNA) COVID-19 vaccines had a prior history of anaphylaxis (15). However, it is not known what proportion of patients who have had a prior history of anaphylaxis, experienced an anaphylactic episode following mRNA vaccination. The task force emphasizes the importance of diagnosing “anaphylaxis” based on objective evidence and strict accordance with the NIAID/FAAN criteria. Also, at present, there was insufficient evidence to recommend any specific cut-off regarding the duration for CS #3 (i.e., time since last anaphylaxis event). Future case-control studies will be essential to ascertain if such association truly exists and to guide further refinements to future recommendations.
The inclusion of a “history of severe immediate-type allergic reactions to multiple foods or more than one class of drugs” was designed to encompass patients with potentially undiagnosed (or “idiopathic”) anaphylaxis and excipient allergies. As mentioned, the term “drugs” was defined to include both non-vaccine and vaccine medications. There is limited evidence to suggest that the presence of drug allergy alone is a risk factor for COVID-19 vaccination-associated allergic reactions. Instead, it is thought that allergic reactions to vaccines are primarily due to adjuvants or excipients in the vaccine and not to the active ingredient itself. Excipients have been implicated as the major allergen for many COVID-19 vaccine-associated allergic reactions, especially following mRNA vaccines (16). It would, therefore, be prudent to avoid vaccinating those with known allergies to the same excipients. However, this is extremely difficult given the current medical infrastructure. In Hong Kong, drugs are not required legally to include excipient lists in product inserts. Therefore, it is near impossible to diagnose a potential excipient allergy if the culprit excipient cannot be identified in the causative drug or vaccine (17). Furthermore, given the unmet provision of allergy services and specialists in Hong Kong, anaphylaxis survivors remain severely underinvestigated and underdiagnosed (18, 19). For example, among Hong Kong adult patients, more than 10% of anaphylaxis survivors did not have an identifiable cause for their allergies and were mistakenly labeled with a diagnosis of multiple food allergies (20).
To prevent patients with excipient allergies to be inadvertently reexposed to the same excipients in COVID-19 vaccines, we advise patients with a history of severe immediate-type allergic reactions to multiple foods or multiple classes of drugs to seek the evaluation of allergists prior to vaccination. Therefore, CS #5 was instigated in hope that full excipient lists in all registered drugs could become mandated in the future so that more people could be safely vaccinated after a proper and comprehensive evaluation.
Skin testing has been a well-established tool for diagnosing various excipient allergies and suggested mRNA COVID-19 vaccine allergy testing (16, 21). However, excipient allergy skin testing, especially with polyethylene glycol (PEG), is not without risk and has been associated with systemic allergic reactions (22). Furthermore, the predictive values of either vaccine- or excipient-based skin testing remain uncertain and carry a risk of false-positive or negative results. Given the potential risks and uncertainties of excipient allergy testing, there was 100% agreement among the task force for CS #6 which explicitly warns that pre-vaccination vaccine or excipient allergy testing should not be routinely performed. Instead, as stated in CS #7, we recommend that those planning to receive COVID-19 vaccination should be screened for factors associated with a higher risk of COVID-19 vaccine-associated allergic reactions (as described previously) based on their clinical history. Specialized allergy testing should be reserved for those deemed at higher risk of COVID-19 vaccine-associated allergic reactions after allergist evaluation and appropriate counseling.
CS #8–10 pertains to VAS throughout the vaccination process, including monitoring requirements and management of potential allergic reactions. Again, these CS are generally in line with the broader DH guidance and current practice for anaphylaxis care. CS #8 supports the Hong Kong Anaphylaxis Consortium recommendation that “adrenaline autoinjectors should be used as first-line treatment for all patients at risk of anaphylaxis” (23). Although autoinjectors could be replaced with adrenaline ampoules (suggested by one task force member who voted “Neither Agree/Disagree” to CS #8), the task force agreed that autoinjectors may limit the risk of incorrect dosing and preferred over ampoules in the community setting if resources allow. An acute serum tryptase level can be of immense benefit to support a diagnosis of anaphylaxis in the subsequent allergy consultation. Therefore, CS #9 reminds all frontline healthcare providers to save the blood for serum tryptase from 30 min to 4 h (preferably within 2 h) of symptom onset when a severe immediate-type allergic reaction following COVID-19 vaccination is suspected. CS #10 recommends routine post-vaccine observation for at least 15 min and extended to 30 min for those at higher risk of COVID-19 vaccine-associated allergic reactions, which arbitrarily balances between safety and practicality. Reassuringly, a 30-min observation period would cover 90% of previously reported anaphylaxis cases based on recently published experiences (15).
Last, but not least, CS #11 emphasizes the importance of an allergist referral for all people with suspected allergic reactions following COVID-19 vaccination. Patients with suspected COVID-19 vaccine-associated allergies must withhold further vaccination prior to appropriate specialist evaluation. If a certain vaccine (or vaccine component) allergy is suspected or confirmed, the allergist may advise further testing and/or propose the use of a suitable alternative vaccine. Optimal allergy testing strategies are still undergoing investigation and are beyond the scope of this document. Despite the persistently low allergist to population ratio of Hong Kong, this has improved slightly in recent years with the advent of new allergist trainees and allied health services (18). Allergy centres are now available in both the public and private sectors, and both local medical universities have dedicated formal allergy-focused teaching and research. A publicly available list of accredited Immunologists and allergists can also be found in the Specialist Register of the Medical Council of Hong Kong (24).
Coronavirus disease 2019 vaccination and approach to VAS is a novel and emerging field of medicine accompanied by challenges and opportunities. With the accumulation of global data and expertise, these statements will likely be superseded by more robust and specific evidence-based guidance soon. Meanwhile, we hope the present CS can help facilitate healthcare providers to make important decisions regarding COVID-19 VAS in Hong Kong.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
VC, MH, GW, and PL contributed to conception and design of the study. PL organized the Delphi Process. VC and PL performed the statistical analysis and wrote the first draft of the manuscript. VC, AL, and PL wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
AW is a consultant of ALK-Abelló A/S and director of Ksena Healthcare Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to acknowledge Dr. Wilfred Wong (Department of Pediatrics & Adolescent Medicine, The University of Hong Kong) for his expertise in technical support and setup of the anonymized questionnaire system.
1. Who.int. Coronavirus Disease (COVID-19) Situation Reports. Who.int. (2021). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (accessed March 14, 2021).
2. Scarbrough Lefebvre CD, Terlinden A, Standaert B. Dissecting the indirect effects caused by vaccines into the basic elements. Hum Vaccin Immunother. (2015) 11:2142–57. doi: 10.1080/21645515.2015.1052196
3. Wong MCS, Wong ELY, Huang J, Cheung AWL, Law K, Chong MKC, et al. Acceptance of the COVID-19 vaccine based on the health belief model: a population-based survey in Hong Kong. Vaccine. (2021) 39:1148–56. doi: 10.1016/j.vaccine.2020.12.083
4. Australasian Society of Clinical Immunology and Allergy (ASCIA). Allergy, Immunodeficiency, Autoimmunity and COVID-19 Vaccination Position Statement. Australasian Society of Clinical Immunology and Allergy (ASCIA). (2021). Available online at: https://www.allergy.org.au/hp/papers/ascia-hp-position-statement-covid-19-vaccination (accessed March 22, 2021).
5. Vander Leek TK, Chan ES, Connors L, Derfalvi B, Ellis AK, Upton JEM, et al. COVID-19 vaccine testing & administration guidance for allergists/immunologists from the Canadian Society of Allergy and Clinical Immunology (CSACI). Allergy Asthma Clin Immunol. (2021) 17:29. doi: 10.1186/s13223-021-00529-2
6. Moh.gov.sg. Ministry of Health Singapore: COVID-19 Vaccination. (2021). Available online at: https://www.moh.gov.sg/covid-19/vaccination (accessed March 22, 2021).
7. GOV.UK. Oxford University/AstraZeneca COVID-19 Vaccine Approved. (2021). Available online at: https://www.gov.uk/government/news/oxford-universityastrazeneca-covid-19-vaccine-approved (accessed March 14, 2021).
8. Cdc.gov. Interim Clinical Considerations for Use of COVID-19 Vaccines | CDC (2021). Available online at: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html (accessed March 14, 2021).
9. Turner PJ, Ansotegui IJ, Campbell DE, Cardona V, Ebisawa M, El-Gamal Y, et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. (2021) 14:100517. doi: 10.1016/j.waojou.2021.100517
10. Hsu C-C, Sandford BA. The delphi technique: making sense of consensus. Pract. Assess. Res. Eval. (2007) 12:10. doi: 10.7275/pdz9-th90
11. Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. (2006) 117:391–7. doi: 10.1016/j.jaci.2005.12.1303
12. Ring J, Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. (1977) 1:466–9. doi: 10.1016/S0140-6736(77)91953-5
13. Doctors' Guide for the Coronavirus Disease 2019. Vaccination Programme at Clinics Under the Vaccination Subsidy Scheme. (2021). Available online at: https://www.covidvaccine.gov.hk/pdf/VSS_DoctorsGuide.pdf (accessed April 4, 2021).
14. Blumenthal KG, Freeman EE, Saff RR, Robinson LB, Wolfson AR, Foreman RK, et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. (2021) 384:1273–7. doi: 10.1056/NEJMc2102131
15. Blumenthal KG, Robinson LB, Camargo CA, Jr., Shenoy ES, Banerji A, et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. (2021) 325:1562–5. doi: 10.1001/jama.2021.3976
16. Banerji A, Wickner PG, Saff R, Stone CA, Jr., Robinson LB, et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. (2020) 9:1423–37. doi: 10.1016/j.jaip.2020.12.047
17. Li PH, Yeung HHF, Lau CS, Au EYL. Excipient allergy and importance of complete allergy histories. J Allergy Clin Immunol Pract. (2020) 8:2122–3. doi: 10.1016/j.jaip.2020.04.010
18. Lee TH, Leung TF, Wong G, Ho M, Duque JR, Li PH, et al. The unmet provision of allergy services in Hong Kong impairs capability for allergy prevention-implications for the Asia Pacific region. Asian Pac J Allergy Immunol. (2019) 37:1–8. doi: 10.12932/AP-250817-0150
19. Li PH, Leung ASY, Li RMY, Leung TF, Lau CS, Wong GWK. Increasing incidence of anaphylaxis in Hong Kong from 2009 to 2019-discrepancies of anaphylaxis care between adult and paediatric patients. Clin Transl Allergy. (2020) 10:51. doi: 10.1186/s13601-020-00355-6
20. Li PH, Thomas I, Wong JC, Rutkowski K, Lau CS. Differences in omega-5-gliadin allergy: east versus West. Asia Pac Allergy. (2020) 10:e5. doi: 10.5415/apallergy.2020.10.e5
21. Li PH, Wagner A, Thomas I, Watts TJ, Rutkowski R, Rutkowski K. Steroid allergy: clinical features and the importance of excipient testing in a diagnostic algorithm. J Allergy Clin Immunol Pract. (2018) 6:1655–61. doi: 10.1016/j.jaip.2018.01.007
22. Sellaturay P, Nasser S, Ewan P. Polyethylene Glycol-Induced Systemic Allergic Reactions (Anaphylaxis). J Allergy Clin Immunol Pract. (2021) 9:670–5. doi: 10.1016/j.jaip.2020.09.029
23. Li PH, Chua GT, Leung ASY, Chan YC, Chan KKL, Cheung KH, et al. Hong Kong anaphylaxis consortium consensus statements on prescription of adrenaline autoinjectors in the acute care setting. Asia Pac Allergy. (2021) 11:e1. doi: 10.5415/apallergy.2021.11.e1
24. Specialist Registration. Available online at: https://www.mchk.org.hk/english/list_register/specialist_list.php (accessed March 22, 2021).
Keywords: COVID19, allergy, vaccine, safety, consensus, Hong Kong
Citation: Chiang V, Leung ASY, Au EYL, Ho MHK, Lee TH, Wu AYY, Wong GWK and Li PH (2021) Consensus Statements on the Approach to COVID-19 Vaccine Allergy Safety in Hong Kong. Front. Allergy 2:690837. doi: 10.3389/falgy.2021.690837
Received: 04 April 2021; Accepted: 15 June 2021;
Published: 14 July 2021.
Edited by:
Cem Akin, University of Michigan, United StatesReviewed by:
Matthew P. Giannetti, Brigham and Women's Hospital and Harvard Medical School, United StatesCopyright © 2021 Chiang, Leung, Au, Ho, Lee, Wu, Wong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philip H. Li, bGlwaGlsaXBAaGt1Lmhr
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.