- Department of Neurology and National Research Center for Aging and Medicine and National Center for Neurological Disorders, State Key Laboratory of Medical Neurobiology, Huashan Hospital, Fudan University, Shanghai, China
Background: Elevated plasma homocysteine (Hcy) has been reported as a risk factor for cognitive impairment in the general population. However, there are conflicting results regarding the relationship between Hcy and cognitive impairment across various cognitive domains in Parkinson’s disease (PD).
Objective: This study aims to explore the association between plasma Hcy levels, cognitive impairment, and dysfunction in various cognitive domains among PD patients with and without mild cognitive impairment (MCI).
Methods: A total of 101 PD patients underwent plasma Hcy measurement, comprising 50 PD-MCI patients and 51 patients with normal cognition (PD-NC). A battery of neuropsychological tests was administered to assess different cognitive domains. Adjusted generalized linear models were used to assess the correlations between Hcy levels and cognitive functions.
Results: As anticipated, PD-MCI patients demonstrated a significant decline in cognitive function across all five cognitive domains (memory, executive function, attention/working memory, language, and visuospatial function). Elevated plasma Hcy levels (≥ 10 μmol/L) were associated with a higher odds of PD-MCI, even within the normal range of Hcy levels (< 15 μmol/L). After adjusting for confounding factors, a negative correlation was observed between plasma Hcy levels and the performance on specific cognitive tests evaluating executive functions in PD, such as the Stroop Color-Word Test-C (β = −1.123, 95% CI = −1.845 ∼−0.401, p = 0.0023).
Conclusion: This study underscores a significant link between plasma Hcy levels and PD-MCI, particularly concerning executive dysfunction, even within the normal range of Hcy levels (< 15 μmol/L).
Highlights
• The plasma homocysteine (Hcy) level is significantly elevated in PD patients with mild cognitive impairment (PD-MCI).
• Plasma Hcy level is significantly associated with the odds of PD-MCI even within the normal range.
• Elevated plasma Hcy level is particularly correlated with executive dysfunction in PD patients.
1 Introduction
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders characterized by motor dysfunction and non-motor symptoms. As the disease progresses, non-motor symptoms such as cognitive impairment, depression, sleep disorders, and autonomic dysfunctions may emerge as significant burdens and become key determinants of the quality of life for PD patients (Schapira et al., 2017). Cognitive impairment is the most prevalent and disabling non-motor symptom in PD, ranging from mild cognitive impairment (PD-MCI) to dementia (PDD) (Jones et al., 2017). During the natural progression of the disease, up to 83% of patients with PD may experience some degree of cognitive dysfunction (Hely et al., 2008). MCI represents a transitional stage between normal aging and dementia (Goldman et al., 2018). A meta-analysis, which included data from over seven thousand PD patients, reported a 40% prevalence of MCI, which could manifest at any time during the disease course (Goldman et al., 2018; Baiano et al., 2020). Despite ongoing efforts to improve early detection and management of MCI, current therapies are limited and primarily focus on symptom relief (Langa and Levine, 2014). Therefore, there is a pressing need to identify modifiable risk factors for this complication.
Homocysteine (Hcy) is an intermediary substance produced within the methionine cycle, which plays a crucial role in maintaining methionine and methylation levels in the human body. Numerous studies have highlighted associations between Hcy and various disorders, including cardiovascular diseases, cancer, and certain neurodegenerative diseases (Zhou, 2023). Elevated plasma Hcy levels, which can be caused by levodopa metabolism and treated with B-vitamin and folic acid, are considered a significant risk factor for PD and cognitive impairment in the general population (Teunissen et al., 2005; Zhou, 2023). However, it has been a topic of investigation for many years with conflicting results regarding the relationship between plasma Hcy levels and cognitive impairment in PD. Some studies have found no association (Hassin-Baer et al., 2006; Camicioli et al., 2009; Rodriguez-Oroz et al., 2009; Song et al., 2013), while others have reported more severe cognitive impairment in PD patients with hyperhomocysteinemia (O’Suilleabhain et al., 2004; Ozer et al., 2006; Zoccolella et al., 2009; Zoccolella et al., 2010; Białecka et al., 2012). Additionally, the link between PD-MCI and Hcy remains unclear. PD-MCI patients often exhibit deficits across multiple cognitive domains, with 65 to 93% experiencing some combination of different cognitive deficits (Goldman et al., 2018). Therefore, investigating the association of Hcy with impairment in various cognitive domains may provide insights into the underlying pathophysiology of PD-MCI.
Given this background, our study aims to explore the association between Hcy levels and cognitive functions in PD patients diagnosed with and without MCI, as well as its relationship to other non-motor symptoms.
2 Materials and methods
2.1 Study participants
All participants were recruited from the Movement Disorders Clinic, Department of Neurology, Huashan Hospital, Fudan University in Shanghai, China, spanning from December 2017 to November 2022. They all fulfilled the Movement Disorder Society (MDS) Clinical Diagnostic Criteria for idiopathic PD (Postuma et al., 2015). Approval for this study was obtained from the Medical Ethics Committee of Huashan Hospital, Fudan University, Shanghai, China. Written informed consent was obtained from all participants and/or their legal representatives prior to their involvement in the study. The study adhered to the principles outlined in the Declaration of Helsinki.
The demographic characteristics were acquired from the participants and/or proxy through a questionnaire, including age, gender, year of education, disease duration, and onset age. Motor symptoms were assessed by two senior investigators specializing in movement disorders. The severity of motor symptoms was evaluated using the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III), items 18–31, after a minimum of 12 h off anti-parkinsonian medications (OFF state). The disease stage of all patients was determined using the Hoehn & Yahr staging scale (H&Y stage). Additionally, participants completed various scales to evaluate non-motor symptoms, including the Geriatric Depression Rating Scale (GDS), Epworth Sleepiness Scale (ESS), Rapid Eye Movement Sleep Behavior Disorder Screening Questionnaire (RBDSQ), Non-Motor Symptoms Scale (NMSS), 39-item Parkinson’s Disease Questionnaire (PDQ-39), and Sniffin’ Sticks Screening 12 Test (SSST-12), as outlined in our previous study (Fan et al., 2020). The dosage of anti-parkinsonian drugs was converted into a total levodopa equivalent daily dose (LEDD) for standardization of medications (Tomlinson et al., 2010).
Cognitive assessments were conducted on patients while they had received their usual anti-parkinsonian medications (in the ON state). Global cognitive ability was assessed using the Mini-Mental State Examination (MMSE) (Zhang et al., 1990). A battery of neuropsychological tests, as detailed in our previous study (Fan et al., 2020), was employed to evaluate five specific cognitive domains: memory, executive function, attention/working memory, language, and visuospatial function. The PD-MCI diagnosis was determined using the more stringent MDS Task Force Level 2 criteria when the PD patients were visited at baseline.
The peripheral blood samples of all patients were obtained from the medial cubital vein after a 12-h fast, on the day of neuropsychological assessment, and Hcy levels were measured using an enzymatic cycling assay conducted by the clinical laboratory of Huashan Hospital.
2.2 Statistical analysis
Continuous variables were presented as means ± standard deviation (SD), while categorical variables were expressed as frequencies (%). The normal distribution of data was assessed using the Kolmogorov-Smirnov test and visual histograms. When the continuous data followed a normal distribution, the Student’s t-test was utilized for comparisons between the two groups. The Wilcoxon rank-sum test was applied for data with a non-normal distribution. The Pearson chi-square test was used for categorical variables. The logistic regression model was employed to investigate the association between Hcy and PD-MCI, with the results reported as odds ratio (OR) and 95% confidence interval (CI). Correlations between Hcy levels and non-motor symptoms or cognitive functions were assessed using the generalized linear model (GLM), adjusting for age, gender, education year, disease duration, and LEDD. Two-tailed p-values were reported, and statistically significant differences were considered as p < 0.05. Data analysis and visualization were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
3 Results
3.1 Demographics and clinical characteristics
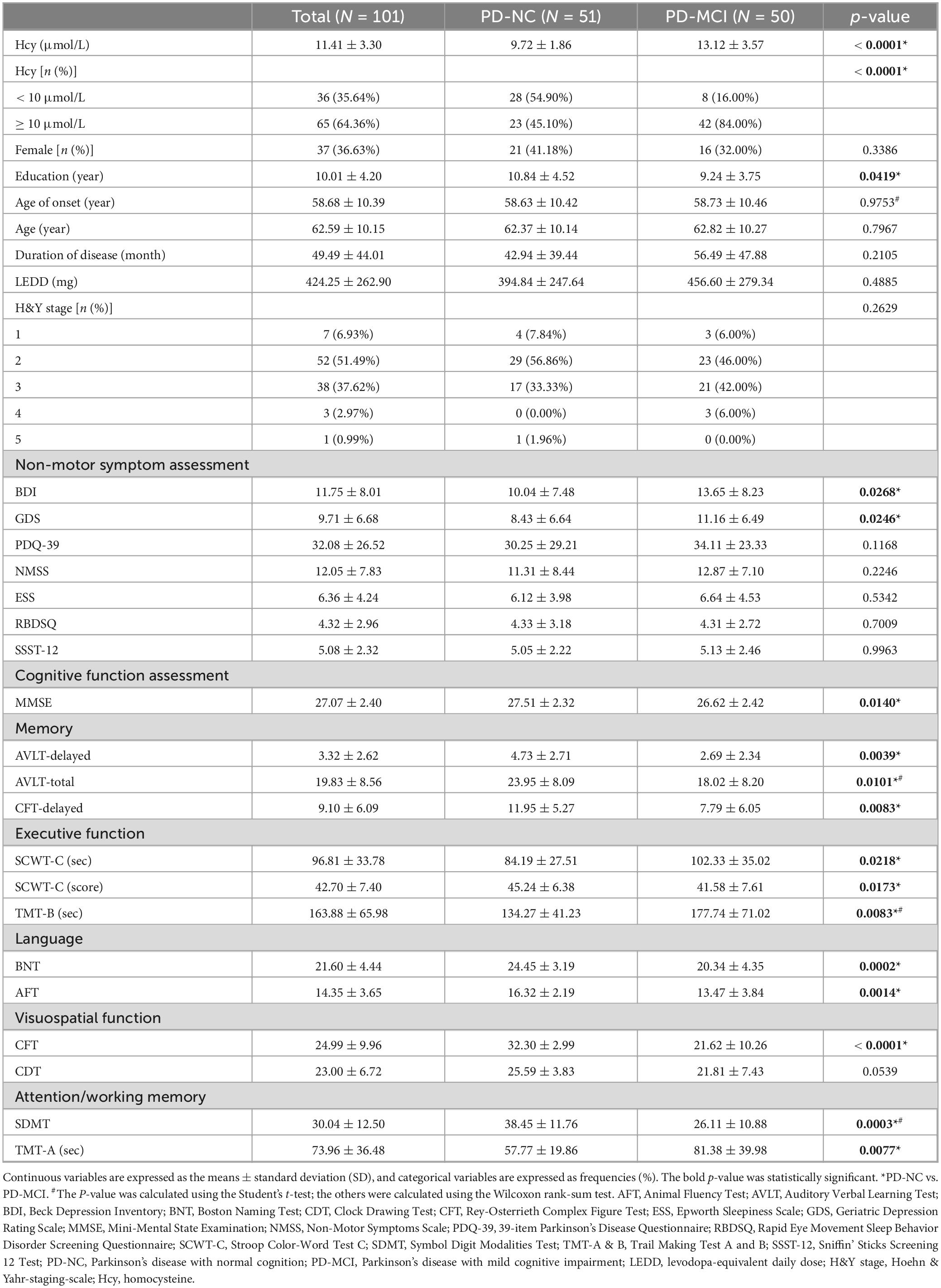
The study enrolled 101 cases, comprising 50 PD-MCI patients and 51 PD patients with normal cognition (PD-NC). Demographic and clinical details are summarized in Table 1. The mean age of all enrolled patients was 62.59 ± 10.15 years, with a mean age of onset of 58.68 ± 10.39 years and a mean disease duration of 49.49 ± 44.01 months. There were no statistically significant differences between these two groups in terms of gender, age, age of onset, disease duration, LEDD, and H&Y stage, except for the education level, which was comparatively lower in PD-MCI patients (p = 0.0419).
As shown in Table 1, PD-MCI patients exhibited a statistically significant decrease in MMSE score (p = 0.0140), along with notably elevated BDI and GDS scores (p = 0.0268 and 0.0246, respectively). Analysis across the five cognitive domains showed a substantial decrease in memory, executive, language, visuospatial, and attention/working memory functions in PD-MCI patients, except for the clock drawing test (CDT) assessing visuospatial function.
3.2 Comparisons of plasma homocysteine levels between groups
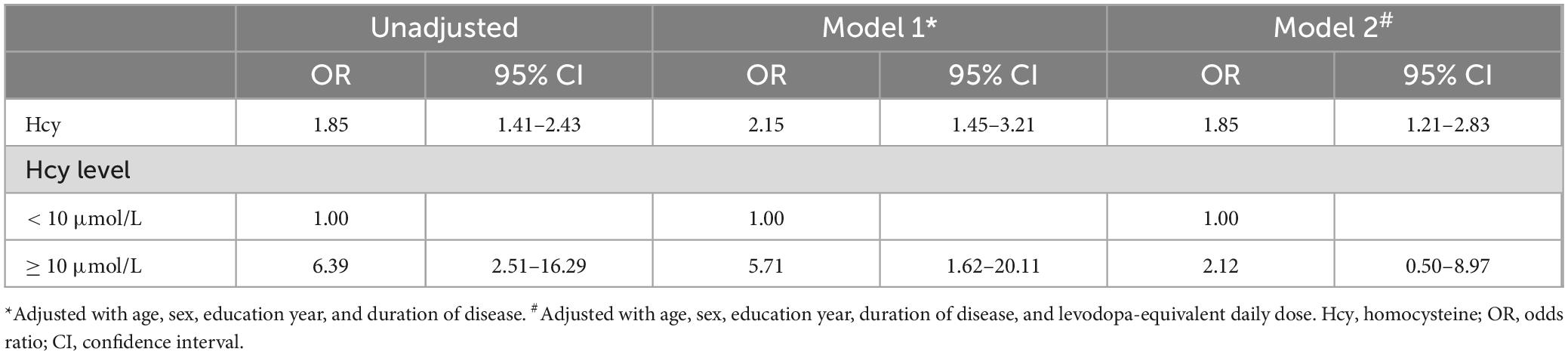
Although the average Hcy levels of all patients fell within the normal range (11.41 ± 3.30 μmol/L, < 15 μmol/L), the Hcy levels of PD-MCI patients (13.12 ± 3.57 μmol/L) were significantly higher than those of PD-NC patients (9.72 ± 1.86 μmol/L, p < 0.0001, Figure 1A). Among the total 65 PD patients with Hcy levels exceeding 10 μmol/L, 42 (64.62%) were in the PD-MCI group, compared to 23 (35.38%) in the PD-NC group. Furthermore, 84.00% of PD-MCI patients had Hcy levels higher than 10 μmol/L, while only 45.10% of PD-NC patients had higher Hcy levels (p < 0.0001, Figure 1B). Additional logistic regression analysis was conducted, controlling for confounding factors, to explore the extent of the increased odds associated with elevated Hcy (Table 2). After correcting for age, gender, years of education, and disease duration, each additional one μmol/L of Hcy was associated with a 115% increase in the odds of PD-MCI (OR = 2.15, 95% CI: 1.45∼3.21), and when Hcy levels were ≥ 10 μmol/L, the odds of PD-MCI increased by 471% (OR = 5.71, 95% CI: 1.62∼20.11).
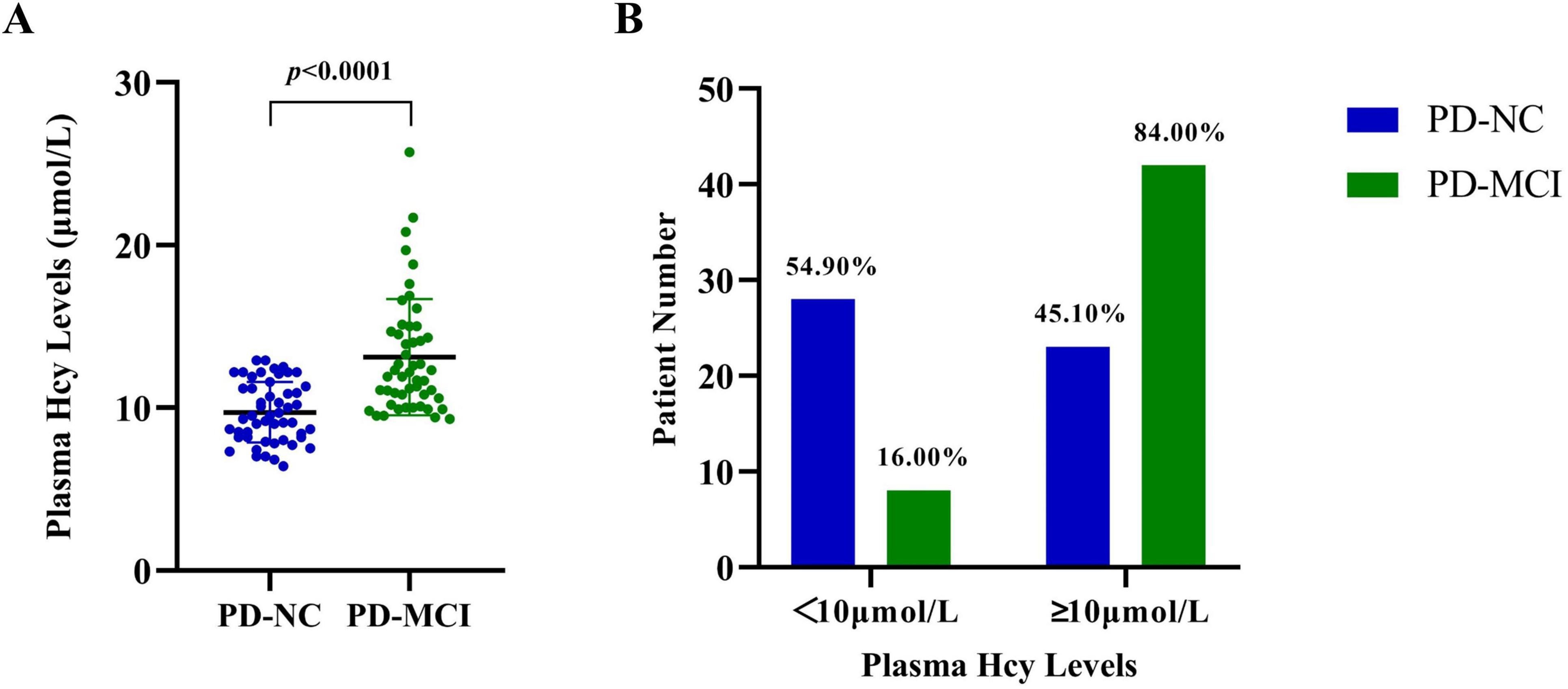
Figure 1. Plasma Hcy levels in PD-NC and PD-MCI patients. (A) The plasma Hcy levels of each patient in the PD-NC and PD-MCI groups. (B) The proportion of PD-NC and PD-MCI patients having Hcy levels higher or lower than 10 μmol/L. PD-NC, Parkinson’s disease with normal cognition; PD-MCI, Parkinson’s disease with mild cognitive impairment; Hcy, homocysteine.
Given the potential homocysteine toxicity resulting from levodopa treatment, correction for LEDD in logistic regression analysis revealed that each additional one μmol/L of Hcy remained associated with an 85% increase in the odds of PD-MCI (OR = 1.85, 95% CI: 1.21∼2.83). When Hcy levels were ≥ 10 μmol/L, the odds of PD-MCI also increased by 112% (OR = 2.12, 95% CI: 0.50∼8.97), though this increase did not reach statistical significance.
3.3 Associations between plasma homocysteine levels and cognitive domains or other non-motor symptoms
Further analysis using a generalized linear model identified significant associations between plasma Hcy levels and cognitive domains, as well as other non-motor symptom rating scales (Table 3). Increased Hcy levels were significantly associated with higher scores in BDI (β = 0.806, 95% CI: 0.359∼1.253, p = 0.0004), GDS (β = 0.440, 95% CI: 0.053∼0.826, p = 0.0257), PDQ-39 (β = 1.826, 95% CI: 0.296∼3.356, p = 0.0193), and ESS (β = 0.373, 95% CI: 0.133∼0.613, p = 0.0023), as well as longer completion time for the SCWT-C (β = 3.813, 95% CI: 1.662∼5.964, p = 0.0005) and TMT-A (β = 2.793, 95% CI: 0.348∼5.238, p = 0.0251).
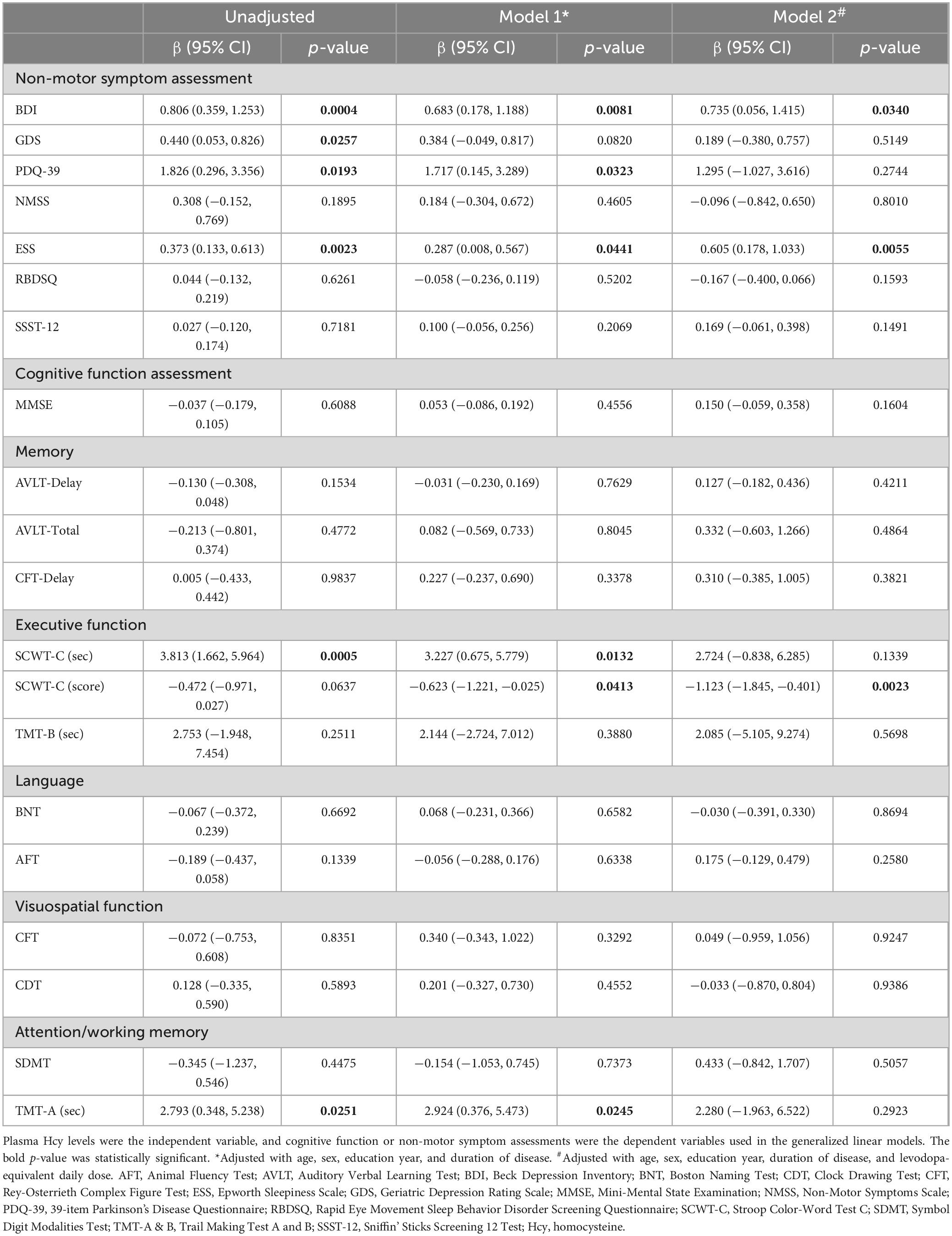
Table 3. The correlation of plasma Hcy levels with cognitive function or non-motor symptom assessments.
After correcting for age, gender, years of education, and disease duration, the associations with BDI, PDQ-39, ESS, SCWT-C completion time, and TMT-A completion time persisted. Hyperhomocysteinemia was negatively associated with the SCWT-C score (β = −0.623, 95% CI: −1.221∼−0.025, p = 0.0413). With additional correction for LEDD, only higher scores in BDI (β = 0.735, 95% CI: 0.056∼1.415, p = 0.0340) and ESS (β = 0.605, 95% CI: 0.178∼1.033, p = 0.0055), along with poorer performance on the SCWT-C (β = −1.123, 95% CI: −1.845∼−0.401, p = 0.0023), which reflected executive function, were significantly associated with elevated Hcy levels.
4 Discussion
In our study, we noted a substantial increase in plasma homocysteine levels among patients with PD-MCI in comparison to those with PD-NC. Interestingly, even within the normal range (< 15 μmol/L), a comparatively higher Hcy level (≥ 10 μmol/L) was linked to a higher odds of PD-MCI, a relationship that remained significant after adjusting for confounding factors. Our results additionally solidify a connection between plasma Hcy levels and the performance on certain cognitive assessments, particularly those evaluating executive functions in PD.
To date, there have been limited studies exploring the association between Hcy and PD-MCI, yielding disparate findings. Rodriguez-Oroz et al. (2009) investigated the role of Hcy among PD-NC, PD-MCI and PDD patients, reporting no significant differences in Hcy levels among these cognitive statuses and no correlation between plasma Hcy levels and performance on any neuropsychological tests. Similarly, Licking et al. (2017) also found no differences in plasma Hcy levels between PD-NC, PD-MCI and PDD patients, however, their results revealed a notable association between plasma Hcy and specific cognitive domains, including language, memory and executive function, as assessed by semantic verbal fluency task, Hopkins verbal learning-delayed test, and digital symbol test. It is worth noting that the participants in these studies were older (mean age of ∼70 years old) and had longer disease duration (mean duration of 10–14 years), which may differ from our cohort. In contrast, a study by Kobak Tur and Ari (2023) enrolled younger PD patients with shorter disease duration. They found higher Hcy levels in PD-MCI patients and revealed a significant association between Hcy levels and visuospatial/executive function using the Montreal Cognitive Assessment Scale (MoCA). These results align with our findings and suggest a specific association between executive function impairment in PD-MCI patients and elevated Hcy levels, as indicated by different neuropsychological tests. Furthermore, a literature review was conducted on the association between Hcy and impaired cognitive domains in PD, even if some studies did not specifically focus on PD-MCI (Table 4). In all studies with positive results, executive function has been found to be associated with plasma Hcy levels, indicating that executive function may be a characteristically affected cognitive area in PD patients with elevated Hcy levels.
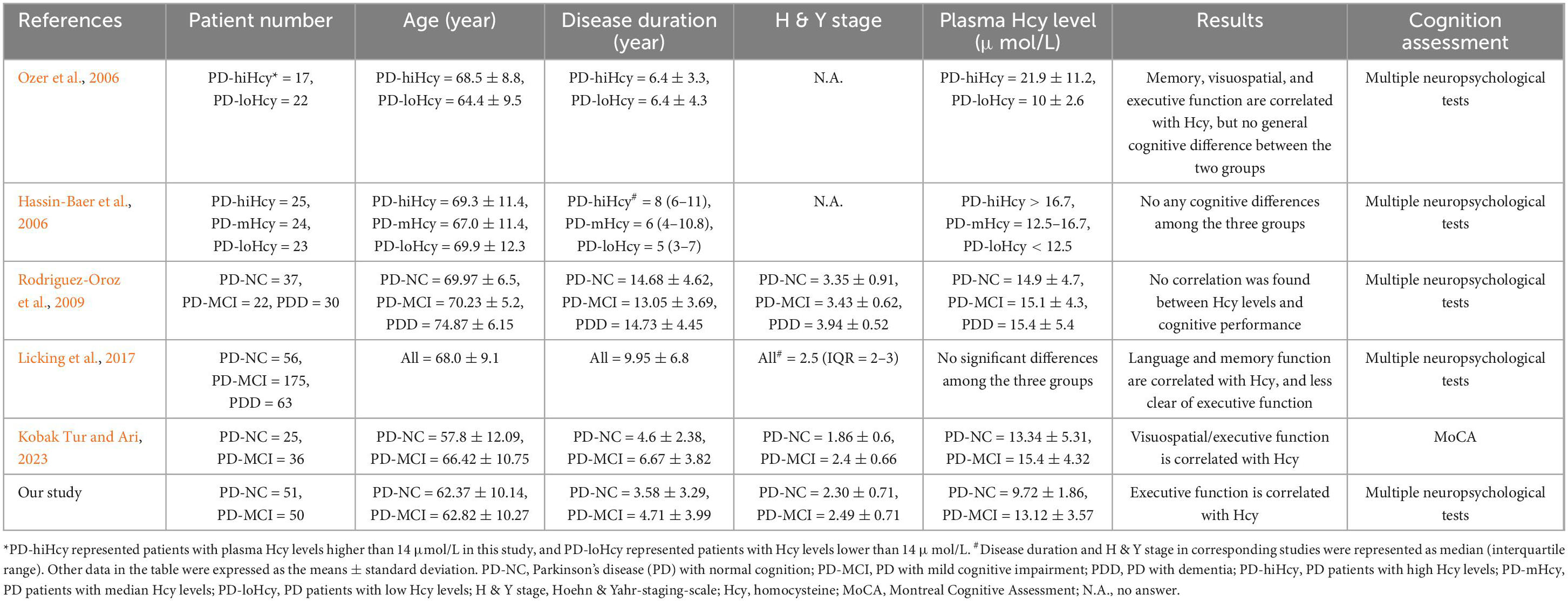
Table 4. Summary of the literature about the association between Hcy and impaired cognitive domains in PD.
Some imaging and metabolomics studies have also explored the role of Hcy in the brains of PD patients. Sampedro et al. (2022) identified a correlation between elevated Hcy levels and cognitive impairment in PD, along with thinning of the frontal cortex and microstructural damage. Additionally, some metabolomics analyses revealed accumulation of Hcy in the frontal cortex of PD subjects, emerging as a prominent characteristic associated with dementia (Kalecký et al., 2022; Kalecký and Bottiglieri, 2023). Given the critical role of the fontal cortex in executive function, the specific targeting of Hcy to the frontal cortex might support the hypothesis that executive function impairment is associated with elevated Hcy levels in PD patients.
As previously mentioned, the variability in age and disease duration among enrolled PD patients in different studies may partially explain the discrepancies in results regarding the association between Hcy and cognitive impairment. Cognitive decline in PD patients with advanced age and longer disease duration could be influenced by numerous factors beyond Hcy, such as aging, the progressive accumulation of Aβ and α-synuclein pathology in the cortex, and the development of motor symptoms (Aarsland et al., 2021). The effect of these confounding factors could gradually intensify and ultimately become the primary contributors to cognitive damage, potentially overshadowing the direct association between Hcy and cognitive impairment. Clinical trials of B-vitamin treatment for hyperhomocysteinemia and cognitive decline have also emphasized that the cognitive benefit of B-vitamin therapy might be expected primarily among patients who are not yet in an advanced disease stage (Ahlskog, 2023). Furthermore, it’s noteworthy that the threshold for defining hyperhomocysteinemia in other studies (> 14 or 15 μmol/L) was considerably higher than that used in our studies (> 10 μmol/L). Although some clinical trials of B-vitamin therapy found cognitive benefits only in subjects with high baseline Hcy levels (de Jager et al., 2012), the results of our study indicate that relatively higher levels of Hcy, even within the normal range, are strongly associated with cognitive impairment in PD patients.
Our study has revealed a significant correlation between elevated levels of Hcy and depression, as well as excessive daytime sleepiness. Depression stands out as the most prevalent emotional disorder among PD patients, with an approximate prevalence rate of 40% (Cummings, 1992). Some studies have found significantly higher incidences of mood symptoms, such as apathy, anxiety, and depression, in older adults with MCI compared to those with normal cognition (Geda et al., 2008). Conversely, mood symptoms have been linked to a heightened risk of subsequent MCI development (Geda et al., 2014). Additionally, previous research has suggested that depression might serve as an early indicator of cognitive impairment, sharing certain neuropathological features with MCI and dementia (Panza et al., 2010). This notion is substantiated by the common impairment of frontal-related cognitive function in PD-MCI and depressive patients (Lockwood et al., 2002; Kalbe et al., 2016). Studies have suggested that elevated Hcy level is an independent risk factor for depression in the general population (Moradi et al., 2021), as well as in PD patients (O’Suilleabhain et al., 2004). Our study found that elevated Hcy levels were associated not only with cognitive dysfunction in PD-MCI but also with increased score on the BDI, suggesting shared neuropathogenesis that may be partially explained by frontal-targeting impairment associated with elevated Hcy levels. Excessive daytime sleepiness has been reported to be related to cognitive decline in PD (Xiang et al., 2019). Kobak Tur and Ari (2023) also described an inverse correlation of ESS scores with MoCA in PD-MCI patients. Furthermore, a cross-sectional study of the general population in China has reported an association of excessive daytime sleepiness with hyperhomocysteinemia (Zhang et al., 2016). Although we described the significant paralleled relationship between excessive daytime sleepiness and elevated Hcy levels in PD patients, further studies are required to validate whether sleepiness is caused by the direct effect of Hcy or an incidental alteration associated with worsened cognition status.
To date, the causal relationship between elevated Hcy levels and cognitive decline in PD remains undetermined. One limitation of our study is that while we have adjusted for many potential confounders presented before, we cannot exclude the possible influence of unmeasured confounders, such as various comorbidities, including chronic inflammation and metabolic diseases (e.g., diabetes, hypertension, coronary artery disease, dyslipidemia), which could significantly be associated with Hcy levels. Another limitation of our study is its cross-sectional nature, which makes it difficult to establish evidence for a causal directionality between PD-MCI and hyperhomocysteinemia. Prospective follow-up of cognitive impairment progression in these patients would aid in clarifying the pathogenic effect of baseline Hcy levels on cognitive deterioration, particularly in early PD cases. A prospective cohort study by Sleeman et al. (2019) revealed that higher baseline Hcy concentrations could predict a decline in MoCA scores over a 54-month follow-up period in newly diagnosed PD patients. Additionally, in certain clinical trials investigating B-vitamin treatment for hyperhomocysteinemia, MRI documentation demonstrated a significantly reduced brain atrophy rate over 24 months, particularly in the top quartile of baseline Hcy levels (Smith et al., 2010). Furthermore, cognitive benefit from B-vitamin therapy were observed solely in participants with elevated baseline Hcy levels (de Jager et al., 2012). All these interconnecting results seemingly indicate a critical role of Hcy in the pathogenesis of cognitive impairment in the general population and PD patients. Although the specific molecular mechanism is still debated and confusing, some well-established mechanisms about the neurotoxicity of Hcy, such as inhibiting neurotransmitters, increasing oxidative stress and mitochondrial dysfunction, promoting inflammation, and inducing neuronal apoptosis, were revealed by dozens of basic research studies (Bhatia and Singh, 2015; Zhou, 2023). A recently published animal study reported that aging and L-methionine administration may increase brain Hcy levels in mice, leading to elevated homocysteinylation of α-synuclein, which facilitated its fibrillization, seeding capacity, and neurotoxicity, exacerbating α-synuclein pathology in the cortex of a mouse model of PD. In contrast, blocking α-synuclein homocysteinylation could ameliorate the toxicity of Hcy in vivo (Zhou et al., 2023). This result may provide an additional evidence of a causal relationship between Hcy and cognitive impairment from the perspective of α-synuclein pathology in the cortex.
5 Conclusion
Our research findings reveal a significant association between plasma Hcy levels and PD-MCI, particularly concerning executive dysfunction, even within a normal range of Hcy levels (< 15 μmol/L). These results suggest that Hcy might represent a potentially treatable risk factor and an interesting target for future intervention studies aimed at preventing cognitive impairment in PD. Routine screening of plasma Hcy levels and cognitive function in early PD patients might be beneficial in this regard.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Huashan Hospital, Fudan University, Shanghai, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YX: Formal analysis, Methodology, Writing – original draft. L-HG: Formal analysis, Methodology, Writing – original draft. X-NL: Formal analysis, Investigation, Methodology, Writing – original draft. Z-HX: Methodology, Software, Writing – review and editing. T-YH: Methodology, Writing – review and editing. X-YL: Validation, Writing – review and editing. Y-LT: Conceptualization, Funding acquisition, Supervision, Writing – review and editing, Investigation. JW: Conceptualization, Funding acquisition, Supervision, Writing – review and editing. Y-QL: Conceptualization, Investigation, Validation, Writing – original draft.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by research funding from National Health Commission of China (Pro20211231084249000238); National Nature Science Foundation of China (82171421, 92249302, and 82371261); and Shanghai Municipal Science and Technology Major Project (21S31902200).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarsland, D., Batzu, L., Halliday, G. M., Geurtsen, G. J., Ballard, C., Ray Chaudhuri, K., et al. (2021). Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers 7:47.
Ahlskog, J. E. (2023). Levodopa, homocysteine and Parkinson’s disease: What’s the problem? Parkinsonism Relat. Disord. 109:105357. doi: 10.1016/j.parkreldis.2023.105357
Baiano, C., Barone, P., Trojano, L., and Santangelo, G. (2020). Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: A meta-analysis. Mov. Disord. 35, 45–54. doi: 10.1002/mds.27902
Bhatia, P., and Singh, N. (2015). Homocysteine excess: Delineating the possible mechanism of neurotoxicity and depression. Fundam. Clin. Pharmacol. 29, 522–528. doi: 10.1111/fcp.12145
Białecka, M., Kurzawski, M., Roszmann, A., Robowski, P., Sitek, E. J., Honczarenko, K., et al. (2012). Association of COMT, MTHFR, and SLC19A1(RFC-1) polymorphisms with homocysteine blood levels and cognitive impairment in Parkinson’s disease. Pharmacogenet. Genomics 22, 716–724. doi: 10.1097/FPC.0b013e32835693f7
Camicioli, R. M., Bouchard, T. P., and Somerville, M. J. (2009). Homocysteine is not associated with global motor or cognitive measures in nondemented older Parkinson’s disease patients. Mov. Disord. 24, 176–182.
Cummings, J. L. (1992). Depression and Parkinson’s disease: A review. Am. J. Psychiatry 149, 443–454.
de Jager, C. A., Oulhaj, A., Jacoby, R., Refsum, H., and Smith, A. D. (2012). Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Geriatr. Psychiatry 27, 592–600. doi: 10.1002/gps.2758
Fan, Y., Liang, X., Han, L., Shen, Y., Shen, B., Chen, C., et al. (2020). Determinants of quality of life according to cognitive status in Parkinson’s disease. Front. Aging Neurosci. 12:269. doi: 10.3389/fnagi.2020.00269
Geda, Y. E., Roberts, R. O., Knopman, D. S., Petersen, R. C., Christianson, T. J., Pankratz, V. S., et al. (2008). Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: Population-based study. Arch. Gen. Psychiatry 65, 1193–1198.
Geda, Y. E., Roberts, R. O., Mielke, M. M., Knopman, D. S., Christianson, T. J., Pankratz, V. S., et al. (2014). Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: A population-based study. Am. J. Psychiatry 171, 572–581.
Goldman, J. G., Holden, S. K., Litvan, I., McKeith, I., Stebbins, G. T., and Taylor, J. P. (2018). Evolution of diagnostic criteria and assessments for Parkinson’s disease mild cognitive impairment. Mov. Disord. 33, 503–510.
Hassin-Baer, S., Cohen, O., Vakil, E., Sela, B. A., Nitsan, Z., Schwartz, R., et al. (2006). Plasma homocysteine levels and Parkinson disease: Disease progression, carotid intima-media thickness and neuropsychiatric complications. Clin. Neuropharmacol. 29, 305–311. doi: 10.1097/01.WNF.0000236763.16032.60
Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M., and Morris, J. G. (2008). The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 23, 837–844. doi: 10.1002/mds.21956
Jones, A. J., Kuijer, R. G., Livingston, L., Myall, D., Horne, K., MacAskill, M., et al. (2017). Caregiver burden is increased in Parkinson’s disease with mild cognitive impairment (PD-MCI). Transl. Neurodegener. 6:17.
Kalbe, E., Rehberg, S. P., Heber, I., Kronenbuerger, M., Schulz, J. B., Storch, A., et al. (2016). Subtypes of mild cognitive impairment in patients with Parkinson’s disease: Evidence from the LANDSCAPE study. J. Neurol. Neurosurg. Psychiatry 87, 1099–1105. doi: 10.1136/jnnp-2016-313838
Kalecký, K., and Bottiglieri, T. (2023). Targeted metabolomic analysis in Parkinson’s disease brain frontal cortex and putamen with relation to cognitive impairment. NPJ Parkinsons Dis. 9:84. doi: 10.1038/s41531-023-00531-y
Kalecký, K., Ashcraft, P., and Bottiglieri, T. (2022). One-carbon metabolism in Alzheimer’s disease and Parkinson’s disease brain tissue. Nutrients 14:599. doi: 10.3390/nu14030599
Kobak Tur, E., and Ari, B. C. (2023). Mild cognitive impairment in patients with Parkinson’s disease and the analysis of associated factors. Neurol. Res. 45, 1161–1168.
Langa, K. M., and Levine, D. A. (2014). The diagnosis and management of mild cognitive impairment: A clinical review. JAMA 312, 2551–2561.
Licking, N., Murchison, C., Cholerton, B., Zabetian, C. P., Hu, S. C., Montine, T. J., et al. (2017). Homocysteine and cognitive function in Parkinson’s disease. Parkinsonism Relat. Disord. 44, 1–5.
Lockwood, K. A., Alexopoulos, G. S., and van Gorp, W. G. (2002). Executive dysfunction in geriatric depression. Am. J. Psychiatry 159, 1119–1126.
Moradi, F., Lotfi, K., Armin, M., Clark, C. C. T., Askari, G., and Rouhani, M. H. (2021). The association between serum homocysteine and depression: A systematic review and meta-analysis of observational studies. Eur. J. Clin. Invest. 51:e13486. doi: 10.1111/eci.13486
O’Suilleabhain, P. E., Sung, V., Hernandez, C., Lacritz, L., Dewey, R. B. Jr., Bottiglieri, T., et al. (2004). Elevated plasma homocysteine level in patients with Parkinson disease: Motor, affective, and cognitive associations. Arch. Neurol. 61, 865–868. doi: 10.1001/archneur.61.6.865
Ozer, F., Meral, H., Hanoglu, L., Aydemir, T., Yilsen, M., Cetin, S., et al. (2006). Plasma homocysteine levels in patients treated with levodopa: Motor and cognitive associations. Neurol. Res. 28, 853–858.
Panza, F., Frisardi, V., Capurso, C., D’Introno, A., Colacicco, A. M., Imbimbo, B. P., et al. (2010). Late-life depression, mild cognitive impairment, and dementia: Possible continuum? Am. J. Geriatr. Psychiatry 18, 98–116. doi: 10.1097/JGP.0b013e3181b0fa13
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601.
Rodriguez-Oroz, M. C., Lage, P. M., Sanchez-Mut, J., Lamet, I., Pagonabarraga, J., Toledo, J. B., et al. (2009). Homocysteine and cognitive impairment in Parkinson’s disease: A biochemical, neuroimaging, and genetic study. Mov. Disord. 24, 1437–1444. doi: 10.1002/mds.22522
Sampedro, F., Martínez-Horta, S., Horta-Barba, A., Grothe, M. J., Labrador-Espinosa, M. A., Jesús, S., et al. (2022). Increased homocysteine levels correlate with cortical structural damage in Parkinson’s disease. J. Neurol. Sci. 434:120148. doi: 10.1016/j.jns.2022.120148
Schapira, A. H. V., Chaudhuri, K. R., and Jenner, P. (2017). Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 18, 435–450.
Sleeman, I., Lawson, R. A., Yarnall, A. J., Duncan, G. W., Johnston, F., Khoo, T. K., et al. (2019). Urate and homocysteine: Predicting motor and cognitive changes in newly diagnosed Parkinson’s disease. J. Parkinsons Dis. 9, 351–359. doi: 10.3233/JPD-181535
Smith, A. D., Smith, S. M., de Jager, C. A., Whitbread, P., Johnston, C., Agacinski, G., et al. (2010). Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS One 5:e12244. doi: 10.1371/journal.pone.0012244
Song, I. U., Kim, J. S., Park, I. S., Kim, Y. D., Cho, H. J., Chung, S. W., et al. (2013). Clinical significance of homocysteine (hcy) on dementia in Parkinson’s disease (PD). Arch. Gerontol. Geriatr. 57, 288–291.
Teunissen, C. E., van Boxtel, M. P., Jolles, J., de Vente, J., Vreeling, F., Verhey, F., et al. (2005). Homocysteine in relation to cognitive performance in pathological and non-pathological conditions. Clin. Chem. Lab. Med. 43, 1089–1095. doi: 10.1515/CCLM.2005.190
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., and Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653.
Xiang, Y. Q., Xu, Q., Sun, Q. Y., Wang, Z. Q., Tian, Y., Fang, L. J., et al. (2019). Clinical features and correlates of excessive daytime sleepiness in Parkinson’s disease. Front. Neurol. 10:121. doi: 10.3389/fneur.2019.00121
Zhang, M. Y., Katzman, R., Salmon, D., Jin, H., Cai, G. J., Wang, Z. Y., et al. (1990). The prevalence of dementia and Alzheimer’s disease in Shanghai, China: Impact of age, gender, and education. Ann. Neurol. 27, 428–437. doi: 10.1002/ana.410270412
Zhang, N., Chen, S., Chen, Y., Guo, X., Sun, G., Qian, H., et al. (2016). Daytime sleepiness is associated with hyperhomocysteinemia in rural area of China: A cross-sectional study. Eur. J. Intern. Med. 35, 73–77. doi: 10.1016/j.ejim.2016.05.029
Zhou, L., Guo, T., Meng, L., Zhang, X., Tian, Y., Dai, L., et al. (2023). N-homocysteinylation of α-synuclein promotes its aggregation and neurotoxicity. Aging Cell 22:e13745. doi: 10.1111/acel.13745
Zoccolella, S., dell’Aquila, C., Abruzzese, G., Antonini, A., Bonuccelli, U., Canesi, M., et al. (2009). Hyperhomocysteinemia in levodopa-treated patients with Parkinson’s disease dementia. Mov. Disord. 24, 1028–1033.
Zoccolella, S., Lamberti, S. V., Iliceto, G., Santamato, A., Lamberti, P., and Logroscino, G. (2010). Hyperhomocysteinemia in L-dopa treated patients with Parkinson’s disease: Potential implications in cognitive dysfunction and dementia? Curr. Med. Chem. 17, 3253–3261. doi: 10.2174/092986710792232012
Keywords: Parkinson’s disease, homocysteine, mild cognitive impairment, cognitive function, executive function
Citation: Xiao Y, Gan L-H, Liang X-N, Xu Z-H, Hu T-Y, Li X-Y, Tang Y-L, Wang J and Liu Y-Q (2024) Association of plasma homocysteine with cognitive impairment in patients with Parkinson’s disease. Front. Aging Neurosci. 16:1434943. doi: 10.3389/fnagi.2024.1434943
Received: 05 June 2024; Accepted: 22 November 2024;
Published: 09 December 2024.
Edited by:
Robert Petersen, Central Michigan University, United StatesReviewed by:
Daniel van Wamelen, King’s College London, United KingdomXiao Deng, National Neuroscience Institute (NNI), Singapore
Copyright © 2024 Xiao, Gan, Liang, Xu, Hu, Li, Tang, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-Qi Liu, bGl1eWlxaV9zaG1jQDE2My5jb20=; Jian Wang, d2FuZ2ppYW5faHNAZnVkYW4uZWR1LmNu; Yi-Lin Tang, dGFuZ3lpbGluQGZ1ZGFuLmVkdS5jbg==
†These authors have contributed equally to this work
 Yan Xiao†
Yan Xiao† Lin-Hua Gan
Lin-Hua Gan Xiao-Niu Liang
Xiao-Niu Liang Zhi-Heng Xu
Zhi-Heng Xu Tian-Yu Hu
Tian-Yu Hu Xiu-Yuan Li
Xiu-Yuan Li Yi-Lin Tang
Yi-Lin Tang Jian Wang
Jian Wang Yi-Qi Liu
Yi-Qi Liu