- 1Department of Veterinary Biosciences, Melbourne Veterinary School, Faculty of Science, The University of Melbourne, Werribee, VIC, Australia
- 2Departamento de Medicina y Zootecnia de Rumiantes, Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de México, Mexico City, Mexico
- 3School of Mathematical and Statistical Sciences, Clemson University, Clemson, SC, United States
- 4Department of Mathematics and Statistics, University of Agriculture, Faisalabad, Pakistan
- 5Goat Veterinary Consultancies- goatvetoz, Keperra, QLD, Australia
Background: Coccidiosis is a protozoal disease caused by Eimeria species, the main symptom of which is diarrhea. Eimeria spp. infection can cause weight loss and ill-thrift in goats, and in severe cases, it can lead to mortality in kids, resulting in economic losses for the goat industry. This study aimed to determine the global prevalence of Eimeria spp. in goats and to identify the possible predictors of heterogeneity among selected studies.
Methods: Data were retrieved from five databases of major global importance (PubMed, Web of Science, CAB Direct, Scopus, and Google Scholar), with 255 studies published between 1963 and 2022 being included. A random-effects model was used to calculate pooled prevalence estimates with 95% confidence intervals (CI), followed by subgroup meta-analysis and meta-regression analysis to identify factors contributing to high prevalence and explore sources of heterogeneity among studies.
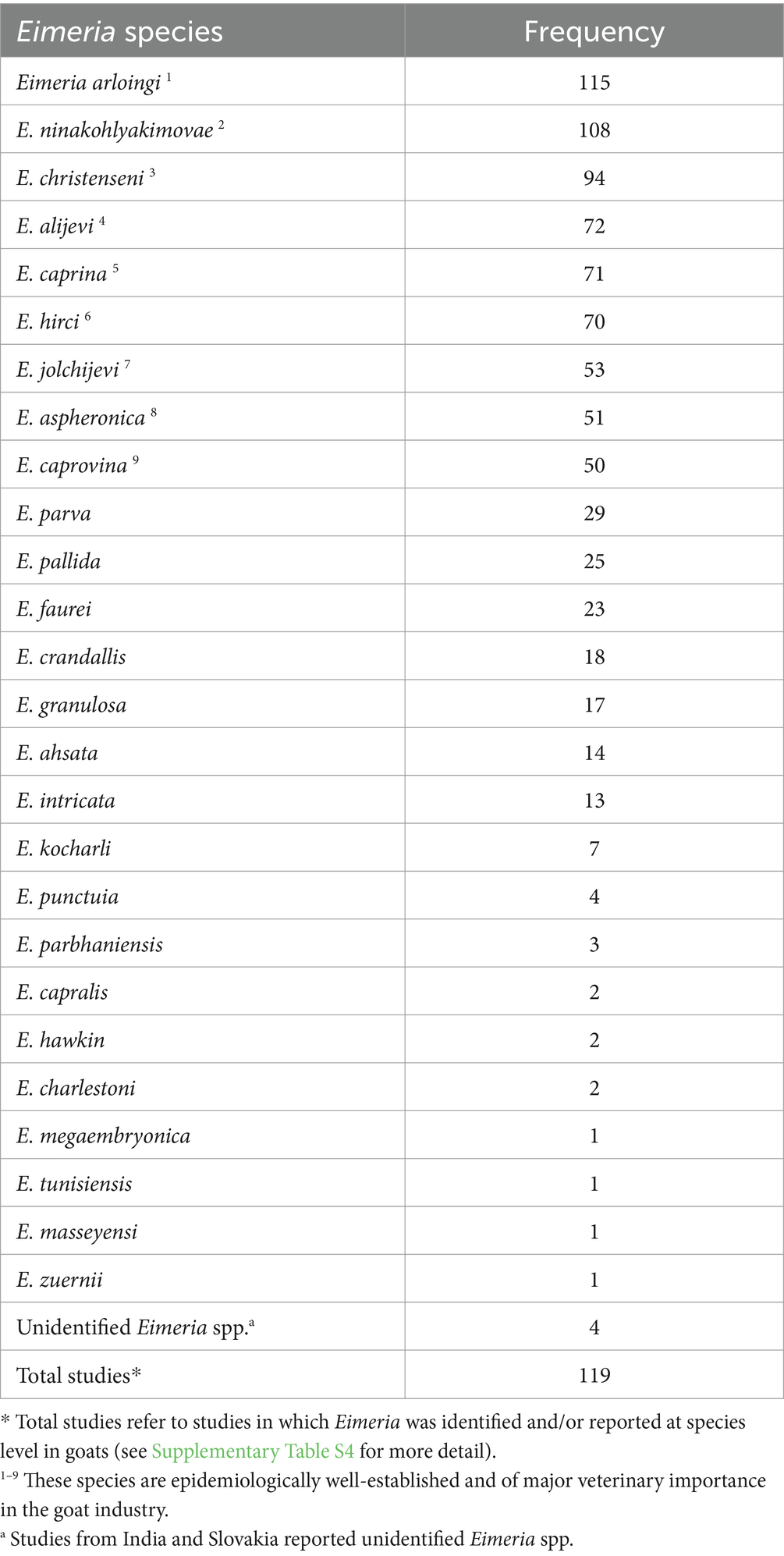
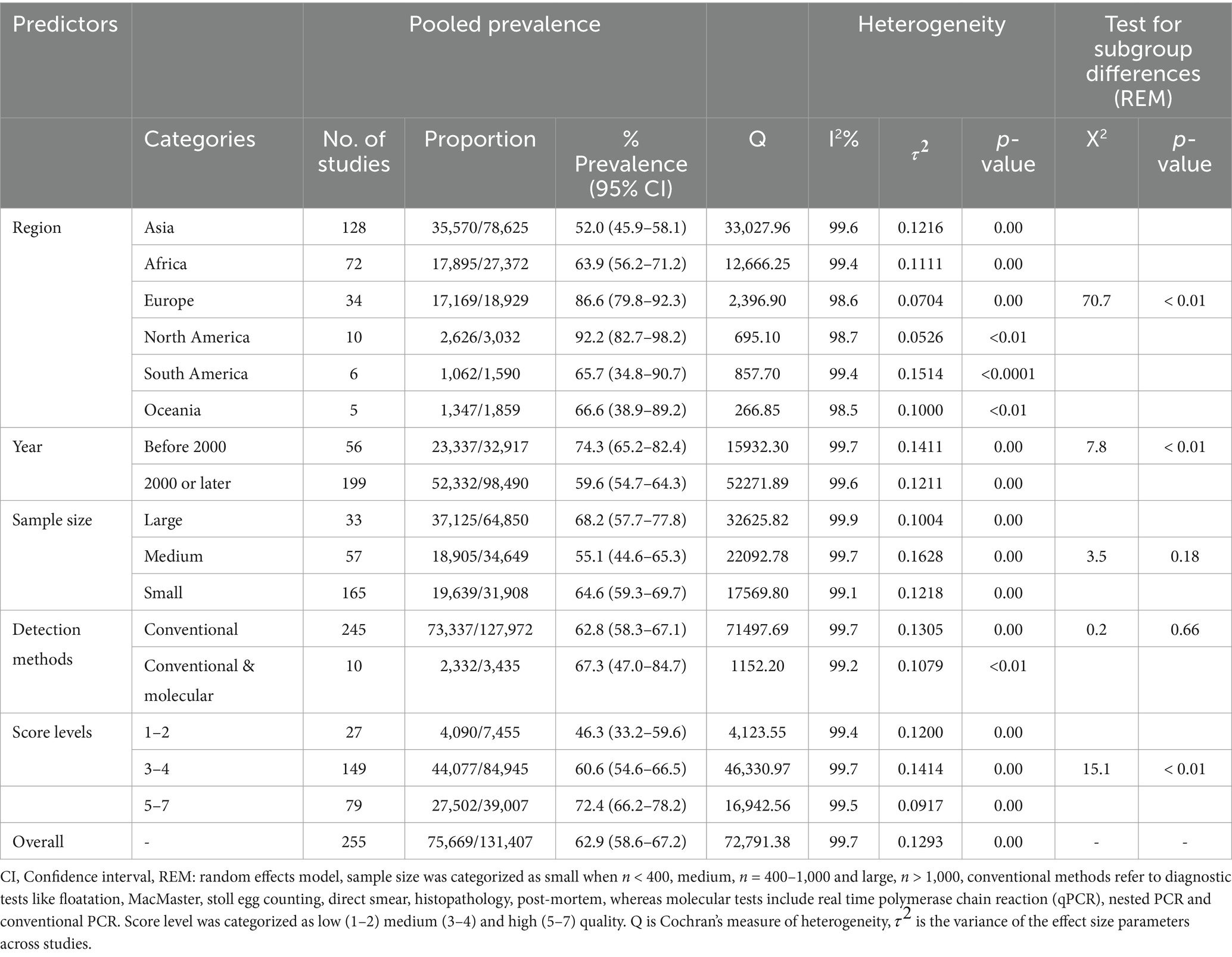
Results: The estimated global prevalence of Eimeria spp. in goats was 62.9% (95% CI: 58.6–67.2). Our results indicated high inter-study variability (inconsistency index (I2) = 99.7%, p < 0.01). Among the variables analyzed, regions and quality of studies were the most significant predictors of heterogeneity. According to the region-based subgroup meta-analysis, North America had the highest estimated prevalence of Eimeria spp. (92.2, 95% CI: 82.7–98.2), followed by Europe (86.6, 95% CI: 79.8–92.3), while Asia had the lowest prevalence (52.0, 95% CI: 45.9–58.1). Most countries (n = 42/56) had an estimated prevalence above the overall pooled estimate (>62.9%). The subgroup of studies conducted in 2000 or later presented a lower prevalence of 59.6% (95% CI: 54.7–64.3). Studies with a score of 5–7 had a significantly higher prevalence (72.4, 95% CI: 66.2–78.2) than studies with low or medium scores (p < 0.01). The prevalence of Eimeria spp. in goats detected with conventional and molecular methods was 67.3% (95% CI: 47.0–84.7). Only 47% (119/255) of the studies provided details on identifying Eimeria at the species level. Overall, more than 26 Eimeria spp. have been identified in goats globally. Among these, the most frequently reported and pathogenic species were E. arloingi (115/119), E. ninakohlyakimovae (108/119), E. christenseni (94/119), and E. caprina (71/119). Other valid species that were reported less frequently include E. alijevi, E. hirci, E. caprovina, E. aspheronica and E. jolchijevi.
Conclusion: These findings suggest that the pathogenic Eimeria spp. are widespread in goats globally. Given the high prevalence and the extensive distribution of pathogenic Eimeria spp. in goats, it is recommended that integrated parasite management approaches be implemented for the effective control of coccidiosis in goats.
1 Introduction
There are estimated to be more than one billion domestic goats worldwide (1), and the global goat population has more than doubled in the last four decades (2). Despite significant growth in the global goat population, the productivity of the goat industry is challenged by health, management and production constraints (3). Among various health concerns, gastrointestinal diseases (e.g., parasitic gastroenteritis) can lead to significant economic losses for the global goat industry (4).
For instance, coccidiosis, a parasitic disease caused by the intracellular protozoan parasites of the genus Eimeria (Apicomplexa: Eimeriidae) (5), is a significant concern for goat farmers due to its economic impact. Globally, several species of Eimeria (also known as coccidia) infect goats (6–16), leading to significant economic losses due to poor growth and lower productivity. Although the economic impact of coccidiosis is believed to be substantial (17), there is a lack of data to substantiate such a statement regarding small ruminant coccidiosis.
To date, the number of Eimeria spp. that are considered parasites of goats remains variable and controversial (14, 18, 19) and depends on the acceptance of the validity of certain Eimeria spp. (12). For instance, Levine (20) reported 13 species of Eimeria as the true parasites of goats, including Eimeria arloingi, E. ninakohlyakimovae, E. christenseni, E. caprina, E. caprovina, E. alijevi, E. hirci, E. jolchijevi and E. aspheronica, which are distributed globally (6–16). Among these species, E. arloingi, E. ninakohlyakimovae, E. christenseni and E. caprina are considered the most pathogenic and prevalent (21), while others are non-pathogenic.
Goats between 1 and 6 months of age are most susceptible to Eimeria spp. which inhabit the small and large intestines (22, 23). The oocysts are passed in feces, infecting other animals after further development (i.e., sporulation – asexual reproduction) in the environment. When a susceptible host ingests a sporulated oocyst, the sporozoites are released in the gastrointestinal tract and invade intestinal epithelial cells. Following a number of predetermined generations of development (schizogony – asexual reproduction) in intestinal cells, the female and male gametes form a zygote, which develops into an oocyst (24). The damage to the host occurs due to cell disruption during schizogony and later during gametogony (sexual reproduction). The more prevalent form of coccidiosis is a subclinical disease resulting in poor growth while the clinical form of the disease is most commonly characterized by diarrhea (25). The economic losses due to coccidiosis (26) are attributed to reduced productivity, reduced weight gain, mortality (22, 23) and treatment costs (27). The damage done to the kid’s intestines may be permanent in severe cases, resulting in kids that exhibit ill-thrift for life, i.e., they remain “poor doers” as they fail to recover fully after treatment (25). These kids are more susceptible to other diseases, such as respiratory infections, due to their lower immunity (28).
Given that the global prevalence of Eimeria spp. in goats is variable (29–35), with more than 90% prevalence reported in some regions (11, 14, 35–39), effective control measures are essential to minimize losses associated with subclinical and clinical coccidiosis (40). Currently, control of coccidiosis is based on sound management, using preventive medications and treating clinical cases using anticoccidial drugs (23). Although coccidiosis is well-studied in poultry, sheep and cattle, our understanding of goat coccidiosis is limited despite the remarkable growth in the global goat industry in recent decades (14). As a first step to ascertain the current state of play, an exploration of the prevalence and geographical distribution of Eimeria spp. in goats could pave the way for global efforts to control goat coccidiosis. Therefore, we conducted a systematic review and meta-analysis to estimate the global prevalence of Eimeria spp. infecting goats, with an emphasis on temporal and spatial trends, frequency and spatial distribution of species, diagnostic methods, sample size and quality of selected studies. The findings of this study could be used by veterinarians, researchers, goat farmers and policymakers to make informed decisions about the effective control of coccidiosis in goats worldwide.
2 Materials and methods
2.1 Search strategy
This study was designed and analyzed according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) protocol (41) (Supplementary Table S1). Goats of any age and sex constituted the study population in this study. Two searches across four electronic databases, including PubMed, Scopus, CAB Direct, and Web of Science were performed to retrieve the maximum number of publications. A manual search was also carried out on Google Scholar and reference lists of reviews and included studies. The search queries were designed based on the medical subject headlines (MeSH) and Boolean logic. The MeSH terms and keywords were used to retrieve all relevant articles from the above databases (Supplementary Table S2). The same keywords were used in all electronic databases, and the final database search was completed on March 09, 2023.
2.2 Inclusion and exclusion criteria
The search results were imported into an online systematic review platform, Covidence.1 Duplicate references were then removed, followed by assessment and screening in two steps (Figure 1). The first screening step involved removing irrelevant studies based on the information available from titles and abstracts. In the next step, full texts of selected studies were retrieved. If full texts were unavailable online, they were obtained through interlibrary loans from the University of Melbourne library and were also subjected to the set assessment criteria. To ensure the quality of included studies, the following inclusion criteria were used where (i) the study defined the number of goats examined and the number testing positive, (ii) the study was published in the English language, (iii) the studies contained a full text, (iv) samples indicated the prevalence of Eimeria in goats and (v) individual samples were taken from each goat (i.e., samples were not pooled). In addition, we excluded those studies that were not original research articles, involved plagiarism or used the same data for multiple publications, exhibited internal data conflict or insufficient data, only reported other parasites or Eimeria reported in host species other than goats or studies with experimental findings only (Figure 1).
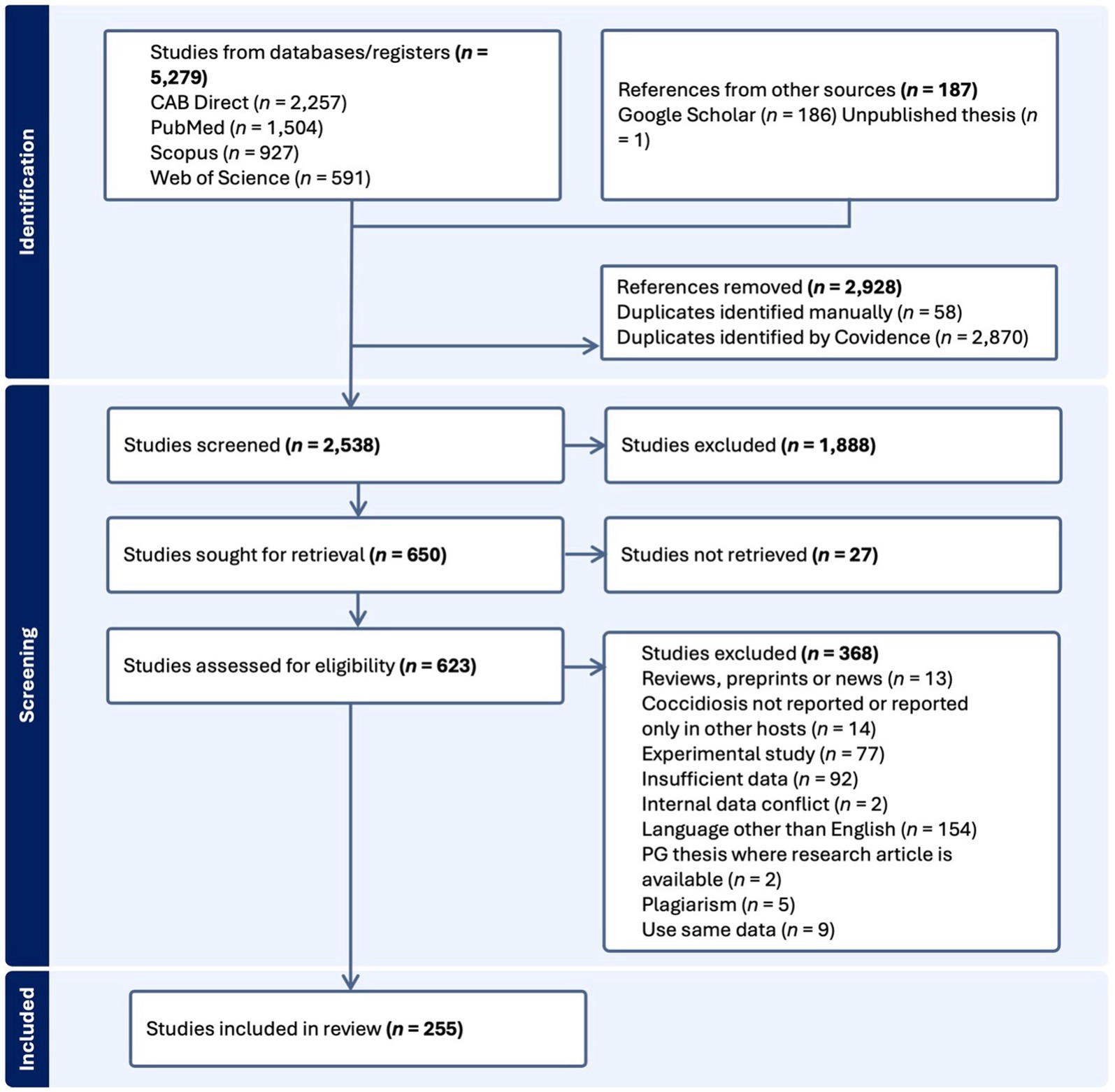
Figure 1. Flow diagram indicating the process of identification, screening and inclusion of studies for the systematic review and meta-analysis according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PG, postgraduate.
2.3 Data extraction
The first author, EA, performed the data extraction. Any discrepancies were discussed with co-authors, who acted as secondary reviewers whenever required. The selected studies were coded, and data were collected using a format prepared in a Microsoft Excel® spreadsheet. The format included the first author’s name, research type, title, year of publication, aims of the study, type of diagnostic sample, country of publication and origin of the sample, continent, diagnostic method, study design, sampling period, sampling method, sample size and methods of sample size calculation, the number of goats tested positive, prevalence, age, sex, method of Eimeria spp. identification, the identification keys used, and the number of Eimeria spp. isolated and/or reported. Data related to molecular methods and markers used for Eimeria spp. identification, and sequencing information were also extracted. During the study period, we contacted the authors of some articles to obtain more information and included unpublished data. The extracted data were checked at least twice for accuracy.
2.4 Quality assessment
We evaluated the quality of the eligible studies using a scoring method described previously (42–45). The method assessed specific points, including (i) random sampling, (ii) clarity of the detection method described, (iii) detailed description of the sampling method, (iv) inclusion of the sampling period, (v) calculation of sample size, (vi) aim of the study and (vii) species-level identification of Eimeria. Each point was scored as one, and articles were assigned to low (0–2 points), medium (3–4), or high (5–7) levels based on their scores (Supplementary Table S3). Data extracted from the studies included were summarized and edited using Microsoft Excel® spreadsheet Version 16.
2.5 Statistical analyses
All the analyses and visualization were performed in RStudio 4.3.1 (46) using the “meta” package (47). The bar plots were built using GraphPad Prism 10.2.0.2 The mean species richness value was calculated at the country level considering the common nine Eimeria spp. of goats reported globally. A random effects model was used to estimate the global pooled prevalence of Eimeria spp. of goats, along with a 95% confidence interval (CI) (48). The estimated pooled prevalence was presented as a percentage [(number of positive samples/total samples tested) *100] and displayed using forest plots. To stabilize the variance, we used the Freeman-Tukey double arcsine transformation (referred to as “PFT” in the “meta” package) (49).
Cochran’s Q values and the inconsistency index (I2) test statistics were used to assess study heterogeneity. The I2 estimates the percentage of variability in effect estimates due to heterogeneity rather than sampling error or chance differences. Therefore, the I2 test measures the level of statistical heterogeneity among studies. I2 scores of 25, 50 and 75% indicate low, moderate and high degrees of heterogeneity, respectively (50, 51). To evaluate the possibility of publication bias, we utilized funnel plots, Egger’s asymmetry test (52) and Begg’s rank correlation test (53). In the funnel plot, we examined the symmetry of the figure, and if the dots (representing included studies) in the funnel plot were symmetrically distributed on both sides of the mid-line, it indicated no publication bias. If they were asymmetric, it suggested publication bias among the included studies. Begg’s and Egger’s significance tests were also employed to determine the presence of bias. The stability of this study was evaluated by the Duval and Tweedie’s trim and fill analysis (54). Sensitivity analysis was conducted to verify the reliability and robustness of the meta-analysis.
The Baujat plot was used to identify sources of heterogeneity. In the Baujat plot, the horizontal (x) axis represents the contribution of each study to the general statistics of the Cochran’s Q test for heterogeneity, while the vertical (y) axis represents the influence of each study on the overall estimate. The most heterogeneous studies are represented in the upper right area of the graph (55). Subgroup and meta-regression analyses were conducted to investigate the possible sources of heterogeneity. The variables included in the subgroup and meta-regression analyses were year of publication (before 2000 and 2000 or later), region (Asia, Africa, Europe, North America, Oceania and South America), diagnostic methods (conventional, and conventional and molecular methods), the score level of studies (1–2, 3–4 and 5–7), sample size (n = <400; n = 400–1,000; n = >1,000) and country (n = 56).
Following the subgroup and univariate meta-regression analyses, a multivariate meta-regression analysis was performed on all response variables to identify the best model that explains the between-study variability in effect size estimates. In the meta-regression, the variable x represents study characteristics (such as region, country, year, sample size, score level and detection method), which are used to predict the study effect size ( ) as shown in the following model (Equation 1):
Where: is the observed effect size, is the intercept, is a predictor (or covariate) with a regression coefficient (fixed effect), is the sampling error and is the between-study error (random effect).
The first step in multivariate meta-regression analysis involved all explanatory variables in a full model. Subsequently, multi-predictor models were manually reduced using backward selection of variables until all predictors were statistically significant (p < 0.05). We constructed mixed-effects regression models (for the meta-regression analysis). We applied Akaike’s information and Bayesian information criteria to compare and select the models. We assessed the goodness of fit for the meta-regression by calculating the correlation analog coefficient (R2) using the following formula (Equation 2):
Where: represents the estimated total heterogeneity based on the random effects model and represents the total heterogeneity of the mixed effects regression model.
In all analyses, p-value <0.05 were used to determine a statistically significant association.
3 Results
3.1 Characteristics of eligible studies
During the literature search, 5,466 studies were retrieved from five databases (CAB Direct = 2,257, PubMed = 1,504, Scopus = 927, Web of Science = 591 and Google Scholar = 186) and one unpublished thesis, and 255 of them met the inclusion criteria (Figure 1). Most of these studies were original research papers (n = 218), followed by short communications (n = 27) and postgraduate theses (n = 4) published from 1963 to 2022 (Figure 2). An evaluation of the quality of 255 studies showed that 79 scored high (5–7), 149 medium (3–4) and 27 low (1–2) points (Supplementary Table S3).
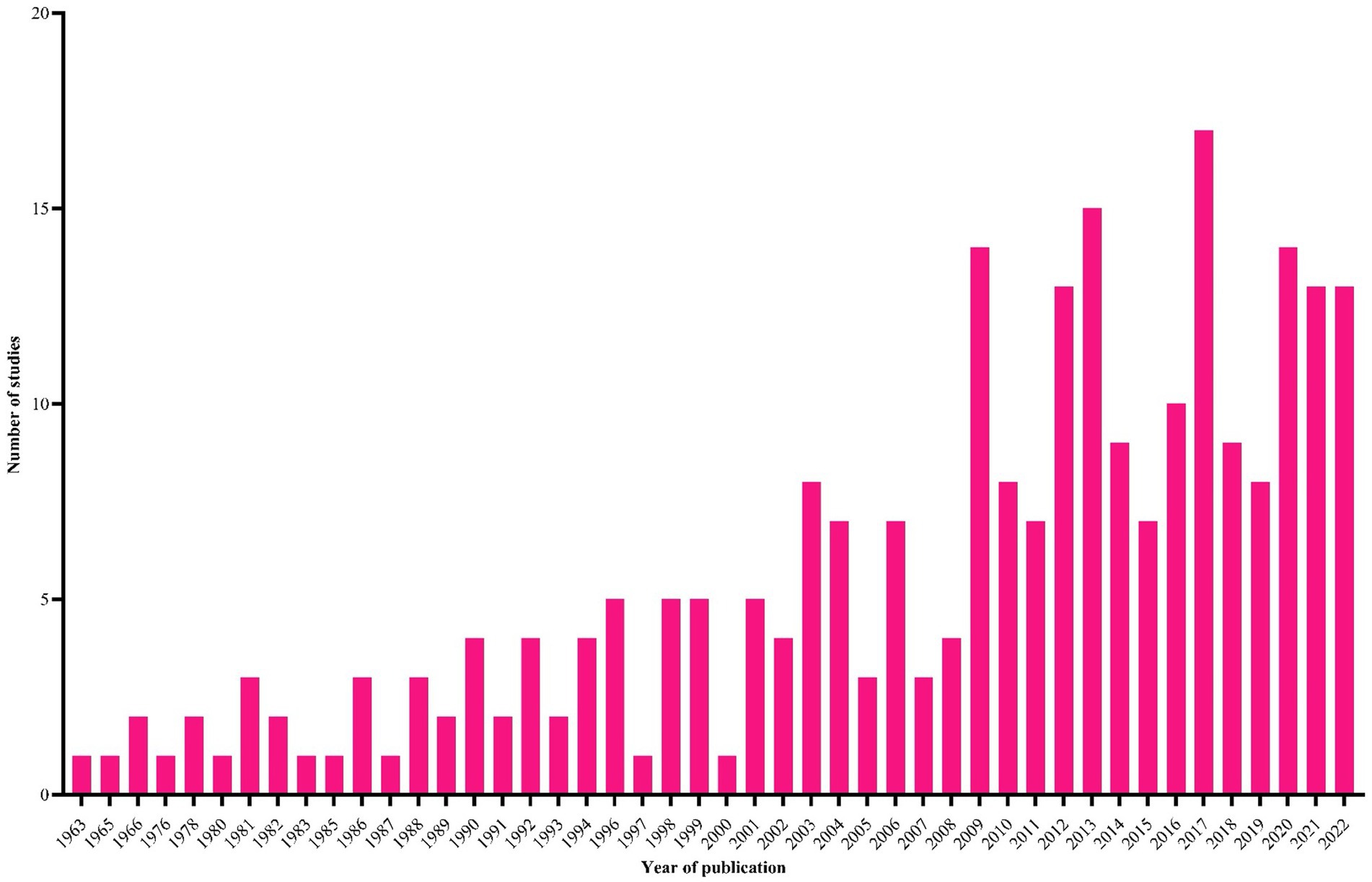
Figure 2. The temporal distribution of studies (n = 255) included in this systematic review and meta-analysis.
Two hundred and fifty-five eligible studies originated from 56 countries across six continents (Figure 3) and most of them were from Asia (n = 128) followed by Africa (n = 72), Europe (n = 34) and others (n = 21). The eligible studies tested 131,407 goat fecal samples and 75,669 were positive for Eimeria. The apparent prevalence in studies ranged from 1.6 to 100% (Supplementary Table S4). Various diagnostic methods were used, including flotation (using saturated sodium chloride, sucrose, sodium nitrate, zinc sulfate and magnesium sulfate), the McMaster method (n = 140) in combination with direct smear (n = 28), histopathology and/or post-mortem examination (n = 11), and molecular tests (n = 10) (real-time polymerase chain reaction (qPCR), nested PCR and conventional PCR) (Supplementary Table S4).
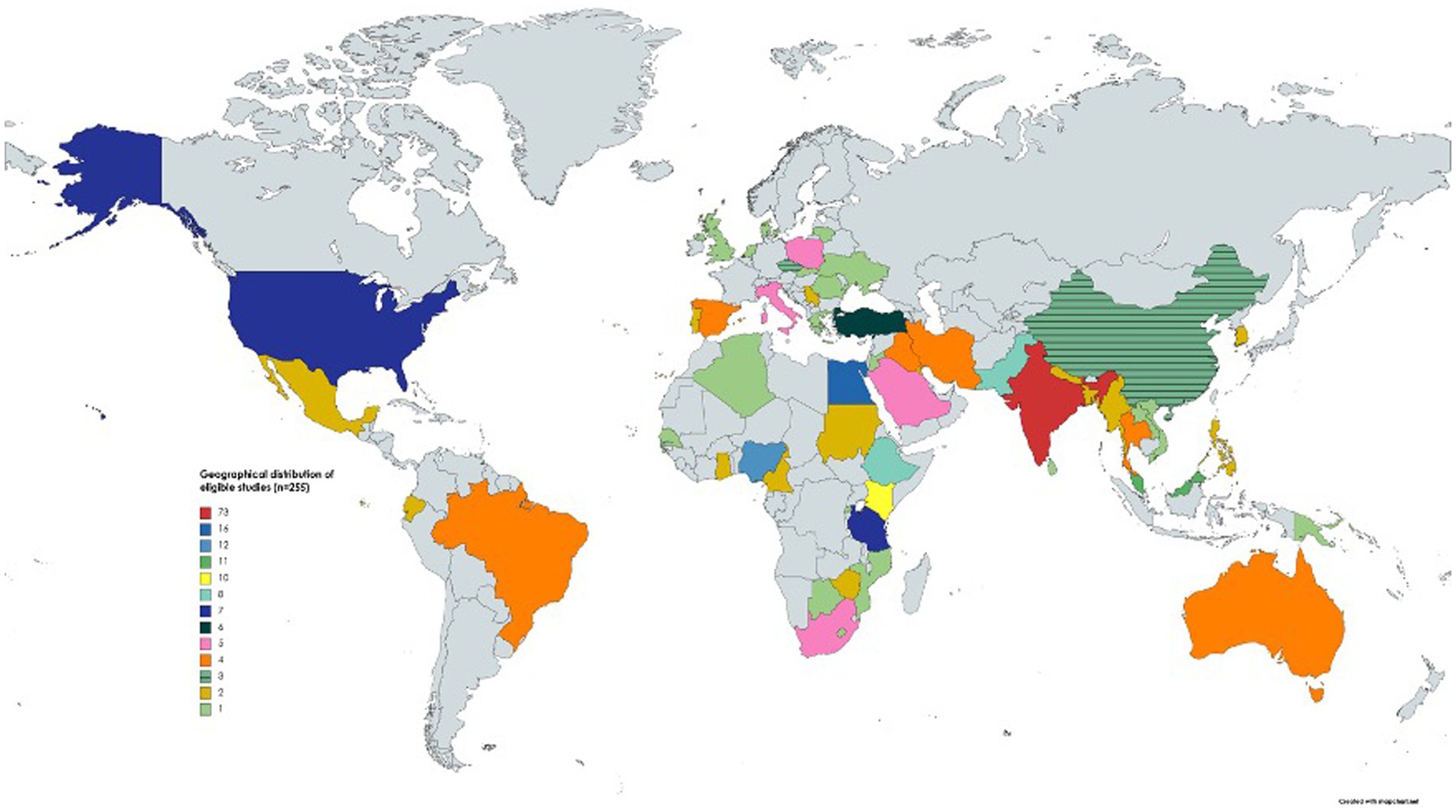
Figure 3. The geographical distribution of studies (n = 255) included in this systematic review and meta-analysis. One study was conducted in two countries (see Supplementary Table S4); hence, 256 studies were included in the map.
Morphological identification of Eimeria spp. was conducted based on the morphology of sporulated oocysts. For this purpose, fecal samples that tested positive for Eimeria were artificially incubated in 2% or 2.5% potassium dichromate for several days (usually 7–10 days) at room temperature. The species of Eimeria were then identified using various parameters, including the size and shape index (SI) of oocysts, the presence/absence of a polar cap and micropyle, and the size and SI of sporocysts along with sporulation time. However, only 119 (47%) studies conducted morphological identification of Eimeria at the species level using sporulated oocysts and histopathological findings. Globally, more than 26 Eimeria spp. (Table 1; Supplementary Table S4) were recorded in goats, with recognized species, including E. arloingi, E. ninakohlyakimovae, E. christenseni, E. alijevi, E. caprina, E. hirci, E. caprovina, E. aspheronica and E. jolchijevi being the most frequently reported (Figure 4), while other species were reported less frequently (Table 1). Mixed species infections were observed in most studies, with an average or mean species richness value of 7.3 and a median of 8 Eimeria spp. reported per study (Figure 5). Only 10 studies from 8 countries corroborated their identification using molecular methods. Molecular-based studies primarily targeted the small subunit of the nuclear ribosomal RNA gene (18S rRNA), followed by mitochondrial cytochrome c oxidase 1 (COX1), and/or the first and second internal transcribed spacers of nuclear ribosomal DNA (ITS-1, ITS-2) to genetically characterize four Eimeria spp. (E. arloingi, E. ninakohlyakimovae, E. christenseni, and E. hirci) (Figure 6). As of June 25th 2024, more than 70 nucleotide sequences belonging to E. arloingi (37 sequences), E. christenseni (30 sequences), E. hirci (8 sequences), E. ninakohlyakimovae (2 sequences), and unidentified Eimeria spp. (4 sequences) were available via GenBank3. However, only a few of these sequences have been published in peer-reviewed journals.
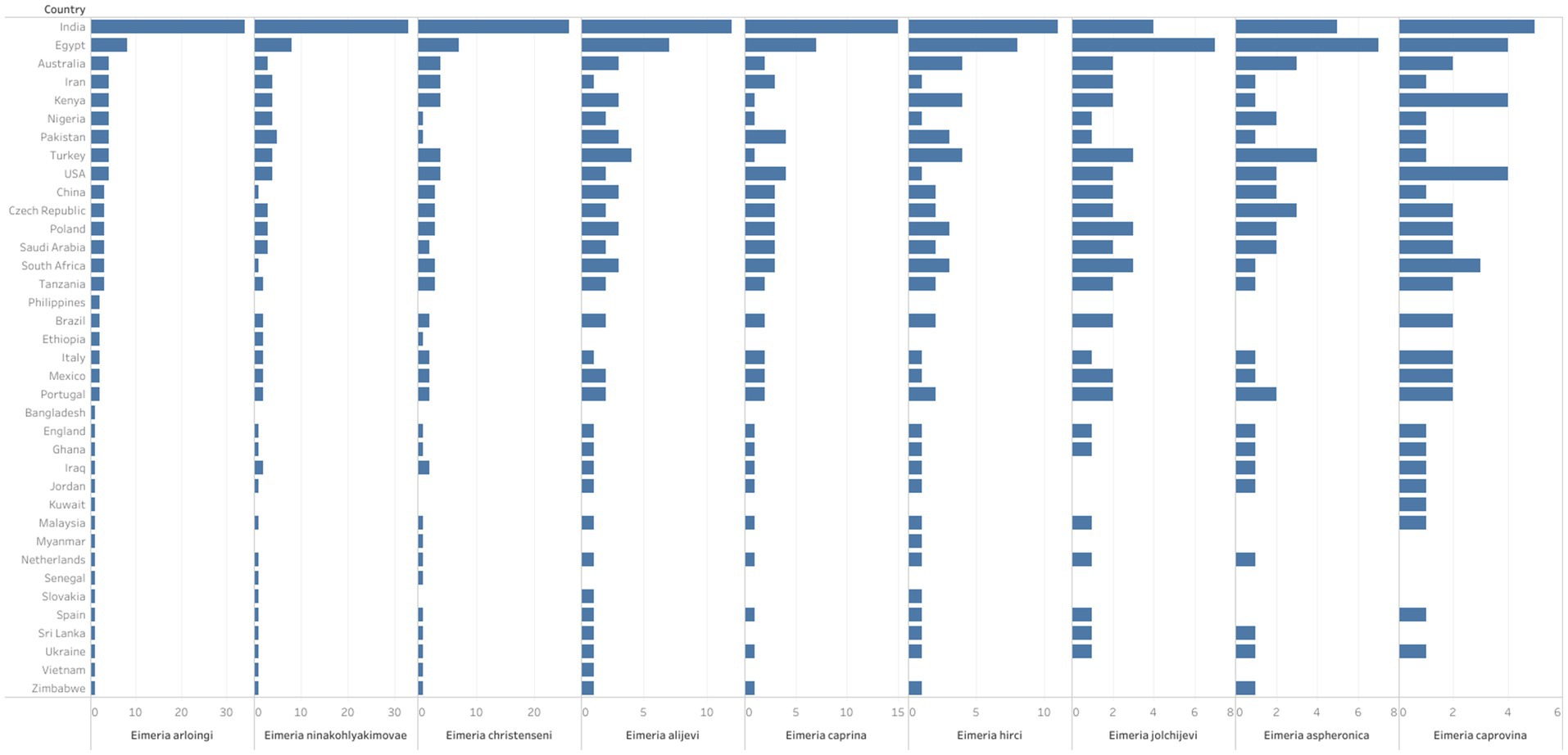
Figure 4. The spatial distribution and diversity of the valid Eimeria species commonly reported in goats globally. The length of each bar indicates the number of studies which reported the respective Eimeria spp. from each country.
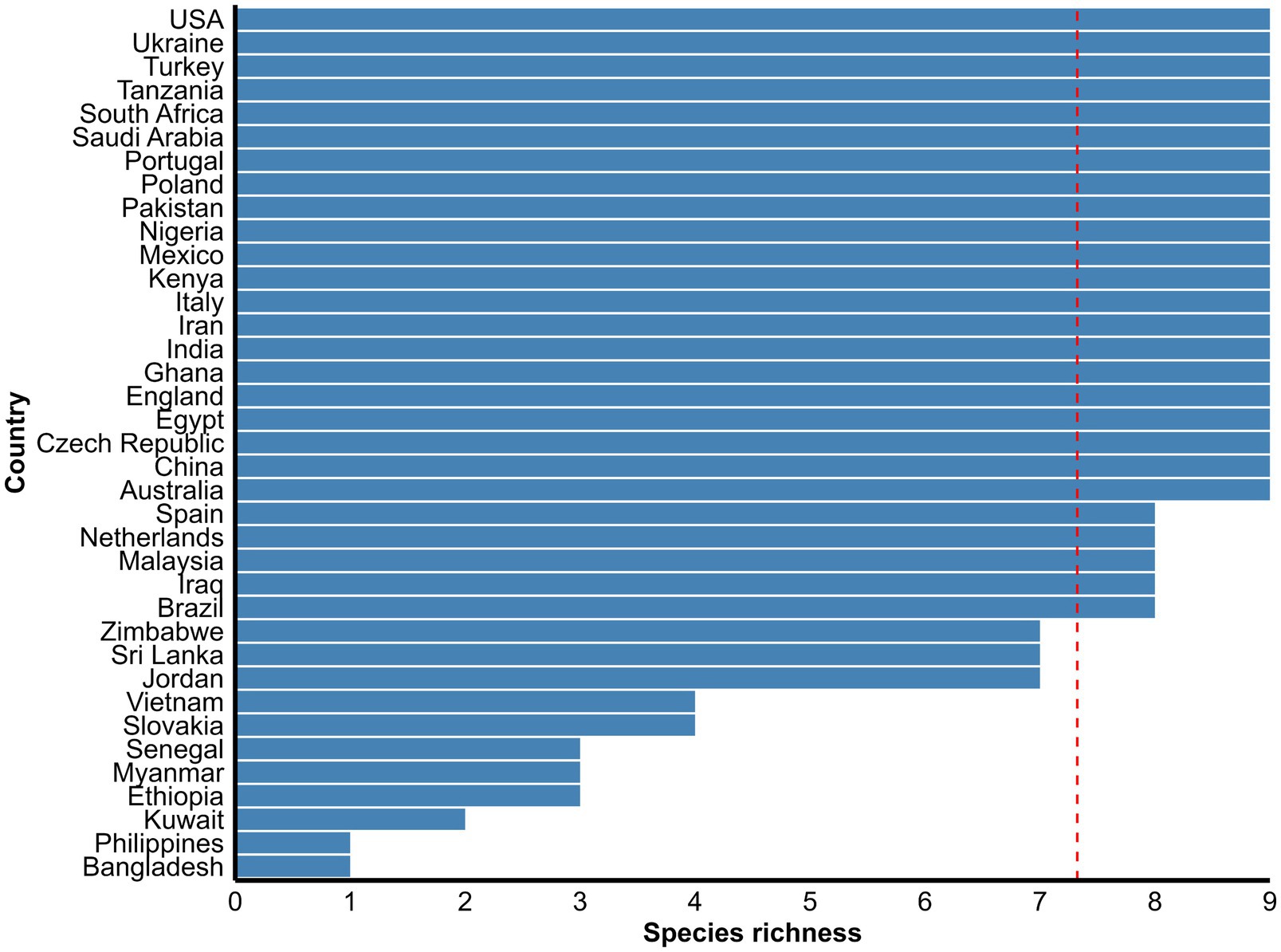
Figure 5. Mean species richness of nine common Eimeria species of goats reported globally. The dashed vertical line is the overall mean species richness value. See Supplementary Table S4 for more detail in each country.
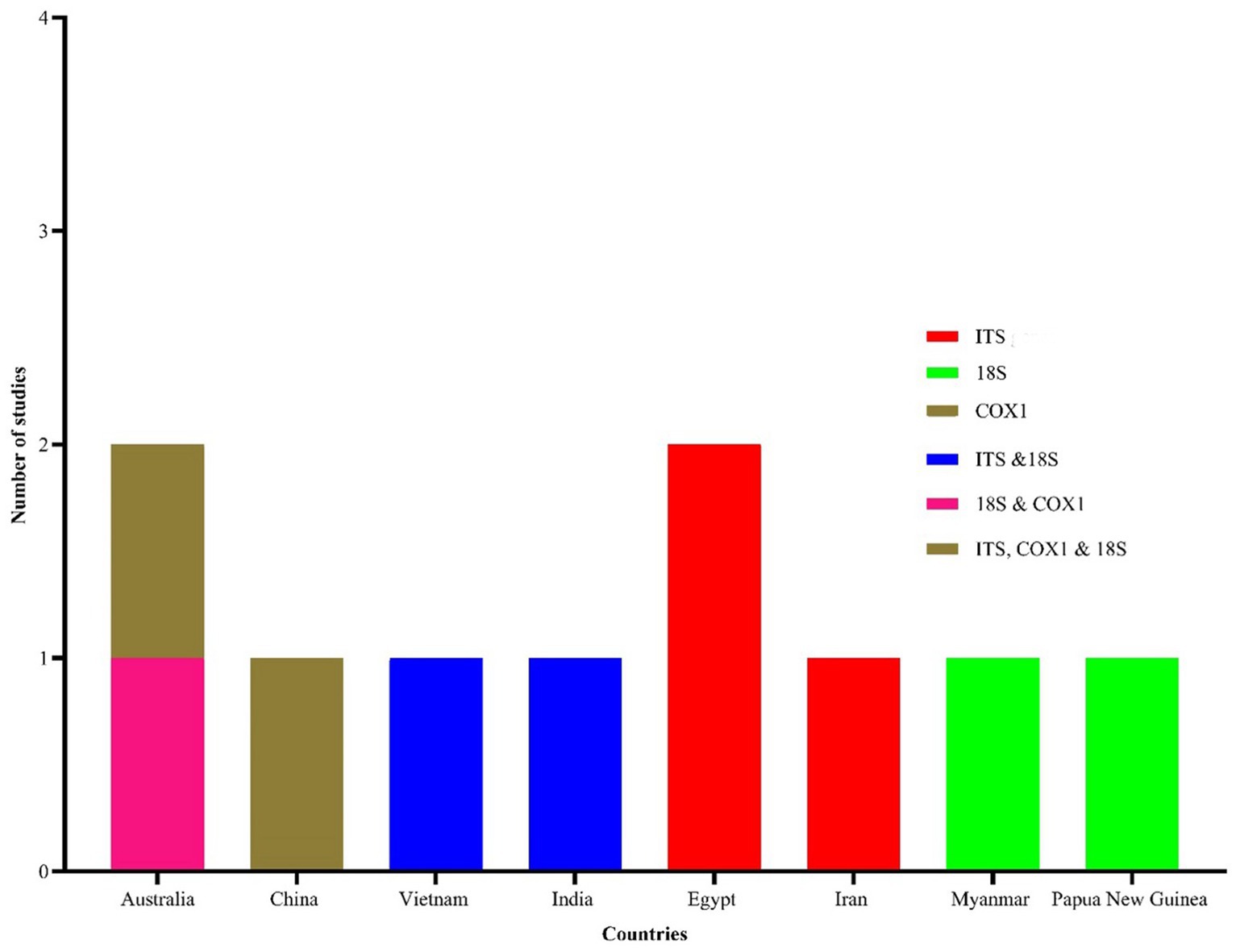
Figure 6. Geographical distribution of studies reporting molecular characterization of Eimeria spp. in goats. Three molecular markers, including 18S (small subunit of the nuclear ribosomal RNA), coxI (mitochondrial cytochrome c oxidase 1) and ITS (the internal transcribed spacer of the nuclear ribosomal DNA) were used in 10 studies.
3.2 Meta-analysis
A random-effects meta-analysis model was computed using the Freeman-Tukey double-arcsine transformed proportion. This model was chosen due to the expected variation among studies. Based on this model, the estimated global prevalence of Eimeria spp. in goats was 62.9% (95% CI: 58.6–67.2) (Figure 7; Table 2). The forest plot revealed high heterogeneity among the studies reporting the prevalence of Eimeria spp. ( = 0.1293, I2 = 99.7%, Q = 72,791.38, degrees of freedom = 254, p < 0.01). The random effects of studies were weighted similarly to the common (fixed) effects of studies, ranging from 0.0 to 9.6% (see Supplementary Figure S1). The Baujat plot shows that the study conducted by Rahman et al. (56) from India significantly impacted the pooled estimate and contributed the most to the overall heterogeneity (Figure 8A).
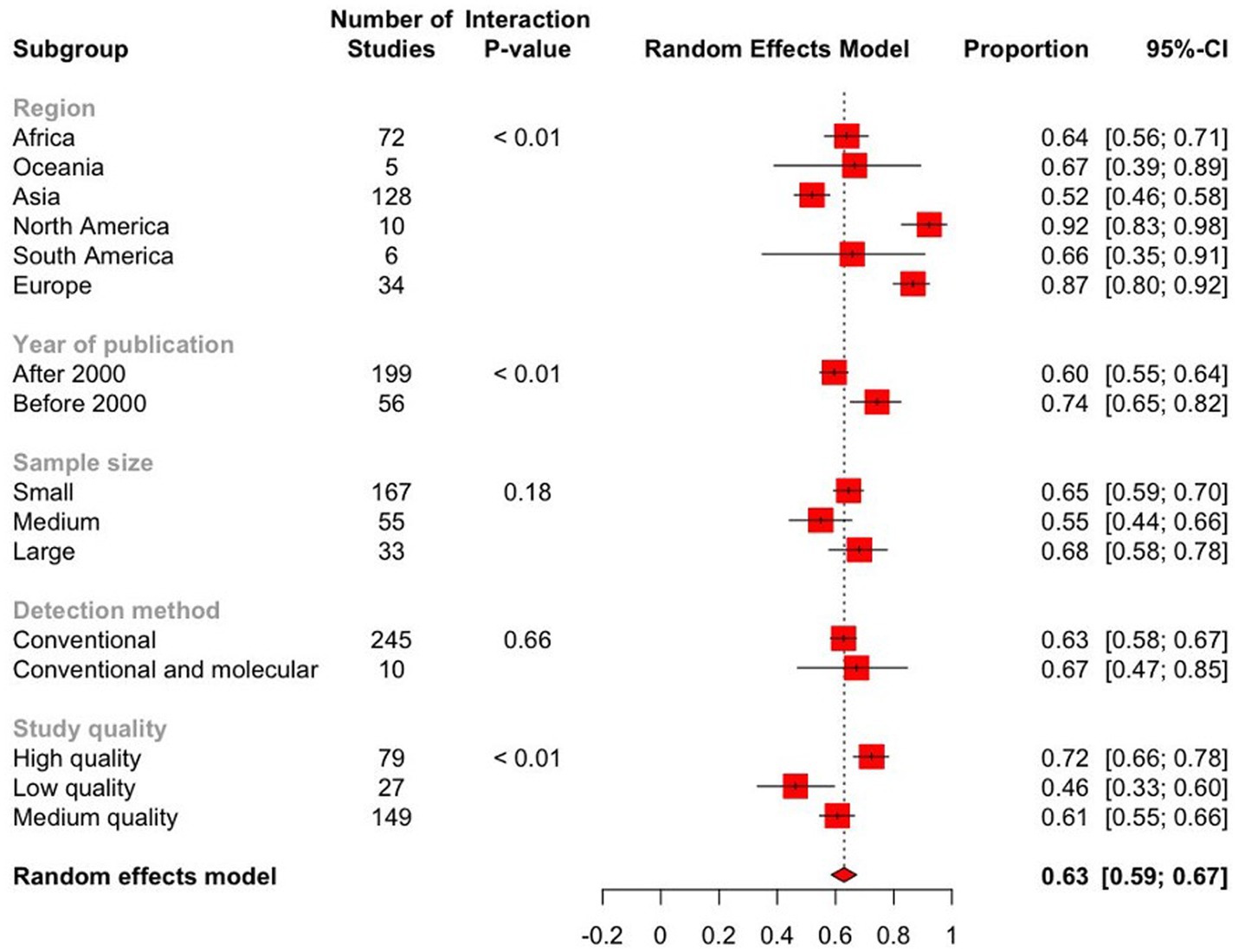
Figure 7. Forest plot displaying the pooled prevalence estimates of Eimeria spp. in goats from subgroup meta-analysis. The “Proportion” column shows the prevalence of Eimeria spp. in each subgroup, while the 95% CI represents the corresponding confidence interval (CI). The dashed line represents the global pooled prevalence estimate based on the random effects model. The length of the horizontal lines represents the 95% CIs. The estimated global prevalence is the red diamond at the bottom of the plot.
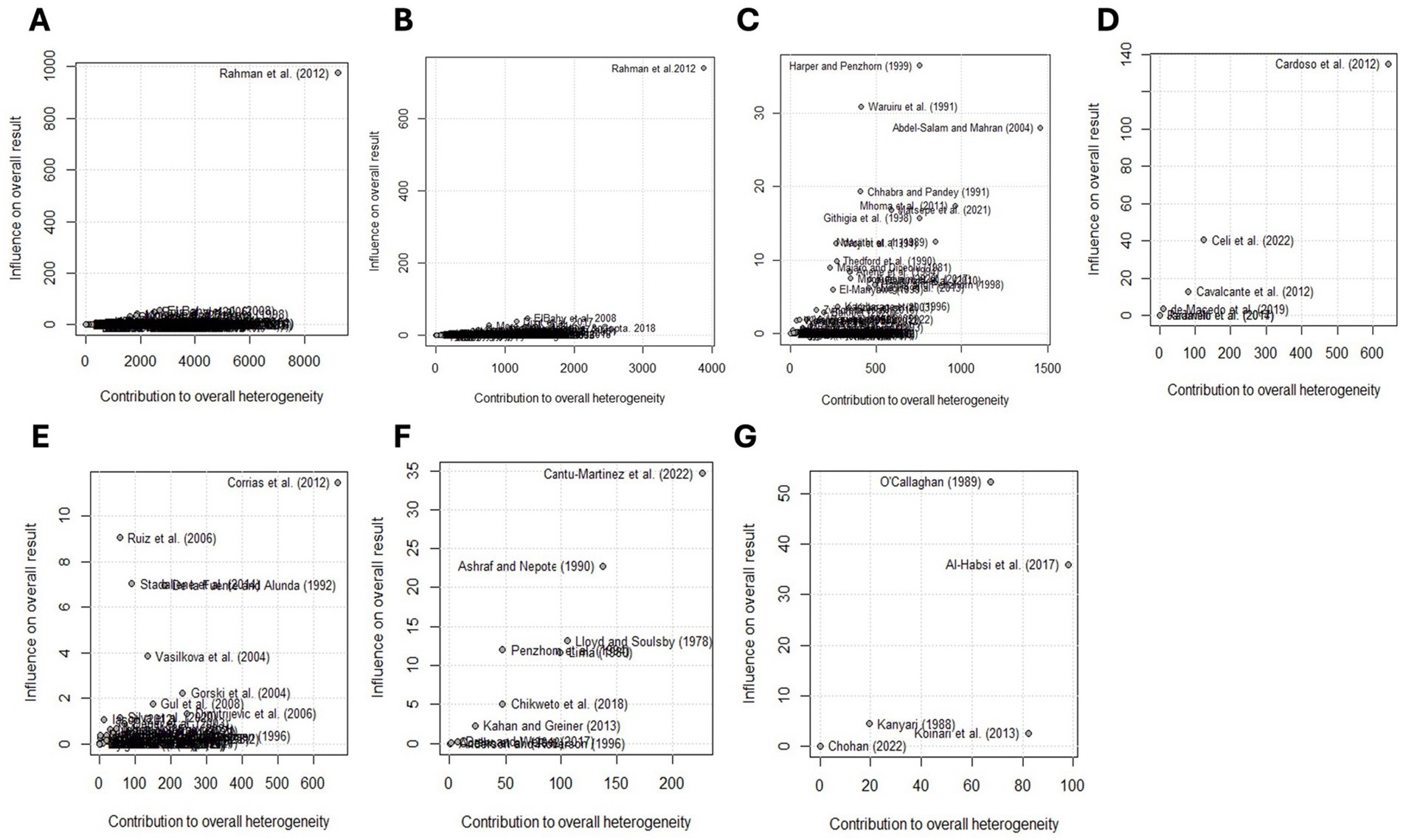
Figure 8. Baujat plots illustrate the contribution of each study to the general statistics of the Q test for heterogeneity. The first graph shows the global pooled prevalence of Eimeria spp. (A) followed by Asia (B), Africa (C), South America (D), Europe (E), North America (F), and Oceania (G). The studies in the upper right corner have the greatest influence on the results and contribute the most to heterogeneity. Heterogeneity was high (t2 = 0.1293; I2 = 99.7%; Cochran’s Q = 72,791.38, p = 0.00) for the global prevalence estimate. See Table 2 for general statistics in each region.
3.3 Subgroup meta-analysis
The subgroup analysis revealed significant heterogeneity between studies in all subgroups. The Baujat plots illustrating the studies that contributed the most to the heterogeneity in each continent are also presented (see Figure 8). The highest prevalence was estimated in North America (92.2, 95% CI: 82.7–98.2) followed by Europe (86.6, 95% CI: 79.8–92.3) while it was the lowest in Asia (52.0, 95% CI: 45.9–58.1) (Figure 7; Table 2). Among the 56 countries included in the eligible studies, 75% (n = 42) of them had an estimated prevalence (63–100%) above the overall pooled estimate (>62.9%). On the other hand, Papua New Guinea, Saudi Arabia, and Kuwait reported the lowest estimates with pooled prevalences of 16.4% (95% CI: 7.8–28.8), 19.8% (95% CI: 18.5–21.0) and 19.8% (95% CI: 15.0–25.4), respectively (Figure 9; Supplementary Figure S2).
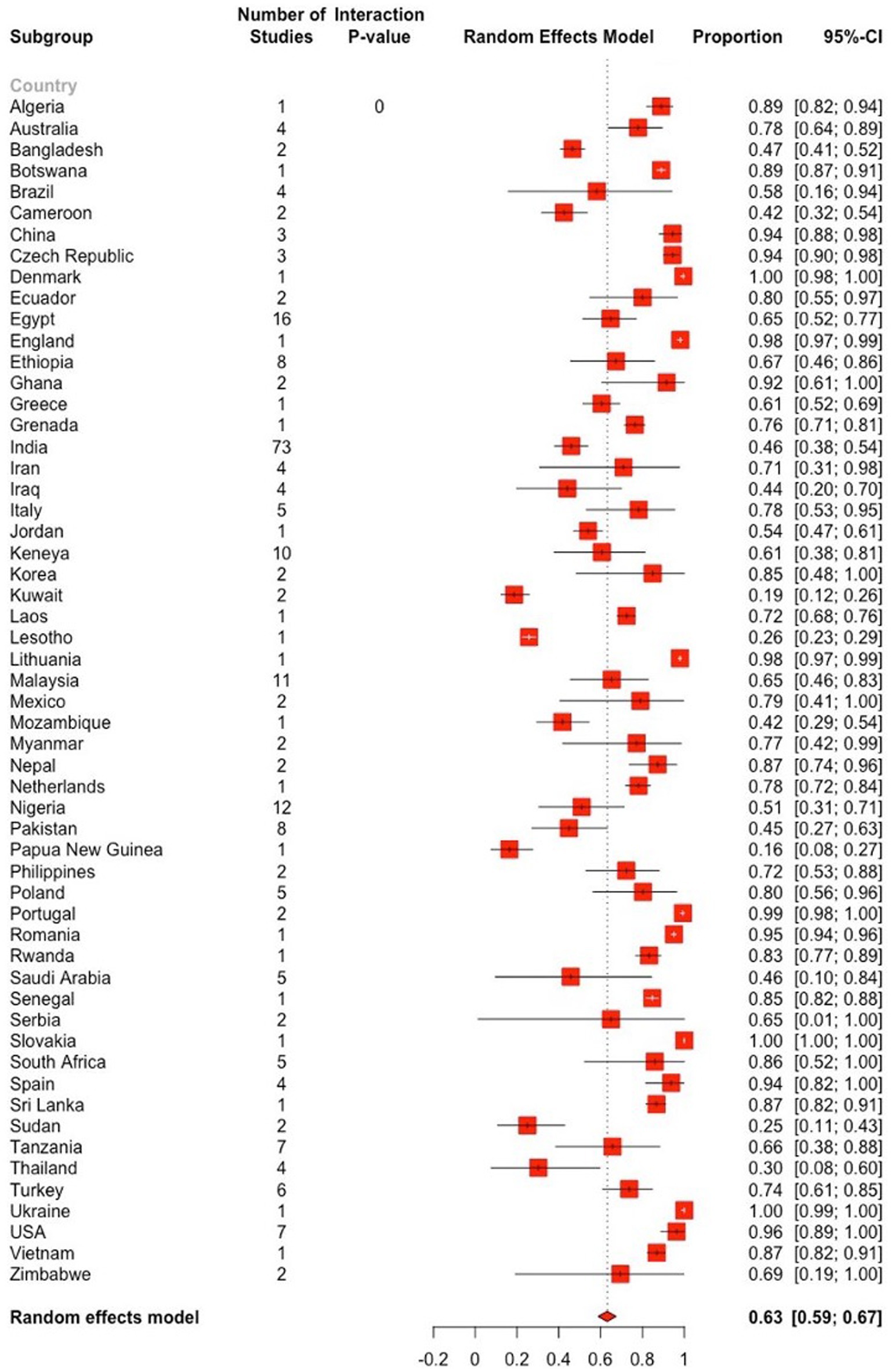
Figure 9. Forest plot displaying the pooled prevalence estimates of Eimeria spp. in goats stratified by country. The “Proportion” column shows the prevalence of Eimeria spp. in respective country, while the 95% CI represents the corresponding confidence interval (CI). The dashed vertical line denotes the global pooled prevalence estimate, derived from the random effects model. The length of the horizontal line represents the 95% CI. The white and black bars, respectively, denotes a very narrow and wide CIs. The estimated global prevalence is the red diamond at the bottom of the plot.
The prevalence of Eimeria spp. in goats sampled before 2000 was 74.3% (95% CI: 65.2–82.4) and 59.6% (95% CI: 54.7–64.3) in 2000 or later, showing a statistically significant downward trend (p < 0.05). Based on sample size, the prevalence of Eimeria spp. was estimated to be 55.1% (95% CI: 44.6–65.3) in studies with a sample size ranging from 400 to 1,000. In studies with a sample size greater than 1,000, the estimated prevalence was 68.2% (95% CI: 57.7–77.8). However, these differences were not statistically significant (p > 0.05). Based on the score level subgrouping, studies with score levels of 5–7 reported the highest prevalences (72.4, 95% CI: 66.2–78.2) compared to low or medium score levels, with statistically significant differences (p < 0.01). The prevalence of Eimeria spp. in goats detected using conventional and molecular methods was 67.3% (95% CI: 47.0–84.7), which was higher than that detected by conventional methods alone, but these differences were statistically non- significant (Table 2).
3.4 Meta-regression models
Univariate and multivariate meta-regression analyses were used to further explore the heterogeneity of data. Among the moderators considered in the univariate analysis, the region, year of publication, sample size and quality level of studies had statistically significant effects on the observed variability between the reports (Table 3). The results of univariate meta-regression indicate that the region was the covariate that contributed the most to explaining the heterogeneity of the total prevalence (R2 = 15.71). In the multivariate meta-regression, the first approach was to build a full model including all moderators, where almost all covariates were significant (p < 0.05) except the detection method. The R2 of the full model was 27.3. The best model included the covariates of region, year, sample size, country and score level with an R2 of 31.7 (Table 3).
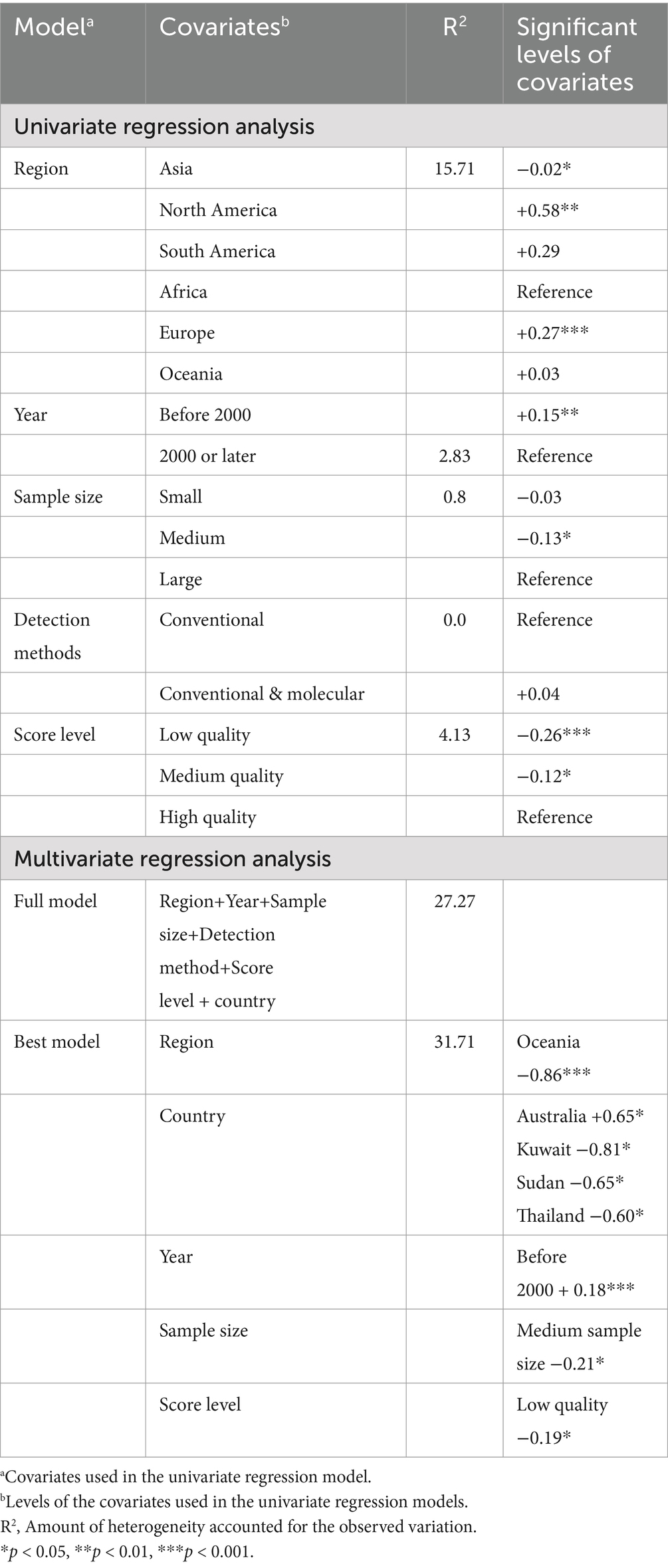
Table 3. Univariate and multivariate approach of meta-regression of estimated global pooled prevalence of Eimeria spp. in goats.
In the univariate regression, a strong positive relationship was observed between the prevalence of Eimeria in goats and the European (+0.27***) and North American (+0.58**) regions. Conversely, in the multivariate approach, a negative relationship was found between Eimeria prevalence and the Oceania region (−0.86***). In relation to the year of publication of the studies analyzed, both meta-regression approaches yielded similar results, indicating that studies published before 2000 were associated with a higher prevalence of Eimeria in goats. Interestingly, the sample size of the studies was identified as a significant factor contributing to the heterogeneity observed in both meta-regression approaches. This was evidenced by a negative relationship between the prevalence level and a medium-sized sample. Finally, the univariate (−0.26***) and multivariate (−0.19*) regression analyses indicated that studies with a lower quality tended to report a lower prevalence of Eimeria. However, the univariate regression also identified that medium-quality studies were associated with lower Eimeria prevalence (Table 3).
3.5 Publication bias assessment
In the funnel plot, all studies were symmetrically distributed (Figure 10). Additionally, the results of Egger’s regression (b = 3.87; p = 0.06) and rank correlation tests (z = −1.03; p = 0.30) for funnel plot asymmetry were not statistically significant for the global pooled estimate (Supplementary Tables S5, S6). Egger’s test did not show significance, nor did the funnel plot exhibit asymmetry for the African (b = −4.88; p = 0.166) and North American continents (b = 3.7479; p = 0.736). However, publication bias was significant for the pooled prevalence estimate of Europe (b = −5.59; p = 0.02) and Asia (b = 7.42; p = 0.005) (Supplementary Table S7). Funnel plots for each continent are presented in Supplementary Figure S3. Furthermore, there was evidence of missing studies that could be included using Duval and Tweedie’s trim and fill analysis, which would address the asymmetry seen in the plot (Supplementary Figure S4). Eighty-one studies were found in the trim and fill analysis, resulting in a final change to the pooled estimate (Supplementary Figure S5). A sensitivity test showed that the reconstructed data were not affected by the removal of any study, suggesting the rationality and reliability of our analysis (Supplementary Figure S6).
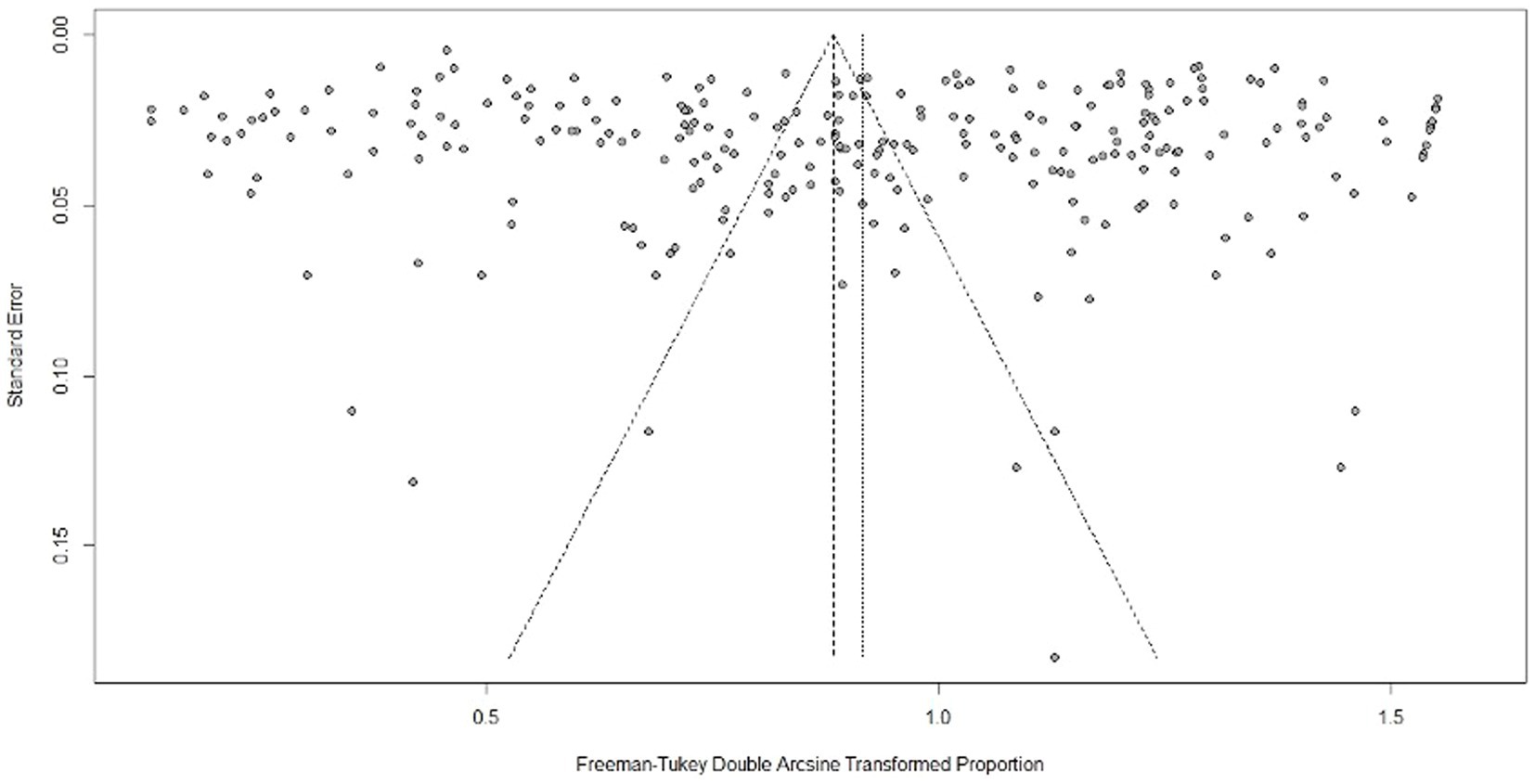
Figure 10. Funnel plot for evaluating publication bias in all included studies. The plot shows each study’s estimated effect plotted against its standard error and evaluates the relationship between study results and their precision.
4 Discussion
This is the first study on the global prevalence of Eimeria spp. infection in goats using a substantial number of studies (n = 255) retrieved from five databases. The overall global prevalence of Eimeria spp. in goats is 62.9%. The 95% prediction interval (PI) is estimated at 4–100%. The highest prevalence was found in North America (92.2%), followed by Europe (86.6%), while it was the lowest in Asia (52.0%). Surprisingly, in 42 out of 56 countries, the estimated prevalence (63–100%) was higher than the overall pooled estimate (62.9%).
The subgroup analysis by region and country showed that the highest pooled prevalence of Eimeria spp. was found in North America (92.2%), and the meta-regression showed a positive beta coefficient (b = 0.58). Within this region, the USA had a higher prevalence of Eimeria spp. in goats (92.4%), whereas it was lowest in Mexico (71.2%). In the USA, the higher prevalence of Eimeria spp. in goats was attributed to risk factors such as season, age, farm management, and the use of deep litter straw bedding materials (57). Additionally, the intensive production system and climate conditions contributed to the higher prevalence of Eimeria spp. in the USA (9). However, no studies reported the prevalence of Eimeria spp. in goats in other North American countries with significant goat populations such as Haiti and Cuba (1). The study conducted by Cantú-Martínez et al. (58) from Mexico contributed most to the observed heterogeneity on the continent (Figure 8F). Interestingly, no publication bias was detected in this region (Supplementary Table S7).
The estimated prevalence of Eimeria spp. in goats in Europe was 86.6%. A meta-regression analysis showed a positive beta coefficient (b = 0.27, p < 0.01) (Table 2). In Europe, 80% (12/15) of countries had a prevalence of Eimeria spp. higher than 70%. The lowest estimated prevalence was 57.1% in Serbia, which is still higher than the estimated prevalence of the Asian continent (52.0%). The goat sector in Europe is specialized in milk production and is highly commercially oriented, dominated by an intensive production system, as reviewed by Miller and Lu (59). This may have contributed to the higher prevalence of Eimeria spp. in goats reported in this region, because the intensification of dairy goat production presents challenges in limiting the spread of infectious and parasitic diseases (e.g., coccidiosis), which are facilitated by environmental stressors such as high stocking density (60, 61). A major challenge in commercially oriented dairy goat production systems arises when multiple kidding events occur throughout the year to maintain a consistent milk supply. If the same pens are used repeatedly for successive batches or if newly born kids are introduced to a pen already housing older animals, the later-born kids are immediately exposed to a heavy challenge. They can develop severe coccidiosis in the first few weeks of life (62). Moreover, herd size, age and climatic conditions were associated with varying levels of Eimeria spp. prevalence in Europe (40). The studies conducted by Ruiz et al. (40) in Spain and Corrias et al. (63) in Italy influenced the estimated prevalence and contributed to the observed heterogeneity (Figure 8E). We also detected the probability of publication bias in Europe (b = −5.59; p = 0.02) (Supplementary Table S7; Supplementary Figure S3), which may have further contributed to the heterogeneity.
In Oceania, the estimated prevalence of Eimeria spp. in goats was 66.6%. However, it is worth mentioning that almost all studies were conducted in Australia, with limited prevalence data available from countries such as Fiji, New Zealand, Vanuatu, and French Polynesia, which have a significant goat population (64). The studies conducted by O’Callaghan (13) and Al-Habsi et al. (65), had an impact on the pooled estimate and contributed to the heterogeneity (Figure 8G). The number of studies accessible in Oceania was insufficient (< 10) to perform Egger’s test.
In South America, the estimated prevalence of Eimeria spp. in goats was 65.7%. The apparent prevalence of Eimeria spp. in goats ranged from 4.0 to 91.2%, and almost all studies were conducted in Brazil. This meta-analysis identified the highest prevalence in southern Ecuador and the lowest in Brazil (Supplementary Table S4). The high prevalence of Eimeria spp. in goats from Ecuador is often related to the animals’ exposure to risk factors such as age, presence of cattle, type of pasture and body condition (66). In addition, this may be due to the typical situation in goat pens in Ecuador with moist and dark environments, ideal conditions for oocyst sporulation to occur (66). However, countries with a goat population greater than 1 million, such as Bolivia, Peru and Venezuela (1), do not have data on the prevalence of Eimeria spp. in goats. A study conducted by Cardoso et al. (67) from Brazil influenced the pooled estimate in this region and contributed to heterogeneity (Figure 8D).
The estimated prevalence of Eimeria spp. in goats in Africa was 63.9%. Despite Africa being home to over 40% of the world’s goat population (1), only 72 studies from 17 countries reported the prevalence of Eimeria spp. in goats in Africa. The majority of these studies were conducted in five countries, including Egypt (n = 16), Nigeria (n = 12), Kenya (n = 10), Ethiopia (n = 8) and Tanzania (n = 7) (Figure 5). The highest estimated prevalence of Eimeria were found in Algeria (89.0%) and Botswana (89.0%), while the lowest estimate was recorded in Lesotho (26.0%). However, it is important to note that the estimates for these countries were based solely on one study, which may not accurately reflect the true prevalence of Eimeria spp. The studies conducted in Africa were more heterogeneous than those of other continents. The heterogeneity was influenced by studies from Egypt (68) and Tanzania (69) (Figure 8C). Egger’s test did not identify publication bias in the prevalence estimates in Africa.
Asia has recognized the importance of dairy goat husbandry in the face of climate change, leading to significant investments in dairy goat projects over the past few decades [see the review by (59)]. This explains the large number of studies (n = 128) that report the prevalence of Eimeria spp. of goat in Asia. Interestingly, the estimated prevalence of Eimeria spp. in Asia, which is home to the world’s largest goat population (1), is 52.0%. This result is significantly lower than the global pooled estimate (Table 2). Moreover, the meta-regression analysis showed a negative beta coefficient (b = − 0.02, p < 0.05). The highest estimated prevalence in Asia was found in China (95.1%), a global leader in goat populations (1). This result differs from the previous study reported by Diao et al. (42), who estimated the pooled prevalence of Eimeria spp. in goats in China to be 78.7% (95% CI: 68.2–87.7%). This discrepancy could be due to the differences in the number of studies (70 studies) and (3 studies in present estimate) included in the meta-analysis and the study periods. Conversely, studies from Saudi Arabia, and Kuwait, Asian countries that reported the lowest prevalence of Eimeria spp., were conducted in extensively managed goat flocks and in regions with higher annual average temperatures and lower relative humidity (arid areas) (70, 71). These environmental conditions are known to negatively affect the sporulation and survival of Eimeria oocysts in the environment which could be the reason for the lower prevalence. The lower prevalence of Eimeria spp. in Saudi Arabia was also attributed to sanitation efforts in management programs introduced by goat producers or ecological differences (8). Most studies used to estimate the prevalence of Eimeria spp. in goats in Asia were conducted in India (n = 73), Malaysia (n = 11) and Pakistan (n = 8). However, countries with large goat populations, such as Bangladesh, Indonesia, Mongolia, Nepal and Myanmar (1), had limited or no available data on the prevalence of Eimeria spp. In this region, Egger’s test identified statistically significant publication bias (b = 7.42; p = 0.005) and the greatest contribution to heterogeneity was shared by Rahman et al. (56) (Figure 8B).
It was believed that Eimeria spp. in goats and sheep were the same for a long time because their oocysts have strikingly similar morphologies (12, 72). However, cross-infection studies disproved this assumption in the late 20th century (73, 74). In this meta-analysis, we conducted a subgroup analysis based on the year of publication to investigate any discrepancies between studies conducted before and after the assumption was made. We also aimed to identify any changes in the temporal trend of reported cases over time. The prevalence of Eimeria spp. in goats sampled before 2000 was 74.3%, whereas in the samples collected in 2000 or later, it was 59.6%, which clearly showed a statistically significant decline (p < 0.05). The rationale behind this trend remains uncertain, but it is possible that the previous misconception concerning Eimeria spp. in goats and sheep contributed to these differences. Further comprehensive research and scientific justifications are needed to determine whether the decrease in Eimeria spp. infection in goats is genuine or not.
The antemortem diagnosis of Eimeria spp. infection traditionally relies on the concentration and/or quantification of Eimeria oocysts per gram (OPG) in the feces through microscopic examination using flotation techniques and/or the morphometry of sporulated oocysts (24, 75). Most studies (n = 202) in this review used fecal flotation techniques to detect and/or enumerate Eimeria fecal oocyst count. However, determining a threshold OPG value that indicates clinical coccidiosis is challenging (76). For instance, while some studies suggest that an OPG of 50,000–100,000 could indicate clinical coccidiosis, non-pathogenic Eimeria spp. can also be excreted in large numbers without clinical signs (76). Therefore, it is crucial to differentiate between pathogenic and non-pathogenic species to confirm clinical coccidiosis (24).
Our systematic literature review shows that morphological identification is the primary method used to differentiate Eimeria spp. in goats. Interestingly, only 47% (119/255) of the studies included in the review documented the identifications of Eimeria spp. worldwide. More than 26 Eimeria spp. have been reported in goats, including recognized/valid species such as E. arloingi, E. ninakohlyakimovae, E. christenseni, E. caprina, E. caprovina, E. alijevi, E. hirci, E. jolchijevi and E. aspheronica as well as other less characterized species. The spatial distribution of the recognized/valid Eimeria spp. shows no distinct regional pattern, suggesting their widespread presence (Figure 4). However, there is considerable variation and controversy regarding the number of Eimeria spp. parasitising goats (14, 18, 19), and this variation depends on the acceptance of certain Eimeria spp. as valid (12). Mixed species infections were commonly observed in most studies, with an average of 7.3 and a median of 8 Eimeria spp. reported per study. This review also supports the ongoing controversy that some studies have reported Eimeria spp. typically found in sheep (E. faurei, E. crandallis, and E. intricata) and cattle (E. zuernii), raising concerns about misinterpretation despite evidence of the absence of cross-host species transmission of Eimeria (73, 74). Recent studies in South Korea (77), India (78), and Slovakia (34) described unspecified Eimeria spp. Moreover, species such as E. masseyensis and E. charlestoni (19) from New Zealand, E. hawkin and E. charlestoni (33, 79) from India, E. minasensis (80) from Brazil, E. sundarbanensis (81) from India, E. megaembryonica (82) from Iraq, and E. tunisiensis and E. masseyensis (83) from Nepal have been reported despite these species never been previously recorded or described globally. Eimeria capralis was first reported in New Zealand (19), with subsequent reports in Iraq (84) and Nepal (83). Furthermore, at least 14 studies reused the same data and/or published their findings twice in different journals, raising significant concerns about the validity of the description of new Eimeria spp. or morphological identification of previously described species.
The accurate identification of Eimeria spp. is paramount for understanding their epidemiology and assessing the effectiveness of anticoccidial drugs, as only a few Eimeria spp. are pathogenic to goats (36, 76, 85). This systematic review showed that the pathogenic species of Eimeria (E. arloingi, E. ninakohlyakimovae, E. christenseni and E. caprina) are widely distributed. For goat farmers, the widespread presence of these pathogenic Eimeria spp., along with a 62.9% estimated prevalence of Eimeria spp. poses significant economic losses (42).
Despite the morphological characterization of sporulated oocysts being the primary method for identifying Eimeria spp. in goats, this approach has notable limitations, including low sensitivity, the extended time required (1–2 weeks) for oocysts to sporulate under varying conditions (86), labor intensive requirements, requires experienced microscopists (87) and difficulty in differentiating morphologically similar oocysts in certain species of Eimeria (12). Although more than 26 Eimeria spp. have been documented globally, molecular characterization has only been partially achieved for a few Eimeria spp., including E. arloingi, E. christenseni, E. hirci, E. ninakohlyakimovae, and one unidentified Eimeria spp. Only a limited number of studies (n = 10) across eight countries have used molecular techniques, primarily using PCR amplification of 18S, and/or COX1, and ITS-1 or ITS-2. The lack of combined morphological and molecular methods could lead to the erroneous identification of certain Eimeria spp. in goats. As of June 25th, 2024, at least 78 nucleotide sequences have been deposited in GenBank4 along with their accession numbers. Most of these sequences were based on partial amplification of 18S gene. Surprisingly, most of these nucleotide sequences (>50%) are not accompanied by peer-reviewed publications, casting doubts on their reliability and validation. Given these challenges, adopting a combined approach using morphological and molecular methods is imperative to accurately identify Eimeria spp. More nucleotide sequencing is needed, particularly for Eimeria spp. that have yet to be characterized. Furthermore, advanced molecular-based studies that include the genetic characterization of new Eimeria spp. and utilizing next-generation sequencing tools could help address the challenges associated with Eimeria.
In this systematic review and meta-analysis, there were only 27 low-quality studies with a score of 1–2, but 149 with a score of 3–4. The reasons for fewer points in some studies were: (1) they needed to clarify whether the sampling was random or not, and the sampling method needed to be detailed. (2) Furthermore, neither was the sample size calculated in most studies nor Eimeria reported at the species level. When investigating the prevalence of Eimeria spp. in goats, we recommend researchers report and/or identify Eimeria spp., calculate the sample size, apply representative sampling techniques, and collect and present as much information as possible. Detailed data on potential risk factors, such as age, production systems, study period, and climatic conditions is also quite important. Such data would enhance the understanding of the factors driving Eimeria spp. prevalence and allow for more robust risk factor analyses in future meta-analyses.
This meta-analysis has shown high I2 and Cochran’s Q statistics, suggesting significant heterogeneity among the studies reporting the prevalence of Eimeria spp. in goats worldwide. The wide range of the 95% PI (4 to 100%) further supports this finding. This variation could be due to several reasons, including geographical factors, differences in production systems, the immune status of the host, differences in the age of goats included in the studies, sample size, diversity of the study populations, breed differences, sampling methods, sex and study periods (88–93). Our meta-regression analyses further showed that region, year of publication, sample size, and the quality level of studies were significant sources of heterogeneity, while the diagnostic methods did not have an impact. Although the improvement in R2 was minimal (4.4%) compared to the full and best models, the full model allows for improved balance, fit and parsimony, making it the preferred model that achieves a good fit with fewer predictors. Additionally, the study by Rahman et al. (56) notably influenced the pooled estimate and contributed the most to the overall heterogeneity.
The estimated pooled prevalence reported in this systematic review and meta-analysis should be interpreted with caution due to the following limitations. Firstly, we found high heterogeneity and publication biases (for the Asian and European continents). Although we applied relevant statistical methods, these may not completely eliminate the impact of heterogeneity and publication bias on the interpretation of the pooled results. Secondly, our database search was conducted only in five databases, and the search strategy might have overlooked some research, particularly that published in languages other than English. Thirdly, we excluded a substantial number of studies published in languages other than English, which could introduce potential bias. However, considering the narrow range of the 95% CI, the missed studies are unlikely to significantly affect the present estimate. Furthermore, the wider range of the 95% PI (4–100%) is likely to encompass future primary studies reporting the prevalence of Eimeria spp. in goats. Despite these limitations, the authors strongly believe that this systematic review and meta-analysis provide a reliable reflection of the true global prevalence of Eimeria spp. in goats.
5 Conclusion and future work
This study presents the global prevalence of Eimeria spp. in goats based on data collected from approximately 30% (n = 56) of countries worldwide. The results of the meta-analysis indicate variations in the prevalence of Eimeria spp. in goats globally, with significant heterogeneity observed between studies. Nevertheless, the narrow range of the 95% CI (58.6–67.2%) suggests a precise and reliable estimate of the pooled prevalence of Eimeria spp. in goats (62.9%). This finding indicates that the included studies reported similar prevalences, instilling high confidence in the accuracy of the current estimate. However, the wide 95% PI (4–100%) reflects substantial heterogeneity and underscores the need for further studies utilizing advanced molecular tools to resolve the ongoing controversy regarding the number of Eimeria spp. parasitising goats. Although a limited number of studies have reported the prevalence of and/or characterized Eimeria spp. using molecular data, this technique is known for its sensitivity and accuracy. More sensitive molecular-based approaches, such as next-generation sequencing and genomic analyses based on single oocyst isolation for mixed infections, could offer more precise insights. Our study provides the first meta-analysis of prevalence data on Eimeria spp. globally, thereby serving as a valuable reference for the prevention and control of Eimeria spp. in goats. Considering the high prevalence and the widespread presence of pathogenic Eimeria spp. in goats globally, it is recommended that integrated parasite management approaches be implemented for the effective control of coccidiosis in goats.
Author contributions
EA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AG: Data curation, Investigation, Writing – review & editing. JA-H: Formal analysis, Writing – review & editing. MY: Formal analysis, Visualization, Writing – review & editing. CG: Resources, Writing – review & editing. IB: Supervision, Writing – review & editing. SB: Writing – review & editing. AJ: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
EA is a grateful recipient of the Australian Government Research Training Scholarship through the University of Melbourne.
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be perceived as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1537171/full#supplementary-material
Footnotes
References
1. FAOSTAT. (2023). In production indices national.. (https://www.fao.org/faostat/en/#data).
2. Utaaker, K, Chaudhary, S, and Kifleyohannes, TR. Global goat! Is the expanding goat population an important reservoir of Cryptosporidium? Front Vet Sci. (2021) 8:648500. doi: 10.3389/fvets.2021.648500
3. Ruvuga, PR, and Maleko, DD. Dairy goats' management and performance under smallholder farming systems in eastern Africa: a systematic review and meta-analysis. Trop Anim Health Prod. (2023) 55:255. doi: 10.1007/s11250-023-03661-w
4. Fthenakis, GC, and Papadopoulos, E. Impact of parasitism in goat production. Small Rumin Res. (2018) 163:21–3. doi: 10.1016/j.smallrumres.2017.04.001
5. Taylor, MA, Coop, RL, and Wall, RL. Veterinary parasitology. Oxford, United Kingdom: Blackwell Publishing (2007).
6. Balicka-Ramisz, A, Ramisz, A, Vovk, S, and Snitynskyj, V. Prevalence of coccidia infection in goats in Western Pomerania (Poland) and West Ukraine region. Ann Parasitol. (2012) 58:167–71.
7. Hassanen, EAA, Anter, RGA, El-Neshwy, WM, and Elsohaby, I. Prevalence and phylogenetic analysis of Eimeria species in sheep and goats in Sharkia governorate, Egypt. Pak Vet J. (2020) 40:437–42. doi: 10.29261/pakvetj/2020.064
8. Ibrahim, MM. Prevalence of Eimeria species of the domestic goats Capra hircus Linnaeus, 1758 in Al-Baha area, Saudi Arabia. Egypt Acad J Biol Sci B Zool. (2012) 4:165–72. doi: 10.21608/eajbsz.2012.14297
9. Kahan, TB, and Greiner, EC. Coccidiosis of goats in Florida, USA. Open J Vet Med. (2013) 3:209–12. doi: 10.4236/ojvm.2013.33033
10. Koudela, B, and Boková, A. Coccidiosis in goats in the Czech Republic. Vet Parasitol. (1998) 76:261–7. doi: 10.1016/S0304-4017(97)00147-7
11. Liang, G, Yang, X, Liu, D, Li, Y, Wang, J, Chen, X, et al. Molecular characterization of 18S rDNA, ITS-1, ITS-2, and COI from Eimeria christenseni and E. Arloingi in goats from Shaanxi Province, northwestern China. Animals. (2022) 12:1340. doi: 10.3390/ani12111340
12. Norton, CC. Coccidia of the domestic goat Capra hircus, with notes on Eimeria ovinoidalis and E. Bakuensis (syn. E. ovina) from the sheep Ovis aries. Parasitology. (1986) 92:279–89. doi: 10.1017/S0031182000064052
13. O'Callaghan, MG. Coccidia of domestic and feral goats in South Australia. Vet Parasitol. (1989) 30:267–72. doi: 10.1016/0304-4017(89)90095-2
14. Silva, LMR, Carrau, T, Vila-Viçosa, MJM, Musella, V, Rinaldi, L, Failing, K, et al. Analysis of potential risk factors of caprine coccidiosis. Vet Parasitol Reg Stud Reports. (2020) 22:100458. doi: 10.1016/j.vprsr.2020.100458
15. Verma, R, Sharma, DK, Gururaj, K, Paul, S, Banerjee, PS, and Tiwari, J. Molecular epidemiology and point mutations in ITS1 and 18S rDNA genes of Eimeria ninakohlyakimovae and E. christenseni isolated from Indian goats. Vet Parasitol Reg Stud Reports. (2017) 9:51–62. doi: 10.1016/j.vprsr.2017.04.008
16. Waruiru, RM, Gichanga, EJ, Kimoro, CO, and Karanu, FN. Prevalence of gastrointestinal nematodes, coccidia and lungworms in Ol'Magogo dairy goats. Bull Anim Health Prod Afr. (1994) 50:291–5.
17. Bangoura, B, and Bardsley, KD. Ruminant coccidiosis. Vet Clin North Am Food Anim Pract. (2020) 36:187–203. doi: 10.1016/j.cvfa.2019.12.006
18. Hashemnia, M, Khodakaram-Tafti, A, Razavi, SM, and Nazifi, S. Experimental caprine coccidiosis caused by Eimeria arloingi: morphopathologic and electron microscopic studies. Vet Res Commun. (2012) 36:47–55. doi: 10.1007/s11259-011-9511-9
19. Soe, AK, and Pomroy, WE. New species of Eimeria (Apicomplexa: Eimeriidae) from the domesticated goat Capra hircus in New Zealand. Syst Parasitol. (1992) 23:195–202. doi: 10.1007/bf00010872
20. Levine, ND. Progress in taxonomy of the apicomplexan protozoa. J Protozool. (1988) 35:518–20. doi: 10.1111/j.1550-7408.1988.tb04141.x
21. Ruiz, A, and Molina, JM. Coccidiosis in goat (Capra hircus) In: JP Dubey, editor. Coccidiosis in livestock, poultry, companion animals, and humans. Boca Raton, FL: CRC Press (2019)
23. Ruiz, A, Guedes, AC, Muñoz, MC, Molina, JM, Hermosilla, C, Martín, S, et al. Control strategies using diclazuril against coccidiosis in goat kids. Parasitol Res. (2012) 110:2131–6. doi: 10.1007/s00436-011-2746-0
24. Keeton, STN, and Navarre, CB. Coccidiosis in large and small ruminants. Vet Clin North Am Food Anim Pract. (2018) 34:201–8. doi: 10.1016/j.cvfa.2017.10.009
26. de Macedo, LO, Bezerra-Santos, MA, de Mendonça, CL, Alves, LC, Ramos, RAN, and de Carvalho, GA. Prevalence and risk factors associated with infection by Eimeria spp. in goats and sheep in northeastern Brazil. J Parasit Dis. (2020) 44:607–12. doi: 10.1007/s12639-020-01235-3
27. Fitzgerald, PR. The economic impact of coccidiosis in domestic animals. Adv Vet Sci Comp Med. (1980) 24:121–43.
28. Andrews, AH. Some aspects of coccidiosis in sheep and goats. Small Rumin Res. (2013) 110:93–5. doi: 10.1016/j.smallrumres.2012.11.011
29. De la Fuente, C, and Alunda, JM. A quantitative study of Eimeria infections of goats from Central Spain. Vet Parasitol. (1992) 41:7–15. doi: 10.1016/0304-4017(92)90003-R
30. Githigia, SM, Munyua, WK, Kyvsgaard, NC, and Thamsborg, SM. Helminth infection levels in goats in a semi arid area of Kenya. Bull Anim Health Prod Afr. (1998) 46:209–10.
31. Harper, CK, and Penzhorn, BL. Seasonal occurrence of coccidia in a mixed herd of sheep and goats at Nebo, Northern Province, South Africa: research communication. J South Afr Vet Assoc. (1998) 69:93–4. doi: 10.4102/jsava.v69i3.824
32. Lima, JD. Prevalence of coccidia in domestic goats from Illinois, Indiana, Missouri and Wisconsin. Int Goat Sheep Res. (1980) 1:234–41.
33. Sharma, RL, Bhattacharya, D, Laha, R, Biswas, JC, and Rangarao, GSC. Preliminary observations on intestinal coccidiosis in pashmina (cashmere) goats in India. J Appl Anim Res. (1997) 12:107–12. doi: 10.1080/09712119.1997.9706193
34. Vasilková, Z, Krupicer, I, Legáth, J, Kovalkovicova, N, and Pet’ko, B. Coccidiosis of small ruminants in various regions of Slovakia. Acta Parasitol. (2004) 49:272–5.
35. Zhao, GH, Lei, L-H, Shang, C-C, Gao, M, Zhao, YQ, Chen, C-X, et al. High prevalence of Eimeria infection in dairy goats in Shaanxi province, northwestern China. Trop Anim Health Prod. (2012) 44:943–6. doi: 10.1007/s11250-011-9997-8
36. Cavalcante, ACR, Teixeira, M, Monteiro, JP, and Lopes, CWG. Eimeria species in dairy goats in Brazil. Vet Parasitol. (2012) 183:356–8. doi: 10.1016/j.vetpar.2011.07.043
37. Juszczak, M, Sadowska, N, and Udala, J. Parasites of the digestive tract of sheep and goats from organic farms in Western Pomerania, Poland. Ann Parasitol. (2019) 65:245–50. doi: 10.17420/ap6503.206
38. Mohamaden, WI, Sallam, NH, and Abouelhassan, EM. Prevalence of Eimeria species among sheep and goats in Suez governorate, Egypt. Int J Vet Sci Med. (2018) 6:65–72. doi: 10.1016/j.ijvsm.2018.02.004
39. Penzhorn, BL, Rognlie, MC, Hall, LL, and Knapp, SE. Enteric coccidia of cashmere goats in southwestern Montana, USA. Vet Parasitol. (1994) 55:137–42. doi: 10.1016/0304-4017(94)90064-7
40. Ruiz, A, González, JF, Rodríguez, E, Martín, S, Hernández, YI, Almeida, R, et al. Influence of climatic and management factors on Eimeria infections in goats from semi-arid zones. J Veterinary Med Ser B. (2006) 53:399–402. doi: 10.1111/j.1439-0450.2006.00985.x
41. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:790–9. doi: 10.1136/bmj.n71
42. Diao, N-C, Zhao, B, Chen, Y, Wang, Q, Chen, Z-Y, Yang, Y, et al. Prevalence of Eimeria spp. among goats in China: a systematic review and meta-analysis. Front Cell Infect Microbiol. (2022) 12:222. doi: 10.3389/fcimb.2022.806085
43. Gong, Q-L, Zhao, W-X, Wang, Y-C, Zong, Y, Wang, Q, Yang, Y, et al. Prevalence of coccidia in domestic pigs in China between 1980 and 2019: a systematic review and meta-analysis. Parasit Vectors. (2021) 14:248. doi: 10.1186/s13071-021-04611-x
44. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
45. Li, X-M, Geng, H-L, Wei, Y-J, Yan, W-L, Liu, J, Wei, X-Y, et al. Global prevalence and risk factors of Cryptosporidium infection in Equus: a systematic review and meta-analysis. Front Cell Infect Microbiol. (2022) 12:1072385. doi: 10.3389/fcimb.2022.1072385
46. RCoreTeam. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing (2023).
47. Balduzzi, S, Rücker, G, and Schwarzer, G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
48. DerSimonian, R, and Laird, N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
49. Freeman, MF, and Tukey, JW. Transformations related to the angular and the square root. Ann Math Stat. (1950) 21:607–11. doi: 10.1214/aoms/1177729756
51. Rücker, G, Schwarzer, G, Carpenter, JR, and Schumacher, M. Undue reliance on I2 in assessing heterogeneity may mislead. BMC Med Res Methodol. (2008) 8:79. doi: 10.1186/1471-2288-8-79
52. Egger, M, Smith, GD, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
53. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
54. Duval, S, and Tweedie, R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
55. Baujat, B, Mahé, C, Pignon, JP, and Hill, C. A graphical method for exploring heterogeneity in meta-analyses: application to a meta-analysis of 65 trials. Stat Med. (2002) 21:2641–52. doi: 10.1002/sim.1221
56. Rahman, H, Papri, P, Bandyopadhyay, S, and Chatlod, LR. Epidemiology of gastrointestinal parasitism in goats in Sikkim. Indian J Anim Sci. (2012) 82:355–8.
58. Cantú-Martínez, MA, González-Sáenz, IS, Pereira-Berto, B, Zamora-Ávila, DE, Ávalos-Ramírez, R, Vázquez-Cisneros, KW, et al. Identificación de especies de Eimeria presentes en caprinos (Capra aegagrus hircus) en Nuevo León, Mexico. Rev MVZ Cordoba. (2022) 27:e2560. doi: 10.21897/rmvz.2560
59. Miller, BA, and Lu, CD. Current status of global dairy goat production: an overview. Asian Australas J Anim Sci. (2019) 32:1219–32. doi: 10.5713/ajas.19.0253
60. Perry, BD, Grace, D, and Sones, K. Current drivers and future directions of global livestock disease dynamics. Proc Natl Acad Sci USA. (2013) 110:20871–7. doi: 10.1073/pnas.1012953108
61. Ridler, A. Disease threats to sheep associated with intensification of pastoral farming. N Z Vet J. (2008) 56:270–3. doi: 10.1080/00480169.2008.36846
63. Corrias, F, Brajon, G, Salari, F, Dal Prà, A, Ragona, G, Lombardo, A, et al. Health evaluation in the native Garfagnina goat. Small Rumin Res. (2012) 104:191–4. doi: 10.1016/j.smallrumres.2011.10.005
64. Asia-Pacific Association of Agricultural Research Institutions. (2021). Sheep and goats in Fiji and Papua New Guinea – success story. Available at: https://apaari.org/web/what-we-do/apcoab/publications/.
65. Al-Habsi, K, Yang, R, Ryan, U, Miller, DW, and Jacobson, C. Morphological and molecular characterization of three Eimeria species from captured rangeland goats in Western Australia. Vet Parasitol Reg Stud Reports. (2017) 9:75–83. doi: 10.1016/j.vprsr.2017.05.001
66. Celi, K, Guzmán, L, and Rey-Valeirón, C. Apicomplexans in goat: prevalence of Neospora caninum, toxoplasma gondii, Cryptosporidium spp., Eimeria spp., and risk factors in farms from Ecuador. Animals. (2022) 12:2224. doi: 10.3390/ani12172224
67. Cardoso, CP, Cardozo, LL, Silva, BF, and Amarante, AFT. Gastrointestinal parasites in goats from Monte Castelo, Santa Catarina, Brazil. Rev Bras Parasitol Vet. (2012) 21:148–50. doi: 10.1590/S1984-29612012000200014
68. Abdel-Salam, M, and Mahran, OM. Some biochemical changes in blood serum of balady goats infested with internal and external parasites at Assiut governorate. Assiut Vet Med J. (2004) 50:145–56. doi: 10.21608/avmj.2004.178957
69. Mhoma, JRL, Kanyari, PWN, and Kagira, JM. The prevalence of gastrointestinal parasites in goats in urban and peri-urban areas of Mwanza City, Tanzania. Sci Parasitol. (2011) 12:191–6.
70. El-Bahy, MM, Omer, OH, and Al-Sadrani, AA. Temperature difference and parasite infection at Qassim region, Saudi Arabia. Res J Parasitol. (2008) 3:114–22. doi: 10.3923/jp.2008.114.122
71. Mottelib, AA, Haroun, EM, Magzoub, M, and El-Basheer, E. The effect of gastro-intestinal parasites on blood picture in sheep and goats at Al-Gassim. Assiut Vet Med J. (1992) 28:215–23.
72. Reeg, KJ, Gauly, M, Bauer, C, Mertens, C, Erhardt, G, and Zahner, H. Coccidial infections in housed lambs: oocyst excretion, antibody levels and genetic influences on the infection. Vet Parasitol. (2005) 127:209–19. doi: 10.1016/j.vetpar.2004.10.018
73. Lima, JD. The Coccidia (Protozoa: Eimeriidae) of the domestic goat, Capra hircus. Illinois: University of Illinois at Urbana-Champaign (1980).
74. McDougald, LR. Attempted cross-transmission of coccidia between sheep and goats and description of Eimeria ovinoidalis sp. n. J Protozool. (1979) 26:109–13. doi: 10.1111/j.1550-7408.1979.tb02741.x
75. Bangoura, B, Bhuiya, MAI, and Kilpatrick, M. Eimeria infections in domestic and wild ruminants with reference to control options in domestic ruminants. Parasitol Res. (2022) 121:2207–32. doi: 10.1007/s00436-022-07564-x
76. Chartier, C, and Paraud, C. Coccidiosis due to Eimeria in sheep and goats: a review. Small Rumin Res. (2012) 103:84–92. doi: 10.1016/j.smallrumres.2011.10.022
77. Roh, SG, Kim, J, Ku, B-K, and Lee, K. Case study: pathological and phylogenetic analysis of coccidiosis in two goats with heavy infection of unrecorded Eimeria sp. Parasitol Int. (2023) 92:102662. doi: 10.1016/j.parint.2022.102662
78. Khillare, BS, and Narladkar, BW. Epidemiology of coccidiosis in caprines of Marathwada region of Maharashtra age, sex, breed and season wise prevalence. J Vet Parasitol. (2014) 28:7–13.
79. Jha, D, and Subramanian, G. Incidence of Eimeria species in goats of Uttar Pradesh. Indian Vet J. (1966) 43:588–91.
80. Silva, AC, and Lima, JD. Eimeria minasensis n. sp. (Apicomplexa: Eimeriidae) in the domestic goat Capra hircus, from Brazil. Mem Inst Oswaldo Cruz. (1998) 93:741–4. doi: 10.1590/s0074-02761998000600009
81. Bandyopadhyay, PK. A new coccidium Eimeria sundarbanensis n. sp. (Protozoa: Apicomplexa: Sporozoea) from Capra hircus (Mammalia: Artiodactyla). Protistology. (2004) 3:223–5.
82. Al-Bayati, SM, Al-Rekani, AM, and Hamed, AA. Diagnosis of Eimeria spp. in Capra ibex (local meriz goat). Iraqi J Vet Med. (2016) 40:47–52. doi: 10.30539/iraqijvm.v40i1.137
83. Ghimire, TR, Adhikari, RB, and Bhattarai, N. Diversity and prevalence of Eimeria species in goats of Nepal. J Hellenic Vet Med Soc. (2021) 72:3299–306. doi: 10.12681/jhvms.29363
84. Shaheed, HA, and Al-Azizz, S. Epidemiological characterization on eimeriosis in small ruminants in Basrah City of southern Iraq. Plant Arch. (2020) 20:6010–4.
85. de Macedo, LO, Santos, MAB, da Silva, NMM, do Rêgo Barros, GMM, Alves, LC, Giannelli, A, et al. Morphological and epidemiological data on Eimeria species infecting small ruminants in Brazil. Small Rumin Res. (2019) 171:37–41. doi: 10.1016/j.smallrumres.2018.12.006
86. Carvalho, FS, Wenceslau, AA, Teixeira, M, Carneiro, JAM, Melo, ADB, and Albuquerque, GR. Diagnosis of Eimeria species using traditional and molecular methods in field studies. Vet Parasitol. (2011) 176:95–100. doi: 10.1016/j.vetpar.2010.11.015
87. Khodakaram-Tafti, A, Hashemnia, M, Razavi, SM, Sharifiyazdi, H, and Nazifi, S. Genetic characterization and phylogenetic analysis of Eimeria arloingi in Iranian native kids. Parasitol Res. (2013) 112:3187–92. doi: 10.1007/s00436-013-3494-0
88. Balicka-Ramisz, A. Studies on coccidiosis in goats in Poland. Vet Parasitol. (1999) 81:347–9. doi: 10.1016/S0304-4017(98)00258-1
89. Harper, CK, and Penzhorn, BL. Occurrence and diversity of coccidia in indigenous, Saanen and crossbred goats in South Africa. Vet Parasitol. (1999) 82:1–9. doi: 10.1016/S0304-4017(98)00266-0
90. Hashemnia, M, Khodakaram-Tafti, A, Razavi, SM, and Nazifi, S. Changing patterns of acute phase proteins and inflammatory mediators in experimental caprine coccidiosis. Korean J Parasitol. (2011) 49:213–9. doi: 10.3347/kjp.2011.49.3.213
91. Khodakaram-Tafti, A, and Hashemnia, M. An overview of intestinal coccidiosis in sheep and goats. Rev Med Vet. (2017) 168:1–3.
92. Ruiz, A, Matos, L, Muñoz, MC, Hermosilla, C, Molina, JM, Andrada, M, et al. Isolation of an Eimeria ninakohlyakimovae field strain (Canary Islands) and analysis of its infection characteristics in goat kids. Res Vet Sci. (2013) 94:277–84. doi: 10.1016/j.rvsc.2012.08.003
Keywords: Eimeria , goat, global prevalence, meta-analysis, systematic review
Citation: Ali EA, Ghafar A, Angeles-Hernandez JC, Yaseen M, Gauci CG, Beveridge I, Baxendell S and Jabbar A (2025) Global prevalence of Eimeria species in goats: a systematic review and meta-analysis. Front. Vet. Sci. 11:1537171. doi: 10.3389/fvets.2024.1537171
Edited by:
Calin Mircea Gherman, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Saw Bawm, University of Veterinary Science, Yezin, MyanmarAdriana Gyorke, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Copyright © 2025 Ali, Ghafar, Angeles-Hernandez, Yaseen, Gauci, Beveridge, Baxendell and Jabbar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul Jabbar, amFiYmFyYUB1bmltZWxiLmVkdS5hdQ==
 Endris A. Ali
Endris A. Ali Abdul Ghafar
Abdul Ghafar Juan C. Angeles-Hernandez
Juan C. Angeles-Hernandez Muhammad Yaseen
Muhammad Yaseen Charles G. Gauci1
Charles G. Gauci1 Abdul Jabbar
Abdul Jabbar