
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Nutr. , 26 March 2021
Sec. Sport and Exercise Nutrition
Volume 8 - 2021 | https://doi.org/10.3389/fnut.2021.652094
This article is part of the Research Topic Polyphenols and their Impact on Human Health And Performance: Plant Power View all 3 articles
Tart cherries (TC) are a rich source of polyphenols that elicit antioxidant and anti-inflammatory effects. As a consequence, the effects of TC derived supplements on markers of human health, exercise performance and sleep have been investigated. Supplementation protocols have been highly variable across studies and the dose of bioactive compounds used has often been poorly characterized. Specific and non-specific analytical methods were employed for measuring the total polyphenol and anthocyanin content in TC supplements. This review critically analyses the supplementation protocols and the analytical methods used for the characterization of TC supplements, culminating in recommendations for good practice in the analysis and reporting of the polyphenol content and profile of TC products. A literature search was conducted using PubMed/Medline and Web of Science up to May 4th, 2020, including studies published in all years prior. Only articles written in English that provided a TC dietary supplement as opposed to fresh whole TC were included in this review. Forty-three studies were identified as eligible and included for analysis in this review. The studies investigated the effects of TC supplementation on various aspects of human health, exercise recovery and performance and sleep. Twenty studies conducted an analysis of TC supplement and reported total polyphenol/anthocyanin content. Six studies did not report the polyphenol content of the TC supplement used. Seventeen studies reported the TC supplement polyphenol content but this was derived from previously published studies and presumably different supplement batches. The duration of the supplementation protocol ranged from acute supplementation to 84 days, meanwhile the total polyphenol and anthocyanin dose ranged from 143 to 2,140 mg/day and 15 to 547 mg/day, respectively. Due to the variety of specific and non-specific analytical methods used, the relative efficacy of different doses and polyphenol blends cannot reliably be extrapolated from critical analysis of the literature. Future studies should conduct an analysis of the study supplement batch. In addition to analysis and reporting of total polyphenol content, specific analytical methods such as HPLC UV/MS should be used to quantify total and individual anthocyanin contents.
Tart cherries (TC) are part of the Prunus species and are predominantly cultivated from the Montmorency cultivar (1). The chemical composition of TC can be affected by many parameters such as cultivar, maturation stage, agricultural practices, and environmental conditions. In general, the level of soluble solids increases as the fruit matures, whereas titratable acidity declines (2). Water and carbohydrates are the major constituents of the fruit. The main sugar in cherries is glucose with no starch present (3) and malic acid at 600–900 mg/100 grams of fresh weight is the dominant organic acid (2). All essential amino acids can be found in TC with an additional high level of melatonin in the Montmorency cultivar (3, 4). Tart cherries are considered a good source of potassium; other minerals exist in low concentrations in the fruit. There are a wide range of vitamins in TC and a noticeably high level of vitamin A. The macro- and micro-nutrient and phytochemical content of TC is compared to other common berry fruits in Table 1 (3, 5). The plant is rich in phenolic compounds which have become the focus of interest for consumers and researchers since these compounds are considered to confer their antioxidant and anti-inflammatory properties (6–10).
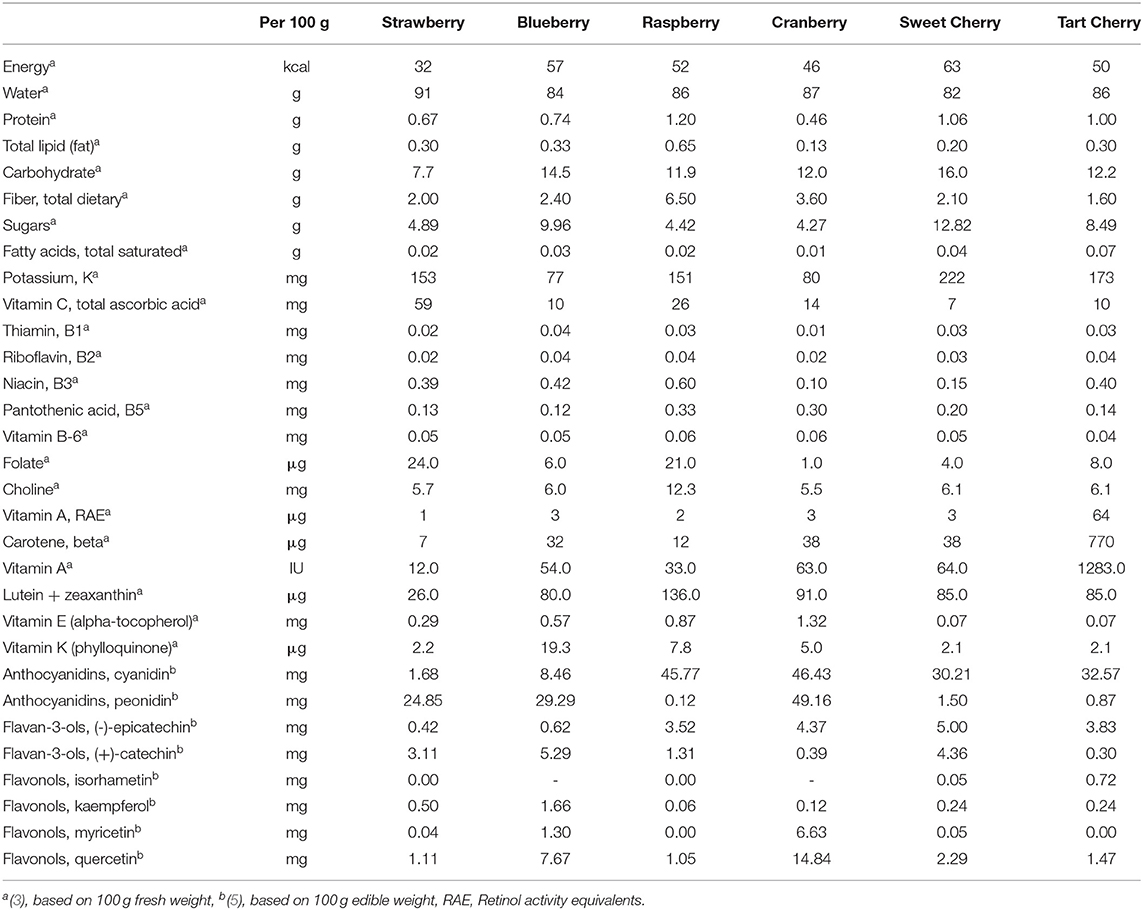
Table 1. Comparison of major nutrients, vitamins and phenolic compounds between tart cherries and other common berries.
At least 24 anthocyanins, 12 phenolic acids, 17 flavanols, and 18 flavones have been identified in tart cherries (11). Collectively this results in a high total polyphenol content, on average 352 mg of total polyphenols per 100 g of fresh weight (fw) (12) and a greatly diversified profile that includes kaempferol, quercetin, cathechins, epicathechins, proanthocyanidins and anthocyanins (13). Although the anthocyanins present in TC have received the highest degree of interest to date, it is possible that the bioactive properties of TC arise from the interaction of the various polyphenols present in this fruit, which may act synergistically in modulating various molecular pathways (14). The commonly detected anthocyanins in tart cherries are cyanidin-3-glucoside, cyanidin-3-glucoslrutinoside, cyanidin-3-rhamnoglucoside, cyanidin-3-sophorside, peonidin-3-glucoside, and peonidin-3-rutinoside (11). The types and the relative abundance of the anthocyanins vary among cultivars (15, 16).
TC polyphenols are likely to exert antioxidant and anti-inflammatory properties by modulating several cell signaling pathways (17, 18). Growing evidence indicates that rather than exerting direct antioxidant effects as radical scavengers, polyphenols upregulate endogenous antioxidant capacity via activation of the transcription factor nuclear related factor 2 (Nrf2)/antioxidant response element (ARE) pathway (19, 20). Signaling is suggested to be induced by quinones, produced via exposure of polyphenols to reactive oxygen species. In vitro, polyphenols have been shown to have the potential to protect the Keap1-Nrf2 complex against ubiquitylation and degradation, and to promote Nrf2 phosphorylation. Phosphorylated Nrf2 translocates to the nucleus resulting in downstream gene and protein expression [for reviews see (21, 22)], culminating with increased synthesis of phase || detoxifying and antioxidant enzymes (22). Furthermore, polyphenols may inhibit the expression and activity of superoxide producing enzymes such as nicotinamide adenine dinucleotide phosphate (NAPDH) oxidase (23–25), thereby reducing the formation of ROS. The anti-inflammatory molecular mechanisms of action for polyphenols have also been explored through in vitro studies. The current body of literature indicates that polyphenols inhibit the enzymatic activity and expression of the two cyclooxygenase (COX) isoforms, COX1 and COX 2 (26, 27), thus preventing the formation of prostaglandins, a group of lipid compounds that are involved in the inflammatory response by promoting swelling and pain (28). Furthermore, polyphenols may also potentially inhibit the activation of nuclear factor-κ B (NF-κB) (29), a transcription factor that modulates the expression of over 200 genes involved in the body's pro-inflammatory response. These bioactive properties provide important potential applications for TC supplementation in the management and treatment of various clinical pathologies which are linked to chronic elevation of oxidative stress and inflammation, such as cardiovascular and metabolic diseases (30–33). Indeed, there is evidence to suggest that TC supplementation is able to reduce pain and other clinical symptoms associated with knee osteoarthritis (34), to improve vascular function and cardio-metabolic markers (35–37) and to reduce uric acid markers, which has important implications for gout management (38, 39). Although, not all studies have found such favorable effects [for a review, see (40)].
Furthermore, the elevated levels of oxidative stress and inflammation identified following intense athletic competitions (7, 41, 42) contribute to the exercise-induced muscle damage (EIMD) that occurs after these events, and negatively impact exercise recovery and subsequent athletic performance. The increased production of ROS during intense exercise impairs blood flow and vasodilation (43) and may also impair calcium handling and sensitivity, and disrupt mitochondrial function (44), resulting in ergolytic effects on exercise performance. Given the antioxidant and anti-inflammatory properties of TC, the potential ergogenic effects of its supplementation for exercise recovery and performance have been investigated. To date, 8 studies showed a beneficial effect of TC supplementation on recovery following various exercise modalities including strenuous resistance-based exercise (45, 46), endurance running (7, 47) and intermittent running/cycling protocols (48–51). In contrast, seven studies found no improvements in muscle function recovery or subsequent physical performance following TC supplementation (52–58). The research on TC supplementation and exercise performance has so far been more limited and focused on endurance exercise only, with three out of four studies finding a beneficial effect of this nutritional strategy (59–61). Lastly, TC supplementation may also enhance sleep in both clinical and athletic environments (62, 63). A limited number of studies have found improvements in sleep duration and/or quality in both young (64) and older (65, 66) subjects. The melatonin content and anti-inflammatory properties of TC have been suggested to drive these beneficial effects on sleep (63, 64). Nevertheless, the mechanisms of action are yet to be established.
To date, there have been multiple narrative reviews exploring the applications of TC and derived dietary supplements for human health (40, 67), exercise recovery and performance (67–70). Furthermore, several systematic reviews have found favorable effects of TC supplementation on systolic blood pressure and systemic markers of inflammation (71), uric acid and gout (72) and endurance exercise performance (73). However, it is hard to compare the dose of bioactive compounds provided across studies since the methods utilized to analyse the composition of the TC supplements provided are highly variable. In some instances, non-specific methods such as antioxidant activity assays, total phenolic content assays, and total anthocyanin assays have been adopted. Alternatively, individual chemical constituents and specific chemical data can be generated by the use of advanced analytical instruments such as high performance liquid chromatography (HPLC), mass spectrometry (MS), and nuclear magnetic resonance (NMR). These approaches have been less commonly employed in the TC supplementation literature, although the adoption of these techniques has increased (Table 2). There is a large variability between sour cherry cultivars in terms of both total polyphenol (from 74 to 754 mg/100 g fw) and anthocyanin (21 and 285 mg/100 g fw) content and/or profile (1, 90). In addition growing conditions and post-harvest processing alter the phenolic composition, such that a wide variation in phenolic dose and blend is anticipated between supplements and studies, which will impact upon supplement efficacy. Accurate quantification and reporting of total polyphenol content and the specific polyphenol subclasses present in the TC supplements is therefore critical to determine the influence of the dose and blend of polyphenols on efficacy. None of the published reviews have attempted such an analysis due to the high degree of variability in the analytical methods used, and a reliance on the measurement of total polyphenol rather than specific anthocyanin content. In this review the TC supplementation protocols and the analytical methods used for the characterization of TC supplements are critically analyzed and best-practice recommendations made for the analysis and reporting of the polyphenol content and profile of TC products.
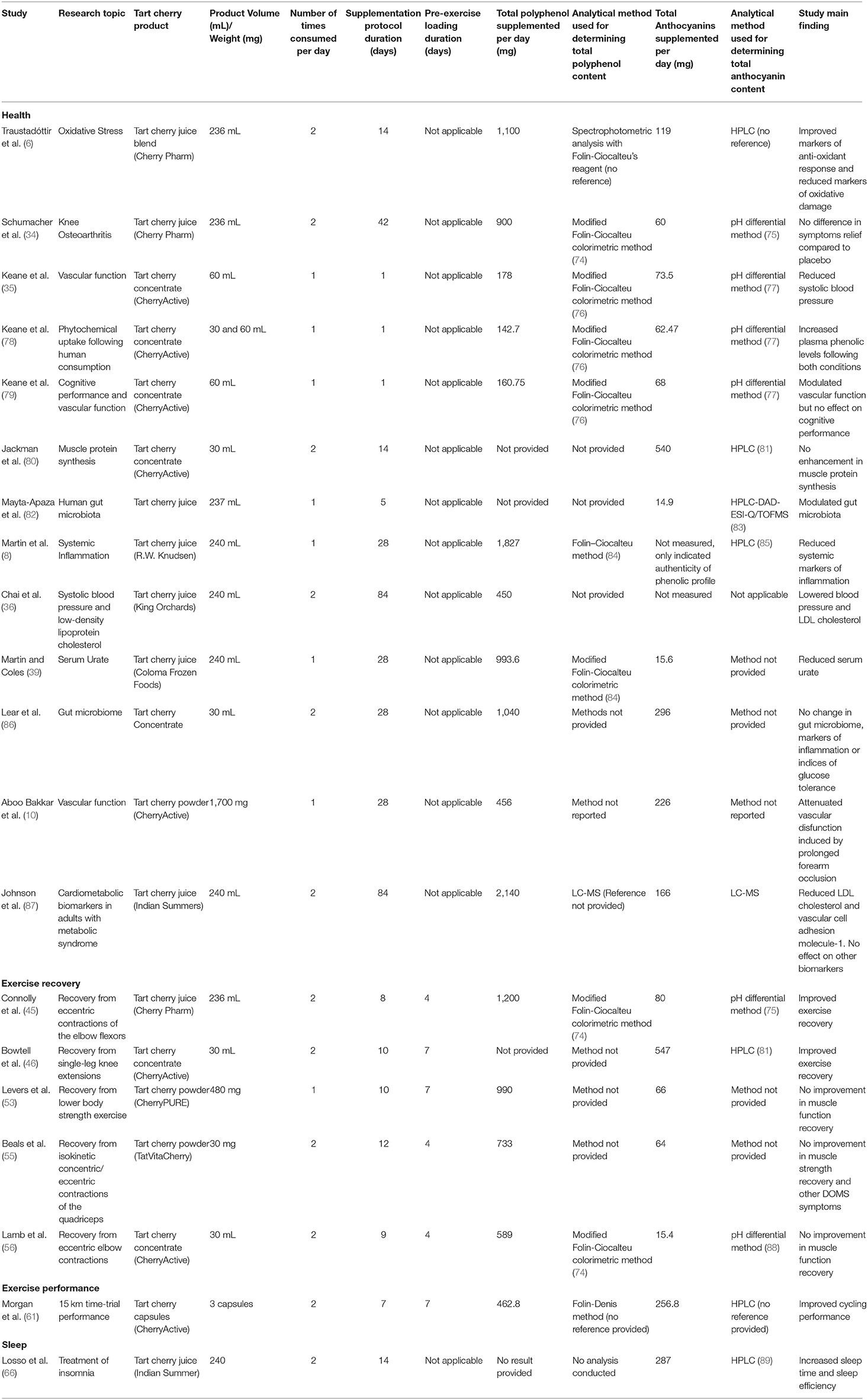
Table 2. Table presenting the extracted data from the tart cherry supplementation studies included in this review that conducted direct analysis on the dietary supplement used in the supplementation protocol.
A literature search was conducted using PubMed/Medline and Web of Science up to May 4th, 2020, including studies published in all years prior. Keywords included in the search were: tart cherry, tart cherry juice, tart cherry concentrate, cardiovascular health, metabolic health, blood pressure, uric acid, gout, gut health, gut microbiome, cognitive performance, exercise recovery, exercise-induced muscle damage, exercise performance, physical performance, ergogenic effects, sleep. No data restrictions were placed for publication date or subjects age, however only articles written in English were eligible for inclusion in this review. Only studies that used a tart cherry dietary supplement (concentrate, juice, powder, capsules or gels) as opposed to fresh whole tart cherry were eligible for this review. Study protocols, abstracts of conference proceedings, animal and in vitro studies were excluded. The forward citation and the reference lists of the articles identified to be eligible for this review have also been screened for additional eligible studies.
A total number of 43 studies were found eligible for this review (Figure 1), ranging in their topic from the application of TC supplementation for various aspects of human health, to exercise recovery, exercise performance and sleep. The following data were extracted from the identified studies: location, topic, participants characteristics, dietary supplement used, dietary supplement volume/weight, number of times the supplement was consumed per day, dietary supplement manufacturer, duration of the supplementation protocol, supplementation protocol pre-exercise loading (where applicable), total polyphenol and anthocyanin dosage per day, analytical methods used for measurement of total polyphenol and anthocyanin content and main study findings.
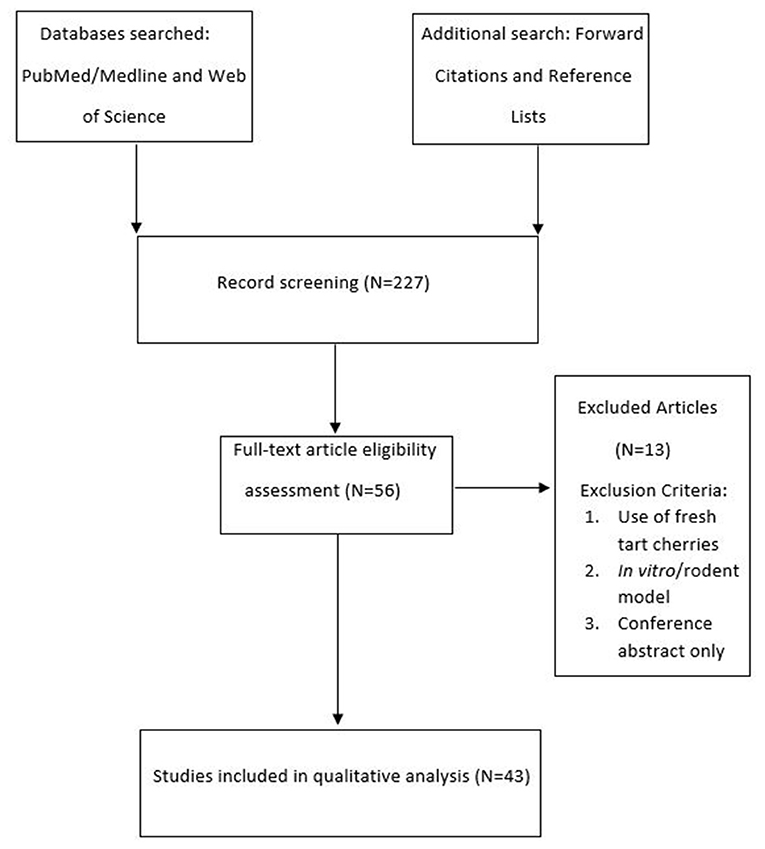
Figure 1. Diagram illustrating the literature search. The number in brackets indicates the total number of studies identified.
Extracted data were grouped based on the analytical methods used for TC analysis and presented in three tables. Table 2 includes the studies where direct primary analysis of total polyphenol and anthocyanin content (20/43) was conducted. Table 3 describes studies where no quantification of total polyphenol and anthocyanin content was performed, but values from previously published articles were reported (17/43). Table 4 describes studies that have reported no total polyphenol and anthocyanin content values (6/43).
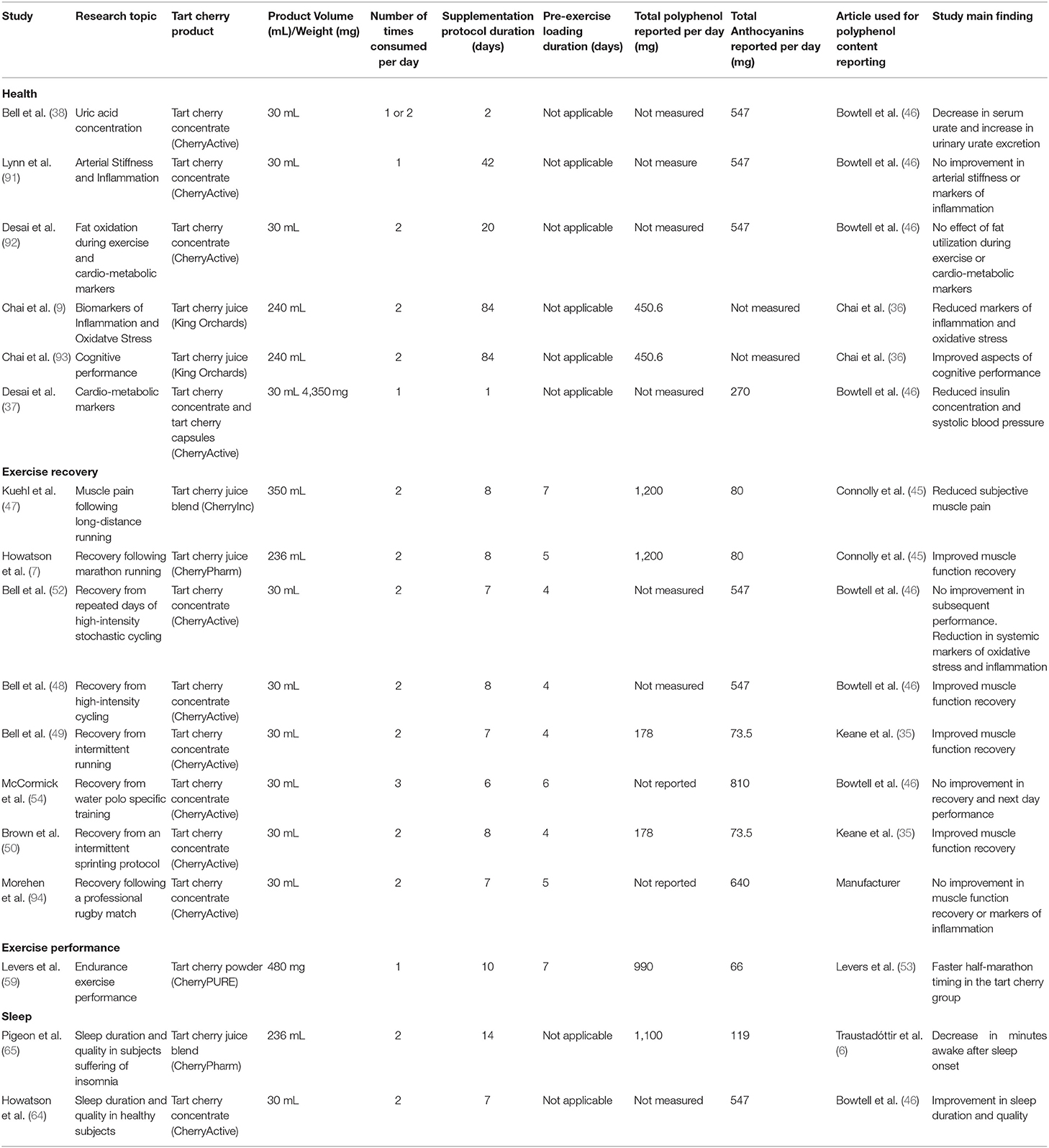
Table 3. Table presenting the extracted data from the tart cherry supplementation studies included in this review that reported the characterization (polyphenol and anthocyanin content) from previously published studies.
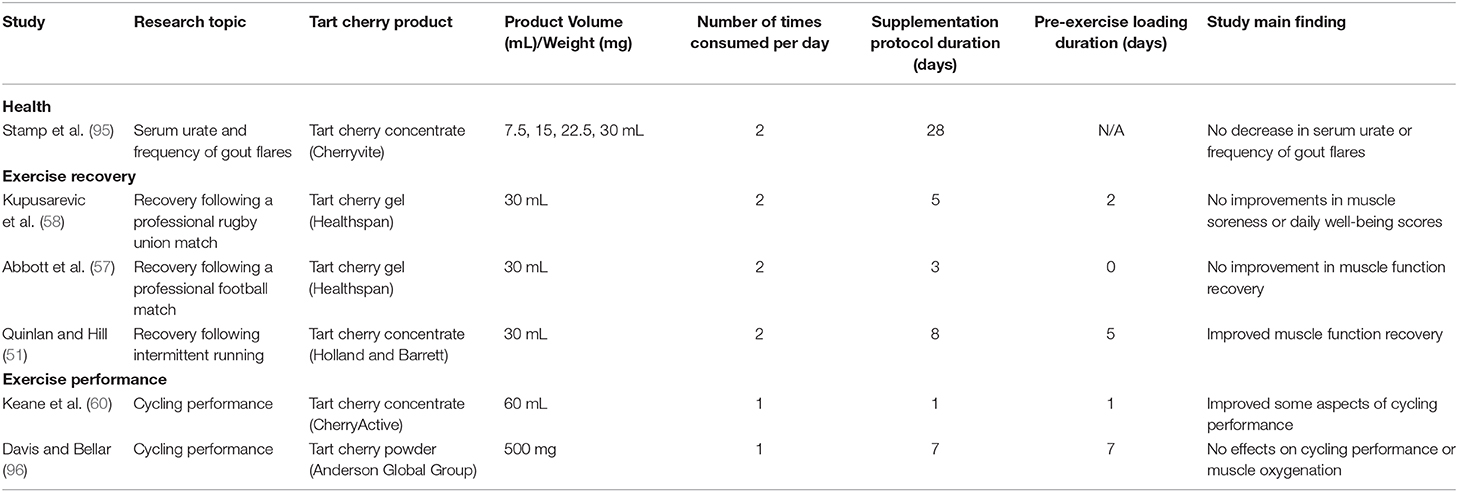
Table 4. Table presenting the extracted data from the tart cherry supplementation studies included in this review that did not include any characterization of the dietary supplement used.
Twenty studies that report conducting an analysis of TC supplement total polyphenol/anthocyanin content are included in Table 2. A large variation was identified in the supplementation protocols used within these studies. The duration of the supplementation protocols ranged from acute supplementation where only one TC dose was provided (35, 78, 79), to 84 days of chronic supplementation (9, 93). The type of dietary supplements were TC juice, concentrate, powder and capsules, produced by various manufacturers. Out of the 20 studies, 16 studies measured the total polyphenol content of the dietary supplement provided, meanwhile the anthocyanin content was measured and indicated in 18 studies. In total, 14 of these 20 studies indicated both the total polyphenol and anthocyanin content of the dietary supplement used. In the studies that conducted direct analysis of the supplement used in the study, the total polyphenol dose supplemented by participants ranged from 143 to 2,140 mg/day, meanwhile the anthocyanin dose ranged from 15 to 547 mg/day. The analytical methods used for determining the total polyphenol content of dietary supplements include several modified colorimetric methods based on the Folin-Ciocalteu or the Folin-Denis reagent and a liquid chromatography-mass spectrometry method, meanwhile several studies did not indicate the analytical method used, despite reporting that an analysis was conducted. The analytical methods used for determining the anthocyanin content included several HPLC-based and pH differential methods. Similarly, several studies provided the anthocyanin content of the dietary supplement used but did not indicate the analytical method used for this measurement.
Seventeen studies are presented in Table 3. Although values for the total polyphenol and/or the anthocyanin content of the dietary supplement used are reported in the studies included in this table, these values were reproduced from previously published studies. The duration of the supplementation protocols ranged from acute supplementation where only one TC dose was served (37), to chronic supplementation with a duration of up to 84 days (36). The type of dietary supplements used were TC juice, concentrate, powder and capsules, produced by various manufacturers. The reported total polyphenol and anthocyanin dose supplemented by participants ranged from 178 to 1,200 mg/day and 66 to 810 mg/day, respectively.
Six studies were included in Table 4. The duration of the supplementation protocol ranged from acute supplementation where only one TC dose was provided (60) to 28 days of supplementation with two daily doses (95). The type of dietary supplements used in these studies were TC concentrate, gel and powder. No values for the total polyphenol or the anthocyanin content of the dietary supplements used were reported in these studies.
The goal of this review was to critically analyse the analytical methods employed to characterize TC supplement phenolic composition with the aim of generating evidence-based recommendations for good practice in the analysis and reporting of the polyphenol content and profile of TC products in future studies.
From the studies included in this review, less than half conducted an analysis of the specific batch of TC supplement used, with an even lower number (~30%) reporting both the total phenolic and anthocyanin content. Furthermore, specific and non-specific analytical methods were used in these studies for the characterization of TC supplements, with the latter being most frequently utilized. Total phenolic content assays, such as Folin-Ciocalteu, and total anthocyanin content assays based on the pH differential methods are common non-specific methods used by researchers for estimating sample phenolics (97–100). These analytical methods were also used frequently in the TC supplementation literature (34, 35, 45, 56, 78, 79). Furthermore, although less frequently encountered in the studies identified in this review, other non-specific analytical methods, for example antioxidant activity measurements, inducing oxygen radical absorbance capacity (ORAC), DDPH, and ABTS, have been previously used for examining the chemistry of TC (101, 102). Nevertheless, in recent years, researchers have come to recognize the limitations of these non-specific methods, as they are susceptible to pH, solvent, and sample matric effects (98, 99, 103). Methods such as Folin-Ciocaleteu, ABTS and ORAC are based on the reductive capacity of the compounds. However, plant extracts contain a wide range of chemicals, many of which exhibit reductive capacity. Especially for fruits, like berries and oranges, strong interferences can be found with components, such as organic acids and reducing sugars, which greatly undermine the reliability of the results (100). It has been long acknowledged that there is a lack of agreement between data obtained from the HPLC methods and the Folin-Ciocaleteu method (85). The Folin-Ciocalteu assay appeared to under-report total phenolic concentration and displayed no significant correlation with total phenolics via HPLC (104). Total anthocyanin content assays based on pH differential methods work under the assumption that all aglycones in solution are the hydrolysis products of anthocyanins, despite the fact that other compounds may have the same aglycone once hydrolysed (105). A comparative study between pH differential and HPLC methods for total anthocyanin contents in tart cherry juice revealed that the difference in data between the two methods can be over 2-fold (106). Even though there was a good correlation between the two methods, the pH differential method tends to underestimate the total anthocyanin content (106–108). This under reporting has also been found in method-comparison studies of other berries and was demonstrated to be a result of the difference in glycone in the berries (108). The types of glycone vary based on berry types and the impact of such variations on the pH differential method data is observed but not well-controlled or understood (108). In addition to this lack of agreement between total anthocyanin measurement methods, a simple total phenolic value not do justice to the complexity of the chemical profile of the plant. Very little chemical information can be obtained from the value. For example, there was little to no difference in the total phenolic content between green cabbage and pear (58 mg vs. 60 GAE/100 g fresh weight), even though their individual phenolic profiles differ substantially (109).
The analysis of anthocyanins is complicated because of their ability to undergo structural transformations, which makes them highly reactive and easily degraded during storage and analysis (110). Recent advances in chromatographic methods, such as HPLC UV/MS, have enabled researchers to provide more specific and accurate quantification of anthocyanin in tart cherries. Individual anthocyanin in tart cherries has been identified and quantified. Such methods are often tailored to the target anthocyanin and sample matrix, with optimized sample preparation and analysis conditions that minimize anthocyanin degradation and transformation. Liquid chromatography has the capability of determining individual anthocyanin levels to mg/kg or μg/kg, depending on the detection method. HPLC was used in a number of studies identified in this review for measuring the total anthocyanin content and the main anthocyanin groups present in TC supplements (6, 46, 61, 66, 80). The use of these analytical methods instead of the pH differential methods represents an important step toward identifying the role played by the individual anthocyanin families in the efficacy of TC supplements. Furthermore, using HPLC UV/MS for measuring the other flavonoids and polyphenol groups present in TC supplements would provide further insight into the importance of the polyphenol profile for various health and exercise related goals. The major challenge of using HPLC, especially HPLC UV, for quantifying individual anthocyanin is the difficulty in obtaining the anthocyanin reference standards. The estimated number of anthocyanins found in nature are over 550 (111); however, only 5% of these are commercially available as reference standards (112). Thus, HPLC coupled to various type of MS is preferable since these approaches allow exact molecular weight determinations (113). HPLC-MS is often used for determination of anthocyanin in TC juice (87), due to the low level of anthocyanin this dilute sample (e.g., vs concentrate); hence there is a need for a more sensitive MS detector. However outside of juice analyses, this HPLC-MS approach is less often encountered compared to HPLC UV. This is due to its high cost and technical difficulty.
Nevertheless, from the studies that did employ HPLC (Table 2), only three studies included a detailed HPLC method. The remaining either provided no information beyond stating that an HPLC method was used or indicated that a third-party company completed a HPLC analysis. Although a published method was used by the third-party company (81) with some method validation information such as recovery and precision, neither the detection limit nor the reproducibility of the method was provided. Method validation is a process of using experimental design to prove that the method fits its intended purposes and produces accurate and precise results, all of which are fundamental to the analysis (114). Evaluating parameters, such as specificity, precision, linearity, accuracy, range, detection limit, quantitation limit, robustness, and system suitability, is a basic requirement of the United States Pharmacopeia and AOAC INTERNATIONAL for analytical methods (114, 115). Inadequate validation or lack of validation, especially transferring methods between sample types, such as from grape juice to tart cherry (8), can compromise the reliability of the data. Tart cherry showed a unique chemical profile and matrix effects in the literature, indicating a strong need for a method validation process to ensure the accuracy of the data. Furthermore, to date, no studies investigating TC supplementation effects on human physiology have used HPLC-MS or HPLC UV for measuring the other flavonoids present in the supplement in addition to the anthocyanin content. LC-MS and UV-visible MS were however previously used for determining the total polyphenol content and the amount of individual polyphenolics, respectively, present in a New Zealand blackcurrant juice (116). A similar approach could be applied for an optimal characterization of TC supplements.
In the studies where direct analysis of the supplement was conducted (Table 2), a large variation in the total polyphenol and the anthocyanin content of the TC supplements used, and thus in the daily supplemented dose, were found. These large differences in the quantity of total polyphenols and anthocyanins supplemented are driven by the different types of TC supplement used (different TC quantity & concentration used for supplement production), the natural variation in the polyphenol content and profile of TC and the different intended daily doses of supplement provided. The different analytical methods used for the characterization of TC supplements also potentially influence the findings. Large differences in the anthocyanin content of TC supplements appear to exist [between the studies that have used HPLC analysis [60 to 273 mg/30 mL; (6, 46, 66, 80)] and those that used various pH differential methods [7.5 to 40 mg/30 mL; (34, 35, 45, 56, 78, 79)]. Furthermore, differences in the total phenolic content of the TC supplements also tend to exist between the studies that used different versions of the Folin-Ciocalteu method, with the modified version based on Shahidi (76), consistently showing lower values (35, 78, 79) compared to the values identified in the studies (34, 39, 45, 56) that used previous versions of the Folin-Ciocalteu method [(74) or (84)]. These data highlight the importance of standardizing the analytical methods used for the characterization of TC supplements, with the goal of allowing effective comparisons among the studies. Ultimately, this would represent an important step toward the development of optimal total polyphenol dose and polyphenol profile recommendations for TC supplementation based on critical analysis of published studies. At present the degree of variability in the methods employed preclude such an approach.
Of the studies included in this review, 40% reported TC supplement phenolic values derived from previously published studies for the same supplement type. An additional study (94) indicated the total anthocyanin content provided by the manufacturer, without batch-testing the TC supplement used. Given the high natural variation in the total polyphenol content and polyphenol profile of TC (1, 90) the values provided in the studies above may not be an accurate representation of the phenolic composition of the consumed TC supplements. As a consequence, any comparison of dose and polyphenol blend across studies to draw conclusions regarding the efficacy and optimal TC polyphenol dose and blend is flawed. Several additional studies did not provide any characterization of the dietary supplements used. These studies compound the lack of clarity surrounding the polyphenol dose and profile provided via TC supplementation.
Alongside these analytical shortcomings within the TC literature, it is important to briefly note that the supplementation protocols used within the studies included in this review also varied considerably. Large differences were identified between studies with regard to both the duration of the supplementation protocol and the intended daily polyphenol and anthocyanin dose provided. Further, there is considerable variability in the type of TC supplements used across the studies. When these sources of heterogeneity are considered in parallel with the inadequate analytical methods employed for characterizing TC supplements, comparisons across research studies are not reliable. There is no standard reference material or consistent sample that could provide a reliable measure for comparison between studies. This raises a substantial challenge to the critical analysis of the literature in order to derive evidence-based recommendations for TC supplementation protocols.
In order to allow for greater confidence in future crossstudy comparisons, better data on supplement composition is required. On the basis of the existing discrepancies in the literature, we recommend that future TC supplementation studies should; (a) use specific analytical methods such as HPLC UV/MS to quantify total and individual anthocyanin content and to characterize other polyphenol contents, (b) provide an analysis of the study supplement batch and appropriate controls, (c) provide multiple timepoint analyses to demonstrate stability of the supplement polyphenol content throughout the study period and (d) provide quantitative data for all of the different formulation types used in the study. A repository of frozen supplement samples could be a useful tool for cross study comparisons. Indeed, samples could be shared between labs for standardization purposes. In the absence of certified reference materials for TC supplements, a standard supplement preparation included with each analytical batch could serve as a measure of method stability, accuracy and precision. Adoption of these recommendations in future studies would allow comparison of polyphenol dose across studies and allow researchers and applied nutrition practitioners to generate evidence-based recommendations for TC supplementation strategies.
In conclusion, there is a high degree of variation in the supplementation protocols and the analytical methods used within the TC supplementation literature. Over 50% of the studies conducted to date reported polyphenol and anthocyanin content of the TC supplement from previously conducted studies rather than the specific study batch, or did not provide any characterization of the supplement used. Where direct analyses were conducted, both specific and non-specific methods were used. However, non-specific assays for measuring total phenolics content and total anthocyanin content predominate and these do not fully reflect the complex chemistry of TC supplements. Using these approaches alone to draw conclusions regarding the relationship between specific chemicals or classes of chemicals and health or performance outcomes is not appropriate. Specific analytical methods are required to better understand how the chemical nature of plants and ultimately how consumption of those chemicals may influence health, exercise and sleep.
VS was responsible for selecting the studies eligible for this review and for extracting the required data for subsequent analysis. All authors were involved in manuscript writing (review and editing), study design and methodology development and agree to be accountable for the content of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Wojdyło A, Nowicka P, Laskowski P, Oszmianski J. Evaluation of sour cherry (Prunus cerasus L.) fruits for their polyphenol content, antioxidant properties, and nutritional components. J Agric Food Chem. (2014) 62:12332–45. doi: 10.1021/jf504023z
2. Serradilla MJ, Hernández A, López-Corrales M, Ruiz-Moyano S, de Guía Córdoba M, Martín A. Composition of the Cherry (Prunus avium L. and Prunus cerasus L.; Rosaceae). In: Nutritional Composition of Fruit Cultivars. London: Academic Press (2016). p. 127–47. doi: 10.1016/B978-0-12-408117-8.00006-4
3. USDA. Food Data Central. (2020). Available online at: https://ndb.nal.usda.gov/fdc-app.html#/food-details/173954/nutrients (accessed December 9, 2020).
4. Burkhardt S, Tan DX, Manchester LC, Hardeland R, Reiter RJ. Detection and quantification of the antioxidant melatonin in Montmorency and Balaton tart cherries (Prunus cerasus). J Agric Food Chem. (2001) 49:4898–902. doi: 10.1021/jf010321+
5. USDA. Database for the Flavonoid Content of Selected Foods. (2015). Available online at: https://libraryguides.vu.edu.au/harvard/internet-websites (accessed December 9, 2020).
6. Traustadóttir T, Davies SS, Stock AA, Su Y, Heward CB, Roberts LJ, et al. Tart cherry juice decreases oxidative stress in healthy older men and women. J Nutr. (2009) 139:1896–900. doi: 10.3945/jn.109.111716
7. Howatson G, McHugh MP, Hill JA, Brouner J, Jewell AP, Van Someren KA, et al. Influence of tart cherry juice on indices of recovery following marathon running. Scand J Med Sci Sports. (2010) 20:843–52. doi: 10.1111/j.1600-0838.2009.01005.x
8. Martin KR, Burrell L, Bopp J. Authentic tart cherry juice reduces markers of inflammation in overweight and obese subjects: a randomized, crossover pilot study. Food Funct. (2018) 9:5290–300. doi: 10.1039/C8FO01492B
9. Chai SC, Davis K, Zhang Z, Zha L, Kirschner KF. Effects of tart cherry juice on biomarkers of inflammation and oxidative stress in older adults. Nutrients. (2019) 11:228. doi: 10.3390/nu11020228
10. Aboo Bakkar Z, Fulford J, Gates PE, Jackman SR, Jones AM, Bond B, et al. Montmorency cherry supplementation attenuates vascular dysfunction induced by prolonged forearm occlusion in overweight, middle-aged men. J Appl Physiol. (2019) 126:246–54. doi: 10.1152/japplphysiol.00804.2018
11. Poonam V, Kumar G, S Reddy LC, Jain R, K Sharma S, K Prasad A, et al. Chemical constituents of the genus Prunus and their medicinal properties. Curr Med Chem. (2011) 18:3758–824. doi: 10.2174/092986711803414386
12. Phenol Explorer. Database on Polpyphenol Content in Foods. (2016). Available online at: http://phenol-explorer.eu/ (accessed June 10, 2020).
13. Chaovanalikit A, Wrolstad RE. Anthocyanin and polyphenolic composition of fresh and processed cherries. J Food Sci. (2004) 69:73–83. doi: 10.1111/j.1365-2621.2004.tb17859.x
14. Kirakosyan A, Seymour EM, Noon KR, Llanes DEU, Kaufman PB, Warber SL, et al. Interactions of antioxidants isolated from tart cherry (Prunus cerasus) fruits. Food Chem. (2010) 122:78–83. doi: 10.1016/j.foodchem.2010.02.017
15. Damar I, Ekşi A. Antioxidant capacity and anthocyanin profile of sour cherry (Prunus cerasus L.) juice. Food Chem. (2012) 135:2910–4. doi: 10.1016/j.foodchem.2012.07.032
16. Kirakosyan A, Seymour EM, Llanes DEU, Kaufman PB, Bolling SF. Chemical profile and antioxidant capacities of tart cherry products. Food Chem. (2009) 115:20–5. doi: 10.1016/j.foodchem.2008.11.042
17. Shukitt-Hale B, Kelly ME, Bielinski DF, Fisher DR. Tart cherry extracts reduce inflammatory and oxidative stress signaling in microglial cells. Antioxidants. (2016) 5:33. doi: 10.3390/antiox5040033
18. Kirakosyan A, Gutierrez E, Solano BR, Seymour EM, Bolling SF. The inhibitory potential of Montmorency tart cherry on key enzymes relevant to type 2 diabetes and cardiovascular disease. Food Chem. (2018) 252:142–6. doi: 10.1016/j.foodchem.2018.01.084
19. Scapagnini G, Sonya V, Nader AG, Calogero C, Zella D, Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol. (2011) 44:192–201. doi: 10.1007/s12035-011-8181-5
20. Zhang Q, Pi J, Woods CG, Andersen ME. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol Appl Pharmacol. (2010) 244:84–97. doi: 10.1016/j.taap.2009.08.018
21. Forman HJ, Davies KJ, Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med. (2014) 66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045
22. Huang Y, Li W, Su ZY, Kong ANT. The complexity of the Nrf2 pathway: beyond the antioxidant response. J Nutr Biochem. (2015) 26:1401–13. doi: 10.1016/j.jnutbio.2015.08.001
23. Dávalos A, de la Pena G, Sanchez-Martin CC, Guerra MT, Bartolome B, Lasunción MA. Effects of red grape juice polyphenols in NADPH oxidase subunit expression in human neutrophils and mononuclear blood cells. Br J Nutr. (2009) 102:1125–35. doi: 10.1017/S0007114509382148
24. Calabriso N, Massaro M, Scoditti E, D'Amore S, Gnoni A, Pellegrino M, et al. Extra virgin olive oil rich in polyphenols modulates VEGF-induced angiogenic responses by preventing NADPH oxidase activity and expression. J Nutr Biochem. (2016) 28:19–29. doi: 10.1016/j.jnutbio.2015.09.026
25. Rodriguez-Mateos A, Rendeiro C, Bergillos-Meca T, Tabatabaee S, George TW, Heiss C, et al. Intake and time dependence of blueberry flavonoid–induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am J Clin Nutr. (2013) 98:1179–91. doi: 10.3945/ajcn.113.066639
26. Corona G, Deiana M, Incani A, Vauzour D, Dessì MA, Spencer JP. Inhibition of p38/CREB phosphorylation and COX-2 expression by olive oil polyphenols underlies their anti-proliferative effects. Biochem Biophys Res Commun. (2007) 362:606–11. doi: 10.1016/j.bbrc.2007.08.049
27. Scoditti E, Calabriso N, Massaro M, Pellegrino M, Storelli C, Martines G, et al. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys. (2012) 527:81–9. doi: 10.1016/j.abb.2012.05.003
28. Burgos-Edwards A, Martín-Pérez L, Jiménez-Aspee F, Theoduloz C, Schmeda-Hirschmann G, Larrosa M. Anti-inflammatory effect of polyphenols from Chilean currants (Ribes magellanicum and R. punctatum) after in vitro gastrointestinal digestion on Caco-2 cells: anti-inflammatory activity of in vitro digested Chilean currants. J Funct Foods. (2019) 59:329–36. doi: 10.1016/j.jff.2019.06.007
29. Karlsen A, Retterstøl L, Laake P, Paur I, Kjølsrud-Bøhn S, Sandvik L, et al. Anthocyanins inhibit nuclear factor-κ B activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J Nutr. (2007) 137:1951–4. doi: 10.1093/jn/137.8.1951
30. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Münzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. (2001) 104:2673–8. doi: 10.1161/hc4601.099485
31. Vaziri ND, Rodríguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. (2006) 2:582. doi: 10.1038/ncpneph0283
32. Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González Á, Esquivel-Chirino C, et al. Inflammation, oxidative stress, and obesity. Int J Mol Sci. (2011) 12:3117–32. doi: 10.3390/ijms12053117
33. Fischer R, Maier O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: role of TNF. Oxidative Med Cell Longevity. (2015) 2015:610813. doi: 10.1155/2015/610813
34. Schumacher HR, Pullman-Mooar S, Gupta SR, Dinnella JE, Kim R, McHugh MP. Randomized double-blind crossover study of the efficacy of a tart cherry juice blend in treatment of osteoarthritis (OA) of the knee. Osteoarthritis Cartilage. (2013) 21:1035–41. doi: 10.1016/j.joca.2013.05.009
35. Keane KM, George TW, Constantinou CL, Brown MA, Clifford T, Howatson G. Effects of Montmorency tart cherry (Prunus cerasus L.) consumption on vascular function in men with early hypertension. Am J Clin Nutr. (2016) 103:1531–9. doi: 10.3945/ajcn.115.123869
36. Chai SC, Davis K, Wright RS, Kuczmarski MF, Zhang Z. Impact of tart cherry juice on systolic blood pressure and low-density lipoprotein cholesterol in older adults: a randomized controlled trial. Food Funct. (2018) 9:3185–94. doi: 10.1039/C8FO00468D
37. Desai T, Roberts M, Bottoms L. Effects of Montmorency tart cherry supplementation on cardio-metabolic markers in metabolic syndrome participants: a pilot study. J Funct Foods. (2019) 57:286–98. doi: 10.1016/j.jff.2019.04.005
38. Bell PG, Gaze DC, Davison GW, George TW, Scotter MJ, Howatson G. Montmorency tart cherry (Prunus cerasus L.) concentrate lowers uric acid, independent of plasma cyanidin-3-O-glucosiderutinoside. J Funct Foods. (2014) 11:82–90. doi: 10.1016/j.jff.2014.09.004
39. Martin KR, Coles KM. Consumption of 100% tart cherry juice reduces serum urate in overweight and obese adults. Curr Develop Nutr. (2019) 3:nzz011. doi: 10.1093/cdn/nzz011
40. Kelley DS, Adkins Y, Laugero KD. A review of the health benefits of cherries. Nutrients. (2018) 10:368. doi: 10.3390/nu10030368
41. Suzuki K, Nakaji S, Yamada M, Liu Q, Kurakake S, Okamura N, et al. Impact of a competitive marathon race on systemic cytokine and neutrophil responses Med Sci Sports Exerc. (2003) 35:348–55. doi: 10.1249/01.MSS.0000048861.57899.04
42. Mohr M, Draganidis D, Chatzinikolaou A, Barbero-Álvarez JC, Castagna C, Douroudos I, et al. Muscle damage, inflammatory, immune and performance responses to three football games in 1 week in competitive male players. Eur J Appl Physiol Occup Physiol. (2016) 116:179–93. doi: 10.1007/s00421-015-3245-2
43. Donato AJ, Uberoi A, Bailey DM, Walter Wray D, Richardson RS. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am J Physiol Heart Circ Physiol. (2010) 298:H671–8. doi: 10.1152/ajpheart.00761.2009
44. Reid MB. Reactive oxygen species as agents of fatigue. Med Sci Sports Exerc. (2016) 48:2239–46. doi: 10.1249/MSS.0000000000001006
45. Connolly DAJ, McHugh MP, Padilla-Zakour O. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br J Sports Med. (2006) 40:679–83. doi: 10.1136/bjsm.2005.025429
46. Bowtell JL, Sumners DP, Dyer A, Fox P, Mileva KN. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med Sci Sport Exerc. (2011) 43:1544–51. doi: 10.1249/MSS.0b013e31820e5adc
47. Kuehl KS, Perrier ET, Elliot DL, Chesnutt JC. Efficacy of tart cherry juice in reducing muscle pain during running: a randomized controlled trial. J Int Soc Sports Nutr. (2010) 7:1–6. doi: 10.1186/1550-2783-7-17
48. Bell PG, Walshe IH, Davison GW, Stevenson EJ, Howatson G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl Physiol Nutr Metab. (2015) 40:414–23. doi: 10.1139/apnm-2014-0244
49. Bell PG, Stevenson E, Davison GW, Howatson G. The effects of montmorency tart cherry concentrate supplementation on recovery following prolonged, intermittent exercise. Nutrients. (2016) 8:441. doi: 10.3390/nu8070441
50. Brown MA, Stevenson EJ, Howatson G. Montmorency tart cherry (Prunus cerasus L.) supplementation accelerates recovery from exercise-induced muscle damage in females. Eur J Sport Sci. (2019) 19:95–102. doi: 10.1080/17461391.2018.1502360
51. Quinlan R, Hill JA. The efficacy of tart cherry juice in aiding recovery after intermittent exercise. Int J Sports Physiol Perform. (2019) 1:1–7. doi: 10.1123/ijspp.2019-0101
52. Bell PG, Walshe IH, Davison GW, Stevenson E, Howatson G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients. (2014) 6:829–43. doi: 10.3390/nu6020829
53. Levers K, Dalton R, Galvan E, Goodenough C, O'Connor A, Simbo S, et al. Effects of powdered Montmorency tart cherry supplementation on an acute bout of intense lower body strength exercise in resistance trained males. J Int Soc Sports Nutr. (2015) 12:1–23. doi: 10.1186/s12970-015-0102-y
54. McCormick R, Peeling P, Binnie M, Dawson B, Sim M. Effect of tart cherry juice on recovery and next day performance in well-trained Water Polo players. J Int Soc Sports Nutr. (2016) 13:41. doi: 10.1186/s12970-016-0151-x
55. Beals K, Allison KF, Darnell M, Lovalekar M, Baker R, Nieman DC, et al. The effects of a tart cherry beverage on reducing exercise-induced muscle soreness. Isokinet Exerc Sci. (2017) 25:53–63. doi: 10.3233/IES-160645
56. Lamb KL, Ranchordas MK, Johnson E, Denning J, Downing F, Lynn A. No effect of tart cherry juice or pomegranate juice on recovery from exercise-induced muscle damage in non-resistance trained men. Nutrients. (2019) 11:1593. doi: 10.3390/nu11071593
57. Abbott W, Brashill C, Brett A, Clifford T. Tart cherry juice: no effect on muscle function loss or muscle soreness in professional soccer players after a match. Int J Sports Physiol Perform. (2020) 15:249–54. doi: 10.1123/ijspp.2019-0221
58. Kupusarevic J, McShane K, Clifford T. Cherry gel supplementation does not attenuate subjective muscle soreness or alter wellbeing following a match in a team of professional rugby union players: a pilot study. Sports. (2019) 7:84. doi: 10.3390/sports7040084
59. Levers K, Dalton R, Galvan E, O'Connor A, Goodenough C, Simbo S, et al. Effects of powdered Montmorency tart cherry supplementation on acute endurance exercise performance in aerobically trained individuals. J Int Soc Sports Nutr. (2016) 13:1–23. doi: 10.1186/s12970-016-0133-z
60. Keane KM, Bailey SJ, Vanhatalo A, Jones AM, Howatson G. Effects of montmorency tart cherry (L. Prunus cerasus) consumption on nitric oxide biomarkers and exercise performance. Scand J Med Sci Sports. (2018) 28:1746–56. doi: 10.1111/sms.13088
61. Morgan PT, Barton MJ, Bowtell JL. Montmorency cherry supplementation improves 15-km cycling time-trial performance. Eur J Appl Physiol. (2019) 119:675–84. doi: 10.1007/s00421-018-04058-6
62. St-Onge MP, Mikic A, Pietrolungo CE. Effects of diet on sleep quality. Adv Nutr. (2016) 7:938–49. doi: 10.3945/an.116.012336
63. Halson SL. Sleep in elite athletes and nutritional interventions to enhance sleep. Sports Med. (2014) 44:13–23. doi: 10.1007/s40279-014-0147-0
64. Howatson G, Bell PG, Tallent J, Middleton B, McHugh MP, Ellis J. Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur J Nutr. (2012) 51:909–16. doi: 10.1007/s00394-011-0263-7
65. Pigeon WR, Carr M, Gorman C, Perlis ML. Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: a pilot study. J Med Food. (2010) 13:579–83. doi: 10.1089/jmf.2009.0096
66. Losso JN, Finley JW, Karki N, Liu AG, Pan W, Prudente A, et al. Pilot study of tart cherry juice for the treatment of insomnia and investigation of mechanisms. Am J Ther. (2018) 25:e194. doi: 10.1097/MJT.0000000000000584
67. Bell PG, McHugh MP, Stevenson E, Howatson G. The role of cherries in exercise and health. Scand J Med Sci Sports. (2014) 24:477–90. doi: 10.1111/sms.12085
68. de Lima LCR, de Oliveira Assumpção C, Prestes J, Denadai BS. Consumption of cherries as a strategy to attenuate exercise-induced muscle damage and inflammation in humans. Nutr Hosp. (2015) 32:1885–93. doi: 10.3305/nh.2015.32.5.9709
69. Vitale KC, Hueglin S, Broad E. Tart cherry juice in athletes: a literature review and commentary. Curr Sports Med Rep. (2017) 16:230–9. doi: 10.1249/JSR.0000000000000385
70. Bowtell J, Kelly V. Fruit-derived polyphenol supplementation for athlete recovery and performance. Sports Med. (2019) 49:3–23. doi: 10.1007/s40279-018-0998-x
71. Han B, Bhagavathula AS, Rashid M, Chhabra M, Clark C, Abdulazeem HM, et al. The effect of sour cherry consumption on blood pressure, IL-6, CRP, and TNF-α levels: a systematic review and meta-analysis of randomized controlled trials sour cherry consumption and blood pressure. J King Saud Univ Sci. (2020) 32:1687–93. doi: 10.1016/j.jksus.2020.01.002
72. Chen PE, Liu CY, Chien WH, Chien CW, Tung TH. Effectiveness of cherries in reducing uric acid and gout: a systematic review. Evid Based Complement Alternat Med. (2019) (2019) 2019:9896757. doi: 10.1155/2019/9896757
73. Gao R, Chilibeck PD. Effect of tart cherry concentrate on endurance exercise performance: a meta-analysis. J Am Coll Nutr. (2020) 39:657–64. doi: 10.1080/07315724.2020.1713246
74. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult. (1965) 16:144–58.
75. Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr Protoc Food Anal Chem. (2001) F1-2.1–13. doi: 10.1002/0471142913.faf0102s00
76. Shahidi F. HCT: Antioxidant Measurement and Applications. Washington, DC: American Chemical Society (2007). doi: 10.1021/bk-2007-0956.ch001
77. Buckow R, Kastell A, Terefe NS, Versteeg C. Pressure and temperature effects on degradation kinetics and storage stability of total anthocyanins in blueberry juice. J Agric Food Chem. (2010) 58:10076–84. doi: 10.1021/jf1015347
78. Keane KM, Bell PG, Lodge JK, Constantinou CL, Jenkinson SE, Bass R, et al. Phytochemical uptake following human consumption of Montmorency tart cherry (L. Prunus cerasus) and influence of phenolic acids on vascular smooth muscle cells in vitro. Eur J Nutr. (2016) 55:1695–705. doi: 10.1007/s00394-015-0988-9
79. Keane KM, Haskell-Ramsay CF, Veasey RC, Howatson G. Montmorency Tart cherries (Prunus cerasus L.) modulate vascular function acutely, in the absence of improvement in cognitive performance. Br J Nutr. (2016) 116:1935–44. doi: 10.1017/S0007114516004177
80. Jackman SR, Brook MS, Pulsford RM, Cockcroft EJ, Campbell MI, Rankin D, et al. Tart cherry concentrate does not enhance muscle protein synthesis response to exercise and protein in healthy older men. Exp Gerontol. (2018) 110:202–8. doi: 10.1016/j.exger.2018.06.007
81. Chandra A, Rana J, Li Y. Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC– MS. J Agric Food Chem. (2001) 49:3515–21. doi: 10.1021/jf010389p
82. Mayta-Apaza AC, Pottgen E, De Bodt J, Papp N, Marasini D, Howard L, et al. Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo. J Nutr Biochem. (2018) 59:160–72. doi: 10.1016/j.jnutbio.2018.04.001
83. Nagy Á, Abrankó L. Profiling of hydroxycinnamoylquinic acids in plant extracts using in-source CID fragmentation. J Mass Spectr. (2016) 51:1130–45. doi: 10.1002/jms.3847
84. Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. (1999) 299:152–78. doi: 10.1016/S0076-6879(99)99017-1
85. Spanos GA, Wrolstad RE. Influence of processing and storage on the phenolic composition of thompson seedless grape juice. J Agric Food Chem. (1990) 38:1565–71. doi: 10.1021/jf00097a030
86. Lear R, O'Leary M, O'Brien Andersen L, Holt CC, Stensvold CR, van der Giezen M, et al. Tart cherry concentrate does not alter the gut microbiome, glycaemic control or systemic inflammation in a middle-aged population. Nutrients. (2019) 11:1063. doi: 10.3390/nu11051063
87. Johnson SA, Navaei N, Pourafshar S, Jaime SJ, Akhavan NS, Alvarez-Alvarado S, et al. Effects of montmorency Tart Cherry juice consumption on cardiometabolic biomarkers in adults with metabolic syndrome: a randomized controlled pilot trial. J Med Food. (2020) 23:1238–47. doi: 10.1089/jmf.2019.0240
88. Lee J, Durst RW, Wrolstad RE, Giusti EE, Hach MM, Hofsommer J, et al. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. (2005) 88:1269–78. doi: 10.1093/jaoac/88.5.1269
89. Kim D, Lee C. Current Protocols in Food Analytical Chemistry. Extraction and Isolation of Polyphenolics. Hoboken, NJ: John Wiley & Sons, Inc. (2002). p. I1.2.1–12. doi: 10.1002/0471142913.fai0102s06
90. Khoo GM, Clausen MR, Pedersen BH, Larsen E. Bioactivity and total phenolic content of 34 sour cherry cultivars. J Food Composition Anal. (2011) 24:772–6. doi: 10.1016/j.jfca.2011.03.004
91. Lynn A, Mathew S, Moore CT, Russell J, Robinson E, Soumpasi V, et al. Effect of a tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: a randomised controlled trial. Plant Foods Human Nutr. (2014) 69:122–7. doi: 10.1007/s11130-014-0409-x
92. Desai T, Bottoms L, Roberts M. The effects of Montmorency tart cherry juice supplementation and FATMAX exercise on fat oxidation rates and cardio-metabolic markers in healthy humans. Eur J Appl Physiol. (2018) 118:2523–39. doi: 10.1007/s00421-018-3978-9
93. Chai SC, Jerusik J, Davis K, Wright RS, Zhang Z. Effect of Montmorency tart cherry juice on cognitive performance in older adults: a randomized controlled trial. Food Funct. (2019) 10:4423–31. doi: 10.1039/C9FO00913B
94. Morehen JC, Clarke J, Batsford J, Barrow S, Brown AD, Stewart CE, et al. Montmorency tart cherry juice does not reduce markers of muscle soreness, function and inflammation following professional male rugby League match-play. Eur J Sport Sci. (2020) 6:1–10. doi: 10.1080/17461391.2020.1797181
95. Stamp LK, Chapman P, Frampton C, Duffull SB, Drake J, Zhang Y, et al. Lack of effect of tart cherry concentrate dose on serum urate in people with gout. Rheumatology. (2020) 59:2374–80. doi: 10.1093/rheumatology/kez606
96. Davis GR, Bellar D. Montmorency cherry supplement does not affect aerobic exercise performance in healthy men. Int J Vitamin Nutr Res. (2020) 90:403–10. doi: 10.1024/0300-9831/a000575
97. Harnly J, Lu Y, Sun J, Chen P. Botanical supplements: detecting the transition from ingredient to product. J Food Composition Anal. (2017) 64:85–92. doi: 10.1016/j.jfca.2017.06.010
98. Apak R, Gorinstein S, Böhm V, Schaich KM, Özyürek M, Güçlü K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl Chem. (2013) 85:957–98. doi: 10.1351/PAC-REP-12-07-15
99. Granato D, Shahidi F, Wrolstad R, Kilmartin P, Melton LD, Hidalgo FJ, et al. Antioxidant activity, total phenolics and flavonoids contents: should we ban in vitro screening methods? Food Chem. (2018) 264:471–5. doi: 10.1016/j.foodchem.2018.04.012
100. Sánchez-Rangel JC, Benavides J, Heredia JB, Cisneros-Zevallos L, Jacobo-Velázquez DA. The Folin–Ciocalteu assay revisited: improvement of its specificity for total phenolic content determination. Anal Met. (2013) 5:5990–9. doi: 10.1039/c3ay41125g
101. Ou B, Bosak KN, Brickner PR, Iezzoni DG, Seymour EM. Processed tart cherry products—Comparative phytochemical content, in vitro antioxidant capacity and in vitro anti-inflammatory activity. J Food Sci. (2012) 77:H105–12. doi: 10.1111/j.1750-3841.2012.02681.x
102. Nawirska-Olszańska A, Kolniak-Ostek J, Oziembłowski M, Ticha A, Hyšpler R, Zadak Z, et al. Comparison of old cherry cultivars grown in Czech Republic by chemical composition and bioactive compounds. Food Chem. (2017) 228:136–42. doi: 10.1016/j.foodchem.2017.01.154
103. Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. (2013) 51:15–25. doi: 10.1016/j.fct.2012.09.021
104. Lester GE, Lewers KS, Medina MB, Saftner RA. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin–Ciocalteu: assay interference by ascorbic acid. J Food Composition Anal. (2012) 27:102–7. doi: 10.1016/j.jfca.2012.05.003
105. Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. (2017) 61:1361779. doi: 10.1080/16546628.2017.1361779
106. Lee J, Renaker C, Wrolstad E, Correlation of two anthocyanin quantification methods: HPLC and spectrophotometric methods. Food Chem. (2008) 110:782–6. doi: 10.1016/j.foodchem.2008.03.010
107. Lao F, Giusti MM. Quantification of purple corn (Zea mays L.) anthocyanins using spectrophotometric and HPLC approaches: method comparison and correlation. Food Anal Methods. (2016) 9:1367–80. doi: 10.1007/s12161-015-0318-0
108. Lee SG, Vance TM, Nam T-G, Kim D-O, Koo SI, Chun OK. Evaluation of pH differential and HPLC methods expressed as cyanidin-3-glucoside equivalent for measuring the total anthocyanin contents of berries. J Food Meas Charact. (2016) 10:562–8. doi: 10.1007/s11694-016-9337-9
109. Proteggente AR, Pannala AS, Paganga G, Buren LV, Wagner E, Wiseman S, et al. The antioxidant activity of regularly consumed fruit and vegetables reflects their phenolic and vitamin C composition. Free Radic Res. (2002) 36:217–33. doi: 10.1080/10715760290006484
110. Welch CR, Wu Q, Simon JE. Recent advances in anthocyanin analysis and characterization. Curr Anal Chem. (2008) 4:75–101. doi: 10.2174/157341108784587795
111. Tiefenbacher K. Chapter Four - Technology of Minor Ingredients for Wafers and Waffles. San Diego, CA: Wafer and Waffles, Academic Press (2017). p. 227–311. doi: 10.1016/B978-0-12-809438-9.00004-1
112. Zhang Z, Kou X, Fugal K, McLaughlin J. Comparison of HPLC methods for determination of anthocyanins and anthocyanidins in bilberry extracts. J Agric Food Chem. (2004) 52:688–91. doi: 10.1021/jf034596w
113. Castaneda-Ovando A, de LourdesPacheco-Hernández M, ElenaPáez-Hernández M, Rodríguez JA, Galán-Vidal CA, et al. Chemical studies of anthocyanins: a review. Food Chem. (2009) 113:859–71. doi: 10.1016/j.foodchem.2008.09.001
114. Bridwell H, Dhingra V, Peckman D, Roark J, Lehman T. Perspectives on method validation: importance of adequate method validation. Qual Assur J. (2010) 13:72–7. doi: 10.1002/qaj.473
115. Liu Y, Lund JA, Murch SJ, Brown PN. Single-lab validation for determination of kavalactones and flavokavains in piper methysticum (Kava). Planta Med. (2018) 84:1213–8. doi: 10.1055/a-0637-2400
116. Lomiwes D, Ha B, Ngametua N, Burr NS, Cooney JM, Trower TM, et al. Timed consumption of a New Zealand blackcurrant juice support positive affective responses during a self-motivated moderate walking exercise in healthy sedentary adults. J Int Soc Sports Nutr. (2019) 16:1–14. doi: 10.1186/s12970-019-0300-0
Keywords: tart cherry, anthocyanins, exercise recovery, sleep, dietary supplement
Citation: Sabou VR, O'Leary MF, Liu Y, Brown PN, Murch S and Bowtell JL (2021) Review of Analytical Methods and Reporting of the Polyphenol Content of Tart Cherry Supplements in Human Supplementation Studies Investigating Health and Exercise Performance Effects: Recommendations for Good Practice. Front. Nutr. 8:652094. doi: 10.3389/fnut.2021.652094
Received: 11 January 2021; Accepted: 02 March 2021;
Published: 26 March 2021.
Edited by:
David Michael Bellar, University of North Carolina at Charlotte, United StatesReviewed by:
María Serrano, Miguel Hernández University of Elche, SpainCopyright © 2021 Sabou, O'Leary, Liu, Brown, Murch and Bowtell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanna L. Bowtell, ai5ib3d0ZWxsQGV4ZXRlci5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.