- 1Division of Cardiology, Department of Medicine, Albert Einstein College of Medicine, New York, NY, United States
- 2ASL Avellino, Avellino, Italy
- 3BIOGEM, Ariano Irpino, Italy
- 4Department of Molecular Pharmacology, Einstein Institute for Aging Research, Einstein-Sinai Diabetes Research Center, Albert Einstein College of Medicine, New York, NY, United States
Emerging evidence has shown that microRNAs (miRNAs) play critical role in the pathogenesis of several disorders. In the present minireview, we focus our attention on the functional role of a specific miRNA, namely miR-34a, in the pathophysiology of frailty and diabetes mellitus. Based on the current literature, we speculate that this miRNA may serve as a potential biomarker of frailty in diabetic older adults. Additionally, its actions on oxidative stress might represent a druggable target to obtain new potentials treatments.
Background
Frailty is a clinical burden which is typical of older adults (Clegg et al., 2013; Hanlon et al., 2018). Frail older adults have a high risk of adverse events such as functional and cognitive impairment, hospitalizations, and death (Puts et al., 2017; Gilbert et al., 2018; Hoogendijk et al., 2019; Pilotto et al., 2020). A prompt diagnosis and a careful management of comorbidities is the first step to avoid adverse outcomes; diabetes is one of these comorbidities and it is very common in frail older adults (Umegaki, 2016; Yarnall et al., 2017; Clegg and Hassan-Smith, 2018; Nagai et al., 2018; Li et al., 2019; Sieber, 2019; Mone et al., 2022a; Mone et al., 2022b; Mone et al., 2022d). Indeed, diabetes leads to a higher risk of cardiovascular complications and functional and physical impairment driving adverse outcomes (Fadini and Avogaro, 2010; Prattichizzo et al., 2018; Yun et al., 2018; Jarvie et al., 2019; Yu et al., 2019; Gulsin et al., 2020).
microRNAs (miRNAs) are small non-coding RNAs that act as post-transcriptional gene regulators (Ambros, 2004; Krol et al., 2010; Santulli, 2015; Ferrante and Conti, 2017; Fridrichova and Zmetakova, 2019; Stavast and Erkeland, 2019; Mone et al., 2021b; Mone et al., Forthcoming 2022c); miRNAs exert their activity in many biological processes and have been proposed as biomarkers and therapeutic strategies (Creemers et al., 2012; Wronska et al., 2015; Barwari et al., 2016; Chen et al., 2018; Wong et al., 2018; Mone et al., 2021b; Fonseca et al., 2021; Mone et al., Forthcoming 2022c). Many miRNAs have been associated to mitochondrial dysfunction, inflammation, and oxidative stress and their concentration may vary in physiological conditions (Zhang et al., 2014; Hathaway et al., 2018; Wei et al., 2018; Rusanova et al., 2019; Song et al., 2019; Gambardella et al., Forthcoming 2022). Interestingly, several investigators evidenced the potential roles of microRNAs (miRNAs) in the pathogenesis of frailty (Rusanova et al., 2019; Carini et al., 2021; Lee et al., 2021; Carini et al., 2022; Dowling et al., 2022).
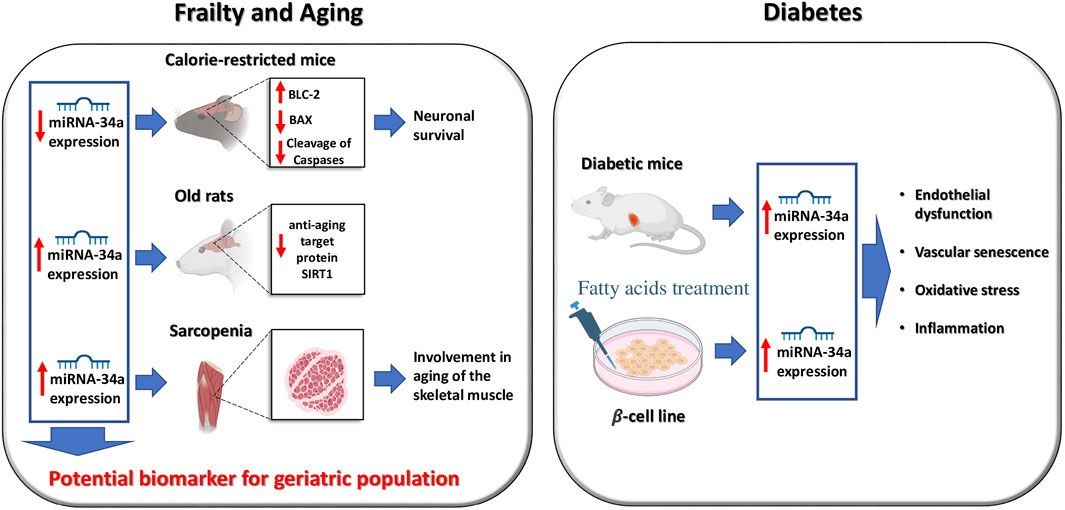
Specifically, miR-34a has been associated to frailty, aging, and diabetes (Figure 1) (Boon et al., 2013; Chakraborty et al., 2014; Rippo et al., 2014; Badi et al., 2018; Thounaojam and Bartoli, 2019; Kukreti and Amuthavalli, 2020; Ni et al., 2020; Manakanatas et al., 2022) and is generally considered a bona fide biomarker of cellular and vascular senescence (Badi et al., 2015; Park et al., 2020; Manakanatas et al., 2022).
Role of miR-34a in frailty and aging
The pathophysiology of frailty includes chronic inflammation, which is prevailing in aging (“inflammaging”), oxidative stress with or without mitochondrial dysfunction, insulin resistance, loss of anabolic hormones, and reduced tolerance to physical exercise with a reduction in muscle strength (Bandeen-Roche et al., 2015; Cruz-Jentoft and Sayer, 2019; Rusanova et al., 2019).
Frailty onset is due to the failure of multiple organs and/or systems and many pathologic conditions have been associated with frailty (Walston et al., 2008; Afilalo et al., 2014; Mone and Pansini, 2020; Mone et al., 2021a; Waite et al., 2021; Mone et al., 2022e). In 2001, Fried et al. (2001) developed the five criteria now routinely used to diagnose frailty. Equally important, the frailty index is another tool to diagnose and manage frailty (Rockwood et al., 2005; Searle et al., 2008).
In 2011, Khanna et al. (2011) observed an age-dependent decreased expression of miR-34a in the brain of calorie-restricted mice, mirrored by an increase in Bcl-2 expression, and a reduced expression of pro-apoptosis genes such as Bax. The authors concluded that this miRNA was involved in the neuronal survival in long-lived calorie-restricted fed mice.
A subsequent investigation by Zheng and collaborators evidenced the involvement of miR-34a in cellular senescence via MAPK: the authors detected its overexpression in sarcopenia, suggesting a role of this miRNA in the aging process of the skeletal muscle (Zheng et al., 2018). Similarly, miR-34a expression was significantly up-regulated in the hearts of aged mice lacking Calstabin 2, the stabilizing protein of the cardiac isoform of Ryanodine Receptor (Yuan et al., 2014). Another investigation revealed that an increased expression of miR-34a in older rats correlates with a concomitant decrease in the brain of the anti-aging target protein SIRT1 (Hu et al., 2017).
Notably, a clinical paper indicated miR-34a as a biomarker of aging/frailty in oncogeriatric populations (Dalmasso et al., 2018). In line with these observations, a very recent paper evidenced that miR-34 regulates protein translation and protein turnover in the aging brain of Drosophila (Srinivasan et al., 2022).
Role of miR-34a in diabetes
Insulin resistance is one of the most important features of Type 2 Diabetes mellitus (T2DM) (Feve and Bastard, 2009; Taylor, 2013; Mastrototaro and Roden, 2021). Of note, miR-34a supports pancreatic development and has been associated to insulin resistance and to the onset of T2DM (Wei et al., 2013; Chakraborty et al., 2014). Intriguingly, previous investigations had highlighted that the expression of miR-34a is increased in islets of diabetic mice (Rottiers and Naar, 2012). The prolonged exposure of saturated fatty acids to MIN6 β-cells and pancreatic islets increased the expression of miR-34a (Lovis et al., 2008). Furthermore, miR-34a leads to endothelial dysfunction and vascular senescence in diabetes (Li et al., 2016; Carracedo et al., 2019; Thounaojam and Bartoli, 2019), increasing the overall risk of oxidative stress and inflammation with or without diabetes (Li et al., 2016; Cheleschi et al., 2019; Xiong et al., 2019; Zimta et al., 2019; Li et al., 2021; Mahjabeen et al., 2021; Zhu et al., 2021).
Conclusion
Herein, we summarized the investigations linking miR-34a and frailty. Furthermore, miR-34 may be linked to diabetes and endothelial dysfunction. Based on the provided evidence, we speculate that this miRNA may serve as a potential biomarker of frailty in diabetic older adults. Additionally, its actions on oxidative stress might represents a druggable target in order to develop new potentials therapeutic options.
Author contributions
Study concept and design: PM and GS. Drafting of the manuscript: PM, AP, and GS. Critical revision of the manuscript for important intellectual content: SJ, FV, UK, and GS. Administrative, technical, or material support: PM, AdD, and AP. Study supervision: PM and GS.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Afilalo, J., Alexander, K. P., Mack, M. J., Maurer, M. S., Green, P., Allen, L. A., et al. (2014). Frailty assessment in the cardiovascular care of older adults. J. Am. Coll. Cardiol. 63, 747–762. doi:10.1016/j.jacc.2013.09.070
Badi, I., Burba, I., Ruggeri, C., Zeni, F., Bertolotti, M., Scopece, A., et al. (2015). MicroRNA-34a induces vascular smooth muscle cells senescence by SIRT1 downregulation and promotes the expression of age-associated pro-inflammatory secretory factors. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1304–1311. doi:10.1093/gerona/glu180
Badi, I., Mancinelli, L., Polizzotto, A., Ferri, D., Zeni, F., Burba, I., et al. (2018). miR-34a promotes vascular smooth muscle cell calcification by downregulating SIRT1 (sirtuin 1) and axl (AXL receptor tyrosine kinase). Arterioscler. Thromb. Vasc. Biol. 38, 2079–2090. doi:10.1161/ATVBAHA.118.311298
Bandeen-Roche, K., Seplaki, C. L., Huang, J., Buta, B., Kalyani, R. R., Varadhan, R., et al. (2015). Frailty in older adults: a nationally representative profile in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1427–1434. doi:10.1093/gerona/glv133
Barwari, T., Joshi, A., and Mayr, M. (2016). MicroRNAs in cardiovascular disease. J. Am. Coll. Cardiol. 68, 2577–2584. doi:10.1016/j.jacc.2016.09.945
Boon, R. A., Iekushi, K., Lechner, S., Seeger, T., Fischer, A., Heydt, S., et al. (2013). MicroRNA-34a regulates cardiac ageing and function. Nature 495, 107–110. doi:10.1038/nature11919
Carini, G., Mingardi, J., Bolzetta, F., Cester, A., Bolner, A., Nordera, G., et al. (2022). miRNome profiling detects miR-101-3p and miR-142-5p as putative blood biomarkers of frailty syndrome. Genes (Basel) 13, 231. doi:10.3390/genes13020231
Carini, G., Musazzi, L., Bolzetta, F., Cester, A., Fiorentini, C., Ieraci, A., et al. (2021). The potential role of miRNAs in cognitive frailty. Front. Aging Neurosci. 13, 763110. doi:10.3389/fnagi.2021.763110
Carracedo, J., Alique, M., Ramirez-Carracedo, R., Bodega, G., and Ramirez, R. (2019). Endothelial extracellular vesicles produced by senescent cells: pathophysiological role in the cardiovascular disease associated with all types of diabetes mellitus. Curr. Vasc. Pharmacol. 17, 447–454. doi:10.2174/1570161116666180820115726
Chakraborty, C., Doss, C. G., Bandyopadhyay, S., and Agoramoorthy, G. (2014). Influence of miRNA in insulin signaling pathway and insulin resistance: micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip. Rev. RNA 5, 697–712. doi:10.1002/wrna.1240
Cheleschi, S., Tenti, S., Mondanelli, N., Corallo, C., Barbarino, M., Giannotti, S., et al. (2019). MicroRNA-34a and MicroRNA-181a mediate visfatin-induced apoptosis and oxidative stress via NF-kappaB pathway in human osteoarthritic chondrocytes. Cells 8, 874. doi:10.3390/cells8080874
Chen, L., Sun, H., Wang, C., Yang, Y., Zhang, M., Wong, G., et al. (2018). miRNA arm switching identifies novel tumour biomarkers. EBioMedicine 38, 37–46. doi:10.1016/j.ebiom.2018.11.003
Clegg, A., and Hassan-Smith, Z. (2018). Frailty and the endocrine system. Lancet. Diabetes Endocrinol. 6, 743–752. doi:10.1016/S2213-8587(18)30110-4
Clegg, A., Young, J., Iliffe, S., Rikkert, M. O., and Rockwood, K. (2013). Frailty in elderly people. Lancet 381, 752–762. doi:10.1016/S0140-6736(12)62167-9
Creemers, E. E., Tijsen, A. J., and Pinto, Y. M. (2012). Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 110, 483–495. doi:10.1161/CIRCRESAHA.111.247452
Cruz-Jentoft, A. J., and Sayer, A. A. (2019). Sarcopenia. Lancet 393, 2636–2646. doi:10.1016/S0140-6736(19)31138-9
Dalmasso, B., Hatse, S., Brouwers, B., Laenen, A., Berben, L., Kenis, C., et al. (2018). Age-related microRNAs in older breast cancer patients: biomarker potential and evolution during adjuvant chemotherapy. BMC Cancer 18, 1014. doi:10.1186/s12885-018-4920-6
Dowling, L., Duseja, A., Vilaca, T., Walsh, J. S., and Goljanek-Whysall, K. (2022). MicroRNAs in obesity, sarcopenia, and commonalities for sarcopenic obesity: a systematic review. J. Cachexia Sarcopenia Muscle 13, 68–85. doi:10.1002/jcsm.12878
Fadini, G. P., and Avogaro, A. (2010). Potential manipulation of endothelial progenitor cells in diabetes and its complications. Diabetes Obes. Metab. 12, 570–583. doi:10.1111/j.1463-1326.2010.01210.x
Ferrante, M., and Conti, G. O. (2017). Environment and neurodegenerative diseases: an update on miRNA role. Microrna 6, 157–165. doi:10.2174/2211536606666170811151503
Feve, B., and Bastard, J. P. (2009). The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 5, 305–311. doi:10.1038/nrendo.2009.62
Fonseca, A., Ramalhete, S. V., Mestre, A., Pires Das Neves, R., Marreiros, A., Castelo-Branco, P., et al. (2021). Identification of colorectal cancer associated biomarkers: an integrated analysis of miRNA expression. Aging (Albany NY) 13, 21991–22029. doi:10.18632/aging.203556
Fridrichova, I., and Zmetakova, I. (2019). MicroRNAs contribute to breast cancer invasiveness. Cells 8, 1361. doi:10.3390/cells8111361
Fried, L. P., Tangen, C. M., Walston, J., Newman, A. B., Hirsch, C., Gottdiener, J., et al. (2001). Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M156. doi:10.1093/gerona/56.3.m146
Gambardella, J., Fiordelisi, A., Sorriento, D., Cerasuolo, F. A., Buonaiuto, A., Avvisato, R., et al. (Forthcoming 2022). Mitochondrial microRNAs are dysregulated in patients with Fabry Disease. J. Pharmacol. Exp. Ther. JPET-AR, 2022-001250. doi:10.1124/jpet.122.001250
Gilbert, T., Neuburger, J., Kraindler, J., Keeble, E., Smith, P., Ariti, C., et al. (2018). Development and validation of a hospital frailty risk score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 391, 1775–1782. doi:10.1016/S0140-6736(18)30668-8
Gulsin, G. S., Henson, J., Brady, E. M., Sargeant, J. A., Wilmot, E. G., Athithan, L., et al. (2020). Cardiovascular determinants of aerobic exercise capacity in adults with type 2 diabetes. Diabetes Care 43, 2248–2256. doi:10.2337/dc20-0706
Hanlon, P., Nicholl, B. I., Jani, B. D., Lee, D., Mcqueenie, R., Mair, F. S., et al. (2018). Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK biobank participants. Lancet. Public Health 3, e323–e332. doi:10.1016/S2468-2667(18)30091-4
Hathaway, Q. A., Pinti, M. V., Durr, A. J., Waris, S., Shepherd, D. L., Hollander, J. M., et al. (2018). Regulating microRNA expression: at the heart of diabetes mellitus and the mitochondrion. Am. J. Physiol. Heart Circ. Physiol. 314, H293–H310. doi:10.1152/ajpheart.00520.2017
Hoogendijk, E. O., Afilalo, J., Ensrud, K. E., Kowal, P., Onder, G., Fried, L. P., et al. (2019). Frailty: implications for clinical practice and public health. Lancet 394, 1365–1375. doi:10.1016/S0140-6736(19)31786-6
Hu, G., Liao, K., Yang, L., Pendyala, G., Kook, Y., Fox, H. S., et al. (2017). Tat-mediated induction of miRs-34a & -138 promotes astrocytic activation via downregulation of SIRT1: implications for aging in HAND. J. Neuroimmune Pharmacol. 12, 420–432. doi:10.1007/s11481-017-9730-0
Jarvie, J. L., Pandey, A., Ayers, C. R., Mcgavock, J. M., Senechal, M., Berry, J. D., et al. (2019). Aerobic fitness and adherence to guideline-recommended minimum physical activity among ambulatory patients with type 2 diabetes mellitus. Diabetes Care 42, 1333–1339. doi:10.2337/dc18-2634
Kukreti, H., and Amuthavalli, K. (2020). MicroRNA-34a causes ceramide accumulation and effects insulin signaling pathway by targeting ceramide kinase (CERK) in aging skeletal muscle. J. Cell. Biochem. 121, 3070–3089. doi:10.1002/jcb.29312
Khanna, A., Muthusamy, S., Liang, R., Sarojini, H., and Wang, E. (2011). Gain of survival signaling by down-regulation of three key miRNAs in brain of calorie-restricted mice. Aging (Albany NY) 3, 223–236. doi:10.18632/aging.100276
Krol, J., Loedige, I., and Filipowicz, W. (2010). The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 11, 597–610. doi:10.1038/nrg2843
Lee, H., Kim, Y. I., Nirmala, F. S., Kim, J. S., Seo, H. D., Ha, T. Y., et al. (2021). MiR-141-3p promotes mitochondrial dysfunction in ovariectomy-induced sarcopenia via targeting Fkbp5 and Fibin. Aging (Albany NY) 13, 4881–4894. doi:10.18632/aging.202617
Li, G., Prior, J. C., Leslie, W. D., Thabane, L., Papaioannou, A., Josse, R. G., et al. (2019). Frailty and risk of fractures in patients with type 2 diabetes. Diabetes Care 42, 507–513. doi:10.2337/dc18-1965
Li, Q., Kim, Y. R., Vikram, A., Kumar, S., Kassan, M., Gabani, M., et al. (2016). P66Shc-Induced MicroRNA-34a causes diabetic endothelial dysfunction by downregulating Sirtuin1. Arterioscler. Thromb. Vasc. Biol. 36, 2394–2403. doi:10.1161/ATVBAHA.116.308321
Li, T., Pang, Q., Liu, Y., Bai, M., Peng, Y., Zhang, Z., et al. (2021). Sulforaphane protects human umbilical vein endothelial cells from oxidative stress via the miR-34a/SIRT1 axis by upregulating nuclear factor erythroid-2-related factor 2. Exp. Ther. Med. 21, 186. doi:10.3892/etm.2021.9617
Lovis, P., Roggli, E., Laybutt, D. R., Gattesco, S., Yang, J. Y., Widmann, C., et al. (2008). Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction. Diabetes 57, 2728–2736. doi:10.2337/db07-1252
Mahjabeen, W., Khan, D. A., Mirza, S. A., and Pervez, M. A. (2021). Effects of delta-tocotrienol supplementation on glycemic control, oxidative stress, inflammatory biomarkers and miRNA expression in type 2 diabetes mellitus: a randomized control trial. Phytother. Res. 35, 3968–3976. doi:10.1002/ptr.7113
Manakanatas, C., Ghadge, S. K., Agic, A., Sarigol, F., Fichtinger, P., Fischer, I., et al. (2022). Endothelial and systemic upregulation of miR-34a-5p fine-tunes senescence in progeria. Aging (Albany NY) 14, 195–224. doi:10.18632/aging.203820
Mastrototaro, L., and Roden, M. (2021). Insulin resistance and insulin sensitizing agents. Metabolism. 125, 154892. doi:10.1016/j.metabol.2021.154892
Mone, P., Gambardella, J., Lombardi, A., Pansini, A., De Gennaro, S., Leo, A. L., et al. (2022a). Correlation of physical and cognitive impairment in diabetic and hypertensive frail older adults. Cardiovasc. Diabetol. 21, 10. doi:10.1186/s12933-021-01442-z
Mone, P., Gambardella, J., Pansini, A., De Donato, A., Martinelli, G., Boccalone, E., et al. (2021a). Cognitive impairment in frail hypertensive elderly patients: role of hyperglycemia. Cells 10, 2115. doi:10.3390/cells10082115
Mone, P., Gambardella, J., Pansini, A., Martinelli, G., Minicucci, F., Mauro, C., et al. (2022b). Cognitive dysfunction correlates with physical impairment in frail patients with acute myocardial infarction. Aging Clin. Exp. Res. 34, 49–53. doi:10.1007/s40520-021-01897-w
Mone, P., Gambardella, J., Wang, X., Jankauskas, S. S., Matarese, A., Santulli, G., et al. (2021b). miR-24 targets the transmembrane glycoprotein neuropilin-1 in human brain microvascular endothelial cells. Noncoding. RNA 7, 9. doi:10.3390/ncrna7010009
Mone, P., Lombardi, A., Kansakar, U., Varzideh, F., Jankauskas, S. S., Pansini, A., et al. (Forthcoming 2022c). Empagliflozin improves the microRNA signature of endothelial dysfunction in patients with HFpEF and diabetes. J. Pharmacol. Exp. Ther.
Mone, P., Pansini, A., Calabro, F., De Gennaro, S., Esposito, M., Rinaldi, P., et al. (2022d). Global cognitive function correlates with P-wave dispersion in frail hypertensive older adults. J. Clin. Hypertens. (Greenwich) 24 (5), 638–643. doi:10.1111/jch.14439
Mone, P., Pansini, A., Frullone, S., De Donato, A., Buonincontri, V., De Blasiis, P., et al. (2022e). Physical decline and cognitive impairment in frail hypertensive elders during COVID-19. Eur. J. Intern. Med. 99, 89–92. doi:10.1016/j.ejim.2022.03.012
Mone, P., and Pansini, A. (2020). Gait speed test and cognitive decline in frail women with acute myocardial infarction. Am. J. Med. Sci. 360, 484–488. doi:10.1016/j.amjms.2020.03.021
Nagai, K., Miyamato, T., Okamae, A., Tamaki, A., Fujioka, H., Wada, Y., et al. (2018). Physical activity combined with resistance training reduces symptoms of frailty in older adults: a randomized controlled trial. Arch. Gerontol. Geriatr. 76, 41–47. doi:10.1016/j.archger.2018.02.005
Ni, T., Lin, N., Lu, W., Sun, Z., Lin, H., Chi, J., et al. (2020). Dihydromyricetin prevents diabetic cardiomyopathy via miR-34a suppression by activating autophagy. Cardiovasc. Drugs Ther. 34, 291–301. doi:10.1007/s10557-020-06968-0
Park, J., Kim, J., Chen, Y., Song, H. C., Chen, Y., Zheng, M., et al. (2020). CO ameliorates cellular senescence and aging by modulating the miR-34a/Sirt1 pathway. Free Radic. Res. 54, 848–858. doi:10.1080/10715762.2019.1710142
Pilotto, A., Custodero, C., Maggi, S., Polidori, M. C., Veronese, N., Ferrucci, L., et al. (2020). A multidimensional approach to frailty in older people. Ageing Res. Rev. 60, 101047. doi:10.1016/j.arr.2020.101047
Prattichizzo, F., De Nigris, V., Spiga, R., Mancuso, E., La Sala, L., Antonicelli, R., et al. (2018). Inflammageing and metaflammation: the yin and yang of type 2 diabetes. Ageing Res. Rev. 41, 1–17. doi:10.1016/j.arr.2017.10.003
Puts, M. T. E., Toubasi, S., Andrew, M. K., Ashe, M. C., Ploeg, J., Atkinson, E., et al. (2017). Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing 46, 383–392. doi:10.1093/ageing/afw247
Rippo, M. R., Olivieri, F., Monsurro, V., Prattichizzo, F., Albertini, M. C., Procopio, A. D., et al. (2014). MitomiRs in human inflamm-aging: a hypothesis involving miR-181a, miR-34a and miR-146a. Exp. Gerontol. 56, 154–163. doi:10.1016/j.exger.2014.03.002
Rockwood, K., Song, X., Macknight, C., Bergman, H., Hogan, D. B., Mcdowell, I., et al. (2005). A global clinical measure of fitness and frailty in elderly people. CMAJ 173, 489–495. doi:10.1503/cmaj.050051
Rottiers, V., and Naar, A. M. (2012). MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 13, 239–250. doi:10.1038/nrm3313
Rusanova, I., Fernandez-Martinez, J., Fernandez-Ortiz, M., Aranda-Martinez, P., Escames, G., Garcia-Garcia, F. J., et al. (2019). Involvement of plasma miRNAs, muscle miRNAs and mitochondrial miRNAs in the pathophysiology of frailty. Exp. Gerontol. 124, 110637. doi:10.1016/j.exger.2019.110637
Santulli, G. (2015). microRNAs distinctively regulate vascular smooth muscle and endothelial cells: Functional implications in angiogenesis, atherosclerosis, and in-stent restenosis. Adv. Exp. Med. Biol. 887, 53–77. doi:10.1007/978-3-319-22380-3_4
Searle, S. D., Mitnitski, A., Gahbauer, E. A., Gill, T. M., and Rockwood, K. (2008). A standard procedure for creating a frailty index. BMC Geriatr. 8, 24. doi:10.1186/1471-2318-8-24
Sieber, C. C. (2019). Malnutrition and sarcopenia. Aging Clin. Exp. Res. 31, 793–798. doi:10.1007/s40520-019-01170-1
Song, R., Hu, X. Q., and Zhang, L. (2019). Mitochondrial MiRNA in cardiovascular function and disease. Cells 8, 1475. doi:10.3390/cells8121475
Srinivasan, A. R., Tran, T. T., and Bonini, N. M. (2022). Loss of miR-34 in Drosophila dysregulates protein translation and protein turnover in the aging brain. Aging Cell 21, e13559. doi:10.1111/acel.13559
Stavast, C. J., and Erkeland, S. J. (2019). The non-canonical aspects of MicroRNAs: many roads to gene regulation. Cells 8, 1465. doi:10.3390/cells8111465
Taylor, R. (2013). Type 2 diabetes: etiology and reversibility. Diabetes Care 36, 1047–1055. doi:10.2337/dc12-1805
Thounaojam, M. C., and Bartoli, M. (2019). MicroRNA-34a and vascular senescence in diabetes. Aging (Albany NY) 11, 11799–11800. doi:10.18632/aging.102625
Umegaki, H. (2016). Sarcopenia and frailty in older patients with diabetes mellitus. Geriatr. Gerontol. Int. 16, 293–299. doi:10.1111/ggi.12688
Waite, S. J., Maitland, S., Thomas, A., and Yarnall, A. J. (2021). Sarcopenia and frailty in individuals with dementia: a systematic review. Arch. Gerontol. Geriatr. 92, 104268. doi:10.1016/j.archger.2020.104268
Walston, J., Fedarko, N., Yang, H., Leng, S., Beamer, B., Espinoza, S., et al. (2008). The physical and biological characterization of a frail mouse model. J. Gerontol. A Biol. Sci. Med. Sci. 63, 391–398. doi:10.1093/gerona/63.4.391
Wei, R., Yang, J., Liu, G. Q., Gao, M. J., Hou, W. F., Zhang, L., et al. (2013). Dynamic expression of microRNAs during the differentiation of human embryonic stem cells into insulin-producing cells. Gene 518, 246–255. doi:10.1016/j.gene.2013.01.038
Wei, Y., Corbalan-Campos, J., Gurung, R., Natarelli, L., Zhu, M., Exner, N., et al. (2018). Dicer in macrophages prevents atherosclerosis by promoting mitochondrial oxidative metabolism. Circulation 138, 2007–2020. doi:10.1161/CIRCULATIONAHA.117.031589
Wong, W. K. M., Sorensen, A. E., Joglekar, M. V., Hardikar, A. A., and Dalgaard, L. T. (2018). Non-coding RNA in pancreas and beta-cell development. Noncoding. RNA 4, E41. doi:10.3390/ncrna4040041
Wronska, A., Kurkowska-Jastrzebska, I., and Santulli, G. (2015). Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol. 213, 60–83. doi:10.1111/apha.12416
Xiong, H., Chen, S., Lai, L., Yang, H., Xu, Y., Pang, J., et al. (2019). Modulation of miR-34a/SIRT1 signaling protects cochlear hair cells against oxidative stress and delays age-related hearing loss through coordinated regulation of mitophagy and mitochondrial biogenesis. Neurobiol. Aging 79, 30–42. doi:10.1016/j.neurobiolaging.2019.03.013
Yarnall, A. J., Sayer, A. A., Clegg, A., Rockwood, K., Parker, S., Hindle, J. V., et al. (2017). New horizons in multimorbidity in older adults. Age Ageing 46, 882–888. doi:10.1093/ageing/afx150
Yu, D., Shang, J., Cai, Y., Wang, Z., Zhang, X., Zhao, B., et al. (2019). Derivation and external validation of a risk prediction algorithm to estimate future risk of cardiovascular death among patients with type 2 diabetes and incident diabetic nephropathy: prospective cohort study. BMJ Open Diabetes Res. Care 7, e000735. doi:10.1136/bmjdrc-2019-000735
Yuan, Q., Chen, Z., Santulli, G., Gu, L., Yang, Z. G., Yuan, Z. Q., et al. (2014). Functional role of Calstabin2 in age-related cardiac alterations. Sci. Rep. 4, 7425. doi:10.1038/srep07425
Yun, J. S., Park, Y. M., Cha, S. A., Ahn, Y. B., and Ko, S. H. (2018). Progression of cardiovascular autonomic neuropathy and cardiovascular disease in type 2 diabetes. Cardiovasc. Diabetol. 17, 109. doi:10.1186/s12933-018-0752-6
Zhang, X., Zuo, X., Yang, B., Li, Z., Xue, Y., Zhou, Y., et al. (2014). MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell 158, 607–619. doi:10.1016/j.cell.2014.05.047
Zheng, Y., Kong, J., Li, Q., Wang, Y., and Li, J. (2018). Role of miRNAs in skeletal muscle aging. Clin. Interv. Aging 13, 2407–2419. doi:10.2147/CIA.S169202
Zhu, H., Lin, Y., and Liu, Y. (2021). miR34a increases inflammation and oxidative stress levels in patients with necrotizing enterocolitis by downregulating SIRT1 expression. Mol. Med. Rep. 24, 664. doi:10.3892/mmr.2021.12303
Keywords: miRNA, miR-34, frailty, diabetes, aging
Citation: Mone P, de Donato A, Varzideh F, Kansakar U, Jankauskas SS, Pansini A and Santulli G (2022) Functional role of miR-34a in diabetes and frailty. Front. Aging 3:949924. doi: 10.3389/fragi.2022.949924
Received: 21 May 2022; Accepted: 29 June 2022;
Published: 18 July 2022.
Edited by:
Manish Kumar Gupta, University of Central Florida, United StatesReviewed by:
Angelica Perna, University of Molise, ItalyAlfonso Baldi, University of Campania Luigi Vanvitelli, Italy
Copyright © 2022 Mone, de Donato, Varzideh, Kansakar, Jankauskas, Pansini and Santulli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pasquale Mone, cGFzcXVhbGUubW9uZUBlaW5zdGVpbm1lZC5lZHU=;, cGFzcXVhbGVtb25lQGhvdG1haWwuaXQ=
 Pasquale Mone
Pasquale Mone Antonio de Donato
Antonio de Donato Fahimeh Varzideh1
Fahimeh Varzideh1 Urna Kansakar
Urna Kansakar