- 1Department of Neurology, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan
- 2Dementia Center, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan
- 3Graduate Institute of Humanities in Medicine, Taipei Medical University, Taipei, Taiwan
- 4Taipei Neuroscience Institute, Taipei Medical University, Taipei, Taiwan
- 5Ph.D. Program for Neural Regenerative Medicine, College of Medical Science and Technology, Taipei Medical University and National Health Research Institutes, Taipei, Taiwan
- 6Department of Neurology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 7Graduate Institute of Neural Regenerative Medicine, College of Medical Science and Technology, Taipei Medical University, Taipei, Taiwan
- 8Graduate Institution of Data Science, College of Management, Taipei Medical University, Taipei, Taiwan
- 9School of Health Care Administration, College of Management, Taipei Medical University, Taipei, Taiwan
- 10Health Data Analytics and Statistics Center, Office of Data Science, Taipei Medical University, Taipei, Taiwan
- 11Institute of Population Health Sciences, National Health Research Institutes, Zhunan, Taiwan
- 12Master Program in Applied Epidemiology, College of Public Health, Taipei Medical University, Taipei, Taiwan
- 13School of Public Health, College of Public Health, Taipei Medical University, Taipei, Taiwan
- 14Ph.D. Program in Biotechnology Research and Development, College of Pharmacy, Taipei Medical University, Taipei, Taiwan
Introduction: Post-stroke cognitive impairment (PSCI) cannot be neglected because it drastically influences the daily life of patients and their families. However, there are no studies exploring the association between preclinical blood biomarkers of neurodegeneration including plasma amyloid-β (Aβ), tau, and brain-derived neurotrophic factor (BDNF) together with the risk of PSCI. This longitudinal study was to investigate whether these blood biomarkers with imaging markers of cerebral small vessel disease can improve the prediction for PSCI. In addition, we also explored the association between blood biomarkers with the trajectories of PSCI.
Methods: Adult patients with first-ever acute ischemic stroke were recruited, and the cognitive and functional abilities of these patients were evaluated. Furthermore, blood biomarkers of neurodegeneration including plasma Aβ-40, Aβ-42, total tau, phosphorylated tau 181 (p-tau181), and BDNF levels and image markers of cerebral small vessel disease were measured. Each patient was followed up at 3 and 12 months at the outpatient department.
Results: Of 136 patients, 40 and 50 patients developed PSCI at 3 and 12 months after stroke, respectively. In functional trajectories, 27 patients did not have PSCI at 3 months but did at 12 months. By contrast, the PSCI status of 17 patients at 3 months was reversed at 12 months. Patients with high-acute plasma p-tau181 had a significantly lower PSCI risk at 3 months (odds ratio [OR] = 0.62, 95% CI = 0.40–0.94, p = 0.0243) and 12 months (OR = 0.69, 95% CI = 0.47–0.99, p = 0.0443) after adjustment for covariates and image biomarkers. Discrimination and reclassification statistics indicated that the p-tau181 level can improve discrimination ability for PSCI at 3 and 12 months, respectively. In addition, the plasma p-tau181 level was the highest in subjects without PSCI followed by those with delayed-onset PSCI and early-onset PSCI with reversal, whereas the lowest plasma p-tau181 level was found among those with persistent PSCI, showing a significant trend test (p = 0.0081).
Conclusion: Plasma p-tau181 is a potential biomarker for predicting early- and delayed-onset PSCI. Future studies should incorporate plasma p-tau181 as an indicator for timely cognitive intervention in the follow-up of patients with stroke.
Introduction
Cognition decline after stroke is not rare. The clinical course of post-stroke cognitive impairment (PSCI) is not a unitary syndrome but varies from individual to individual (Patel et al., 2002). Cognitive impairment assessment is often performed at 3–6 months after acute stroke to provide sufficient time for delirium resolution and neurological stability. Notably, stroke patients free of early-onset PSCI (3–6 months after stroke) are still at risk of delayed-onset PSCI (>6 months after stroke), which suggests an underlying pathological process beyond 3 months after stroke (Wagle et al., 2011). Many risk factors for PSCI have been proposed based on observational studies, including age, sex, educational attainment, stroke severity, stroke histories, and cardiovascular risk factors—particularly diabetes mellitus and hypertension (Sun et al., 2014). Brain image risk factors include white matter hyperintensities and gray matter and hippocampal volumes loss (Dichgans and Leys, 2017; Mok et al., 2017; Ding et al., 2019). However, there is still a lack of precise blood biomarkers for predicting PSCI.
Accumulation of amyloid-beta (Aβ) peptides and phosphorylated tau (p-tau) in the brain are both key pathological features of the Alzheimer’s disease (Glenner and Wong, 1984; Grundke-Iqbal et al., 1986; Jack et al., 2018; Drummond et al., 2020). Several previous studies implicated plasma Aβ40 and Aβ42 levels as associated with cognitive decline among older adults and stroke patients (Tang et al., 2018; Giudici et al., 2020). Our previous work also showed plasma Aβ42 and tau levels at 3 months were lower in the patients with PSCI at 1 year than in those without PSCI (Chi et al., 2019).
Some cohorts found that plasma tau phosphorylated at threonine 181 (p-tau181) was associated with cognitive decline (Karikari et al., 2020). In addition, brain-derived neurotrophic factor (BDNF) is an important neurotrophin in the adult brain, which can help the brain to repair (Liu et al., 2020). A previous study further found that serum BDNF levels are decreased in the acute phase of stroke, and lower circulating concentrations of BDNF protein are associated with poor long-term functional outcomes (Stanne et al., 2016).
Since these key peptides and proteins play important roles in cognitive performance, and there are few studies to examine the plasma levels of Aβ42, tau, BDNF, and p-tau181 in patients after stroke through longitudinal follow-up, the purpose of this study was to investigate whether these blood with imaging markers can improve the prediction for PSCI. In addition, the association between blood biomarkers with the trajectories of PSCI was also examined.
Materials and Methods
Study Participants
Patients aged ≥ 20 years who were admitted to Shuang-Ho Hospital, Taipei Medical University within 7 days of acute ischemic stroke were screened for enrollment eligibility between 2015 and 2018. Patients with known premorbid cognitive impairment, mood disorders, or neurodegenerative diseases that have impaired daily activities were excluded. In order to focus on the cognitive trajectory after stroke, we excluded the major cognitive impairment of stroke per se: large infarcts that cause immediate consciousness impairment; strategic infarcts involving the hippocampus or medial frontal cortex; and severe language or physical disabilities that hinder neuropsychological testing. Each patient was evaluated in the hospital within 7 days of the stroke and followed up at the outpatient department at 3 and 12 months post-stroke. The Institutional Review Board of the Taipei Medical University approved the study. Written informed consent was obtained from all patients or their legal guardians.
Data Collection
Brain magnetic resonance images were obtained once at admission, including T1- and T2-weighted images, T2 fluid-attenuated inversion recovery (T2 FLAIR) images, diffusion-weighted images (DWI), apparent diffusion coefficient (ADC) maps, T2 star-weighted angiography (SWAN), and time-of-flight magnetic resonance angiograms. Acute ischemic brain infarction was confirmed with hyperintensity on DWI with corresponding ADC maps. Visual ratings of white matter hyperintensities (WMHs) were performed by an investigator who was blinded to the clinical details by applying the Fazekas rating scale (Fazekas et al., 1987). Microbleeds were rated as round- or oval-shaped dark blooming signals (2–10 mm in diameter) using the Microbleeds Anatomical Rating Scale (MARS) on the SWAN image and were classified into deep, lobar, and infratentorial categories (Gregoire et al., 2009). Stroke severity was assessed using the National Institute of Health Stroke Scale (NIHSS) at admission. NIHSS is a 15-item impairment scale, each of which scores a specific ability between 0 and 4. Total Score ranges from 0 to 42. The stroke etiological subtype was classified according to The Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification: (1) large-artery atherosclerosis, (2) cardioembolism, (3) small-vessel occlusion, (4) stroke of other determined etiology, and (5) stroke of undetermined etiology (Adams et al., 1999) by an experienced neurologist who was unaware of the cognition outcomes of patients. A total of two neuropsychologists blinded to patients’ plasma biomarker data conducted cognitive function assessments using the Taiwanese version of the Montreal Cognitive Assessment (MoCA) screening instrument (Tsai et al., 2012) and the Clinical Dementia Rating (CDR) global score, Sum of Boxes (CDR-SB; range: 0–18). The MoCA showed a low ceiling effect with high sensitivity and specificity when used for assessing cognitive impairment after stroke (Pendlebury et al., 2012). The CDR–SB comprises six cognitive and functional domains (memory, orientation, judgment, and problem-solving, community affairs, home and hobbies, and personal care), which yield additional information, particularly regarding mild impairment (Lynch et al., 2006). In this study, we defined PSCI as a CDR-SB > 0 when the patient presented with functional impairment after stroke in either one or more of the six domains. Given the diverse clinical presentation of PSCI, in addition to the memory domain, other cognitive functions must also be assessed (Skrobot et al., 2018). We further classified patients based on the 3 and 12-month CDR-SB assessments to understand the trajectory of PSCI after stroke. Persistent non-PSCI is defined as 3 and 12-month CDR-SB = 0 (n = 69), delayed-onset PSCI is CDR-SB = 0 at 3 months while CDR-SB ≥ 0.5 at 12 months (n = 27), early PSCI with reversal is 3-month CDR-SB ≥ 0.5 and 12-month CDR-SB = 0 (n = 17), and persistent PSCI is CDR-SB ≥ 0.5 at 3 and 12 months (n = 23).
Measurement of Plasma Biomarkers
A total of two 10 ml non-fasting venous blood was collected within 7 days of stroke onset. The blood samples were centrifuged at 1,500 × g for 15 min at room temperature and the plasma in the EDTA tube was transferred and aliquoted into 0.5-ml microcentrifuge tubes stored at −80°C until biomarker assays. Plasma Aβ40, Aβ42, total tau, and p-tau181 were analyzed using immunomagnetic reduction (IMR) assays manufactured by MagQu Co. Ltd. (New Protein Analysis Taipei City, Taiwan). Technical details of IMR assays have been described in previous studies (Tang et al., 2018; Yang et al., 2018; Chi et al., 2019). The BDNF was quantified through enzyme-linked immunosorbent assay by using cytokine detection kits (DY248; R&D Systems, Minneapolis, MN, United States) according to the manufacturer’s protocol. Absorbance at 450 nm was measured with a SpectraMax microplate reader (Molecular Devices, San Jose, CA, United States). All the samples were analyzed in duplicate.
Statistical Analysis
Continuous variables are presented as mean and SD, and data with non-normal distribution are expressed as medians with interquartile ranges. Categorical variables are presented in terms of the frequency with percentage. Univariate logistic regression was used to estimate the odds ratios (ORs) of PSCI at 3 and 12 months based on clinical characteristics and laboratory data. They identified important covariates with borderline significance in the univariate analysis were then verified in the multivariate logistic model by using automatic forward selection methods. Receiver operating characteristic (ROC) analysis was conducted to estimate the performance of plasma biomarkers combined with important covariates for differentiating PSCI risk at 3 and 12 months. In addition, the net reclassification index (NRI) and integrated discrimination improvement (IDI) were computed to evaluate the incremental prognostic value of plasma biomarkers beyond conventional risk factors as well as image biomarkers. All statistical analyses were performed using SAS (version 9.4, Cary, NC, United States). A two-tailed p < 0.05 was considered statistically significant.
Results
All 173 participants completed initial clinical and neuropsychological examinations as well as plasma biomarker assays and a brain MRI scan within 7 days after stroke. Each individual was given a standardized treatment scheme during hospitalization. No patient died during the follow-up time of 1 year. Attrition was due to loss to follow-up in 37 patients at 3 months. No significant difference between the baseline profiles of participants involved in this study and those lost in follow-up was found. Data from 136 patients were processed in the final analysis. At 3 months after stroke, 40 patients met the criteria for PSCI according to CDR-SB > 0, whereas at 12 months after stroke, 50 patients had PSCI (Figure 1).
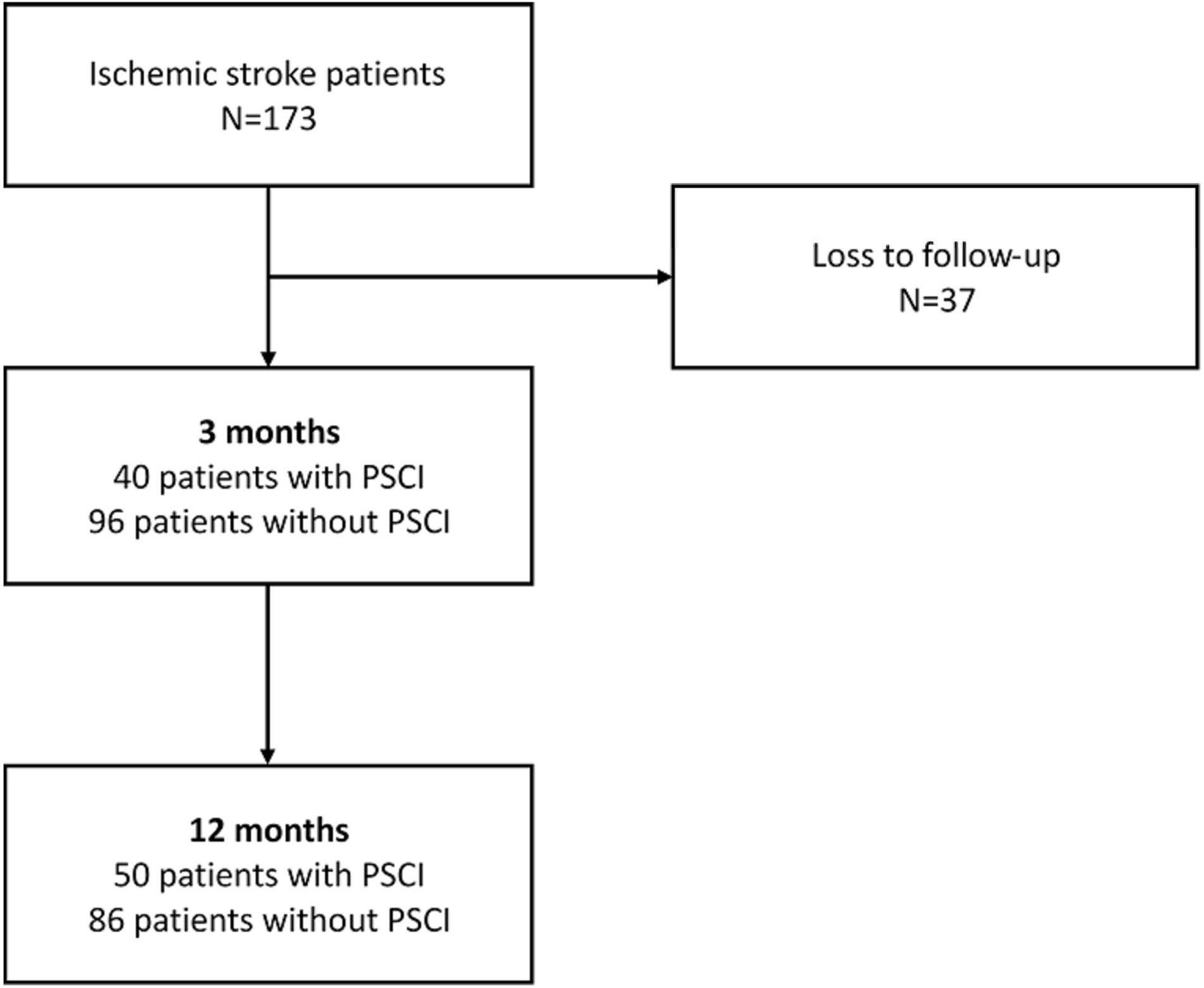
Figure 1. Flowchart of patient enrollment and cognitive function changes during the 12 months’ follow-up.
Characteristics of Study Subjects
Demographic data, brain MRI visual rating scores, and plasma biomarkers are summarized in Table 1. Dividing into two groups according to whether positive of PSCI at 3 months after stroke, the mean ages of patients with and without PSCI were 61.43 ± 12.57 and 57.73 ± 12.66 years, respectively. A total of 40% of patients with PSCI have an education level of >9 years, which is significantly lower than that of patients with non-PSCI. A remarkably high frequency of hypertension was observed among patients with PSCI compared with those without PSCI. Most patients had mild stroke severity, and median NIHSS scores within 7 days for patients with and without PSCI were 4 and 3, respectively. Periventricular white matter Fazeka scale was significantly different between the two groups. MoCA scores at 3 and 12 months were significantly lower in patients with PSCI than in those without PSCI. Furthermore, the plasma p-tau181 level in patients with PSCI (3.19 ± 1.77 pg/ml) was significantly lower than in those without PSCI (4.16 ± 2.18 pg/ml).
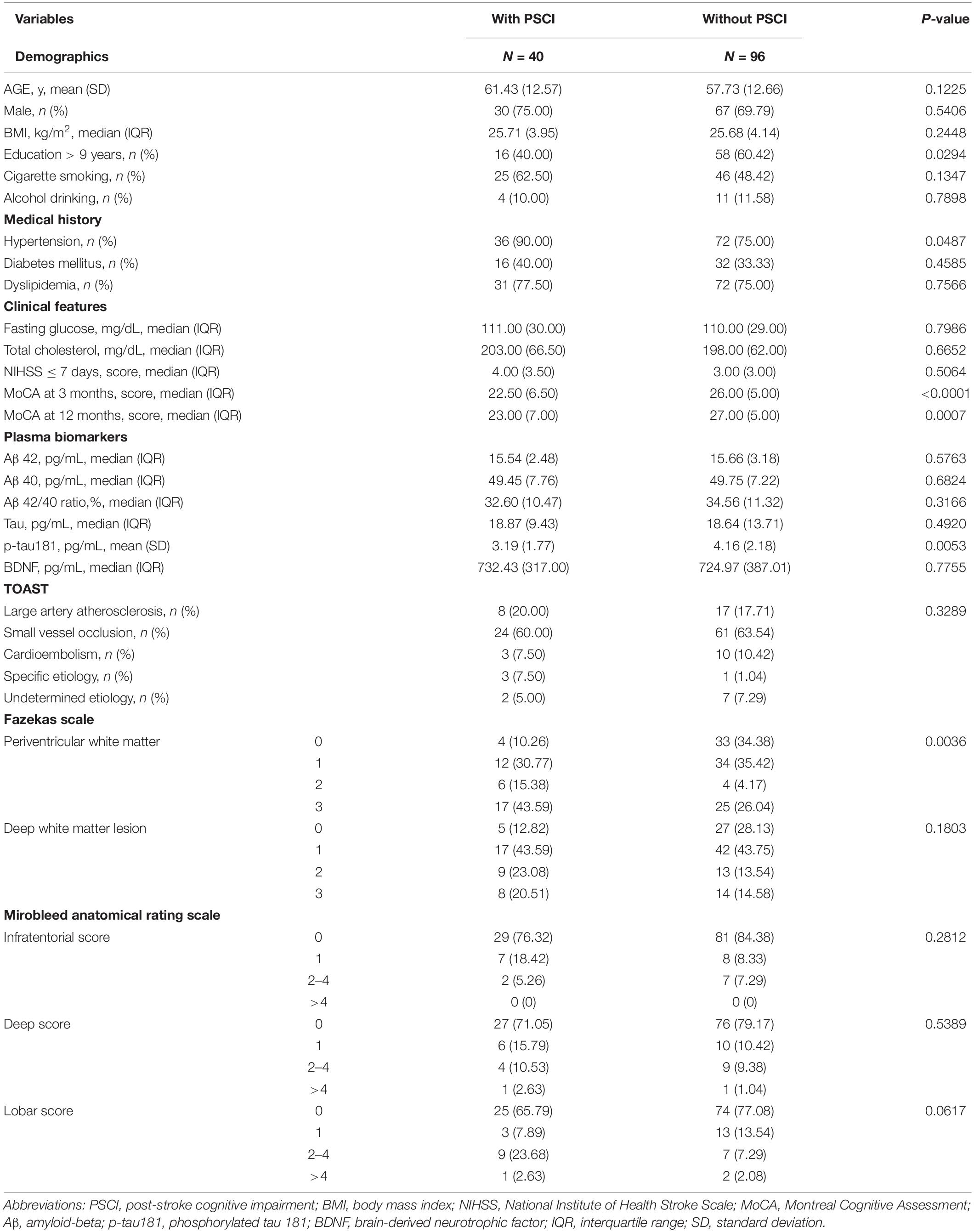
Table 1. Basic characteristics of study subjects with and without PSCI defined at 3 months after stroke.
Univariate and Multivariate Regression Analyses of Post-stroke Cognitive Impairment Risk
Table 2 presents the results of univariate and multivariate logistic regression analyses for patients with and without PSCI at both 3 and 12 months, respectively. For PSCI at 3 months, education level, plasma p-tau181 level, periventricular white matter Fazekas scale, and MARS lobar score were significant factors in the univariate model. These identified important covariates together with hypertension were then verified in the multivariate analysis. After forward selection, education level, hypertension, and plasma p-tau181 level were significantly independent factors of PSCI, showing the patients having an increased p-tau181 level had a significantly lower risk of PSCI at 3 months (OR = 0.62, 95% CI = 0.40–0.94, p = 0.0243). Similar findings were found when the patients were followed up for 12 months. Age, periventricular white matter Fazeka scale were significant factors in the univariate model. After forward selection, the plasma p-tau181 level was a significant independent factor of PSCI. For patients with increment of p-tau181 level had a 0.69-fold risk of PSCI at 12 months (95% CI = 0.47–0.99, p = 0.0443). However, plasma tau, Aβ42, Aβ40, and BDNF were not predictors of PSCI at 3 or 12 months.
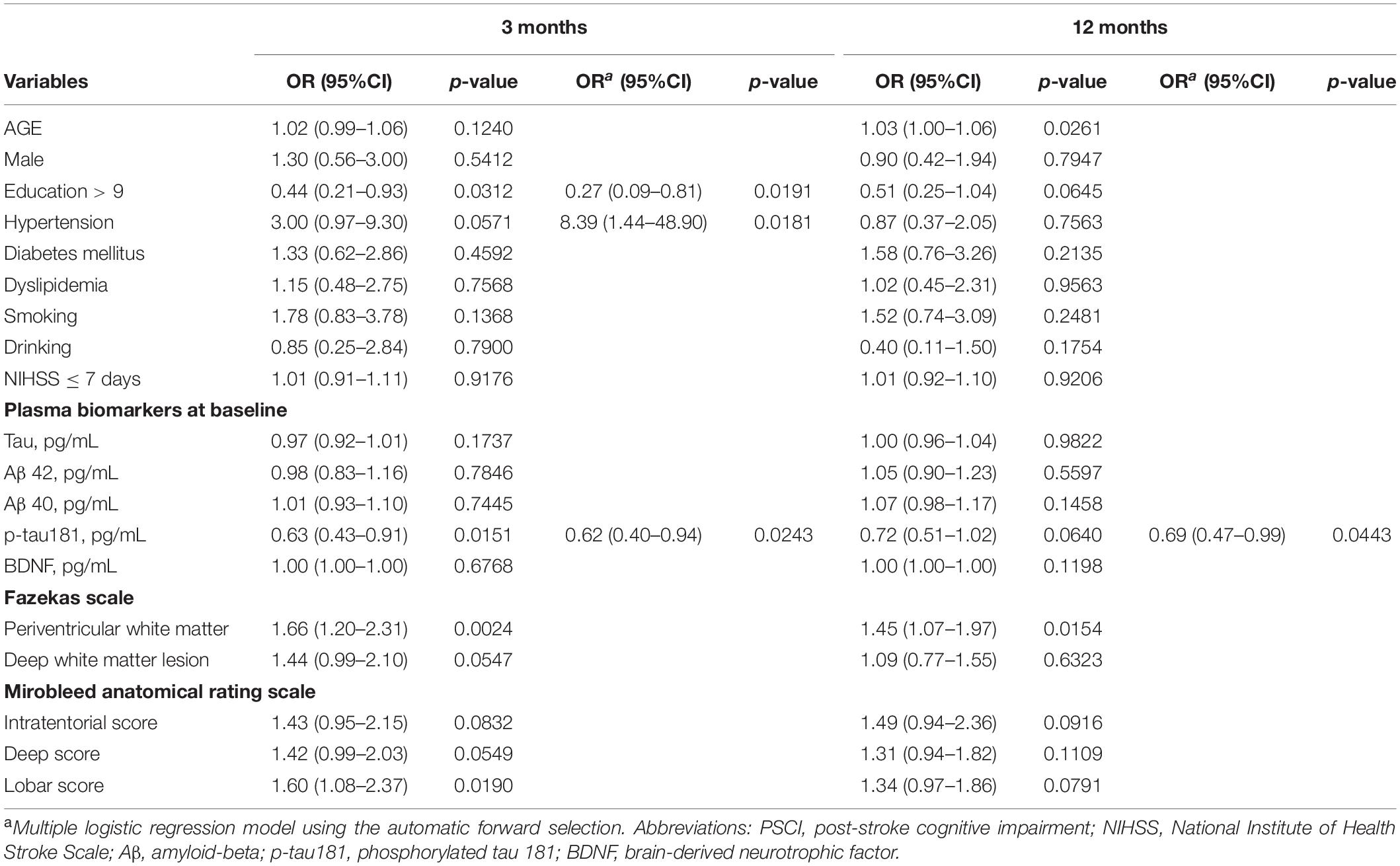
Table 2. Univariate and multivariate logistic regression analyses for patients with PSCI and without PSCI at 3 and 12 months, respectively.
Predictive Accuracy of p-tau181 for Assessing Post-stroke Cognitive Impairment
To examine the effect of p-tau181 on PSCI at 3 and 12 months, respectively, in addition to conventional risk factors, discrimination and reclassification statistics were calculated. Figure 2A illustrates the area under the curve (AUC) of the model at 3 months based only on conventional risk factors was 0.7632 (95% CI = 0.6520–0.8744), but it slightly increased to 0.7655 (95%CI = 0.6549–0.8761) when image biomarkers were added. After integrating plasma p-tau181 level, the performance has been greatly improved, which achieved good discrimination ability (AUC = 0.8067; 95% CI = 0.7066–0.9067). Similar findings were also found when analyzing the predictive effects at 12 months (Figure 2B). According to the NRI and IDI indexes, adding the p-tau181 level to the model containing conventional risk factors and image biomarkers significantly improves the measure of reclassification and discrimination for PSCI at 3 and 12 months (Table 3).
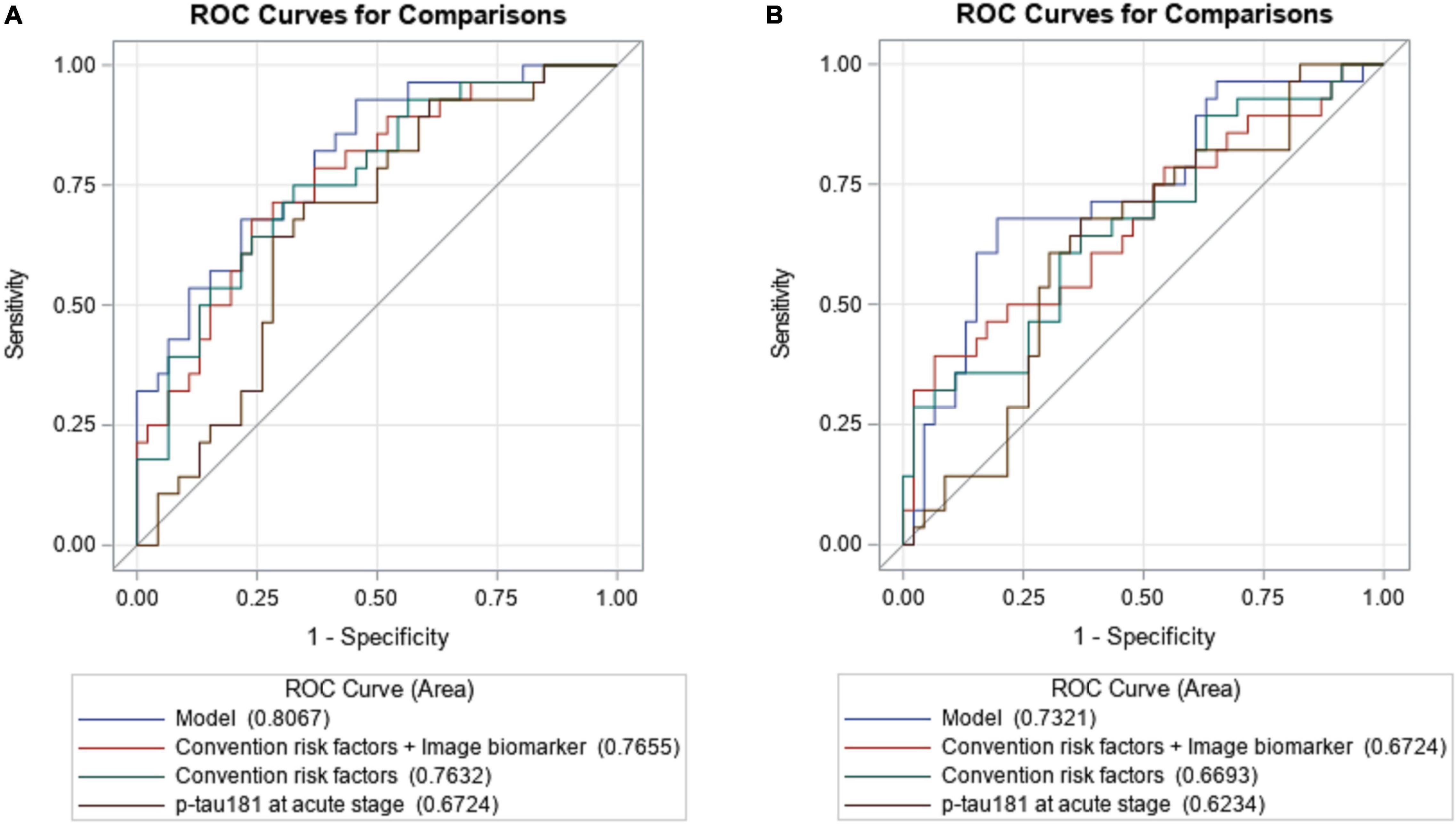
Figure 2. The area under the receiver operating characteristic of the p-tau181 predicting PSCI at 3 (A) and 12 months (B), respectively, when compared to conventional risk factors with image markers.
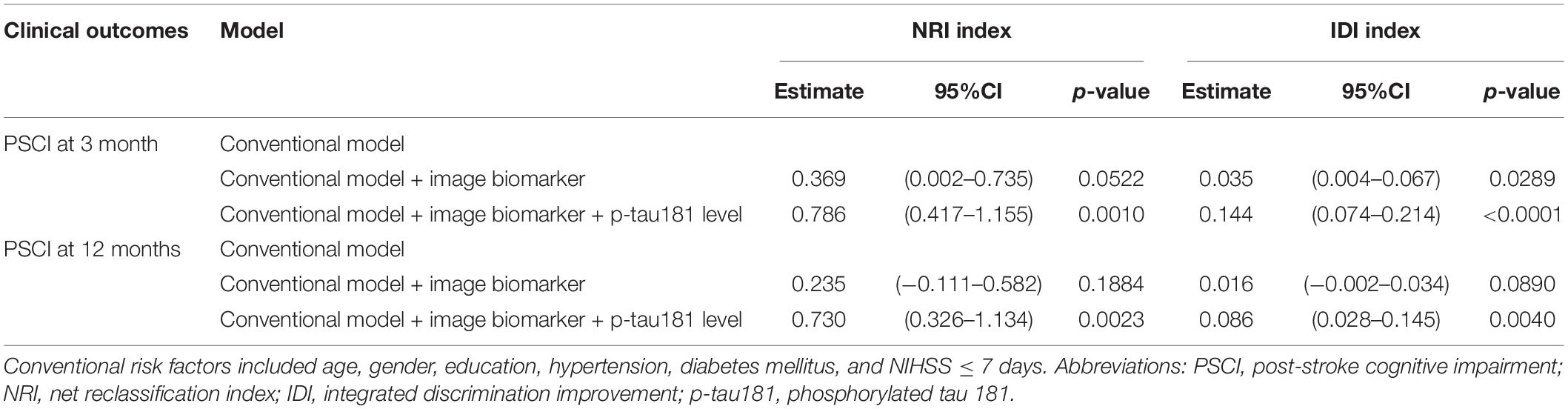
Table 3. Discrimination and reclassification statistics of p-tau181 level for patients with PSCI at 3 and 12 months, respectively.
Association Between Plasma Biomarkers and Different Persistent Cognitive Impairment Status
Plasma biomarkers were analyzed according to the trajectory of cognitive impairment status defined by the CDR-SB at 3 and 12 months. Table 4 presents the levels of plasma tau, Aβ42, Aβ40, Aβ42/40 ratio, p-tau181, and BDNF among the four subgroups. The results indicated that only plasma p-tau181 levels differed among the four groups, with the highest level in the persistent non-PSCI group (4.40 ± 1.77 pg/ml) followed by the delayed-onset PSCI (3.65 ± 1.41 pg/ml), early PSCI with reversal (3.36 ± 1.38 pg/ml) groups, and the lowest level was in the persistent PSCI group (3.12 ± 0.78 pg/ml), showing a significant trend test (p = 0.0081).
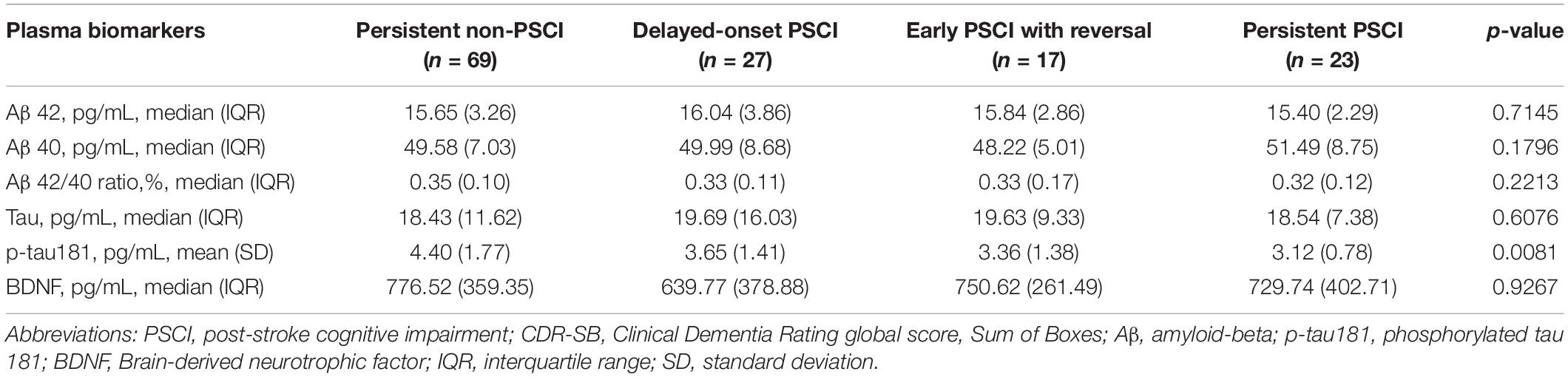
Table 4. Association between plasma biomarkers and different persistent cognitive impairment statuses according to CDR-SB at 3 and 12 months.
Discussion
This study investigated 136 patients with acute ischemic stroke and followed them up for consequential cognitive function trajectory for 1 year. The results demonstrated that a high plasma p-tau181 level in the acute post-stroke stage is related to a low PSCI risk at 3 (OR = 0.62, 95% CI = 0.40–0.94, p = 0.0243) and 12 (OR = 0.69, 95% CI = 0.47–0.99, p = 0.0443) months. Integrating p-tau181 to the model containing conventional risk factors (age, education, hypertension, diabetes mellitus, and NIHSS score at ≤7 days) and image biomarker significantly improves prediction.
Cognitive improvement after stroke often occurs within 6 months, and early recognition of PSCI is necessary for assessing the need for rehabilitation (Turunen et al., 2018). In the case of cerebral ischemia, hyperphosphorylated tau protein may play a protective role by promoting β-catenin and other proteins to inhibit cell apoptosis, suggesting that neurons survive apoptotic attacks and achieve self-repair (Wang et al., 2010). Phosphorylation of tau results in reduced binding to microtubules and potentially enhances plasticity, as observed during brain development, which is also important in neural repair after stroke (Alonso et al., 2001, 2006; Wang et al., 2007; Morris et al., 2011). In Alzheimer’s disease (AD), plasma p-tau181 is a prognostic and confirmatory biomarker (Bateman et al., 2020). Hyperphosphorylated tau protein forms paired helical filaments and progressively aggregates to form the main component of neurofibrillary tangles in AD pathology (Pevalova et al., 2006). However, unlike in AD pathology, neurofibrillary tangles are rarely seen in the brain after minor stroke. Exceptional case series of NFTs in the ipsilateral basal nucleus of Meynert (BNM) associated with a massive cerebral infarct in the MCA territory or a putaminal hemorrhage, speculated to form within the 5–10 years after stroke onset (Hatsuta et al., 2019). Studies of cerebrospinal fluid (CSF) samples from patients with acute stroke showed that CSF p-tau did not increase, whereas CSF total tau increased and returned to normal levels at 3–5 months after stroke, suggesting different pathogenic processes from AD (Hesse et al., 2001; Hjalmarsson et al., 2014; Hagberg et al., 2020). Therefore, the diagnostic criteria for vascular cognitive disorders from the International Society of Vascular Behavioral and Cognitive Disorders statement suggest excluding CSF p-tau when diagnosing vascular cognitive impairment in research (Sachdev et al., 2014), whereas a persistent increase in CSF p-tau is considered a defining biomarker of AD in the National Institute on Aging—Alzheimer’s Association Research Framework (Albert et al., 2011). In addition, plasma p-tau181 predicted PSCI in a dose-dependent manner in our longitudinal cohort study, and the p-tau181 level was the highest in the persistent non-PSCI group and the lowest in the persistent PSCI group. A protective role for tau phosphorylation at threonine 181 could facilitate its binding to exosomes and the release of excess tau (Avila et al., 2012). Further studies are warranted to understand the mechanism underlying this finding.
Previous studies have revealed that plasma Aβ42/40 ratios are surrogate biomarkers of cortical Aβ deposition (Fandos et al., 2017). High-plasma concentrations of Aβ40, especially when combined with low concentrations of Aβ42, indicate an increased dementia risk (van Oijen et al., 2006). A previous publication by our group using the same cohort but few participants showed Aβ42 and tau levels at 3 months were lower in the patients with PSCI at 1 year than in those without PSCI, which may reveal AD pathology one mechanism of PSCI development after 3 months of stroke and decreased levels of plasma tau could be explained by its association with the decreased plasma Aβ42 levels (Chi et al., 2019). In this study, Aβ42, Aβ40, and Aβ42/40 ratios were not different between patients with and without PSCI, which suggests the involvement of additional processes other than amyloid pathology. In addition, BDNF was found to be an indicator of long-term functional outcomes after ischemic stroke, although the additional predictive value of BNDF was modest according to clinical data (Stanne et al., 2016). Our data showed that there was no significant difference in circulating BDNF levels regardless of whether the patient had PSCI or not, which may be due to its limited impact on cognitive outcomes. In this study, a low-education level, hypertension, and pre-existing periventricular white matter disease were also the major risk factors for PSCI at 3 months. This finding is consistent with those of previous studies (Zhou et al., 2005; Sun et al., 2014; Ding et al., 2019).
Clinical Dementia Rating global score, Sum of Boxes was adopted to define PSCI in our study. Given the diverse clinical presentation of PSCI, not only memory but also other cognitive domains should be evaluated (Skrobot et al., 2018). The global CDR is weighted more on memory dysfunction, whereas CDR-SB is weighted equally for all domains (Wyman-Chick and Scott, 2015). In addition, CDR-SB has been considered an effective and reliable assessment method, which combines two sets of questions, one set is for the insider and the other is for the subject. This implies the use of CDR-SB to define the PSCI has a clinically significant impact on the follow-up after stroke. In our study, none of the patients had dementia before the stroke. However, the prevalence of PSCI at 3 and 12 months after stroke was 29.4 and 36.8%, respectively, during the longitudinal follow-up. The reported prevalence varied among previous studies depending on divergent estimates of PSCI according to the population under study and the methods of defining PSCI, suggesting a need for diagnosis criteria consensus (del Ser et al., 2005; Abzhandadze et al., 2019).
Notably, in our cohort, 27 patients without PSCI at 3 months developed PSCI at 12 months after stroke (late PSCI: 19.9%), and 17 patients reversed from PSCI at 3 months to non-PSCI at 12 months (reversal: 42.5%). Our findings further revealed that a dose-dependent trend of plasma p-tau181 level existed among patients with various persistent cognitive impairment statuses, with the highest level in the non-PSCI group followed by the early PSCI with a reversal and delayed-onset PSCI groups; the lowest level was in the persistent PSCI group, which implicated the protective effect on longitudinal cognitive function after a minor ischemic stroke.
This study has several limitations. First, there was no formal test conducted for measuring baseline cognitive function before stroke in our participants. However, patients with known cognitive impairment or neurodegeneration that impaired daily activities before stroke were excluded based on their medical histories at the screening phase. In addition, this study adopted the CDR-SB to define PSCI in order to distinguish functional changes after stroke since we relied on informant-based evidence rather than performance-based tests. Second, the severity of stroke in the patients in this study is relatively small and mainly involves small vessel diseases which might influence the generalizability of the findings to large brain infarction or intracranial hemorrhage. Future studies will be needed to elucidate the natural course of other types of brain insults. Third, in our study, no further AD diagnosis was performed; however, previous findings indicated that after ischemia with reperfusion in the brain, secondary neurodegeneration of AD type may occur (Pluta, 2000). Further longitudinal studies regarding biomarkers are needed to distinguish between PSCI and AD processes.
In conclusion, PSCI is not uncommon in the population with minor stroke. Using plasma p-tau181 as a surrogate biomarker for predicting early- and delayed-onset PSCI is helpful in interventional studies and clinical follow-up.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Taipei Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
L-KH wrote the manuscript with support from Y-CH. Y-CH, S-PC, and L-NC verified the analytical methods. C-JH and H-YC conceived the study and were in charge of overall direction and planning. Y-CL, S-PC, and L-NC aided in interpreting the results and worked on the manuscript. All authors discussed the results and contributed to the final manuscript.
Funding
This study was supported by the Academia Sinica Stroke Biosignature Project (BM10701010021), Taiwan Ministry of Science and Technology (MOST) Clinical Trial Consortium for Stroke (MOST 107-2321-B-039-004, 106-2314-B-038-001, and 107-2314-B-038-050), and National Health Research Institutes (NHRI-EX111-11132HT).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This manuscript was edited by Wallace Academic Editing.
References
Abzhandadze, T., Rafsten, L., Lundgren Nilsson, ÅC., Palstam, A., and Sunnerhagen, K. S. (2019). Very early MoCA can predict functional dependence at 3 months after stroke: a longitudinal, cohort study. Front. Neurol. 10:1051. doi: 10.3389/fneur.2019.01051
Adams, H. P., Davis, P., Leira, E., Chang, K.-C., Bendixen, B., Clarke, W., et al. (1999). Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 53, 126–131. doi: 10.1212/wnl.53.1.126
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279. doi: 10.1016/j.jalz.2011.03.008
Alonso, A. C., Li, B., Grundke-Iqbal, I., and Iqbal, K. (2006). Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc. Natl. Acad. Sci. U.S.A. 103, 8864–8869. doi: 10.1073/pnas.0603214103
Alonso, A. C., Zaidi, T., Novak, M., Grundke-Iqbal, I., and Iqbal, K. (2001). Hyperphosphorylation induces self-assembly of τ into tangles of paired helical filaments/straight filaments. Proc. Natl. Acad. Sci. U.S.A. 98, 6923–6928. doi: 10.1073/pnas.121119298
Avila, J., León-Espinosa, G., García, E., García-Escudero, V., Hernández, F., and DeFelipe, J. (2012). Tau phosphorylation by GSK3 in different conditions. Int. J. Alzheimers Dis. 2012:578373. doi: 10.1155/2012/578373
Bateman, R. J., Barthélemy, N. R., and Horie, K. (2020). Another step forward in blood-based diagnostics for Alzheimer’s disease. Nat. Med. 26, 314–316. doi: 10.1038/s41591-020-0797-4
Chi, N.-F., Chao, S.-P., Huang, L.-K., Chan, L., Chen, Y.-R., Chiou, H.-Y., et al. (2019). Plasma amyloid beta and tau levels are predictors of post-stroke cognitive impairment: a longitudinal study. Front. Neurol. 10:715. doi: 10.3389/fneur.2019.00715
del Ser, T., Barba, R., Morin, M. M., Domingo, J., Cemillan, C., Pondal, M., et al. (2005). Evolution of cognitive impairment after stroke and risk factors for delayed progression. Stroke 36, 2670–2675. doi: 10.1161/01.STR.0000189626.71033.35
Ding, M.-Y., Xu, Y., Wang, Y.-Z., Li, P.-X., Mao, Y.-T., Yu, J.-T., et al. (2019). Predictors of cognitive impairment after stroke: a prospective stroke cohort study. J. Alzheimers Dis. 71, 1139–1151. doi: 10.3233/JAD-190382
Drummond, E., Pires, G., MacMurray, C., Askenazi, M., Nayak, S., Bourdon, M., et al. (2020). Phosphorylated tau interactome in the human Alzheimer’s disease brain. Brain 143, 2803–2817. doi: 10.1093/brain/awaa223
Fandos, N., Pérez-Grijalba, V., Pesini, P., Olmos, S., Bossa, M., Villemagne, V. L., et al. (2017). Plasma amyloid β 42/40 ratios as biomarkers for amyloid β cerebral deposition in cognitively normal individuals. Alzheimers Dement. 8, 179–187. doi: 10.1016/j.dadm.2017.07.004
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., and Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am. J. Roentgenol. 149, 351–356. doi: 10.2214/ajr.149.2.351
Giudici, K. V., de Souto Barreto, P., Guyonnet, S., Li, Y., Bateman, R. J., and Vellas, B. (2020). Assessment of plasma amyloid-β42/40 and cognitive decline among community-dwelling older adults. JAMA Netw. Open 3:e2028634. doi: 10.1001/jamanetworkopen.2020.28634
Glenner, G. G., and Wong, C. W. (1984). Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890. doi: 10.1016/s0006-291x(84)80190-4
Gregoire, S. M., Chaudhary, U. J., Brown, M. M., Yousry, T. A., Kallis, C., Jäger, H. R., et al. (2009). The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology 73, 1759–1766. doi: 10.1212/WNL.0b013e3181c34a7d
Grundke-Iqbal, I., Iqbal, K., Tung, Y.-C., Quinlan, M., Wisniewski, H. M., and Binder, L. I. (1986). Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U.S.A. 83, 4913–4917. doi: 10.1073/pnas.83.13.4913
Hagberg, G., Ihle-Hansen, H., Fure, B., Thommessen, B., Ihle-Hansen, H., Øksengård, A. R., et al. (2020). No evidence for amyloid pathology as a key mediator of neurodegeneration post-stroke-a seven-year follow-up study. BMC Neurol. 20:174. doi: 10.1186/s12883-020-01753-w
Hatsuta, H., Takao, M., Nogami, A., Uchino, A., Sumikura, H., Takata, T., et al. (2019). Tau and TDP-43 accumulation of the basal nucleus of Meynert in individuals with cerebral lobar infarcts or hemorrhage. Acta Neuropathol. Commun. 7:49. doi: 10.1186/s40478-019-0700-z
Hesse, C., Rosengren, L., Andreasen, N., Davidsson, P., Vanderstichele, H., Vanmechelen, E., et al. (2001). Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci. Lett. 297, 187–190. doi: 10.1016/s0304-3940(00)01697-9
Hjalmarsson, C., Bjerke, M., Andersson, B., Blennow, K., Zetterberg, H., Åberg, N. D., et al. (2014). Neuronal and glia-related biomarkers in cerebrospinal fluid of patients with acute ischemic stroke. J. Cent. Nerv. Syst. Dis. 6, 51–58. doi: 10.4137/JCNSD.S13821
Jack, C. R. Jr., Bennett, D. A., Blennow, K., Carrillo, M. C., Dunn, B., Haeberlein, S. B., et al. (2018). NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562. doi: 10.1016/j.jalz.2018.02.018
Karikari, T. K., Pascoal, T. A., Ashton, N. J., Janelidze, S., Benedet, A. L., Rodriguez, J. L., et al. (2020). Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 19, 422–433. doi: 10.1016/S1474-4422(20)30071-5
Liu, W., Wang, X., O’Connor, M., Wang, G., and Han, F. (2020). Brain-derived neurotrophic factor and its potential therapeutic role in stroke comorbidities. Neural Plast. 2020:1969482. doi: 10.1155/2020/1969482
Lynch, C. A., Walsh, C., Blanco, A., Moran, M., Coen, R. F., Walsh, J. B., et al. (2006). The clinical dementia rating sum of box score in mild dementia. Dement. Geriatr. Cogn. Disord. 21, 40–43. doi: 10.1159/000089218
Mok, V. C., Lam, B. Y., Wong, A., Ko, H., Markus, H. S., and Wong, L. K. (2017). Early-onset and delayed-onset poststroke dementia – revisiting the mechanisms. Nat. Rev. Neurol. 13, 148–159. doi: 10.1038/nrneurol.2017.16
Morris, M., Maeda, S., Vossel, K., and Mucke, L. (2011). The many faces of tau. Neuron 70, 410–426. doi: 10.1016/j.neuron.2011.04.009
Patel, M. D., Coshall, C., Rudd, A. G., and Wolfe, C. D. (2002). Cognitive impairment after stroke: clinical determinants and its associations with long-term stroke outcomes. J. Am. Geriatr. Soc. 50, 700–706. doi: 10.1046/j.1532-5415.2002.50165.x
Pendlebury, S. T., Mariz, J., Bull, L., Mehta, Z., and Rothwell, P. M. (2012). MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke–Canadian Stroke Network vascular cognitive impairment harmonization standards neuropsychological battery after TIA and stroke. Stroke 43, 464–469. doi: 10.1161/STROKEAHA.111.633586
Pevalova, M., Filipcik, P., Novak, M., Avila, J., and Iqbal, K. (2006). Post-translational modifications of tau protein. Bratisl. Lek. Listy 107, 346–353.
Pluta, R. (2000). The role of apolipoprotein E in the deposition of beta-amyloid peptide during ischemia-reperfusion brain injury. A model of early Alzheimer’s disease. Ann. N. Y. Acad. Sci. 903, 324–334. doi: 10.1111/j.1749-6632.2000.tb06383.x
Sachdev, P., Kalaria, R., O’Brien, J., Skoog, I., Alladi, S., Black, S. E., et al. (2014). Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis. Assoc. Disord. 28, 206–218. doi: 10.1097/wad.0000000000000034
Skrobot, O. A., Black, S. E., Chen, C., DeCarli, C., Erkinjuntti, T., Ford, G. A., et al. (2018). Progress toward standardized diagnosis of vascular cognitive impairment: guidelines from the vascular impairment of cognition classification consensus study. Alzheimers Dement. 14, 280–292. doi: 10.1016/j.jalz.2017.09.007
Stanne, T. M., Åberg, N. D., Nilsson, S., Jood, K., Blomstrand, C., Andreasson, U., et al. (2016). Low circulating acute brain-derived neurotrophic factor levels are associated with poor long-term functional outcome after ischemic stroke. Stroke 47, 1943–1945. doi: 10.1161/STROKEAHA.115.012383
Sun, J. H., Tan, L., and Yu, J. T. (2014). Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann. Transl. Med. 2:80. doi: 10.3978/j.issn.2305-5839.2014.08.05
Tang, S.-C., Yang, K.-C., Chen, C.-H., Yang, S.-Y., Chiu, M.-J., Wu, C.-C., et al. (2018). Plasma β-amyloids and tau proteins in patients with vascular cognitive impairment. Neuromol. Med. 20, 498–503. doi: 10.1007/s12017-018-8513-y
Tsai, C. F., Lee, W. J., Wang, S. J., Shia, B. C., Nasreddine, Z., and Fuh, J. L. (2012). Psychometrics of the Montreal Cognitive Assessment (MoCA) and its subscales: validation of the Taiwanese version of the MoCA and an item response theory analysis. Int. Psychogeriatr. 24, 651–658. doi: 10.1017/S1041610211002298
Turunen, K. E., Laari, S. P., Kauranen, T. V., Uimonen, J., Mustanoja, S., Tatlisumak, T., et al. (2018). Domain-specific cognitive recovery after first-ever stroke: a 2-year follow-up. J. Int. Neuropsychol. Soc. 24, 117–127. doi: 10.1017/S1355617717000728
van Oijen, M., Hofman, A., Soares, H. D., Koudstaal, P. J., and Breteler, M. M. (2006). Plasma Abeta(1-40) and Abeta(1-42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 5, 655–660. doi: 10.1016/S1474-4422(06)70501-4
Wagle, J., Farner, L., Flekkoy, K., Bruun Wyller, T., Sandvik, L., Fure, B., et al. (2011). Early post-stroke cognition in stroke rehabilitation patients predicts functional outcome at 13 months. Dement. Geriatr. Cogn. Disord. 31, 379–387. doi: 10.1159/000328970
Wang, H. H., Li, H. L., Liu, R., Zhang, Y., Liao, K., Wang, Q., et al. (2010). Tau overexpression inhibits cell apoptosis with the mechanisms involving multiple viability-related factors. J. Alzheimers Dis. 21, 167–179. doi: 10.3233/JAD-2010-091279
Wang, J. Z., Grundke-Iqbal, I., and Iqbal, K. (2007). Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur. J. Neurosci. 25, 59–68. doi: 10.1111/j.1460-9568.2006.05226.x
Wyman-Chick, K. A., and Scott, B. (2015). Development of clinical dementia rating scale cut-off scores for patients with Parkinson’s disease. Mov. Disord. Clin. Pract. 2, 243–248. doi: 10.1002/mdc3.12163
Yang, C.-C., Chiu, M.-J., Chen, T.-F., Chang, H.-L., Liu, B.-H., and Yang, S.-Y. (2018). Assay of plasma phosphorylated tau protein (threonine 181) and total tau protein in early-stage Alzheimer’s disease. J. Alzheimers Dis. 61, 1323–1332. doi: 10.3233/JAD-170810
Keywords: early-onset PSCI, delayed-onset PSCI, p-tau181, ischemic stroke, biomarker
Citation: Huang L-K, Chao S-P, Hu C-J, Chien L-N, Chiou H-Y, Lo Y-C and Hsieh Y-C (2022) Plasma Phosphorylated-tau181 Is a Predictor of Post-stroke Cognitive Impairment: A Longitudinal Study. Front. Aging Neurosci. 14:889101. doi: 10.3389/fnagi.2022.889101
Received: 03 March 2022; Accepted: 24 March 2022;
Published: 29 April 2022.
Edited by:
Stephen D. Ginsberg, Nathan Kline Institute for Psychiatric Research, United StatesReviewed by:
Khalid Iqbal, Independent Researcher, New York, NY, United StatesSylvain Lehmann, Université de Montpellier, France
Copyright © 2022 Huang, Chao, Hu, Chien, Chiou, Lo and Hsieh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Chun Lo, aricalo@tmu.edu.tw; Yi-Chen Hsieh, ychsieh@tmu.edu.tw
†These authors have contributed equally to this work
 Li-Kai Huang
Li-Kai Huang