- 1Division of Child Health and Pediatrics, Moi Teaching and Referral Hospital (MTRH), Eldoret, Kenya
- 2Division of Surgery, Moi Teaching and Referral Hospital (MTRH), Eldoret, Kenya
- 3Division of Internal Medicine, Moi Teaching and Referral Hospital (MTRH), Eldoret, Kenya
- 4School of Public Health, Moi University, Eldoret, Kenya
- 5Department of Surgery, Moi University, Eldoret, Kenya
- 6Division of Health Records and Informatics, Moi Teaching and Referral Hospital (MTRH), Eldoret, Kenya
- 7Moi Teaching and Referral Hospital (MTRH), Eldoret, Kenya
Introduction: We describe the clinical spectrum of COVID-19 cases in western Kenya from 6 April 2020 to 31 May 2021, providing baseline data for further studies into COVID-19 in Kenya.
Methods: We did a retrospective chart review of laboratory and inpatient files of patients diagnosed and managed for COVID-19 at the Moi Teaching and Referral Hospital in Kenya and analyzed the data using Stata® version 16 (StataCorp LP, College Station, TX, USA) and calculated measures of association at 95% CI.
Results: The patients (n = 1,770) had a mean age of 43 years (SD 20 years) and 55.4% were male. Close to 70% had asymptomatic disease, with the symptomatic cases largely being respiratory in nature. One-quarter had comorbidities. The case fatality rate was 13.6% (n = 240). Male sex increased the odds of mortality by 1.69 (95% CI 1.27–2.25; p ≤ 0.001), and the presence of comorbidities increased the odds of mortality by 3.16 (95% CI 2.38–4.18; p ≤ 0.001). Those aged 59 years and above were 18 times more likely to die from COVID-19 than those below 15 years of age (95% CI 1.61–90.66; p = 0.015).
Conclusion: COVID-19 had a significantly high mortality rate in western Kenya. Male sex and the presence of comorbidities increased the risk of severe disease and mortality.
Background
The novel coronavirus disease (COVID-19), which was first reported in China in December 2019, resulted in over 304 million infections and over 5.4 million deaths globally between December 2019 and January 2022 (1). Approximately 70% of these cases were reported in Europe and the Americas, and 14% were reported in Asia. Those reported in Africa contributed to less than 2% of the global case load (2). Although there has been a massive increase in COVID-19-related information and research globally, little has been generated in Africa. The clinical spectrum of the disease is largely undescribed in Africa.
Methodology
This is a cross-sectional study involving a retrospective patients’ chart review of all patients managed for COVID-19 at Moi Teaching and Referral Hospital (MTRH). MTRH is the second-largest public hospital in Kenya, with a catchment population of 24 million, and was designated to be a COVID-19 testing point for the larger western Kenya (3) area. The MTRH testing center used a cobas® 8800 machine using reverse transcription-PCR (RT-PCR) diagnostics for the detection of COVID-19. The data were linked to the Kenyan National Database at the Ministry of Health Headquarters.
The study population comprised patients that tested positive for COVID-19 between 6 April 2020 and 31 May 2021 and who were managed by MTRH either through home-based care (HBC) or admitted to the COVID-19 isolation ward. The REDCap database was used to register all patients who were evaluated and tested. The data collected included sociodemographic details, signs and symptoms at evaluation, comorbidities, and test results. We retrieved the outcome of treatment data from in-patients’ files. Data were collected using a structured data abstraction form. Test statistics were carried out at 95% CI.
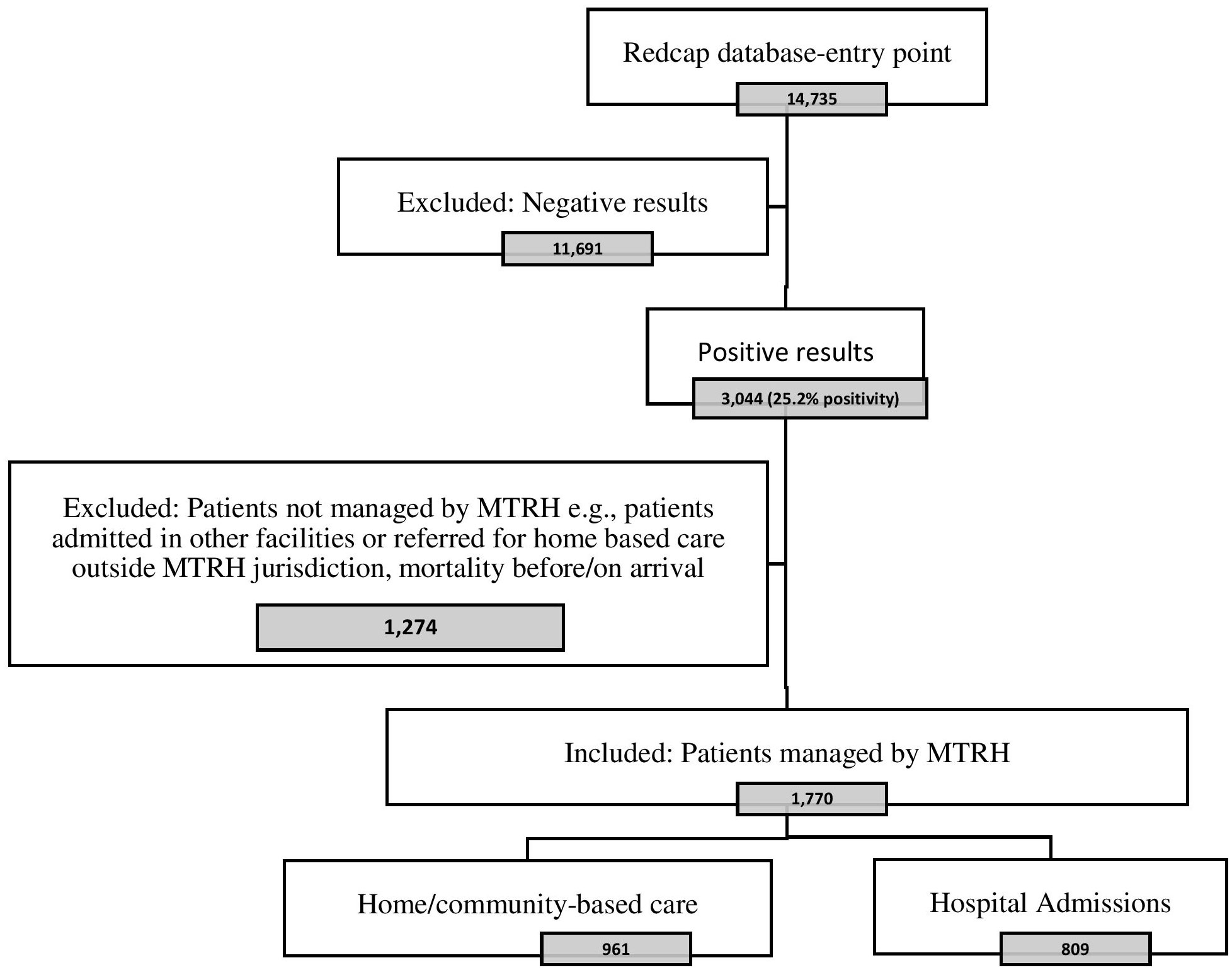
Of the 14,735 patients tested during the study period, 3,044 (25.2%) were positive, of whom 1,770 were managed by MTRH. Of these, 809 were admitted to the isolation ward, and 961 were managed through HBC. Below is a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of our selection of the study population (Figure 1).
Those with asymptomatic disease were marked as asymptomatic and/or no symptoms were listed. Those with mildly symptomatic disease were marked as symptomatic and/or had any symptoms marked but had a pulse oximetry reading of > 90% and had no difficulties in breathing/dyspnea/respiratory distress. Those with moderate/severe disease were classified as cases who had a pulse oximetry reading of < 90% and/or difficulties in breathing/dyspnea/respiratory distress.
At the beginning of the pandemic, patients were hospitalized for observation/isolation even if they were asymptomatic. Later, the asymptomatic and mildly symptomatic patients were managed through HBC. Those hospitalized (ward) also included patients who had COVID-19 tests done while they were admitted for other conditions such as pre surgery.
Data from the patient files and the laboratory data sheet were de-identified when entered into the study’s Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) sheet. The de-identified data were password protected by the research assistant, who only shared the passwords with the statistician. We obtained ethics clearance to conduct the study from the MTRH/MU Ethics Review Committee (approval no. 0003767). Data were analyzed using Stata® version 16 (StataCorp LP, College Station, TX, USA) and measures of association were calculated at 95% CI.
Results
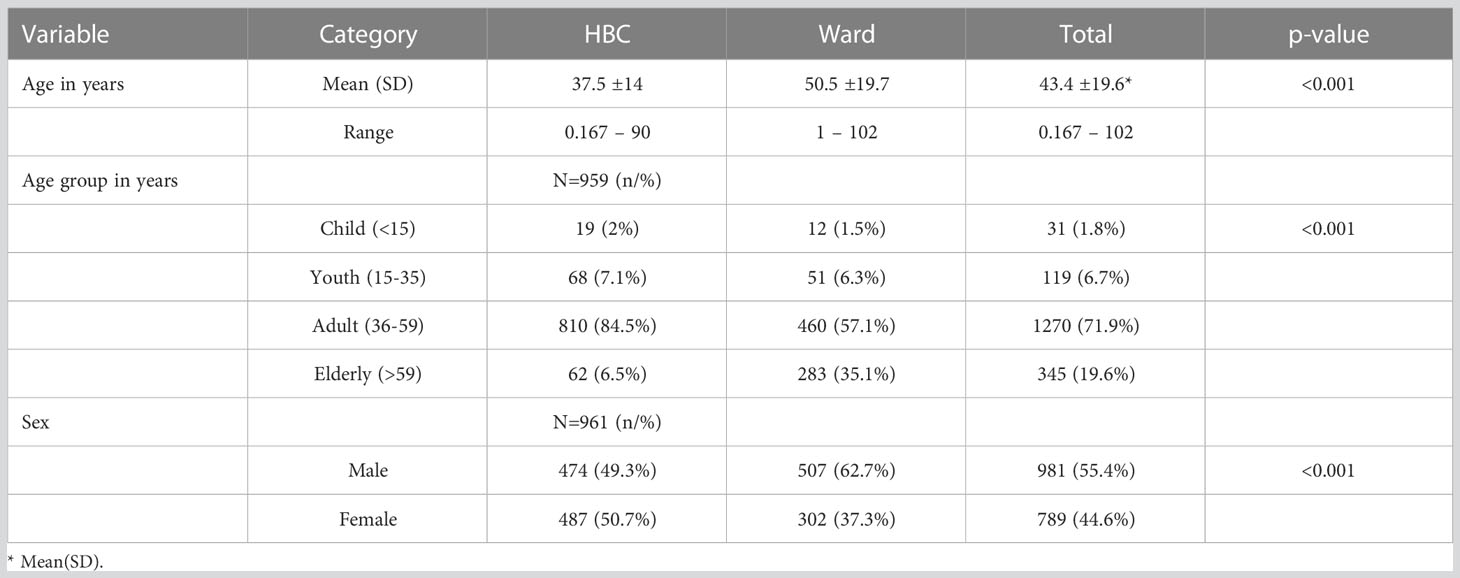
The patients diagnosed with COVID-19 had a mean age of 43.4 years, 71.9% were aged 36–59 years, and 55.4% were male (see Table 1). Sixty-three percent (63%) of the patients were managed through HBC, 92% of whom had asymptomatic disease and 65% of those managed in the wards had moderate to severe disease. Overall, 69.1% of the patients had asymptomatic disease, 11.4% had moderate disease, and 5.9% had severe disease. The case fatality rate was 13.6% (n = 240).
Among those who were symptomatic, coughing, difficulties in breathing, fatigue, myalgia, fever, and chest pain were the commonest presenting complaints, whereas tachypnea and respiratory distress were the commonest physical signs.
One-quarter (25%) of the patients had comorbidities. The commonest comorbidities were hypertension (9.9%, n = 175), diabetes (8.3%, n = 147), and chronic cardiac disease (3.9%, n = 70). The other comorbidities included asthma, chronic obstructive pulmonary disease, chronic kidney disease, chronic hematologic diseases, rheumatologic disorders, liver disease, obesity, and dementia.
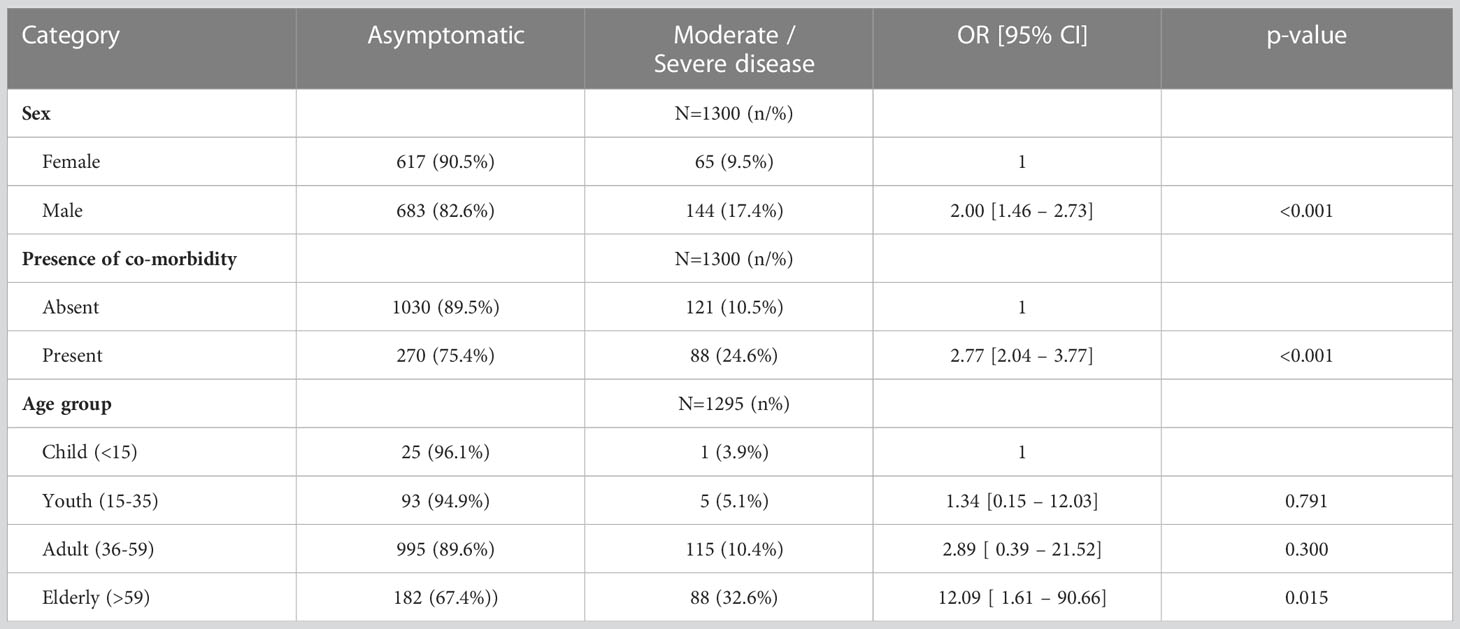
Males were twice as likely to get severe disease than females (95% CI 1.46–2.73, p ≤ 0.001), and the presence of comorbidities increased the likelihood of severe disease 2.77 times (95% CI 2.04—3.77 times; p ≤ 0.001). Persons aged 59 years and above were 12 times more likely to have severe disease than children below 15 years (95% CI 1.61–90.66 times; p = 0.015) (see Table 2).
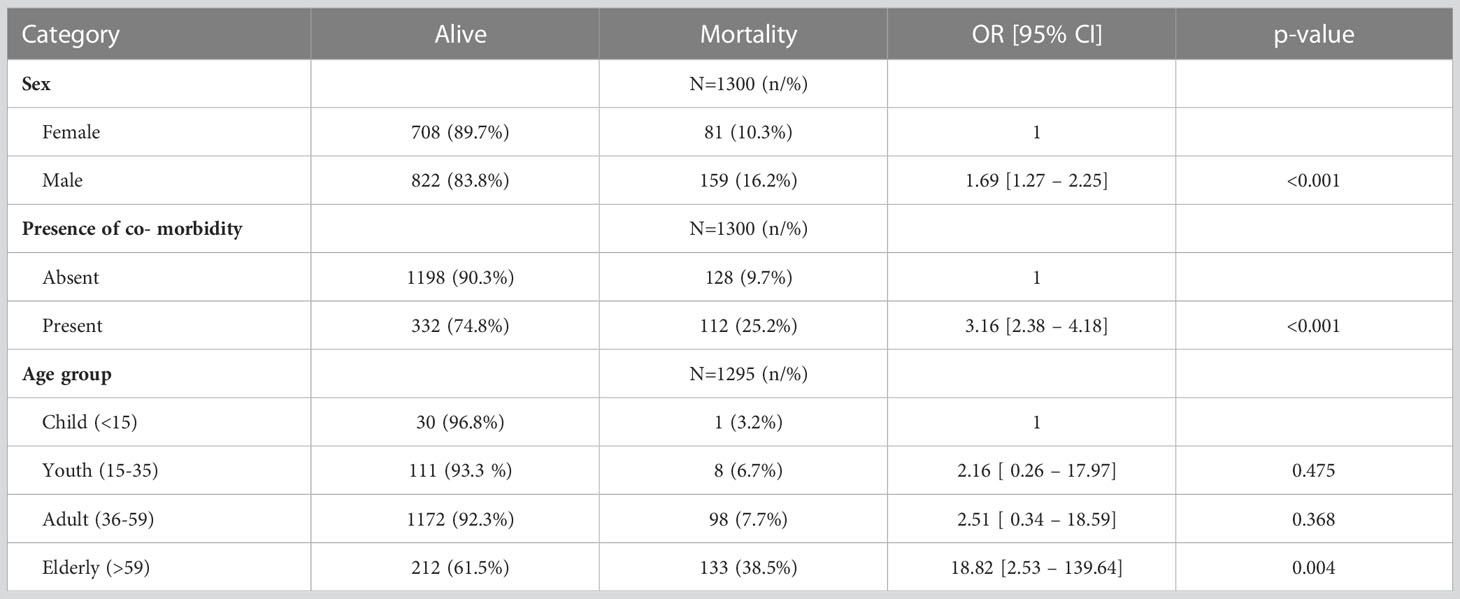
Male sex increased the odds of mortality by 1.69 (95% CI 1.27–2.25; p ≤ 0.001) while the presence of comorbidity increased the odds of mortality by 3.16 (95% CI 2.38–4.18; p ≤ 0.001). Those aged 59 years and above were 18 times more likely to die from COVID than those below 15 years (95% CI 2.53–139.64; p = 0.004) (see Table 3).
Discussion
The COVID-19 epidemic has been observed as being significantly smaller in sub-Saharan Africa (SSA) than had been initially modeled and forecasted (3). In Kenya, the first case was diagnosed in March 2020, 1 month after the first African case, and by December 2021 there were 25,028 cases and 5,375 deaths (4). The MTRH, the second-largest public hospital in Kenya, was designated to be a testing point for the larger western and South Rift areas of Kenya as well as an isolation center, initially for all cases and then later for those that clinically needed admission. We describe the spectrum of the COVID-19 disease in the population managed by MTRH, thus a representation of COVID-19 cases in this part of Kenya.
The hospital had a 25% COVID-19 test positivity rate. Some of the tests were done pre surgery and did not meet the criteria for suspected COVID-19. Eighty percent of the positive cases had either asymptomatic or mildly symptomatic disease, in keeping with data from most of SSA and from China (5). Most of these patients were managed through HBC. As has been described in other settings, the disease in the study population was largely respiratory in nature, with significant constitutional symptomatology such as fever, myalgia, fatigue, headache, and joint pains. We found a few cases with gastrointestinal and neurological symptomatology.
Hypertension and diabetes were the commonest comorbidities. Obesity was not routinely measured, and it is possible that cases of this were underreported. Although HIV infections were verbally reported by the patients, the HIV prevalence was comparable with the prevalence throughout Kenya, where the prevalence among those aged 15–49 years is 4.5% (6). Comorbidities increased the odds of severe disease by more than 2.7 times, and the case fatality was relatively high for all the comorbidities. Comorbidities have been hypothesized to worsen COVID-19 due to the pre-existent chronic inflammation, reduction in adaptive and innate immunity, overexpression of the angiotensin-converting enzyme 2 (ACE2) receptor, which is responsible for the successful entry of the virus into the body, and pre-existent organ dysfunction (7–9).
In this population, the odds of having moderate/severe disease increased by 5% for every 1-year increase in age, while the odds of mortality increased by 6% for every 1-year increase in age. COVID-19 has been documented to adversely affect the elderly globally, especially for those above 65 years (10). The advance in age is associated with a decay of the immune system, as memory cells outnumber naive cells at about 37 years of age (11).
Similar to other regions, we found higher odds of severe disease and mortality among males (12, 13). It has been postulated that the sex sensitivity of COVID-19 is related to the sex differences in the expression of the ACE2 receptor and the transmembrane protease serine 2 (TMPRSS2), both of which are responsible for the successful entry of SARS-CoV-2 into the body (18). These receptors are regulated by estrogen, thus likely conferring protection to females. In addition, females have a more rigorous humoral- and cell-mediated immunity, as well as innate immune reactions such as through nitric oxide (NO) due to enhancement by estrogen (18, 19). Moreover, it is thought that behavioral differences between sex, such as smoking, may contribute to the sex differences.
We reported a higher case fatality rate than that reported in China and other countries in the developed world, probably due to the late presentation of cases to hospitals and the poor availability of intensive care unit (ICU) services in the region (20).
Limitations
This study suffers from Berkson’s selection bias, given that only those patients managed by the hospital were included for analysis. In addition, this study has the limitations of being a retrospective chart review and the association with severe disease and mortality cannot be directly inferred. The comorbidities may not have been reported for all cases as they were dependent on patients’ verbal reports. Vital measurements, such as weight and body mass index (BMI), were not routinely collected. This article, however, forms a baseline for a more in-depth analysis of the patients managed for COVID-19 in SSA.
Conclusion
Although the majority of the patients had asymptomatic disease, the mortality rate was significantly high. Male sex and the presence of comorbidity increased the risk of severe disease and mortality. COVID-19 presents similarly in Africa as in other continents.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Moi Teaching and Referral Hospital Ethics Review Board (IREC/2020/222 and approval number 003767). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
EO, EG, and DK participated in proposal write up, EO, EG, TA, SN, TS, RO, and HM participated in data collection. HM participated in data analysis with EO, and EG reviewing the analytics. All the authors participated in manuscript write up. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Moi Teaching and Referral Intramural Research Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Weekly epidemiological update on COVID-19 - 11 January 2022. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—11-january-2022 (Accessed January 30, 2022).
2. WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. Available at: https://covid19.who.int/ (Accessed January 30, 2022).
3. Tessema SK, Nkengasong JN. Understanding COVID-19 in Africa. Nat Rev Immunol (2021) 21(8):1. doi: 10.1038/S41577-021-00579-Y
4. Muchiri SK, Muthee R, Kiarie H, Sitienei J, Agweyu A, Atkinson PM, et al. Unmet need for COVID-19 vaccination coverage in Kenya. Vaccine (2022) 40:2011–9. doi: 10.1016/j.vaccine.2022.02.035
5. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA (2020) 323(13):1239–42. doi: 10.1001/JAMA.2020.2648
6. National AIDS Control Council. Available at: www.nacc.or.ke (Accessed January 30, 2022).
7. WHO Coronavirus Disease (COVID-19) Dashboard | WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int/ (Accessed October 6, 2020).
8. Lima-Martínez MM, Carrera Boada C, Madera-Silva MD, Marín W, Contreras M. COVID-19 and diabetes: A bidirectional relationship. Clin Investig Arterioscler (2021) 33(3):151–7. doi: 10.1016/J.ARTERI.2020.10.001
9. Han HJ, Nwagwu C, Anyim O, Ekweremadu C, Kim S. COVID-19 and cancer: From basic mechanisms to vaccine development using nanotechnology. Int Immunopharmacol (2021) 90:107247. doi: 10.1016/J.INTIMP.2020.107247
10. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera A, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis (2020) 34:101623. doi: 10.1016/J.TMAID.2020.101623
11. Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev (2006) 127(3):274–81. doi: 10.1016/J.MAD.2005.11.001
12. Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect (2020) 80(5):e1–6. doi: 10.1016/j.jinf.2020.03.004
13. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (2020) 395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3
18. Mukherjee S, Pahan K. Is COVID-19 Gender-sensitive?. J Neuroimmune Pharmacol (2021) 16(1):1. doi: 10.1007/S11481-020-09974-Z
19. Foresta C, Rocca MS, Di Nisio A. Gender susceptibility to COVID-19: a review of the putative role of sex hormones and X chromosome. J Endocrinol Invest (2021) 44(5):951. doi: 10.1007/S40618-020-01383-6
Keywords: COVID-19, western Kenya, asymptomatic, comorbidities, retrospective study
Citation: Ogalo EA, Gudu E, Andale T, Korir D, Ndege S, Simiyu T, Olekuyo R, Mwangi H, Kimaiyo S and Aruasa W (2023) Clinical spectrum of COVID-19 at a national referral hospital in western Kenya during the period 2020–2021. Front. Virol. 3:1202742. doi: 10.3389/fviro.2023.1202742
Received: 09 April 2023; Accepted: 25 July 2023;
Published: 12 October 2023.
Edited by:
Yoon-Seok Chung, Korea Disease Control and Prevention Agency, Republic of KoreaReviewed by:
Mohamed Farouk Allam, Ain Shams University, EgyptNirmal Kumar Ganguly, Indraprastha Apollo Hospitals, India
Copyright © 2023 Ogalo, Gudu, Andale, Korir, Ndege, Simiyu, Olekuyo, Mwangi, Kimaiyo and Aruasa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edith A. Ogalo, ZWRpdGhhcG9uZGlAZ21haWwuY29t
 Edith A. Ogalo
Edith A. Ogalo Edwin Gudu2
Edwin Gudu2 Sylvester Kimaiyo
Sylvester Kimaiyo