- 1Directorate of Veterinary Services, Nairobi, Kenya
- 2International Livestock Research Institute, Nairobi, Kenya
- 3Department of Medical Microbiology and Immunology, Faculty of Health Sciences, University of Nairobi, Nairobi, Kenya
- 4Centre for Epidemiological Modelling and Analysis, Institute of Tropical and Infectious Diseases, University of Nairobi, Nairobi, Kenya
- 5Institute of Immunology and Infection Research, University of Edinburgh, Edinburgh, United Kingdom
- 6Paul G. Allen School for Global Health, Washington State University, Pullman, WA, United States
- 7County Directorate of Veterinary Services, Kajiado, Kenya
- 8County Directorate of Veterinary Services, Busia, Kenya
- 9Institute of Infection Veterinary and Ecological Sciences, University of Liverpool, Neston, United Kingdom
Introduction: Animal health surveillance systems in Kenya have undergone significant changes and faced various challenges throughout the years.
Methods: In this article, we present a comprehensive overview of the Kenya animal health surveillance system (1944 to 2024), based on a review of archived documents, a scoping literature review, and an examination of past surveillance assessments and evaluation reports.
Results: The review of archived documents revealed key historical events that have shaped the surveillance system. These include the establishment of the Directorate of Veterinary Services in 1895, advancements in livestock farming, the implementation of mandatory disease control interventions in 1944, the growth of veterinary services from a section to a ministry in 1954, the disruption caused by the Mau Mau insurrection from 1952 to 1954, which led to the temporary halt of agriculture in certain regions until 1955, the transition of veterinary clinical services from public to private, and the progressive privatization plan for veterinary services starting in 1976. Additionally, we highlight the development of electronic surveillance from 2003 to 2024. The scoping literature review, assessments and evaluation reports uncovered several strengths and weaknesses of the surveillance system. Among the strengths are a robust legislative framework, the adoption of technology in surveillance practices, the existence of a formal intersectoral coordination platform, the implementation of syndromic, sentinel, and community-based surveillance methods, and the presence of a feedback mechanism. On the other hand, the system’s weaknesses include the inadequate implementation of strategies and enforcement of laws, the lack of standard case definitions for priority diseases, underutilization of laboratory services, the absence of formal mechanisms for data sharing across sectors, insufficient resources for surveillance and response, limited integration of surveillance and laboratory systems, inadequate involvement of private actors and communities in disease surveillance, and the absence of a direct supervisory role between the national and county veterinary services.
Discussion and recommendations: To establish an effective early warning system, we propose the integration of surveillance systems and the establishment of formal data sharing mechanisms. Furthermore, we recommend enhancing technological advancements and adopting artificial intelligence in surveillance practices, as well as implementing risk-based surveillance to optimize the allocation of surveillance resources.
1 Introduction
Emerging Infectious Diseases (EID) pose a global challenge to economies and public health (1). The majority (60%) of these EID events are zoonotic, with nearly three out of every four events (72%) originating from wildlife (1–3). The drivers of emergence are multifaceted including environmental, ecological, and socio-economic changes that facilitate novel or increased contact between wildlife, livestock and humans leading to the transmission of pathogens between hosts (1). Therefore, One Health (OH) approaches that involve collaboration between animal, human, and environmental sectors are critical for understanding the emergence of zoonotic infections, their early detection before spilling over to humans, and for timely response to control their spread and impact (4).
Early warning systems for health-related events should provide timely information and be sensitive enough to capture and analyze any unusual patterns in the occurrence of health events, animal diseases, and diseases transmissible between humans and animals (zoonoses) for prompt epidemiological response actions (5). To be useful for early warning systems, the surveillance system ought to employ an OH approach, to foster collaborations and preparedness across the sectors (6, 7) and align with the current global focus on deep prevention which emphasizes on mid-stream and upstream prevention of zoonoses (8). The system should utilize real-time digital tools in data collection and reporting (9, 10), utilize risk assessment and modeling techniques to enhance forecasting of events of public health and economic importance (10) and strengthen laboratory capacity for both human and animal cases (10–12). The surveillance system should also benefit from syndromic surveillance (13), operate within a robust legal framework (10) and financing mechanism (14), and continuously build capacity of surveillance officers across the sectors at all levels of government (10, 15).
Animal disease surveillance safeguards the health and welfare of animals and public health, ensures the safety of foods of animal origin, and provides quality assurance for trade in animals and animal products. Importantly animal health data also provides useful information for the timely detection of potential hazards and ensuring appropriate actions can be taken to safeguard public health and decision-making and priority setting for control measures (16).
Kenya, like the rest of the East Africa region, carries a large burden or is at risk of multiple EID of zoonotic origin including Rift Valley Fever (RVF), dengue, and yellow fever. It also experiences endemic zoonoses such as anthrax, rabies, brucellosis, trypanosomiasis, bovine tuberculosis, cysticercosis, leishmaniasis, echinococcosis, and other transboundary animal diseases (17). Surveillance of these diseases in the animal population may support forecasting of disease risks to humans (18).
Animal health surveillance systems in Kenya have evolved based on the changing needs. This evolution provides important lessons that are critical for the development and maintenance of a robust system. In this manuscript, we review the evolution of animal health surveillance in Kenya over the last 80 years, examine the evaluations undertaken on Kenya’s animal health surveillance system, and provide a perspective on the opportunities and challenges of the current Kenyan animal health surveillance system in providing early warning for infectious disease threats of zoonotic origin.
2 Methods
This manuscript provides a synthesis of Animal Health (AH) surveillance systems and tools in Kenya from 1944 to 2024 drawn from a three-stage review: stage one reviewed documents held at the Directorate of Veterinary Services (DVS), specifically: transcripts from historical government reports, disease control strategies, and reports of consultative forums involving national and county surveillance officers generated during the Kenya Animal Bio-surveillance System (KABS) rollout and training workshops (19). Secondly, we conducted a scoping review of the literature. The first author identified and reviewed articles on animal health surveillance in Kenya during the study period using the PubMed database. The search syntax used was “((Kenya[Title]) AND (surveillance[Title/Abstract])) AND (disease[Title/Abstract]).” We included articles that contained information relevant to Kenya animal health surveillance system and excluded articles that were not related to animal health events or surveillance, as well as articles that did not contain any information about Kenya animal health surveillance. The selected articles were then reviewed based on specific attributes of a surveillance system including usefulness, simplicity, flexibility, acceptability, positive predictive value, representativeness, timeliness, and collaborations (20). The first author extracted relevant data into a Word document categorizing it according to the attribute it addressed. This data was then then refined through consensus with the other authors.
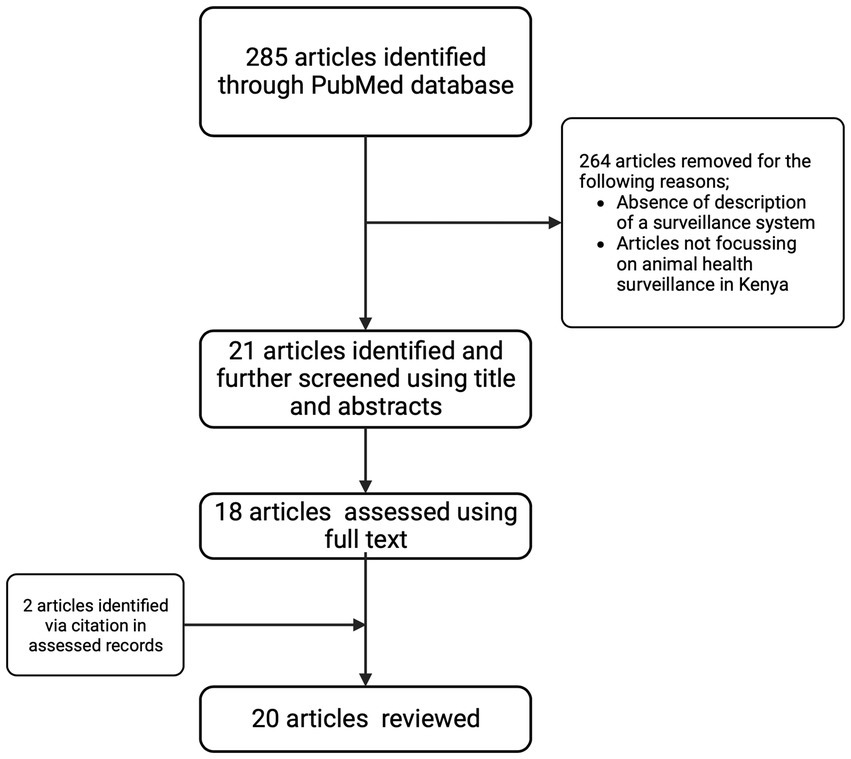
Finally, we reviewed reports on assessments and evaluations of animal health surveillance systems undertaken in Kenya for the period between 2011 to 2023. Additionally, we present the perspective of the authors, who have diverse backgrounds ranging from governmental officers involved in developing and utilizing surveillance tools to researchers and academics who have implemented surveillance programs across various regions in Kenya. The information gathered in this study was utilized to enhance our understanding and document the evolution of animal health surveillance in Kenya, starting from 1944 up to the present. Furthermore, it provides insights into the strengths, identified gaps, and potential solutions, as well as assesses the reporting rates and trends over time. Lastly, the retrospective analysis of the system against key surveillance attributes will inform its capacity for early detection of zoonotic diseases and offer valuable lessons for strengthening the system. The summary of the process is outlined in the flow diagram in Figure 1.
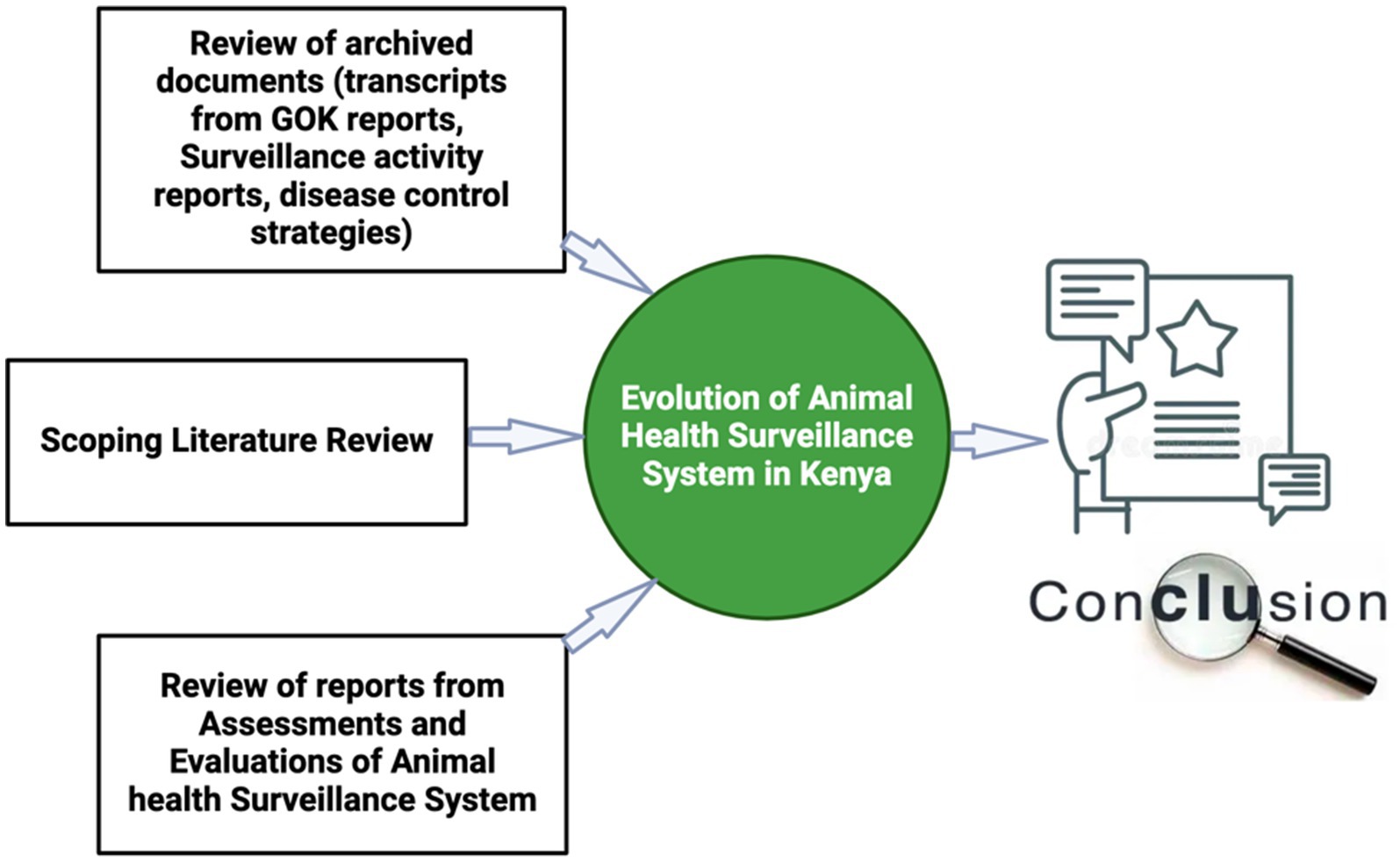
Figure 1. Summary of the approach used to collect information used to document the evolution of animal surveillance systems in Kenya for the period of 1944 – to-date.
3 Results
The review of archived documentation captured 20 records. The documents are provided for in the supplementary materials. Key information regarding surveillance was extracted and included in our record. Our scoping review identified 21 relevant articles from which key findings were extracted having direct or indirect implications on the surveillance systems (21–40).
The Figure 2 outlines the process used in identifying the articles reviewed.
Six reports were identified from assessments and evaluations missions undertaken in Kenya in the last decade. These reports include the performance of Veterinary Services (PVS) by the World Organization for Animal Health (WOAH) in 2011, 2017 and 2022 (41, 42); the Joint External Evaluation (43); the assessment of animal disease surveillance capacity by the Food and Agriculture Organization (FAO) in 2017 (44); and the evaluation of surveillance systems relevant to zoonotic diseases in Kenya in 2015 (45).
3.1 Animal health surveillance in Kenya 1944-to date
3.1.1 Evolution of the systems to date
Directorate of Veterinary Services (DVS) was established during colonial rule in 1895. It was among the first directorates to be created (21, 46). Around 1944, the European dominated areas of Colony and Protectorate of Kenya had begun to improve and diversify farming unlike the native Africans dominated areas where there were no efforts toward modernization since most pastoralists were seen to be less responsive to educational and missionary influence (21). The government during this time had a plan for compulsory dipping and immunization against certain priority diseases like rinderpest and rabies, introduction of improved animals, provision of sufficient dips and application of fencing ordinance. The government planned to improve native animal husbandry through propaganda, and education due to the anticipated slow adoption (21). Gene multiplication was undertaken in Ngong, Maseno, Baraton, Sangalo and Machakos which are currently Efficacy trial centers. During the same period a Central Artificial Insemination Station was established for semen production and distribution. The station would be supported by government until it become self-sustaining (21). Disease surveillance and diagnostic services in Kenya were governed by Diseases of animal Ordinance. The ordinance also guided application of quarantine rules and prophylaxis by vaccination and dipping as the key mechanisms for control of the diseases (21).
Between 1945 to 1958, total staff of veterinary department increased from 291 to 892 and headquarter grew from a small section to a separate ministry in 1954 and integrated on United Kingdom pattern in 1956 following the constitutional development of the colony. The ministry’s expenditure during the period was 10 per cent of the total government budget since the aim of the government was to maintain stable agriculture while conserving and developing land in accordance with the good husbandry practice.
Following insurrection of Mau Mau in 1952 to 1954, and subsequent detention of huge numbers of people in central province and surrounding areas, agriculture almost ran to a standstill as the government focused on restoring security until 1955 when Agriculture Ordinance organized markets and fixing of producer prices of major animal products leading to successful emergence of farmers from the mau Mau ordeal. Later in 1957, major decisions were made on animal husbandry and livestock improvement in African areas including establishment of District and provincial agricultural committees where African were members and allocation of different animal breeds to different regions in the colony and protectorate of Kenya (22).
The major diseases of concern during this time were FMD Type O and A and SAT 2 which appeared in 1957 in Samburu District among the African herds. FMD type C appeared in Machakos late 1957. The country required capacity for Research into FMD and this led to establishment of current FMD laboratory and vaccine production. Institute (Kenya Vaccine Production Institute - KEVEVAPI) in 1956 (22).
Veterinary services were offered as an essential service and the Director of Veterinary Service was an ex-official member of the parliament due to the crucial role played by the directorate (21). Before independence in 1963, clinical services were provided by private veterinarians with the Directorate of Veterinary Services (DVS) providing a regulatory role. Surveillance was predominately focused on notifiable diseases and used a passive structure, relying on reports from private sector veterinarians that served commercial ranches and dairy farms, being passed manually to the DVS (23, 46).
Shortly after independence in 1963, a decision was made to temporarily transfer the provision of clinical services to the public sector through the DVS. Plan to privatize veterinary services in Kenya commenced in 1976 with establishment of nine veterinary clinical stations (Tongaren, Karatina, Olkarau, Kericho, Sotik, Kakamega, Machakos, Thika, Nyahururu) (23) and six Regional Veterinary Investigative Laboratories (RVILs) were established between 1973 and 1987 (47) with a mandate to support veterinary diagnostic services in the field. These infrastructures were intended to gradually be privatized and become self-sustainable. During this period disease reports were in the form of narrative reports which generally lacked important epidemiological information, hindering epidemiological investigations and disease control efforts.
In 1981, the Veterinary Epidemiology and Economics Units (VEEU) was established with donor support and mandated to manage animal health data and disseminate relevant information (46). The VEEU developed enhanced tools to capture animal health events with sufficient spatial, temporal, and species data. These included the Notifiable Disease form (ND1) and Zero Report forms (18). In 1983, the government implemented a “District Focus” plan which established District treasuries to finance the operations at the district level. National level was left with the responsibility for general policy and planning of multi-district and national programs. This also shifted the district veterinarians financing model from Local Purchasing Orders at their disposal to an authority to incur expenditure with a budget ceiling (24).
The VEEU became dormant after the end of donor support around the mid-1980s other than a restricted remit supporting the Rinderpest eradication campaign (1987-2009) (26, 27). In 1988 the government dropped the ‘full employment policy for veterinary doctors’ which had previously provided public sector recruitment of veterinary graduates on an annual basis. Consequently, surveillance and disease control were negatively affected particularly in the Arid and Semi-Arid Lands (ASAL) where private veterinary practice were less viable (46). This preceded the full implementation of the donor-dictated Structural Adjustment Program (SAP) in 1990 (48) which decreased government involvement in the delivery of animal health services resulting in the collapse of most services including disease surveillance. This collapse and lack of government support to cushion private practitioners working in the ASAL regions may have contributed to the spread of several animal diseases across the country resulting in their endemic status (47).
In 2010 constitutional change led to the formation of 47 counties in 2013 with devolved powers (49). The division of responsibility between the two levels of government, was such that counties were mandated to provide county health services, including veterinary services while the national government provided national-level referral health services, disaster management, and formulating health and veterinary policies (50). A previous proposal by health professionals during the constitutional development stage to create a health service commission to secure the chain of command was rejected at the final stages of the constitutional review process and all services proposed under the commission, including veterinary services, were automatically placed under county health services (50). Notwithstanding the provision of the constitution, veterinary services remained domiciled in the Agriculture Departments and ministries even after the promulgation of the constitution. Following this, the direct reporting lines between the field surveillance officers and the DVS were hampered (45). While the DVS is the competent authority for veterinary services in Kenya, implementation of disease prevention control activities is a preserve of county governments, and this presented challenges in the coordination of disease control (45).
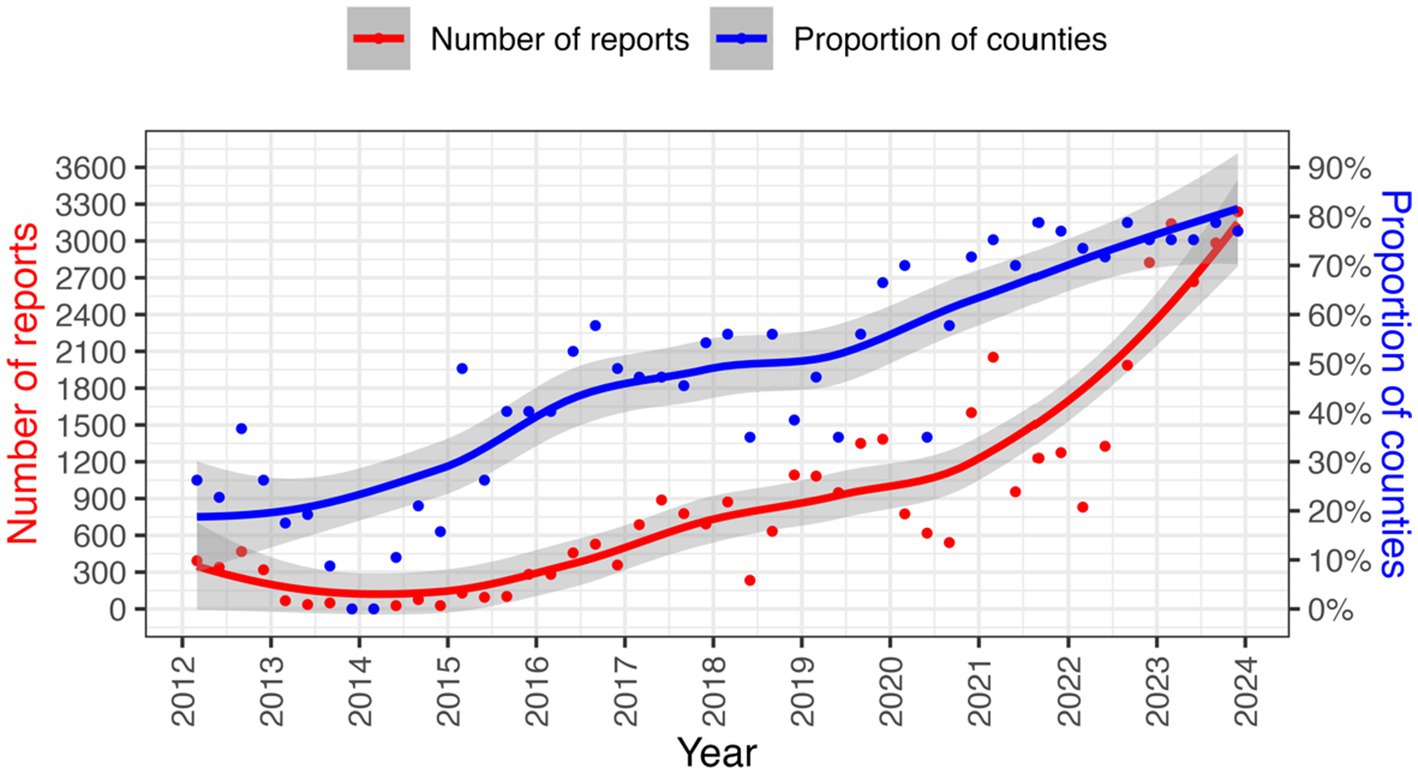
Disease reporting to the DVS sharply declined after the promulgation of the Kenyan Constitution in 2010 with the biggest impact being felt in 2014 as shown in Figure 3. Consequently, the VEEU team sought collaborations with AU-IBAR through the Standards Methods and Procedures in Animal Health (SMP-AH) to hold a consultative meeting with key stakeholders from the counties on challenges affecting surveillance. Complex and unharmonized reporting systems, complex data collection tools, inadequate feedback from stakeholders, inadequate capacity building for technical personnel, and low prioritization for disease surveillance activities were reported as key issues associated with the decline (51).
Following this feedback, the VEEU team instituted corrective measures which included the creation of an email group (VETINFO) for data sharing, simplifying, and standardizing reporting tools and uploading the standardized tools into a harmonized mobile application (Epicollect), creation of WhatsApp groups and google group for communication and capacity building of technical personnel in counties on the electronic surveillance systems. The DVS also developed guidelines for the delivery of veterinary services under the devolved system. Though not legally binding, the guideline outlined obligations for the counties, especially on their responsibility to report animal diseases and related events to the Directorate of Veterinary Services to comply with international treaties ratified by Kenya on the application of sanitary and phytosanitary measures (34).
Efforts to streamline animal health surveillance in Kenya and adoption of technology in surveillance resulted in improved reporting performance from 2014 onwards as seen in Figure 3.
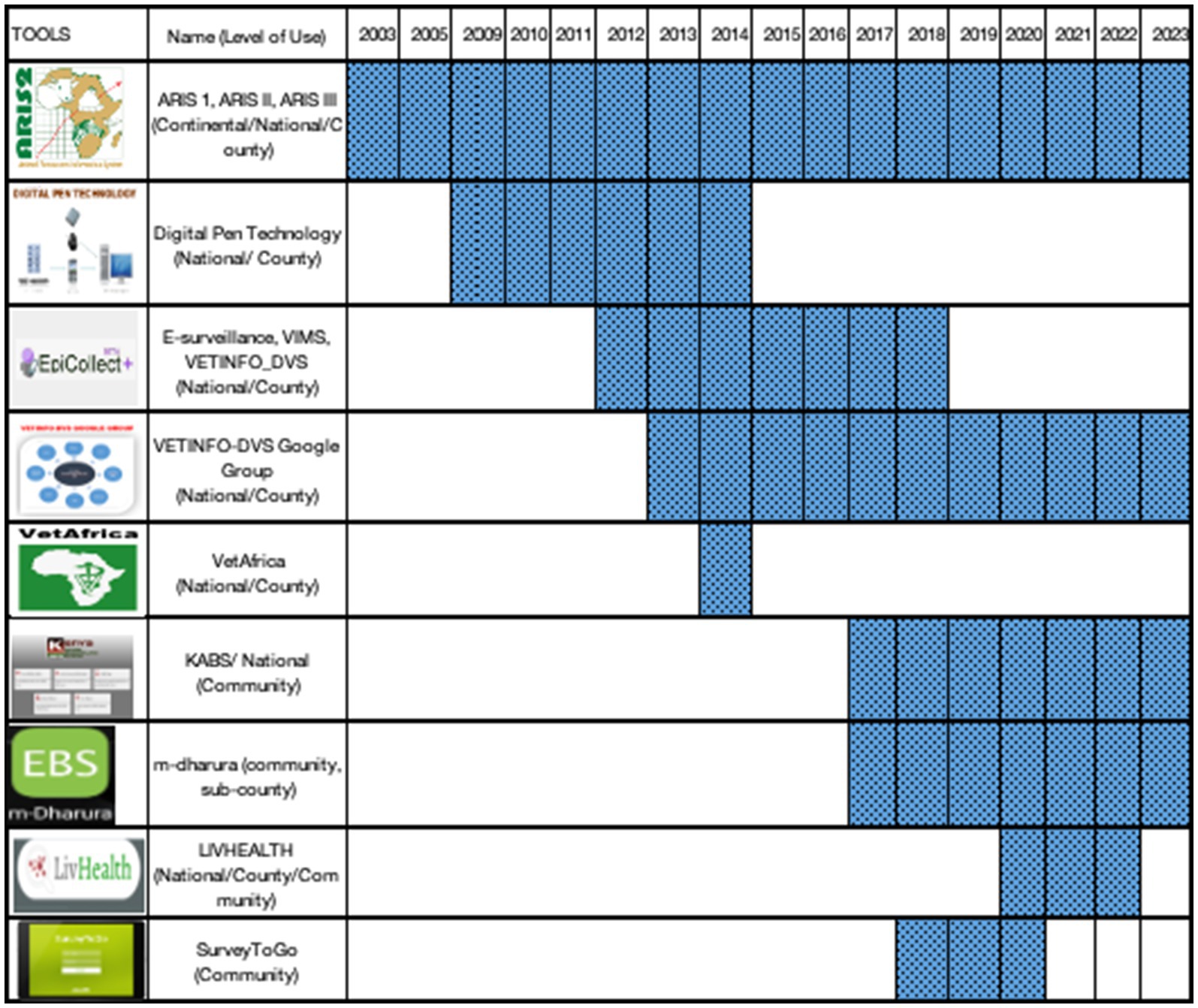
The Epicollect surveillance system, however, presented several limitations including data access issues resulting from the absence of a local server and a limited capacity for customization according to the needs of the country, and was therefore only used as a stop-gap measure. Previously, from 2003, Kenya piloted various electronic disease reporting tools including Digital Pen Technology, Epicollect (Veterinary Information Management System (VIMS), Epicollect plus, Epicollect Beta plus, and Epicollect 5), VETINFO-DVS google group, VetAfrica, and ODK collect (Liv Health). The evolution of electronic tools for animal Health surveillance in Kenya is outlined in Figure 4.
Epicollect was used as the official reporting tool for the DVS until the Kenya Animal Bio-surveillance System (KABS) was developed (52). This is a mobile-based technology that provides for the creation of forms for data collection, data analysis, and feedback. Data is stored in the DVS server for security. KABS introduced syndromic surveillance to the previously existing surveillance tools. The progressive roll-out of KABS commenced in 2017 and was taken up by all 47 counties by the end of 2021 and was confirmed to process capacity to enhance preparedness for epidemics of zoonotic diseases (19). During the same period, Kenya customized wildlife surveillance tools and incorporated them in the KABS to capture events in the wild populations (19). The tools were to be used by wildlife veterinarians, researchers, wardens, and others who interacted with sick wild animals. All the target users in the wildlife sector were also trained on the use of the system. However, only 18% (22/120) of the trained users submitted at least one report through the system from 2017 to 2021 (38). In 2017 mDharura was developed jointly by human and animal health sector and undergoing progressive roll out in counties to date. The system aims to support event-based surveillance where signals in both sectors are collected by members of community and shared across the sectors. The signals undergo verification and response depending on whether they are animal only, human only or zoonotic in nature. After verification, the event is reported through KABS or Kenya Health Information System (KHIS) as appropriate.
3.1.2 Describing the flow of surveillance information in the current system
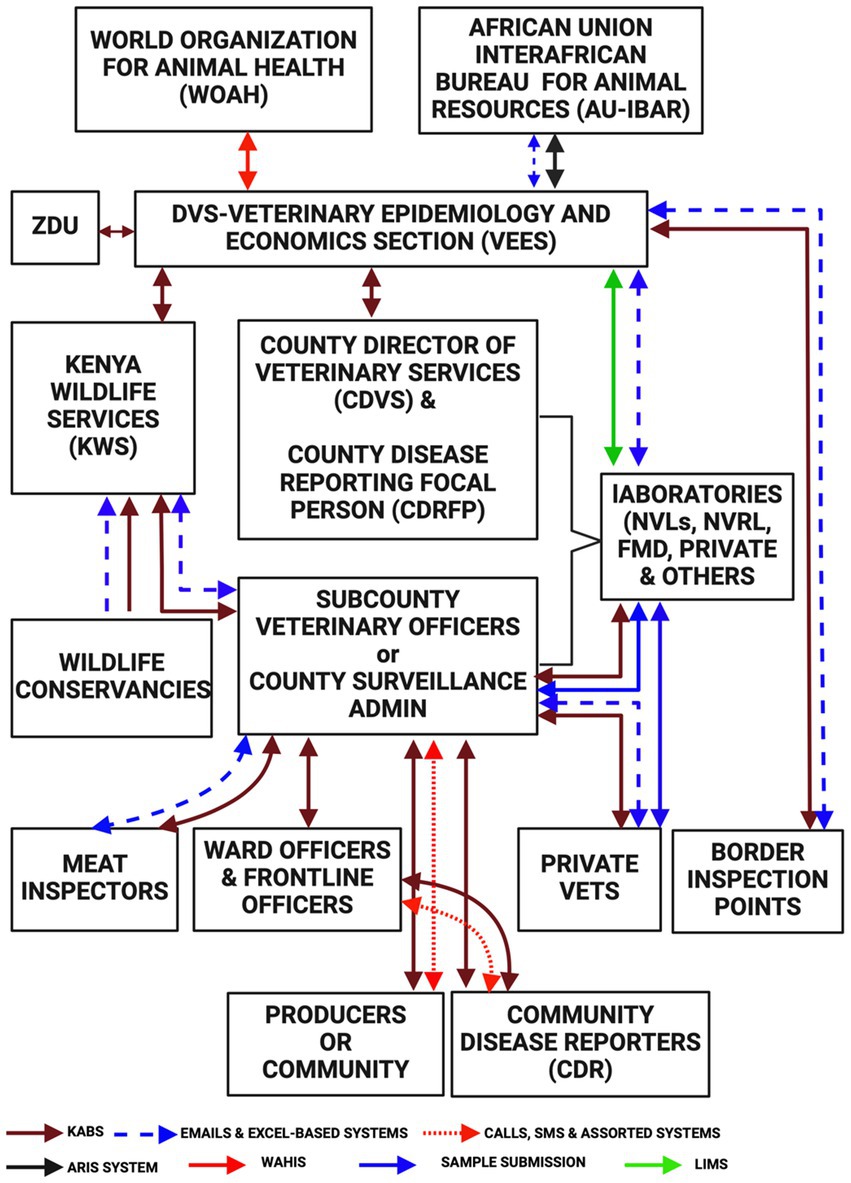
Here we describe the current functioning of the animal health surveillance system in terms of the flow of information through the system. Frontline animal health workers receive information from livestock keepers (passive surveillance) and undertake routine active surveillance at the villages, abattoirs, and livestock sale yards. In some counties where community disease reporting and event-based surveillance has been adopted (53) the Community Disease Reporters (CDRs) and/or Community Health Promoters (CHP) send information on the observed clinical signs or events which are verified by the frontline animal health workers, responded to where possible, and reported via KABS. The frontline animal health workers including private practitioners report disease occurrences using their mobile phones. The data in KABS is accessible to the sub-county and county administrators who can download data through the dashboard and use it to make their own disease control decisions. This information is also concurrently accessible to the VEES (the unit was changed to a section in 2021) (28). Currently AH surveillance in Kenya predominantly relies on passively collected clinical data with limited (7%) laboratory-diagnosed data. Additionally, the data also mainly (94%) comes from public sector practitioners, with only 6% coming from private practitioners (53).
The regional and national veterinary laboratories submit data to VEES every week through the VETINFO mailing group. Most laboratories use a standard Excel spreadsheet for reporting, while three of them utilize Laboratory Information Management Systems (LIMS). Data is downloaded in Excel format from LIMS, KABS, and the VETINFO group, then cleaned, and collated by a VEES epidemiologist. This process generates immediate notifications, quarterly feedback bulletins and monthly reports for international reporting to the World Organization for Animal Health (WOAH) and the African Union Inter African Bureau for Animal Resources (AU-IBAR).
The WOAH utilizes the World Animal Health Information System (WAHIS) to capture disease outbreaks (54). Regionally, the AU-IBAR uses the Animal Resource Information System (ARIS) which collects AH information from the member states monthly (55).
The Zoonotic Disease Unit (ZDU) was established in March 2012, comprising officers from the Ministry of Health and the DVS. The unit was created to establish a framework for collaboration at the animal, human, and ecosystem interfaces for the management of zoonotic diseases (17). The unit has been instrumental in coordinating zoonotic disease response activities and officers in the unit have access rights to data in the KABS system. The data flow within the current AH surveillance systems is illustrated in Figure 5.
3.1.3 Current diagnostic capacity linked to animal health surveillance in Kenya
Veterinary diagnostic services in Kenya are mainly a national government function under the DVS. The diagnostic services comprise six National Veterinary laboratories (NVLs) each serving a block of counties (56). There also exists reference laboratory services with the NVRL (National Veterinary Reference Laboratory at Kabete and the FMD Laboratory at Embakasi). A new Biosecurity Level Three (BSL3) Laboratory is also in the process of being established in the country. Various counties have also made attempts to establish basic laboratory testing to supplement the national laboratory network (42).
In March 2022, the National Reference Laboratory achieved ISO 17025 accreditation and proficiency testing which provides the DVS with the ability to certify animals and animal products per importing trading partner requirements as well as relevant international standards (57). The capacity of the veterinary diagnostic services is negatively affected by insufficient resources to conduct outbreak investigations and submit samples from suspected priority disease events, inadequate personnel, and inadequate supplies (42, 44). The structure of the veterinary laboratory system is currently not anchored in a formal legal instrument.
3.1.4 Governance and funding of current animal health surveillance in Kenya
Kenya has a devolved system of government (50) which when properly implemented, the structure of administration brings decision-making closer to the actors thus allowing for the allocation of funds to meet the needs of local communities. Many success stories have been documented in some counties including the employment of adequate surveillance officers, and customized approaches to prevalent animal health events among others (45). On the other hand, the creation of semi-autonomous surveillance planning can present many difficulties. The lack of a direct reporting line from the CDVSs to the DVS presents a challenge in the coordination of the surveillance activities around the country leading to heterogeneous disease surveillance efforts between counties.
Surveillance activities often rely on resources that are also used for other animal health activities and in many cases, there are no resources specifically designated for surveillance (14). This lack of dedicated resources extends to the means of transport for surveillance teams as most counties do not have dedicated vehicles that can be used in emergencies (44, 45). Furthermore, in the event of an animal health-related emergency, government funding for surveillance and response is often delayed due to a lack of a proper contingency fund. This delay has the potential to hinder the effectiveness of the control program. As a result, there is a heavy reliance on project funding which compromises the long-term sustainability of the surveillance efforts.
3.2 Retrospective analysis of the surveillance system attributes
The data extracted from publications and reports regarding surveillance system attributes is summarized in the Table 1. The usefulness of the system has been experienced in some cases such as an enhanced syndromic surveillance to make decisions on RFV prevention and control (14). However, the system lacks a reliable early warning system which is due to limited resources (58). The usefulness of the system declined especially after devolution when it could not detect events of public health importance in a timely manner (45). The current surveillance system has been friendly, cost-effective and simple to use (19, 59). A previous evaluation also confirmed the flexibility of the system (60). On the contrary, the system was slow to adapt to devolution changes (45). To improve the acceptability of the system, there is a need to cultivate political goodwill and engage stakeholders as well as provision of timely feedback that can inform local decision-making (14, 61). The positive predictive value of the system is negatively affected by the minimal utilization of the laboratory in diagnosis due to the costs involved and sometimes inadequate capacity of the available laboratories (41, 43). The system was representative as it used different data sources and different systems such as syndromic surveillance, sentinel surveillance and adopted the use of a real time electronic reporting system which improved reporting rates and spatial distribution of reports. However, this is negated by reporting gaps experienced (43, 45, 62), weak active surveillance (41, 44, 74), and inadequate involvement of private practitioners and the community in surveillance (43, 44, 60, 66). Timeliness of the system received a major boost following the introduction of syndromic surveillance and a mobile-based electronic reporting system (19, 58, 69). Collaborations in the system are enhanced by presence of ZDU platform (44, 71) but there is a weak intra and inter-county collaboration as well as collaboration between the public and private sectors (44).
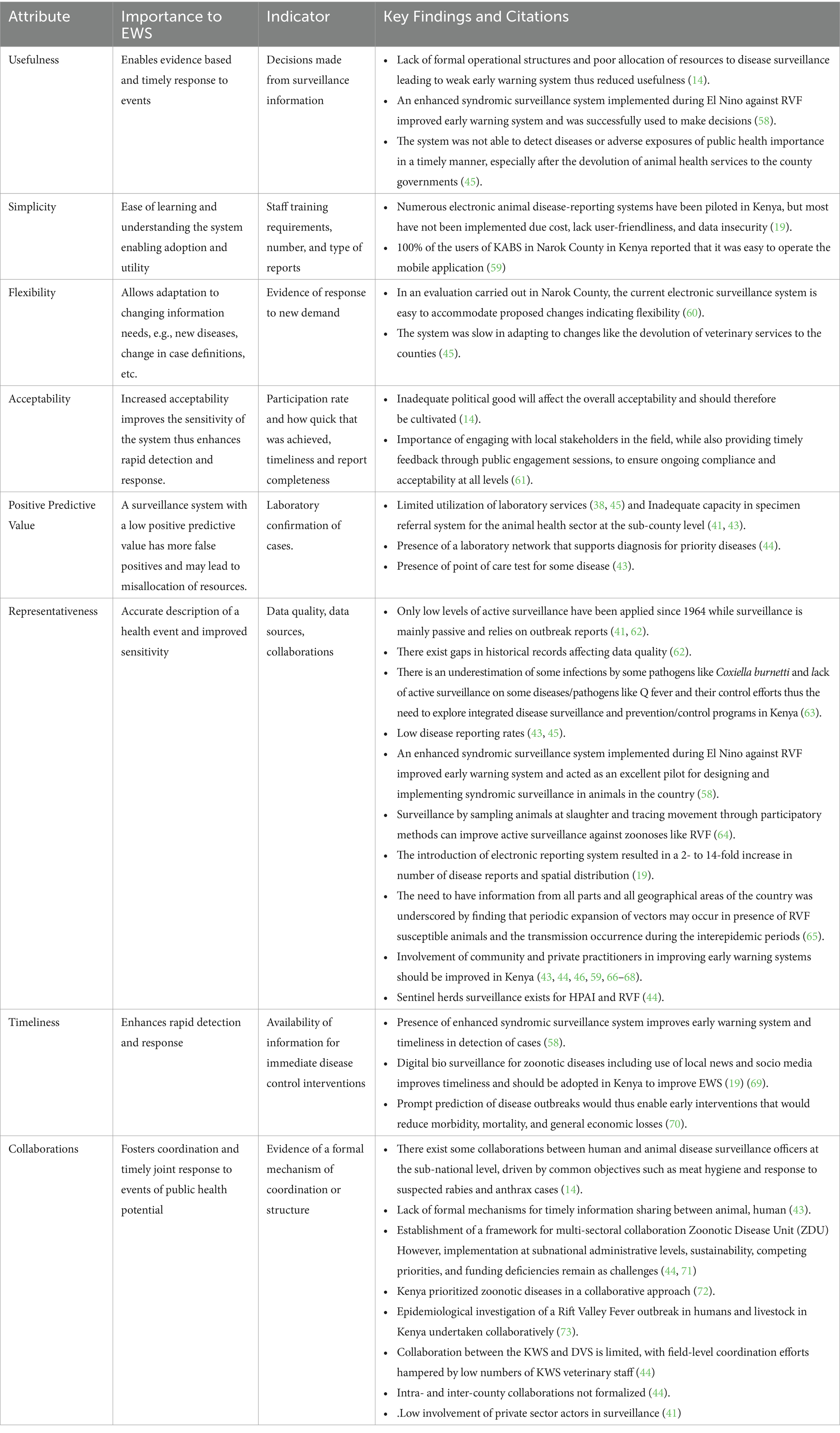
Table 1. Summary of the surveillance system attributes for the animal health surveillance systems currently active in Kenya articles identified and the key findings with effect on surveillance.
3.3 Assessment of strengths, weaknesses, and recommendations from recent external evaluations
The animal health surveillance systems in Kenya have undergone various evaluations each providing key insights into their strengths and weaknesses (14–18). These evaluations form a good basis for prioritizing interventions to improve the surveillance systems.
Table 2 outlines all the identified strengths, weaknesses, and recommendations highlighted in the evaluation exercises for the animal health surveillance systems in Kenya over the past 10 years.
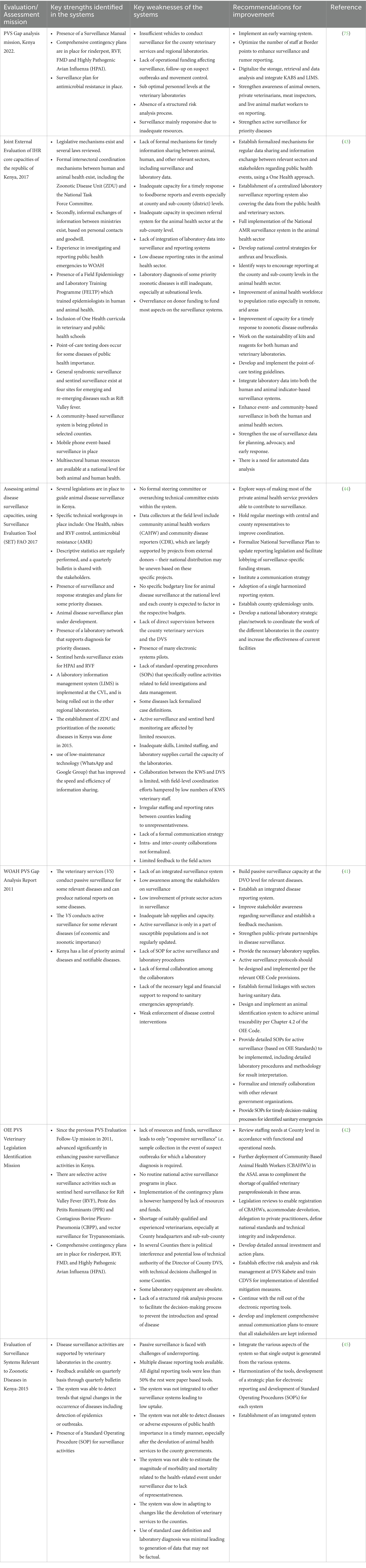
Table 2. Assessment of the key strengths, shortcomings, and recommendations as outlined in the recent evaluations of the animal health surveillance system.
Key strengths identified include: a strong legislative framework for disease surveillance and control (41–44), a formal intersectoral coordination mechanism between the human and animal health sectors (43–45), presence of adequate experience in investigating and reporting public health emergencies to WOAH, WHO,AU-IBAR, FAO and Africa CDC, provision of field epidemiology trainings to build local capacities, and inclusion of one health in school curricula (43). Other strengths include the availability of a laboratory network to support surveillance (43–45), syndromic and sentinel surveillance for emerging and re-emerging diseases (41–44), community disease reporting (43), electronic disease reporting systems and LIMS (43–45), comprehensive contingency plans, specific disease control strategies as well as use of low maintenance technological initiatives like WhatsApp and Google groups for information sharing (41–45) (44). The is also feedbacking mechanism through a quarterly bulletin shared with field actors and stakeholders (45), as well as considerable capacity for data analysis (44). The PVS follow-up evaluation indicated significant improvement in passive surveillance following recommendations from previous evaluations (42), underscoring the importance of regular evaluations.
The evaluations also identified several shortcomings and gaps within the surveillance systems. These include the absence of a formal mechanism for data sharing across collaborating sectors due to the lack of a legal framework for ZDU establishment (41, 43), inadequate capacity for timely response to reported events due to insufficient funding for surveillance (42–44), insufficient diagnostic capacity due to unreliable supplies, low staffing, obsolete equipment and inadequate skills (42, 43), lack of integration of surveillance systems across sectors including laboratory systems (41, 43, 45), low disease reporting rates in the country leading to unrepresentative data (43–45), limited utilization of diagnosis (43), inadequate involvement of private sector actors and the community in disease surveillance (41, 42, 44), limited use of rapid diagnostic kits to support detection (43), lack of a direct supervisory role between the DVS and CDVS causing complications in coordinating surveillance activities (44), and potential political interference threatening the technical authority of the CDVSs (42). Most evaluations also acknowledged weaknesses in active surveillance which largely relied on the availability of resources and partner organization activities (41, 42, 44). Lack of stakeholder awareness, weak implementation of strategies and enforcement of laws, and the lack of standard case definitions for priority diseases were also key shortcomings hindering the capacity of the animal health surveillance systems in Kenya (41–44). Furthermore, the surveillance systems faced challenges such as staff shortages and a lack of a structured risk analysis process to facilitate decision-making and the implementation of preventive and control measures (42, 44).
4 Discussion: utility of animal health surveillance in Kenya for efficient detection of zoonotic diseases
The animal health surveillance in Kenya has evolved and experienced growth and challenges over the last 80 years. Major changes occurred during key periods such as pre-independence, the independence period, government programs like rinderpest eradication, the structural adjustment program around 1990, devolution of veterinary services and the roll out of the electronic reporting systems in the country. This study provides a summary of these experiences, highlights identified gaps and aims to improve the systems for integrated surveillance systems and a public health early warning system.
Surveillance approaches for early warning are complemented by effective laboratory testing to diagnose the underlying infectious causes of emerging trends and alarms (76). However, accurate diagnosis of animal health events in Kenya is greatly hindered by the sparse distribution and limited capacities of veterinary laboratories (31, 45). Field surveillance officers bear the cost of sample collection and submission leading to a majority opting for clinical diagnosis. This compromises data quality and the positive predictive value of the surveillance system (45).
The current national surveillance may not accurately reflect the true burden of diseases in the country. Consequently, mapping disease risk using this surveillance data may be challenging and less accurate. This is due to low representativeness of the data which arises from inadequate participation by some stakeholders and low reporting rates (20). After the privatization of veterinary services, most of the sick animals are now treated by private practitioners (14) and veterinary medicine shops (5). However, contrary to this, the private sector only contributes only about 6% of the data in the current animal health surveillance systems (53). To improve reporting rates, it is crucial to involve all stakeholders in surveillance. Although the involvement of the community in disease reporting has gained traction in the country, it is still inadequate. Only 10% of the counties have made attempts to roll out (53) community disease reporting. Previous studies have emphasized the importance of community information and ongoing surveillance in EWS (59, 67). However, the adoption and utilization of wildlife surveillance tools in KABS has been lacking, leading to minimal complementarity from other data sources (5). This lack of adoption may be attributed to the possible requirement for wildlife veterinarians to send reports in other different templates, leading to duplication and user fatigue. Therefore, continuous capacity building and awareness creation are needed in this area. In addition to current data sources, it is important to consider other sources such as livestock producers, livestock markets, abattoirs, zoo-sanitary checkpoints, dips/ crushes, veterinary laboratories, and veterinary medicine shops (77). These sources can provide valuable data for disease surveillance and important components in the big data for surveillance.
Currently surveillance experts are concerned with multivariate surveillance systems which entails monitoring multiple variables and indicators from different data sources. This increases the probability of detecting important events as a single data source may miss crucial aspects of an outbreak (66, 77). The current systems, which mainly focus on a univariate approach, should adopt the application of big data, including syndromic surveillance data, community disease reports, production data, wildlife surveillance data, climatic data, animal treatment records, livestock identification data and socio-economic factors coupled with Artificial Intelligence (AI) as demonstrated in previous studies to improve efficiency in the management of animal health and zoonotic related risks (78, 79). This will enable the surveillance system to provide reliable information from complex analytical models for decision making thus partially mitigating effects of inadequate staffing and analytical capacity throughout the country.
Active surveillance for most priority diseases in Kenya has been happening at very low levels since independence (62). Most active surveillance activities mainly depend on donor-funded projects or occur on need basis (41, 42, 44). However, incorporating sentinels into surveillance systems has increased the likelihood of detecting of the first incursion of a particular disease in the shortest time possible (76). Therefore, utilizing early warning systems, such as sentinel surveillance in vectors, wildlife, companion animals, and zoological parks, has been recognized as the key method for improving surveillance of emerging diseases (80) and could be strengthened in Kenya. Participatory surveillance is also an active surveillance approach that can result in enhanced collaboration and communication among different sectors and institutions. This can help better understand the causes of diseases, determine the success or failure of surveillance programs, contribute to policy reforms, or provide a quick overview of the epidemiological situation in an area (76). Active surveillance at abattoirs could also leverage livestock movement and employ participatory methods to improve active surveillance for zoonotic diseases like Rift Valley Fever (64). The increasing use of electronic data collection and electronic data interchange by surveillance systems promotes timeliness and increases the usefulness of reporting (20). Mobile-based surveillance systems are known to capture higher numbers of AH events compared to traditional surveillance systems (68, 81). This is evident from the progressive improvement in reporting rates following the progressive roll-out of KABS, as seen in Figure 3. The real-time nature of the mobile technologies allows for constant update thus improving EWS for rapid response. The roll out of electronic reporting tools should leverage on the widespread use of mobile phones in sub-Saharan Africa, which is estimated to be 67% (82). Therefore, use of mobile technologies is prerequisite for efficient EWS. However, for the system to be efficient the key capabilities to consider include sustainability in resource-limited environments, a mobile application for field data collection, have local capacity for maintenance and capacity building, integration with laboratory and human health surveillance systems and the ability to analyze and import data from other sources.
Currently in Kenya, there is lack of integration between the epidemiological data collections system (KABS) and LIMS. As a result, duplication of work and complex management of data from various sources are occurring. To effectively protect public health, trade, and animal health and welfare its crucial to establish data sharing and collaboration mechanisms (63, 83). This will create synergies among relevant sectors and facilitate efficient management of EID EWS (17, 84) particularly in resource-limited countries like Kenya (14). The surveillance systems should be integrated with other health information systems allowing for data exchange and sharing in multiple formats, as well as data transformation. By doing so, individual systems can meet specific data collection needs without duplicating effort or causing disharmony in data management (20).
There is a need for continuous review and updating of the existing legislation that governs the surveillance systems in Kenya to create a common objective and to enhance structured collaborations among stakeholders (14). Therefore, constant advocacy and review of legislation are key for OH’s approach toward improving the sustainability of EWS. This should be coupled with modern innovative mechanisms for data sharing.
External evaluations are key in identifying the key intervention areas for improving of the surveillance system. Due to the availability of many surveillance evaluation tools or guidelines, Kenya should develop and implement an internal surveillance evaluation guideline with country-specific indicators. To operate optimally, these systems should be regularly monitored and evaluated for continuous improvement. The surveillance and Information Sharing Operational Tool (SIS-OT) could also be considered to identify gaps in multisectoral surveillance and information sharing for zoonotic diseases for improvement (85).
The provision of veterinary services as a private good especially in ASALs where there are limited numbers of private practitioners may not be feasible. Therefore, animal disease surveillance and control activities should be offered as a public good to minimize the spread of animal diseases.
5 Conclusion
In conclusion, the animal health surveillance systems in Kenya have evolved over time. This study provides valuable insights into the strengths, weaknesses, and opportunities of the system at different stages of development. These insights can be used to improve the current early warning systems for rapid disease detection and response. Several potential areas of improvement have been identified, including adopting of harmonized reporting tools, establishing a clear chain of command across all levels of government, implementing electronic-based surveillance systems, integrating Artificial Intelligence in surveillance, developing of case definitions for priority diseases, incentivizing disease reporting, regularly evaluating the systems, involving the private sector players, wildlife and community in surveillance, strengthening legal framework for collaboration mechanisms, and providing regular support.
However, it is important to acknowledge the limitations of this study. It primarily relied on secondary data which may have resulted to overlooking certain aspects of the surveillance system evaluation. Therefore, further studies are recommended to comprehensively evaluate the current systems. The use of tools like the Surveillance and Information Sharing Operational Tool (SIS-OT) is recommended for this purpose.
In summary, this research contributes to the advancement of animal health surveillance in Kenya by highlighting the areas that require the attention from the government. By addressing the identified shortcomings, the effectiveness and resilience of animal health surveillance in Kenya can be enhanced, leading to improved timely disease detection, response, and control.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The requirement of ethical approval was waived by ILRI Institutional Review and Ethics Committee for the studies involving animals because the study used retrospective data mainly and did not involve primary data collection and sample collection. However the use of the data was approved by the Directorate of Veterinary Services. The studies were conducted in accordance with the local legislation and institutional requirements.
Author contributions
SK: Supervision, Software, Resources, Funding acquisition, Writing – review & editing, Writing – original draft, Visualization, Validation, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. ST: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. BB: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation. MWM: Project administration, Investigation, Writing – review & editing, Validation, Supervision, Methodology. NN: Writing – review & editing, Methodology. AO: Writing – review & editing, Methodology. MM: Writing – review & editing, Methodology. LT: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the German Federal Ministry for Economic Cooperation and Development through the One Health Research, Education and. Outreach Center in Africa (OHRECA) and Bill and Melinda Gates Foundation through the Centre for Epidemiological Modeling and Analysis (INV-044079). The funders had no role in the decision to publish or the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jones, KE, Patel, NG, Levy, MA, Storeygard, A, Balk, D, Gittleman, JL, et al. Global trends in emerging infectious diseases. Nature. (2008) 451:990–3. doi: 10.1038/nature06536
2. Leslie, MJ, and Kazmierczak, JJ. Surveillance for zoonotic diseases In: MJ Leslie , editor. Infectious disease surveillance. Oxford, UK: John Wiley and Sons Ltd. (2013). 143–56.
3. Horby, PW, Hoa, NT, Pfeiffer, DU, and Wertheim, HFL. Drivers of emerging zoonotic infectious diseases. In: Horby . Confronting emerging Zoonoses: The one health paradigm. Japan: Springer, pp. 13–26. (2014).
4. Jeggo, M, and Mackenzie, JS. Defining the future of one health. Microbiol Spectr. (2014) 2:OH-0007-2012. doi: 10.1128/microbiolspec.OH-0007-2012
5. George, J, Häsler, B, Komba, E, Sindato, C, Rweyemamu, M, and Mlangwa, J. Towards an integrated animal health surveillance system in Tanzania: making better use of existing and potential data sources for early warning surveillance. BMC Vet Res. (2021) 17:2789. doi: 10.1186/s12917-021-02789-x
6. Bordier, M, Delavenne, C, Nguyen, DTT, Goutard, FL, and Hendrikx, P. One health surveillance: a matrix to evaluate multisectoral collaboration. Front Vet Sci. (2019) 6:1–12. doi: 10.3389/fvets.2019.00109/full
7. Bordier, M, Goutard, FL, Antoine-Moussiaux, N, Pham-Duc, P, Lailler, R, and Binot, A. Engaging stakeholders in the Design of one Health Surveillance Systems: a participatory approach. Front Vet Sci. (2021):8. doi: 10.3389/fvets.2021.646458/full
8. Vinuales, J, Moon, S, Le Moli, G, and Burci, GL. A global pandemic treaty should aim for deep prevention. Lancet. (2021) 397:1791–2. doi: 10.1016/S0140-6736(21)00948-X
9. Fletcher-Lartey, SM, and Caprarelli, G. Application of GIS technology in public health: Successes and challenges. Parasitology. (2016) 143:401–15.
10. Ruckert, A, Zinszer, K, Zarowsky, C, Labonté, R, and Carabin, H. What role for one health in the COVID-19 pandemic? Special section on Covid (1997) 19:409. doi: 10.17269/s41997-020-00409-z,
11. Godfroid, J, Nielsen, K, and Saegerman, C. Diagnosis of brucellosis in livestock and wildlife. Croat Med J. (2010) 51:296–305. doi: 10.3325/cmj.2010.51.296
12. FAO . Conclusions and recommendations 2 FAO-APHCA/OIE regional workshop on brucellosis diagnosis and control with an emphasis on 2009. pp. 4–6. (2009).
13. Gardy, JL, and Loman, NJ. Towards a genomics-informed, real-time, global pathogen surveillance system. Nat Rev Genet. (2018) 19:9–20. doi: 10.1038/nrg.2017.88
14. Thomas, LF, Rushton, J, Bukachi, SA, Falzon, LC, Howland, O, and Fèvre, EM. Cross-sectoral zoonotic disease surveillance in Western Kenya: identifying drivers and barriers within a resource constrained setting. Front Vet Sci. (2021) 8:658454. doi: 10.3389/fvets.2021.658454/full
15. Buregyeya, E, Atusingwize, E, Nsamba, P, Musoke, D, Naigaga, I, Kabasa, JD, et al. Operationalizing the one health approach in Uganda: challenges and opportunities. J Epidemiol Glob Health. (2020) 10:250–7. doi: 10.2991/jegh.k.200825.001
16. Drewe, JA, Hoinville, LJ, Cook, AJC, Floyd, T, Gunn, G, and Stärk, KDC. SERVAL: a new framework for the evaluation of animal health surveillance. Transbound Emerg Dis. (2015) 62:33–45. doi: 10.1111/tbed.12063
17. Mbabu, M, Njeru, I, File, S, Osoro, E, Kiambi, S, Bitek, A, et al. Establishing a one health office in Kenya. Pan Afr Med J. (2014) 19:106. doi: 10.11604/pamj.2014.19.106.4588
18. Robertson, C, Sawford, K, Daniel, SLA, Nelson, TA, and Stephen, C. Mobile phone-based infectious disease surveillance system. Emerg Infect Dis: Sri Lanka (2010).
19. Njenga, K, Kemunto, N, Kahariri, S, Holmstrom, L, Oyas, H, Biggers, K, et al. High real-time reporting of domestic and wild animal diseases following rollout of Mobile phone reporting system in Kenya. PLoS One. (2020) 16:e0244119. doi: 10.1371/journal.pone.0244119
20. Armstrong, G, Birkhead, GS, Horan, JM, Herrera, G, Lee, LM, Milstein, RL, et al. Updated guidelines for evaluating public health surveillance systems recommendations from the guidelines working group the following CDC staff members prepared this report. (2001).
22. Government printer N . Three year report 1955-1957 by Ministry of Agriculture, animal husbandry and water resources. Nairobi (1958).
23. DVS Kenya . Government sponsored clinical service statement to KVA by DVS. The Kenya Veterinarian (1975).
24. Government printer N . District focus for rural development. Government printer, Nairobi Kenya: Government printer, Nairobi (1983).
27. Kithinji Kiragu . Animal health rehabilitation project technical proposal. Nairobi: Kithinji Kiragu (1993).
29. DVS Kenya . Contingency plan for Rift Valley fever. Nairobi: Contingency Plan for Rift Valley Fever, Directorate of Veterinary Services (2008).
32. Zoonotic Disease Unit K . Kenya brucellosis control strategy. Kenya: Government of Kenya (2022).
33. DVS Kenya . One health strategic plan for the prevention and control of zoonotic diseases in Kenya. Kenya: DVS Kenya (2021).
36. DVS K . Kenya Animal Bio-surveillance User Manual. Kenya animal bio-surveillance user manual. Directorate of Veterinary Services (2019).
37. DVS K . County surveillance consultative and capacity building workshops. Nairobi: DVS K (2023).
38. KABS Account Portal . (2024). Available at: https://kabs.kilimo.go.ke/kabs/account/index.html#/manage. (Accessed Jan 15, 2024).
43. WHO . Republic of Kenya. Joint external evaluation of IHR Core capacities of the Republic of Kenya. Geneva: World Health Organization (2017).
45. Omondi, M, Ngere, I, and Ndeta, C. Report on the evaluation of surveillance systems relevant to zoonotic diseases in Kenya-2015: A basis for Design of an Integrated Human Livestock Surveillance System Evaluation Report. (2016). Available at: www.zoonotic-diseases.org.
46. Chema, S, and Gathuma, JM. Kenya: the development of private services and the role of the Kenya veterinary association. Rev Sci Tech. (2004) 23:331–40. doi: 10.20506/rst.23.1.1483
47. Bitek, AO, Osoro, E, Munyua, PM, Nanyingi, M, Muthiani, Y, Kiambi, S, et al. A hundred years of rabies in Kenya and the strategy for eliminating dog-mediated rabies by 2030. AAS Open Res. (2018) 1:23. doi: 10.12688/aasopenres.12872.1
48. Gauthier, J, Siméon, M, and de Haan, C. The effect of structural adjustment PROGRAMMES on the delivery of veterinary services in Africa. (1999).
50. National Council for Law . The Constitution of Kenya. Kenya Law Reports Chief Registrar of the Judiciary (2010).
52. Epicollect5 - VETINFODVS . (2024). Available at: https://five.epicollect.net/project/vetinfodvs. (Accessed Jan 29, 2024).
54. Disease Data Collection . Disease Data Collection–WOAH–World Organisation for Animal Health. (2024). Available at: https://www.woah.org/en/what-we-do/animal-health-and-welfare/disease-data-collection/.
55. Establishment of National Animal Resources Data Management and Sharing Platforms The African Union–Interafrican Bureau for Animal Resources (AU-IBAR). (2024). Available at: https://www.au-ibar.org/node/34. (Accessed Jan 14, 2024).
57. FAO and USAID Support Kenya Central Veterinary Lab . To have Testing and Calibration Competence FAO in Kenya. (2024). Available at: https://www.fao.org/kenya/news/detail-events/zh/c/1480543/. (Accessed Jan 14, 2024).
58. Oyas, H, Holmstrom, L, Kemunto, NP, Muturi, M, Mwatondo, A, Osoro, E, et al. Enhanced surveillance for Rift Valley fever in livestock during El Niño rains and threat of RVF outbreak, Kenya, 2015-2016. PLoS Negl Trop Dis. (2018) 12:e0006353. doi: 10.1371/journal.pntd.0006353
59. Kang'ethe, EK, Ekuttan, CE, Kimani, VN, and Kiragu, MW. Investigations into the prevalence of bovine brucellosis and the risk factors that predispose humans to infection among urban dairy and non-dairy farming households in Dagoretti division, Nairobi, Kenya. East Afr Med J. (2007) 84:S96–100.
60. Nakadio, E, Kahariri, S, and Owiny, M. Evaluation of the Kenya Livestock and Wildlife Syndromic Surveillance System for Rift (2018).
61. Falzon, LC, Alumasa, L, Amanya, F, Kang’ethe, E, Kariuki, S, Momanyi, K, et al. One health in action: operational aspects of an integrated surveillance system for Zoonoses in Western Kenya. Front Vet Sci. (2019) 6:1–13. doi: 10.3389/fvets.2019.00252/full
62. Compston, P, Limon, G, Sangula, A, Onono, J, King, DP, and Häsler, B. Understanding what shapes disease control: An historical analysis of foot-and-mouth disease in Kenya. Prev Vet Med. (2021) 190:105315. doi: 10.1016/j.prevetmed.2021.105315
63. Njeru, J, Henning, K, Pletz, MW, Heller, R, and Neubauer, H. Q fever is an old and neglected zoonotic disease in Kenya: A systematic review. BMC Public Health. (2016) 16:297. doi: 10.1186/s12889-016-2929-9
64. Gerken, KN, Ndenga, BA, Owuor, KO, Winter, CA, Seetah, K, and LaBeaud, AD. Leveraging livestock movements to urban slaughterhouses for wide-spread Rift Valley fever virus surveillance in Western Kenya. One Health. (2022) 15:100457. doi: 10.1016/j.onehlt.2022.100457
65. Lichoti, JK, Kihara, A, Oriko, AA, Okutoyi, LA, Wauna, JO, Tchouassi, DP, et al. Detection of rift valley fever virus interepidemic activity in some hotspot areas of Kenya by sentinel animal surveillance, 2009-2012. Vet Med Int. (2014) 2014:10. doi: 10.1155/2014/379010
66. Owange, NO, Ogara, WO, Affognon, H, GP, B, Okuthe, S, Onyango-Ouma, W, et al. Occurrence of rift valley fever in cattle in Ijara district, Kenya. Prev Vet Med. (2014) 117:121–8. doi: 10.1016/j.prevetmed.2014.08.008
67. Jost, CC, Nzietchueng, S, Kihu, S, Bett, B, Njogu, G, Swai, ES, et al. Epidemiological assessment of the Rift Valley fever outbreak in Kenya and Tanzania in 2006 and 2007. Am J Trop Med Hyg. (2010) 83:66–72. doi: 10.4269/ajtmh.2010.09-0290
68. Wamwenje, SAO, Wangwe, II, Masila, N, Mirieri, CK, Wambua, L, and Kulohoma, BW. Community-led data collection using open data kit for surveillance of animal African trypanosomiasis in Shimba hills, Kenya. BMC Res Notes. (2019) 12:4198. doi: 10.1186/s13104-019-4198-z
69. Keshavamurthy, R, Thumbi, SM, and Charles, LE. Digital biosurveillance for zoonotic disease detection in Kenya. Pathogens. (2021) 10:783. doi: 10.3390/pathogens10070783
70. Matete, GO . Occurrence, clinical manifestation and the epidemiological implications of naturally occurring canine trypanosomiasis in western Kenya. Onderstepoort J Vet Res. (2003) 70:317–23. doi: 10.4102/ojvr.v70i4.296
71. Munyua, PM, Njenga, MK, Osoro, EM, Onyango, CO, Bitek, AO, Mwatondo, A, et al. Successes and challenges of the one health approach in Kenya over the last decade. BMC Public Health. (2019) 19:465. doi: 10.1186/s12889-019-6772-7
72. Munyua, P, Bitek, A, Osoro, E, Pieracci, EG, Muema, J, Mwatondo, A, et al. Prioritization of zoonotic diseases in Kenya, 2015. PLoS One. (2016) 11:e0161576. doi: 10.1371/journal.pone.0161576
73. Hassan, A, Muturi, M, Mwatondo, A, Omolo, J, Bett, B, Gikundi, S, et al. Epidemiological investigation of a rift valley fever outbreak in humans and livestock in Kenya, 2018. Am J Trop Med Hyg. (2020) 103:1649–55. doi: 10.4269/ajtmh.20-0387
74. Schneider, HP . OIE standards and tools on the quality of veterinary services: evaluation of the quality and performance of veterinary services using the OIE PVS tool. First OIE/FAO global conference on foot and mouth disease: the way towards global control, Asunción, Paraguay, 2009, pp. 24–26. (2011).
76. Rodríguez-Prieto, V, Vicente-Rubiano, M, Sánchez-Matamoros, A, Rubio-Guerri, C, Melero, M, Martínez-López, B, et al. Systematic review of surveillance systems and methods for early detection of exotic, new and re-emerging diseases in animal populations. Epidemiol Infect. (2015) 143:2018–42. doi: 10.1017/S095026881400212X
77. Dórea, FC, and Vial, F. Animal health syndromic surveillance: a systematic literature review of the progress in the last 5 years (2011–2016). Vet Med. (2016) 7:157–70.
78. Barrett, D . The potential for big data in animal disease surveillance in Ireland. Front Vet Sci. (2017) 4:276434. doi: 10.3389/fvets.2017.00150
79. Jiao, Z, Ji, H, Yan, J, and Qi, X. Application of big data and artificial intelligence in epidemic surveillance and containment. Intell Med. (2023) 3:36–43. doi: 10.1016/j.imed.2022.10.003
80. Lynn, T, Marano, N, Treadwell, T, and Bokma, B. Linking human and animal health surveillance for emerging diseases in the United States: achievements and challenges. Ann N Y Acad Sci. (2006) 108:108–11. doi: 10.1196/annals.1373.011
81. Thumbi, SM, Njenga, MK, Otiang, E, Otieno, L, Munyua, P, Eichler, S, et al. Mobile phone-based surveillance for animal disease in rural communities: implications for detection of zoonoses spillover. Philos Trans R Soc B Biol Sci. (2019) 374:20190020. doi: 10.1098/rstb.2019.0020
82. Karimuribo, ED, Mutagahywa, E, and Sindato, C. A smartphone app (AfyaData) for innovative one health disease surveillance from community to National Levels in Africa: Intervention in disease surveillance. JMIR Public Health Surveill. (2017) 3:e94. doi: 10.2196/publichealth.7373
83. Zinsstag, J, Utzinger, J, Probst-Hensch, N, Shan, L, and Zhou, XN. Towards integrated surveillance-response systems for the prevention of future pandemics. Infect Dis Poverty. (2020) 9:140. doi: 10.1186/s40249-020-00757-5
84. Bordier, M, Uea-Anuwong, T, Binot, A, Hendrikx, P, and Goutard, FL. Characteristics of one health surveillance systems: a systematic literature review. Prev Vet Med. (2020) 2018:104560. doi: 10.1016/j.prevetmed.2018.10.005
85. WHO . Surveillance and information sharing operational tool. (2024). Available at: https://www.who.int/initiatives/tripartite-zoonosis-guide/surveillance-and-information-sharing-operational-tool.
Keywords: surveillance, one health, early warning, animal health, integration
Citation: Kahariri S, Thumbi SM, Bett B, Mureithi MW, Nyaga N, Ogendo A, Muturi M and Thomas LF (2024) The evolution of Kenya’s animal health surveillance system and its potential for efficient detection of zoonoses. Front. Vet. Sci. 11:1379907. doi: 10.3389/fvets.2024.1379907
Edited by:
Taran Rai, University of Surrey, United KingdomReviewed by:
Kennedy Kapala Mwacalimba, Zoetis (United States), United StatesChrisborn Mubamba, Ministry of Fisheries and Livestock, Zambia
Copyright © 2024 Kahariri, Thumbi, Bett, Mureithi, Nyaga, Ogendo, Muturi and Thomas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samuel Kahariri, s.kahariri@cgiar.org; Lian F. Thomas, lthomas8@ed.ac.uk
†PRESENT ADDRESS: Lian F. Thomas Royal (Dick) School of Veterinary Studies, University of Edinburgh, Edinburgh, United Kingdom
†ORCID: Samuel Kahariri, https://orcid.org/0000-0002-3190-8543
Lian F. Thomas, https://orcid.org/0000-0001-8447-1210
 Samuel Kahariri
Samuel Kahariri