
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 21 February 2025
Sec. Veterinary Epidemiology and Economics
Volume 12 - 2025 | https://doi.org/10.3389/fvets.2025.1502933
This article is part of the Research Topic Utilizing Real World Data and Real World Evidence in Veterinary Medicine: Current Practices and Future Potentials View all 10 articles
Introduction: Chronic kidney disease (CKD) in canines is a progressive condition characterized by a gradual decline in kidney function. There are significant gaps in understanding how CKD is managed in canines and the full extent of its impact. This study aimed to characterize disease management of CKD and its impact on dogs, their owners and the veterinary healthcare system in the United States of America (United States).
Methods: Data were drawn from the Adelphi Real World Canine CKD Disease Specific Programme™, a cross-sectional survey of veterinarians, pet owners and their dogs with CKD in the United States from December 2022 to January 2024. Veterinarians reported demographic, diagnostic, treatment, and healthcare utilization data, for dogs with CKD. Owners voluntarily completed questionnaires, providing data about their dog, as well as quality of life and work-related burden using the Dog Owners Quality of Life, and the Work Productivity and Activity Impairment questionnaires. Analyses were descriptive and Cohen’s Kappa was used to measure agreement between owners and veterinarians.
Results: A total of 117 veterinarians provided data for 308 dogs, of which 68 owners also reported information. Discrepancies in recognizing symptoms of CKD in dogs, particularly excessive water consumption and urination, were identified between veterinary professionals and owners. Interventions for managing CKD in dogs focused on controlling symptoms and supporting kidney function through dietary modifications and medication. Owners of dogs with CKD reported minimal impact to overall work and activity impairment (10 and 14%, respectively). At diagnosis, 78.6% of dogs were International Renal Interest Society Stage I-II, and 21.5% were Stage III-IV. Regardless of CKD stage, owners strongly agreed that ownership provided them with emotional support and companionship. Regarding veterinary healthcare utilization, 95% of dogs were seen in general veterinary practices.
Discussion: These findings emphasize the value of real-world evidence in enhancing our understanding of CKD in companion animals and informs future strategy for the real-world diagnosis and treatment of CKD. The results also provide insights to the potential burden experienced by owners of dogs with CKD.
Chronic kidney disease (CKD) is a common renal disease in older dogs (1, 2), with an estimated prevalence of up to 3% in the general population (1–3) and up to 10% in hospitalized dogs (4). CKD is characterized by structural and/or functional impairment of one or both kidneys for at least 3 months that is irreversible and progressive (1, 4, 5). The International Renal Interest Society (IRIS) staging system defines 4-stages of CKD, ranging from stage I (mild loss of kidney function) to stage IV (end stage renal failure), which aids in diagnosis, management, and assessment of response to treatment (6).
In the early stages of the disease, increased urination and increased water consumption (polyuria and polydipsia, respectively) can occur due to the inability of the kidneys to regulate water homeostasis (2, 6). As the disease progresses, further symptoms develop such as loss of body condition, weight, and muscle mass, an unkempt appearance, hyporexia, anorexia, vomiting, lethargy, diarrhea, halitosis, azotemia, and ulcerative stomatitis and gastroenteritis, as well as electrolyte and acid–base imbalances (2–4, 7, 8). Disease progression varies, with factors such as anemia, low body condition score, proteinuria, hypertension, hypoalbuminemia, hyperphosphatemia, and IRIS stage playing key roles in prognosis (1, 2, 9–11). CKD may be diagnosed at a later stage, when renal damage is already severe and the prognosis is more guarded, due to limitations of renal function biomarkers, non-compliance with the recommended frequency of check-ups, the difficulty owners have in identifying early clinical signs, and their respective delay in seeking veterinary assistance (4, 6, 12, 13). It is thought that earlier diagnosis and treatment of clinical signs may slow progression and improve quality of life for canine patients (2).
Interventions for managing CKD in dogs focus on controlling symptoms and supporting kidney function. For example, the primary recommendation for management of CKD in dogs is dietary modification, with low-protein and phosphate-restricted diets being commonly recommended to reduce workload on the kidneys (14–16). In addition to this, fluid therapy and antiemetic use are recommended to maintain hydration and to treat vomiting and nausea, respectively (16). Similarly, other medications such as antihypertensive and antiproteinuric drugs can be prescribed to address potential CKD complications, such as systemic hypertension and renal proteinuria, respectively (17–20).
Currently, little is known about the burden experienced by owners of dogs with CKD, despite caregiver burden being a well-researched concept in human medicine. To date, studies have focused on the comparison between the burden carried by owners of ill pets and those with healthy pets, suggesting that increased stress, symptoms of anxiety and depression, and reduced quality of life were more prevalent in the former (21–23). These findings were, however, limited due to the lack of disease-specific data included in each study. It is reasonable to assume that the need for frequent veterinarian visits, testing and diagnostic exams, dietary modifications, lifestyle changes and medication administration could impact an owner’s time, mental load, and finances. However, data on this issue is still scarce in the literature, and our understanding of the owners’ burden related to specific canine diseases, such as CKD, remains limited.
Although randomized clinical trials (RCTs) are crucial to determine the effects of treatment in controlled settings, they often lack external validity (24, 25). Real-world evidence (RWE) can externally validate beyond RCT populations, increasing understanding of the clinical management of diseases compared to guideline recommendations (24–26), as well as the benefits and risks of treatments within a real-world patient population (27).
The Adelphi Real World Disease Specific Program™ (DSP) is a validated source of real-world data (28–31) and has been used to generate RWE in over 100 human disease areas. Findings from the DSP have furthered our understanding of disease management and treatment patterns (32, 33), patient quality of life (34), physician-patient disconnect (35), caregiver burden (36), and health-care resource utilization (37).
Although RWE generation is routinely used in human health, there is a lack of similar research in animal health, based on information provided by veterinarians and pet owners. To the authors’ knowledge, this study is the first of its kind in the animal health space, aiming to apply the DSP methodology to demonstrate the relevance of RWE in assessing the impact of canine CKD on affected dogs, their owners and the veterinary healthcare system.
Data were extracted from the Adelphi Real World Canine CKD DSP, a large cross-sectional survey, with retrospective data collection, of veterinarians, owners and their dogs with CKD and conducted in the United States of America (United States) between December 2022 and January 2024. The DSP methodology has been previously described (28, 29) and validated (30) and has shown to be representative and consistent over time (31).
Veterinarians were recruited to participate in the DSP following completion of a short screening questionnaire. Veterinarians were eligible if they were personally responsible for treatment decisions and management of a minimum of 1–4 dogs with CKD per month. Veterinarian participation was financially incentivized, with reimbursement upon survey completion according to fair market research rates.
Dogs were eligible for inclusion if they had been examined by a veterinarian, were at least 3 years old, had a confirmed diagnosis of CKD, and their primary caregiver was at least 18 years of age.
Upon recruitment, veterinarians completed a survey which included demographic data. Following this, veterinarians were invited to complete a questionnaire for their next five consecutively consulting canine patients, who visited them for routine care and met the eligibility criteria. The veterinarian-reported questionnaire contained questions on canine patient demographics, consultation history, diagnostic tests and monitoring of CKD, clinical signs, IRIS stage, prescribed CKD treatment, satisfaction and adherence to prescribed treatment, concomitant conditions, emergency visits, hospitalizations and other recommendations given.
Completion of the veterinarian-reported questionnaires was undertaken through consultation of existing canine patient clinical records, as well as the judgment and diagnostic skills of the respondent veterinarian, consistent with decisions made in routine real-world clinical practice.
The owner of each canine patient for whom the veterinarian completed a veterinarian-reported questionnaire was invited to voluntarily complete an owner-reported questionnaire. Owner-reported questionnaires were completed independently of the veterinarian and returned in a sealed envelope, ensuring the owners responses were kept confidential.
Owner-reported questionnaires contained questions on owner demographics, insurance details, positive and negative aspects of owning a dog with CKD using the Dog Owner Quality of Life (DOQOL) questionnaire (38), work-related burden using the Work Productivity and Activity Impairment (WPAI) questionnaire (39) and information about their dog’s condition, including clinical signs and their severity, level of satisfaction with their dog’s treatment, and other recommendations.
The DOQOL is a 10-item questionnaire that includes statements relating to three different factors: emotional wellbeing, social and physical activities, and stress and responsibility of dog ownership (38). Responses to each item are measured using a 7-point Likert scale which includes the following choices: strongly disagree, mostly disagree, somewhat disagree, neither agree or disagree, somewhat agree, mostly agree, and strongly agree (38). Results are reported on an item-by-item basis.
The WPAI measured impairment using four domains: absenteeism (work time missed), presenteeism (impairment at work), overall work impairment, and overall activity impairment (activities that do not include work) over the 7 days prior to survey (39). Scores are expressed as impairment percentages and range from 0 to 100, with higher scores indicating greater impairment (39).
Using a checkbox, owners provided consent to take part in the survey. No potential identifiable information was extracted during the study, so neither the veterinarians nor the pet owners could be directly identified. In addition, the generated data were aggregated prior to analysis and publication.
Data collection was undertaken in line with European Pharmaceutical Marketing Research Association guidelines and, therefore, did not require ethics committee approval (40). In addition, each survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act 1996 (41), and Health Information Technology for Economic and Clinical Health Act legislation (Health Information Technology (42).
Mean and standard deviation (SD) were calculated for continuous variables, and frequency and percentages for categorical variables.
Cohen’s Kappa was used to measure agreement of owner-reported and veterinarian-reported clinical signs. The Kappa statistic (κ) was determined between −1 and 1, depending on whether the level of agreement was interpreted as poor (<0.00), slight (0.00–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), or almost perfect (0.81–1.00) (43).
Descriptive analyses were performed on the positive and negative aspects of owning a dog with CKD. Dogs were grouped into two groups based on their current IRIS stage: early (I and II) and late (III and IV) stages.
All descriptive analyses were conducted in UNICOM Intelligence Survey Reporter version 7.5.1 (44) and all statistical analyses were conducted in Stata v18 (45). Missing data were not considered and, therefore, not all patients had information regarding all variables, leading to each one being described and analyzed separately. A significance level of p < 0.05 was used for all statistical analyses.
A total of 117 veterinarians from the United States participated in this study: 13 from the Northwest, 23 from the Midwest, 39 from the South, and 22 from the West. For the remaining 20 veterinarians, region was not stated. Most veterinarians had qualified between 1986 and 2019 and provided data on a total of 308 dogs with CKD. All owners were offered the chance to complete an owner-reported questionnaire, of which 68 agreed. Whenever relevant, the results are presented on a matched basis, associating data from veterinarian questionnaires and owner questionnaires for the same dogs. All other data is presented for the full dataset, unless stated otherwise (Table 1).
Of the 308 dogs included in this analysis, 173 were female, and mean (SD) age was 11.4 (3.4) years. Weight groupings were defined via the American Kennel Club guidelines and body condition groupings were taken from the World Small Animal Veterinary Association Body Condition Score Chart. Guidelines for weight groupings of mixed breed dogs were unavailable, which prevented 53 dogs being included in the weight analysis. Weight groupings for 255 dogs showed that 18% (n = 46) were underweight, 43% (n = 109) had ideal weight, and 39% (n = 100) were overweight. Body condition scores for 253 dogs showed 21% (n = 53) were below the ideal body condition score, 53% (n = 133) were in ideal body condition score and 27% (n = 67) were above the ideal body condition score (Table 2).
IRIS stage data were available for the full dataset (n = 308). At CKD diagnosis, 50% (n = 154) of all dogs were at IRIS stage II, with 29% (n = 88) at IRIS Stage I, 18% (n = 55) at IRIS Stage III and 4% (n = 11) at IRIS Stage IV. At the time of survey completion, the mean (SD) time since diagnosis (n = 295) was 11.7 (15.6) months and the proportion of dogs at IRIS stages I, II, III and IV was 21% (n = 66), 49% (n = 150), 21% (n = 66) and 8% (n = 26), respectively. Since first diagnosis, 79% (n = 244) of dogs had not progressed in IRIS stage (Table 3).
The mean (SD) number of comorbidities experienced per dog was 2.0 (1.6), with 18% (n = 56) of dogs having no comorbidities. The most reported comorbidities were periodontal disease (37%), degenerative joint disease (33%), and mitral valve disease (16%; Table 4).
Data on clinical signs were available for 253 dogs. Veterinarians reported that 42% of dogs experienced increased water consumption, 37% experienced increased or excessive urination, and 25% experienced unexplained weight loss (Table 5; Supplementary Table S1).
Owners of dogs with CKD also reported on clinical signs, resulting in 59 dogs having matched-base data available from veterinarians and owners (Table 5).
The mean (SD) number of clinical signs was 3.0 (3.1) and 2.8 (2.8) for veterinarians and owners, respectively. Both veterinary professionals and owners reported weakness/fatigue, increased urination, and increased water consumption as common clinical manifestations. Veterinarians also reported unexplained weight loss as a common finding, while owners listed loss of appetite and lethargy as the other most frequent abnormalities. Veterinary professionals and owners reported that 36% (n = 21) and 14% (n = 8) of dogs, respectively, showed no clinical signs suggestive of CKD (Table 5).
Cohen’s Kappa analysis identified a poor level of agreement between veterinarians and owners for osteoporosis and urinary tract infections, halitosis, and blood in urine. A slight level of agreement was identified between veterinarians and owners for increased/excessive water consumption (κ = 0.04), increased/excessive urination (κ = 0.09) and diarrhea (κ = 0.11). A fair level of agreement was identified for gastroenteritis (κ = 0.21), problems with vision (κ = 0.21), lethargy (κ = 0.22), hyporexia (κ = 0.26), vomiting (κ = 0.26), dehydration (κ = 0.30), weakness/fatigue (κ = 0.32), depression (κ = 0.37), and no clinical signs (κ = 0.27). Unexplained weight loss (κ = 0.44), anorexia (κ = 0.44) and unkempt appearance (κ = 0.45) had moderate levels of agreement between veterinarians and owners. There was a substantial level of agreement between veterinarians and owners for pale gums (κ = 0.66).
Owners reported clinical signs when veterinarians did not more often than the opposite. This was observed in the following clinical signs: osteoporosis, urinary tract infections, halitosis, increased water consumption, increased urination, diarrhea, gastroenteritis, problems with vision, lethargy, hyporexia, vomiting, weakness/fatigue, depressed mood, unexplained weight loss, unkempt appearance, and pale gums (Figure 1). In contrast, veterinarians reported more frequently dehydration, anorexia, blood in urine, and no clinical signs when owners did not, compared to the inverse situation (Figure 1).
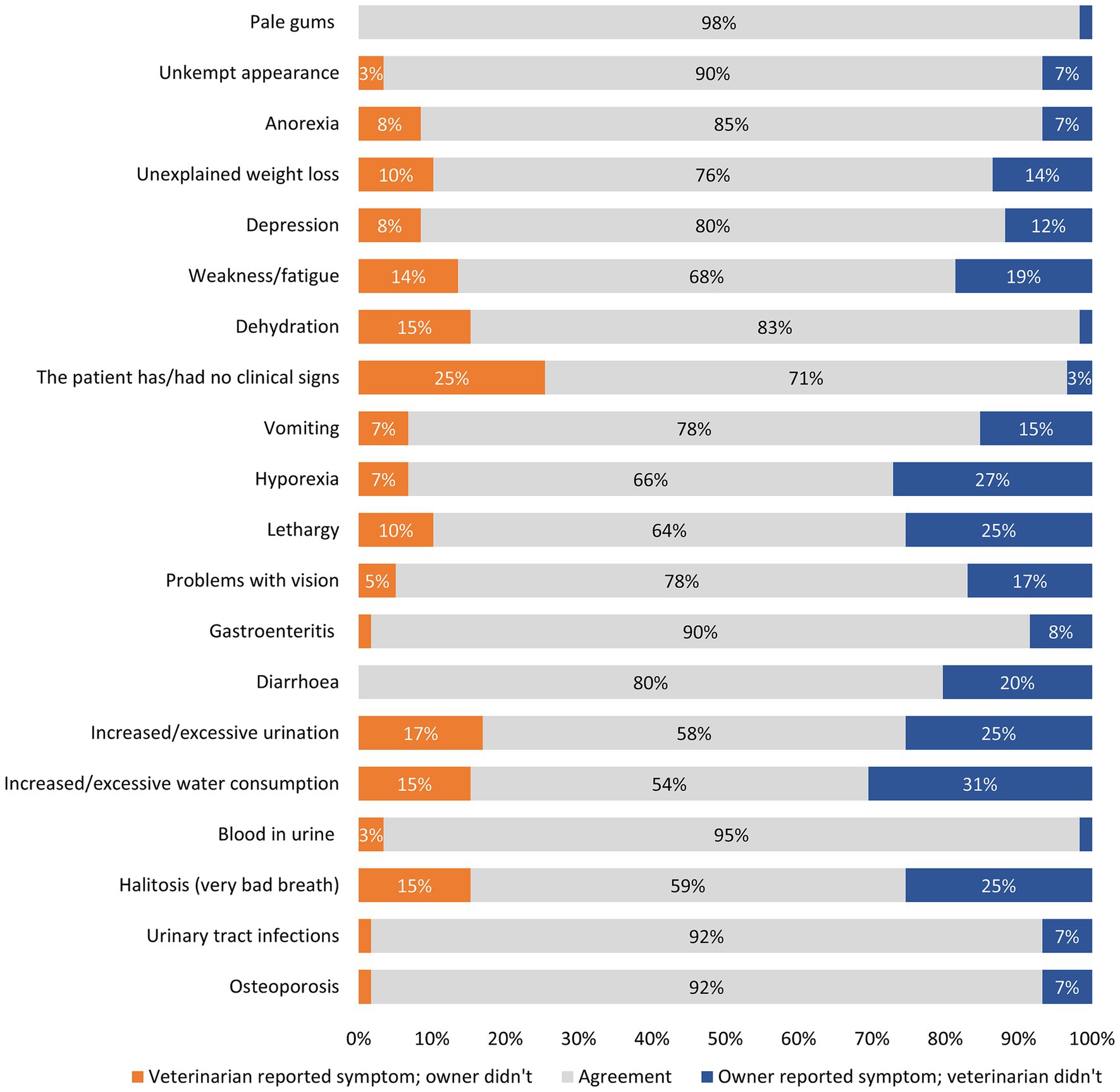
Figure 1. Level of agreement between veterinarian-reported and owner-reported clinical signs in dogs with CKD. *Please note that data points of less than 3% have been removed.
Data on veterinary and owner perceptions of the most problematic clinical signs were available on a matched base (n = 33): weakness/fatigue was reported for 30% of dogs by veterinarian professionals and for 42% of dogs by owners. Veterinarian professionals also recorded unexplained weight loss (30%) and dehydration (27%) as the other most problematic clinical signs for dogs, whereas owners felt that hyporexia (42%) and lethargy (39%) were the most challenging.
Data on treatment status is summarized in Table 6. Veterinarian-reported treatment status was available for 253 dogs and, among these, 36% (n = 90) were receiving treatment at the time of survey and 54% (n = 137) had never received treatment. Of the 90 dogs receiving treatment at the time of survey, the most prescribed therapies were maropitant (34%), subcutaneous fluid therapy (23%), aluminium hydroxide (18%), and capromorelin (18%). Other treatments received at the time of survey included benazepril (17%), enalapril (13%), omeprazole (12%), and telmisartan (11%). Treatment was initiated based on both the presence of clinical signs and test results in 71% (n = 64) of dogs, and the mean (SD) treatment duration was 7.3 (10.7) months (n = 80) at the time of survey. The most common issue with current treatment was progressive CKD (reported by 22% of veterinarians). Of 87 owners with veterinarian-perceived owner compliance, 28% were deemed to be completely adherent, 44% mostly adherent, 22% somewhat adherent, 5% little adherent, and 2% not adherent at all. A total of 30 owners reported information on adherence and medication administration, of which 80% stated they were always adherent, with 37 and 27% further reporting that administering the medication was easy or very easy, respectively. Of 6 owners who had difficulty administering medication to their dogs, 50% justified it by stating that their dog did not like the medication.
Overall, data regarding veterinarian-recommended changes were available for 253 dogs. Among these, a renal diet was recommended to 74%, increased water intake to 48%, and adding water to kibble to 34% (Table 7; Supplementary Table S1).
Matched-base data on dietary changes were available for 60 dogs. Veterinarians and owners reported that the dogs were prescribed a renal diet (82 and 88%, respectively), recommended to increase water intake (53 and 57%, respectively), and advised to avoid foods high in sodium (42 and 50%, respectively). The implementation of these recommendations were also evaluated: veterinarians and owners reported implementing a renal diet in 80 and 79%, respectively, increasing water intake in 91 and 74%, respectively, and avoiding foods high in sodium in 96 and 73%, respectively (Table 7).
Of the 68 owners who completed the questionnaire, the majority were female (71%), with a mean (SD) age of 51.5 (16.1) years and among those for whom employment status (n = 67) was available, 52% were in full-time employment (Table 8). Of all owners who responded, 8% (n = 5) had pet insurance that provided coverage for their dog’s CKD.
Matched-base data regarding the most challenging clinical signs according to the owners’ perspective (n = 33) are reported in Table 9. Veterinarians reported weakness/fatigue as the most problematic in 46% (n = 15) of cases. Increased urination (39%), unexplained weight loss (30%), and lethargy (30%) were also reported by veterinarians as difficult clinical signs for the owners’ management. Owners reported hyporexia, increased urination and weakness/fatigue as the most challenging to care for in 64, 42 and 39% of cases, respectively.
Work Productivity and Activity Impairment (WPAI) scores are reported in Table 10. Mean (SD) presenteeism (impairment at work) was 9% (13.8%), absenteeism (work time missed) was 2% (9.9%), and overall work impairment was 10% (17.4%). Mean (SD) overall activity impairment (activities that do not include work) was 14% (17.2%).
The positive and negative aspects of owning a dog with CKD were also explored based on IRIS Stage, so the sample was divided into IRIS Stage I-II (n = 44) and IRIS Stage III-IV (n = 20; Figure 2). Owners reported their level of agreement with each statement, ranging from strongly agree to strongly disagree.
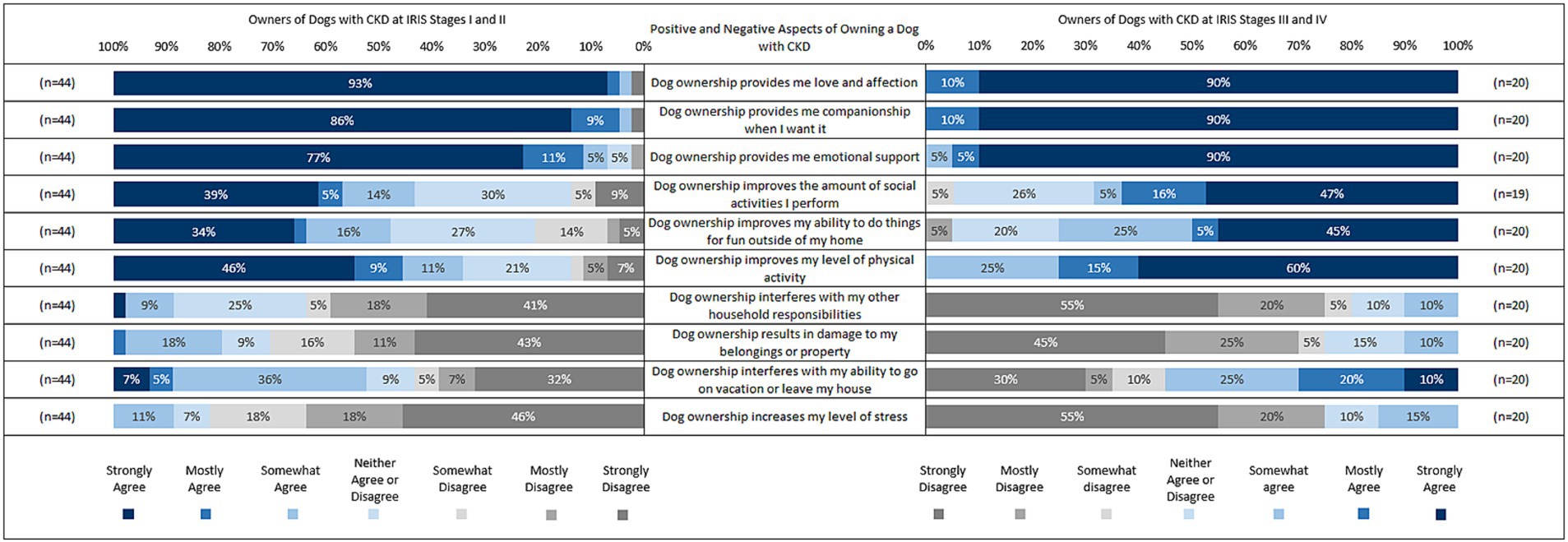
Figure 2. Positive and negative aspects of owning a dog with CKD by IRIS stage. *Please note that data points of less than 3% have been removed.
In relation to the emotional wellbeing aspects of dog ownership, owners of dogs at IRIS I-II reported agreement that dog ownership provided them with love and affection (98%), companionship when wanted (98%), and emotional support (93%), with 93, 86 and 77% reporting strong agreement, respectively. All owners of dogs at IRIS III-IV agreed that dog ownership provides them with love and affection, companionship when wanted, and emotional support, with 90% reporting strong agreement with each item, respectively.
In relation to the social and physical aspects of dog ownership, owners of dogs at IRIS I-II reported agreement that dog ownership improves the amount of social activities they do (57%), improves their ability to do things for fun outside the house (52%), and improves their level of physical activity (66%), with 39, 34, and 46% reporting strong agreement, respectively. Owners of dogs at IRIS III-IV reported agreement that dog ownership improves the amount of social activities they perform (69%), improves their ability to do things for fun outside the house (75%), and improves their level of physical activity (100%), with 47, 45, and 60% reporting strong agreement, respectively.
In relation to stress and responsibilities of dog ownership, owners of dogs at IRIS I-II reported disagreement that dog ownership interferes with their household responsibilities (64%), results in damage to their belongings/property (71%), interferes with their ability to go on vacation or leave their home (43%), and increases their level of stress (82%), with 41, 43, 32, and 46% reporting strong disagreement, respectively. Owners of dogs at IRIS III-IV reported disagreement that dog ownership interferes with their household responsibilities (80%), results in damage to their belongings/property (75%), interferes with their ability to go on vacation or leave their home (45%), and increases their level of stress (75%), with 55, 45, 30, and 55% reporting strong disagreement, respectively.
Data on veterinary healthcare resource utilization is summarized in Table 11. Most dogs were enrolled at general practice veterinary clinics and hospitals (95%) and the remainder were enrolled at private specialty referral veterinary clinics/hospitals, academic specialty referral veterinary clinics/hospitals, veterinary emergency clinics/hospitals and telemedicine.
In a 12-month period, the mean (SD) number of appointments per dog was 3.9 (6.5) and these appointments were most frequently with a veterinarian. Data on diagnostic tests/assessments were available for 253 dogs. The most frequently used tests/assessments to aid CKD diagnosis were serum creatinine (93%), blood urea nitrogen (86%) and complete blood count (75%) (Table 11). Other tests/assessments used to aid diagnosis included serum phosphorous (71%), serum calcium (61%), symmetric dimethylarginine (60%), urine sediment examination (45%), urine dipstick test for protein (40%), urine protein: creatinine ratio (23%), and blood pressure (20%) (Supplementary Table S1). Of 238 responses, the mean (SD) number of tests/assessments used to aid diagnosis of CKD was 8.3 (4.0). Data on tests/assessments used to monitor CKD were available for 240 dogs. A mean (SD) of 7.3 (4.8) tests/assessments were used, with serum creatinine used in 81% of cases, blood urea nitrogen in 73%, and clinical signs and physical examinations in 63% of patients. Other tests/assessments used to monitor included serum phosphorus (61%), symmetric dimethylarginine (50%), serum calcium (49%), blood pressure (25%), and urine protein:creatinine ratio (24%) (Supplementary Table S1).
Mean (SD) hospital admissions per dog in the last 12 months were 0.3 (0.6) as reported for 238 dogs (Table 11). Respondents reported the number of hospital admissions per dog as follows: 0 admissions for 80% of dogs, 1 admission for 16%, 2 admissions for 4%, and ≥ 3 admissions for less than 1%. Most inpatient hospitalizations (n = 48) were non-emergencies (63%), although 55% were unplanned (n = 42). Veterinarians reported that 67% (n = 30) of hospitalizations were day cases that did not require an overnight stay, and 83% (n = 39) of patients did not require intensive care during their stay.
To the authors’ knowledge, the Adelphi Real World Canine CKD DSP is the first study to apply this methodology in the veterinary healthcare setting, using RWE to characterize the management of CKD in canines, as well as the impact the disease has on owners and veterinary healthcare resource utilization.
In our study, the average age of dogs at the time of treatment and/or monitoring was 11.4 years, which is characteristic of this disease arising in older patients. Most dogs were diagnosed at IRIS stages I and II, with only 21% reported to have progressed since diagnosis. This is potentially due to CKD being identified at such early stages, allowing for prompt interventions that can slow progression (46, 47). Furthermore, although weight loss was reported in approximately 30% of dogs, most were either at ideal weight or overweight, and had an ideal or over ideal body condition. This could be explained due to most dogs included in the study being at IRIS stage I-II, as significant weight loss typically occurs at more advanced stages of CKD. Common clinical signs reported in this study included polydipsia, polyuria, weight loss, lethargy, and vomiting. This signalment is in line with previous research which showed that affected dogs tend to be diagnosed at an older age (1–3), in early IRIS stages (12), and with clinical manifestations and outcomes similar to those described in the present study (3, 7, 8). The authors recommend further research into the association between weight, body condition and IRIS staging to gain a more comprehensive understanding.
The findings of the current study, which highlight discrepancies in the identification and reporting of clinical signs between veterinarians and dog owners, align with existing research on veterinarian-client interactions. For example, among dogs that were considered dehydrated by veterinarians, only one-quarter were identified with this condition by the owners, highlighting gaps in owner awareness and understanding of clinical signs. This is significant as the failure to identify dehydration can lead to further disease complications such as the worsening of pre-renal azotemia (48), and clinical signs such as polydipsia and lethargy. Additionally, our results also indicate that veterinarians under-reported the number of dogs with clinical signs as well as the number of dogs experiencing problematic clinical signs, with owners reporting vomiting, hyporexia, and diarrhea more frequently than veterinarians. As current guidelines recommend that treatments should be prescribed for clinical signs management (16), it is important for veterinarians to correctly identify patients with these conditions, so that the appropriate treatment can be received. These discrepancies may reflect differences in how clinical signs are observed, prioritized, and discussed by veterinarians and owners. Previous studies have shown that veterinarians often employ a paternalistic communication style in veterinary practice (49), focusing on providing information rather than engaging owners in discussions, which can limit the owners’ ability to recognize subtle clinical signs (50, 51). Effective communication is crucial for improving patient outcomes. Motivational interviewing, a client-centered communication approach, has been recommended to improve alignment between veterinarians and owners by encouraging empathy, active listening and collaboration (52). Incorporating this communication style into veterinary consultation could address gaps in owner education and improve reporting of clinical signs. Taken collectively, these findings highlight the need for further owner education regarding the important clinical signs of CKD, and improved methods of communication between veterinary professionals and owners’ during appointments to improve outcomes for dogs suffering from CKD.
This study also gathered valuable real-world data on the management of canine CKD. Firstly, recommendations provided by veterinary professionals were consistent with those recommended within the literature and current guidelines (14–16). Furthermore, our data showed that owners were implementing the recommendations made by veterinarians, such as the use of a renal diet, which is considered paramount to CKD management (16). A previous study has demonstrated that feeding a renal diet significantly improves survival times and reduces the risk of uremic crises in dogs with CKD (15). However, adherence to renal diets can be challenging due to palatability issues or food refusal, which may limit the effectiveness of this intervention (19). When considering treatments, most dogs in this study had never received treatment for their disease. Of those who were receiving treatment, maropitant – typically used to treat nausea and vomiting – was the most common. Other common treatments were fluid therapy, aluminum hydroxide, and capromorelin. These interventions align with what is recommended in the treatment guidelines (16); however, it is important to note that evidence of efficacy or effectiveness of these interventions is often variable or lacking (20). For example, while controlled clinical studies have established the value of using aluminum hydroxide or other intestinal phosphate-binding agents in dogs with CKD, some aluminum-containing phosphate binders are not always well tolerated and must be given several times a day with meals which may impact on the acceptance of food (20) and exacerbate any adherence issues. Indeed, half of owners in the current study who reported difficulty administering medication, reported that this was due to their dogs’ refusal. To address these challenges, veterinary professionals could consider strategies such as educating owners on medication administration techniques, exploring alternate therapies with less frequent dosing schedules, and using compounded formulations or flavored medications (13, 53).
Previous studies have documented that owners often experience heightened stress, symptoms of anxiety and depression, and a reduced quality of life when managing the care of an ill pet (21–23). This burden can be exacerbated by the financial costs of treatment, time demands, and the emotional impacts of the pets’ decline in health (22). In this study, we found that owners experienced the greatest difficulty with their dog not eating (hyporexia). However, this was not identified as a most problematic aspect of care by veterinarians, highlighting a previously unknown aspect of burden for owners of dogs with CKD. Time constraints during veterinary appointments may further intensify this burden by preventing owners from fully discussing their concerns, leaving them feeling unheard and unsupported (49). Limited consultation time can also hinder veterinarians from addressing the emotional aspects of caregiving, such as guilt or anxiety, which are common among pet owners. While veterinarians are trained to focus on clinical outcomes and treatment protocols, these findings emphasize the need to place greater importance on understanding and addressing the emotional challenges faced by pet owners. Improving appointment structures to allow for more comprehensive discussions could help alleviate this burden and provide owners with support during disease management. Overall, this represents an important opportunity for veterinary practices to enhance both clinical care and owner wellbeing.
When work productivity and activity impairment were considered, no significant decline was observed in the surveyed owners. However, it is important to note that the sample sizes for this analysis were low and that further studies with larger sample sizes are recommended to increase our understanding on how CKD in dogs impacts the owners’ ability to work and perform daily activities.
Regarding owner attitudes and perceptions, according to our findings, owning a dog with CKD did not substantially impact on the owners’ dispositions, regardless of IRIS stage. Therefore, it can be considered that the bond an owner has with their dog and the joy associated with it (54, 55) outweighs most of the negative aspects of owning a dog with illness (21–23). Furthermore, previous research has also reported that owners who can anticipate their pet’s death typically view their pet more positively and less negatively (56). As CKD is a progressive and terminal condition in dogs, this may explain why owner’s attitudes toward their pet remain positive in the current study, regardless of IRIS stage. These findings suggest that owners may require additional guidance and support with managing challenging clinical signs, and further research within this topic may provide additional insights into the best approach to support the owners of these patients.
The utilization of veterinary healthcare resources for CKD primarily occurred in general veterinary practices, with most appointments taking place in this setting. Irrespective of appointment location, veterinary healthcare utilization was evident, with an average of 3.9 appointments and 7.3 tests administered per dog over a 12-month period. According to our findings, the number of hospitalizations for CKD was low, with most dogs not requiring inpatient care. However, it is important to note that the majority of dogs were diagnosed at IRIS stage I-II and were IRIS stage I-II at the time of survey, so therefore may not have reached the stage of disease where hospitalization was necessary. Hospitalization is typically reserved for stabilizing clinical signs, managing complications such as dehydration or uremia, and providing supportive care (4). A previous study demonstrated that all acute cases of CKD required hospitalization, with a median hospitalization time of 5 days, underscoring the need for inpatient care during acute exacerbations or in advanced stages of disease (4). However, to reduce the likelihood of hospitalization in dogs with CKD, proactive management strategies are essential. These include implementing dietary changes, ensuring consistent hydration, scheduling regular veterinary check-ups to allow for the early detection of complications, and educating owners on the warning signs of deterioration (6, 16). Therefore, by prioritizing proactive care and early intervention, veterinary professionals and pet owners can work together to mitigate the risks associated with CKD, reduce the need for hospitalization, and alleviate the burden on the veterinary healthcare sector.
This study had a number of strengths and limitations. First of all, although the eligibility criteria for participating veterinarians were broad and inclusive, their enrollment was influenced by willingness to complete the survey, as is the case in all studies of this nature. Additional research with a larger sample would be useful to explore relevant associations with WPAI and IRIS stage identified in this study and help to further understanding of the impact of owning a dog with CKD. The cross-sectional design of this study prevents any conclusions about causal relationships, even though identification of significant associations was possible. While recall bias is a common limitation of surveys, the data for these analyses were collected at the time of each patient’s appointment, which is expected to reduce this likelihood. In addition, veterinarians had access to patient medical records for data extraction. Despite such limitations, real-world studies play an important part in highlighting areas of concern that are not addressed in clinical trials, with the data in this study representative of current clinical practice at the time the survey was conducted.
In conclusion, RWD has the power to overcome a lack of external validity in clinical trials and, ultimately, enhance the management of a disease in a clinical setting. There were notable differences between veterinarians and dog owners in identifying the clinical signs of CKD, highlighting essential areas for improvement in owner awareness and education and veterinarian-owner communication. Caring for a dog with CKD did not substantially influence the owners’ attitudes toward their dog, regardless of disease stage, reflecting the strong emotional connection owners form with their pets. As a first-of-its kind animal RWE study using the approved DSP methodology, this study highlights the value of gathering RWD. The insights gained from this research will enhance our understanding of CKD in dogs, as well as the impact the condition has on owners, ultimately guiding improved management strategies for both veterinarians and pet owners.
The datasets presented in this article are not readily available because all data, i.e., methodology, materials, data and data analysis, that support the findings of this survey are the intellectual property of Adelphi Real World. Requests to access the datasets should be directed to James Jackson, amFtZXMuamFja3NvbkBhZGVscGhpZ3JvdXAuY29t.
Data collection was undertaken in line with European Pharmaceutical Marketing Research Association guidelines and, therefore, did not require ethics committee approval. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AW: Conceptualization, Writing – review & editing. DT: Conceptualization, Writing – review & editing. ML: Conceptualization, Writing – review & editing. SB: Formal analysis, Writing – review & editing. JJ: Writing – review & editing, Conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
Medical writing and editorial support on behalf of Adelphi Real World (Bollington, United Kingdom) was provided by Kate Revill of Kate Revill Medical Communications Ltd. and Charlotte Ensor of Adelphi Real World, under the guidance of the authors and in accordance with Good Publication Practice 2022 (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).
JJ, ML, SB, and DT are employees of Adelphi Real World.
The authors declare that this study received funding from Zoetis, Inc and analysis for this manuscript used data derived from the Adelphi Real World Canine Chronic Kidney Disease DSP which is proprietary to Adelphi Real World. The funder had the following involvement in the study: The funder reviewed the study documents to ensure they were appropriate for veterinary clinics and reviewed the final paper for publication and decision to publish.
AW declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1502933/full#supplementary-material
1. Polzin, DJ. Chronic kidney disease in small animals. Vet Clin North Am Small Anim Pract. (2011) 41:15–30. doi: 10.1016/j.cvsm.2010.09.004
2. Bartges, JW. Chronic kidney disease in dogs and cats. Vet Clin North Am Small Anim Pract. (2012) 42:669–92. doi: 10.1016/j.cvsm.2012.04.008
3. O'Neill, DG, Elliott, J, Church, DB, McGreevy, PD, Thomson, PC, and Brodbelt, DC. Chronic kidney disease in dogs in UK veterinary practices: prevalence, risk factors, and survival. J Vet Intern Med. (2013) 27:814–21. doi: 10.1111/jvim.12090
4. Dunaevich, A, Chen, H, Musseri, D, Kuzi, S, Mazaki-Tovi, M, Aroch, I, et al. Acute on chronic kidney disease in dogs: etiology, clinical and clinicopathologic findings, prognostic markers, and survival. J Vet Intern Med. (2020) 34:2507–15. doi: 10.1111/jvim.15931
5. Brown, CA, Rissi, DR, Dickerson, VM, Davis, AM, Brown, SA, and Schmiedt, CW. Chronic renal changes after a single ischemic event in an experimental model of feline chronic kidney disease. Vet Pathol. (2019) 56:536–43. doi: 10.1177/0300985819837721
6. International Renal Interest Society (IRIS). IRIS staging of CKD (modified 2023). (2023). Available at: http://www.iris-kidney.com/pdf/2_IRIS_Staging_of_CKD_2023.pdf (Accessed Aug 15, 2024).
7. Nelson, RW, and Couto, CG. Small animal internal medicine-E-book: Small animal internal medicine-E-book. Amsterdam, Netherlands: Elsevier Health Sciences (2019).
8. Bartlett, PC, Van Buren, JW, Bartlett, AD, and Zhou, C. Case-control study of risk factors associated with feline and canine chronic kidney disease. Vet Med Int. (2010) 2010:957570. doi: 10.4061/2010/957570
9. Rudinsky, AJ, Harjes, LM, Byron, J, Chew, DJ, Toribio, RE, Langston, C, et al. Factors associated with survival in dogs with chronic kidney disease. J Vet Intern Med. (2018) 32:1977–82. doi: 10.1111/jvim.15322
10. Parker, VJ, and Freeman, LM. Association between body condition and survival in dogs with acquired chronic kidney disease. J Vet Intern Med. (2011) 25:1306–11. doi: 10.1111/j.1939-1676.2011.00805.x
11. Jacob, F, Polzin, DJ, Osborne, CA, Neaton, JD, Kirk, CA, Allen, TA, et al. Evaluation of the association between initial proteinuria and morbidity rate or death in dogs with naturally occurring chronic renal failure. J Am Vet Med Assoc. (2005) 226:393–400. doi: 10.2460/javma.2005.226.393
12. Perini-Perera, S, Del-Ángel-Caraza, J, Pérez-Sánchez, AP, Quijano-Hernández, IA, and Recillas-Morales, S. Evaluation of chronic kidney disease progression in dogs with therapeutic management of risk factors. Front Vet Sci. (2021) 8:621084. doi: 10.3389/fvets.2021.621084
13. Maddison, J, Cannon, M, Davies, R, Farquhar, R, Faulkner, B, Furtado, T, et al. Owner compliance in veterinary practice: recommendations from a roundtable discussion. Companion Anim. (2021) 26:S1–S12. doi: 10.12968/coan.2021.0029
14. Chew, DJ. Chronic kidney disease (CKD) in dogs & cats-staging and management strategies. Va Vet Med Assoc. (2015) 1:1–22.
15. Jacob, F, Polzin, DJ, Osborne, CA, Allen, TA, Kirk, CA, Neaton, JD, et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic renal failure in dogs. J Am Vet Med Assoc. (2002) 220:1163–70. doi: 10.2460/javma.2002.220.1163
16. International Renal Interest Society (ISIS). IRIS treatment recommendations for CKD in dogs (modified 2023). (2023). Available at: http://www.iris-kidney.com/pdf/IRIS-DOG-Treatment_Recommendations_2023.pdf (Accessed Aug 15, 2024).
17. De Santis, F, Boari, A, Dondi, F, and Crisi, PE. Drug-dosing adjustment in dogs and cats with chronic kidney disease. Animals. (2022) 12:262. doi: 10.3390/ani12030262
18. Sparkes, AH, Caney, S, Chalhoub, S, Elliott, J, Finch, N, Gajanayake, I, et al. ISFM consensus guidelines on the diagnosis and Management of Feline Chronic Kidney Disease. J Feline Med Surg. (2016) 18:219–39. doi: 10.1177/1098612X16631234
19. Polzin, DJ. Evidence-based step-wise approach to managing chronic kidney disease in dogs and cats. J Vet Emerg Crit Care. (2013) 23:205–15. doi: 10.1111/vec.12034
20. Roudebush, P, Polzin, DJ, Adams, LG, Towell, TL, and Forrester, SD. An evidence-based review of therapies for canine chronic kidney disease. J Small Anim Pract. (2010) 51:244–52. doi: 10.1111/j.1748-5827.2010.00932.x
21. Spitznagel, MB, Gober, MW, and Patrick, K. Caregiver burden in cat owners: a cross-sectional observational study. J Feline Med Surg. (2023) 25:835. doi: 10.1177/1098612X221145835
22. Spitznagel, MB, Cox, MD, Jacobson, DM, Albers, AL, and Carlson, MD. Assessment of caregiver burden and associations with psychosocial function, veterinary service use, and factors related to treatment plan adherence among owners of dogs and cats. J Am Vet Med Assoc. (2019) 254:124–32. doi: 10.2460/javma.254.1.124
23. Spitznagel, MB, Jacobson, DM, Cox, MD, and Carlson, MD. Caregiver burden in owners of a sick companion animal: a cross-sectional observational study. Vet Rec. (2017) 181:321. doi: 10.1136/vr.104295
24. Rothwell, PM. Treating individuals 1 external validity of randomised controlled trials:“to whom do the results of this trial apply?”. Lancet. (2005) 365:82–93. doi: 10.1016/S0140-6736(04)17670-8
25. Bothwell, LE, Greene, JA, Podolsky, SH, and Jones, DS. Assessing the gold standard—lessons from the history of RCTs. N Engl J Med. (2016) 374:2175–81. doi: 10.1056/NEJMms1604593
26. Sherman, RE, Anderson, SA, Dal Pan, GJ, Gray, GW, Gross, T, Hunter, NL, et al. Real-world evidence—what is it and what can it tell us. N Engl J Med. (2016) 375:2293–7. doi: 10.1056/NEJMsb1609216
28. Anderson, P, Benford, M, Harris, N, Karavali, M, and Piercy, J. Real-world physician and patient behaviour across countries: disease-specific programmes - a means to understand. Curr Med Res Opin. (2008) 24:3063–72. doi: 10.1185/03007990802457040
29. Anderson, P, Higgins, V, Courcy, JD, Doslikova, K, Davis, VA, Karavali, M, et al. Real-world evidence generation from patients, their caregivers and physicians supporting clinical, regulatory and guideline decisions: an update on disease specific Programmes. Curr Med Res Opin. (2023) 39:1707–15. doi: 10.1080/03007995.2023.2279679
30. Babineaux, SM, Curtis, B, Holbrook, T, Milligan, G, and Piercy, J. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the disease specific Programme. BMJ Open. (2016) 6:e010352. doi: 10.1136/bmjopen-2015-010352
31. Higgins, V, Piercy, J, Roughley, A, Milligan, G, Leith, A, Siddall, J, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. (2016) 9:371–80. doi: 10.2147/DMSO.S120101
32. Vilarrasa, E, Molina-Leyva, A, Martín, V, Coak, E, Truman, I, Wallinger, H, et al. Unmet clinical needs, burden of disease and treatment patterns in hidradenitis suppurativa: real-world experience from Spain. JEADV Clin Pract. (2024) 3:347–57. doi: 10.1002/jvc2.237
33. Anstee, QM, Hallsworth, K, Lynch, N, Hauvespre, A, Mansour, E, Kozma, S, et al. Real-world management of non-alcoholic steatohepatitis differs from clinical practice guideline recommendations and across regions. JHEP Rep. (2022) 4:100411. doi: 10.1016/j.jhepr.2021.100411
34. Cooper, N, Kruse, A, Kruse, C, Watson, S, Morgan, M, Provan, D, et al. Immune thrombocytopenia (ITP) world impact survey (I-WISh): impact of ITP on health-related quality of life. Am J Hematol. (2021) 96:199–207. doi: 10.1002/ajh.26036
35. Penner, IK, Heras, VD, Jones, E, Hetherington, S, Karu, H, Chetlangia, R, et al. The presence and burden of cognitive issues: discordance between the perception of neurologists and people living with multiple sclerosis. Eur J Neurol. (2024) 32:e16234. doi: 10.1111/ene.16234
36. Martinez-Martin, P, Skorvanek, M, Henriksen, T, Lindvall, S, Domingos, J, Alobaidi, A, et al. Impact of advanced Parkinson’s disease on caregivers: an international real-world study. J Neurol. (2023) 270:2162–73. doi: 10.1007/s00415-022-11546-5
37. Giunti, P, Greenfield, J, Stevenson, AJ, Parkinson, MH, Hartmann, JL, Sandtmann, R, et al. Impact of Friedreich’s Ataxia on health-care resource utilization in the United Kingdom and Germany. Orphanet J Rare Dis. (2013) 8:38. doi: 10.1186/1750-1172-8-38
38. Oyama, MA, Citron, L, Shults, J, Cimino Brown, D, Serpell, JA, and Farrar, JT. Measuring quality of life in owners of companion dogs: development and validation of a dog owner-specific quality of life questionnaire. Anthrozoös. (2017) 30:61–75. doi: 10.1080/08927936.2016.1228774
39. Reilly, MC, Zbrozek, AS, and Dukes, EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics. (1993) 4:353–65. doi: 10.2165/00019053-199304050-00006
40. EphMRA Code of Conduct. (2019). Available at: https://www.ephmra.org/media/2811/ephmra-2019-code-of-conduct-doc-f.pdf (Accessed Jun 20, 2024).
41. Summary of the HIPAA Security Rule. (2024). Available at: https://www.hhs.gov/hipaa/for-professionals/security/laws-regulations/index.html (Accessed Jun 20, 2024).
42. HITECH Act Enforcement Interim Final Rule. (2024). Available at: https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcementinterim-fnal-rule/index.html. (Accessed Jun 20, 2024).
43. Landis, JR, and Koch, GG. The measurement of observer agreement for categorical data. Biometrics. (1997) 33:159–74. doi: 10.2307/2529310
46. Leibetseder, JL, and Neufeld, KW. Effects of medium protein diets in dogs with chronic renal failure. J Nutr. (1991) 121:S145–9. doi: 10.1093/jn/121.suppl_11.S145
47. Finco, DR, Brown, SA, Crowell, WA, Duncan, RJ, Barsanti, JA, and Bennett, SE. Effects of dietary phosphorus and protein in dogs with chronic renal failure. Am J Vet Res. (1992) 53:2264–71. doi: 10.2460/ajvr.1992.53.12.2264
48. Foster, JD. Canine chronic kidney disease. Current Diagnostics and Goals for Long-Term Management Today’s Veterinary Practice, pp. 21–26. (2013).
49. Janke, N, Coe, JB, Bernardo, TM, Dewey, CE, and Stone, EA. Pet owners’ and veterinarians’ perceptions of information exchange and clinical decision-making in companion animal practice. PLoS One. (2021) 16:e0245632. doi: 10.1371/journal.pone.0245632
50. Groves, CNH, Coe, JB, Sutherland, KA, Bauman, C, and Grant, LE. Clients prefer collaborative decision-making with veterinarians regardless of appointment type. J Am Vet Med Assoc. (2024) 263:1–11. doi: 10.2460/javma.24.06.0421
51. Janke, N, Coe, JB, Sutherland, KA, Bernardo, TM, Dewey, CE, and Stone, EA. Evaluating shared decision-making between companion animal veterinarians and their clients using the observer OPTION instrument. VetRecord. (2021) 189:e778. doi: 10.1002/vetr.778
52. Enlund, KB, Jennolf, E, and Pettersson, A. Small animal veterinarians’ communication with dog owners from a motivational interviewing perspective. Front Vet Sci. (2021) 8:772589. doi: 10.3389/fvets.2021.772589
53. Odom, TF, Riley, CB, Benschop, J, and Hill, KE. Factors associated with medication noncompliance in dogs in New Zealand. Animals. (2024) 14:2557. doi: 10.3390/ani14172557
54. Janevic, M, Solway, E, Malani, P, Kirch, M, Kullgren, J, and Connell, C. National poll on healthy aging: How pets contribute to healthy aging. Available at: https://deepblue.lib.umich.edu/bitstream/handle/2027.42/148428/NPHA_Pets-Report_FINAL-040319.pdf?sequence=3&isAllowed=y (Accessed Aug 15, 2024).
55. Kushner, RF, Blatner, DJ, Jewell, DE, and Rudloff, K. The PPET study: people and pets exercising together. Obesity. (2006) 14:1762–70. doi: 10.1038/oby.2006.203
56. Brkljačić, T, Sučić, I, Lučić, L, Glavak Tkalić, R, and Kaliterna, LL. The beginning, the end, and all the happiness in between: pet owners’ wellbeing from pet acquisition to death. Anthrozoös. (2020) 33:71–87. doi: 10.1080/08927936.2020.1694313
Keywords: real-world, canine, chronic kidney disease, owner burden, disease management, cross-sectional study, animal health, veterinarian-owner communication
Citation: Wright A, Taylor D, Lowe M, Barlow S and Jackson J (2025) Replicating the real-world evidence methods available in human health to assess burden and outcomes for dogs with chronic kidney disease, their owners, and the veterinary healthcare system in the United States of America. Front. Vet. Sci. 12:1502933. doi: 10.3389/fvets.2025.1502933
Received: 27 September 2024; Accepted: 28 January 2025;
Published: 21 February 2025.
Edited by:
Ioannis Magouras, University of Bern, SwitzerlandReviewed by:
David S. Gardner, University of Nottingham, United KingdomCopyright © 2025 Wright, Taylor, Lowe, Barlow and Jackson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James Jackson, SmFtZXMuSmFja3NvbkBhZGVscGhpZ3JvdXAuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.