- 1Department of Neurology, Yijishan Hospital, The First Affiliated Hospital of Wannan Medical College, Wuhu, Anhui, China
- 2Department of Neurology, Provincial Hospital Affiliated to Anhui Medical University, Hefei, Anhui, China
- 3Institute of Food and Biological Engineering, Hefei University of Technology, Hefei, Anhui, China
- 4Department of Neurology, The First Affiliated Hospital of University of Science and Technology of China, Hefei, Anhui, China
Background: Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by memory and cognitive impairments. Previous studies have shown neuronal death in the brains of AD patients, but the role of cuproptosis and its associated genes in AD neurons remains unclear.
Methods: Intersection analysis was conducted using the AD transcriptome dataset GSE63060, neuron dataset GSE147528, and reported cuproptosis-related genes to identify the cuproptosis key gene FDX1 highly expressed in AD. Subsequently, cell experiments were performed by treating SH-SY5Y cells with Aβ25-35 to establish AD cell model. The real-time reverse transcriptase-polymerase chain reaction (RT-qPCR) and western blotting (WB) assays were employed to detect the expression levels of FDX1, DLAT, and DLST. Cell proliferation was analyzed by counting Kit-8 (CCK8), mitochondrial ROS levels were analyzed using flow cytometry. shRNA was used to downregulate FDX1 expression, followed by repetition of the aforementioned experiments. Clinical experiments utilized qPCR to detect FDX1 mRNA levels in peripheral venous blood of patients, and analyzed FDX1 expression differences in different APOE genotypes of AD patients. Finally, a protein–protein interaction (PPI) network of FDX1 was constructed based on the GeneMANIA database, immune infiltration analysis was conducted using R language, and transcription factors prediction for FDX1 was performed based on the ENCODE database.
Results: The cuproptosis key gene FDX1 showed significantly higher expression in peripheral blood and neuron models of AD compared to non-AD individuals, with significantly higher expression in APOE ε4/ε4 genotype than other APOE genotype of AD patients. Knockdown of FDX1 expression reduced the lipidation levels of DLAT and DLST in neurons, alleviated ROS accumulation in mitochondria, improved cell viability, and mitigated cuproptosis. Immune infiltration analysis results indicated a high enrichment of peripheral blood γδ-T lymphocytes in AD, and FDX1 was significantly associated with the infiltration of four immune cells and may be regulated by three transcription factors.
Conclusion: The cuproptosis key gene FDX1 is highly expressed in AD and may promote cuproptosis in AD neurons by regulating the lipidation levels of DLAT and DLST, thereby participating in the onset and development of AD. This provides a potential target for the diagnosis and treatment of AD.
Introduction
Alzheimer’s disease is currently the most common type of dementia, accounting for approximately 60–70% of all dementia cases (Gao et al., 2022; Huang, 2023), affecting around 47 million people worldwide (Oboudiyat et al., 2013). Its clinical features include impairments in executive and visuospatial functions, as well as short-term memory deficits (Scheltens et al., 2021). The exact etiology of AD remains incompletely understood. Currently, the leading pathological hypotheses revolve around the deposition of amyloid-β (Aβ) forming senile plaques and abnormal p-Tau protein forming neurofibrillary tangles, leading to neuronal loss and death (Bharadwaj et al., 2009; Van der Kant et al., 2020). However, numerous targeted drug clinical trials globally based on these hypotheses have ended in failure (Lam et al., 2013; Byun et al., 2015), and there is still no effective treatment available to slow down, treat, or reverse AD (Lam et al., 2013; Byun et al., 2015). The exact causes and pathogenic mechanisms of AD remain to be further elucidated, with research focusing on alternative or innovative fields (Lai et al., 2022).
Long-term observational studies have found widespread neuronal loss in the brains of AD patients. Neuronal death is the direct cause of neurodegenerative changes, resulting in irreversible damage to the nervous system, leading to memory deficits and cognitive impairments (Yan et al., 2021; Duan et al., 2016). However, previous relevant studies mainly focused on apoptosis (Mangalmurti and Lukens, 2022).
Regulated cell death (RCD), as determined by genes, plays a crucial role in the orderly active death of cells, regulating homeostasis (Peng et al., 2022). As research by domestic and international scholars deepens, regulated cell death modes such as ferroptosis, pyroptosis, and cuproptosis have been shown to play important roles in diseases (Wang et al., 2023).
In March 2022, Tsvetkov et al. first proposed and confirmed cuproptosis as a novel form of cell death (Tsvetkov et al., 2022). Unlike other known forms of cell death such as apoptosis, pyroptosis, and necroptosis, cuproptosis, similar to ferroptosis and zinc-induced cell death, is a regulated cell death induced by metal ion overload. When copper directly binds to the lipoyl moiety of the tricarboxylic acid cycle, it triggers the loss of iron–sulfur cluster proteins, protein toxicity stress, and ultimately leads to cell death (Tsvetkov et al., 2022). Among them, FDX1 serves a key regulator of cuproptosis and a direct target of elesclomol (ES), a copper ionophore that induces cuproptosis by promoting oxidative stress (Tsvetkov et al., 2019). FDX1 is directly related to protein lipoylation and significantly associated with ATP, ROS, etc. (Zhang et al., 2021; Zhang et al., 2022), thereby playing a crucial role in cuproptosis. Knocking out the key upstream regulator FDX1 of lipoylation proteins or lipoylation-related enzymes can block cuproptosis (Tsvetkov et al., 2022).
There have been preliminary studies exploring the correlation between cuproptosis-related genes and immune cells in AD (Nie et al., 2023; Zhang et al., 2023), and experimental evidence showing that Cu overload can induce extensive neuronal cell death in the hippocampus of mice (Zhang et al., 2023), but the role of cuproptosis in the pathogenesis of AD is still unclear.
Based on this, we reasonably speculate that the cuproptosis mechanism may be involved in the pathological process of AD. However, the role of cuproptosis and its related gene regulation in AD remains to be determined. This study aims to screen for differentially expressed genes related to cuproptosis in AD neurons by biological information technology, verify their expression in peripheral blood and neurons of AD, and explore their impact on the activity of AD neurons to provide insights for effective treatment strategies.
Materials and methods
Data download and processing
The transcriptome dataset GSE63060 for AD patients (n = 145) and healthy subjects (n = 184) was downloaded from the GEO database1 and analyzed for differential expression genes (DEGs) using the Limma (Ritchie et al., 2015) package in R language. The single-cell dataset GSE147528 was also obtained and processed using the Seurat package version 3.2.2. Principal component analysis (PCA) was performed, and key principal components (PCs) were selected by executing JackStraw and PCEIbowPlot functions. The FindAllMarkers function in Seurat was used to accurately identify genes specific to each cell subtype, followed by UMAP algorithm-based cell clustering and visualization analysis using the RunUMAP method. Annotation of marker genes was done using the singleR package, and feature calibration was performed using CellMarker. The corrected DEGs, neuron cell-related genes, and collected cuproptosis genes were intersected using an online Venn diagram tool to obtain the differential expression cuproptosis-related genes (DE-CRG): FDX1.
Cell culture and transfection
The human neuroblastoma cell line SH-SY5Y was obtained from the Cell Bank of the Chinese Academy of Sciences. Cells were cultured in DMEM medium supplemented with 10% fetal bovine serum and 1% antibiotics (penicillin–streptomycin mixture) at 37°C and 5% CO2.
Given that the generation, aggregation, and deposition of Aβ are considered pivotal initiating events in the pathological cascade of AD, with the Aβ25-35 peptide segment being one of the most neurotoxic fragments (Wang et al., 2023), it is capable of inducing oxidative cellular damage, increasing the production of reactive oxygen species (ROS) within cells, and leading to apoptosis, thereby simulating key pathological processes of AD in cellular models (Arispe et al., 1993; Changhong et al., 2021; Limón et al., 2009). Consequently, this study employed a concentration of 20 μmol/L of Aβ25-35 to treat SH-SY5Y cells for 36 h in order to establish an AD cellular model.
Short hairpin RNA (shRNA) targeting FDX1 (pGPU6-shFDX1-1 and pGPU6-shFDX1-2) were purchased from Shanghai Jierui Biotechnology Co., Ltd., containing two different FDX1 shRNA sequences (shFDX1-1: 5’-GGACAAUAUGACUGUUCGAGU-3’, shFDX1-2: 5’-AGUUGGUGAUUCUCUGCUAGA-3’). The plasmids were transfected into SH-SY5Y cells (density: 2 × 10^5/ml in 6-well plates) using Lipofectamine 2000 Reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions, with pGPU6 empty vector used as a negative control.
Clinical data and sample collection
Study subjects
The study subjects were patients admitted to the Neurology Department of Anhui Provincial Hospital from 2021 to 2023. The inclusion criteria for the disease group followed the core clinical criteria for AD dementia from the National Institute on Aging and Alzheimer’s Association (NIAAA-2011) (McKhann et al., 2011), and 30 typical AD patients were included based on cerebrospinal fluid and imaging biomarkers. The control group consisted of 20 hospitalized patients with normal cognitive function during the same period. All subjects signed informed consent forms for the study and received approval from the hospital’s Medical Ethics Committee, with ethics approval number: 2019KY Ethics Review No. 79.
Data collection
Patient information including name, gender, age, medical history, laboratory tests, and MMSE scores were collected through electronic medical records and on-site questionnaires. MMSE scores of ≤19 (illiterate), ≤22 (primary education), and ≤ 27 (middle school education and above) were considered indicative of cognitive impairment.
Sample collection
Peripheral venous blood was collected from all subjects around 6 a.m. on the day after admission (fasting for at least 8 h) into EDTA-K2 anticoagulant tubes. Within 2 h, the blood was aliquoted into centrifuge tubes (EP tubes) at 400 μL per tube, stored in −80°C, and avoided repeated freeze–thaw cycles.
Real-time reverse transcriptase-polymerase chain reaction
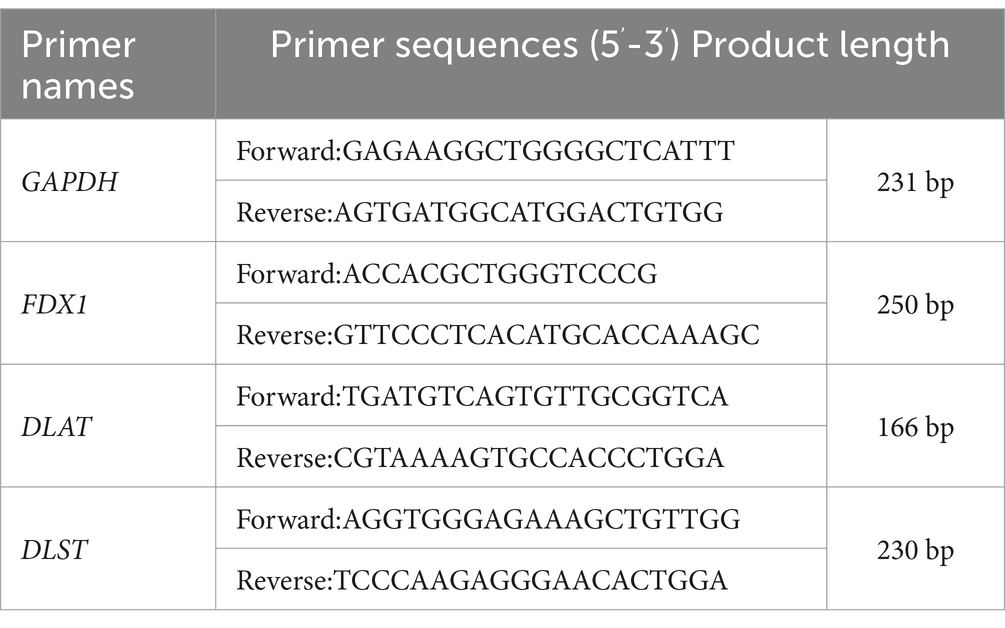
Total RNA was extracted and purified from cells/whole blood using TRIzol reagent according to the manufacturer’s instructions. The extracted RNA was reverse transcribed into cDNA, followed by RT-qPCR amplification normalizing all samples to GAPDH. The gene primers were synthesized by Shanghai Jierui Biotechnology Co., Ltd., which sequences are listed in Table 1. The corresponding Ct values and melting curves were recorded after the reaction, and the 2-ΔΔCt method was used for relative quantitative analysis of the target genes (Yildiz Gulhan et al., 2022).
Western blotting
Cells were separated using a cell scraper and then incubated on ice for 30 min. Protein lysates were prepared by adding 4:1 volume ratio of 5× reducing protein loading buffer, followed by denaturation in a boiling water bath for 15 min. Total protein content was separated by SDS-PAGE electrophoresis, and 20 μg of protein samples were loaded. Electrophoresis was conducted initially at 80 V for 20 min and then increased to 120 V for an additional 60 min. Subsequently, proteins were transferred onto PVDF membranes at 25 V for 30 min. After blocking in skim milk, the membranes were incubated overnight at 4°C with primary antibodies against the target proteins (FDX1, DLAT, and DLST) and the internal reference (GAPDH). The next day, the membranes were incubated with goat anti-rabbit HRP-conjugated secondary antibodies and goat anti-mouse HRP-conjugated secondary antibodies for 30 min. After incubation, the membranes were washed and visualized, and band grayscale values were calculated using Image J imaging analysis software.
Cell proliferation assay
The effect of FDX1 downregulation on cell viability was determined by CCK-8. SH-SY5Y cells and transfected cells were treated at 0, 24, 48, and 72 h. CCK-8 and serum-free essential culture medium were mixed at a 1:10 volume ratio, and 100 μL of the mixture was added to each well. Cells were then incubated at 37°C and 5% CO2 for 2 h, and absorbance at 450 nm wavelength was measured using a microplate reader.
Flow cytometry
Total mitochondrial ROS in SH-SY5Y cells was detected using the DCFH-DA probe (Beyotime Biotechnology). Cells were incubated with diluted DCFH-DA probe (1 mL) in serum-free culture medium for 20 min, followed by three washes with serum-free culture medium. Fluorescence intensity was measured by flow cytometry at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Protein–protein interaction network
The PPI network of FDX1 was automatically constructed on the GeneMANIA database2 online website, and functional and enrichment pathway analyses were also performed. The more connecting lines between proteins in the network diagram, the stronger the correlation.
Immune infiltration analysis
Based on the gene expression profile of AD patients from the GSE63060 transcriptome dataset, the CIBERSORT algorithm was used to calculate the proportions of various cell types (p < 0.05). The “pheatmap” package was used to create a heatmap of 22 immune cell types, and the “corrplot” package was used to create a correlation heatmap to visualize the correlation between 22 different infiltrating immune cells. The relationship between infiltrating immune cells and FDX1 was analyzed by Spearman rank correlation test in R package.
Transcription factors prediction
FDX1-related transcription factors binding data were retrieved from the ENCODE database. Through bioinformatics analysis tools, the binding strength and differential expression patterns of transcription factors were compared to predict which transcription factors might affect FDX1 expression.
Statistical analysis
Statistical analysis was performed using SPSS 25.0 software. Count data were expressed as numbers of cases, and qualitative data were compared using the χ2 test. Quantitative data following a normal distribution were expressed as (x̄ ± s), and comparisons between two groups were performed using independent sample t-tests. Non-normally distributed data were expressed as median (upper quartile, lower quartile), and comparisons between two groups were performed using Mann–Whitney U test. p-value <0.05 was used as the filtering condition. Experimental data were plotted using GraphPad Prism 10.0.
Results
Dataset information and DE-CRG screening results
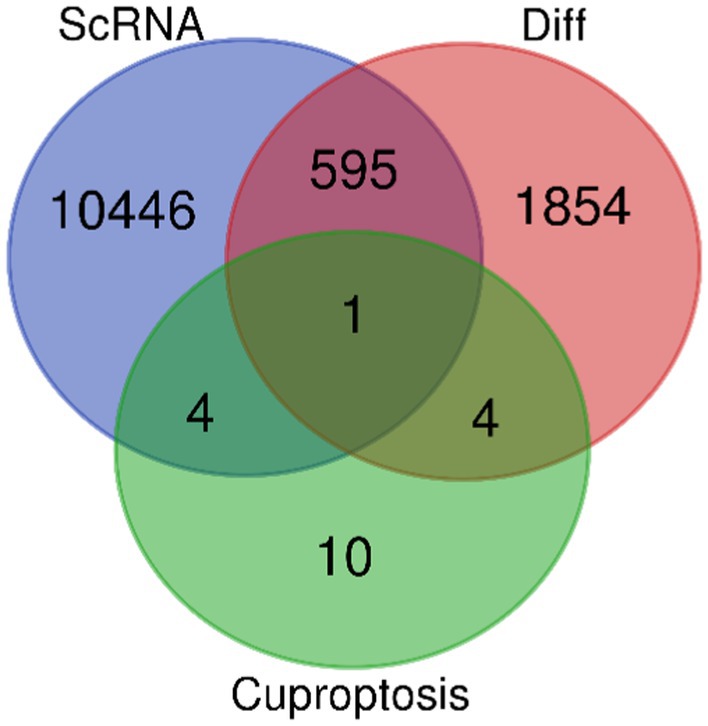
After careful screening, the original files of GSE63060 were downloaded. DEGs were filtered with criteria of p < 0.05 and |logFC| > 1, resulting in 21,684 genes meeting the requirements, with 11,043 genes upregulated and 10,641 genes downregulated. The volcano plot and heatmap of DEGs were generated using the ggplot2 and pheatmap packages (Figure 1). T-SNE and UMAP plots were used to visualize the expression distribution of neurons in clusters, demonstrating the heterogeneity of neurons in the GSE147528 dataset (Figure 2). Finally, a Venn diagram was constructed to intersect the processed DEGs, neuron cell-related genes, and cuproptosis-related genes, obtaining DE-CRG: FDX1 (Figure 3).
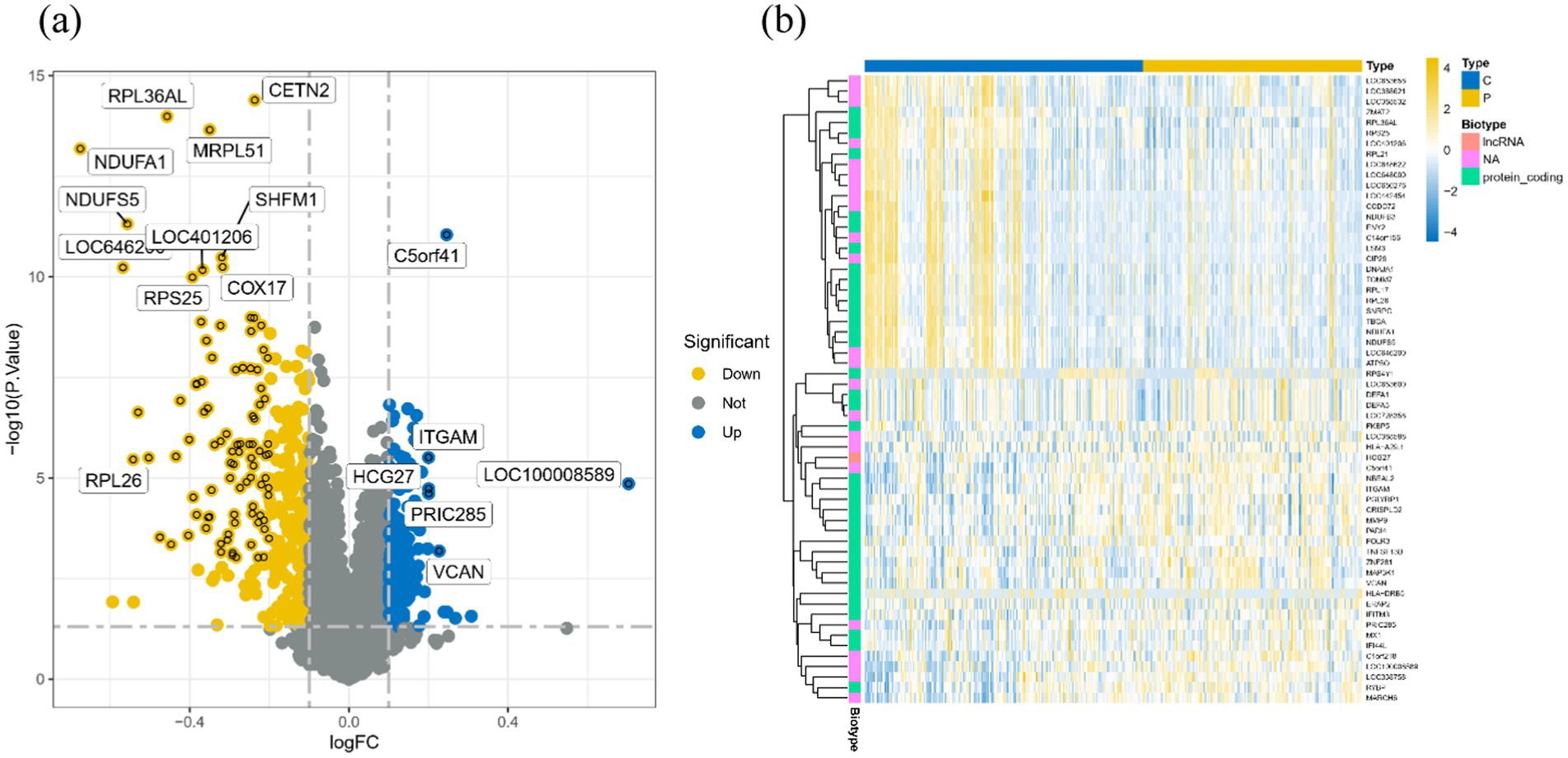
Figure 1. Volcano map and heat map of DEGs: (A) Volcano map of DEGs, blue for up-regulated gene, yellow for down-regulated gene, gray for no significant difference; (B) Heat maps of DEGs in different groups, blue for healthy controls and yellow for disease groups.
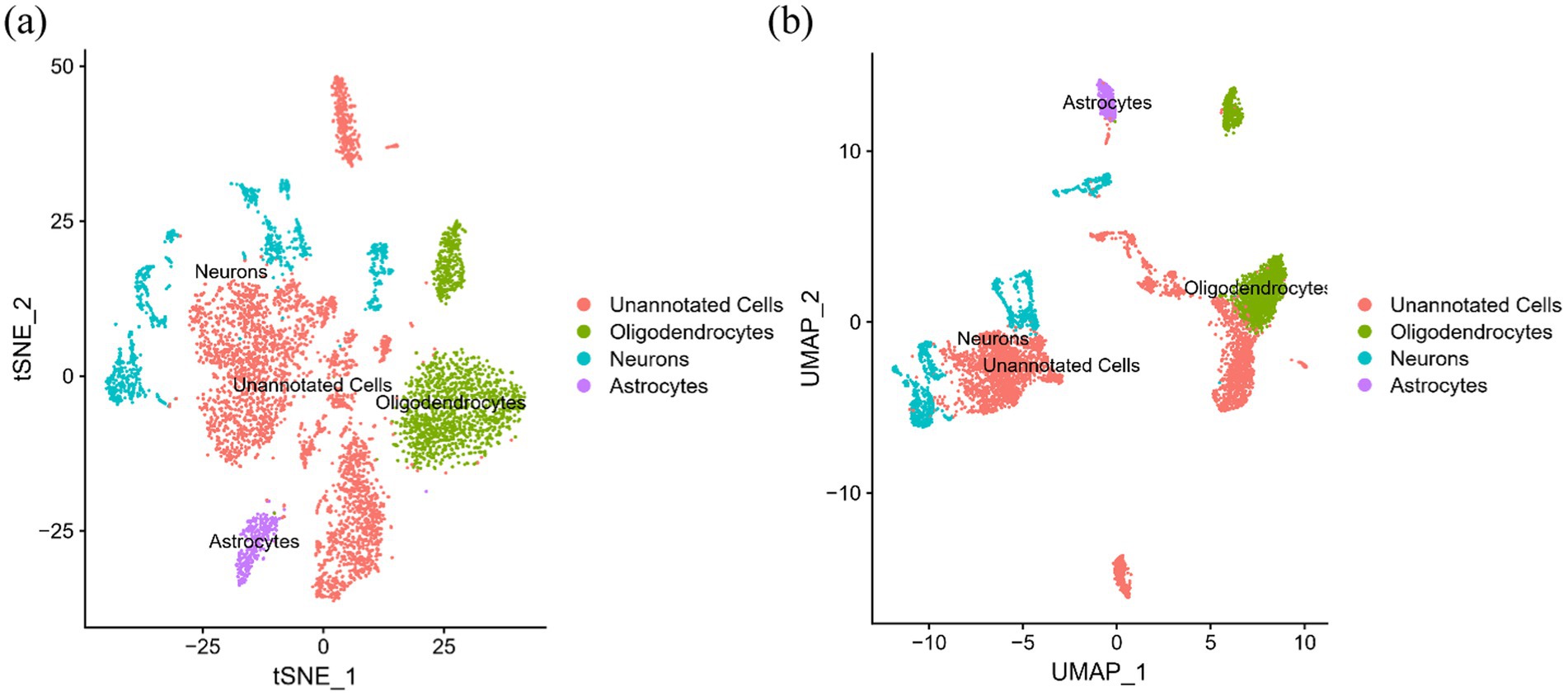
Figure 2. T-SNE and UMPA atlas: (A) T-SNE projection of 3,000 cells from all brain tissues; (B) UMPA projection of 3,000 cells from all brain tissues.
High expression of FDX1 in AD model cells
The relative expression levels of genes and proteins in cells were detected using RT-qPCR and WB assays, with results depicted in Figure 4. In the Aβ25-35-induced AD model SH-SY5Y cells, the relative expression levels of FDX1, DLAT, and DLST mRNA and proteins were significantly increased compared to the control group.
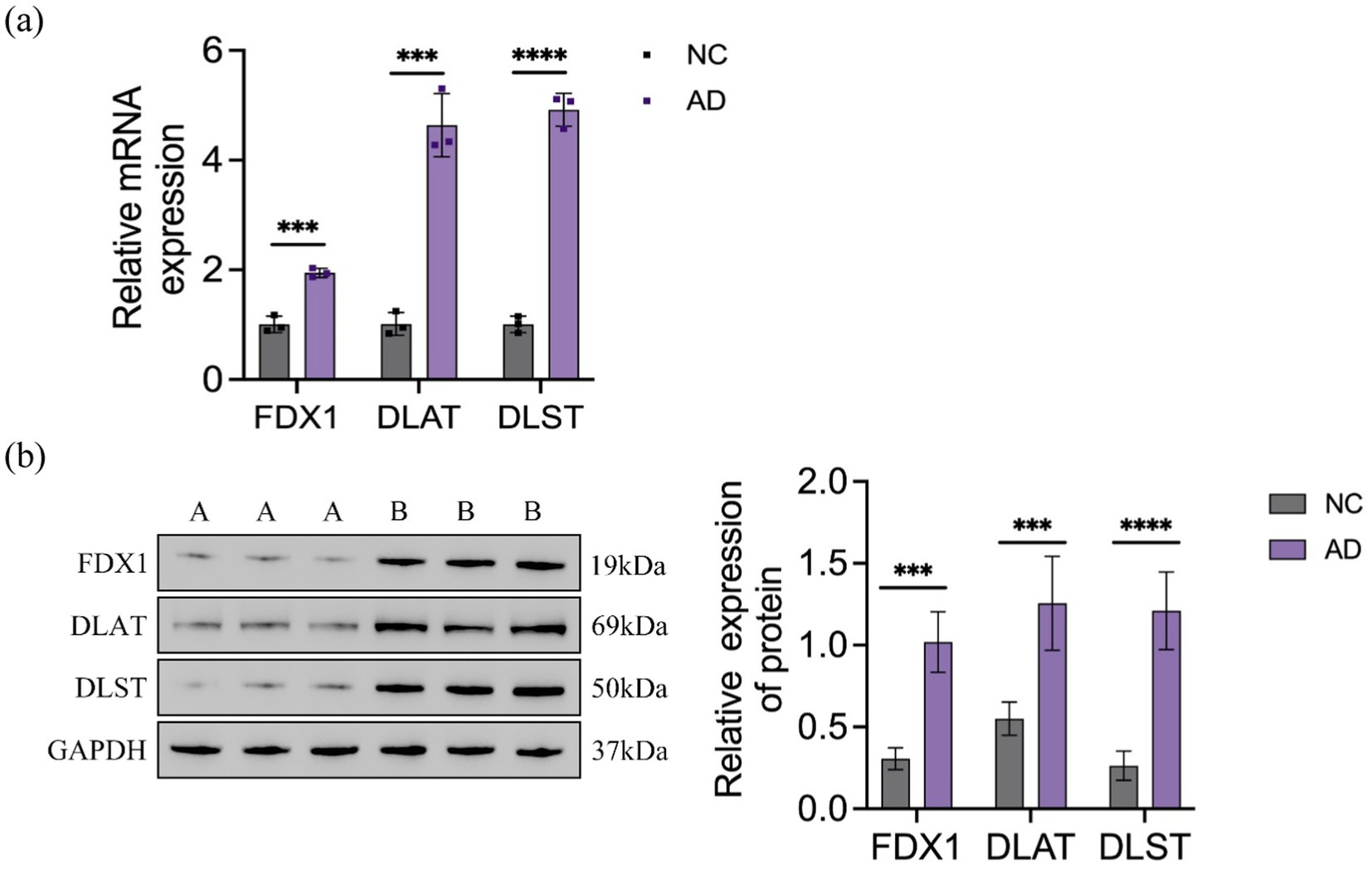
Figure 4. (A) Relative expression of FDX1 mRNA, DLAT mRNA, DLST mRNA in NC and AD model SH-SY5Y cells; (B) Relative expression of FDX1, DLAT, DLST protein levels in NC and AD model SH-SY5Y cells (***p < 0.001, ****p < 0.0001). A: control group, B: model group.
Decreased expression of DLAT and DLST after FDX1 knockdown
After downregulation of FDX1 expression, the relative expression levels of DLAT and DLST mRNA and proteins in the AD + shFDX1-1 and AD + shFDX1-2 model SH-SY5Y cells were markedly reduced compared to the control group, as shown in Figure 5.
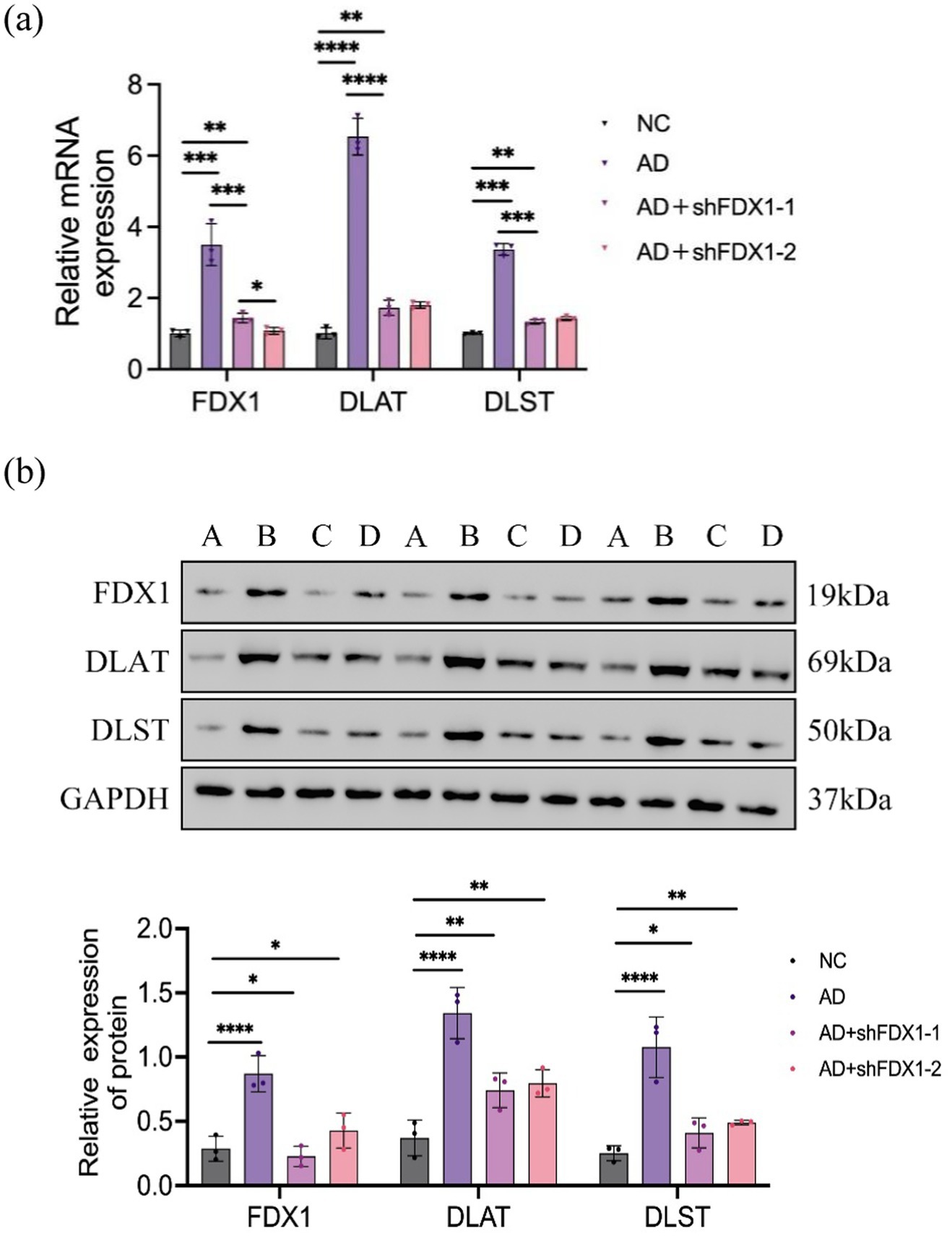
Figure 5. (A) Relative expression of FDX1 mRNA, DLAT mRNA and DLST mRNA in SH-SY5Y cells of NC, AD, AD + shFDX1-1 and AD + shFDX1-2 models; (B) Relative expression of FDX1, DLAT and DLST in SH-SY5Y cells of NC, AD, AD + shFDX1-1 and AD + shFDX1-2 models. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). A: control group, B: model group, C: model group + shFDX1-1, D: model group + shFDX1-2.
Increased cell proliferation activity after FDX1 knockdown
Cell proliferation activity of SH-SY5Y cells was measured by CCK8 assay at 0, 24, 48, and 72 h. Compared to the control group, cell proliferation activity significantly decreased after Aβ25-35 treatment (Figure 6A). After FDX1 knockdown, cell proliferation activity significantly increased compared to the non-knockdown group (Figure 6B). At 48 h, cell viability recovered to 71.41% (p < 0.0001).
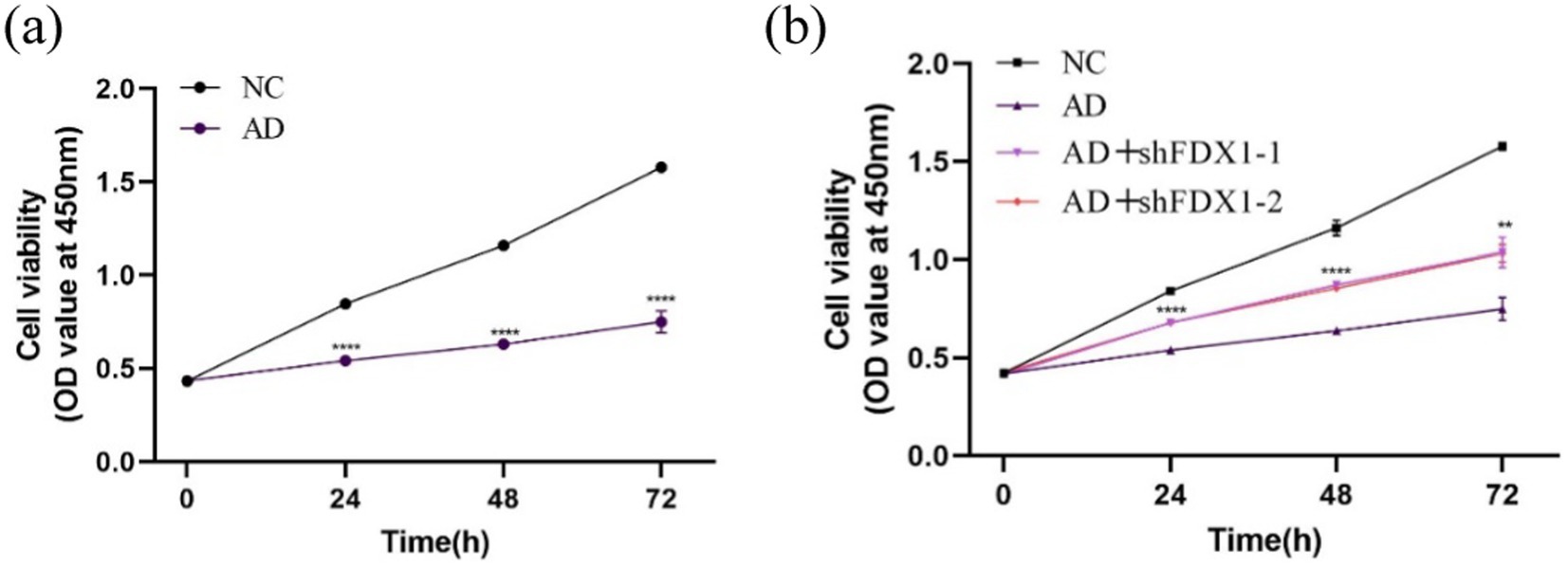
Figure 6. (A) The effect of Aβ25-35 on the viability of SH-SY5Y cells; (B) The effect of knocking down FDX1 expression on the viability of SH-SY5Y cells in AD model. (**p < 0.01, ***p < 0.001, ****p < 0.0001).
Reduced cell damage after FDX1 mRNA knockdown
Mitochondrial ROS levels in SH-SY5Y cells were measured using flow cytometry. Compared to the control group, mitochondrial ROS accumulation in SH-SY5Y cells significantly increased after Aβ25-35 treatment (Figure 7A). After FDX1 knockdown, mitochondrial ROS levels in SH-SY5Y cells decreased significantly (p < 0.001) (Figure 7B), indicating alleviated cell damage.
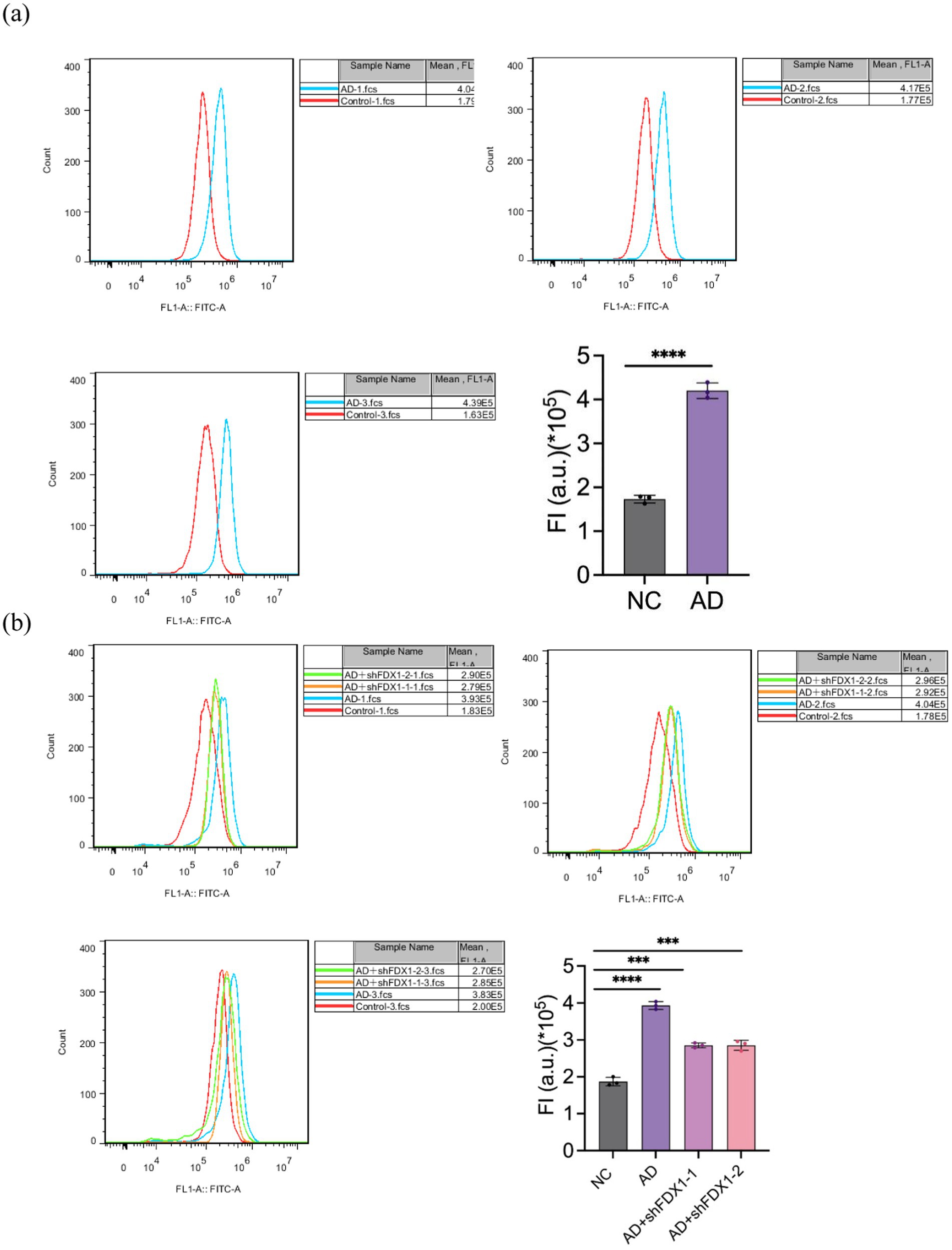
Figure 7. (A) Effect of Aβ25-35 on mitochondrial ROS content in SH-SY5Y cells. (B) The effect of knocking down FDX1 expression on mitochondrial ROS content in SH-SY5Y cells of AD model. (***p < 0.001, ****p < 0.0001).
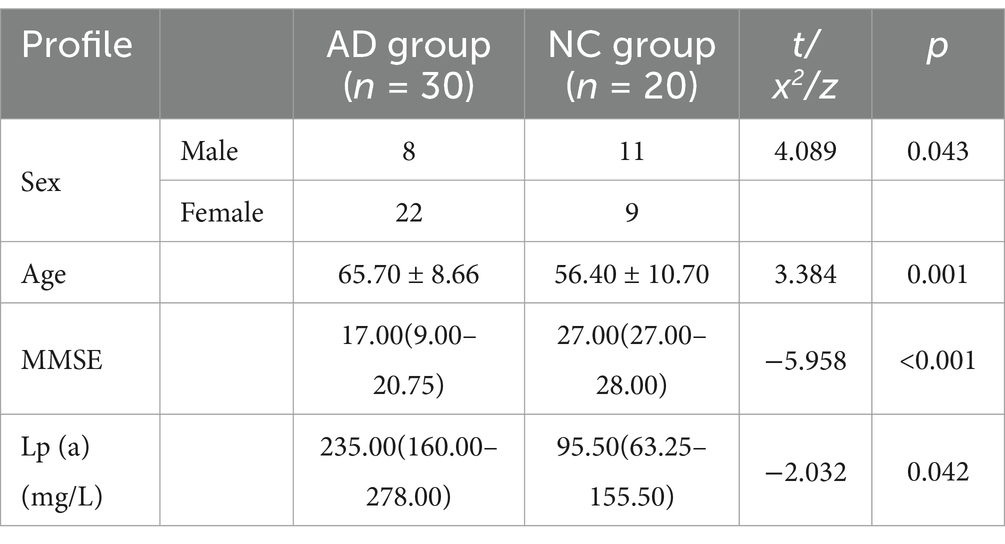
Comparison of general information and FDX1 expression in peripheral blood between AD and control group
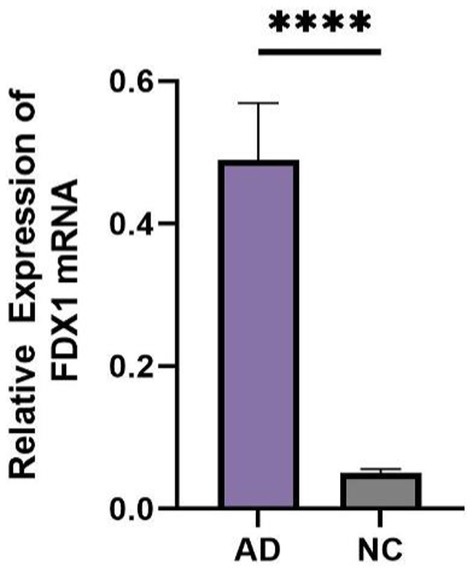
There were statistical differences in gender, age, MMSE, and lipoprotein(a) (Lp(a)) between the two groups (p < 0.05) (Table 2). The relative expression of FDX1 mRNA in the peripheral blood of AD patients was significantly increased compared to non-AD patients (p < 0.0001) (Figure 8).
Comparison of FDX1 expression among AD patients with different APOE genotypes
In AD patients, there was no statistical difference in FDX1 mRNA relative expression levels between APOE ε4+ and APOE ε4− individuals, but there was differential expression between APOE ε4/ε4 and APOE ε2, ε3/ε4 individuals (p < 0.05) (Figure 9). FDX1 mRNA relative expression levels in peripheral blood of AD patients with the APOE ε4/ε4 genotype were significantly higher.
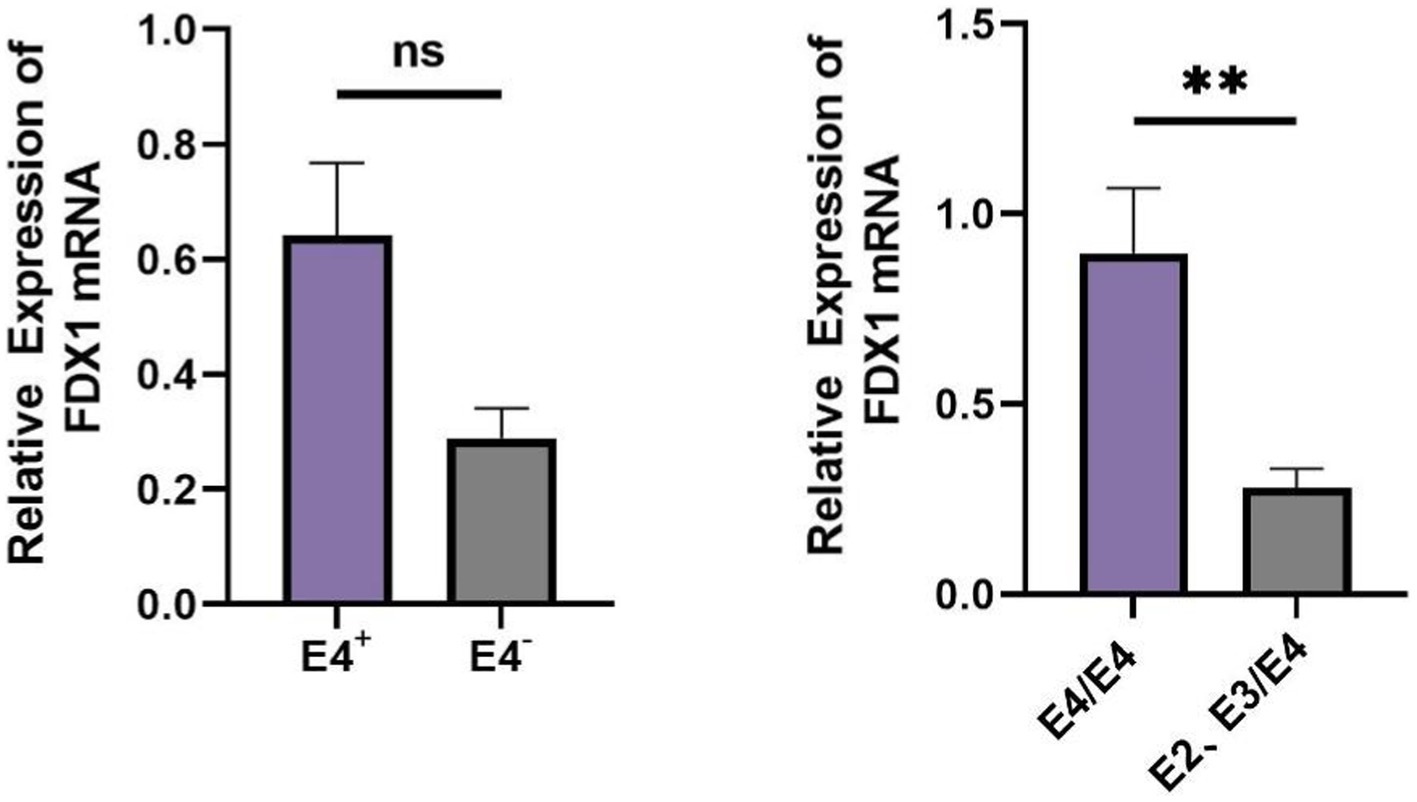
Figure 9. (A) Difference analysis of relative expression of FDX1 mRNA in APOE ε4+/APOE ε4− of AD patients; (B) Difference analysis of relative expression of FDX1 mRNA in APOE ε4/ε4 and APOE ε2 ε3/ε4 of AD patients (**p < 0.01).
PPI analysis results of FDX1
The PPI network showed that FDX1 primarily interacts with FDXR, FDX2, CYP27C1, CYP27B1, and CYP24A1. Functional predictions indicated that FDX1 is involved in hormone biosynthesis along with FDXR, FDX2, and CYP27B1, and also participates in the binding process of iron–sulfur clusters and metal clusters (Figure 10).

Figure 10. PPI network of FDX1. The colors in the circle represent the different functions of the proteins corresponding to each gene, and the colors of the lines represent the different interactions between the proteins. The more lines there are, the closer the connections are.
High infiltration of γδ-T cells in AD
In differential analysis of immune cell infiltration (Figure 11), there was a significant difference in γδ-T cell infiltration expression between AD patients and healthy controls (p < 0.01). γδ-T cells were enriched and increased in AD patients, indicating possible immune inflammatory reactions in AD.
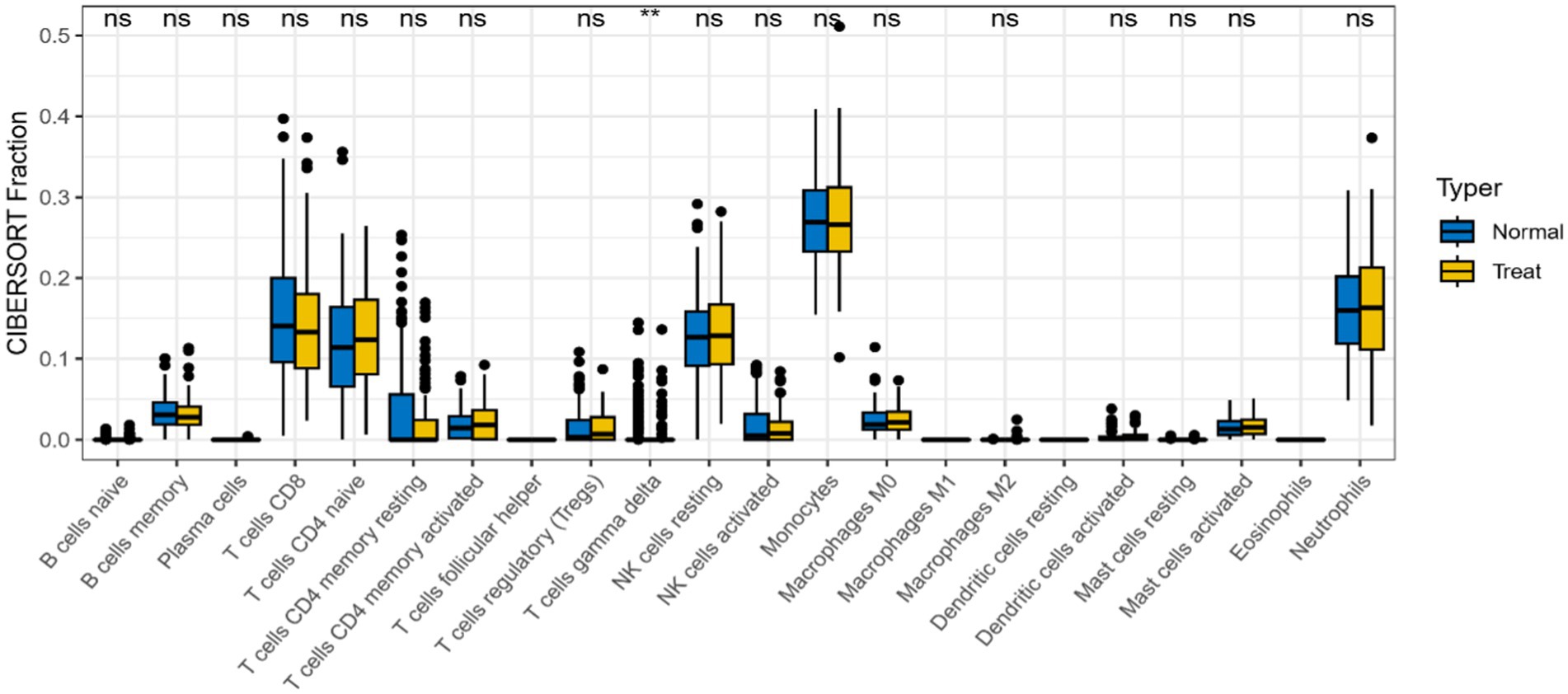
Figure 11. Differences in expression levels of immune cells in AD group and control group (*p < 0.05, **p < 0.01).
Significant correlation between FDX1 and four immune cells
FDX1 were significantly correlated with four immune cells in the analysis of the correlation between 22 immune cells and it (Figure 12). Specifically, FDX1 was negatively correlated with M0 macrophages (p < 0.001), activated mast cells (p < 0.01), and resting NK cells (p < 0.05), and positively correlated with CD4 memory resting T cells (p < 0.05).
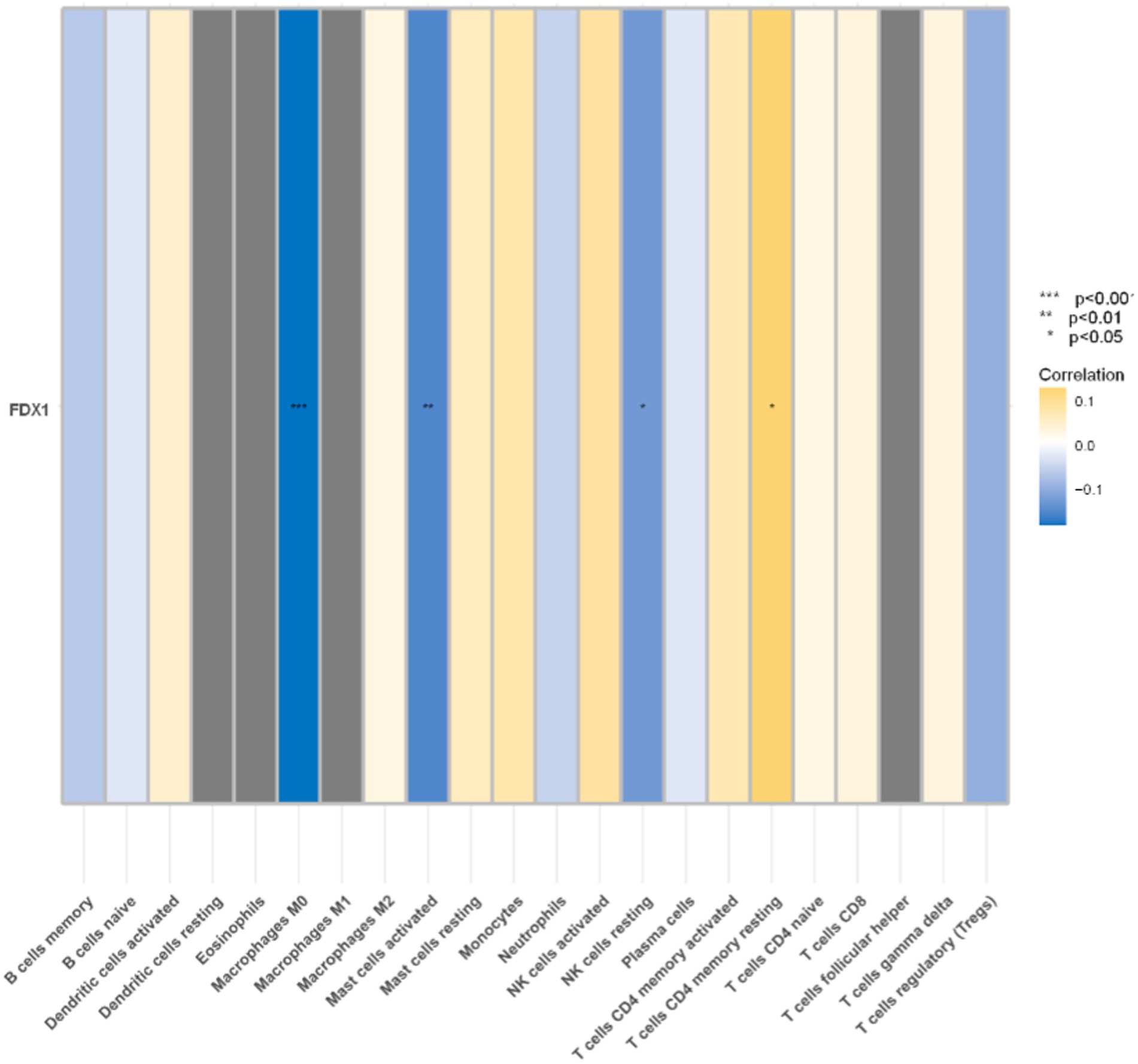
Figure 12. Correlation analysis between immune cells and FDX1 (*p < 0.05,**p < 0.01,***p < 0.001). Blue represents negative correlation, yellow represents positive correlation.
Existence of three transcription factors for FDX1
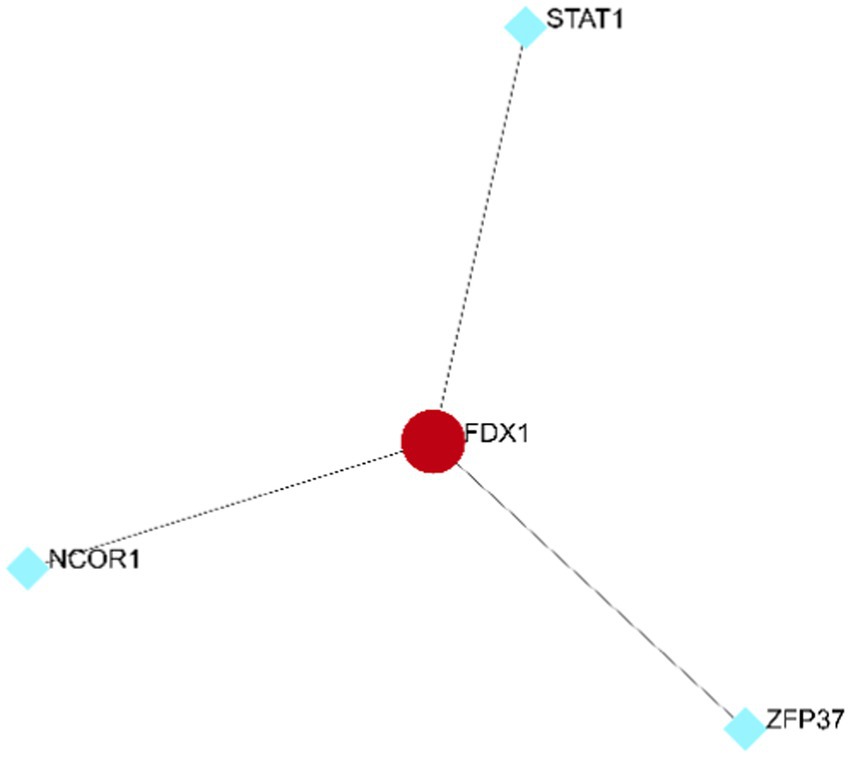
Based on the ENCODE database, three transcription factors related to FDX1 were predicted (Figure 13): Signal Transducer and Activator of Transcription 1 (STAT1), Zinc Finger Protein 37 (ZFP37), and Nuclear Receptor Corepressor 1 (NCOR1).
Discussion
Currently, research on cuproptosis in AD is still in its infancy, lacking direct experimental validation of the relevance between cuproptosis and AD. Therefore, this study conducted preliminary research on the cuproptosis mechanism in AD based on FDX1 by biological information technology and experimental verification. The results indicate that FDX1 is highly expressed in AD neurons. After downregulation of FDX1 expression, the expression of acylated proteins DLAT and DLST in cuproptosis also decreased. Neuronal oxidative stress damage was significantly alleviated, and proliferation activity was markedly improved. Subsequently, using peripheral blood samples from some AD clinical patients, we found that FDX1 was also highly expressed in AD patients, with higher expression levels in patients carrying the APOE ε4/ε4 genotype. The experiments verified the high expression of FDX1 in the peripheral and central nervous systems of AD patients, which is associated with the APOE ε4/ε4 risk gene and may regulate neurons through the cuproptosis pathway. Inhibition of FDX1 expression can alleviate cuproptosis in AD neurons, thereby delaying the progression of AD.
This study’s results demonstrate that after downregulation of FDX1 expression, the expression of DLAT and DLST also decreases, potentially affecting the cuproptosis process through the FDX1-DLAT/DLST regulatory axis (Belloy et al., 2019), consistent with previous research findings. The loss of FDX1 disrupts the acylation of proteins such as DLAT and DLST (Nie et al., 2023), resulting in loss of their ability to bind to copper, thus easing the cuproptosis process in neurons. Therefore, subsequent gene expression regulation can be achieved through FDX1 transcription factors STAT1, ZFP37, and NCOR1, providing new strategies for AD treatment.
APOE mainly functions as a lipid transport protein, playing a key role in certain transport between neurons (Yamazaki et al., 2019). It can also affect the development of AD by blocking Aβ clearance, accelerating Aβ aggregation, influencing Tau pathology and Tau-mediated neurodegeneration, and damaging synaptic integrity and plasticity (Komai et al., 2024; Strittmatter et al., 1993). In 1993, APOE isoforms were first confirmed as risk factors for AD (Strittmatter and Roses, 1996), among which the ε4 isoform increases the risk of developing AD and lowers the age of onset. One ε4 allele can increase the risk of developing AD by 2- to 4-fold, while homozygosity for the allele can increase the risk to 8–12 times (Gao et al., 2022; Strittmatter and Roses, 1996).
This study showed that FDX1 is more highly expressed in patients with AD carrying the APOE ε4/ε4 genotype, suggesting that FDX1 may interact with the APOE ε4/ε4 genotype, jointly affecting the progression of the disease.
Clinical data show differences in age, gender, and lipoprotein between the two groups of patients. Age is one of the most significant risk factors for cognitive decline in AD (Liu et al., 2024). The older the age, the higher the probability of developing AD. Women are more likely to develop AD than men (Alzheimer’s Disease International, 2018), with a 19–29% higher incidence rate (ADI, n.d.), possibly due to women having a higher Tau protein load (Buckley et al., 2019; Babapour Mofrad et al., 2020), measured at higher levels of AD pathology (Oveisgharan et al., 2018; Biechele et al., 2024). Related experiments have shown that the effects of Cu on memory function and oxidative stress in rats are also gender-related, with slightly greater effects observed in female rats (Lamtai et al., 2020), which may be due to estrogen enhancing copper retention, making females more susceptible to its neurotoxic effects (Amtage et al., 2014). Therefore, further research is necessary to clarify the potential gender differences in the effects of copper homeostasis on neurocognitive function.
Lp(a) is a distinctive hepatic lipoprotein (Kamstrup, 2021), and there is evidence indicating a high correlation between serum Lp(a) levels and the severity of AD (Larsson et al., 2020; Merched et al., 2000), which suggests that clinical measurement of Lp(a) could be utilized for assessing the risk and severity of AD. Nevertheless, the relationship between Lp(a) and AD remains elusive. Some studies have demonstrated a significant positive correlation between serum concentrations of Lp(a) and increased risk of AD (Solfrizzi et al., 2002), while some studies have indicated a negative correlation (Gong et al., 2022), and others have found no significant difference in Lp(a) concentrations between AD patients and healthy controls (Ray et al., 2013; Ban et al., 2009). This current study reveals that the Lp(a) concentration in the AD patient group is higher than that in the control group, implying a potential positive correlation between Lp(a) and the risk of AD onset. However, due to the small sample size of this study, the statistical results may be subject to certain biases. Therefore, whether reducing Lp(a) levels would increase the risk of dementia requires further investigation with an expanded sample size (Larsson et al., 2020).
Immunocytes, particularly microglia and astrocytes, play a pivotal role in the pathogenesis of AD by modulating neuroinflammation (Princiotta Cariddi et al., 2022), Aβ clearance (Ennerfelt et al., 2022; Hur et al., 2020), and tau pathology (Rajesh and Kanneganti, 2022). Impaired immunocyte function can lead to reduced phagocytosis and accumulation of Aβ, while their activation results in the release of pro-inflammatory cytokines, neuronal damage, and disruption of synaptic integrity. Additionally, this activation may facilitate the spread of pathological Tau, induce neuronal apoptosis, and accelerate the decline in cognitive abilities in AD patients (da Mesquita et al., 2018). Recent investigative findings suggest that γδ-T cells, functioning as potential regulatory or pathogenic entities, are capable of infiltrating the brain (Aliseychik et al., 2020). During the early stages of AD, the IL-17 produced by γδ-T cells accumulates in the central nervous system in significant quantities and persists throughout the disease’s progression. IL-17 can cause in synaptic dysfunction and impairments in short-term memory in AD mouse models, which neutralization was sufficient to rescue Aβ-induced neuroinflammation and hippocampal glutamatergic dysfunction in early stages of disease with a mechanism that is independent of Aβ and Tau pathology or blood–brain barrier (BBB) disruption (Brigas et al., 2021; Cristiano et al., 2019). This is consistent with the findings of the current study, which observed a high enrichment of γδ-T cells in AD, potentially offering new avenues for the diagnosis and therapeutic intervention of AD.
In the analysis of the correlation between FDX1 and immune cells in AD, we found that FDX1 is closely associated with the infiltration of four types of immune cells, among which M0 macrophages were the most strongly associated. According to research reports, Chronic M0-differentiation may lead Triggering Receptor Expressed on Myeloid Cells 2 (TREM2), which serves as a putative therapeutic target for AD, to increased synthesis in AD-derived cells (Cosma et al., 2023). This suggests that the high expression of FDX1 is correlated with immune pathways (Wang et al., 2024), and may participate in the occurrence and development of AD by altering the composition of the immune microenvironment.
There have been reports on the development of functional drugs targeting cuproptosis pathways and their application in cancer (Tong et al., 2022), but the role of cuproptosis and its related genes in AD has not been thoroughly studied. Therefore, developing new therapies for AD based on the cuproptosis is of great significance and deserves further investigation.
However, this study has certain limitations. Firstly, our study used a public dataset with limitations, which sample size is mainly composed of gene expression data, omitting other biological data like proteomics or metabolomics. Sample diversity and platform differences could bias our analysis and affect the findings’ generalizability. Diverse samples and multi-omics data should be utilized in future research to better understand disease mechanisms. Secondly, the sample size for clinical validation is relatively small, which may limit the universality and reliability of the research outcomes. Further validation and exploration are needed through the expansion of sample size and in vitro experiments. Thirdly, whether FDX1 exclusively affects AD through the cuproptosis and the specific effects on AD after cuproptosis in neurons remain to be explored further.
Conclusion
The key gene FDX1 involved in cuproptosis is highly expressed in neurons and peripheral blood of AD, and it can affect the status of neurons by regulating the expression of DLAT and DLST. Thus, we speculate that FDX1 may participate in the occurrence and development of AD through the cuproptosis. Exploring how to reduce FDX1 expression to inhibit cuproptosis in neurons may become a new strategy for developing anti-AD drugs or delaying the progression of AD.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Anhui Provincial hospital’s Medical Ethics Committee, with ethics approval number: 2019KY Ethics Review No. 79. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GC: Data curation, Methodology, Validation, Writing – original draft, Project administration. EX: Data curation, Resources, Writing – original draft. XG: Methodology, Resources, Writing – review & editing. HW: Methodology, Resources, Supervision, Writing – review & editing. QT: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Strategic Pilot Science and Technology Project (Class B), Chinese Academy of Sciences, XDB39040400.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
ADI. (n.d.) Dementia facts & figures. https://www.alzint.org/about/dementia-facts-figures (Accessed October 8, 2023).
Aliseychik, M., Patrikeev, A., Gusev, F., Grigorenko, A., Andreeva, T., Biragyn, A., et al. (2020). Dissection of the human T-cell receptor γ gene repertoire in the brain and peripheral blood identifies age- and Alzheimer's disease-associated Clonotype profiles. Front. Immunol. 11:12. doi: 10.3389/fimmu.2020.00012
Alzheimer’s Disease International. (2018). World Alzheimer Report 2018. The state of the art of dementia research: new frontiers. Available at: https://www.alzint.org/u/WorldAlzheimerReport2018.pdf (Accessed October 8, 2023).
Amtage, F., Birnbaum, D., Reinhard, T., Niesen, W. D., Weiller, C., Mader, I., et al. (2014). Estrogen intake and copper depositions: implications for Alzheimer's disease? Case Rep. Neurol. 6, 181–187. doi: 10.1159/000363688
Arispe, N., Rojas, E., and Pollard, H. B. (1993). Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc. Natl. Acad. Sci. USA 90, 567–571. doi: 10.1073/pnas.90.2.567
Babapour Mofrad, R., Tijms, B. M., Scheltens, P., Barkhof, F., van der Flier, W., Sikkes, S. A. M., et al. (2020). Sex differences in CSF biomarkers vary by Alzheimer disease stage and APOE ε4 genotype. Neurology 95, e2378–e2388. doi: 10.1212/WNL.0000000000010629
Ban, Y., Watanabe, T., Suguro, T., Matsuyama, T. A., Iso, Y., Sakai, T., et al. (2009). Increased plasma urotensin-II and carotid atherosclerosis are associated with vascular dementia. J. Atheroscler. Thromb. 16, 179–187. doi: 10.5551/jat.E608
Belloy, M. E., Napolioni, V., and Greicius, M. D. (2019). A quarter century of APOE and Alzheimer’s disease: Progress to date and the path forward. Neuron 101, 820–838. doi: 10.1016/j.neuron.2019.01.056
Bharadwaj, P. R., Dubey, A. K., Masters, C. L., Martins, R. N., and Macreadie, I. G. (2009). Abeta aggregation and possible implications in Alzheimer's disease pathogenesis. J. Cell. Mol. Med. 13, 412–421. doi: 10.1111/j.1582-4934.2009.00609.x
Biechele, G., Rauchmann, B. S., Janowitz, D., Buerger, K., Franzmeier, N., Weidinger, E., et al. (2024). Associations between sex, body mass index and the individual microglial response in Alzheimer's disease. J. Neuroinflammation 21:30. doi: 10.1186/s12974-024-03020-y
Brigas, H. C., Ribeiro, M., Coelho, J. E., Gomes, R., Gomez-Murcia, V., Carvalho, K., et al. (2021). IL-17 triggers the onset of cognitive and synaptic deficits in early stages of Alzheimer’s disease. Cell Rep. 36:109574. doi: 10.1016/j.celrep.2021.109574
Buckley, R. F., Mormino, E. C., Rabin, J. S., Hohman, T. J., Landau, S., Hanseeuw, B. J., et al. (2019). Sex differences in the Association of Global Amyloid and Regional tau Deposition Measured by positron emission tomography in clinically Normal older adults. JAMA Neurol. 76, 542–551. doi: 10.1001/jamaneurol.2018.4693
Byun, M. S., Kim, S. E., Park, J., Yi, D., Choe, Y. M., Sohn, B. K., et al. (2015). Alzheimer’s Disease Neuroimaging Initiative heterogeneity of regional brain atrophy patterns associated with distinct progression rates in Alzheimer's disease. PLoS One 10:e0142756. doi: 10.1371/journal.pone.0142756
Changhong, K., Peng, Y., Yuan, Z., and Cai, J. (2021). Ginsenoside Rb1 protected PC12 cells from Aβ25-35-induced cytotoxicity via PPARγactivation and cholesterol reduction. Eur. J. Pharmacol. 893:173835. doi: 10.1016/j.ejphar.2020.173835
Cosma, N. C., Eren, N., Üsekes, B., Gerike, S., Heuser, I., Peters, O., et al. (2023). Acute and chronic macrophage differentiation modulates TREM2 in a personalized Alzheimer's patient-derived assay. Cell. Mol. Neurobiol. 43, 3047–3060. doi: 10.1007/s10571-023-01351-7
Cristiano, C., Volpicelli, F., Lippiello, P., Buono, B., Raucci, F., Piccolo, M., et al. (2019). Neutralization of IL-17 rescues amyloid-β-induced neuroinflammation and memory impairment. Br. J. Pharmacol. 176, 3544–3557. doi: 10.1111/bph.14586
da Mesquita, S., Louveau, A., Vaccari, A., Smirnov, I., Cornelison, R. C., Kingsmore, K. M., et al. (2018). Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature 560, 185–191. doi: 10.1038/s41586-018-0368-8
Duan, W., Zhang, Y. P., Hou, Z., Huang, C., Zhu, H., Zhang, C. Q., et al. (2016). Novel insights into NeuN: from neuronal marker to splicing regulator. Mol. Neurobiol. 53, 1637–1647. doi: 10.1007/s12035-015-9122-5
Ennerfelt, H., Frost, E. L., Shapiro, D. A., Holliday, C., Zengeler, K. E., Voithofer, G., et al. (2022). SYK coordinates neuroprotective microglial responses in neurodegenerative disease. Cell 185, 4135–4152.e22. doi: 10.1016/j.cell.2022.09.030
Gao, D., Li, P., Gao, F., Feng, Y., Li, X., Li, D., et al. (2022). Preparation and multitarget anti-AD activity study of chondroitin sulfate Lithium in AD mice induced by combination of D-gal/AlCl3. Oxidative Med. Cell. Longev. 2022, 1–22. doi: 10.1155/2022/9466166
Gong, J., Harris, K., Peters, S. A. E., and Woodward, M. (2022). Serum lipid traits and the risk of dementia: a cohort study of 254,575 women and 214,891 men in the UK biobank. EClinicalMedicine 54:101695. doi: 10.1016/j.eclinm.2022.101695
Huang, X. (2023). A concise review on oxidative stress-mediated Ferroptosis and Cuproptosis in Alzheimer's disease. Cells 12:1369. doi: 10.3390/cells12101369
Hur, J. Y., Frost, G. R., Wu, X., Crump, C., Pan, S. J., Wong, E., et al. (2020). The innate immunity protein IFITM3 modulates γ-secretase in Alzheimer's disease. Nature 586, 735–740. doi: 10.1038/s41586-020-2681-2
Kamstrup, P. R. (2021). Lipoprotein(a) and cardiovascular disease. Clin. Chem. 67, 154–166. doi: 10.1093/clinchem/hvaa247
Komai, M., Noda, Y., Ikeda, A., Kaneshiro, N., Kamikubo, Y., Sakurai, T., et al. (2024). Nuclear SphK2/S1P signaling is a key regulator of ApoE production and Aβ uptake in astrocytes. J. Lipid Res. 65:100510. doi: 10.1016/j.jlr.2024.100510
Lai, Y., Lin, C., Lin, X., Wu, L., Zhao, Y., and Lin, F. (2022). Identification and immunological characterization of cuproptosis-related molecular clusters in Alzheimer's disease. Front. Aging Neurosci. 14:932676. doi: 10.3389/fnagi.2022.932676
Lam, B., Masellis, M., Freedman, M., Stuss, D. T., and Black, S. E. (2013). Clinical, imaging, and pathological heterogeneity of the Alzheimer's disease syndrome. Alzheimers Res. Ther. 5:1. doi: 10.1186/alzrt155
Lamtai, M., Zghari, O., Ouakki, S., Marmouzi, I., Mesfioui, A., el Hessni, A., et al. (2020). Chronic copper exposure leads to hippocampus oxidative stress and impaired learning and memory in male and female rats. Toxicol Re 36, 359–366. doi: 10.1007/s43188-020-00043-4
Larsson, S. C., Gill, D., Mason, A. M., Jiang, T., Bäck, M., Butterworth, A. S., et al. (2020). Lipoprotein(a) in Alzheimer, atherosclerotic, cerebrovascular, thrombotic, and Valvular disease: Mendelian randomization investigation. Circulation 141, 1826–1828. doi: 10.1161/CIRCULATIONAHA.120.045826
Limón, I. D., Díaz, A., Mendieta, L., Chamorro, G., Espinosa, B., Zenteno, E., et al. (2009). Amyloid-beta(25-35) impairs memory and increases NO in the temporal cortex of rats. Neurosci. Res. 63, 129–137. doi: 10.1016/j.neures.2008.11.006
Liu, Y., Tan, Y., Zhang, Z., Yi, M., Zhu, L., and Peng, W. (2024). The interaction between ageing and Alzheimer's disease: insights from the hallmarks of ageing. Transl. Neurodegener. 13:7. doi: 10.1186/s40035-024-00397-x
Mangalmurti, A., and Lukens, J. R. (2022). How neurons die in Alzheimer's disease: implications for neuroinflammation. Curr. Opin. Neurobiol. 75:102575. doi: 10.1016/j.conb.2022.102575
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Jr, Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 263–269. doi: 10.1016/j.jalz.2011.03.005
Merched, A., Xia, Y., Visvikis, S., Serot, J. M., and Siest, G. (2000). Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer's disease. Neurobiol. Aging 21, 27–30. doi: 10.1016/S0197-4580(99)00103-7
Nie, B., Duan, Y., Xie, X., Qiu, L., Shi, S., Fan, Z., et al. (2023). Systematic analysis of cuproptosis-related genes in immunological characterization and predictive drugs in Alzheimer's disease. Front. Aging Neurosci. 15:1204530. doi: 10.3389/fnagi.2023.1204530
Oboudiyat, C., Glazer, H., Seifan, A., Greer, C., and Isaacson, R. (2013). Alzheimer's disease. Semin. Neurol. 33, 313–329. doi: 10.1055/s-0033-1359319
Oveisgharan, S., Arvanitakis, Z., Yu, L., Farfel, J., Schneider, J. A., and Bennett, D. A. (2018). Sex differences in Alzheimer's disease and common neuropathologies of aging. Acta Neuropathol. 136, 887–900. doi: 10.1007/s00401-018-1920-1
Peng, F., Liao, M., Qin, R., Zhu, S., Peng, C., Fu, L., et al. (2022). Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct. Target. Ther. 7:286. doi: 10.1038/s41392-022-01110-y
Princiotta Cariddi, L., Mauri, M., Cosentino, M., Versino, M., and Marino, F. (2022). Alzheimer's disease: from immune homeostasis to Neuroinflammatory condition. Int. J. Mol. Sci. 23:13008. doi: 10.3390/ijms232113008
Rajesh, Y., and Kanneganti, T. D. (2022). Innate immune cell death in Neuroinflammation and Alzheimer's disease. Cells 11:1885. doi: 10.3390/cells11121885
Ray, L., Khemka, V. K., Behera, P., Bandyopadhyay, K., Pal, S., Pal, K., et al. (2013). Serum homocysteine, Dehydroepiandrosterone Sulphate and lipoprotein (a) in Alzheimer's disease and vascular dementia. Aging Dis. 4, 57–64
Ritchie, M. E., Phipson, B., Wu, D., Hu, Y., Law, C. W., Shi, W., et al. (2015). Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43:e47. doi: 10.1093/nar/gkv007
Scheltens, P., de Strooper, B., Kivipelto, M., Holstege, H., Chételat, G., Teunissen, C. E., et al. (2021). Alzheimer's disease. Lancet 397, 1577–1590. doi: 10.1016/S0140-6736(20)32205-4
Solfrizzi, V., Panza, F., D'Introno, A., Colacicco, A. M., Capurso, C., Basile, A. M., et al. (2002). Lipoprotein(a), apolipoprotein E genotype, and risk of Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry 72, 732–736. doi: 10.1136/jnnp.72.6.732
Strittmatter, W. J., and Roses, A. D. (1996). Apolipoprotein E and Alzheimer's disease. Annu. Rev. Neurosci. 19, 53–77. doi: 10.1146/annurev.ne.19.030196.000413
Strittmatter, W. J., Saunders, A. M., Schmechel, D., Pericak-Vance, M., Enghild, J., Salvesen, G. S., et al. (1993). Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl. Acad. Sci. USA 90, 1977–1981. doi: 10.1073/pnas.90.5.1977
Tong, X., Tang, R., Xiao, M., Xu, J., Wang, W., Zhang, B., et al. (2022). Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J. Hematol. Oncol. 15:174. doi: 10.1186/s13045-022-01392-3
Tsvetkov, P., Coy, S., Petrova, B., Dreishpoon, M., Verma, A., Abdusamad, M., et al. (2022). Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 375, 1254–1261. doi: 10.1126/science.abf0529
Tsvetkov, P., Detappe, A., Cai, K., Keys, H. R., Brune, Z., Ying, W., et al. (2019). Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol. 15, 681–689. doi: 10.1038/s41589-019-0291-9
Van der Kant, R., Goldstein, L. S. B., and Ossenkoppele, R. (2020). Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 21, 21–35. doi: 10.1038/s41583-019-0240-3
Wang, Q., Sun, J., Chen, T., Song, S., Hou, Y., Feng, L., et al. (2023). Palmatine protects PC12 cells and mice from Aβ 25-35-induced oxidative stress and Neuroinflammation via the Nrf2/HO-1 pathway. Molecules 28:7955. doi: 10.1021/acschemneuro.3c00343
Wang, Q., Su, Z., Zhang, J., Yan, H., and Zhang, J. (2024). Unraveling the copper-death connection: decoding COVID-19‘s immune landscape through advanced bioinformatics and machine learning approaches. Hum. Vaccin. Immunother. 20, 20:2310359. doi: 10.1080/21645515.2024.2310359
Wang, Q., Sun, J., Chen, T., et al. (2023). Ferroptosis, Pyroptosis, and Cuproptosis in Alzheimer's disease. ACS Chem. Neurosci. 14, 3564–3587.
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C. C., and Bu, G. (2019). Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 15, 501–518. doi: 10.1038/s41582-019-0228-7
Yan, W., Fan, J., Zhang, X., Song, H., Wan, R., Wang, W., et al. (2021). Decreased neuronal synaptosome associated protein 29 contributes to poststroke cognitive impairment by disrupting presynaptic maintenance. Theranostics 11, 4616–4636. doi: 10.7150/thno.54210
Yildiz Gulhan, P., Eroz, R., Ataoglu, O., İnce, N., Davran, F., Öztürk, C. E., et al. (2022). The evaluation of both the expression and serum protein levels of Caspase-3 gene in patients with different degrees of SARS-CoV2 infection. J. Med. Virol. 94, 897–905. doi: 10.1002/jmv.27362
Zhang, E., Dai, F., Chen, T., Liu, S., Xiao, C., and Shen, X. (2023). Diagnostic models and predictive drugs associated with cuproptosis hub genes in Alzheimer's disease. Front. Neurol. 13:1064639. doi: 10.3389/fneur.2022.1064639
Zhang, Z., Ma, Y., Guo, X., du, Y., Zhu, Q., Wang, X., et al. (2021). FDX1 can impact the prognosis and mediate the metabolism of lung adenocarcinoma. Front. Pharmacol. 12:749134. doi: 10.3389/fphar.2021.749134
Zhang, C., Zeng, Y., Guo, X., Shen, H., Zhang, J., Wang, K., et al. (2022). Pan-cancer analyses confirmed the cuproptosis-related gene FDX1 as an immunotherapy predictor and prognostic biomarker. Front. Genet. 13:923737. doi: 10.3389/fgene.2022.923737
Keywords: Alzheimer’s disease, cuproptosis, FDX1 , DLAT , DLST
Citation: Chen G, Xi E, Gu X, Wang H and Tang Q (2024) The study on cuproptosis in Alzheimer’s disease based on the cuproptosis key gene FDX1. Front. Aging Neurosci. 16:1480332. doi: 10.3389/fnagi.2024.1480332
Edited by:
Xuemin Xu, University of Texas Permian Basin, United StatesReviewed by:
Mariana Toricelli, Association for Research Incentive Fund (AFIP), BrazilErdong Zhang, Guizhou Medical University, China
Copyright © 2024 Chen, Xi, Gu, Wang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huili Wang, d2FuZ2hsQGhmdXQuZWR1LmNu; Qiqiang Tang, dHFxMTk5NUB1c3RjLmVkdS5jbg==
 Guilin Chen1
Guilin Chen1 Huili Wang
Huili Wang Qiqiang Tang
Qiqiang Tang