
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 03 March 2025
Sec. Alzheimer's Disease and Related Dementias
Volume 17 - 2025 | https://doi.org/10.3389/fnagi.2025.1543282
 Muhammad Danish Mujib1
Muhammad Danish Mujib1 Ahmad Zahid Rao1,2
Ahmad Zahid Rao1,2 Muhammad Fahim Ul Haque3
Muhammad Fahim Ul Haque3 Ahmad O. Alokaily4,5*
Ahmad O. Alokaily4,5* Syeda Sehar Hussain1
Syeda Sehar Hussain1 Ahmed A. Aldohbayb4,5
Ahmed A. Aldohbayb4,5 Saad Ahmed Qazi6,7
Saad Ahmed Qazi6,7 Muhammad Abul Hasan1,7*
Muhammad Abul Hasan1,7*Introduction: Alzheimer’s disease (AD) affects 50 million individuals worldwide, a number projected to triple by 2050. Due to discomfort through electrical and magnetic neuromodulation technologies, this is the first study to propose the potential of auditory binaural beat (BB) stimulation at an alpha frequency (10 Hz) for enhancing cognitive and neurological outcomes in AD patients.
Methods: Twenty-five patients were divided into the experimental-Group (n = 15) and control-Group (n = 10). Psychometric and neurological assessments were conducted Pre-Treatment (Day 1) and Post-Treatment (Day 14) following consecutive days of binaural beats (BB) or auditory tone stimulation administered from Day 2 to Day 13.
Results: A two-way ANOVA revealed a significant main effect of group (F = 6.087, p = 0.016) and session (F = 3.859, p = 0.024) on MMSE scores, with the experimental group showing significant improvement in MMSE scores (t = 7.33, p = 0.00000012) compared to the control group (p = 0.2306). Paired t-tests revealed a significant reduction in depression scores (DASS-21, t = 1.701, p = 0.0253) in the experimental group, while no significant improvements were noted in the control group. EEG recordings revealed significant changes in α-band, β-band, and γ-band power (p < 0.05). Moreover, The correlation between EEG bands and MMSE subparts showed that increased θ-band power in the experimental group was positively correlated (p < 0.05) with the frontal region during language tasks and in the frontal and central regions during registration and orientation tasks, indicating potential neurocognitive benefits.
Discussion: The results of this research imply that BB stimulation has untapped potential as a non-invasive therapy for patients with AD, hence there is the need for further studies to manage the dementia epidemic.
Alzheimer’s disease (AD) is a neurological disorder that progressively impairs cognition, attention span, and memory and hinders the ability to perform everyday activities (Okabe et al., 2020; Ott et al., 1996; Parasuraman and Haxby, 1993). Behavioral and neurological symptoms of AD are associated with aging, as it is more prevalent in the elderly (Galasko et al., 1990; Turner et al., 2012). This condition progresses through three stages starting with memory loss in the early phase and learning difficulties in the intermediate stage and ultimately leading to complete debilitation in the severe stage (Agrawal et al., 2020; Galasko et al., 2005). According to the World Health Organization (WHO), around 50 million individuals worldwide are currently affected by AD and related dementia issues and this number is expected to triple by 2050 (World Health Organization, 2021).
Dementia in AD patients is a leading cause of dependency on professional caregivers for performing everyday activities (Bell et al., 2001; Galasko et al., 2005; González-Salvador et al., 1999). This dependency and inability to execute tasks on their own may have negative psychological effects on AD patients in the long term and thus lead to serious mental health issues such as anxiety, stress, and depression (Botto et al., 2022; Gallagher et al., 2011). According to previous studies, the Depression Anxiety Stress Scale (DASS-21) serves as a neuropsychological assessment tool to screen AD patients for signs of depression, stress, and anxiety (Ali et al., 2022). Effective assessment techniques for diagnosing the severity of AD include the Mini-Mental State Examination (MMSE) (Arevalo-Rodriguez et al., 2015), the Clinical Dementia Rating (CDR) (Morris et al., 2001), the Sum of Boxes (CDR-SB) (Williams et al., 2013), and the CDR orientation score (Kim et al., 2017). MMSE assesses a person’s cognitive abilities, specifically orientation, attention and recall, registration, calculation, language, and visual construction (MacKenzie et al., 1996; Sposito et al., 2015).
Aside from behavioral and neuropsychological assessments of AD patients, recent studies have also utilized neuroimaging techniques to investigate neurological changes. These include Positron Emission Tomography scan (Nordberg et al., 2010), Magnetic Resonance Imaging (Teipel et al., 2013), functional Magnetic Resonance Imaging (Machulda et al., 2003), Computed Tomography-scan (Cuttler et al., 2016), and electroencephalography (EEG) (Bennys et al., 2001). EEG provides frequency-specific changes in the cortex (García Domínguez et al., 2013; Soininen et al., 2020). Power spectrum density (PSD) is one of the features of EEG that may be used to compare the neurological changes that occur in AD (Liu et al., 2016; Wang et al., 2015). Increased θ-band power while decreasing α- and β-bands power is mainly reported specifically in the temporal and posterior/occipital brain regions (Azami et al., 2023; Babiloni et al., 2021; Bruña et al., 2023; Cassani et al., 2018; Horvath et al., 2018; López-Sanz et al., 2016; López-Sanz et al., 2019; Maestú and Fernández, 2020; Moretti et al., 2004; Musaeus et al., 2018; Roh et al., 2011).
Electrical and magnetic neuromodulation have been clinically proven as an effective means of treatment for the improvement of AD symptoms (Bentwich et al., 2011; Gangemi et al., 2021; Rajji, 2019; Scherder et al., 1995). Several studies have demonstrated, improved cognitive scores measured with MMSE following two to four weeks of neuromodulation with rTMS and tDCS (Im et al., 2019; Wei et al., 2022). In addition to potential risks to body tissues and feelings of discomfort, trained personnel are required to operate these neuromodulation techniques (Johnson, 2001; Nikolin et al., 2018; Russo et al., 2013). Therefore, it is impractical for family caregivers to provide these stimulations at home.
Auditory stimulation such as binaural beat (BB) is cost-effective and easy to use (Parodi et al., 2021; Tani et al., 2022). BB stimulation is provided through earphones with slightly different frequencies presented separately to each ear. The brain perceives a sound with a frequency corresponding to the difference between these two frequencies. Its origin is subcortical, specifically in the pons, within the medial nucleus of the superior olivary complex (Sadeghijam et al., 2023). BB stimulation has been shown to modulate brain activity and improve cognitive functions such as working memory and attention (Beauchene et al., 2017; Mujib et al., 2021). Several studies have explored the effects of binaural beats (BB) stimulation on memory and cognitive function in both healthy and clinical populations. Research suggests that BB stimulation enhances episodic and working memory by modulating neural oscillations (Jirakittayakorn and Wongsawat, 2017; Reedijk et al., 2015). For instance, Jirakittayakorn and Wongsawat (2017) demonstrated that BB increased theta power in EEG recordings, which correlated with improved working memory performance. Similarly, alpha-frequency BB has been associated with increased alpha power, promoting attentional focus and relaxation, which may be beneficial for AD patients (Crespo et al., 2013; Park et al., 2018). Reedijk et al. found that alpha-frequency BB improved sustained attention and task performance, likely by enhancing alpha synchronization (Gao et al., 2014; Reedijk et al., 2015). Research also indicates that BB may play a role in cognitive enhancement by promoting neural synchrony (Park et al., 2018). Garcia-Argibay et al. (2019) suggested that BB stimulation could enhance cognitive flexibility and executive function.
However, some studies report no significant cognitive improvements following BB exposure, attributing the discrepancies to variations in individual variability, stimulation frequency, and differences in study design, including variations in EEG recording protocols, cognitive assessment tools, stimulation duration, and control conditions (Chaieb et al., 2015; Vernon et al., 2014). For instance, Goodin et al. (2019) reported no significant effects of BB stimulation on cognitive function, highlighting the need for standardized methodologies in future research. Despite limited research, some preliminary studies suggest that binaural beats BB may influence EEG-derived biomarkers of cognitive decline, such as changes in theta and alpha power, in Alzheimer’s disease (AD) patients. However, the long-term effects and underlying mechanisms of BB in AD remain underexplored (Garcia-Argibay et al., 2019; McMurray, 2006). By employing strict inclusion criteria to ensure participant homogeneity, we hypothesized that BB-induced neurological changes, as measured through EEG analysis, would correlate with significant improvements in cognitive performance in AD patients.
The study was conducted at two locations: Dar-ul-Sakoon and Gills Shelter Center. The consent was achieved before the trial from both the administrations of the two facilities. Dar-ul-Sakoon and Gills Shelter Center directed all the related information to their in-charge officials and they were able to assess before and after the experiment which was in line with our ethics guidelines. This approach proactively sought to build on a foundation of collaboration, integrity, and safety by prioritizing the stakeholders’ welfare and success during the research process. The research was approved by the “Research Ethics Committee” at NED University of Engineering & Technology.
A total of 107 Alzheimer’s disease (AD) patients at Dar-ul-Sukun, Karachi, Pakistan and Gill Shelter Center, Karachi, Pakistan were initially screened for potential inclusion in the study. The experimental procedures were thoroughly explained to all patients by the administration at both centers, along with our research team. The inclusion criteria for the study included a diagnosis of Alzheimer’s disease based on the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria and the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition DSM-IV, age 60 years or older, a history of Alzheimer’s disease for at least 5 years, Mini-Mental State Examination (MMSE) scores between 10 and 24 or Clinical Dementia Rating (CDR) scores between 1 and 2, and the ability to provide informed consent, or consent obtained from a legal guardian if the participant was unable.
Exclusion criteria included a diagnosis of other neurological or psychiatric disorders, severe auditory impairments that could interfere with participation in auditory stimulation, the use of medications or therapies that could significantly affect cognitive function, such as nootropic drugs, a history of epilepsy or other seizure disorders, and a lack of interest or refusal to participate in the study.
The participant selection criteria for the study are shown in Figure 1. Of the initial 107 patients, 67 were excluded based on the above criteria, including a lack of interest in participating, having an AD history of less than five years, or the presence of other neurological disorders. The remaining patients were then evaluated using the NINCDS-ADRDA criteria (McKhann et al., 1984) and the DSM-IV (American Psychiatric Association, 1994). Following this evaluation, 30 patients met the inclusion criteria; however, 5 of these were further excluded based on their Mini-Mental State Examination (MMSE) or Clinical Dementia Rating (CDR) scores. Ultimately, 25 patients were selected to participate in the study.
Participants were randomly allocated to the experimental and control groups prior to administering the MMSE for unbiased distribution. The MMSE was then conducted to assess baseline cognitive function. The inclusion/exclusion criteria were pre-defined, as given in Figure 1, to ensure that all participants met the cognitive baseline required for meaningful comparisons and validity of results. A modified minimization approach was employed to allocate participants to either the Experimental or Control group.
The study’s sample size was determined using an interim analysis conducted on the first six participants in each group (12 total), resulting in high effect size (d = 1.72) and statistical power (SP = 90%) for significant changes in MMSE scores. The Experimental Group exhibited significant improvements in MMSE scores (Pre-MMSE mean score = 11.57 ± 3.25, Post-MMSE mean score = 17.10 ± 3.18), highlighting the effectiveness of the intervention. Based on these promising results, a maximum sample size of 11 participants per group (total of 22) was calculated to achieve p < 0.025 and SP = 80%, ensuring a balance between precision and feasibility.
The interim analysis approach allowed for adaptive refinement of the sample size, optimizing resource utilization while maintaining high statistical rigor. By employing a stricter significance threshold of p < 0.025 instead of the conventional p < 0.05, the robustness and reliability of the findings were further enhanced, effectively mitigating the potential for false positives. Five participants from the control group did not meet the minimum MMSE qualification criteria necessary for inclusion in the study and were thus excluded thereafter. Despite these exclusions resulting in slightly unequal sample sizes, the study design maintained its robustness due to the randomized allocation process and high statistical power for the sample size. The integrity of the analysis was preserved by adhering strictly to the inclusion and exclusion criteria, ensuring that the findings are both valid and generalizable.
The included 25 AD patients were randomly assigned to either the Experimental-Group (n = 15) or the Control-Group (n = 10). The Experimental-Group was provided alpha BB stimulation of 10 Hz difference (Left ear: 400 Hz and Right ear: 410 Hz) whereas Control-Group received a created tone comprising the frequency of 400 Hz in both left and right ears. Although the researchers were aware of the group assignments, the administration and the participants in both centers were blinded to their allocation to prevent any bias in their responses.
There were 16 males and 9 females, with an average age of 69.96 ± 8.67 years and an average history of AD of 6.36 ± 1.35 years. The males had a mean age of 71.19 ± 8.28 years and an average history of AD of 6.34 ± 1.52 years, while the female AD patients had mean age of 67.78 ± 9.40 years and an average history of AD of 6.39 ± 1.05 years.
As shown in Figure 2, the experimental procedure involved a 12-day treatment period with assessments conducted at three key stages: Pre-Treatment (Day 1), Post-Treatment (Day 14), and follow-up session. The Pre-Treatment assessment was carried out one day prior to the start of the treatment, while the Post-Treatment assessment took place one day after the treatment concluded. The follow-up assessment was conducted two weeks later to evaluate the patients’ cognitive progress using the MMSE.
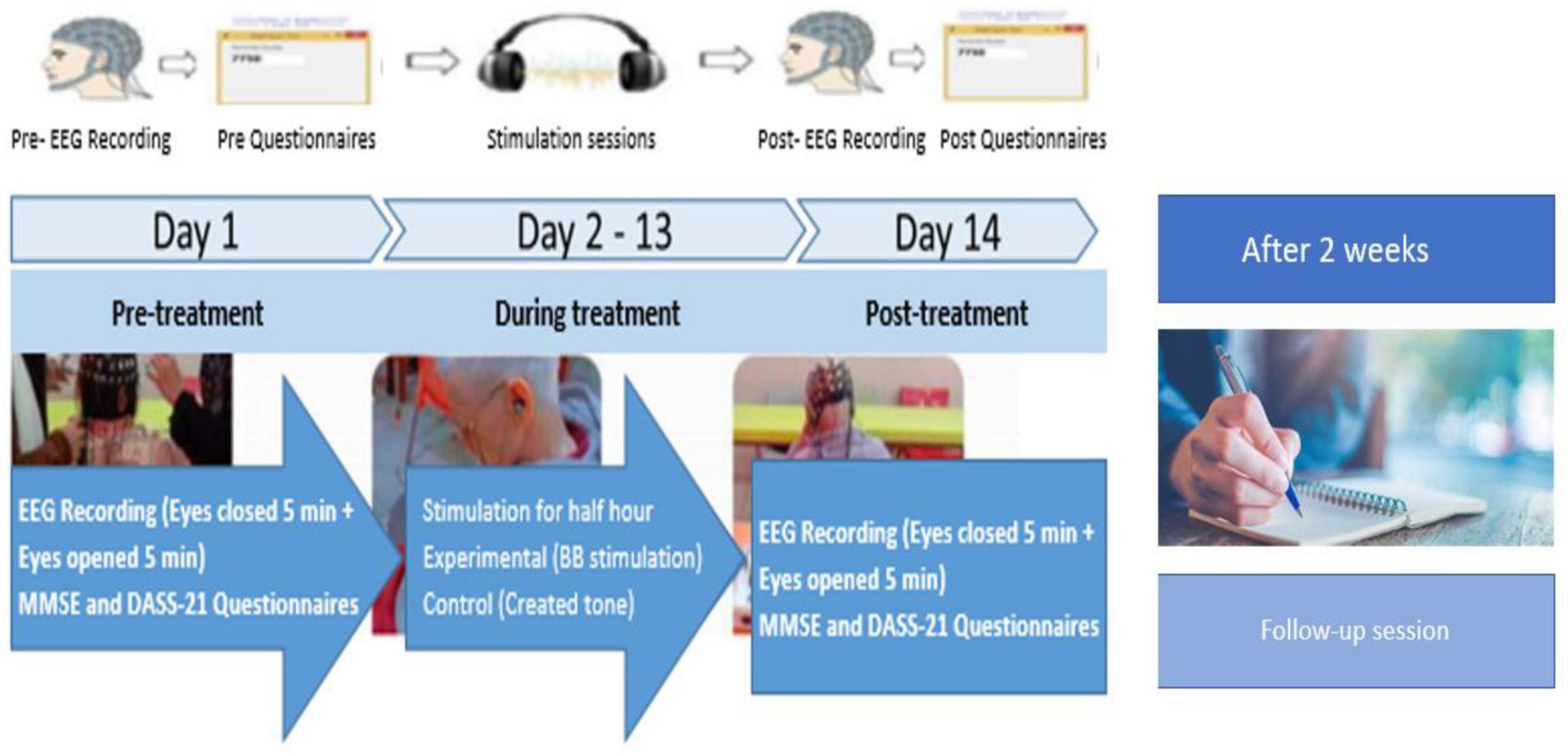
Figure 2. Experiment protocol for experimental- and control groups of AD patients. Pre-Treatment represents psychometric assessments and neurological recording on day 1, During treatment represents BB stimulation days from day 2 to 13 and Post-Treatment represents psychometric assessments and neurological recording on day 14. A follow-up session was taken after two weeks of Post-Treatment, focusing solely on psychometric assessments (MMSE scores).
During the treatment period (from Day 2 to Day 13), both groups underwent half-hour daily stimulation sessions. At the Pre-Treatment (Day 1) and Post-Treatment (Day 14) phases, both neurological assessments (EEG recordings) and psychometric assessments (MMSE and Depression Anxiety Stress Scales-21 [DASS-21]) (Al-Shargie and Goh, 2019; Coker et al., 2018; Insel et al., 2006; Pillai et al., 2014; Shigemori et al., 2010; Tierney et al., 2000) were performed. The follow-up session focused solely on MMSE scores. Additionally, participants were asked general questions about their health, daily routine, sleep schedule, activities, and diet to conduct a thorough behavioral and psychometric analysis. These assessments were designed to establish a baseline for each participant’s cognitive and emotional state at the study’s outset.
Some challenges were encountered when preparing and attending to most elderly patients, particularly their resistance and anxiety toward the experimental procedures. To address this, we made multiple efforts to build trust and provide reassurance before beginning the experiment. We prioritized clear communication, taking time to thoroughly explain the procedures and address any concerns to ensure the patients felt comfortable and ready to participate. This approach was crucial in upholding ethical considerations and ensuring the patient’s willingness to engage in the study. Throughout the experiment, participants adhered strictly to the protocol under the supervision of qualified neurologists, psychologists, and the nursing staff.
During the test, each participant’s hearing threshold was assessed in a soundproof room using calibrated stereo headphones. The procedure involved presenting pure tones (400 Hz and 410 Hz) at low-intensity levels, starting below the expected threshold (e.g., 0–10 dB), and gradually increasing the intensity in 5 dB increments until the participant could reliably detect the sound. The lowest intensity level at which the participant could detect the tone 50% of the time was recorded as their hearing threshold for that frequency.
The device threshold was calibrated to present the stimuli at a level 50 dB above the hearing threshold. For instance, if the given criteria for 400 Hz for a particular subject was 15 dB, the sound was produced at 65 dB (15+50). Subsequent changes were made to both amplification levels based on participant input to maintain the volume at a loud but comfortable level. This process ensures that the auditory stimuli were both detectable and comfortably loud, tailored to each participant’s auditory sensitivity. This auditory test was conducted on Day 1.
BB is created by presenting two slightly different-frequency sound waves to each ear separately. The brain then perceives a third sound, which is the difference between the two frequencies. For example, if one ear hears a 400 Hz tone and the other ear hears a 410 Hz tone, the brain perceives a beat at a frequency of 10 Hz (Mujib et al., 2021; Sadeghijam et al., 2023). Both types of stimulation tones (BB/Standard auditory tone stimulation) were created using Adobe Audition v3.0 (Tierney et al., 2000). The stimuli were presented at a minimum intensity of 50 dB above each participant’s hearing threshold, determined through standardized pure-tone audiometry (Goodin et al., 2019).
The study utilized MMSE and DASS-21 questionnaires to assess the cognitive and psychometric effects of BB and standard auditory stimulation, respectively. The MMSE is a widely used screening tool for assessing cognitive characteristics (Al-Shargie and Goh, 2019; American Psychiatric Association, 1994; Shigemori et al., 2010) and assessing areas of cognitive functioning including orientation, language, attention, calculation, registration, recall, and copying (Pillai et al., 2014; Shigemori et al., 2010; Tierney et al., 2000). Each task is scored based on the individual’s performance, with a maximum score of 30 points indicating normal cognitive function. The MMSE questions were asked orally and the particular questions involving writing, reading, and drawing were carried out on paper, with the total duration for each patient ranging from 30 to 40 min. MMSE is categorized into different levels of cognitive impairment. The scores 21–26 represent mild impairment, 10–20 indicate moderate impairment, 10–14 suggest moderately severe impairment, and scores below 10 signify severe impairment (Al-Shargie and Goh, 2019; Pillai et al., 2014; Shigemori et al., 2010; Tierney et al., 2000). The DASS-21 is a 21-item questionnaire that measures symptoms associated with depression, anxiety, and stress (Insel et al., 2006). The predictive validity of the DASS-21 has also been studied in AD patients (Tierney et al., 2000). This test was conducted verbally and lasted approximately half an hour. The degree of application of each statement is indicated by a rating on a scale from 0 to 3. Scores range from 0 for “not applied” to 3 for “sometimes applied” (Coker et al., 2018; Insel et al., 2006; Pillai et al., 2014).
To explore the usability of the Binaural Beat delivered through stereo headsets, participants were given the System Usability Scale (SUS) questionnaire at the end of the evaluation (Marijanović et al., 2021). The SUS itself, consisting of 10 items with Likert scale responses ranging from 1 (strongly disagree) to 5 (strongly agree), was implemented. If the SUS score is 66 there is no reason to worry because it indicates good usability. To SUS (system usability scale) rating’s calculation is done by the sum of the odd-numbered questions rating and then five is subtracted from it. Then the sum of the even-numbered questions rating minus twenty-five is taken to find out the SUS rating. In addition, the total of the first and last SUS ratings from both odd and even-numbered questions is multiplied by 2.5 to discover the final SUS composite rating (Eq. 1) (Brooke, 1996; Mujib et al., 2023).
To investigate brain activity, this study used an EEG Mitsar-NVX from 38 scalp locations (FP1, FPZ, FP2, F7, F3, FZ, F4, F8, FT7, FC3, FCZ, FC4, FT8, T3, TP7, T5, T4, TP8, T6, C3, CZ, C4, CP3, CPZ, CP4, P3, PZ, P4, P5, PO3, POZ, PO4, P6, PO7, PO8, O1, OZ, O2) with two reference electrodes (A1, A2) following the standardized 10–20 international system (Rao and Hasan, 2021). EEG recordings were obtained during phases on day 1 and 14. The sampling frequency is set at 500 Hz. The saline liquid is used as an electrolytic gel to obtain good conductivity. Data is acquired in a quiet and ventilated room to minimize noise.
Raw EEG data was subjected to detrending to remove a DC offset and thereby adjust the baseline. A 5th-order Butterworth band-pass IIR filter (1–45 Hz) was used to eliminate the 50 Hz artifact. The sampling frequency is set at 500 Hz. By adjusting the filter order n, frequency range Xa, and filter type ftype, the MATLAB command “butter (a, Xa, ftype)” was used to calculate the filter coefficients. The required filtered output was then obtained using the command “filter (b, c, x),” where b and c are filter coefficients and x is raw EEG data as input. Based on a visual inspection, EEG data with eye blinks, ocular movements, and EMG artifacts were eliminated. The power spectral density of EEG signals was determined by utilizing Welch’s method carried out in Matlab. To enhance estimation quality by controlling spectral leakage and data variance sliding Hanning window of 4 s, with a cross-over of 2 s, was applied. The relative EEG power was computed by normalizing the absolute power within each frequency band (θ: 4–8 Hz, α: 8–12 Hz, β: 13–30 Hz, and γ:30–45 Hz) using the total absolute power ranging across all channels within 2 to 45 Hz.
The demographic and baseline performance data of all 25 participants which include participants’ age, MMSE score, and DASS-21 score in Pre-Treatment (Day-1) were compared between both groups (Experimental- and Control-Group) using an unpaired t-test (p < 0.05). The Wilcoxon signed-rank test was applied to compare changes in MMSE score and DASS-21 score between Post-Treatment (Day 14) and Pre-Treatment (Day 1) for both groups (Experimental- and Control-Group). A two-way ANOVA was conducted to examine the effects of groups (Experimental- and Control-Group) and Sessions (Pre-Treatment, Post-Treatment, and follow-up session) on the MMSE score. The student t-test was applied to SUS scores of Post-Treatment against the acceptable usability level of 66 for both groups (experimental- and control groups). However, a paired t-test was applied to compare the relative EEG power recorded in Pre-Treatment (Day 1) with the power recorded in Post-Treatment (Day 14) in both eyes opened and eyes closed states.
The Cohens method was applied to find the effect size and to demonstrate whether the effects of training have practical importance; ensuring that significant behavioral and neurological changes in Post-Treatment (Day 14) are not due to false positives (Homan, 1988). The effect size was calculated for changes in MMSE scores and EEG relative power. The mean of the two groups were subtracted and divided with a pooled standard deviation [see Eq. (2)]. The values of the effect size larger than 0.8 were considered as large while values between 0.4 and 0.8, and less than 0.4 were considered as medium and low effect sizes.
Where are mean values, {SD}1,2 are standard deviations, and n1,2 are sample sizes of two variables.
Statistical Pearson correlation tests were computed to examine the correlation between MMSE Components (orientation, language, attention, calculation, registration, recall, and copying) and PSD of each EEG channel for four frequency (θ, α, β, and γ) bands using Eq. 3, for both Experimental- and Control-Groups. Statistical significance was set at p < 0.05 for all tests, and multiple comparisons were corrected to control the expected proportion of false positives using the False Discovery Rate (FDR) method. All the statistical analyses were carried out in MATLAB software.
Where, r represents the correlation coefficient, Xi and Yi are individual data points, and X‘ and Y‘ are means of X and Y, respectively.
The baseline demographic data (age, MMSE, and DASS-21 scores) of both the Experimental and Control groups were compared using an unpaired t-test (p < 0.05) to confirm no significant differences at the start of the study. This ensures that any observed effects can be attributed to the treatment rather than pre-existing group differences.
Table 1 shows a comprehensive analysis of the demographic characteristics of the AD patients’ cohort, including age, gender distribution, and duration of AD. Table 2 presents the MMSE scores for participants in both the experimental group (patients # 1 to 15) and Control-Group (patients # 16 to 25) across three time points; Pre-Treatment, Post-Treatment, and follow-up sessions. Patients # 26 to 30 were assessed but did not qualify to receive further interventions (stimulation) due to their low baseline scores, which excluded them from subsequent sessions.

Table 2. Change in MMSE scores across sessions for AD patients, showing Pre-Treatment, Post-Treatment, and follow-up.
A two-way ANOVA (as shown in Table 3) was conducted to examine the effects of groups (Experimental- and Control-Group) and Sessions (Pre-Treatment, Post-Treatment, and follow-up session) on the MMSE score. The analysis revealed a statistically significant main effect of the group, F = 6.08, p = 0.016, with a partial eta-squared (h2) of 0.063 indicating that 6.3% of the variance in the MMSE score can be attributed to differences between Experimental and Control-Group. Similarly, there was a significant main effect of session, F = 3.859, p = 0.024, with partial eta-squared (h2) of 0.079, accounting for 7.9% of the variance. However, the interaction between the group and the session was not significant.
Figure 3 displays the total score of MMSE within the group (for both Experimental- and Control-Group). In the Experimental-Group, the Post-Treatment MMSE score significantly increased (p = 0.00000012, t-value = 7.33) as compared to the Pre-Treatment MMSE score while no significant improvements were noted in the Control-Group (p = 0.2306). Similarly, Follow-up MMSE scores in the experimental group (p = 0.00000077, t = 7.27) significantly increased compared to Pre-Treatment scores, with no significant improvement observed in the control group (p = 0.4236).

Figure 3. Comparison of MMSE total within group scores between Pre- and Post-Treatment phases. Bars represent the mean and SD of Pre-Treatment score (blue bars), Post-Treatment score (orange bar), and Follow-up score (gray bar). * Indicate the significant difference (p < 0.025) in MMSE score between the Post-Treatment (Day 14) and follow-up sessions as compared to Pre-Treatment (Day 1) in both groups.
Figure 4 shows a comparison of MMSE mean scores of both groups (Experimental- and Control groups) with the Pre-Treatment session for all MMSE sections (Orientation, Registration, Recall, Attention, Language, and Copying). In the experimental group, significant improvement was observed in the patients’ orientation (p = 0.00015, t-value = 1.687), registration (p = 0.0213, t-value = 1.688), and language (p = 0.0182, t-value = 1.703). However, other parts of the MMSE, such as attention, recall, and copy, did not reveal significant improvement after the BB session. In the control group, a significant decline in activity was observed only in the patients’ registration (p = 0.04122, t-value = −1.705) but, no significant changes were observed in other sections.
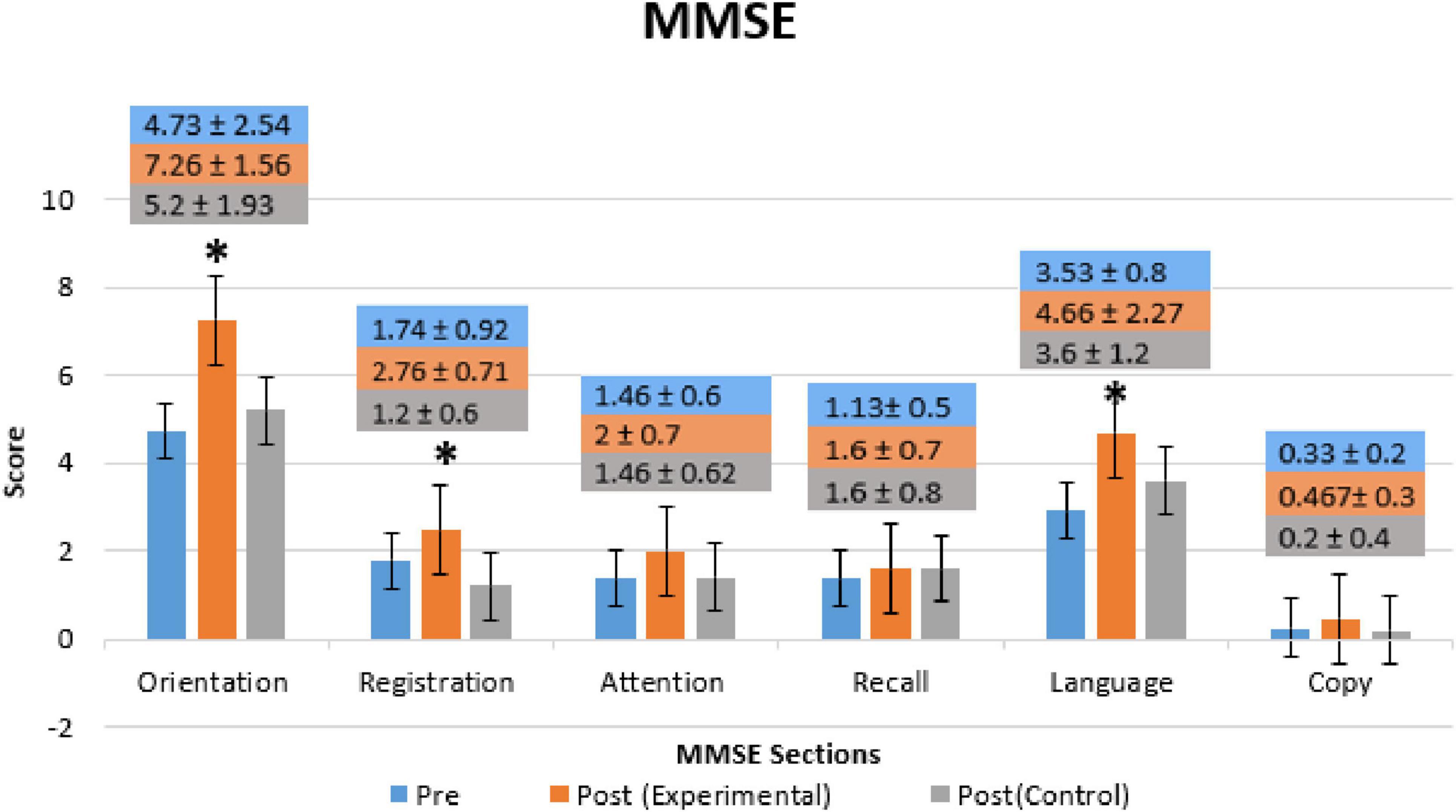
Figure 4. Mini-Mental State Exam (MMSE) mean scores of each section (Orientation, Registration, Recall, Attention, Language, and Copying) for Pre- and Post-Treatment (Day 14) of Experimental- and Control-Group. Bars represent the mean and SD of Pre-Treatment (blue bars), Post-Treatment of the Experimental-Group (orange bars), and Post-Treatment of Control-Group (gray bars). * Indicate the significant difference (p < 0.025) in MMSE score between the Post-Treatment (Day 14) as compared to Pre-Treatment (Day 1) in both groups.
Figure 5 shows the comparison of DASS-21 within (for both the experimental and control groups) in both Pre- and Post-Treatment for all subparts of DASS-21’s score (Depression, Anxiety, and stress). Depression significantly decreased in the experimental group (p = 0.0235, t = 1.701) while no significant improvements were observed in the control group or the other subparts of both groups. A significant level was set at p < 0.05.
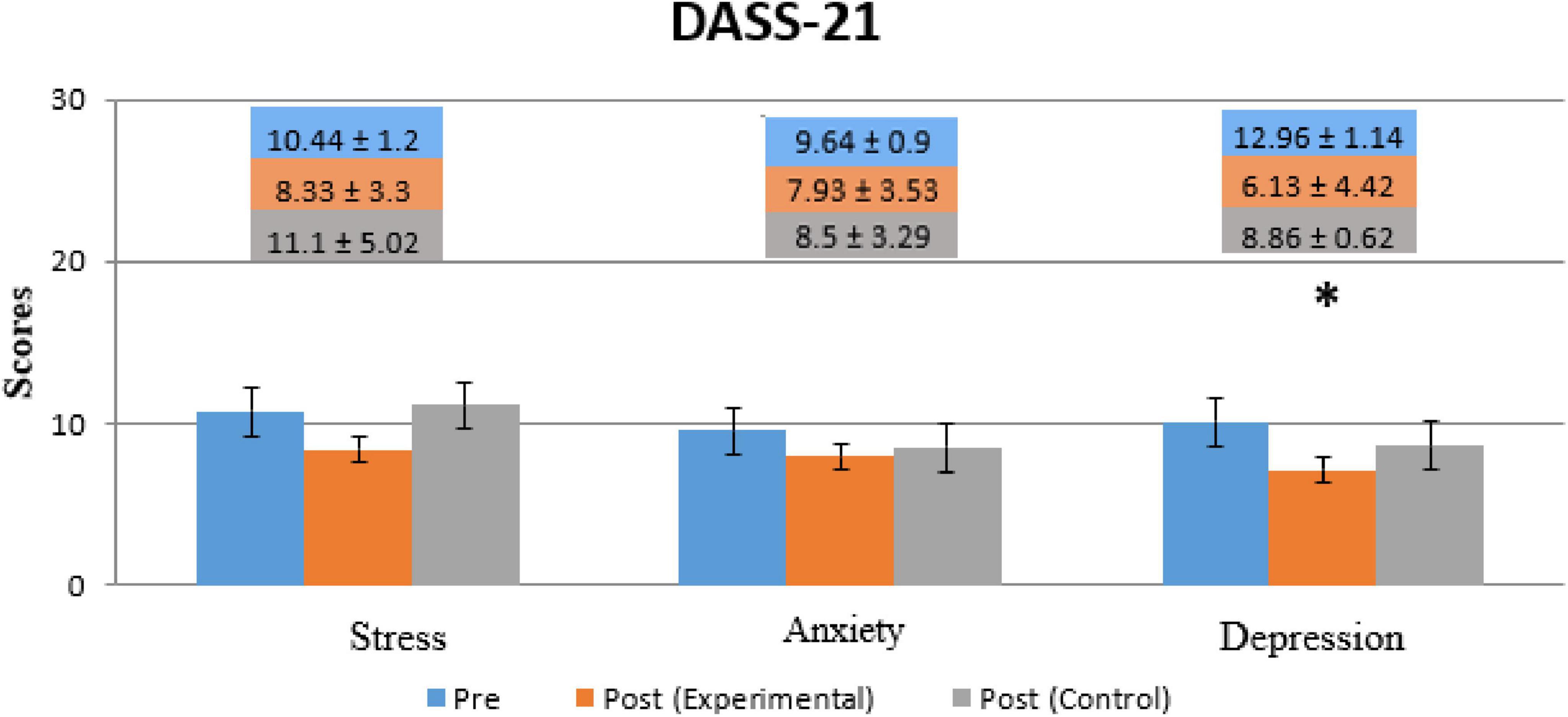
Figure 5. Comparison of patients’ scores for the DASS-21 questionnaire for all three conditions (Depression, Anxiety, and Stress). The Blue Bar represents Pre-Treatment, the orange bar represents Post-Treatment (Experimental group) and the Gray bar represents Post-Treatment (Control group). * Indicates the significant difference (p < 0.05) in DAAS score between Post-Treatment (Day 14) as compared to Pre-Treatment (Day 1) in both groups.
Figure 6 shows EEG power spectrums for the experimental group in both eyes opened (EO) and eyes closed (EC). Rows represent frequency bands (first row: θ-band, second row: α-band, third row: β-band and fourth row: 7- band). θ-band power increased significantly (p < 0.05) with high effect size in the temporal, parietal, and occipital region in the Post-Treatment EO BB stimulation session which significantly increased only in the occipital region in the Post-Treatment EC BB stimulation session. A decrease in power was detected in the α-band during the Post-Treatment EO BB state compared to the Pre-Treatment EO BB state specifically in the temporal region. Conversely, an increase in power was observed in the γ-band, primarily localized to the frontal regions in the Post-Treatment EC BB stimulation session. No changes were observed in β-band in both Pre- and Post-Treatment BB stimulation.
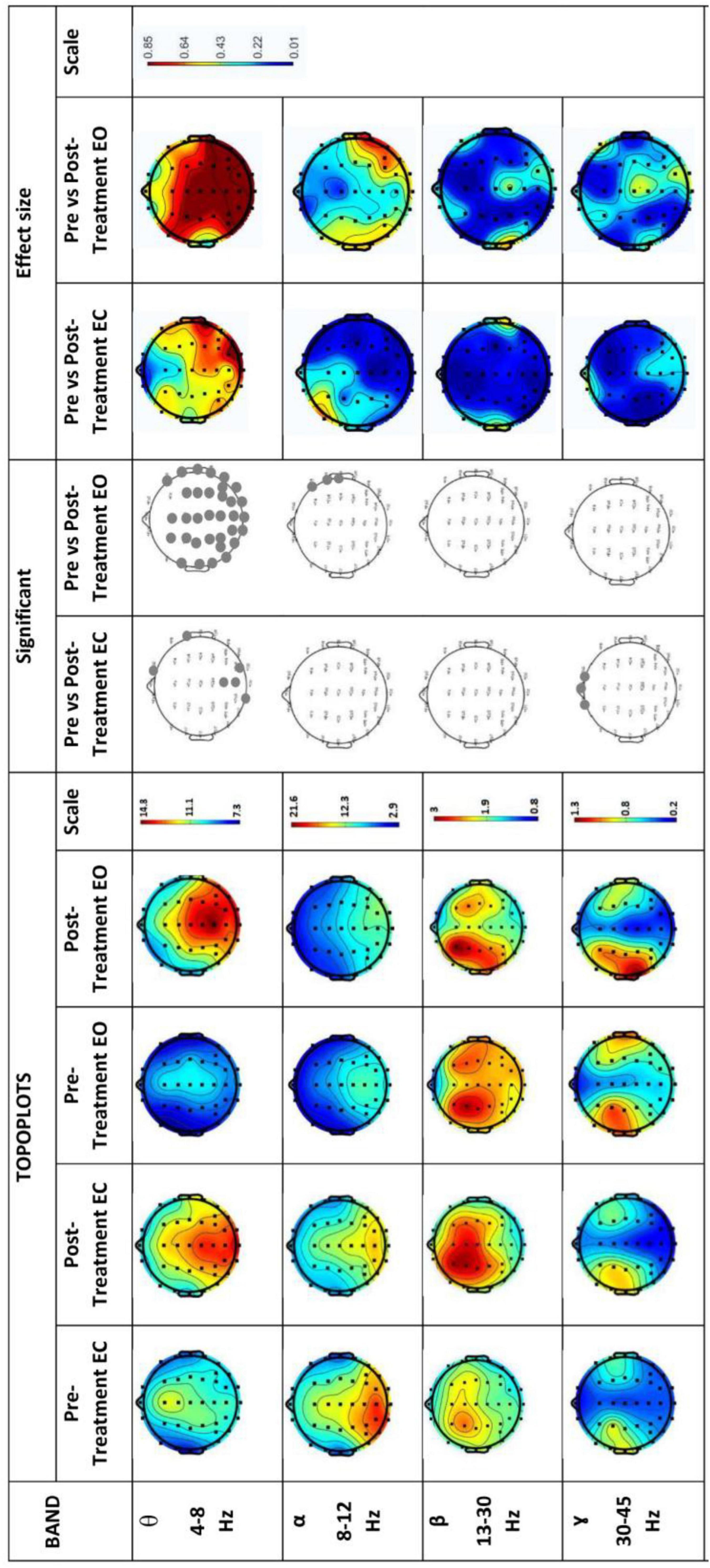
Figure 6. EEG power spectrums for Experimental-Group and statistical comparisons between states in four frequency bands (θ, α, β, and γ). Rows represent frequency bands (first row: θ-band, second row: α-band, third row: β-band, fourth row: 7-band). Columns represent the power spectrum in four states (Pre-Treatment EC; first column, Post-Treatment EC; second column, Pre-Treatment EO; third column and fourth column; Post-Treatment EO), statistical comparisons between Pre-Treatment EC vs. Post-Treatment EC and Pre-Treatment EO vs. Post-Treatment EO states (significant changes in column 5 and 6, respectively) and effect size between Pre-Treatment EC vs. Post-Treatment EC and Pre-Treatment EO vs. Post-Treatment EO states (column 7 and 8, respectively). Gray dots represent a significant increase and black dots represent a significant decrease.
Figure 7 shows EEG power spectrums for the control group. Rows represent frequency bands (first row: θ-band, second row: α-band, third row: β-band and fourth row: 7- band). In contrast to the experimental group, the neurological findings in the control group indicated no observable changes in all bands.
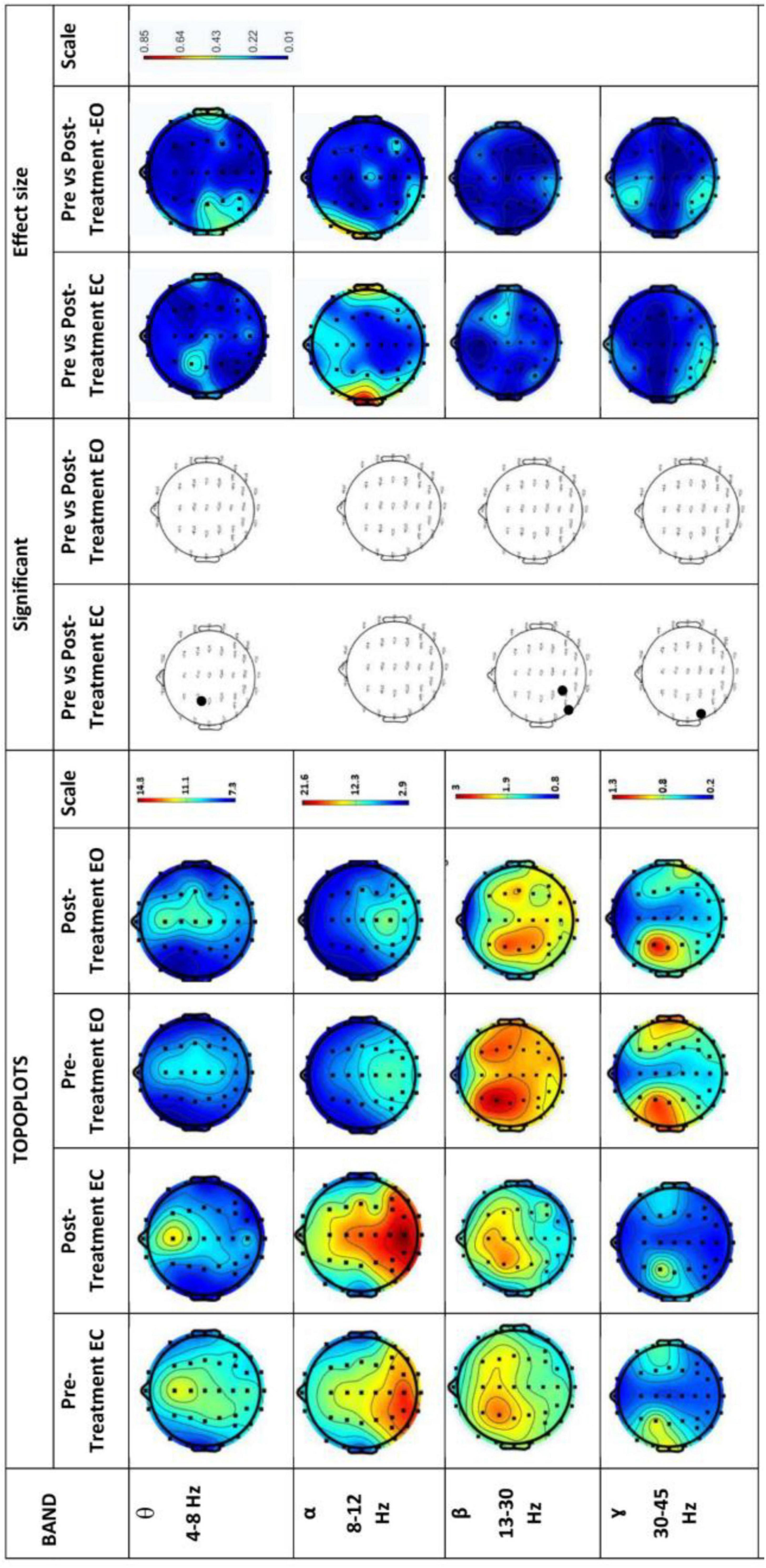
Figure 7. EEG power spectrums for Control group and statistical comparisons between states in four frequency bands (θ, α, β, and γ). Rows represent frequency bands (first row: θ-band, second row: α-band, third row: β-band, fourth row: 7-band). Columns represent the power spectrum in four states (Pre-Treatment EC; first column, Post-Treatment EC; second column, Pre-Treatment EO; third column and fourth column; Post-Treatment EO), statistical comparisons between Pre-Treatment EC vs. Post-Treatment EC and Pre-Treatment EO vs. Post-Treatment EO states (significant changes in column 5 and 6, respectively) and effect size between Pre-Treatment EC vs. Post-Treatment EC and Pre-Treatment EO vs. Post-Treatment EO states (column 7 and 8, respectively). Gray dots represent a significant increase and black dots represent a significant decrease.
A correlation analysis was subsequently performed between the MMSE subtest scores and the EEG θ-band and α-band activity. Figure 8 shows the correlation between the subtest scores on MMSE and activity in specific frequency bands of the Pre-Treatment EO state, and Post-Treatment EO states of the experimental group.
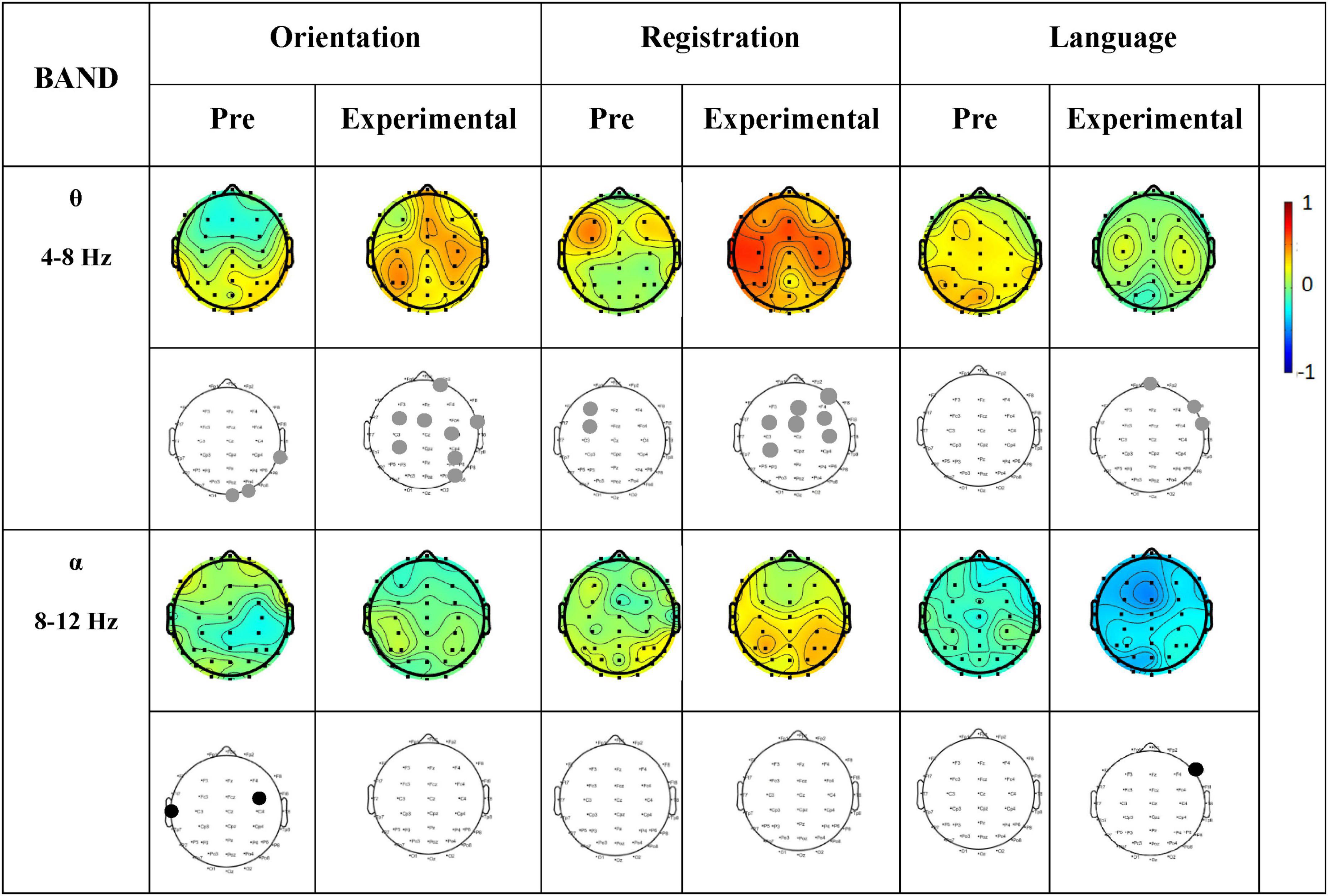
Figure 8. Correlation between MMSE scores (Orientation, Registration, and Language) and PSD of each EEG channel for Pre-Treatment and Post-Treatment (Experimental group). Columns represent the sessions (Pre-Treatment and Post-Treatment experimental group) of MMSE score (Orientation, Registration, and Language) of frequency bands (θ and α). The first row in each frequency band represents the correlation values, while the second row shows the significance values. Gray dots indicate a significant positive correlation, and black dots indicate a significant negative correlation. The scale from –1 to 0 represents a negative correlation, while the scale from 0 to 1 represents a positive correlation.
We found a positive correlation (significantly p < 0.05) in θ-band in the frontal region of the brain during language tasks and in the frontal and central regions during registration and orientation tasks. In addition, no correlation was observed in the α- band.
The SUS scores information shows that 86% of AD patients had positive feedback which was above the threshold of 66 which is the acceptable value. Besides, a significant difference in SUS average scores was determined in both the experimental- and control groups, which were widely beyond the acceptable usability level. Both the experimental (p = 0.024, t-value = 2.88) and the control (p = 0.023, t-value = 2.667) groups produced significant differences.
This study aimed to investigate the effects of BB stimulation on enhancing cognitive functions of AD patients. BB stimulations corresponding to θ and α- brain waves have shown a beneficial influence on cognitive processes (Cohen, 1988; Cruceanu and Alpha, 2013; Wianda and Ross, 2019). Previous studies have recommended BB stimulation at a frequency of 10 Hz yielded a positive effect on the working memory capacity. Consequently, in this study, the AD patients in the Experimental-Group received 10 Hz BB stimulation. We found improved cognitive and psychometric scores followed by increased θ band activity and a modified correlation between MMSE scores and global θ-band activity.
The observed behavioral outcomes in the experimental group which underwent BB stimulation demonstrated significant improvements. The substantial decrease in depression and stress scores, as indicated by the DASS-21 scale, aligns with research suggesting that auditory interventions can positively impact emotional wellbeing (Le Scouarnec et al., 2001; Puzi et al., 2013; Sung et al., 2017). These findings highlight the potential of BB stimulation to reduce emotional distress in AD patients experiencing severe mood disturbances as part of their dementia-related symptoms (Babulal et al., 2016).
Furthermore, behavioral changes were also observed in the experimental group by MMSE indicating a meaningful impact of the BB stimulation. The consequences of AD include cognitive decline, behavioral change, memory loss, and communication. The improved score in components of the MMSE examination following intervention demonstrates that BB has the potential to facilitate advancements in orientation, registration and language. These findings align with studies conducted on non-AD patients which explore the cognitive benefits of BB interventions in dementia-related disorders (Beauchene et al., 2016; Beauchene et al., 2017; Kraus and Porubanová, 2015). However, there were no significant impacts in the control group either in depression, anxiety, and stress levels or in any part of MMSE.
The EEG recordings in the experimental group provide insight into the underlying neurological mechanisms contributing to the observed behavioral improvements. The significant increase in θ- band power in the occipital region of the brain coincides with research (Sarnthein et al., 1998; Sauseng et al., 2010; Zhang et al., 2016) suggested showing θ- oscillations have been connected to cognitive functions like attention, and memory. θ- activity has been reported to play a major role in working memory functions (Gevins and Smith, 2000; Kraus and Porubanová, 2015; Zhang et al., 2016) and in the integration of different neural circuits during memory processes (Kraus and Porubanová, 2015). The results of previous research (Sammer et al., 2007) have shown that an increase in the synchronization of oscillatory phases between different brain regions supports working memory and acts by facilitating neural connections. Phase synchronization in the θ- frequency range persists between the prefrontal and parietal brain regions throughout the stages of a working memory task, including encoding, maintenance, and retrieval (Fell and Axmacher, 2011).
The observed decrease in α-band power, despite the use of α-frequency BB stimulation may indicate that the brain is reallocating cognitive resources to more demanding tasks. A reduction in α-band power is often associated with increased cognitive engagement, suggesting the brain enters a more active state that facilitates processes such as attention and memory (Beauchene et al., 2016; Cruceanu and Alpha, 2013; McKhann et al., 1984; Sauseng et al., 2004). This shift in neural activity could account for the simultaneous increase in θ-band power, which is linked to memory and attentional processes, reflecting an adaptive response to BB stimulation aimed at enhancing cognitive function (Chaieb et al., 2015; Garcia-Argibay et al., 2019).
The results of this study align with those stated by Christopher (Desai et al., 2015), providing further support in the role of θ-band activity modulation in enhancing cognitive functions through binaural beats stimulation. While our study did not observe significant evidence of improvement in attention, as noted in prior studies, we found significant changes in orientation, registration, and language in the Experimental Group, suggesting that θ-band activity modulation contributes to specific cognitive improvements. Our findings follow the path taken by the abovementioned variation wherein the frontal and temporal regions show an increase in correlation score after the BB stimulation. Moreover, in AD patients, BB stimulation appeared to have a positive effect on neural network efficiency with θ-band correlation increasing during cognitive tasks, which was particularly prominent in brain regions related to learning and memory (Benwell et al., 2020; Klimesch, 1999). The prevalence of left hemisphere activity in the θ-band during cognitive tasks underlines the high importance of BB stimulation on speech, meaning processing, and concentration in AD subjects (Fonseca et al., 2011; Gianotti et al., 2007; Karakaş et al., 2003; Nokia et al., 2012).
The findings of this study hold significant implications for diagnosing AD and identifying its biomarkers. By analyzing MMSE scores, brainwave activity (including changes in α- and θ-band power), and cognitive performance across various tasks, the study uncovers specific EEG patterns linked to cognitive decline in AD patients. These neurophysiological markers can help distinguish AD patients from healthy controls. By demonstrating the potential of BB to improve cognitive function, as shown by enhanced MMSE scores, this research provides valuable insights into non-invasive treatment options for AD patients. Moreover, the methods used to implement BB stimulation in this study offer a practical framework that can be adopted by other clinics to incorporate BB into patient care protocols, potentially improving treatment outcomes.
This study was a single-blind pilot/feasibility investigation, and as such, effect sizes may be smaller in larger, more rigorously controlled studies. Therefore, the findings should be regarded as preliminary and as a foundation for future research. To enhance scientific rigor and expand understanding, future studies could explore the combination of therapies such as tDCS (Brown, 2007), transcutaneous electrical nerve stimulation (Abul Hasan et al., 2023; Mujib et al., 2024), and neurofeedback (Zafar et al., 2025), along with the integration of machine learning approaches (Ather et al., 2024; Marappan et al., 2022; Zahid Rao et al., 2024).
In conclusion, this research investigated the BB effect on cognitive scores, particularly attention and working memory, in patients with AD. Further research is warranted to elucidate its therapeutic mechanisms comprehensively and explore its applicability.
The datasets generated and/or analyzed during the current study are not publicly available due to privacy and ethical concerns but are available from the corresponding author on reasonable request. Requests to access the datasets should be directed to YWJ1bGhhc2FuQG5lZHVldC5lZHUucGs=.
The studies involving humans were approved by the Research Ethics Committee at NED University of Engineering & Technology. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. AR: Conceptualization, Formal Analysis, Methodology, Writing – original draft. MFH: Conceptualization, Investigation, Writing – original draft. AOA: Conceptualization, Resources, Validation, Visualization, Writing – review and editing. SH: Conceptualization, Investigation, Writing – original draft. AAA: Formal Analysis, Funding acquisition, Writing – review and editing. SQ: Conceptualization, Project administration, Resources, Writing – review and editing. MAH: Conceptualization, Supervision, Validation, Writing – review and editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the King Salman Center for Disability Research (KSRG-2023-299).
We extend our appreciation to the King Salman Center For Disability Research for funding this work through Research Group no KSRG-2023-299. Additionally, we acknowledge Dar-ul-Sukun, Karachi, Pakistan and Gills Shelter Center, Karachi, Pakistan for facilitating access to AD patients.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abul Hasan, M., Shahid, H., Ahmed Qazi, S., Ejaz, O., Danish Mujib, M., and Vuckovic, A. (2023). Underpinning the neurological source of executive function following cross hemispheric tDCS stimulation. Int. J. Psychophysiol. 185, 1–10. doi: 10.1016/j.ijpsycho.2023.01.004
Agrawal, Y., Smith, P. F., and Rosenberg, P. B. (2020). Vestibular impairment, cognitive decline and Alzheimer’s disease: Balancing the evidence. Aging Ment. Health 24, 705–708. doi: 10.1080/13607863.2019.1566813
Ali, A. M., Alameri, R., Hendawy, A., Al-Amer, R., Shahrour, G., Ali, E., et al. (2022). Psychometric evaluation of the depression anxiety stress scale 8-items (DASS-8)/DASS-12/DASS-21 among family caregivers of patients with dementia. Front. Public Health 10:1012311. doi: 10.3389/fpubh.2022.1012311
Al-Shargie, F., Tariq, U., Mir, H., Alawar, H., Babiloni, F., and Al-Nashash, H. (2019). Vigilance decrement and enhancement techniques: A review. Brain Sciences 9:178.
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4. Virginia: American Psychiatric Association.
Arevalo-Rodriguez, I., Smailagic, N., Roqué, I., Figuls, M., Ciapponi, A., Sanchez-Perez, E., et al. (2015). Mini-Mental state examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2015:CD010783. doi: 10.1002/14651858.CD010783.pub2
Ather, M., Ejaz, O., Rao, A., Mujib, M., Raees, F., Qazi, S., et al. (2024). Efficacy of audiovisual neurofeedback training for attention enhancement: A multimodal approach. NeuroReport 35, 721–728. doi: 10.1097/WNR.0000000000002063
Azami, H., Zrenner, C., Brooks, H., Zomorrodi, R., Blumberger, D., Fischer, C., et al. (2023). Beta to theta power ratio in EEG periodic components as a potential biomarker in mild cognitive impairment and Alzheimer’s dementia. Alzheimers Res. Ther. 15:133. doi: 10.1186/s13195-023-01280-z
Babiloni, C., Arakaki, X., Azami, H., Bennys, K., Blinowska, K., Bonanni, L., et al. (2021). Measures of resting state EEG rhythms for clinical trials in Alzheimer’s disease: Recommendations of an expert panel. Alzheimers Dement. 17, 1528–1553. doi: 10.1002/alz.12311
Babulal, G., Ghoshal, N., Head, D., Vernon, E., Holtzman, D., Benzinger, T., et al. (2016). Mood changes in cognitively normal older adults are linked to Alzheimer disease biomarker levels. Am. J. Geriatr. Psychiatry 24, 1095–1104. doi: 10.1016/j.jagp.2016.04.004
Beauchene, C., Abaid, N., Moran, R., Diana, R. A., and Leonessa, A. (2017). The effect of binaural beats on verbal working memory and cortical connectivity. J. Neural Eng. 14:026014. doi: 10.1088/1741-2552/aa5d67
Beauchene, C., Abaid, N., Moran, R., Diana, R. A., and Leonessa, A. (2016). The effect of binaural beats on visuospatial working memory and cortical connectivity. PLoS One 11:e0166630. doi: 10.1371/journal.pone.0166630
Bell, C. M., Araki, S. S., and Neumann, P. J. (2001). The association between caregiver burden and caregiver health-related quality of life in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 15, 129–136. doi: 10.1097/00002093-200107000-00004
Bennys, K., Rondouin, G., Vergnes, C., and Touchon, T. (2001). Diagnostic value of quantitative EEG in Alzheimer’s disease. Neurophysiol. Clin. 31, 153–160. doi: 10.1016/s0987-7053(01)00254-4
Bentwich, J., Dobronevsky, E., Aichenbaum, S., Shorer, R., Peretz, R., Khaigrekht, M., et al. (2011). Beneficial effect of repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer’s disease: A proof of concept study. J. Neural Transm. (Vienna) 118, 463–471. doi: 10.1007/s00702-010-0578-1
Benwell, C., Davila-Pérez, P., Fried, P., Jones, R., Travison, T., Santarnecchi, E., et al. (2020). EEG spectral power abnormalities and their relationship with cognitive dysfunction in patients with Alzheimer’s disease and type 2 diabetes. Neurobiol. Aging 85, 83–95. doi: 10.1016/j.neurobiolaging.2019.10.004
Botto, R., Callai, N., Cermelli, A., Causarano, L., and Rainero, I. (2022). Anxiety and depression in Alzheimer’s disease: A systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurol. Sci. 43, 4107–4124. doi: 10.1007/s10072-022-06068-x
Brown, P. (2007). Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr. Opin. Neurobiol. 17, 656–664. doi: 10.1016/j.conb.2007.12.001
Bruña, R., López-Sanz, D., Maestú, F., Cohen, A., Bagic, A., Huppert, T., et al. (2023). MEG oscillatory slowing in cognitive impairment is associated with the presence of subjective cognitive decline. Clin. EEG Neurosci. 54, 73–81. doi: 10.1177/15500594221072708
Cassani, R., Estarellas, M., San-Martin, R., Fraga, F. J., and Falk, T. H. (2018). Systematic review on resting-state EEG for Alzheimer’s disease diagnosis and progression assessment. Dis. Markers 2018:5174815. doi: 10.1155/2018/5174815
Chaieb, S., Wilpert, T., Reber, H. C., and Fell, J. (2015). Auditory beat stimulation and its effects on cognition and mood states. Front. Psychiatry 6:70. doi: 10.3389/fpsyt.2015.00070
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Science. Mahwah, NJ: Lawrence Erlbaum, 75–108.
Coker, A. O., Coker, O. O., and Sanni, D. (2018). Psychometric properties of the 21-item depression anxiety stress scale (DASS-21). Afr. Res. Rev. 12, 135–142. doi: 10.4314/afrrev.v12i2.13
Crespo, A., Recuero, M., Galvez, G., and Begoña, A. (2013). Effect of binaural stimulation on attention and EEG. Arch. Acoustics 38, 517–528.
Cruceanu, V. D., and Rotarescu, V. (2013). Alpha brainwave entrainment as a cognitive performance activator. Cogn. Brain Behav. 17, 249–261.
Cuttler, J. M., Moore, E. R., Hosfeld, V. D., and Nadolski, D. L. (2016). Treatment of Alzheimer disease with CT scans: A case report. Dose-Response 14:1559325816640073. doi: 10.1177/1559325816640073
Desai, R., Tailor, A., and Bhatt, T. (2015). Effects of yoga on brain waves and structural activation: A review. Complement. Ther. Clin. Pract. 21, 112–118. doi: 10.1016/j.ctcp.2015.02.002
Fell, F., and Axmacher, N. (2011). The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 12, 105–118. doi: 10.1038/nrn2979
Fonseca, L. C., Tedrus, G., Prandi, L. R., Almeida, A. M., and Furlanetto, D. S. (2011). Alzheimer’s disease: Relationship between cognitive aspects and power and coherence EEG measures. Arq. Neuro Psiquiatr. 69, 875–881. doi: 10.1590/s0004-282x2011000700005
Galasko, D., Kwo-On-Yuen, P. F., Klauber, M. R., and Thal, L. J. (1990). Neurological findings in Alzheimer’s disease and normal aging. Arch. Neurol. 47, 625–627. doi: 10.1001/archneur.1990.00530060033012
Galasko, D., Schmitt, F., Thomas, S. J., and Bennett, D., Alzheimer’s Disease, and Cooperative Study. (2005). Detailed assessment of activities of daily living in moderate to severe Alzheimer’s disease. J. Int. Neuropsychol. Soc. 11, 446–453. doi: 10.1017/s1355617705050502
Gallagher, D., Ni Mhaolain, A., Crosby, L., Ryan, D., Lacey, L., Coen, R., et al. (2011). Dependence and caregiver burden in Alzheimer’s disease and mild cognitive impairment. Am. J. Alzheimers Dis. Other Dement. 26, 110–114. doi: 10.1177/1533317510394649
Gangemi, A., Colombo, B., and Fabio, R. A. (2021). Effects of short- and long-term neurostimulation (tDCS) on Alzheimer’s disease patients: Two randomized studies. Aging Clin. Exp. Res. 33, 383–390. doi: 10.1007/s40520-020-01546-8
Gao, X., Cao, H., Ming, D., Qi, H., Wang, X., and Wang, X. (2014). Analysis of EEG activity in response to binaural beats with different frequencies. Int. J. Psychophysiol. 94, 399–406.
García Domínguez, L., Stieben, J., Pérez Velázquez, J. L., and Shanker, S. (2013). The imaginary part of coherency in autism: Differences in cortical functional connectivity in preschool children. PLoS One 8:e75941. doi: 10.1371/journal.pone.0075941
Garcia-Argibay, M., Miguel, S. A., and Reales, J. (2019). Efficacy of binaural auditory beats in cognition, anxiety, and pain perception: A meta-analysis. Psychol. Res. 83, 357–372.
Gevins, A., and Smith, M. (2000). Neurophysiological measures of working memory and individual differences in cognitive ability and cognitive style. Cereb. Cortex 10, 829–839. doi: 10.1093/cercor/10.9.829
Gianotti, L., Künig, G., Lehmann, D., Faber, P., Pascual-Marqui, R., Kochi, K., et al. (2007). Correlation between disease severity and brain electric LORETA tomography in Alzheimer’s disease. Clin. Neurophysiol. 118, 186–196. doi: 10.1016/j.clinph.2006.09.007
González-Salvador, M. T., Arango, C., Lyketsos, C. G., and Barba, A. C. (1999). The stress and psychological morbidity of the Alzheimer patient caregiver. Int. J. Geriatr. Psychiatry 14, 701–710. doi: 10.1002/(SICI)1099-1166(199909)14:9
Goodin, P., Lamp, G., Hughes, M. E., Rossell, S. L., and Ciorciari, J. (2019). Decreased response to positive facial affect in a depressed cohort in the dorsal striatum during a working memory task-A preliminary fMRI study. Front. Psychiatry 10:60. doi: 10.3389/fpsyt.2019.00060
Homan, R. W. (1988). The 10–20 electrode system and cerebral location. Am. J. EEG Technol. 28, 269–279. doi: 10.1080/00029238.1988.11080272
Horvath, A., Szucs, A., Csukly, G., Sakovics, A., Stefanics, G., and Kamondi, A. (2018). EEG and ERP biomarkers of Alzheimer’s disease: A critical review. Front. Biosci. (Landmark Ed.) 23:183–220. doi: 10.2741/4587
Im, J., Jeong, H., Bikson, M., Woods, A., Unal, G., Oh, J., et al. (2019). Effects of 6-month at-home transcranial direct current stimulation on cognition and cerebral glucose metabolism in Alzheimer’s disease. Brain Stimul. 12, 1222–1228. doi: 10.1016/j.brs.2019.06.003
Insel, K., Morrow, D., Brewer, B., and Figueredo, A. (2006). Executive function, working memory, and medication adherence among older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 61, 102–107. doi: 10.1093/geronb/61.2.p102
Jirakittayakorn, N., and Wongsawat, Y. (2017). Brain responses to a 6-Hz binaural beat: Effects on general theta rhythm and frontal midline theta activity. Front. Neurosci. 11:365. doi: 10.3389/fnins.2017.00365
Johnson, M. (2001). Transcutaneous electrical nerve stimulation (TENS) and TENS-like devices: Do they provide pain relief? Pain Rev. 8, 121–158. doi: 10.1191/0968130201pr182ra
Karakaş, S., Bekçi, A., and Erzengin, O. U. (2003). Early gamma response in human neuroelectric activity is correlated with neuropsychological test scores. Neurosci. Lett. 340, 37–40. doi: 10.1016/s0304-3940(03)00073-9
Kim, J. W., Byun, M., Sohn, B., Yi, D., Seo, E., Choe, Y., et al. (2017). Clinical dementia rating orientation score as an excellent predictor of the progression to Alzheimer’s disease in mild cognitive impairment. Psychiatry Investig. 14, 420–426. doi: 10.4306/pi.2017.14.4.420
Klimesch, W. (1999). EEG Alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Brain Res. Rev. 29, 169–195. doi: 10.1016/s0165-0173(98)00056-3
Kraus, J., and Porubanová, M. (2015). The effect of binaural beats on working memory capacity. Stud. Psychol. 57, 135–145. doi: 10.21909/sp.2015.02.689
Le Scouarnec, R. P., Poirier, R. M., Owens, J. E., Gauthier, J., Taylor, A. G., and Foresman, P. A. (2001). Use of binaural beat tapes for treatment of anxiety: A pilot study of tape preference and outcomes. Altern. Ther. Health Med. 7, 58–63.
Liu, X., Zhang, C., Ji, Z., Ma, Y., Shang, X., Zhang, Q., et al. (2016). Multiple characteristics analysis of Alzheimer’s electroencephalogram by power spectral density and Lempel–Ziv complexity. Cogn. Neurodyn. 10, 121–133. doi: 10.1007/s11571-015-9367-8
López-Sanz, B., Bruña, A., de Frutos-Lucas, F., and Maestú, F. (2019). Magnetoencephalography applied to the study of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 165, 25–61. doi: 10.1016/bs.pmbts.2019.04.007
López-Sanz, D., Garces, P., Camara, C., Serrano, N., Delgado, M., Montenegro, M., et al. (2016). Alpha band disruption in the AD-continuum starts in the subjective cognitive decline stage: A MEG study. Sci. Rep. 6:37685. doi: 10.1038/srep37685
Machulda, M. M., Ward, H., Borowski, B., Gunter, J., Cha, R., O’Brien, P., et al. (2003). Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology 61, 500–506. doi: 10.1212/01.WNL.0000079052.01016.78
MacKenzie, D. M., Copp, P., Shaw, R. J., and Goodwin, G. M. (1996). Brief cognitive screening of the elderly: A comparison of the Mini-mental state examination (MMSE), Abbreviated mental test (AMT) and Mental status questionnaire (MSQ). Psychol. Med. 26, 427–430. doi: 10.1017/s0033291700034826
Maestú, F., and Fernández, A. (2020). Role of magnetoencephalography in the early stages of Alzheimer disease. Neuroimaging Clin. N. Am. 30, 217–227. doi: 10.1016/j.nic.2020.01.003
Marappan, S., Mujib, M. D., Siddiqui, A. A., Aziz, A., Khan, S., and Singh, M. (2022). Lightweight deep learning classification model for identifying low-resolution CT images of lung cancer. Comput. Intell. Neurosci. 2022:3836539. doi: 10.1155/2022/3836539
Marijanović, I., Kraljević, M., Buhovac, T., Cerić, T., Mekić Abazović, A., Alidžanović, J., et al. (2021). Use of the depression, anxiety and stress scale (DASS-21) questionnaire to assess levels of depression, anxiety, and stress in healthcare and administrative staff in 5 oncology institutions in Bosnia and Herzegovina during the 2020 COVID-19 pandemic. Med. Sci. Monit. 27:e930812. doi: 10.12659/MSM.930812
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology 34, 939–944. doi: 10.1212/wnl.34.7.939
McMurray, J. C. (2006). Binaural Beats Enhance Alpha Wave Activity, Memory, and Attention in Healthy-Aging Seniors. Las Vegas, NV: University of Nevada Las Vegas.
Moretti, D., Babiloni, C., Binetti, G., Cassetta, E., Dal Forno, G., Ferreric, F., et al. (2004). Individual analysis of EEG frequency and band power in mild Alzheimer’s disease. Clin. Neurophysiol. 115, 299–308. doi: 10.1016/s1388-2457(03)00345-6
Morris, J. C., Storandt, M., Miller, J., McKeel, D., Price, J., Rubin, E., et al. (2001). Mild cognitive impairment represents early-stage Alzheimer disease. Arch. Neurol. 58, 397–405. doi: 10.1001/archneur.58.3.397
Mujib, M. D., Hasan, M. A., Qazi, S. A., and Vuckovic, A. (2021). Understanding the neurological mechanism involved in enhanced memory recall task following binaural beat: A pilot study. Exp. Brain Res. 239, 2741–2754. doi: 10.1007/s00221-021-06132-6
Mujib, M. D., Rao, A. Z., Hasan, M. A., Ikhlaq, A., Buzdar, S. A., and Qazi, S. A. (2023). Frontal cortex cooling and modulation of brain frequencies using a wearable Peltier device. Phys. B 652:414641. doi: 10.1016/j.physb.2023.414641
Mujib, M., Rao, A., Hasan, M., Ikhlaq, A., Shahid, H., Bano, N., et al. (2024). Comparative neurological and behavioral assessment of central and peripheral stimulation technologies for induced pain and cognitive tasks. Biomedicines 12:1269. doi: 10.3390/biomedicines12061269
Musaeus, C., Engedal, K., Høgh, P., Jelic, V., Mørup, M., Naik, M., et al. (2018). EEG theta power is an early marker of cognitive decline in dementia due to Alzheimer’s disease. J. Alzheimers Dis. 64, 1359–1371. doi: 10.3233/JAD-180300
Nikolin, S., Huggins, C., Martin, D., Alonzo, A., and Loo, C. K. (2018). Safety of repeated sessions of transcranial direct current stimulation: A systematic review.”. Brain Stimul. 11, 278–288. doi: 10.1016/j.brs.2017.10.020
Nokia, M. S., Sisti, H. M., Choksi, M. R., and Shors, T. J. (2012). Learning to learn: Theta oscillations predict new learning, which enhances related learning and neurogenesis. PLoS One 7:e31375. doi: 10.1371/journal.pone.0031375
Nordberg, A., Rinne, J. O., Kadir, A., and Långström, B. (2010). The use of PET in Alzheimer disease. Nat. Rev. Neurol. 6, 78–87. doi: 10.1038/nrneurol.2009.217
Okabe, K., Nagata, T., Shinagawa, S., Inamura, K., Tagai, K., Nukariya, K., et al. (2020). Effects of neuropsychiatric symptoms of dementia on reductions in activities of daily living in patients with Alzheimer’s disease. Geriatr. Gerontol. Int. 20, 584–588. doi: 10.1111/ggi.13918
Ott, B. R., Lafleche, G., Whelihan, X., Buongiorno, G. W., Albert, M. S., and Fogel, B. S. (1996). Impaired awareness of deficits in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 10, 68–76. doi: 10.1097/00002093-199601020-00003
Parasuraman, R., and Haxby, J. V. (1993). Attention and brain function in Alzheimer’s disease: A review. Neuropsychology 7, 242–272. doi: 10.1037/0894-4105.7.3.242
Park, J., Kwon, H., Kang, S., and Lee, Y. (2018). “The effect of binaural beat-based audiovisual stimulation on brain waves and concentration,” in Proceedings of the 2018 International Conference on Information and Communication Technology Convergence (ICTC), (Piscataway, NJ: IEEE), 420–423.
Parodi, A., Fodde, P., Pellecchia, T., Puntoni, M., Fracchia, E., and Mazzella, M. (2021). A randomized controlled study examining a novel binaural beat technique for treatment of preoperative anxiety in a group of women undergoing elective Caesarean section. J. Psychosom. Obstet. Gynaecol. 42, 147–151. doi: 10.1080/0167482X.2020.1751607
Pillai, J. A., Bonner-Jackson, A., Walker, E., Mourany, L., and Cummings, J. L. (2014). Higher working memory predicts slower functional decline in autopsy-confirmed Alzheimer’s disease. Dem. Geriatr. Cogn. Disord. 38, 224–233. doi: 10.1159/000362715
Puzi, N. M., Jailani, R., Norhazman, H., and Zaini, N. M. (2013). “Alpha and beta brainwave characteristics to binaural beat treatment,” in Publications 9th International Colloquium on Signal Processing and its Applications, (Piscataway, NJ: IEEE), 344–348.
Rajji, T. K. (2019). Transcranial magnetic and electrical stimulation in Alzheimer’s disease and mild cognitive impairment: A review of randomized controlled trials. Clin. Pharmacol. Ther. 106, 776–780. doi: 10.1002/cpt.1574
Rao, A. Z., and Hasan, M. A. (2021). Evaluation of a chair-mounted passive trunk orthosis: A pilot study on able-bodied subjects. Sensors (Basel) 21:8366. doi: 10.3390/s21248366
Reedijk, S. A., Bolders, A., Lorenza, C. S., and Hommel, B. (2015). Eliminating the attentional blink through binaural beats: A case for tailored cognitive enhancement. Front. Psychiatry 6:82. doi: 10.3389/fpsyt.2015.00082
Roh, J., Park, M., Ko, D., Park, K., Lee, D., Han, C., et al. (2011). Region and frequency specific changes of spectral power in Alzheimer’s disease and mild cognitive impairment. Clin. Neurophysiol. 122, 2169–2176. doi: 10.1016/j.clinph.2011.03.023
Russo, R., Wallace, D., Fitzgerald, P. B., and Cooper, N. R. (2013). Perception of comfort during active and sham transcranial direct current stimulation: A double blind study. Brain Stimul. 6, 946–951. doi: 10.1016/j.brs.2013.05.009
Sadeghijam, M., Moossavi, A., Akbari, M., Haghani, H., Yousefi, A., and Mohsen, S. (2023). An increase in the auditory steady-state response amplitudes after a period of listening to binaural beat stimuli in tinnitus patients: A pilot study. Egypt. J. Otolaryngol. 39:1. doi: 10.1186/s43163-023-00402-6
Sammer, G., Blecker, C., Gebhardt, H., Bischoff, M., Stark, R., Morgen, K., et al. (2007). Relationship between regional hemodynamic activity and simultaneously recorded EEG-theta associated with mental arithmetic-induced workload. Hum. Brain Mapp. 28, 793–803. doi: 10.1002/hbm.20309
Sarnthein, J., Petsche, H., Rappelsberger, P., Shaw, G. L., and Von Stein, A. (1998). Synchronization between prefrontal and posterior association cortex during human working memory. Proc. Natl. Acad. Sci. U. S. A. 95, 7092–7096. doi: 10.1073/pnas.95.12.7092
Sauseng, P., Griesmayr, B., Freunberger, R., and Klimesch, W. (2010). Control mechanisms in working memory: A possible function of EEG theta oscillations. Neurosci. Biobehav. Rev. 34, 1015–1022. doi: 10.1016/j.neubiorev.2009.12.006
Sauseng, P., Klimesch, W., Doppelmayr, M., Hanslmayr, S., Schabus, M., and Gruber, W. R. (2004). Theta coupling in the human electroencephalogram during a working memory task. Neurosci. Lett. 354, 123–126. doi: 10.1016/j.neulet.2003.10.002
Scherder, E. J. A., Bouma, A., and Steen, A. M. (1995). Effects of short-term transcutaneous electrical nerve stimulation on memory and affective behaviour in patients with probable Alzheimer’s disease. Behav. Brain Res. 67, 211–219. doi: 10.1016/0166-4328(94)00115-v
Shigemori, K., Ohgi, S., Okuyama, E., Shimura, T., and Schneider, E. (2010). The factorial structure of the Mini-mental state examination (MMSE) in Japanese dementia patients. BMC Geriatr. 10:36. doi: 10.1186/1471-2318-10-36
Soininen, H., Riekkinen, P., Partanen, V. J., Pääkkönen, A., Helkala, E. L., and Laulumaa, V. (2020). EEG in the Diagnosis of Early Alzheimer Disease. Boca Raton, FL: CRC Press, 159–169.
Sposito, G., Neri, A. L., and Yassuda, M. S. (2015). Cognitive performance and engagement in physical, social and intellectual activities in older adults: The FIBRA study. Dement. Neuropsychol. 9, 270–278. doi: 10.1590/1980-57642015DN93000010
Sung, H., Lww, L., Li, M., Li, C., Wu, Y., Wang, C., et al. (2017). Familiar music listening with binaural beats for older people with depressive symptoms in retirement homes. Neuropsychiatry 7, 347–353.
Tani, A., Tartarisco, G., Vagheggini, G., Vaccaro, C., Campana, S., and Tomaiuolo, F. (2022). Binaural beats reduce feeling of pain and discomfort during colonoscopy procedure in not-sedated patients: A randomized control trial. Complement. Ther. Clin. Pract. 48:101605. doi: 10.1016/j.ctcp.2022.101605
Teipel, S. J., Grothe, M., Lista, S., Toschi, N., Garaci, F. G., and Hampel, H. (2013). Relevance of magnetic resonance imaging for early detection and diagnosis of Alzheimer disease. Med. Clin. North Am. 97, 399–424. doi: 10.1016/j.mcna.2012.12.013
Tierney, M. C., Szalai, J. P., Dunn, E., Geslani, D., and McDowell, I. (2000). Prediction of probable Alzheimer disease in patients with symptoms suggestive of memory impairment. Value of the Mini-mental state examination. Arch. Fam. Med. 9, 527–532. doi: 10.1001/archfami.9.6.527
Turner, R. C., Seminerio, M., Naser, Z., Ford, J., Martin, S., Matsumoto, R., et al. (2012). Effects of aging on behavioral assessment performance: Implications for clinically relevant models of neurological disease. J. Neurosurg. 117, 629–637. doi: 10.3171/2012.5.JNS112224
Vernon, D., Peryer, G., Louch, J., and Shaw, M. (2014). Tracking EEG changes in response to Alpha and beta binaural beats. Int. J. Psychophysiol. 93, 134–139. doi: 10.1016/j.ijpsycho.2012.10.008
Wang, R., Wang, J., Yu, H., Wei, X., Yang, C., and Deng, B. (2015). Power spectral density and coherence analysis of Alzheimer’s EEG. Cogn. Neurodyn. 9, 291–304. doi: 10.1007/s11571-014-9325-x
Wei, L., Zhang, Y., Wang, J., Xu, L., Yang, K., Lv, X., et al. (2022). Parietal-hippocampal rTMS improves cognitive function in Alzheimer’s disease and increases dynamic functional connectivity of default mode network. Psychiatry Res. 315:114721. doi: 10.1016/j.psychres.2022.114721
Wianda, E., and Ross, B. (2019). The roles of alpha oscillation in working memory retention. Brain Behav. 9:e01263. doi: 10.1002/brb3.1263
Williams, M. M., Storandt, M., Roe, C. M., and Morris, J. C. (2013). Progression of Alzheimer’s disease as measured by clinical dementia rating sum of boxes scores. Alzheimers Dement. 9, S39–S44. doi: 10.1016/j.jalz.2012.01.005
World Health Organization (2021). Global Status Report on the Public Health Response to Dementia. Geneva: World Health Organization.
Zafar, A., Khan, M., Mujib, M., Arif, H., Khan, W., and Hasan, M. (2025). Design and evaluation of a smart electrotherapy unit to optimize rehabilitation: A clinical trial. Pak. J. Rehabil. 14, 25–38.
Zahid Rao, A., Shahid Siddique, S., Danish Mujib, M., Abul Hasan, O., Alokaily, A. O., and Tahira, T. (2024). Sensor fusion and machine learning for seated movement detection with trunk orthosis. IEEE Access 12, 41676–41687. doi: 10.1109/ACCESS.2024.3377111
Keywords: Alzheimer’s disease, binaural beat, cognitive score, correlation, EEG
Citation: Mujib MD, Rao AZ, Haque MFU, Alokaily AO, Hussain SS, Aldohbayb AA, Qazi SA and Hasan MA (2025) Modulated theta band frequency with binaural beat stimulation correlates with improved cognitive scores in Alzheimer’s patients. Front. Aging Neurosci. 17:1543282. doi: 10.3389/fnagi.2025.1543282
Received: 11 December 2024; Accepted: 12 February 2025;
Published: 03 March 2025.
Edited by:
Enzo Emanuele, 2E Science, ItalyReviewed by:
James Stone, Brighton and Sussex Medical School, United KingdomCopyright © 2025 Mujib, Rao, Haque, Alokaily, Hussain, Aldohbayb, Qazi and Hasan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmad O. Alokaily, YWFsb2thaWx5QGtzdS5lZHUuc2E=; Muhammad Abul Hasan, YWJ1bGhhc2FuQG5lZHVldC5lZHUucGs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.