
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Vet. Sci. , 10 January 2024
Sec. Animal Reproduction - Theriogenology
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1347482
This article is part of the Research Topic Perspectives in The Biotechnology of Artificial Insemination in Ruminants View all 7 articles
Male infertility is frequently caused by idiopathic or unexplained reasons, resulting in an increase in demand for assisted reproductive technologies. In buffaloes, more than in other animals due to reproductive hardiness, successful fertilization needs spermatozoa to effectively transit the female reproductive system to reach the oocyte. This mechanism naturally picks high-quality sperm cells for conception, but when artificial reproductive technologies such as in vitro fertilization, intracytoplasmic sperm injection, or intrauterine insemination are utilized, alternative techniques of sperm selection are necessary. Currently, technology allows for sperm sorting based on motility, maturity, the lack of apoptotic components, proper morphology, and even sex. This study provides current knowledge on all known techniques of sperm cell sorting in buffaloes, evaluates their efficiency, and discusses the benefits and drawbacks of each approach.
The domestic buffaloes are a vital livestock resource with soaring economic importance especially for developing countries. The world’s buffalo population grew by 0.8% annually between 1991 and 2002 and by 1.3% annually between 2002 and 2017, suggesting a recent rise in interest in buffalo husbandry (1). However, reproduction techniques applied in this sector are facing several challenges such as: silent heat (2, 3) and unsatisfactory oestrus detection (4–6), anestrus (2), (7) seasonal infertility (8, 9), longer calving intervals (10, 11), delayed puberty (12, 13) and specific low number of primordial follicles (14) along with the high rate of atresia and apoptosis (13, 15). While these limiting characteristics specific to the female component can be diminished by applying different synchronization protocols (16–18), the use of high-quality semen has to be also addressed for the optimisation of fixed time insemination. Recently, important countries for the buffalo industry have raised concerns regarding the decreased availability of sires with high genetic quality and poor capability of some individuals to qualify for semen collection (19), highlighting the importance of semen selection and preservation in this sector. Along with several enrichment protocols (20–23), sperm separation techniques have emerged as valuable tools in the field of reproductive biology and assisted reproduction. Furthermore, the development of techniques that can effectively separate the motile sperm fraction from the other components of semen is essential to the success of assisted reproductive technology (ART). Reproductive biotechnologies have a unique role in improving livestock herds in contemporary high-efficiency livestock breeding. They also provide global access to new genetics, which benefits biodiversity conservation in animal research (24).
Even though both buffaloes and cattle bulls are classified as large ruminants (25), there are some anatomical differences and variations in terms of sperm characteristics, concerning the volume (mL) (2.958 ± 0.18, respectively 4.038 ± 0.22), concentration (1.678 × 109, respectively 1.736 × 109) (26), pH (6.79 ± 0.01, respectively 6.80 ± 0.01), sperm density (2.63 ± 0.08, respectively 2.57 ± 0.07) and abnormal sperm (7.64 ± 0.36, respectively 8.86 ± 0.38) (27). Moreover, the capacity to fertilize oocytes in vitro and the subsequent in vitro growth of embryos vary significantly between buffalo and cattle bulls’ sperm (28). The normal colour of buffalo semen is creamy white with a clean aspect (29). The mean volume of the ejaculate in Romanian buffalos, already genetically characterized (30) was found to be 4.07 (± 0.02) mL (29), which is less than in other indigenous buffalo breeds: Banni 4.09 (± 1.59), Bhadawari 4.11 (± 1.57), Jaffarabadi 5.10 (± 1.80), Murrah 4.48 (± 1.87), Pandharpuri 4.79 (± 1.80), Surti 4.68 (± 1.73) (31). An important difference was observed between the sperm pH of Romanian buffalo (29) that was 5.81 (± 0.06) and the swamp buffalo bulls in Thailand which was 7 during winter and summer and 6.9 during the raining season (32). The mean motility sperm percentage in Romanian buffalo was 71.5 (± 0.03) (33), being slightly lower than in Asian buffalo (75.2 ± 1.3) (32). In contrast to the motility, sperm concentration in the local buffalo (33) is higher (1.65 × 109 per mL) than that of Thai (1.1 × 109 per mL) (32).
The swim-up method (SU) along with the density gradient centrifugation (DGC) represent the most commonly used separation methods for livestock ART applications (33). Both of them are able to quickly and economically eliminatethe low-quality spermatozoa, unwanted cells, and bioactive particles, resulting in the isolation of longer telomere spermatozoa (34) that can represent an indicator of unimpaired spermatogenesis (35).
Glass wool filtration (GWF) uses tightly packed glass wool fibers to separate immotile sperm cells from motile spermatozoa, the self-propelled mobility of the spermatozoa and the filtering effect of the glass wool being the fundamental components of this sperm separation technology (36).
Sephadex gel filtration (SGF) demonstrates the ability to confine spermatozoa with compromised acrosomes or damaged membranes inside a particular dextran gel column (37).
Magnetic-activated cell sorting (MACS) is one of the most efficient methods of isolating the spermatozoa with deteriorated membranes (apoptosis) in order to provide a high-quality sperm fraction (38). This paper depicts the proposed sperm separation methods utilized in buffaloes along with their effect on semen quality and ART success.
A systematic exploration of the literature was conducted using Pubmed database (1975-2022). The following search strategy was implemented into Pubmed: “semen separation techniques buffalo,” “assisted reproductive technology animals,” “swim-up method buffalo density gradient centrifugation buffalo semen,” “glass wool filtration buffalo,” “sephadex gel filtration buffalo,” and “magnetic-activated cell sorting sperm.”
The electronic search of Pubmed returned 297 papers. Following reading the titles and abstracts, 107 were found to be related to semen assessment and different techniques separation. Tabel 1 provides the descriptive data of all studies for the review highlighting the semen separation methods, species, in vivo, and in vitro semen evaluation.
Different density gradients have been tested, in order to determine their suitability for bovine sperm selection, retrieving higher motility samples with preserved gene expression, acrosomal membrane and DNA integrity (33). Percoll gradient centrifugation relies on the higher density of the nucleus within normal spermatozoa which permits further deposition of the stated in the elevated density region (39). Moreover, it was proven in bovines that highly motile spermatozoa will deposit faster due to the alignment to the centrifugal forces (40).
The ability of Percoll density gradient method to augment X-bearing viable sperm by up to 70% (41, 42) while preserving the sperm membrane and acrosome intact (43), supported the use of density gradient for sexed semen in livestock reproductive biology. In buffaloes, enriched semen after the use of Percoll was trialed for X and Y sperm separation (39, 44, 45) as well, the consequent effect on sperm quality being studied in comparison with filtration techniques (46) or classic swim-up (47).
Through the use of 45 and 90% Percoll solutions, buffalo thawed frozen semen were processed resulting in a low recovery rate with concentrations after centrifugation reaching 4.7 ± 1.5 × 106/mL, which is corresponding to 29.57 times decrease compared to mean original post thaw motility (47). However, the mean motility of the isolated spermatozoa was higher edging 63.1 ± 9.0 %, compared to pre-centrifugation motility rates of 38.5 ± 4.9 % (47). Moreover, significant enhancement of membrane integrity (MI) and acrosome integrity (ACI) were noted (70.5 ± 7.6 % MI; 70.6 ± 11.1 % ACI) (47) (see Table 1).
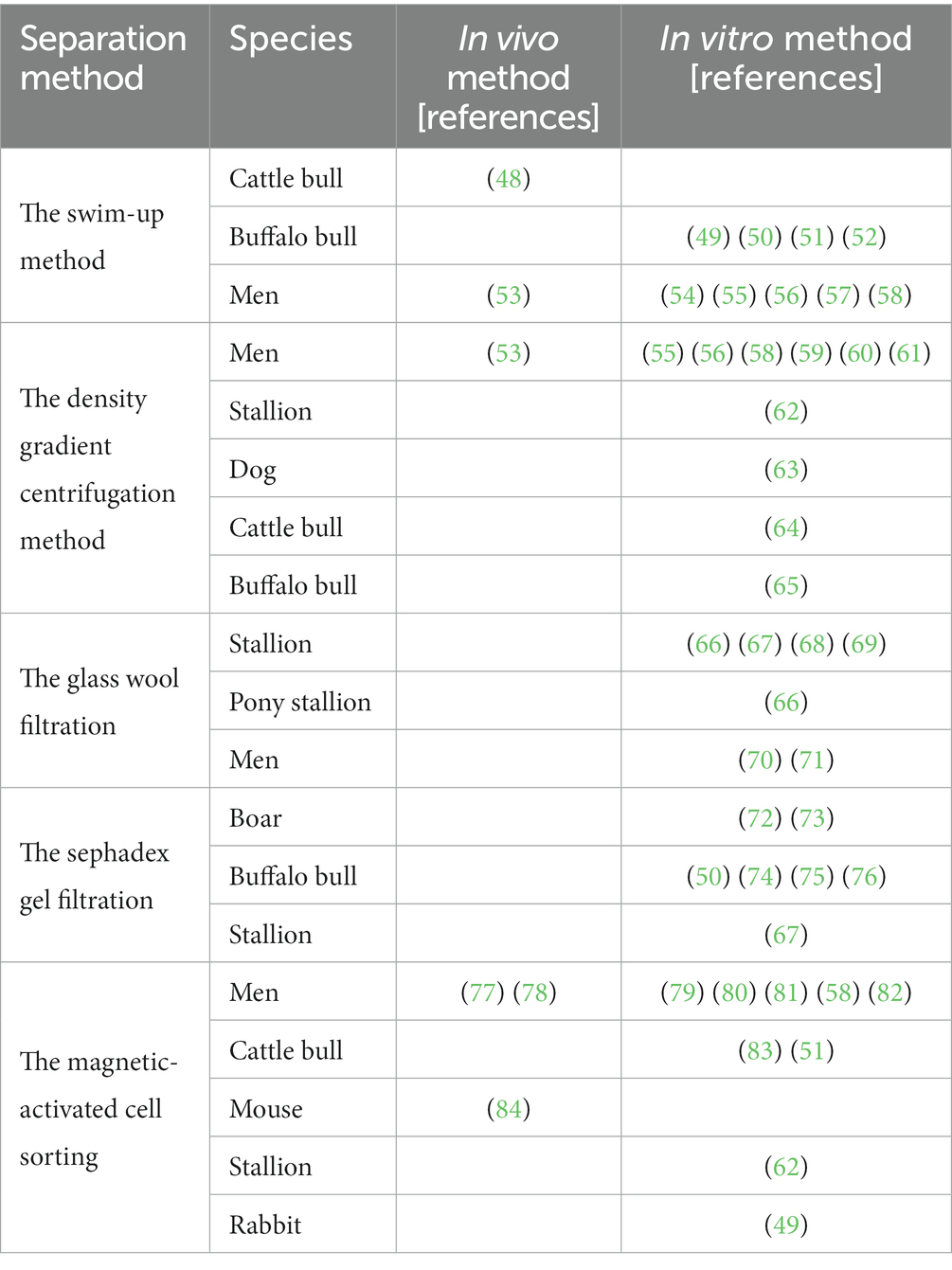
Table 1. Classification of scientific publications on sperm separation techniques according to the method used, the species, and the in vivo or in vitro method.
Similarly, using colloidal suspensions of silica particles, three discontinuous gradient preparations (45% & 95, 65% & 95 and 45%, 65, 95%) were investigated in order to assess the effectiveness of separation (85). For all the gradients formely listed, the values for the motility rates were 77.13 ± 7.9%, 87.38 ± 7.9%, respectively 96.40 ± 1.9% and the recovery rates with concentrations of 15.00 ± 0.8%, 10.46 ± 5.6%, respectively 9.00 ± 4.9. It was observed that the motility rates were higher when using three-layer centrifugation (96.40 ± 1.9%) while the recovery rate was better for the 45-95% formulationpossibly showing more exclusive isolation ability of the three-layered method in terms of motility (85).
The effect of density gradient separation on in vitro fertilization (IVF) was surveyed through the oocyte cleavage rate of the obtained samples. Both experiments (47, 85) discussed also the bull effect when interpreting results. This impact might be generated by changes in sperm capacitation across bulls throughout the fertilization process (86), with differences in fertilizing capability dependent on sperm penetration kinetics (87). Spermatozoa that capacitated and fertilized more quickly produced zygotes that cleaved and matured more quickly than those that cleaved later (88). However, cleavage rates varied subsequently to the gradient formulation from 55.6 % for 45-90 Percoll (86) to 69.1 ± 2.04 % for three layered 45-65-95 preparation (86) (Table 2).
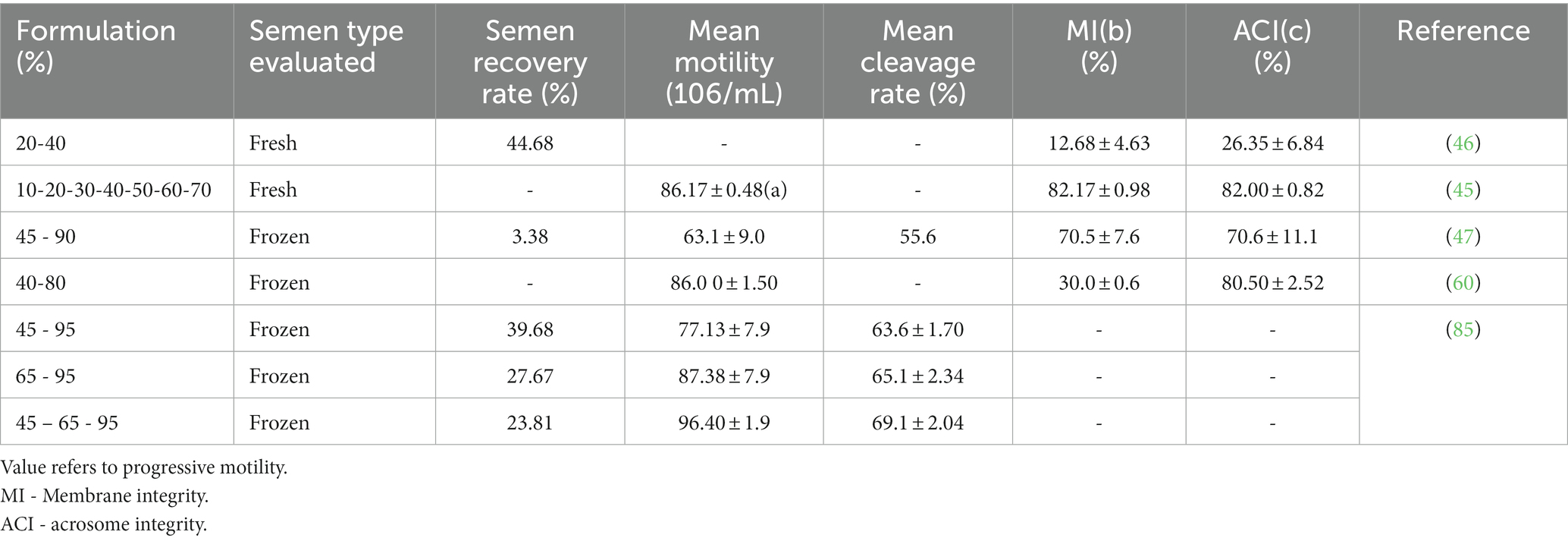
Table 2. Effect of different colloidal preparations on semen quality and fertilizing ability of buffalo semen.
A seven layered preparation (70, 60, 50, 40 30, 20 and 10%) was proposed for fresh, highly mobile (mass motility > + 3 and progressive motility >70%) buffalo semen in order to obtain sexed material (the percentage of female fetuses increased by 66.66%) (45). Secondary to the stated experiments, while selecting X bearing spermatozoa, the protocol was beneficial also for the overall quality of the semen, higher progressive motility, and membrane and acrosome integrity (45).
In terms of morphology, while using Percoll as a 40-80% double layered method on frozen semen, lower abnormalities were observed (7.8 ± 0.7 %) in comparison to the control groups (18.8 ± 1.94 %) and even to other separation techniques such as swim up (8.8 ± 0.57 %) or Sperm/Sperm-Tyrode’s Albumin Lactate Pyruvate (sp-TALP) washing (13.4 ± 1.23 %) (60).
It is noteworthy that there are studies in which volume and density of Percoll gradient used was much higher (2 mL Percoll 90% solution) (44) than in others (0.5 mL Percoll 45% solution) (48). Therefore, it’s possible that the higher height and density of the column provided an additional obstacle to the sperm cells’ movement, causing them to come into prolonged contact with Percoll (44). This might have caused certain changes, like a higher rate of capacitation and an earlier acrosome reaction (89). Thus, it is plausible to hypothesize that the use of Percoll gradients with smaller volumes actually results in lesser acrosomal damages. Therefore, while using density gradients with high volumes in assisted reproduction, we need to take into account the possibility of such acrosomal damage (44). If a double DGC is used, the first DGC in a normal semen readily separates motile sperm, whereas the second DGC leads to the assembly of sperm with a restricted ability to move, including immotile sperm. Therefore, it is more essential and more effective to use a second DGC to separate sperm from inadequate semen samples (56).
Primarily described in 1984 (90), SU follows a basic principle regarding the capacity of motile spermatozoa to migrate toward a cell-free medium usually placed above the sample (91). The SU’s applicability and effect on sperm quality was assessed in buffaloes, some important traits for ART applications being even superior to other separation techniques (50, 60, 85, 92).
Previous experiments showed that SU separated sperm had superior motility (69.1 ± 8.0 %) and significantly higher MI (77.3 ± 8.9 %) when compared to DGC, but the recovery rates were generally lower (85). Moreover, based on cleavage rate analysis SU proved to be more feasible for IVF (significant differences between cleavage rate and cleavage index p < 0.05) (85). Another paper depicted SU as being deficient in ACI preservation, registering a lower percentage of total intact acrosomes in comparison with DGC (68.2 ± 3.21 % vs. 80.5 ± 2.52 %) (60).
In terms of progressive motility, SU was detrimental to different filter separation techniques such as glass wool filtration (GWF) or Sephadex gel filtration (SGF), returning lower values in post-thaw buffalo sperm samples 55.83 ± 1.53 % (SGF 68.33 ± 1.05 %; GWF 65.83 ± 1.54 %) (92). The same pattern was observed when assessing the effect of the three methods on sperm viability and further livability (92). Secondary to oocyte insemination, SU cleavage rates were this time approximatively similar to filter separation techniques, bordering the control samples (SU 21.33 ± 1.94 %; control 21.98 ± 3.00 %) (92). Similar results were reported by older data as well, the recovery rate of motile spermatozoa after SGF being significantly higher than after SU (50).
For the means of sperm sexing, a modified SU method has been validated, the recovery rate for X bearing spermatozoa (5.19 ± 2.04 %) being significantly superior to Y chromosome bearing spermatozoa (0.70 ± 0.15 %) (52). Although MI and AI rates were higher in both X and Y categories when compared to the control, the progressive motility of the non-separated samples was higher (85.00 ± 0.57 % > 76.33 ± 1.11 % X-sorted and 69.67 ± 0.66 Y-sorted) (52). However, those results were obtained prior to freezing, and post thawed samples which were subjects to the modified SU were, in fact, superior in terms of progressive motility (54).
The percentages of DNA fragmentation were 18.30 ± 10.8 in raw samples, 6.6 ± 5.7 after direct SU, 12.9 ± 9.9 after density gradient (DG), 3.7 ± 4.0 after density gradient followed by swim-up (DG-SU), and 4.2 ± 3.8 after pellet SU, the last one being one of the best options in the treatment of semen during IVF/ICSI due to the low cost and reduced time (54).
The protocol of GWF consists in taking a tuberculin syringe or cutting a 1 mL plastic syringe was cut at the 0.6 mL mark and adding 15 mg of glass wool. In order to remove loose glass fibers or any debris, the syringe was flushed with different medium (Ham’s F-10 or TH3) after being positioned perpendicularly in a test tube. An aliquot of the ejaculate was centrifuged to wash it with medium after it had been liquefied. Re-suspended in 1 mL medium, the pellet was laid onto the wet glass wool and allowed to gravity-filter itself without the use of suction or pressure (93, 94).
When spermatozoa are dead or damaged, their plasma membranes alter, which is followed by their binding with glass fibers, producing the desired effect in GWF (95). The type of glass wool used has a direct impact on how well this technique works (36). This method has been proven to be adequate for the recovery of high-quality semen in stallions, results being similar or even better when compared to colloid centrifugation (96). A similar outcome was reported in buffaloes, GWF being able to retrieve actually higher cleavage rates (28.97 ± 4.07 %) than the SU method (21.33 ± 1.94 %), in embryos generated after oocyte insemination (92). Older data states that using GWF more motile spermatozoa may be recovered detrimental to SU (95 ± 3.7 vs. 33 ± 5.5 %) (50). Moreover, the general recovery rate of total and motile spermatozoa was higher for GWF than SGF or SU (92). In addition, a larger proportion of live cells with increased mitochondrial activity and a functioning membrane could be chosen thanks to the usage of glass wool (66).
By combining standard GWF with annexin V binding, its effectiveness can be further enhanced (97). Glass wool filtration, like density gradient centrifugation, uses the entire volume of the ejaculate in comparison to swim-up or migration-sedimentation methods, yielding a far higher total number of motile spermatozoa (36, 70). Furthermore, given its affordability, ease of use, and superior responsiveness to viable spermatozoa of equine semen, its usage ought to be promoted (66).
Filtration using Sephadex columns is an additional method of sperm separation, which was trialed in different species including rams (98), bulls (41), buffaloes (49, 76), boars (49) and stallions (67, 74). With this purpose different pore sizes were used ranging from G-10 to G-200, SGF exhibiting the capacity to trap the spermatozoa with damaged membranes or defective acrosomes within the specific dextran gel column (37, 73). Depending on the size of the molecules, there are more types of Gel (G-10, 15, 25, 50, 75, 100, 150, 200), G-10 being for small molecules (<700 Da) and G-200 for larger molecules (5000-250000 Da).
In buffaloes, comparing filtered and unfiltered semen, it has been shown that the percentage of progressive motile sperm and live sperm increased significantly thanks to the use of SGF (49, 74, 76, 99).
Comparative surveys between separation methods or even distinct Sephadex grades were carried out in buffaloes during the last 20 years, the results being still subject to discussion. Even if G-75 has been recommended for wider use in buffaloes (99), some authors considered G-100 as also suitable, being superior in improving semen quality when compared to G-200 or G-15 (37). Moreover, it was proven that the use of G-75 and G-100 columns did not result in significant variations regarding the mean recovery rate (79%) (100). Conversely, other publications suggested that using the G-15 formulation, higher sperm viability could be obtained in thawed buffalo semen (52), G-75 being more effective than G-100 thanks to superior acrosome integrity rate, motility and morphology (101). However, the results of Sephadex filtering may be influenced by the type of buffer that is used, tris citric acid buffers being more appropriate when compared to N-tris-(hydroxymethyl) methyl-2-aminoethanesulfonic acid (TES) or sodium citrate (37).
An additional approach was represented by the use of Sephadex filters enriched with ion-exchangers (76). Comparative use of the conventional G10 filter alone and the up-stated modified technique, at different stages of the freezing cycle, revealed significant differences regarding certain characteristics of the recovered semen such as: mean individual motility, total sperm abnormalities and plasma membrane and acrosome integrity, in favor of the ion-exchanging units (76).
In terms of fertilizing ability, GWF (28.97 ± 4.07 %) and SU (21.33 ± 1.94 %) returned inferior cleavage rates from embryos produced following oocyte insemination with spermatozoa selected after S-G15 filtration (49). Furthermore, when conception rates were analysed, significantly higher results were obtained from buffalo cows inseminated with G-75 filtered semen compared to the use of unfiltered material (83).
Sephadex filtration produced greater total/motile sperm recovery rates compared to swim-up (SU) and better post-filter quality (progressive motility, plasma membrane integrity, viability and cleavage rate) than both swim-up (SU) and glass wool filtration (GWF) in buffalo (49), bovine (99) and boar (72). In terms of fertilization rates (cleavage rate) of in vitro matured/fertilized oocytes (73), Sephadex and glass wool filtration (40) yielded better results.
The lack of correlation between sperm density and apoptosis in DGC could potentially lead to unsuccessful fertilization. Phosphatidylserine (PS) externalisation to the outer membrane leaflet is the basis for the successful separation of apoptotic and non-apoptotic spermatozoa by MACS employing annexin V-conjugated (a 35-kDa protein) microbeads. To improve the quality and function of sperm, MACS and DGC can be combined as a sperm preparation procedure (102). PS, which is negatively charged and is located on the inner leaflet of the plasma membrane in viable cells, is bound by annexin V. However, early in apoptosis, PS externalises to the outer leaflet of the membrane, a change that is positively correlated with damage to nuclear DNA and has implications for fertilization and pregnancy failure after ART (103).
MACS is performed immediately after DGC in order to evaluate the sperm recovery rate. This rate is calculated by dividing the total motile sperm after MACS by the total motile sperm before MACS (102).
From all the studied combinations of sperm separation techniques within the annexin-negative fraction separated by MACS + DGC the sperm quality is improved and there is very little cell loss (77, 79). This approach had a favorable effect especially on sperm motility and viability, and so, this is why this combination is regarded as a successful sperm preparation method (77, 102). Moreover, this fraction expressed the fewest apoptotic markers (active capase-3, integrity of membrane mitochondrial potential, phospholipid phosphatidylserine) (77). The proportion of sperm DNA fragmentation (9.2 ± 0.7% vs. 12.5 ± 1.0%), mitochondrial membrane potential disruption (18.7 ± 1.9% vs. 27.2 ± 3.0%) and the externalisation of phosphatidylserine (5.9 ± 1.3% vs. 8.2 ± 2.0%) subsequent to MACS was significantly reduced compared to the DGC (78).
The poor rates of fertilization and implantation observed in assisted reproduction may be partially explained by the presence of deregulated apoptosis in spermatozoa (77). Sperm recovery rates in MACS+DGC (73.8 ± 12.1%) was higher than if only DGC (66.7 ± 19.1%) was used (38, 102).
In terms of producing motile, viable, and non-apoptotic spermatozoa, the combination of density gradient centrifugation with annexin-V magnetic cell sorting was superior to all other sperm preparation techniques (101, 102). After MACS + DGC, the rates of sperm recovery were slightly higher (73.83 ± 12.08 vs. 66.67 ± 19.12) than after DGC alone (102). Moreover, using this technique even in teratospermic asthenozoospermic and oligoasthenozoospermic men, the DNA integrity and the functionality will be excellent and it may improve the number of good quality embryos (104, 105).
Significant advancements in sperm analysis have been made in the last decade in buffalo reproduction, opening up new paths for subfertility diagnosis and therapy. Traditional sperm sorting methods rely on centrifugation processes, which are known to produce oxidative stress and, as a result, cell damage. Quantitative examination of sperm motility, morphology, and genetics gives useful information for diagnosing male infertility and allowing ideal sperm for ART selection. The various approaches described in this review for buffaloes’ sperm selection have pros and cons, and, as described, several of these methods have yielded contradictory results, and their clinical relevance is therefore still in question. In terms of motility, SU offered a significantly higher MI comparing to DGC, but the recovery rates were generally lower. Additionally, using a MACS and DGC protocol the sperm recovery rates were higher than using only DGC.
The most effective way to increase freezability and cryopreserve low-quality buffalo bull ejaculates is by sperm separation techniques. While in human medicine, these methods are more investigated, in veterinary medicine, there are still some limitations. Even if several studies have been carried out on other species (dog, cattle bull, boar), buffalo things are still not fully elucidated and they deserve to be further researched for a better thoroughness.
Considering these questionable results, we can conclude that semen separation techniques, both in buffaloes and men, are useful tools for reducing fertility problems, but require much more research to enter into common practice.
CA: Writing – review & editing. FP: Conceptualization, Writing – original draft, Writing – review & editing. NC: Conceptualization, Data curation, Writing – review & editing. IM: Data curation, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hegde, NG . Buffalo husbandry for sustainable development of small farmers in India and other developing countries. Asian J Res Animal and Veterinary Sci. (2019) 3:1–20. no.AJRAVS.47246, Available at https://ssrn.com/abstract=4345431
2. Kumar, PR , Shukla, SN , Shrivastava, OP , and Purkayastha, RD . Incidence of postpartum anestrus among buffaloes in and around Jabalpur. Vet World. (2013) 6:716–9. doi: 10.14202/vetworld.2013.716-719
3. Shashikumar, NG , Baithalu, RK , Bathla, S , Ali, SA , Rawat, P , Kumaresan, A, et al. Global proteomic analysis of water buffalo (Bubalus bubalis) saliva at different stages of estrous cycle using high throughput mass spectrometry. Theriogenology. (2018) 110:52–60. doi: 10.1016/j.theriogenology.2017.12.046
4. Ciornei, S , Drugociu, D , Roșca, P , and Ghinet (Ciornei), L . Caesarean section in uterine torsion at buffalo (Romanian indigenous buffalo) – a case study. Revista Română de Medicină Veterinară. (2020) 30:53–6.
5. Srinivasan, M , Muthukumar, S , Saibaba, G , Manikkaraja, C , Abdulkader Akbarsha, M , and Archunan, G . Salivary luteinizing hormone: an open window to detect oestrous period in buffalo. Reprod Domest Anim. (2020) 55:647–51. doi: 10.1111/rda.13649
6. Selvam, RM , and Archunan, G . A combinatorial model for effective estrus detection in Murrah buffalo. Vet World. (2017) 10:209–13. doi: 10.14202/vetworld.2017.209-213
7. Thakor, D , and Patel, D . Incidence of infertility problems in cattle and buffaloes, dairy cattle. (2023), Available at: https://en.engormix.com
8. Ciornei, S , Drugociu, D , Roșca, P , and Ghinet (Ciornei), L . Ovarian hypofunction in the Carpathian indigenous buffalo, as infertility factor. Revista Română de Medicină Veterinară. (2021) 31:67–73.
9. Nardone, A , Ronchi, B , Lacetera, N , Ranieri, MS , and Bernabucci, U . Effects of climate changes on animal production and sustainability of livestock systems. Livest Sci. (2010) 130:57–69. doi: 10.1016/j.livsci.2010.02.011
10. Devkota, B , Shah, S , and Gautam, G . Reproduction and fertility of buffaloes in Nepal. Animals. (2022) 13:70. doi: 10.3390/ani13010070
11. Devkota, B , Nakao, T , Kobayashi, K , Sato, H , Sah, SK , Singh, DK, et al. Effects of treatment for anestrus in water buffaloes with PGF2α and GnRH in comparison with vitamin-mineral supplement, and some factors influencing treatment effects. J Vet Med Sci. (2013) 75:1623–7. doi: 10.1292/jvms.12-0515
12. Abulaiti, A , Hua, G , Ahmad, HI , and Yang, L . Puberty, ovarian cycle, ovulation and post-partum uterus recovery in river and swamp type crossbred buffaloes. Int J Agric Biol. (2018) 20:1839–45. doi: 10.17957/IJAB/15.0717
13. Abulaiti, A , Riaz, U , Naseer, Z , Ahmed, Z , Hua, G , and Yang, L . Follicular dynamics during estrous cycle of pubertal, mature and postpartum crossbred (Nili Ravi × Jianghan) buffaloes. Animals. (2022) 12:1208. doi: 10.3390/ani12091208
14. Manik, RS , Palta, P , Singla, SK , and Sharma, V . Folliculogenesis in buffalo (Bubalus bubalis): a review. Reprod Fertil Dev. (2002) 14:315. doi: 10.1071/RD01126
15. Cao, L , Li, S , Huang, S , Shi, D , and Li, X . AQP8 participates in oestrogen-mediated buffalo follicular development by regulating apoptosis of granulosa cells. Reprod Domest Anim. (2021) 56:812–20. doi: 10.1111/rda.13921
16. Srirattana, K , Hufana-Duran, D , Atabay, EP , Duran, PG , Atabay, EC , Lu, K, et al. Current status of assisted reproductive technologies in buffaloes. Anim Sci J. (2022) 93:e13767. doi: 10.1111/asj.13767
17. Abulaiti, A , El-Qaliouby, HS , Bahgy, HEK , Naseer, Z , Ahmed, Z , Hua, G, et al. GPGMH, a new fixed timed-ai synchronization regimen for swamp and river crossbred buffaloes (Bubalus bubalis). Front Vet Sci. (2021) 8:646247. doi: 10.3389/fvets.2021.646247
18. Ciornei, SG , and Roşca, P . Upgrading the fixed-time artificial insemination (FTAI) protocol in Romanian buffaloes. Front Vet Sci. (2023) 10:1265060. doi: 10.3389/fvets.2023.1265060
19. Almeida, J , Brito, MF , Neves, BP , Becerra, VAB , Auler, PA , Hadad, JP, et al. Use of cooled buffalo semen as a strategy to increase conception rates in fixed-time artificial insemination programs during unfavorable reproductive periods. Arq Bras Med Vet Zootec. (2021) 73:560–70. doi: 10.1590/1678-4162-12142
20. Hozyen, HF , El Shamy, AA , El Fattah, EMA , and Sakr, AM . Facile fabrication of zinc oxide nanoparticles for enhanced buffalo sperm parameters during cryopreservation. Journal of Trace Elements and Minerals. (2023) 4:100058. doi: 10.1016/j.jtemin.2023.100058
21. Rajoriya, JS , Prasad, JK , Ramteke, SS , Perumal, P , Ghosh, SK , Singh, M, et al. Enriching membrane cholesterol improves stability and cryosurvival of buffalo spermatozoa. Anim Reprod Sci. (2016) 164:72–81. doi: 10.1016/j.anireprosci.2015.11.014
22. El-Sisy, GA , Shahba, MI , and El-Sheshtawy, RI . Freezability of buffalo semen with TRIS extender enriched with disaccharides (trehalose or sucrose) and different glycerol concentrations. Asian Pacific Journal of Reproduction. (2016) 5:416–8. doi: 10.1016/j.apjr.2016.07.007
23. Binsila, BK , Archana, SS , Ramya, L , Swathi, D , Selvaraju, S , Gowa, NKS, et al. Elucidating the processes and pathways enriched in buffalo sperm proteome in regulating semen quality. Cell Tissue Res. (2021) 383:881–903. doi: 10.1007/s00441-020-03303-9
24. Tobă, GF , Ciornei, ȘG , Paraschivescu, MT , Tobă, GF , Ciornei, L , and Bănățeanu, F . Identification, monitoring and conservation of the biodiversity of the national heritage of the Romanian buffalo breed, using breeding biotechnologies. Harnessing Tangible and Intangible Assets in the context of European Integration and Globalization Challenges ahead. (2021) 2:1127–39. doi: 10.3726/978-3-653-06574-9
25. Constantin, NT , Bercea-Strugariu, CM , Bîrțoiu, D , Posastiuc, FP , Iordache, F , and Bîlteanu, L . Șerban Andreea Iren. Predicting pregnancy outcome in dairy cows: the role of IGF-1 and progesterone. Animals, Special Issue: Advances in Reproduction and Nutrition Management in Dairy Cattle. (2023) 13:1579. doi: 10.3390/ani13101579
26. Dixit, S , Pandey, V , Swain, DK , Nigam, R , Sharma, A , Sharma, D, et al. Seminal plasma and sperm membrane proteins of buffalo and cattle bulls: a comparative study. Buffalo Bulletin. (2016) 35:437–43.
27. Dhami, AJ , and Sahni, KL . Comparative appraisal of physicomorphological and enzymatic attributes of semen and their interrelationships in ox and buffalo bulls. J Appl Anim Res. (1994) 5:13–20. doi: 10.1080/09712119.1994.9705992
28. Misra, AK , Rao, LMM , Kasiraj, R , Reddy, NSR , and Pant, HC . Bull-specific effect on fertilization rate and viable embryo recovery in the superovulated buffalo (Bubalus bubalis). Theriogenology. (1999) 52:701–7. doi: 10.1016/s0093-691x(99)00163-6
29. Tăpăloagă, D , Al Dulaimi, MKH , and Tăpăloagă, PR . Quality and quantity parameters in buffalo semen production. Scientific papers. Series D. Anim Sci. (2018) LXI:154–62.
30. Noce, A , Qanbari, S , Gonzalez-Prendes, R , Brenmoehl, J , Luigi-Sierra, MG , Theerkorn, M, et al. Genetic diversity of bubalus bubalis in Germany and global relations of its genetic background. Front. Genet. (2020) 11:610353. doi: 10.3389/fgene.2020.610353
31. Bhave, K , Koilpillai, TPJ , Ragothaman, V , Sontakke, S , Joshi, G , and Ducrocq, V . Semen production and semen quality of indigenous buffalo breeds under hot semiarid climatic conditions in India. Trop Anim Health Prod. (2020) 52:2529–39. doi: 10.1007/s11250-020-02284-9
32. Koonjaenak, S , Chanatinart, V , Aiumlamai, S , Pinyopumimintr, T , and Rodriguez-Martinez, H . Seasonal variation in semen quality of swamp buffalo bulls (Bubalus bubalis) in Thailand. Asian J Androl. (2007) 9:92–101. doi: 10.1111/j.1745-7262.2007.00230.x
33. Arias, ME , Andara, K , Briones, E , and Felmer, R . Bovine sperm separation by swim-up and density gradients (Percoll and BoviPure): effect on sperm quality, function and gene expression. Reprod Biol. (2017) 17:126–32. doi: 10.1016/j.repbio.2017.03.002
34. Zhao, F , Yang, Q , Shi, S , Luo, X , and Sun, Y . Semen preparation methods and sperm telomere length: density gradient centrifugation versus the swim up procedure. Sci Rep. (2016) 6:39051. doi: 10.1038/srep39051
35. Rocca, MS , Speltra, E , Menegazzo, M , Garolla, A , Foresta, C , and Ferlin, A . Sperm telomere length as a parameter of sperm quality in normozoospermic men. Hum Reprod. (2016) 31:1158–63. doi: 10.1093/humrep/dew061
36. Henkel, RR , and Schill, WB . Sperm preparation for ART. Reprod Biol Endocrinol. (2003) 1:108. doi: 10.1186/1477-7827-1-108
37. Maurya, VP , Tuli, RK , and Goyal, RL . Effect of buffer composition, sephadex grade and column size on filtration-based quality improvement of semen from Murrah buffalo bull. Asian Australas J Anim Sci. (2003) 16:165–71. doi: 10.5713/ajas.2003.165
38. Said, TM , Agarwal, A , Grunewald, S , Rasch, M , Glander, HJ , and Paasch, U . Evaluation of sperm recovery following annexin V magnetic-activated cell sorting separation. Reprod Biomed Online. (2006) 13:336–9. doi: 10.1016/S1472-6483(10)61437-X
39. Mota, AV , Oba, E , Castro, A , Araujo, GHM , Stella, E , and Ramos, AA . Selection of X chromosome of buffaloes’ sperm with Percoll gradients. Ital J Anim Sci. (2007) 6:807–9. doi: 10.4081/ijas.2007.s2.807
40. Lee, HL , Kim, SH , Ji, DB , and Kim, YJ . A comparative study of Sephadex, glass wool and Percoll separation techniques on sperm quality and IVF results for cryopreserved bovine semen. J Vet Sci. (2009) 10:249. doi: 10.4142/jvs.2009.10.3.249
41. Hossepian de Lima, V . Enrichment of bovine semen with X-bearing spermatozoa using Percoll? And Optiprep? Discontinuous gradients. Animal and Veterinary Sci. (2015) 3:11. doi: 10.11648/j.avs.20150301.11
42. de Lima, VFMH . Avanços metodológicos na seleção do sexo de espermatozóides bovinos para utilização no melhoramento genético e na produção animal. Rev Bras Zootec. (2007) 36:219–28. doi: 10.1590/S1516-35982007001000020
43. Oliveira, LZ , Arruda, RP , Celeghini, ECCC , de Andrade, AFC , Prini, AP , Resende, MV, et al. Effects of discontinuous Percoll gradient centrifugation on the quality of bovine spermatozoa evaluated with computer-assisted semen analysis and fluorescent probes association. Andrologia. (2012) 44:9–15. doi: 10.1111/j.1439-0272.2010.01096.x
44. Rawat, M , and Sharma, M . Effect of percoll density gradient separation of x and y sperm on buffalo bull semen quality. J Experimental Zoology India. (2020) 23:623–30.
45. Sharma, M , and Rawat, M . Effect of percoll density gradient separation of x and y sperm on buffalo bull semen quality. J Experimental Zoology India. (2020) 23:623–30.
46. Bisla, A . Comparative efficacy of Percoll™ discontinuous density gradient centrifugation and glass wool filtration techniques for spermatozoa selection in Buffalo (Bubalus bubalis). Journal of. Anim Res. (2020) 10:3. doi: 10.30954/2277-940x.02.2020.3
47. Mehmood, A , Anwar, M , and Naqvi, SMS . Motility, acrosome integrity, membrane integrity and oocyte cleavage rate of sperm separated by swim-up or Percoll gradient method from frozen-thawed buffalo semen. Anim Reprod Sci. (2009) 111:141–8. doi: 10.1016/j.anireprosci.2008.02.011
48. Cesari, A , Kaiser, GG , Mucci, N , Mutto, A , Vincenti, A , Fornes, MW, et al. Integrated morphophysiological assessment of two methods for sperm selection in bovine embryo production in vitro. Theriogenology. (2006) 66:1185–93. doi: 10.1016/j.theriogenology.2006.03.029
49. Husna, AU , Azam, A , Qadeer, S , Ejaz, R , and Akhter, S . Pregnancy and calving rates improved using modified swim-up method for buffalo semen sexing. Reprod Domest Anim. (2022) 57:798–801. doi: 10.1111/rda.14112
50. Mustafa, G , Anzar, M , and Arslan, M . Separation of motile spermatozoa from frozen-thawed buffalo semen: swim-up vs filtration procedures. Theriogenology. (1998) 50:205–11. doi: 10.1016/S0093-691X(98)00127-7
51. Faezah, SSM , Zuraina, FMY , Farah, JHF , Khairul, O , Hilwani, NI , Iswadi, MI, et al. The effects of magnetic separation on cryopreserved bovine spermatozoa motility, viability and cryo-capacitation status. Zygote. (2014) 22:378–86. doi: 10.1017/S0967199412000597
52. Asma-ul-Husna, AMA , Mehmood, A , Sultana, T , Shahzad, Q , Ansari, MS , Rakha, BA, et al. Sperm sexing in Nili-Ravi buffalo through modified swim up: validation using SYBR ® green real-time PCR. Anim Reprod Sci. (2017) 182:69–76. doi: 10.1016/j.anireprosci.2017.04.011
53. Siesto, G , Bulletti, C , Ieda, N , Accardi, A , and Vitobello, D . Robotic assisted approach for rectosigmoid resection in patients with deep infiltrating endometriosis. Placenta. (2011) 32:S279–80. doi: 10.1016/j.placenta.2011.07.023
54. Volpes, A , Sammartano, F , Rizzari, S , Gullo, S , Marino, A , and Allegra, A . The pellet swim-up is the best technique for sperm preparation during in vitro fertilization procedures. J Assist Reprod Genet. (2016) 33:765–70. doi: 10.1007/s10815-016-0696-2
55. Muratori, M , Tarozzi, N , Carpentiero, F , Danti, S , Perrone, FM , Cambi, M, et al. Sperm selection with density gradient centrifugation and swim up: effect on DNA fragmentation in viable spermatozoa. Sci Rep. (2019) 9:1–12. doi: 10.1038/s41598-019-43981-2
56. Dai, X , Wang, Y , Cao, F , Yu, C , Gao, T , Xia, X, et al. Sperm enrichment from poor semen samples by double density gradient centrifugation in combination with swim-up for IVF cycles. Sci Rep. (2020) 10. doi: 10.1038/s41598-020-59347-y
57. Gandini, L , Lenzi, A , Lombardo, F , Pacifici, R , and Dondero, F . Immature germ cell separation using a modified discontinuous Percoll gradient technique in human semen (1999) 14:1022–7. doi: 10.1093/humrep/14.4.1022,
58. Cakar, Z , Cetinkaya, B , Aras, D , Koca, B , Ozkavukcu, S , Kaplanoglu, I, et al. Does combining magnetic-activated cell sorting with density gradient or swim-up improve sperm selection? J Assist Reprod Genet. (2016) 33:1059–65. doi: 10.1007/s10815-016-0742-0
59. Fernandes, DNS , Da Silva, CG , Panizzon, GP , Cerialle, PMA , Câmara, VCM , Daraelli, MR, et al. Comparative sperm recovery rate after density gradient centrifugation with two media for in vitro fertilization. J Bras Reprod Assist. (2023) 27:25–8. doi: 10.5935/1518-0557.20220008
60. Abdel-Razek, K , Hussien, HA , Senosy, W , and Yousef, MS . Effect of sperm separation methods on morphology and functions of frozen buffalo spermatozoa. J Adv Vet Res. (2017) 7:18–23.
61. Tavalaee, M , Deemeh, MR , Arbabian, M , and Nasr-Esfahani, MH . Density gradient centrifugation before or after magnetic-activated cell sorting: which technique is more useful for clinical sperm selection? J Assist Reprod Genet. (2012) 29:31–8. doi: 10.1007/s10815-011-9686-6
62. Assumpção, TI , Lançoni, R , Foschini, M , and Vieira, CS . Equine spermatozoa selection by magnetic activation for use in assisted reproduction. J Equine Vet. (2023):123. doi: 10.1016/j.jevs.2023.104245
63. Phillips, TC , Dhaliwal, GK , Verstegen-Onclin, KM , and Verstegen, JP . Efficacy of four density gradient separation media to remove erythrocytes and nonviable sperm from canine semen. Theriogenology. (2012) 77:39–45. doi: 10.1016/j.theriogenology.2011.07.012
64. Rawat, M , and Sharma, M . Effect of percoll density gradient separation of x and y sperm on buffalo bull semen quality. J Exp Zool India. (2021) 23:000–08. www.researchgate.net/publication/349337491
65. Vasicek, J , Pivko, J , and Chrenek, P . Reproductive performance of New Zealand white rabbits after depletion of apoptotic spermatozoa. Folia Biologica (Poland). (2014) 62:109–17. doi: 10.3409/fb62_2.109
66. Pessoa, GA , Martini, AP , Trentin, JM , Minela, T , Fiorenza, MF , and Rubin, MIB . Response to cooling of pony stallion semen selected by glass wool filtration. Andrologia. (2017) 49:1–12. doi: 10.1111/and.12771
67. Sieme, H , Martinsson, G , Rauterberg, H , Walter, K , Aurich, C , Petzoldt, R, et al. Application of techniques for sperm selection in fresh and frozen-thawed stallion semen. Reprod Domest Anim. (2003) 38:134–40. doi: 10.1046/j.1439-0531.2003.00416.x
68. Casey, PJ , Robertson, KR , Liu, IKM , Espinoza, SB , and Drobnis, EZ . Column separation of motile sperm from stallion semen. J Androl. (1993) 14:142–8. doi: 10.1002/j.1939-4640.1993.tb01669.x
69. Klinc, P , Kosec, M , and Majdic, G . Freezability of equine semen after glass beads column separation. Equine Vet J. (2005) 37:43–7. doi: 10.2746/0425164054406810
70. Henkel, RR , Franken, DR , Lombard, CJ , and Schill, WB . Selective capacity of glass-wool filtration for the separation of human spermatozoa with condensed chromatin: a possible therapeutic modality for male-factor cases? J Assist Reprod Genet. (1994) 11:395–400. doi: 10.1007/BF02211725
71. Calamera, JC , Quiros, MC , Brugo, S , and Nicholson, RF . Comparison between swim-up and glass bead column techniques for the separation of human spermatozoa. Andrologia. (1991) 23:259–61. doi: 10.1111/j.1439-0272.1991.tb02554.x
72. Fayemi, EO , Crabo, BG , and Graham, EF . Assay of frozen boar semen with Sephadex filtration. Theriogenology. (1979) 12:13–7. doi: 10.1016/0093-691x(79)90053-0
73. Bussalleu, E , Pinart, E , Rivera, MM , Arias, X , Briz, M , Sancho, S, et al. Effects of filtration of semen doses from subfertile boars through neuter sephadex columns. Reprod Domest Anim. (2008) 43:48–52. doi: 10.1111/j.1439-0531.2007.00853.x
74. Scholkamy, TH , Mahmoud, KGM , El Zohery, FA , and Ziada, MS . Evaluation of sephadex filtration for freezability and in vitro fertilizing ability of buffalo semen. Glob Vet. (2009) 3:144–50.
75. Goyal, RL , Tuli, RK , Georgie, GC , and Chand, D . Comparison of quality akid freezability of water buffalo semen after washing or sephadex filtration. Theriogenology. (1996) 46:679–86. doi: 10.1016/0093-691X(96)00219-1
76. Ahmad, Z , Anzar, M , Shahab, M , Ahmad, N , and Andrabi, SMH . Sephadex and sephadex ion-exchange filtration improves the quality and freezability of low-grade buffalo semen ejaculates. Theriogenology. (2003) 59:1189–202. doi: 10.1016/S0093-691X(02)01159-7
77. Said, TM , Grunewald, S , Paasch, U , Glander, H-J , Baumann, T , Kiegel, CK, et al. Advantage of combining magnetic cell separation with sperm preparation techniques. Reprod Biomed Online. (2005) 10:740–6. doi: 10.1016/S1472-6483(10)61118-2
78. Lee, TH , Liu, CH , and Lee, MS . Sperm preparation by magnetic-activated cell sorting (MACS) reduced apoptotic sperm and improved acrosome reaction for unexplained infertility. Fertil Steril. (2009) 92:S143. doi: 10.1016/j.fertnstert.2009.07.1234
79. Bertelli, TS , Da Broi, MG , Navarro, PA , Martins, W , and Ferriani, R . Magnetic activated cell sorting performed before double gradient centrifugation improve the recovery of good quality spermatozoa. Fertil Steril. (2016) 106:e290–1. doi: 10.1016/j.fertnstert.2016.07.827
80. Lepine, S , McDowell, S , Searle, LM , Kroon, B , Glujovsky, D , and Yazdani, A . Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst Rev. (2019) 2019. doi: 10.1002/14651858.CD010461.pub3
81. Mei, J , Chen, L-J , Zhu, X-X , Yu, W , Gao, Q-Q , Sun, H-X, et al. Magnetic-activated cell sorting of nonapoptotic spermatozoa with a high DNA fragmentation index improves the live birth rate and decreases transfer cycles of IVF/ICSI. Asian J Androl. (2022) 24:367–72. doi: 10.4103/aja202161
82. Pacheco, A , Blanco, A , Bronet, F , Cruz, M , García-Fernández, J , and García-Velasco, JA . Magnetic-activated cell sorting (MACS): a useful sperm-selection technique in cases of high levels of sperm DNA fragmentation. J Clin Med. (2020) 9:1–9. doi: 10.3390/jcm9123976
83. Herrid, M , Davey, RJ , Hutton, K , Colditz, IG , and Hill, JR . A comparison of methods for preparing enriched populations of bovine spermatogonia. Reprod Fertil Dev. (2009) 21:393–9. doi: 10.1071/RD08129
84. Khalid, SN , and Qureshi, IZ . Effect of magnetically selected sperm on fertilization and embryo development: an animal model study. Fertil Steril. (2011) 96:S169. doi: 10.1016/j.fertnstert.2011.07.659
85. Hufana-Duran, D , Duran, PG , Kanai, Y , Takahashi, Y , and Cruz, LC . Effect of density-gradient sperm separation technique on in vitro fertilization potential of water buffalo semen with low post-thaw motilities. Philipp Agric Sci. (2005) 88
86. Parrish, JJ , Susko-Parrish, JL , Leibfried-Rutledge, ML , Critser, ES , Eyestone, WH , and First, NL . Bovine in vitro fertilization with frozen-thawed semen. Theriogenology. (1986) 25:591–600. doi: 10.1016/0093-691x(86)90143-3
87. Otavă, G , Squicciarini, S , Marc, S , Suici, T , Onan, GW , Hușu, I, et al. Effects of age and season on conception rate of Mediterranean Italian dairy Buffalo (Bubalus bubalis) following oestrus synchronization and fixed-time artificial insemination. Reprod Domest Anim. (2021) 56:1511–8. doi: 10.1111/rda.14013
88. Ward, F , Rizos, D , Boland, MP , and Lonergan, P . Effect of reducing sperm concentration during IVF on the ability to distinguish between bulls of high and low field fertility: work in progress. Theriogenology. (2003) 59:1575–84. doi: 10.1016/s0093-691x(02)01202-5
89. Chamberland, A , Fournier, IV , Tardff, S , Sirard, MA , Sulhvan, IR , and Badey, JL . The effect of heparin on motility parameters and protein phosphorylation during bovine sperm capacitation. Theriogenology. (2001) 55:823–35. doi: 10.1016/s0093-691x(01)00446-0
90. Mahadevan, M , and Baker, G . Assessment and preparation of semen for in vitro fertilization In: Clinical In Vitro Fertilization. London: Springer London (1984). 83–97.
91. Oseguera-López, I , Ruiz-Díaz, S , Ramos-Ibeas, P , and Pérez-Cerezales, S . Novel techniques of sperm selection for improving IVF and ICSI outcomes. Front. Cell Dev. Biol. (2019) 7:298. doi: 10.3389/fcell.2019.00298
92. Husna, AU , Ejaz, R , Qadeer, S , Azam, A , Rakha, BA , Ansari, MS, et al. A comparative analysis of sperm selection procedures prior to cryopreservation for Nili-Ravi buffalo bull (Bubalus bubalis) semen-: assessment of its impact on post-thaw sperm functional quality. Anim Reprod Sci. (2016) 174:29–36. doi: 10.1016/j.anireprosci.2016.08.015
93. Sterzik, K , De Santo, M , Uhlich, S , Gagsteiger, F , and Strehler, E . Glass wool filtration leads to a higher percentage of spermatozoa with intact acrosomes: an ultrastructural analysis. Hum Reprod. (1998) 13:2506–11. doi: 10.1093/humrep/13.9.2506
94. de Carvalho, FM , Ramsey, C , Hanna, CB , do Valle, RR , Nichi, M , Binelli, M, et al. Cryopreservation and preparation of thawed spermatozoa from rhesus macaques (Macaca mulatta) for in vitro fertilization. J Am Assoc Lab Anim Sci. (2021) 60:396–406. doi: 10.30802/AALAS-JAALAS-20-000028
95. Morrell, JM , Johannisson, A , Dalin, AM , and Rodriguez-Martinez, H . Single-layer centrifugation with Androcoll-E can be scaled up to allow large volumes of stallion ejaculate to be processed easily. Theriogenology. (2009) 72:879–84. doi: 10.1016/j.theriogenology.2009.05.015
96. Zevallos Valenzuela, GE , Ferrante, A , Verón, GL , Miragaya, M , Marín-Briggiler, CI , and Vazquez-Levin, MH . Glass wool column filtration for stallion sperm selection: a comparative analysis with the single-layer colloid centrifugation. Reprod Domest Anim. (2023) 58:1244–50. doi: 10.1111/rda.14424
97. Grunewald, S , Miska, W , Miska, G , Rasch, M , Reinhardt, M , Glander, H-J, et al. Molecular glass wool filtration as a new tool for sperm preparation. Hum Reprod. (2007) 22:1405–12. doi: 10.1093/humrep/dem015
98. Galarza, DA , López-Sebastián, A , Woelders, H , Blesbois, E , and Santiago-Moreno, J . Sephadex filtration as successful alternative to density-gradient centrifugation procedures for ram sperm selection with improved kinetics. Anim Reprod Sci. (2018) 192:261–70. doi: 10.1016/j.anireprosci.2018.03.022
99. Ziada, MS , Alaa El Deen, MM , Zaky, AAH , and Hassanin, KD . Assessment of the efficacy of sephadex filtration of buffalo spermatozoa for cryopreservation. Veterinary Medical Journal. (2006) 54:295–307.
100. Tiwari, S , Srivastava, R , Kulkarni, NA , Raval, K , Patidar, P , Fernandes, A, et al. Filtration techniques are advantageous over colloidal centrifugation in improving freezability of low-quality buffalo bull (Bubalus bubalis) ejaculates. Anim Biotechnol. (2023) 34:2835–45. doi: 10.1080/10495398.2022.2121715
101. El-Siefy, E , Hussein, A , and El-Sharawy, M . Effect of filtration of post-diluted semen by sephadex on freezing ability and fertilizing capacity of buffalo semen. J Animal and Poultry Prod. (2013) 4:647–57. doi: 10.21608/JAPPMU.2013.71599
102. Said, M , Paasch, U , Grunewald, S , Rasch, M , Glander, H , and Agarwal, CA . Sperm recovery evaluation following magnetic cell sorting. Male Factor ART. (2005) 13:336–9. doi: 10.1016/j.fertnstert.2005.07.524
103. Mehta, A , and Sigman, M . Identification and preparation of sperm for art. Urol Clin N Am. (2014) 41:169–80. doi: 10.1016/j.ucl.2013.08.005
104. Notrica, JA , Vazquez-Levin, MH , Bossi, NM , Notrica, DE , Granados, MC , and Polak de Fried, E . Use of annexin v- macs in ICSI infertile couples: effect on fertilization rate (FR), pregnancy rate (PR) and embryo quality. Fertil Steril. (2014) 102(3):196. doi: 10.1016/j.fertnstert.2014.07.660
Keywords: assisted reproductive technology, buffalo, semen separation methods, centrifugation, filtration
Citation: Andrei CR, Posastiuc FP, Constantin NT and Mitrea IL (2024) New insights into semen separation techniques in buffaloes. Front. Vet. Sci. 10:1347482. doi: 10.3389/fvets.2023.1347482
Received: 30 November 2023; Accepted: 26 December 2023;
Published: 10 January 2024.
Edited by:
Mihai Cenariu, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, RomaniaReviewed by:
Simona Marc, Banat University of Agricultural Sciences and Veterinary Medicine, RomaniaCopyright © 2024 Andrei, Posastiuc, Constantin and Mitrea. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florin Petrișor Posastiuc, florin.posastiuc@gmail.com; Nicolae Tiberiu Constantin, tiberiu.constantin@fmvb.usamv.ro
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.