- 1Jiujiang Key Laboratory of Rare Disease Research, Jiujiang University, Jiujiang, China
- 2Jiangxi Provincial Key Laboratory of Cell Precision Therapy, School of Basic Medical Sciences, Jiujiang University, Jiujiang, China
- 3Jiujiang Innovation Center of Biosensor Technology and Application, Jiujiang University, Jiujiang, China
- 4School of Tropical Agriculture and Forestry (School of Agricultural and Rural Affairs, School of Rural Revitalization), Hainan University, Haikou, China
- 5School of Stomatology and Medicine, Foshan University, Foshan, China
- 6Nanchang Police Dog Base of the Ministry of Public Security of China, Nanchang, China
Cherry eye, the common name for the prolapse of the third eyelid gland in dogs, is a widespread ophthalmic disease affecting dogs of various breeds. This condition severely affects the quality of life of affected dogs, and its underlying cause remains unresolved. In this study, 170K SNP microarray data were collected from 653 brachycephalic dogs and 788 brachycephalic and mesocephalic dogs. These two datasets were analyzed separately in genome-wide association studies (GWAS) involving 12 dog breeds affected by cherry eye. The GWAS analysis of 653 short-headed dogs revealed that four SNPs in the CFA3:15627075-15983629 bp region exceeded the genome-level significance threshold. Association analysis of this region also indicated that these four SNPs were strongly associated. Gene annotation showed that the region contained genes such as KIAA0825, FAM172A, and NR2F1, of which NR2F1 was associated with eye development. The results showed that GWAS analysis performed on 788 short- and medium-headed dogs identified five SNPs in the CFA22:15627075-15983629 bp region that exceeded the genome-level significance threshold, and association analysis was performed in this region, which showed that these five SNPs were strongly associated. In addition, 104 annotated genes were identified in both GWAS. To explore the genes involved in cherry eyes, we performed GO functional enrichment analysis. The genes involved in the high pathway were DIO3 and TTC8. In addition, an in-depth analysis revealed 33 genes associated with eye development and diseases. Our study provides new perspectives for further understanding cherry eye in dogs.
1 Introduction
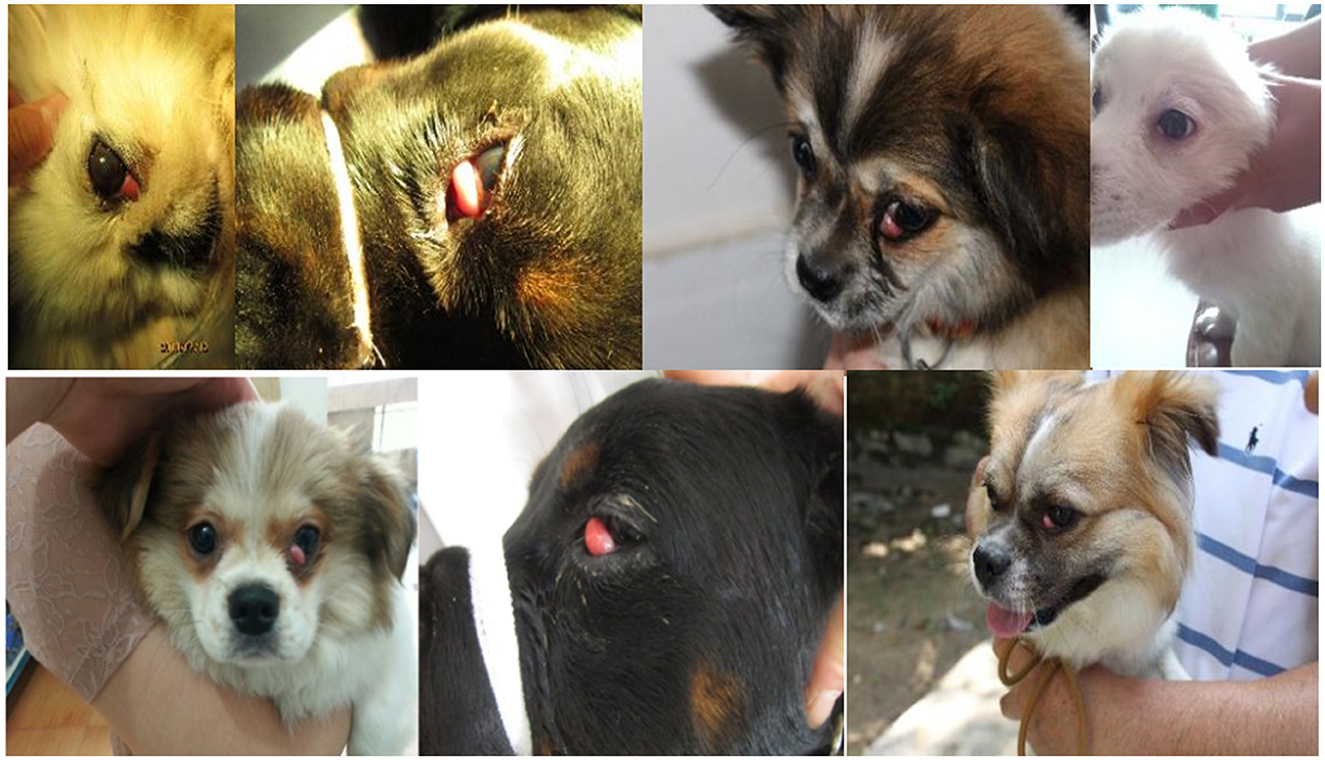
Prolapse of the third eyelid gland in dogs, characterized by a protuberance of the gland at the free edge of the papillae, results in hypertrophy, hyperplasia, or adenoma, forming a prominent red fleshy mass, often referred to as the cherry eye (Figure 1) (1). The cherry eye may be large, covering a large portion of the cornea, or it may be small and appear only periodically (2). Dogs have three eyelids, two of which are easily visible, and an additional eyelid, the third eyelid, which is usually hidden below the inner corner of the eye (2, 3). The third meibomian gland is located at the base of the T-shaped hyaline cartilage in the roughly triangular conjunctival fold at the corner of the eye and it produces 30–60% of tears (4, 5).
The cause of cherry eyes is currently unknown and is hypothesized to be the laxity of the connective tissue that anchors the third eyelid gland, usually on a genetic basis (6–8). Some researchers have suggested that gland enlargement (lymphoid hyperplasia) is caused by antigenic stimulation due to the exposure of puppies to environmental allergens (5, 7, 9). It can be caused by dysplasia or congenital defects in all current breeds but is more common in brachycephalic dogs, such as American and English Cocker Spaniels, Boston Terriers, Peking Pugs, Beagles, and Bulldogs (8). This is mainly because the facial and orbital appearances of brachycephalic dogs are characterized by a rounded cranial shape and flat orbits, which potentially lead to physiological protrusion of the eyeballs, failing to adequately cover the eyeballs, provide lubrication, and make the cornea less sensitive, leading to a variety of ocular disorders (10). Studies have shown that males have a higher incidence than females and the age of onset is between 3 and 12 months (6, 8, 11). Research has indicated that this disease is most common in dogs that usually eat meat, chicken liver, ham sausage, etc., and are well nourished, which may be due to glandular secretions and sticky glandular excretory ducts caused by poor excretion during the development of complete obstruction (12). Symptomatically, the prolapsed gland grows from small to large slowly in 3–5 days, quickly in 1–2 days, and does not continue to develop after swelling to its maximum size. In most dogs, ocular inflammation is not obvious, and in a few cases, it is mainly caused by ocular discomfort, scratching, or friction from an object. Tumors and inflammation can be ruled out from the limited enlargement of the prolapsed gland and the inflammatory manifestations in the affected eye; however, obstruction of the glandular excretory ducts can explain the above symptoms. The degree of obstruction in the glandular excretory ducts determines the disease course's progression rate, and the gland's histological structure determines the limit to its enlargement (6, 11, 12). Pathophysiologically, the glands are enlarged (6), the follicles are dilated (11), the epithelium of the follicles is detached (11, 12), and the nictitating gland dry (13, 14).
This disease is most common in monocular prolapses, a small number of both eyes prolapse at the same time; there are also eye prolapses after the cure of the prolapse of the other eye, followed by prolapse (11, 13). Dogs with third eyelid glandular hyperplasia are initially enlarged due to infection, and gradually, a red fleshy lump appears at the inner corner of the dog's lower eyelid, which increases in size in a short period (13). Sick dogs have conjunctival redness, tearing, and obvious signs of ocular inflammation and discomfort, as well as a marked increase in ocular discharge. Simultaneously, some sick dogs often scratch their eyes with their front paws; therefore, there is a possibility of mechanical damage to the cornea or aggravation of ocular inflammation, which seriously affects the health and lifespan of domestic dogs. Third eyelid gland prolapse is common in domestic dogs and seriously affects the quality of life of the affected dogs (14).
Cherry eye is usually not painful and does not affect a dog's vision; however, if left untreated, it can cause irritation and inflammation (9). Total glandular excision is commonly used to remove the third meibomian gland or to reposition the gland in domestic dogs affected by cherry eye disease (15). Studies have shown that after the removal of the desmoid gland, there is a decrease in tear production, leading to dry keratoconjunctivitis (KCS) (16). KCS is a chronic inflammatory disease that may result from ductal obstruction and is more likely to occur in females compared to males, mainly due to more intense lymphocytic infiltration of the third oculocutaneous gland, with less secretion (3, 17). KCS recurrence is usually associated with repositioning surgery (6, 18). The current popular treatment is the modified Morgan Pocket technique, which is simpler and less prone to recurrence and complications than total adenomectomy (19, 20).
Although more attention has been paid to the treatment of such diseases in domestic dogs, the genetic background of third eyelid gland prolapse in domestic dogs remains of interest. In this study, we performed a genome-wide association studies (GWAS) to understand the genetic mechanism underlying third eyelid gland prolapse in domestic dogs with ophthalmoplegia.
2 Materials and methods
2.1 Data
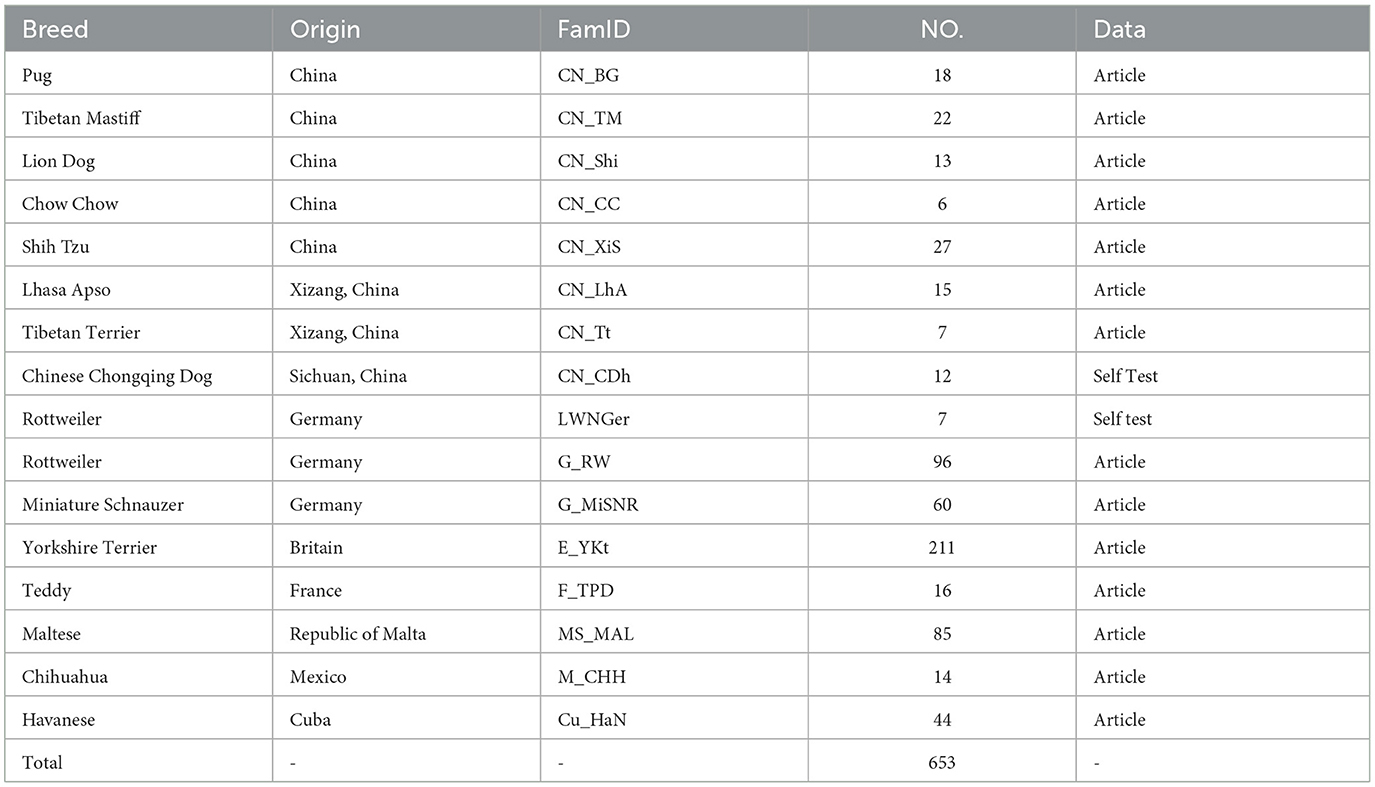
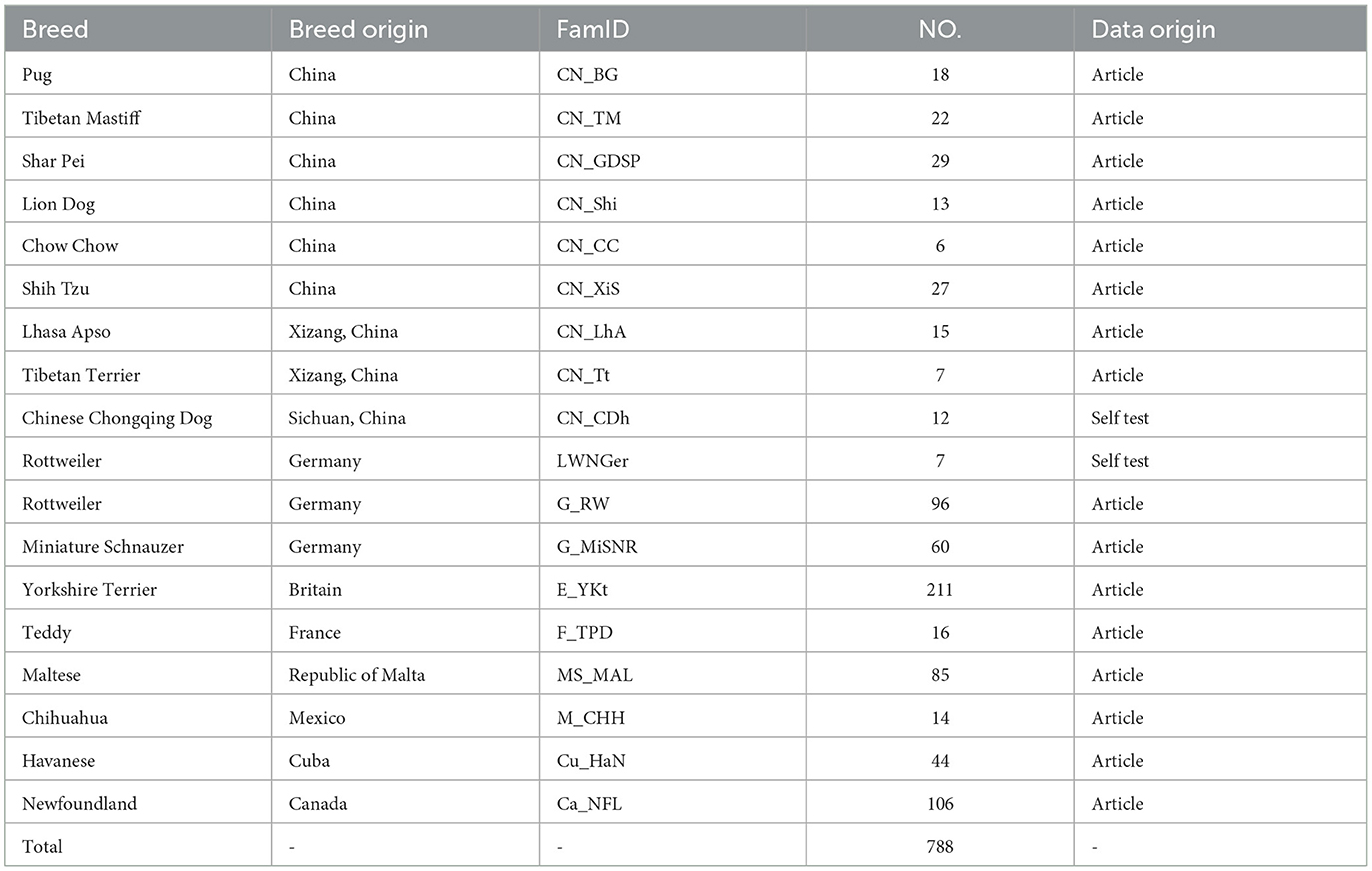
The 12 dogs of different breeds suffering from third eyelid prolapse in the experimental animals came from the Police Dog Hospital at the Nanchang Police Dog Base of the Ministry of Public Security, and these diseased dogs were brought to the hospital by nearby pet breeders, the dog suffering from prolapse of the third eyelid is shown in Figure 1. The breeds of these domestic dogs were Pekingese, Rottweiler, and Butterfly, totaling 12. In this study, we also collected 170 K SNP microarray data from 653 dogs with brachycephalic characteristics (Table 1) and 788 brachycephalic and mesocephalic special dogs (Table 2), such as Newfoundlands, which were previously published as 170K high-density SNP microarray data (21).
2.2 Genomic DNA sample preparation
Blood samples were collected from the place of origin of each local dog, using EDTA-K2 anticoagulation negative pressure tubes, and peripheral venous anticoagulated blood was collected by professional veterinary staff, and couriered to the Nanchang Police Dog Base of the Ministry of Public Security in low-temperature refrigeration using a foam insulated box and preserved at −80°C. All samples were collected following the regulations of the Ministry of Agriculture on the protection of test animals and guidelines for their use.
Genomic DNA was extracted using the SE Blood DNA Kit (OMEGA, USA) kit from OMEGA concerning the instruction manual, and the DNA was dissolved into TE buffer, and the concentration of DNA was determined and quality checked using the Nanodrop-1000 (Thermo Scientific, USA) Nucleic Acid Protein Analyzer, and the qualified The DNA samples were required to have A260/230 ratio of 1.7~1.9 and A260/A280 ratio of 1.8–2.0. After uniformly diluted to 100 ng/μL, agarose gel electrophoresis was performed, which required bright bands and no protein and RNA contamination or DNA degradation and was placed in a −20°C refrigerator for backup for a short period.
2.3 Genome-wide association studies
These two datasets were used to perform a GWAS based on a case-control study with 12 cherry-eyed dogs. We set the phenotype of dogs with cherry-eyed dogs with phenotype set to 1 and other dogs to 0. The genomic significance level was set to -log(1/Nsite), where Nsite denotes the number of SNPs, and the highly significant level was -log(1/Nsite)+2. Visualization was performed using R language, and LD block analysis was performed using LDBlockMovie software (21).
3 Results and analysis
In this study, GWAS results from 12 dogs with cherry eye compared to 653 brachycephalic dogs showed a total of 52 SNPs exceeding the threshold of significance at the chromosomal level, with 16 SNPs exceeding the threshold of significance at the genomic level (Figure 2A). We genetically annotated SNPs and their upstream and downstream 5Kb regions that exceeded the significance threshold at the chromosome level, and identified a total of 240 genes (Supplementary Table S1), which were analyzed for functional enrichment, and the results showed that these genes were involved in multiple biological functions (Figure 2B). Notably, we identified four SNPs in the CFA3:15627075-15983629 bp region that exceeded the genome-level significance threshold. Chaining analysis of this region revealed that these four SNPs were strongly interlinked (Figure 2C), and gene annotation revealed that this region contained three genes, including KIAA0825, FAM172A, and NR2F1, of which NR2F1 is associated with eye development (22). We found an SNP on CFA22 well above the significance threshold, and chaining analysis of this SNP and its upstream and downstream regions showed that this SNP did not appear to be chained to nearby regions (Figure 2D).
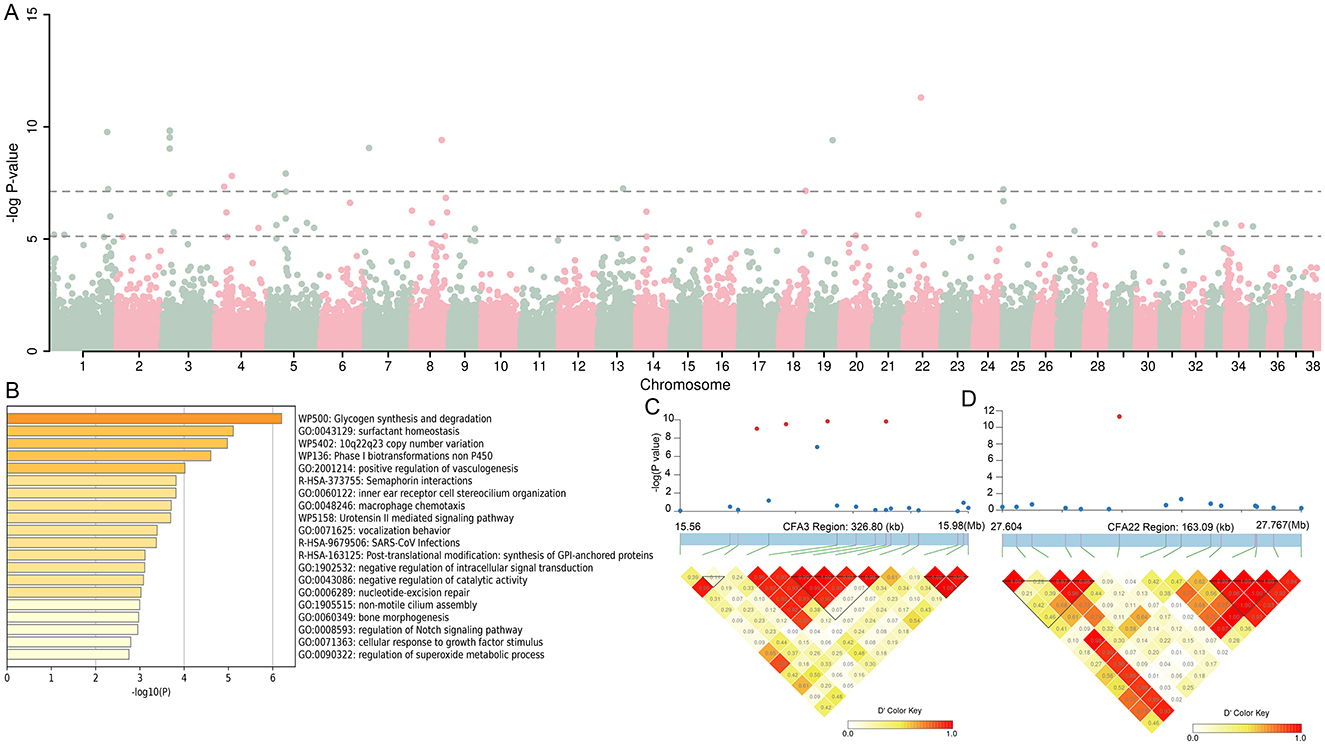
Figure 2. Genomic association analysis (GWAS) between 12 infected dogs and 653 dogs with brachycephalic characteristics. (A) Manhattan map of GWAS. (B) The function of the 240 genes enrichment analysis. (C, D) LD block analysis of the two regions on CFA3 and CFA22.
GWAS analysis of 12 cherry-eyed affected dogs vs. 788 brachycephalic and mesocephalic dogs showed 40 SNPs exceeding the threshold of significance at the chromosomal level and 11 SNPs exceeding the significance threshold at the genomic level (Figure 3A). SNPs exceeding the significance threshold at the chromosomal level and their upstream and downstream 5Kb regions were genetically annotated, and a total of 180 genes (Supplementary Table S2) were identified and subjected to functional enrichment analysis, which showed that these genes were involved in multiple biological functions (Figure 3B). Similar to the above results, we found five SNPs in the CFA22:15627075-15983629 bp region that exceeded the significance threshold at the genome level, and chaining analysis was performed on this region, and these five SNPs were found to be strongly chained (Figure 3C).
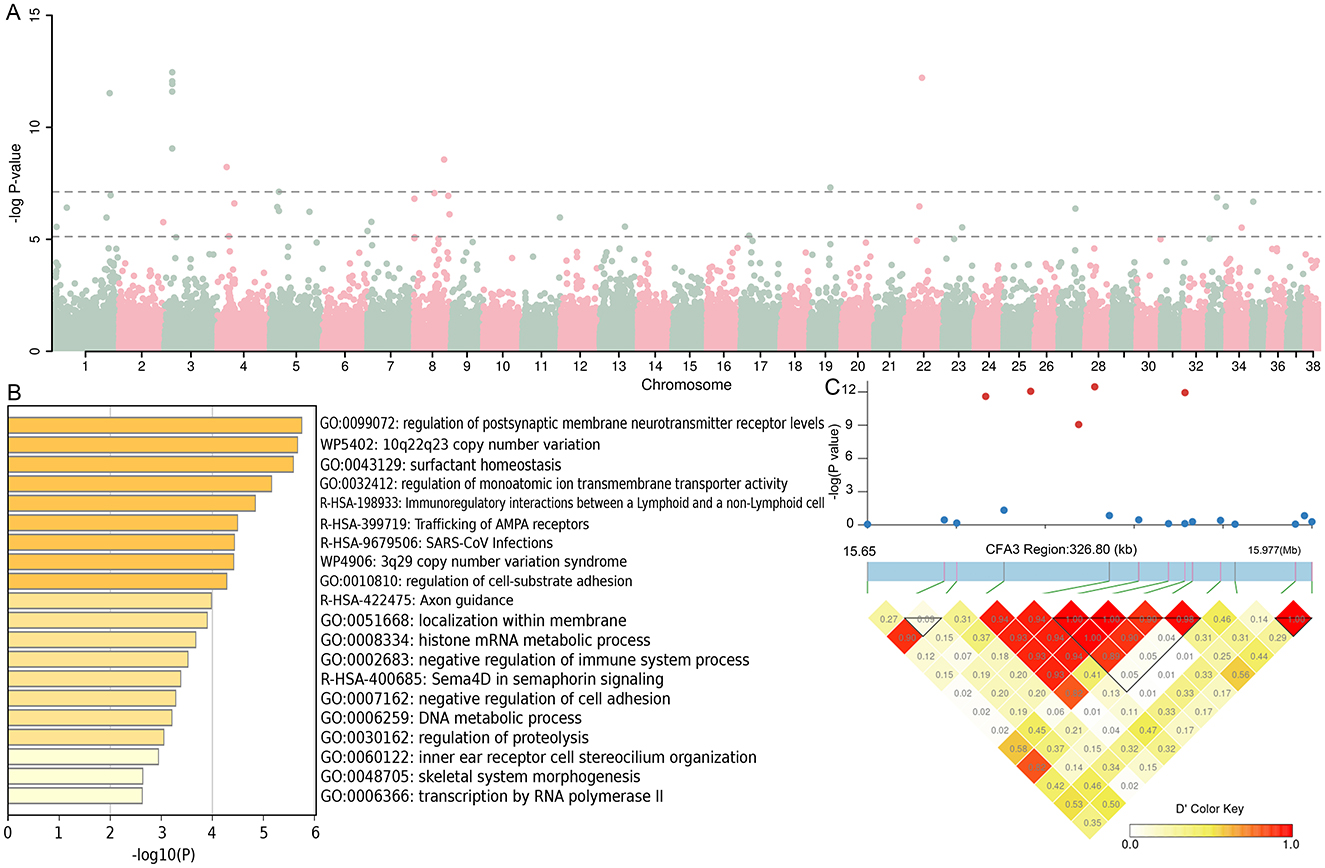
Figure 3. Genomic association analysis between 12 infected dogs and 788 dogs with brachycephalic and mesocephalic characteristics. (A) Manhattan map of GWAS. (B) The function of the 180 genes enrichment analysis. (C) LD block analysis of the two regions on CFA3.
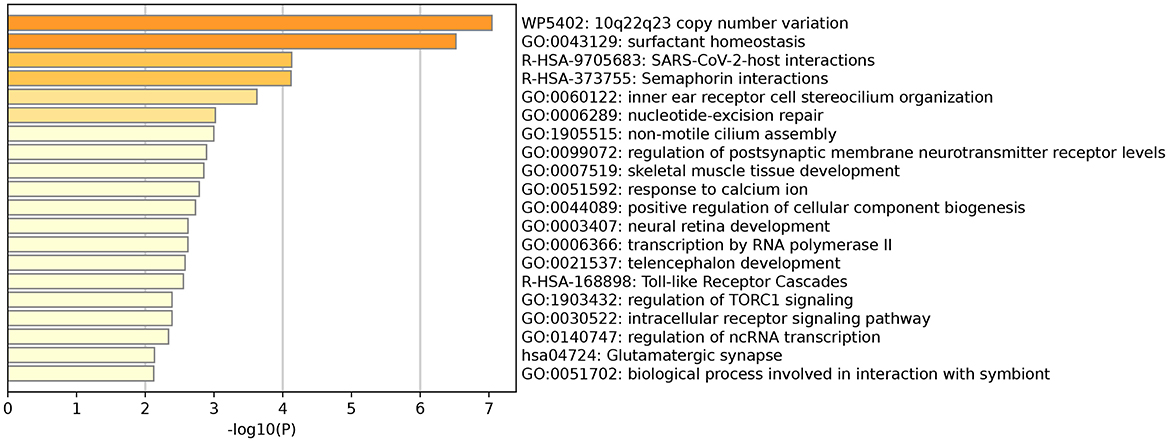
The results showed that a total of 104 annotated genes were identified in both GWAS analyses, and we further performed functional enrichment analysis on these 104 genes, which showed that there were multiple genes involved in neurodevelopment and other processes. It is worth mentioning that the GO term “GO:0003407” pathway is involved in the neural retinal development pathway (Figure 4), and the genes involved in the high pathway include DIO3 and TTC8 (23–25). Therefore, these 104 genes were analyzed in depth, and 33 were found to be associated with eye development or eye diseases (Supplementary Table S3).
4 Discussion
As human companion animals, dogs have the advantages of a large number of naturally occurring genetic diseases with similar body sizes and diets, the same living environment, and a unique population structure that provides high-quality genome-wide reference sequences. Moreover, dog and human genomes have a high degree of similarity (75%), making them ideal animal models for studying spontaneous genetic diseases in humans (26–29). According to the online Human Mendelian Inheritance Database (https://omim.org/home/), as of 2020, ~700 dogs with monogenic diseases or complex traits have been identified, of which at least 230 have known disease-causing mutations and 340 dogs are potential models for human disease (30). The number of spontaneous genetic diseases in other domesticated animals was significantly lower than that in dogs. A large number of dogs with spontaneous genetic diseases can be used as animal models for research, providing more possibilities for studying and treating human genetic diseases (31–33). Third eyelid gland prolapse is a relatively common disease in domesticated animals and has diverse causes (34). A total of 1,802 cases of third eyelid prolapse were identified in 905,543 dogs, with an annual prevalence of 0.20% (35). However, most studies on third eyelid gland prolapse in domestic dogs have focused on the treatment of this disease and the underlying genetic mechanisms have not yet been analyzed. The present study aimed to contribute to the understanding of ocular tumors in humans by analyzing the mechanism of third eyelid gland prolapse.
In this study, we performed GWAS analysis based on 12 dogs with cherry eyes and brachycephalic dogs and identified SNPs. Their upstream and downstream regional gene annotations revealed that some genes are indeed involved in ocular development or are associated with ocular diseases in brachycephalic dogs, NR2F1 may be associated with optic nerve atrophy syndrome. GWAS was also performed on both brachycephalic and mesocephalic dogs, and 33 genes were found to be associated with ocular development or disease in breeds with different cranial structures, including genes associated with diabetic retinopathy, such as SYT3, MYBPC2, and POLD1 (36, 37). These findings are important for exploring the causative genes of third eyelid protrusions in dogs.
In addition to common dog diseases, dogs can also suffer from diseases similar to humans, such as tumors like eye tumors and psychiatric disorders like autism, and studies have shown a strong association between SHANK3 and autism in humans (38, 39). By analyzing the whole genome sequence of domestic dogs, it was found that the structure of the SHANK3 gene in dogs was similar to that of humans. Therefore, the researchers carried out gene editing in dogs, and the results showed that the mutant dogs all showed different degrees of abnormal motor ability, repetitive stereotypy, as well as the presence of severe social abnormalities and cognitive deficits between dogs and dogs, and between dogs and humans, and other clinical symptoms of autism. There are no ideal treatments for fatal human genetic diseases, mainly because of the limited research on the natural history of the disease, insufficient understanding of the correlation between genotype and disease, lack of effective alternative markers, and lag in translational research on effective animal models (40). Therefore, it is necessary to study the natural occurrence and molecular mechanisms of human genetic diseases and evaluate the safety and effectiveness of gene therapy in large animal models (41).
In this study, although we did not fully elucidate the pathogenesis of third eyelid gland prolapse in dogs, this limitation may be attributed to the small sample size or the genetic diversity of the breeds collected. However, we genetically annotated SNPs and their upstream and downstream 5Kb regions associated with canine third eyelid gland ptosis that exceeded the significance threshold at the chromosome level and identified some genes associated with ocular development or disease. This represents a significant step forward in understanding the pathogenesis of third eyelid gland prolapse in dogs.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The animal studies were approved by Medical Ethics Review Committee of Jiujiang University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
YZ: Conceptualization, Investigation, Methodology, Software, Validation, Writing – original draft. CF: Conceptualization, Data curation, Formal analysis, Writing – original draft. ZJ: Data curation, Formal analysis, Investigation, Writing – original draft. WD: Project administration, Supervision, Writing – review & editing. SH: Supervision, Writing – review & editing. XL: Project administration, Supervision, Writing – review & editing. YF: Investigation, Writing – original draft. XO: Investigation, Writing – original draft. BH: Conceptualization, Writing – original draft. YS: Conceptualization, Writing – original draft. SW: Formal analysis, Writing – review & editing. RW: Supervision, Writing – review & editing. ZD: Supervision, Writing – review & editing. PJ: Supervision, Writing – review & editing. JL: Project administration, Supervision, Writing – review & editing. QY: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (32460226).
Acknowledgments
We are very grateful to Dr. Huang for her assistance with this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1520155/full#supplementary-material
Supplementary Table 1 | 240 genes identified by GWAS between 12 dogs with cherry eye and 653 brachycephalic dogs.
Supplementary Table 2 | 180 genes identified by GWAS from 12 cherry eyed dogs and 788 brachycephalic and mesocephalic dogs.
Supplementary Table 3 | Total of 33 genes were identified that were associated with eye development or disease.
References
1. Thamizharasan A, Murugan M, Parthiban S. Surgical management of cherry eye in a dog. Intas Polivet. (2016) 17:420–1.
2. Dugan SJ, Severin GA, Hungerford LL, Whiteley HE, Roberts SM. Clinical and histologic evaluation of the prolapsed third eyelid gland in dogs. J Am Vet Med Assoc. (1992) 201:1861–7. doi: 10.2460/javma.1992.201.12.1861
3. Ioannides J, Parker J, Kumaratunga V, Preston J, Donaldson D, MacFarlane P, et al. A prospective, masked, randomized, controlled superiority study comparing the incidence of corneal injury following general anesthesia in dogs with two methods of corneal protection. Vet Ophthalmol. (2022) 25:291–6. doi: 10.1111/vop.12991
4. Saito A, Izumisawa Y, Yamashita K, Kotani T. The effect of third eyelid gland removal on the ocular surface of dogs. Vet Ophthalmol. (2001) 4:13–8. doi: 10.1046/j.1463-5224.2001.00122.x
5. Lone JM, Nabi B, Asfar A, Javaid F, Bhat MA, Malik FA, et al. Surgical correction of a unilateral cherry eye in a bull dog using Morgan's pocket technique. Pharm Innov J. (2020) 9:243–5. doi: 10.22271/tpi.2020.v9.i7Se.4988
6. Multari D, Perazzi A, Contiero B, De Mattia G, Iacopetti I. Pocket technique or pocket technique combined with modified orbital rim anchorage for the replacement of a prolapsed gland of the third eyelid in dogs: 353 dogs. Vet ophthalmol. (2016) 19:214–9. doi: 10.1111/vop.12286
7. Edelmann ML, Miyadera K, Iwabe S, Komáromy AM. Investigating the inheritance of prolapsed nictitating membrane glands in a large dogs pedigree. Vet ophthalmol. (2013) 16:416–22. doi: 10.1111/vop.12015
9. Freyer J, Labadie JD, Huff JT, Denyer M, Forman OP, Chodroff Foran R, et al. Association of FGF4L1 retrogene insertion with prolapsed gland of the nictitans (cherry eye) in dogs. Genes. (2024) 15:198. doi: 10.3390/genes15020198
10. Sebbag L, Sanchez RF. The pandemic of ocular surface disease in brachycephalic dogs: The brachycephalic ocular syndrome. Vet Ophthalmol. (2023) 26:31–46. doi: 10.1111/vop.13054
11. Mazzucchelli S, Vaillant M, Wéverberg F, Arnold-Tavernier H, Honegger N, Payen G, et al. Retrospective study of 155 cases of prolapse of the nictitating membrane gland in dogs. Vet Rec. (2012) 170:443. doi: 10.1136/vr.100587
12. Crispin S. Ocular lipid deposition and hyperlipoproteinaemia. Progr Ret Eye Res. (2002) 21:169–224. doi: 10.1016/S1350-9462(02)00004-6
13. Park SA, Komáromy AM. Biomechanics of the optic nerve head and sclera in dogs glaucoma: a brief review. Vet ophthalmol. (2021) 24:316–25. doi: 10.1111/vop.12923
14. Hussein K, Hussein MT, Attaai A, Ragab L, Semieka M. Effect of Nictitans gland and third eyelid excisions on ocular surface integrity, pH, and tear production in dogs. J Adv Vet Res. (2022) 12:90–8.
15. Arora N, Chaudhary R, Pandey AK, Potliya S, Singh K. Surgical management of cherry eye in dogs. Intas Polivet. (2014) 15:129−30.
16. Almeida DEd, Mamede FV, Duque Ortiz JP, Laus JL. Iatrogenic keratoconjunctivitis sicca in a dog. Ciênc Rural. (2004) 34:921–4. doi: 10.1590/S0103-84782004000300041
17. Cabral VP, Laus JL, Dagli MLZ, Pereira GT, Talieri IC, Monteiro ER, et al. Dogs lacrimal and third eyelid superficial glands' macroscopic and morphometric characteristics. Ciênc Rural. (2005) 35:391–7. doi: 10.1590/S0103-84782005000200023
18. Trimarchi M, Vinciguerra A, Resti AG, Giordano L, Bussi M. Multidisciplinary approach to lacrimal system diseases. Acta Otorhinolaryngol Ital. (2021) 41:102. doi: 10.14639/0392-100X-suppl.1-41-2021-10
19. Singh K, Gopinathan A, Sangeetha P, Sarangom SB, Kallianpur N, Shivaraju S, et al. Morgan's pocket technique for the surgical management of cherry eye in dogs: a report of 14 cases. Indian J Anim Res. (2017) 51:795–97. doi: 10.18805/ijar.9373
20. Dameski P, Karabolovski N, Zdraveski I, Dodovski P, Pejcinovska N, Hristovska T, et al. Clinical evaluation of surgical treatment of cherry eye with modified morgan pocket technique. Horizons Int Sci J. (2024) 1:7–13.
21. Yang QY, Chen H, Ye JH, Liu CL, Wei RX, Chen CY, et al. Genetic diversity and signatures of selection in 15 Chinese indigenous dog breeds revealed by genome-wide SNPs. Front Genet. (2019) 10:1714. doi: 10.3389/fgene.2019.01174
22. Dong SS, He WM Ji JJ, Zhang C, Guo Y, Yang TL. LDBlockShow: a fast and convenient tool for visualizing linkage disequilibrium and haplotype blocks based on variant call format files. Brief Bioinform. (2021) 22:227. doi: 10.1093/bib/bbaa227
23. Collin GB, Gogna N, Chang B, Damkham N, Pinkney J, Hyde LF, et al. Mouse models of inherited retinal degeneration with photoreceptor cell loss. Cells. (2020) 9:931. doi: 10.3390/cells9040931
24. Mäkeläinen S, Hellsand M, van der Heiden AD, Andersson E, Thorsson E, Holst BS, et al. Deletion in the Bardet-Biedl syndrome gene TTC8 results in a syndromic retinal degeneration in dogs. Genes. (2020) 11:1090. doi: 10.3390/genes11091090
25. Nicolini G, Casini G, Posarelli C, Amato R, Lulli M, Balzan S, et al. Thyroid hormone signaling in retinal development and function: implications for diabetic retinopathy and age-related macular degeneration. Int J Mol Sci. (2024) 25:7364. doi: 10.3390/ijms25137364
26. Switonski M. Dog as a model in studies on human hereditary diseases and their gene therapy. Reprod Biol. (2014) 14:44–50. doi: 10.1016/j.repbio.2013.12.007
27. Domínguez-Oliva A, Hernández-Ávalos I, Martínez-Burnes J, Olmos-Hernández A, Verduzco-Mendoza A, Mota-Rojas D. The importance of animal models in biomedical research: current insights and applications. Animals. (2023) 13:1223. doi: 10.3390/ani13071223
28. Sykes N, Beirne P, Horowitz A, Jones I, Kalof L, Karlsson E, et al. Humanity's best friend: a dog-centric approach to addressing global challenges. Animals. (2020) 10:502. doi: 10.3390/ani10030502
29. Shaffer LG. Special issue on dogs genetics: animal models for human disease and gene therapies, new discoveries for dogs inherited diseases, and standards and guidelines for clinical genetic testing for domestic dogs. Hum Genet. (2019) 138:437–40. doi: 10.1007/s00439-019-02025-5
30. Switonski M. Impact of gene therapy for dogs monogenic diseases on the progress of preclinical studies. J Appl Genet. (2020) 61:179–86. doi: 10.1007/s13353-020-00554-8
31. Ostrander EA, Franklin H. Epstein Lecture Both ends of the leash–the human links to good dogs with bad genes. N Engl J Med. (2012) 367:636–46. doi: 10.1056/NEJMra1204453
32. Plassais J, Kim J, Davis BW, Karyadi DM, Hogan AN, Harris AC, et al. Whole genome sequencing of canids reveals genomic regions under selection and variants influencing morphology. Nat Commun. (2019) 10:1489. doi: 10.1038/s41467-019-09373-w
33. Donner J, Freyer J, Davison S, Anderson H, Blades M, Honkanen L, et al. Genetic prevalence and clinical relevance of dogs Mendelian disease variants in over one million dogs. PLoS Genet. (2023) 19:e1010651. doi: 10.1371/journal.pgen.1010651
34. Williams D, Middleton S, Caldwell A. Everted third eyelid cartilage in a cat: a case report and literature review. Vet Ophthalmol. (2012) 15:123–7. doi: 10.1111/j.1463-5224.2011.00945.x
35. O'Neill DG, Yin Y, Tetas Pont R, Brodbelt DC, Church DB, Pegram C, et al. Breed and conformational predispositions for prolapsed nictitating membrane gland (PNMG) in dogs in the UK: a VetCompass study. PLoS ONE. (2022) 17:e0260538. doi: 10.1371/journal.pone.0260538
36. Xu H, Zhang LB, Luo YY, Wang L, Zhang YP, Chen PQ, et al. Synaptotagmins family affect glucose transport in retinal pigment epithelial cells through their ubiquitination-mediated degradation and glucose transporter-1 regulation. World J Diabetes. (2024) 15:958–76. doi: 10.4239/wjd.v15.i5.958
37. Zhou L, Lv Z, Tian X, Zhang Y, Xu Z. Eye pain and blurred vision as main complaints in a new case with MDPL syndrome. Eur J Ophthalmol. (2022) 32:Np82–6. doi: 10.1177/11206721211009179
38. Uchino S, Waga C. as an autism spectrum disorder-associated gene. Brain Dev. (2013) 35:106–10. doi: 10.1016/j.braindev.2012.05.013
39. Odendaal JSJ, Meintjes RA. Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet J. (2003) 165:296–301. doi: 10.1016/S1090-0233(02)00237-X
40. Claussnitzer M, Cho JH, Collins R, Cox NJ, Dermitzakis ET, Hurles ME, et al. A brief history of human disease genetics. Nature. (2020) 577:179–89. doi: 10.1038/s41586-019-1879-7
Keywords: cherry eye, third eyelid gland, animal model, canine, eye diseases, genetics
Citation: Zeng Y, Feng C, Jiang Z, Du W, He S, Li X, Fan Y, Ouyang X, Huang B, Su Y, Wang S, Wei R, Dai Z, Jin P, Liu J and Yang Q (2025) Genome-wide association studies with prolapsed gland of the third eyelid in dogs. Front. Vet. Sci. 11:1520155. doi: 10.3389/fvets.2024.1520155
Received: 30 October 2024; Accepted: 30 December 2024;
Published: 24 January 2025.
Edited by:
Jun-Mo Kim, Chung-Ang University, Republic of KoreaReviewed by:
Herman Revelo, Fundación Universitaria San Martín, ColombiaGuillermo Giovambattista, CONICET Institute of Veterinary Genetics (IGEVET), Argentina
Seung-Hoon Lee, Chung-Ang University, Republic of Korea
Copyright © 2025 Zeng, Feng, Jiang, Du, He, Li, Fan, Ouyang, Huang, Su, Wang, Wei, Dai, Jin, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qianyong Yang, eWFuZ3FpYW55b25nQDE2My5jb20=
†These authors have contributed equally to this work
 Yu Zeng1,2,3†
Yu Zeng1,2,3† Weian Du
Weian Du Jianyun Liu
Jianyun Liu Qianyong Yang
Qianyong Yang