
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 30 May 2022
Sec. Alzheimer's Disease and Related Dementias
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.844066
This article is part of the Research Topic Translational Advances in Alzheimer's, Parkinson's, and other Dementia: Molecular Mechanisms, Biomarkers, Diagnosis, and Therapies, Volume II View all 58 articles
 Jiajia Fu1
Jiajia Fu1 Yan Huang2
Yan Huang2 Ting Bao2
Ting Bao2 Ruwei Ou1
Ruwei Ou1 Qianqian Wei1
Qianqian Wei1 Yongping Chen1
Yongping Chen1 Jing Yang1
Jing Yang1 Xueping Chen1*
Xueping Chen1* Huifang Shang1*
Huifang Shang1*Background: Sex is an important factor in studying the relationship between the APOE gene, lipid profiles, and AD. However, few studies have focused on the effect of sex on lipids in AD and normal controls with different APOE genes.
Materials and Methods: A total of 549 participants, including 298 AD patients and 251 body mass index (BMI)-matched healthy controls (HCs), were enrolled. Lipid profiles and APOE genes in both AD patients and HCs were determined.
Results: (1) TC and LDL were higher in AD patients than in HCs, only in APOEε4 carrying populations, but not in non-carrying populations. (2) TC and LDL were higher in APOEε4 allele carriers than in non-carriers, only in AD populations, but not in HCs. (3) The TC of APOEε2 carriers was lower than that of non-carriers in the male AD population, but not in the female AD population, female HCs, and male HCs. (4) The increased LDL level may increase the risk of AD in female people carrying APOEε4.
Conclusion: The TC and LDL levels of APOEε4 carriers were higher than those of non-carriers, and the effect was more significant in the female AD population. The TC levels in APOEε2 carriers were lower than those in non-carriers, which was more significant in the male AD population.
Alzheimer’s disease (AD) is the most common neurodegenerative dementia disease which is characterized by insidious onset, progressive memory failure, cognitive impairment, and behavioral and psychological manifestations. The etiology and pathogenesis of AD are still unclear, and the development of AD could be the result of interaction between multiple genetic and environmental risk factors (Poirier et al., 2014). The most specific genetic risk factor for late-onset AD is the Apolipoprotein (APO) E gene (Corder et al., 1993). The ε2, ε3, and ε4 alleles of the APOE gene, located on chromosome 19q13.2, constitute a common polymorphism in most populations (Corder et al., 1993). The APOEε4 allele is shown to be associated with a higher risk of AD and greater disease severity, whereas the APOEε2 allele has an opposite role (Corder et al., 1993). Patients with AD have a higher frequency of APOEε4 allele than control participants (Isbir et al., 2001). An epidemiology study showed that the frequency of the APOEε4 allele varied drastically among different populations; it occurs in about 25% African Americans, 15% Caucasians, and 7% Chinese (Corbo and Scacchi, 1999).
There are plenty of studies focusing on the correlation between dyslipidemia and AD, but most of them are trying to explore the effect of cholesterol (TC) on AD. A previous study has shown that high serum TC level in middle age is a risk factor for AD and AD-related pathology (Anstey et al., 2008). Cerebrovascular risk factors, such as high cholesterol, had a mild combined effect on the earlier onset of AD (de Oliveira et al., 2014). High TC level in the brain was proven to play an important role in the process of amyloid-β (Aβ)-induced AD (Refolo et al., 2000). TC and low-density lipoprotein (LDL) were shown to be involved in the pathogenesis of AD by increasing amyloid accumulation and disrupting the cell cycle (Wu et al., 2019), but late-life hypercholesterolemia might also slow cognitive decline, particularly when in combination with other cerebrovascular risk factors, possibly due to enhanced cerebral perfusion (de Oliveira et al., 2015). However, a longitudinal study did not show significant associations of high cholesterol with cognitive or functional changes in AD (de Oliveira et al., 2018). Some studies have found that serum HDL levels were lower in AD patients (Merched et al., 2000), and were inversely associated with cognitive impairment, but opposite reports also existed (Launer et al., 2001).
The lipid profiles were also found to be associated with the APOE, but the results were inconsistent (Isbir et al., 2001; Hoshino et al., 2002). Previous studies found that the levels of LDL and TC in APOEε4 carriers increased or tended to be increased compared with non-carriers (Sun et al., 2014). However, Isbir et al. (2001) showed a decreasing trend, and Hall et al. (2006) found no statistical differences. It was also reported that AD patients carrying the APOEε2 allele had lower TC and LDL levels and higher HDL levels than AD patients carrying the APOEε4 allele (Sabbagh et al., 2006), but other studies did not find statistical significance (Isbir et al., 2001). de Oliveira et al. (2017) considered that APOEε4 non-carriers might enhance lipid availability to protect neuronal membranes, thus overcoming their supposed dysfunction in cholesterol metabolism, while APOEε4 carriers have inefficient neural repair mechanisms.
In addition, sex can affect APOEε4 allele-associated cognitive impairment. The risk of AD or MCI conversion was higher in female APOEε4 allele carriers than that in male APOEε4 allele carriers (Kim et al., 2015). There was a stronger correlation between APOEε4 and CSF Tau levels in women than in men (Liu et al., 2021). The gender-specific APOE haplotype interactions can alter the response to anticholinesterase therapy (MacGowan et al., 1998). Among the females treated with anticholinesterase, the individuals carrying the APOEε4 allele presented a poor response to treatment than those carrying other APOE alleles, and the anticholinesterase reactivity in the males was superior to that in the females (MacGowan et al., 1998). Some studies found that TC was significantly higher in women with AD (Oliveira et al., 2016). However, few studies have focused on the effect of sex on lipids in AD and normal controls with different APOE genes.
Therefore, sex is an important factor affecting the interaction between the APOE gene and lipid profiles in AD. The sex-related difference is also crucial for precision therapy. Few studies have clearly elucidated the effect of sex on lipids in AD and normal controls with different APOE genes. In order to explore the relationship between lipid profiles, APOE gene, and sex in AD, we examined the lipid profiles in AD patients and healthy controls (HCs) with different APOE alleles and analyzed the effect of sex on lipid profiles in both AD patients and HCs with different APOE genes.
Alzheimer’s disease patients admitted to West China Hospital of Sichuan University from January 2020 to January 2021 were recruited, and trained doctors diagnosed AD according to the NINCDS-ADRDA and DSM V (McKhann et al., 1984; dermann and Fleischhacker, 2016). Detailed medical history-taking and physical examination were performed. Individuals without any disease in the central nervous system and normal cognitive function were recruited as healthy controls (HCs) during the same period, and they were matched for body mass index (BMI) to the AD group. The patients received the standardized assessments, including the Mini-mental State Examination (MMSE), the Montreal Cognitive Assessment (MoCA), and magnetic resonance imaging (MRI). AD patients with MMSE scores higher than 25 were excluded. Since MMSE has shown not to be adequate in detecting MCI and clinical signs of dementia, and MoCA is superior to MMSE in identifying MCI (Pinto et al., 2019), HCs with MoCA scores higher than 22 were included in the present study. All participants with vascular dementia (VaD), cardiopathy, hypertension, diabetes mellitus, demyelinating diseases, white matter lesions, obesity, fatty liver and other diseases closely related to blood lipids were excluded. The study was approved by the ethics committee of West China Hospital of Sichuan University. All AD patients and control participants gave their written informed consent to participate in the investigation.
All blood samples were routinely collected in the early morning when patients were fasting. The serum lipid profiles, including TC, triglycerides (TG), LDL, and HDL, were measured by homogeneous enzyme colorimetry on Roche/Hitachi Cobas C analyzer. DNA was isolated from blood cells.
Samples were amplified by the polymerase chain reaction amplified samples (ABI 7500 FAST, Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, United States). APOE haplotypes were determined according to the manufacturer’s instruction using an APOE haplotype determinating kit (Memorigen Biotech, Xiamen, China). The kit is based on fluorescent PCR technology, using three pairs of detection reagents that can specifically recognize two single nucleotide polymorphisms (RS429358 and RS7412) of APOE gene type 2, 3 and 4, respectively, to identify and amplify the samples. When the sample contained APOE allele matched with the amplification system, PCR amplification reaction would take place in the system. The exonuclease activity at the 5–3 end of DNA polymerase would degrade the fluorescent-labeled DNA molecular probe by enzyme digestion. After degradation by enzyme digestion, the probe could be stimulated with a fluorescence signal and detected by the monitoring system.
SPSS software 26.0 version (IBM, Armonk, NY, United States) was used for data analysis. The χ2 test was used to compare allele frequencies among groups. An independent t-test was used to compare lipid profiles in patients with different APOE haplotypes or sex. For those groups with significant age differences, age was adjusted by covariance analysis, for non-normal distribution data were used for non-parametric ANOVA (Kruskal–Wallis) and a non-parametric Mann–Whitney U test. Logistic regression was used to analyze the influences of various variables on the risk of disease. Two-tailed p < 0.05 was considered statistically significant. Table data are expressed as the means ± standard deviation (SD), and image values are expressed as the mean (standard error).
A total of 549 participants (298 AD patients, 251 HCs) were included in the study. The mean (SD) age of AD patients was 76.07 (7.12) years old, and the HCs were 65.85 (11.33) years old, so the age factor was adjusted in the subsequent lipid analyses (Supplementary Material 1 – adjusting for age). There were 113 male (185 female) people in AD and 103 male (148 female) people in HCs. In AD patients, the mean (SD) course of the disease was 2.5 (2.52) years, the mean (SD) of MMSE score was 18 (5.21), the mean (SD) of MOCA was 12.48 (3.56) (Table 1). There was no significant difference in statin use between the AD and the HCs groups (Table 1). Additional adjustments for the statin use did not change the statistical significance of the results of lipid analyses (Supplementary Material 2 – adjusting for age and the stain use).
There were significant differences in the haplotype frequency of APOEε4, APOEε2, and APOEε3 between the AD and HCs (Table 1). A significantly higher proportion of APOEε4 carriers and a lower proportion of APOEε2 carriers were found in the AD group than in the HCs (Table 1). There was no significant difference in the proportion of APOEε3 carriers between AD group and HCs group, and the state of APOEε3 allele carrying had no effect on lipid profiles (Supplementary Material 3).
The levels of TG were insignificant between AD and HCs groups (Figure 1). The levels of TC and LDL in the AD group were higher than those in the HCs group (Figures 2A, 3A). In the subgroup analysis based on APOE alleles, AD patients carrying APOEε4 had higher levels of TC and LDL than HCs with APOEε4 allele; AD patients without APOEε2 allele had increased TC and LDL levels than HCs without APOEε2 allele (Figures 2B, 3B). In subgroup analysis based on sex, TC and LDL levels in the AD group were significantly higher than in the HCs group in both sexes (Figures 2B, 3B). A subgroup analysis combined sex and APOE haplotypes showed that TC and LDL levels were higher in AD patients than in HCs, which were only found in female APOEε4 carriers or male APOEε2 non-carriers (Figures 2D, 3D). In addition, serum HDL level in the AD group was higher than those in the HCs group (Figure 4A). Subgroup analysis showed that the change of HDL level was found in populations with APOEε4 allele and without APOEε2 allele (Figure 4B), as well as in male non-APOEε2 carriers and male APOEε4 carriers (Figure 4D).

Figure 1. The levels of TG in different populations. *P < 0.05; Values are expressed as mean (standard error). AD, Alzheimer’s disease; HCs, healthy controls; APOEε4+, APOEε4 carriers; APOEε2+, APOEε2 carriers.
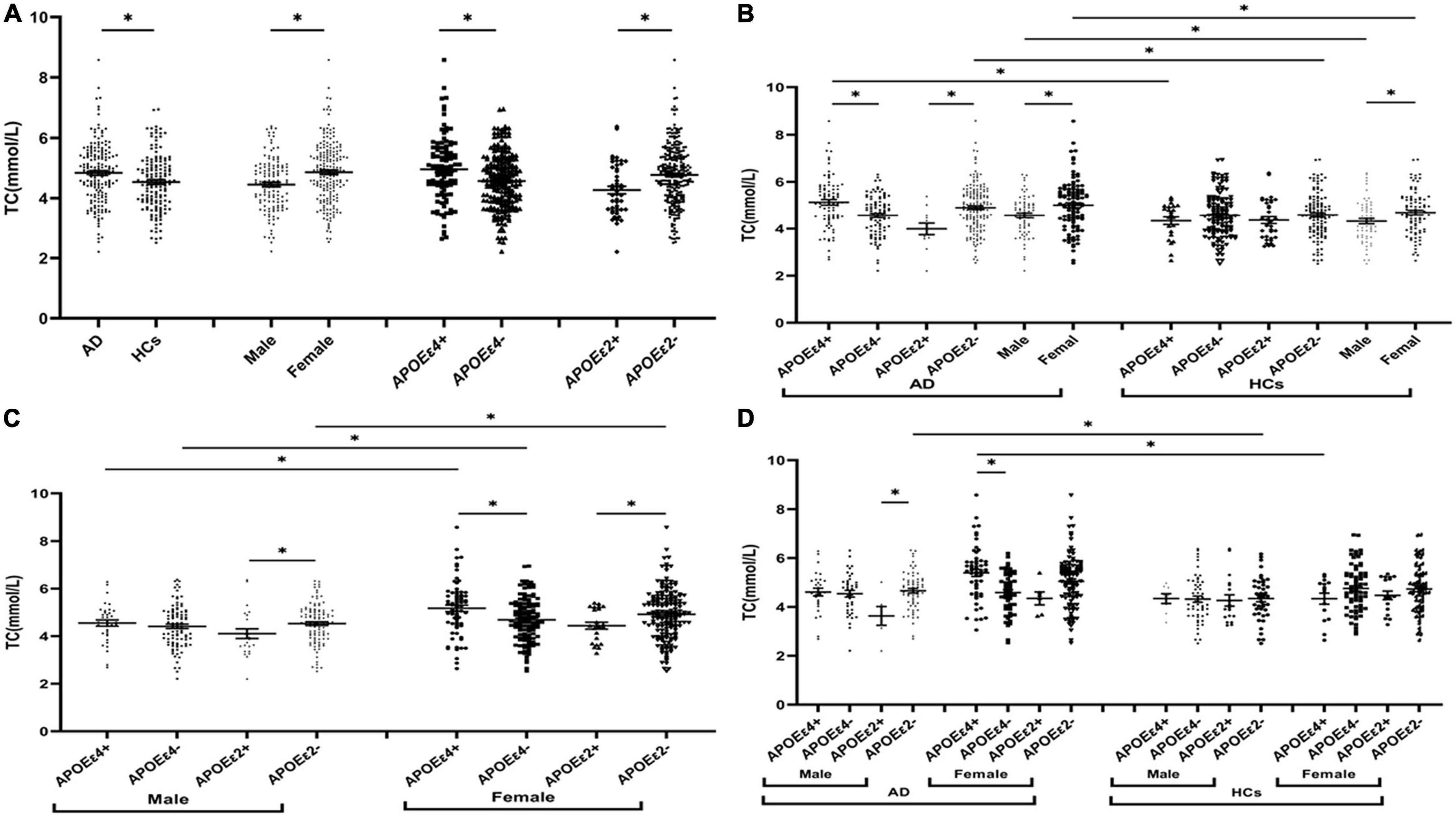
Figure 2. The levels of TC in different populations. *P < 0.05; Values are expressed as mean (standard error). AD, Alzheimer’s disease; HCs, healthy controls; APOEε4+, APOEε4 carriers; APOEε2+, APOEε2 carriers.
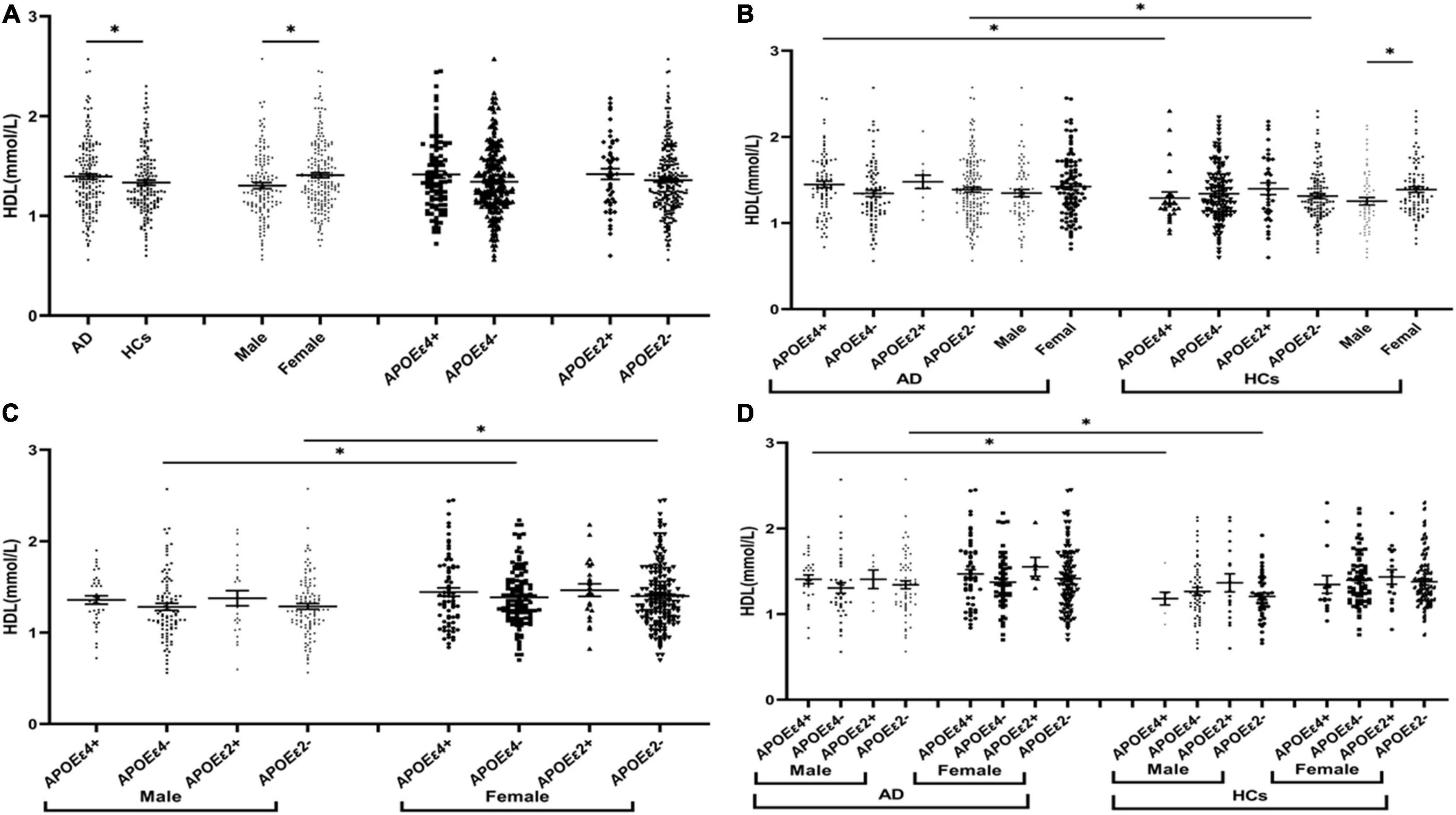
Figure 3. The levels of LDL in different populations. *P < 0.05; Values are expressed as mean (standard error). AD, Alzheimer’s disease; HCs, healthy controls; APOEε4+, APOEε4 carriers; APOEε2+, APOEε2 carriers.
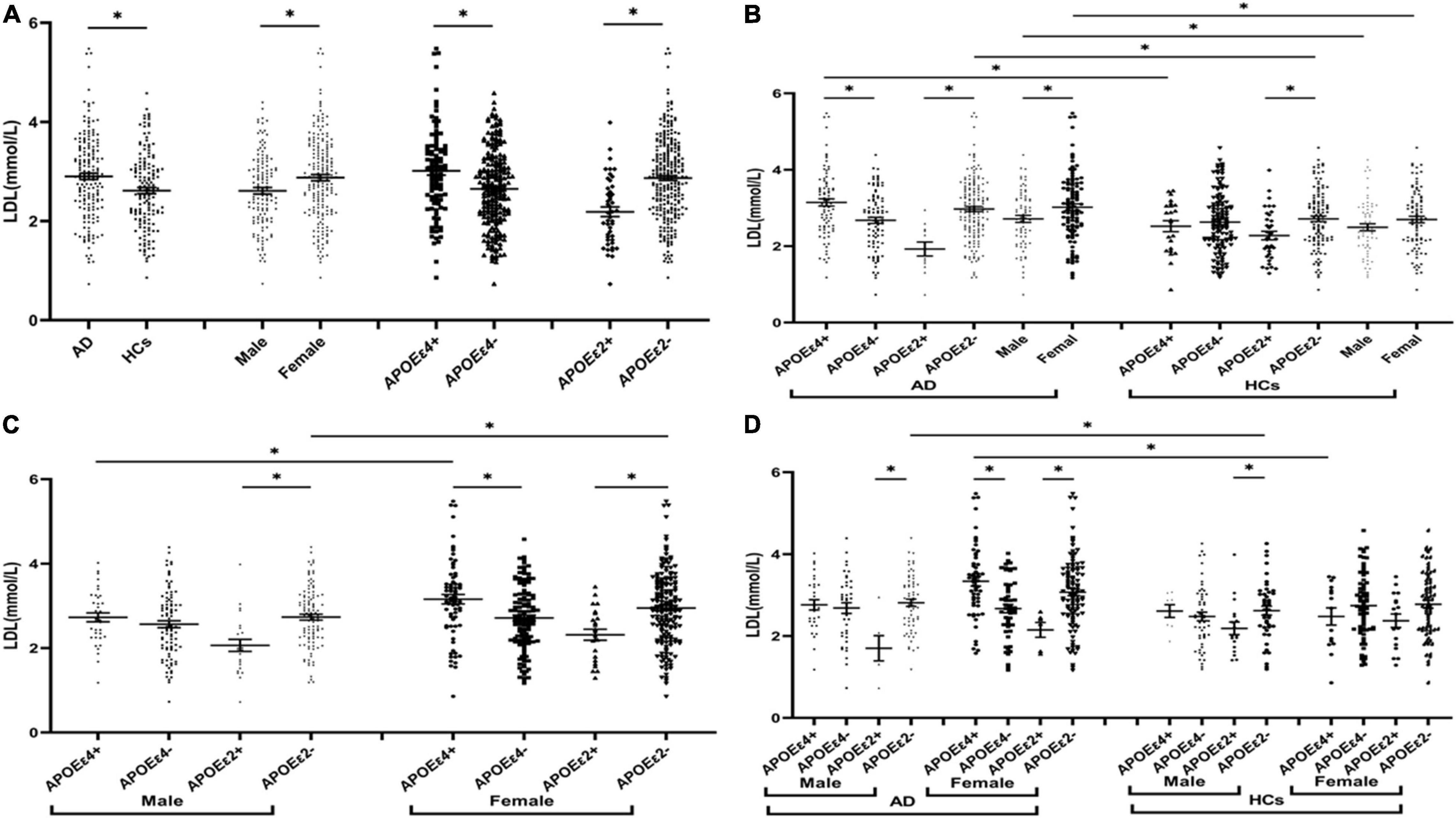
Figure 4. The levels of HDL in different populations. *P < 0.05; Values are expressed as mean (standard error). AD, Alzheimer’s disease; HCs, healthy controls; APOEε4+, APOEε4 carriers; APOEε2+, APOEε2 carriers.
The levels of TC and LDL in the APOEε4 carriers were higher than those in non-APOEε4 carriers (Figures 2A, 3A). In the subgroup analysis based on disease, we found that AD patients with APOEε4 had significantly higher levels of TC and LDL than AD patients without APOEε4 (Figures 2B, 3B). No differences in the levels of TC and LDL between HCs with and without APOEε4 (Figures 2B, 3B). Furthermore, In the subgroup analysis based on sex, significantly higher levels of TC and LDL were only found in female AD patients with APOEε4 than without APOEε4 female AD patients (Figures 2D, 3D). However, no differences were found between male AD patients with and without APOEε4 (Figures 2D, 3D).
The levels of TC and LDL in the APOEε2 carriers were lower than those in non-APOEε2 carriers (Figures 2A, 3A). In the subgroup analysis based on sex and disease, similar changes in LDL levels were found in almost all subgroups except the female control population (Figures 3B–D). The TC levels were lower in AD patients with the APOEε2 allele than in AD patients without the APOEε2 allele, but no such change was observed in the HCs (Figure 2B). In addition, the TC of APOEε2 carriers was lower than that of non-carriers in the male AD population, but there was no such change in the female AD population, female HCs, and male HCs (Figure 2D).
The TC, HDL, and LDL levels in the female population were higher than the male population (Figures 2A, 3A, 4A). In order to exclude the effect of disease and APOE gene, subgroup analyses were performed, and the results showed that sex had similar effects on TC and LDL in AD patients, APOEε4 carriers, and APOEε2 non-carriers (Figures 2B,C, 3B,C). In the control participants and APOEε4 non-carriers, TC and HDL in the female population were higher than the male population (Figures 2B,C, 4B,C).
Age, gender, disease course of AD, APOEε4, and blood lipids level were considered influencing factors for Logistics regression analysis, and we found that age and APOE4 were important risk factors, while the blood lipids were not significantly related with AD. When TG, TC, HDL, and LDL were taken as targets to study the relationship with AD, no correlation was found between lipid profiles and AD through logistics regression analysis. However, in the subgroup analysis, adjusted APOE and sex, we found that LDL increased the risk of AD in females with the APOEε4 allele, that is, for every 1 unit increase in LDL, the risk of AD increased 898.46 times in the female population with APOEε4 (P = 0.04). Therefore, the increased LDL level may increase the risk of AD in female people carrying APOEε4.
This study found that LDL and TC serum levels in AD patients were higher than those in HCs, consistent with previous studies (Wang et al., 2020). In addition, we found higher HDL levels in AD patients compared to controls. A prospective study published in 2021 reported very high serum HDL cholesterol levels as an independent risk factor for either dementia or AD and suggested that elevated HDL may serve as a serum biomarker for assessing the risk of dementia (Kjeldsen et al., 2021). However, a prospective study with approximately 7,000 French people found that HDL was not associated with AD (Schilling et al., 2017). A meta-analysis that combined all relevant studies before 2017 showed that HDL was not associated with AD in later life (Anstey et al., 2017). Marsillach et al. (2020) point out that functional HDL is more important in disease rather than HDL cholesterol levels. Therefore, more attention should be paid to the interaction between HDL functional subtypes and AD.
In the AD population, the TC and LDL levels were increased in the APOEε4 carriers compared to the non-APOEε4 carriers, and this alteration was not found in the control population. In APOEε4 allele carriers, the TC and LDL levels in the AD patients were higher than those in control participants, but these differences in TC and LDL were not found in non-APOEε4 carriers. These findings indicated that the involvement of APOEε4 in AD could be associated with lipid profiles. Similarly, Wang et al. (2020) found that the levels of TC in the AD population carrying APOEε4 were higher than those without APOEε4, while no such change was observed in healthy controls. However, Raygani et al. (2006) found these lipid changes between APOEε4 carriers and non-APOEε4 carriers in both AD and controls. In addition, we also found that LDL may increase the risk of AD in females with the APOEε4 allele. A previous study showed that the association of elevated midlife TC level with late-life AD was not altered after adjusting for the APOEε4 allele (Kivipelto et al., 2002), but another study showed decreasing TC after midlife may represent a risk marker for late-life cognitive impairment (Solomon et al., 2007), and these studies did not take into account the role of sex. Therefore, further research and exploration are needed to verify if the effect of APOEε4 on lipid profiles is gender-specific in AD.
In subgroup analyses based on sex showed that TC and LDL in the AD group were higher than those of the control group in both male and female populations. In the female population, TC and LDL were higher in APOEε4 carriers than in non-APOEε4 carriers, but no change was found in the male population. Several recent studies have shown that sex can alter the risk of the APOEε4 allele, and female people with APOEε4 have a higher risk of AD than male carriers (Kim et al., 2015). Women aged 65–75 with APOEε4 had a higher risk of AD than men and had higher levels of tau in the cerebrospinal fluid (CSF) (Liu et al., 2021). A significant sex-specific association was found between CSF apolipoprotein E and AD biomarkers in Liu’s study. In women, baseline CSF apolipoprotein E was significantly associated with longitudinal changes in baseline CSF Aβ and tau, but no longitudinal association was observed in men (Liu et al., 2021). In addition, a recent study on brain metabolism found that the exponential function of brain metabolism in females declines more rapidly than the linear function in males when young and more slowly in old age. The explanation for this could be a greater brain reserve in men, indicating that men have a greater capacity to withstand more pathology than women (Zhang et al., 2017). Our study found that the effect of the APOEε4 allele on TC and LDL metabolism is significantly altered in female AD patients, but not in male AD patients, so we think the difference may also be related to metabolic reserve.
The effect of the APOEε4 allele on lipid profiles in women was greater than that in men, but the effect of the APOEε2 allele on lipid profiles was different from the APOEε4 allele. In this study, the individuals were stratified according to the presence of the APOEε2 allele, and we found that the role of the APOEε2 allele in lipid levels was affected by the disease of AD. In the AD population, the TC level in APOEε2 carriers was lower than those in non-carriers, but these changes were not found in the HCs. Although the TC level of APOEε2 carriers was lower than that of non-carriers in both male and female populations, such change was only found in the male AD population, but not in the male HCs, female HCs, and female AD population. Therefore, we hypothesized that reducing TC by APOEε2 allele seems to be more biased in male AD populations. Human studies have shown that the APOEε2 allele is associated with decreased Aβ deposition in the brain of non-dementia elderly individuals and AD patients, and protects against cognitive impairment in individuals over 90 years of age with high levels of Aβ in the brain (Serrano-Pozo et al., 2015). In vitro and in vivo studies have shown that APOEε2 provides protection independent of Aβ pathology through multiple potential pathways, including the regulatory role of APOEε2 in lipid metabolism (Huang et al., 2019).
It is well known that the main component of senile plaques is Aβ, which is the main pathological basis of AD. Aβ is produced by the double cleavage of amyloid precursor protein (APP) by β-secretase and γ-secretase. APP, β-secretase and γ-secretase are located in lipid rafts, a type of membrane-bound cholesterol, where APP metabolism occurs. The level of 27-OH cholesterol circulating in the blood is proportional to the level of cholesterol, and there is a concentration-driven flux of 27-OH cholesterol circulating into the brain. The accumulation of 27-OH cholesterol was found to be the most significant change in the brain cholesterol profile of AD patients, and part of this accumulation may be influenced by circulating cholesterol levels. High levels of 27-OH cholesterol increase the formation of Aβ by antagonizing the inhibition of 24S-hydroxyl cholesterol (Feringa and van der Kant, 2021). It is speculated that the increase of tag-rich lipoprotein in the ε4 vector APOE may increase its affinity for LDLR, which ultimately reduces LDL intake and increases circulating plasma cholesterol. APOEε4 has been shown to contribute to AD susceptibility by disrupting lipid and cholesterol levels. One study used correlation analysis to detect gene expression patterns in APOEε4-positive and APOEε4-negative elderly men and women. A large number of genes with the same APOE expression pattern were found in APOEε4-positive individuals but not in APOEε4-negative individuals. APOEε4-positive women tended to be larger than APOEε4-positive men and APOEε4-negative controls. The classification of genes is concerned with hormones involved in oxidation, inflammatory lipid metabolism, and immune processes. A significant triple interaction was observed in the brain region/sex/genotype of γ-secretase, which is closely associated with AD and is thought to play a role in APP processing (Hsu et al., 2019). This may explain the finding in this study that high LDL levels may increase the risk of AD in female people with the APOEε4 allele. Therefore, more attention should be paid to lipid profiles of different genders and APOE genotypes in the future study of AD, especially women who carry the APOE4 allele.
In general, cholesterol and APOEε4 gene are major factors affecting the development of AD, but current studies have found that statins may have a protective effect before the onset of AD, and once the onset of the disease, statins become inefficient or ineffective (Li et al., 2010). A longitudinal study in 2021 evaluated the association between statin use (the time-varying status and the dose-response relationship) and the incidence of AD, and found that statin use was not associated with an increased incidence of AD (Jeong et al., 2021). In this study, The proportion of statin use was small and adjustment for statin use did not significantly affect lipid profile results. However, this study was a cross-sectional result, and it is worthwhile to further explore whether statins benefit female AD patients with APOE4 allele in longitudinal follow-up because these subpopulations have more significant changes in TC and LDL.
The TC and LDL levels of APOEε4 allele carriers were higher than those of non-carriers, and the effect was more significant in the AD and female population. High LDL levels may increase the risk of AD in female people with the APOEε4 allele. The TC levels in APOEε2 allele carriers were lower than those in non-carriers, and the effect was more significant in the male AD population. Further prospective studies focusing on the relationship between the APOE gene, sex, lipid profiles, and AD are essential to confirm our findings, and special attention should be paid to female AD patients with the APOEε4 allele and male AD patients carrying the APOEε2 allele when regulating the blood lipids.
This study has the following limitations. First, due to the large difference in whether AD patients take medication, type of medication, and dose, there is no stratification according to medication. Second, this study did not analyze the relationship between lipid profiles and AD severity due to limited data. Thirdly, This study was cross-sectional and lacks the dynamic changes and correlation of lipid profiles with time and disease. Fourthly, this study is a single-center, small-sample study, which needs to be verified by a larger sample and multi-center study.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Committee of West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
JF contributed to the compilation of articles and data analysis. YH and TB contributed to the selection and data entry of healthy controls. RO, QW, YC, and JY contributed to the screening and data entry of AD patients. XC and HS contributed to the review, editing and scientific research thinking, and methods. All authors read and approved the manuscript.
This study was supported by grant from the National Key Research and Development Program of China (Grant No. 2017YFC09007703), grant from Science and Technology Planning Project in Sichuan Province (Grant No. 2020YJ0281), 1⋅3⋅5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYJC18038), and Cadres Health Care Project in Sichuan Province (Grant No. 2019-112).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We gratefully acknowledge the AD patients for their participation in this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.844066/full#supplementary-material
Supplementary Material 1
Supplementary Table 1 | Comparison of lipids between AD and HCs groups (Adjusting for age); Values are expressed as mean (standard deviation); AD, Alzheimer’s disease; HCs, healthy controls; APOEε4+, APOEε4 carriers; APOEε2+, APOEε2 carriers; Male and APOEε4+, male APOEε4 carriers; Male and APOEε4−, male APOEε4 non-carriers; Female and APOEε4+, female APOEε4 carrier; Female and APOEε4−, female APOEε4 non-carrier; Male and APOEε2+, male APOEε2 carriers; Male and APOEε2−, male APOEε2 non-carriers; Female and APOEε2+, female APOEε2 carrier; Female and APOEε2−, female APOEε2 non-carrier.
Supplementary Table 2 | Comparison of lipids between APOEε4+ group and APOEε4− group (Adjusting for age); Values are expressed as mean (standard deviation); AD, Alzheimer’s disease; HCs, healthy controls; Male and AD, male patients with Alzheimer’s disease; Female and AD, female patients with Alzheimer’s disease; Male and HCs, male healthy controls; Female and HCs, healthy female controls.
Supplementary Table 3 | Comparison of lipids between APOEε2+ group and APOEε2− group (Adjusting for age); Values are expressed as mean (standard deviation); AD, Alzheimer’s disease; HCs, healthy controls; Male and AD, male patients with Alzheimer’s disease; Female and AD, female patients with Alzheimer’s disease; Male and HCs, male healthy controls; Female and HCs, healthy female controls.
Supplementary Table 4 | Comparison of lipids between male and female (Adjusting for age); Values are expressed as mean (standard deviation); AD, Alzheimer’s disease; HCs, healthy controls; APOEε4+, APOEε4 carrier.
Supplementary Material 2
Supplementary Table 1 | Comparison of lipids between AD and HCs groups (Adjusting for age and the stain use); Values are expressed as mean (standard deviation); AD, Alzheimer’s disease; HCs, healthy controls; APOEε4+, APOEε4 carriers; APOEε2+, APOEε2 carriers; Male and APOEε4+, male APOEε4 carriers; Male and APOEε4−, male APOEε4 non-carriers; Female and APOEε4+, female APOEε4 carrier; Female and APOEε4−, female APOEε4 non-carrier; Male and APOEε2+, male APOEε2 carriers; Male and APOEε2−, male APOEε2 non-carriers; Female and APOEε2+, female APOEε2 carrier; Female and APOEε2−, female APOEε2 non-carrier.
Supplementary Table 2 | Comparison of lipids between APOEε4+ group and APOEε4− group (Adjusting for age and the stain use); Values are expressed as mean (standard deviation); AD, Alzheimer’s disease; HCs, healthy controls; Male and AD, male patients with Alzheimer’s disease; Female and AD, female patients with Alzheimer’s disease; Male and HCs, male healthy controls; Female and HCs, healthy female controls.
Supplementary Table 3 | Comparison of lipids between APOEε2+ group and APOEε2− group (Adjusting for age and the stain use); Values are expressed as mean (standard deviation); AD, Alzheimer’s disease; HCs, healthy controls; Male and AD, male patients with Alzheimer’s disease; Female and AD, female patients with Alzheimer’s disease; Male and HCs, male healthy controls; Female and HCs, healthy female controls.
Supplementary Table 4 | Comparison of lipids between male and female (Adjusting for age and the stain use); Values are expressed as mean (standard deviation); AD, Alzheimer’s disease; HCs, healthy controls; APOEε4+, APOEε4 carrier.
Supplementary Material 3
Supplementary Table 1 | Comparison of lipids between AD and HCs groups (Adjusting for age); Values are expressed as mean (standard deviation); AD, Alzheimer’s disease; HCs, healthy controls; APOEε3+, APOEε3 carriers; Male and APOEε3+, male APOEε3 carriers; Male and APOEε3−, male APOEε3 non-carriers; Female and APOEε3+, female APOEε3 carrier; Female and APOEε3−, female APOEε3 non-carrier.
Supplementary Table 2 | Comparison of lipids between APOEε3+ group and APOEε3− group (Adjusting for age); Values are expressed as mean (standard deviation); AD, Alzheimer’s disease; HCs, healthy controls.
Anstey, K. J., Ashby-Mitchell, K., and Peters, R. (2017). Updating the Evidence on the Association between Serum Cholesterol and Risk of Late-Life Dementia: review and Meta-Analysis. J. Alzheimers Dis. 56, 215–228. doi: 10.3233/JAD-160826
Anstey, K. J., Lipnicki, D. M., and Low, L. F. (2008). Cholesterol as a risk factor for dementia and cognitive decline: a systematic review of prospective studies with meta-analysis. Am. J. Geriatr. Psychiatry 16, 343–354. doi: 10.1097/JGP.0b013e31816b72d4
Corbo, R. M., and Scacchi, R. (1999). Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann. Hum. Genet. 63(Pt 4), 301–310. doi: 10.1046/j.1469-1809.1999.6340301.x
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G. W., et al. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. doi: 10.1126/science.8346443
de Oliveira, F. F., Bertolucci, P. H., Chen, E. S., and Smith, M. C. (2014). Risk factors for age at onset of dementia due to Alzheimer’s disease in a sample of patients with low mean schooling from São Paulo. Brazil. Int. J. Geriatr. Psychiatry 29, 1033–1039. doi: 10.1002/gps.4094
de Oliveira, F. F., Chen, E. S., Smith, M. C., and Bertolucci, P. H. F. (2017). Longitudinal lipid profile variations and clinical change in Alzheimer’s disease dementia. Neurosci. Lett. 646, 36–42. doi: 10.1016/j.neulet.2017.03.003
de Oliveira, F. F., de Almeida, S. S., Chen, E. S., Smith, M. C., Naffah-Mazzacoratti, M. D. G., and Bertolucci, P. H. F. (2018). Lifetime Risk Factors for Functional and Cognitive Outcomes in Patients with Alzheimer’s Disease. J. Alzheimers Dis. 65, 1283–1299. doi: 10.3233/JAD-180303
de Oliveira, F. F., Pivi, G. A., Chen, E. S., Smith, M. C., and Bertolucci, P. H. (2015). Risk factors for cognitive and functional change in one year in patients with Alzheimer’s disease dementia from São Paulo, Brazil. J. Neurol. Sci. 359, 127–132. doi: 10.1016/j.jns.2015.10.051
dermann, F., and Fleischhacker, W. W. (2016). Psychotic disorders in DSM-5 and ICD-11. CNS Spectr. 21, 349–354. doi: 10.1017/S1092852916000316
Feringa, F. M., and van der Kant, R. (2021). Cholesterol and Alzheimer’s Disease; From Risk Genes to Pathological Effects. Front. Aging Neurosci. 13:690372. doi: 10.3389/fnagi.2021.690372
Hall, K., Murrell, J., Ogunniyi, A., Deeg, M., Baiyewu, O., Gao, S., et al. (2006). Cholesterol, APOE haplotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology 66, 223–227. doi: 10.1212/01.wnl.0000194507.39504.17
Hoshino, T., Kamino, K., and Matsumoto, M. (2002). Gene dose effect of the APOE-epsilon4 allele on plasma HDL cholesterol level in patients with Alzheimer’s disease. Neurobiol. Aging. 23, 41–45. doi: 10.1016/s0197-4580(01)00252-4
Hsu, M., Dedhia, M., Crusio, W. E., and Delprato, A. (2019). Sex differences in gene expression patterns associated with the APOE4 allele. F1000Res 8:387. doi: 10.12688/f1000research.18671.2
Huang, Y. A., Zhou, B., Nabet, A. M., Wernig, M., and Südhof, T. C. (2019). Differential Signaling Mediated by APOEε2, APOE3, and APOEε4 in Human Neurons Parallels Alzheimer’s Disease Risk. J. Neurosci. 39, 7408–7427. doi: 10.1523/JNEUROSCI.2994-18.2019
Isbir, T., Agaçhan, B., Yilmaz, H., Aydin, M., Kara, I., Eker, E., et al. (2001). Apolipoprotein-E gene polymorphism and lipid profiles in Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 16, 77–81. doi: 10.1177/153331750101600207
Jeong, S. M., Shin, D. W., Yoo, T. G., Cho, M. H., Jang, W., Lee, J., et al. (2021). Association between statin use and Alzheimer’s disease with dose response relationship. Sci. Rep. 11:15280. doi: 10.1038/s41598-021-94803-3
Kim, S., Kim, M. J., Kim, S., Kang, H. S., Lim, S. W., Myung, W., et al. (2015). Gender differences in risk factors for transition from mild cognitive impairment to Alzheimer’s disease: a CREDOS study. Compr. Psychiatry 62, 114–122. doi: 10.1016/j.comppsych.2015.07.002
Kivipelto, M., Helkala, E. L., Laakso, M. P., Hänninen, T., Hallikainen, M., Alhainen, K., et al. (2002). Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann. Intern. Med. 137, 149–155. doi: 10.7326/0003-4819-137-3-200208060-00006
Kjeldsen, E. W., Thomassen, J. Q., Juul Rasmussen, I., Nordestgaard, B. G., Tybjærg-Hansen, A., Frikke-Schmidt, R., et al. (2021). Plasma HDL cholesterol and risk of dementia - observational and genetic studies. Cardiovasc. Res. 2021:cvab164. doi: 10.1093/cvr/cvab164
Launer, L. J., White, L. R., Petrovitch, H., Ross, G. W., and Curb, J. D. (2001). Cholesterol and neuropathologic markers of AD: a population-based autopsy study. Neurology 57, 1447–1452. doi: 10.1212/wnl.57.8.1447
Li, G., Shofer, J. B., Rhew, I. C., Kukull, W. A., Peskind, E. R., McCormick, W., et al. (2010). Age-varying association between statin use and incident Alzheimer’s disease. J. Am. Geriatr. Soc. 58, 1311–1317. doi: 10.1111/j.1532-5415.2010.02906.x
Liu, Y., Song, J. H., Xu, W., Hou, X. H., Li, J. Q., Yu, J., et al. (2021). The Associations of Cerebrospinal Fluid APOE and Biomarkers of Alzheimer’s Disease: exploring Interactions With Sex. Front. Neurosci. 15:633576. doi: 10.3389/fnins.2021.633576
MacGowan, S. H., Wilcock, G. K., and Scott, M. (1998). Effect of gender and apolipoprotein E haplotype on response to anticholinesterase therapy in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 13, 625–630. doi: 10.1002/(sici)1099-1166(199809)13:9<625::aid-gps835<3.0.co;2-2
Marsillach, J., Adorni, M. P., Zimetti, F., Papotti, B., Zuliani, G., Cervellati, C., et al. (2020). HDL Proteome and Alzheimer’s Disease: evidence of a Link. Antioxidants 9:1224. doi: 10.3390/antiox9121224
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. doi: 10.1212/wnl.34.7.939
Merched, A., Xia, Y., Visvikis, S., Serot, J. M., and Siest, G. (2000). Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol. Aging. 21, 27–30. doi: 10.1016/s0197-4580(99)00103-7
Oliveira, F. F., Chen, E. S., Smith, M. C., and Bertolucci, P. H. (2016). Predictors of Cognitive and Functional Decline in Patients With Alzheimer Disease Dementia From Brazil. Alzheimer Dis. Assoc. Disord. 30, 243–250. doi: 10.1097/WAD.0000000000000117
Pinto, T. C. C., Machado, L., Bulgacov, T. M., Rodrigues-Júnior, A. L., Costa, M. L. G., et al. (2019). Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int. Psychogeriatr. 31, 491–504. doi: 10.1017/S1041610218001370
Poirier, J., Miron, J., Picard, C., Gormley, P., Théroux, L., Breitner, J., et al. (2014). Apolipoprotein E and lipid homeostasis in the etiology and treatment of sporadic Alzheimer’s disease. Neurobiol. Aging 35, (Suppl. 2), S3–S10. doi: 10.1016/j.neurobiolaging.2014.03.037
Raygani, A. V., Rahimi, Z., Kharazi, H., Tavilani, H., and Pourmotabbed, T. (2006). Association between apolipoprotein E polymorphism and serum lipid and apolipoprotein levels with Alzheimer’s disease. Neurosci. Lett. 408, 68–72. doi: 10.1016/j.neulet.2006.08.048
Refolo, L. M., Malester, B., LaFrancois, J., Bryant-Thomas, T., Wang, R., et al. (2000). Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 7, 321–331. doi: 10.1006/nbdi.2000.0304
Sabbagh, M. N., Sandhu, S., Kolody, H., Lahti, T., Silverberg, N. B., and Sparks, D. L. (2006). Studies on the effect of the apolipoprotein E haplotype on the lipid profile in Alzheimer’s disease. Curr. Alzheimer Res. 3, 157–160. doi: 10.2174/156720506776383013
Schilling, S., Tzourio, C., Soumaré, A., Kaffashian, S., Dartigues, J. F., Ancelin, M. L., et al. (2017). Differential associations of plasma lipids with incident dementia and dementia subtypes in the 3C Study: A longitudinal, population-based prospective cohort study. PLoS Med. 14:e1002265. doi: 10.1371/journal.pmed.1002265
Serrano-Pozo, A., Qian, J., Monsell, S. E., Betensky, R. A., Hyman, B. T., et al. (2015). APOEε2 is associated with milder clinical and pathological Alzheimer disease. Ann. Neurol. 77, 917–929. doi: 10.1002/ana.24369
Solomon, A., Kåreholt, I., Ngandu, T., Winblad, B., Nissinen, A., Tuomilehto, J., et al. (2007). Serum cholesterol changes after midlife and late-life cognition: twenty-one-year follow-up study. Neurology 68, 751–756. doi: 10.1212/01.wnl.0000256368.57375.b7
Sun, L., Hu, C., Zheng, C., Huang, Z., Lv, Z., Huang, J., et al. (2014). Gene-gene interaction between CETP and APOE polymorphisms confers higher risk for hypertriglyceridemia in oldest-old Chinese women. Exp. Gerontol. 55, 129–133. doi: 10.1016/j.exger.2014.04.003
Wang, P., Zhang, H., Wang, Y., Zhang, M., and Zhou, Y. (2020). Plasma cholesterol in Alzheimer’s disease and frontotemporal dementia. Transl. Neurosci. 11, 116–123. doi: 10.1515/tnsci-2020-0098
Wu, Y., Wang, Z., Jia, X., Zhang, H., Zhang, H., Li, J., et al. (2019). Prediction of Alzheimer’s disease with serum lipid levels in Asian individuals: a meta-analysis. Biomarkers 24, 341–351. doi: 10.1080/1354750X.2019.1571633
Keywords: Alzheimer’s disease, lipid profiles, sex, APOEε4, APOEε2
Citation: Fu J, Huang Y, Bao T, Ou R, Wei Q, Chen Y, Yang J, Chen X and Shang H (2022) Effects of Sex on the Relationship Between Apolipoprotein E Gene and Serum Lipid Profiles in Alzheimer’s Disease. Front. Aging Neurosci. 14:844066. doi: 10.3389/fnagi.2022.844066
Received: 27 December 2021; Accepted: 08 April 2022;
Published: 30 May 2022.
Edited by:
Kuangyu Shi, University of Bern, SwitzerlandReviewed by:
Nutjaree Jeenduang, Walailak University, ThailandCopyright © 2022 Fu, Huang, Bao, Ou, Wei, Chen, Yang, Chen and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueping Chen, Y2hlbnh1ZXBpbmcwNjA2QHNpbmEuY29t; Huifang Shang, aGZzaGFuZzIwMDJAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.