
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 22 June 2022
Sec. Parasitology
Volume 9 - 2022 | https://doi.org/10.3389/fvets.2022.927185
Toxoplasma gondii and Toxocara spp. are the most critical parasites common between humans and cats. The close association of cats with humans in urban areas persuaded us to investigate the prevalence of these parasites in stray and household cats and their possible role in the owners' infection. Herein, 132 and 33 fecal samples of stray and household cats, respectively, and 33 blood samples of their owners were collected in Tehran, Iran. The prevalence of T. gondii was determined by targeting the B1 gene in the feces of stray and household cats and the blood of cat owners. Furthermore, genotypes of T. gondii were identified based on the multilocus genotyping of BTUB, GRA6, SAG3, and APICO loci. Toxocara spp. were detected by targeting the second internal transcribed spacer (ITS-2) of the ribosomal DNA of these parasites in the cats' feces and the humans' blood. Also, Toxocara IgG was assessed in the human serum samples. The B1 gene amplification showed that 15.2% of stray cats, 18.2% of household cats, and 51.5% of cat owners were infected with T. gondii. The multilocus sequence analysis revealed the predominance of genotype I of T. gondii in stray cats and genotype II of T. gondii in household cats and cat owners. The amplifying of ITS-2 revealed a high prevalence of T. cati infection (47.0%) in stray cats, whereas no infection was found in the feces of household cats or the serum of cat owners. Likewise, Toxocara IgG was not detected in the serum of humans. The lower prevalence of T. gondii in stray/household cats than in the cat owners indicates the limited impact of close contact with infected cats in human toxoplasmosis. However, the high prevalence of T. cati infection in stray cats can cause contamination of the environment by excreting eggs that may lead to infecting humans through soil or water. Therefore, public health education in urban management planning is necessary for routine urban cat deworming programs and for training the healthcare workers to prevent, control, and treat these infections.
Cats (Felis catus) have lived in human societies for thousands of years, with a population of over 600 million worldwide. They are the most popular companion animal globally. Approximately 373 million cats were kept as pets in the world in 2018. (https://www.statista.com/statistics/1044386/dog-and-cat-pet-population-worldwide/). However, it is difficult to determine the number of cats that live freely in human societies (1). Cats harbor some important zoonotic parasites that can affect public health. Toxoplasmosis and toxocariasis are the most important parasitic diseases common between humans and cats (2).
Toxoplasmosis, caused by Toxoplasma gondii, is one of the most common protozoan parasitic infections in humans and animals. It is estimated that about two billion of the world's population are chronically infected with this obligate intracellular protozoan parasite (3). Toxoplasmosis generally remains asymptomatic in people with effective immune responses. However, the acute disease may manifest symptoms such as fever, lymphocytosis, and lymphadenitis. The severe form of the disease rarely leads to encephalitis, pneumonia, muscle disorders, and death. Furthermore, early infection during pregnancy may cause abortion (3). The T. gondii infections are acquired by consuming undercooked meat containing tissue cysts or contaminated food and water with oocysts defecated from cats that sporulate in the soil during 1–5 days (4, 5).
The oocysts of Toxoplasma, Neospora, and Hammondia parasites are morphologically indistinguishable. Consequently, molecular methods apply to differentiate Hammondia-like organisms in the feline feces (6). Molecular analysis has categorized T. gondii into three genotypes I, II, and III. Genotype II is the most prevalent genotype in humans (7, 8), although animal toxoplasmosis is more closely related to genotypes II and III (9). Whereas genotype I of T. gondii is more implicated in acute and lethal infections in humans and animals (10).
Stray and household cats, as the definitive hosts of T. gondii in urban communities, through a close relationship with humans, seem to be the leading cause of human infection by passing oocysts in feces (5). Hence, information about the prevalence of T. gondii in stray and household cats and cat owners is necessary to identify the possible role of cats in infecting humans.
Toxocariasis, caused by zoonotic Toxocara species, is one of the most frequently reported parasitic zoonosis in humans worldwide (11). Members of the Toxocara genus are among the most common helminths in canids and felids. They include essential human and animal health species, such as Toxocara cati, Toxocara canis, and Toxascaris leonina. Epidemiological studies indicate 1–100% of T. canis in dogs and 3.2–91% of T. cati in cats (12). The high amount of adult nematodes in the intestines of the definitive hosts leads to the contamination of playgrounds and urban areas that increases the risks of toxocariasis in humans, especially in children. Humans are infected by accidental ingestion of embryonic eggs or by ingesting undercooked meat containing encysted larvae (12). Toxocariasis clinical manifestations are visceral larva migrans, ocular larva migrans, latent toxocariasis, and neurological toxocariasis (12). The close association of cats with humans in urban areas makes them an important source of Toxocara infection. Therefore, knowing the exact number of infected cats is very effective for making the necessary decisions to prevent and control this disease. Limited studies on the prevalence of Toxocara spp. in Iran (13–16) persuaded us to investigate the prevalence of these parasites in stray and household cats and their possible role in the owners' infection.
We applied the molecular analyses approach to fill the gap in our understanding of the prevalence of T. gondii and Toxocara spp. in stray and household cats and determine the possible role of household cats in the infection of their owners. To reach these purposes, we determined the prevalence of T. gondii and Toxocara spp. by molecular detection of the B1 gene of Toxoplasma and the ITS-2 region of the ribosomal DNA of Toxocara spp. in the feces of stray and household cats and the blood of cat owners in Tehran, Iran. In addition, genotypes of T. gondii were determined to identify the possible role of cats in infecting humans based on the multilocus genotyping of BTUB, GRA6, SAG3, and APICO loci.
The study area was in Tehran (35.6892° N, 51.3890° E), the largest city and the capital of Iran (Figure 1). It covers an area of more than 730 km2 and has a population of 8.694 million. The climate of Tehran is generally mild and pleasant in spring and autumn, hot and dry in summer, and cold and wet in winter. The average annual temperature in Tehran is 16.4°C, and the mean annual rainfall is 220 mm.
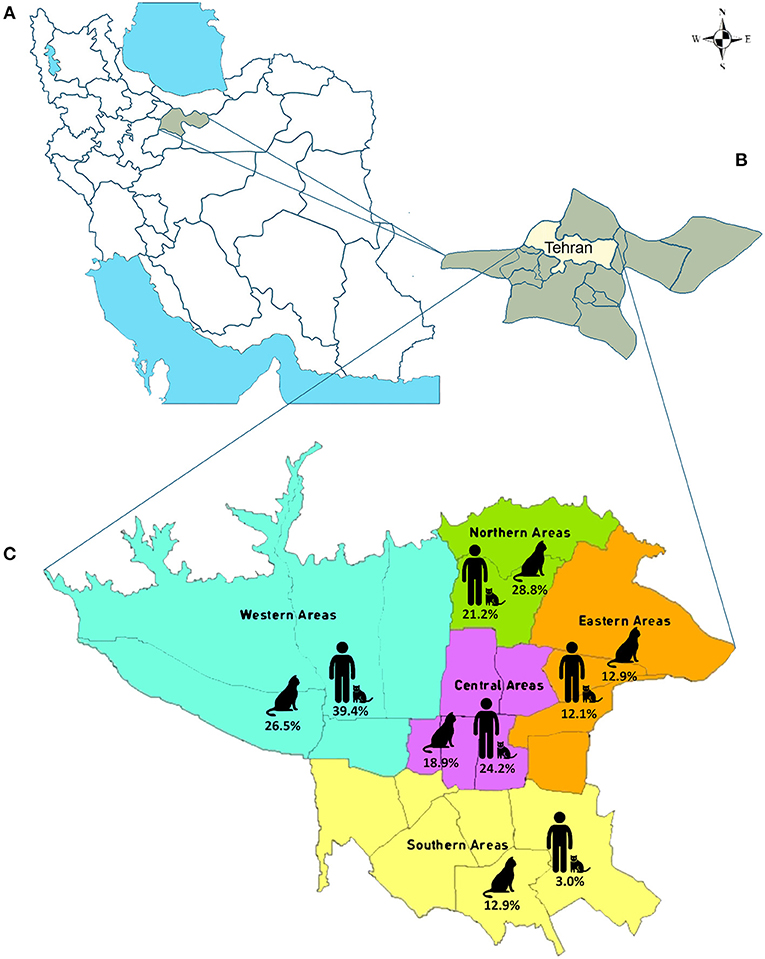
Figure 1. The map of the study area. (A) Map of Iran. Tehran Province is indicated by green. (B) Map of Tehran Province. Tehran is highlighted in yellow. (C) Map of Tehran. The five study areas are indicated, and the percentage of the stray and household cat fecal samples and blood of cat owners collected from each region are shown.
The cat questionnaire was designed to include information on cat demographic data (sex, age, breed, weight, and residence area). There was no history of anti-parasitic treatments in stray cats, although they confidently did not take these drugs at the sampling time. The questionnaire of household cat owners collected information on demographic data (gender, age, occupation, residence area, and education). The cat owners declared that their cats had received anthelmintic treatments six months before sampling. The questionnaires are available in the Supplementary Material.
The stray cats injured in accidents or diseases have been transferred to veterinary clinics or pet hospitals in different parts of Tehran by animal-loving people or animal welfare associations. From 132 stray cats referred to these centers from different regions of Tehran's open public areas (north, south, east, west, and center), visibly-excreted fecal pellets were collected from October 2017 through March 2018. Thirty-three fecal samples were collected from household cats, and 33 blood samples were taken from their owners from January through September 2019. Six and seven milliliters of venous blood were collected in two tubes with and without EDTA to obtain the buffy coat and serum. Collected samples were transferred immediately to the research laboratory of the Department of Parasitology and Mycology, Iran University of Medical Sciences, for further analysis. Serum and buffy coats were stored at −80°C until further investigation. The Toxocara IgG in serum samples was evaluated with an ELISA commercial kit (Toxocara IgG ELISA kit; NovaTec Immunodiagnostic GmbH, Germany) following the manufacturer's instructions.
Fecal samples of stray and household cats (n = 165) were submitted to the sucrose flotation procedure for concentrating cysts/oocysts of parasites (17). Six to eight grams of each sample was dissolved into 50 mL PBS until a homogeneous suspension was prepared. Then, the suspension was passed through three layers of gauze to eliminate large particles. The fecal suspension was centrifuged at 800×g for 5 min. For isolating cysts/oocysts, 50 mL PBS was added to the sediment. After complete mixing, 25 mL of suspension was layered gently over 20 mL of 1 M sucrose solution (specific gravity 1.13) in a 50 mL cleaned conical tube to form two completely distinct phases. The tubes were centrifuged at 800 × g for 5 min at 4°C. The interface and the upper layer of the sucrose were collected by a disposable pipette to a 15 mL clean conical tube. Then, the collected supernatant containing the purified cysts/oocysts of parasites was recentrifuged and washed three times with PBS to remove the residual sucrose. Finally, 100 mg of the sediment was re-suspended in 200 μL 2% polyvinylpolypyrrolidone (PVPP) in PBS, mixed thoroughly, and after keeping at −20°C for 24 h, stored at −80°C until submitted to DNA extraction procedures.
The genomic DNA of parasites was extracted from fecal samples using the QIAamp DNA Mini Kit (QIAGEN Ltd., Hilden, Germany cat. no. 51306) and from serum or buffy coat using a QIAamp DNA Blood Minikit (QIAGEN Ltd., Hilden, Germany cat. no. 51104) according to the manufacturer's instructions. The isolated DNA was stored at −20°C until used in PCR reactions.
Molecular identification of T. gondii was performed by a PCR assay based on the 194-bp fragment amplification of the B1 gene to screen the T. gondii DNA in the cat fecal samples and the buffy coat of blood samples of household cat owners. The B1 gene of T. gondii is a multi-copy sequence-specific and highly conserved gene in all T. gondii strains, making it highly useful in the molecular detection of the parasite (18). The PCR amplification was conducted by the primers designed by Schwab and McDevitt (19) and the PCR procedures introduced in our previous report (18). In addition, the multi-locus genotyping of T. gondii in the B1-positive samples was performed based on the BTUB, GRA6, SAG3, and APICO genetic markers (4, 20) by primers designated by Su et al. (4). The nested PCR was performed with Taq DNA Polymerase Master Mix (Amplicon III, Denmark, cat. no. 180301) in the conditions described by Arshadi et al. (18) (Table 1). The genotype of T. gondii was determined after sequencing the amplified fragment of each marker by internal amplification primers in both directions (Macrogen Inc., Seoul, South Korea).
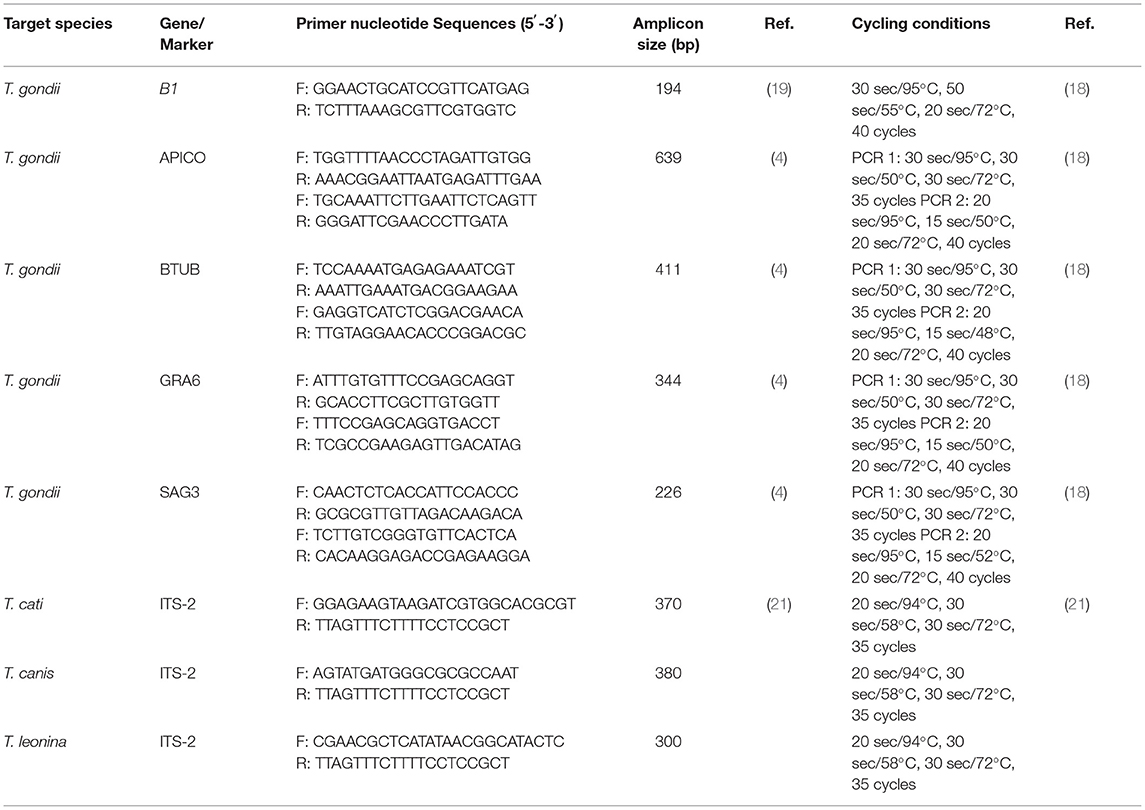
Table 1. Primer sequences and PCR conditions were used for the molecular identification and characterization of Toxoplasma gondii, Toxocara spp., and Toxascaris leonina in the present study.
Molecular identification of Toxocara spp. and T. leonina was performed by species identification PCR procedures to target unique regions of the second internal transcribed spacer (ITS-2) of the ribosomal DNA of T. canis, T. cati, and T. leonina. The amplification of ITS-2 was performed according to the primers and PCR conditions specified by Jacobs et al. (21) in the cat fecal samples and the serum samples of household cat owners. The PCR product of 370 bp from T. cati, 380 bp from T. canis, and 300 bp from T. leonina were amplified by the Tcat1-NC2, Tcan1-NC2, and Tleo1-NC2 primer sets, respectively (Table 1).
PCR reactions were conducted on the samples using primer pairs and conditions listed in Table 1, along with the genomic DNA of the related parasite as a positive control and sterile nuclease-free water as a negative control. The PCR products were analyzed by electrophoresis on 1.5% (w/v) agarose gel.
The PCR-positive amplification products were analyzed and excised from 1% (w/v) agarose gels after electrophoresis to purify using the MinElute gel extraction kit (QIAGEN Ltd., Hilden, Germany), directly sequenced in both directions using forward and reverse primers (Macrogen Inc., Seoul, South Korea). The obtained sequences were verified by the Chromas software (Technelysium Pty Ltd., Queensland, Australia). Then, each parasite sample's corresponding forward and reverse sequences were aligned and assembled using MEGA X software (22). Finally, the final sequences were blasted (http://blast.ncbi.nlm.nih.gov) to identify the genotype of T. gondii, confirm the PCR-positive samples of T. cati, and compare the homology of the obtained sequences of each parasite isolate with the corresponding sequences earlier deposited in the GenBank database.
The obtained sequences were deposited in GenBank under accession numbers LC700048–LC700062, LC700063–LC700066, LC700067–LC700080, and LC700081–LC700088 for BTUB, GRA6, SAG3, and APICO loci of T. gondii, respectively, and LC700099–LC700103 for the ITS-2 of T. cati. The Phylogenetic trees were constructed by maximum likelihood (ML) algorithm with evolutionary distances calculated using the Kimura-2 parameter model and 1000 bootstrap values in the MEGA X software. Figure 2 represents a conceptual overview of sample analysis procedures.
Figure 2. Sample analysis procedures. Image created in Biorender.com.
The descriptive analysis evaluated the prevalence of parasites based on age, sex, breed, weight, and the urban region in cats and age, gender, occupation, residence area, and education in cat owners. Potential associations between qualitative variables were estimated by Chi-square tests with a 95% confidence interval (CIs) in SPSS 24.0 (SPSS Inc., Chicago, IL, USA) to reveal statistically significant differences (P-value <0.05).
The demographic characteristics of 165 cats enrolled in the study, 132 strays and 33 household cats, were categorized into two age groups under or over 1 year old, three breeding groups of DSH (Domestic Short Hair), DLH (Domestic Long Hair), and the Persian cat, three weight groups of <2 kg, between 2 and 4 kg and >4 kg, and five geographic locations of north, center, south, west, and east of Tehran (Table 2). Thirty-three cat owners participated in the investigation, 57.6% men and 42.4% women. The mean age was 38.1 ± 2.15 (M ± SEM) years, ranging from 25 to 68 years. The demographic data of participants included in this study are shown in Table 3.
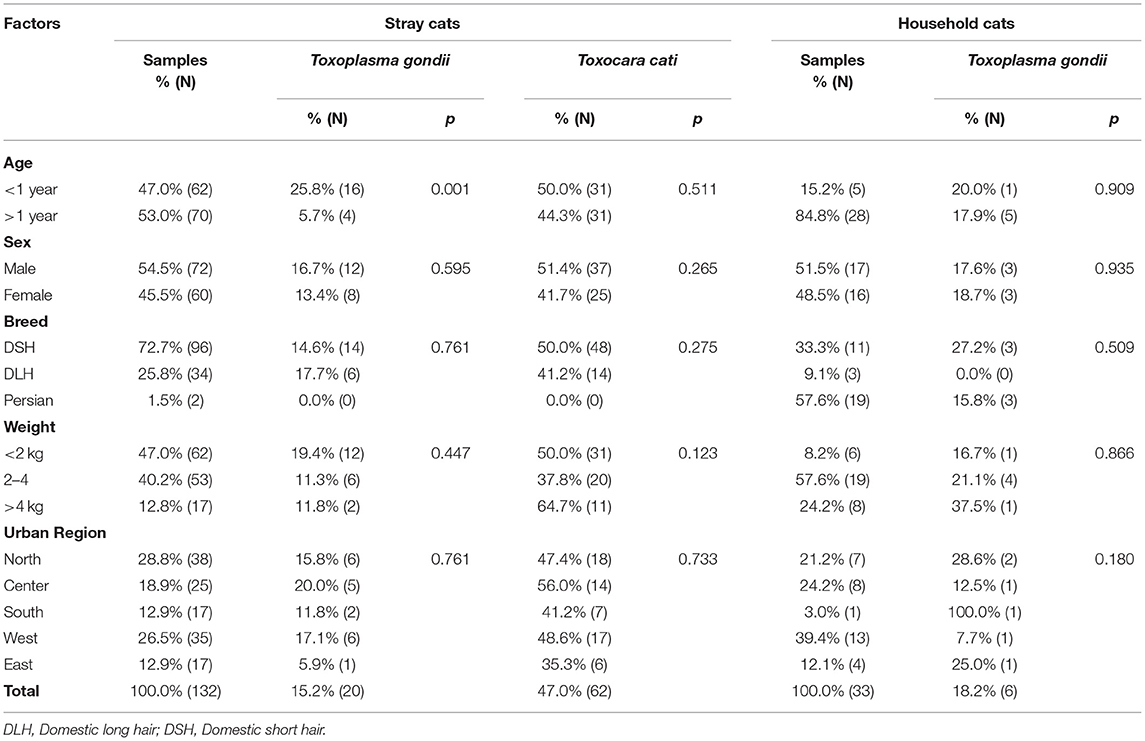
Table 2. Demographic characteristics of the stray and household cats and the prevalence (%) and the number positive (N) of Toxoplasma gondii and Toxocara cati in Tehran.
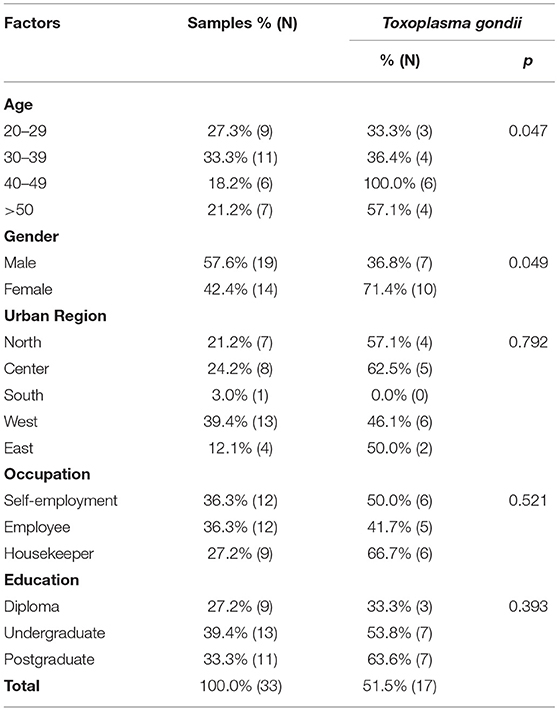
Table 3. Demographic characteristics of the cat owners in Tehran and the prevalence (%) and the number positive (N) of Toxoplasma gondii.
Monitoring of the T. gondii B1 gene showed that 15.2% (20/132; 95% CI 10.0–22.2) of stray cats, 18.2% (6/33; 95% CI 8.6–34.4) of household cats, and 51.5% (17/33; 95% CI 35.2–67.5) of cat owners were infected with T. gondii. There were no statistical differences between the prevalence of T. gondii in stray and household cats. However, the prevalence of T. gondii in cat owners was statistically higher than in both groups of cats. The prevalence of T. gondii infection relative to demographic variables of stray cats, household cats, and cat owners are shown in Tables 2, 3. There was no statistically significant relationship between sex, breed, weight, urban region, and infection with T. gondii in the stray and household cats. Moreover, statistical analysis found no association between urban region, occupation, and education of cat owners and T. gondii infection. However, age affected the prevalence of T. gondii in stray cats and cat owners. The highest prevalence of T. gondii infection was detected in stray cats under 1 year old (25.8%; 95% CI 10.0–22.2; p = 0.001) and cat owners aged 40–49 years (100.0%; 95% CI 61.0–100.0; p = 0.047). Furthermore, the prevalence of T. gondii in the women cat owners 71.4% (10/14; 95% CI 45.4–88.3) was higher than in the men 36.9% (7/19; 95% CI 19.1–59.0) (p = 0.049).
The species identification PCRs of Toxocara spp. and T. leonina revealed a high prevalence of T. cati infection (62/132; 47.0%; 95% CI 38.7–55.4) and no T. canis or T. leonina in stray cats. The ribosomal DNA of T. canis, T. cati, and T. leonina was not detected in the feces of household cats or the serum of cat owners (0/33; 0.0%; 95% CI 0.0–10.4). In addition, the ELISA showed 33 sera samples of cat owners to be Toxocara IgG negative. Consequently, the T. cati infections in stray cats were significantly higher than in household cats and cat owners. Table 2 shows the prevalence of T. cati infection relative to demographic variables of stray cats. There was no statistically significant relationship between any demographic variables and infection with T. cati in the stray cats.
Overall, molecular examination showed at least one T. gondii or T. cati infection occurred in 73 of 132 (55.3%; 95% CI: 47.7–63.6%) stray cats. In addition, coinfections with both parasites were observed in 9 of 132 stray cats (6.8%; 95% CI: 3.0–11.4%). However, coinfections did not ensue in household cats or their owners.
The multi-locus genotyping of 43 B1 positive isolates of T. gondii were performed based on the BTUB, GRA6, SAG3, and APICO genetic markers. The genotype of 26 (60.5%; 95% CI 45.6–73.6) B1 positive isolates were determined with at least one locus, 12, 4, and 10, from stray cats, household cats, and cat owners, respectively. The BTUB, GRA6, SAG3, and APICO loci were amplified and successfully sequenced in 15 (34.9%), 4 (9.3%), 14 (32.6%), and 8 (18.6%) of the B1 positive isolates, respectively (Table 4). The sequencing analysis of four markers showed 9 (34.6%) genotype I, 12 (46.2%) genotype II, and one (3.8%) genotype III (Table 4). Four (14.5%) isolates were inconsistently identified as the genotypes I or II with two markers (Table 4; Supplementary Table S1).
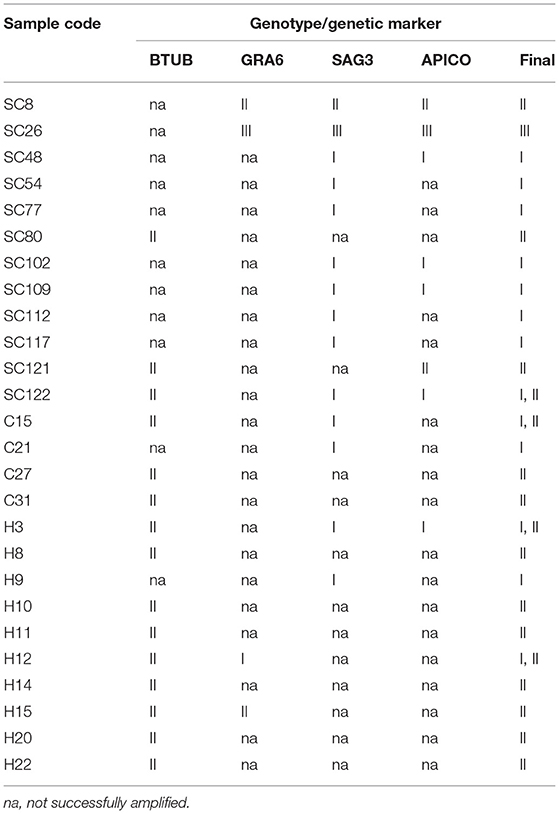
Table 4. Multi-locus genotyping of Toxoplasma gondii isolates from B1 positive samples of stray cats (SC), household cats (C), and cat owners (H).
The multiple alignments of BTUB gene sequences identified the 15 amplified isolates as genotype II from stray cats (three isolates), household cats (three isolates), and cat owners (nine isolates). In addition, sequencing analysis revealed 100% homology between 15 BTUB genotype II isolates and the reference sequence of AF249702 (BEVERLEY) and AY143118 (B7) for genotype II (Supplementary Table S1). Only one isolate from the household cat (C15) and the corresponding owner (H15) were amplified and successfully sequenced at the BTUB locus. Phylogenetic analysis of the BTUB gene located all 15 BTUB amplified isolates in one cluster with genotype II sequence references (AF249702 and AY143118), with a 98% bootstrap value (Figure 3A). Sequencing analysis revealed that four GRA6 amplified isolates belonged to genotype I (one cat owner isolate), II (one stray cat and one cat owner isolate), and III (one stray cat isolate). Moreover, the nucleotide sequence alignment and phylogenetic analysis of GRA6 isolates with the corresponding retrieved genotype I, II, and III sequences of GenBank showed 100% homology (Supplementary Table S1), with 71% or 88% bootstrap value (Figure 3B). Further sequence alignment and phylogenetic analysis of the SAG3 locus identified genotypes I (8 isolates), II (one isolate), and III (one isolate) in stray cats. In contrast, only genotype I was verified in household cats (two isolates) and cat owners (two isolates). Figure 3C illustrates the identified isolates of the SAG3 locus classified in three clusters with sequence references representing genotypes I, II, and III with 89%, 99%, and 96% bootstrap support, respectively. The nucleotide sequence alignment revealed eight APICO amplified isolates belonged to genotype I (four stray cat isolates and one cat owner isolate), II (two stray cat isolates), and III (one stray cat isolate) with 100% homology with sequence references representing genotype I, II and III (Supplementary Table S1). The phylogenetic analysis of APICO isolates was clustered with the corresponding retrieved genotype I, II, and III sequences of GenBank with high bootstrap values (Figure 3D).
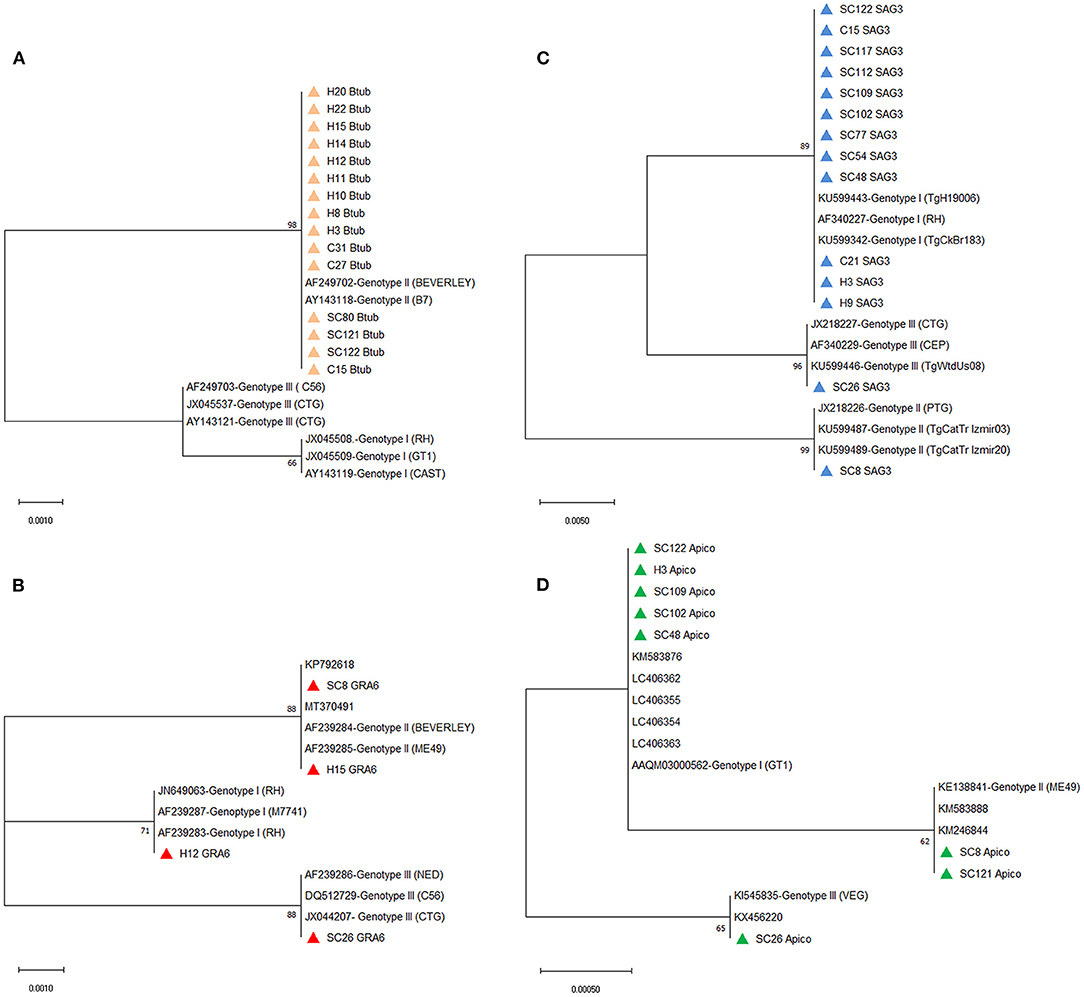
Figure 3. Phylograms of Toxoplasma gondii genotypes were inferred based on the nucleotide sequences of the (A) BTUB, (B) GRA6, (C) SAG3, and (D) APICO. The evolutionary relationship of T. gondii genotypes was constructed by the Maximum Likelihood method and Kimura 2-parameter model, based on the nucleotide sequences of (A) BTUB, (B) GRA6, (C) SAG3, and (D) APICO genetic markers of T. gondii isolated from stray cats [SC], household cats [C], and cat owners [H] retrieved from this study (colored triangles) compared with reference sequences of T. gondii genotype I, II, and III from GenBank. Bootstrap values obtained from 1,000 replicates are indicated on branches in percentage; only bootstrap values >50% are displayed. Evolutionary analyses were conducted in MEGA X.
The multilocus sequence analysis of 26 Toxoplasma-positive isolates revealed genotype I of T. gondii was predominant in stray cats (7/12; 58.3%; 95% CI 32.0–80.7), although genotype II of T. gondii was predominant in household cats (2/4; 50.0%; 95% CI 15.0–85.0) and cat owners (7/10; 70.0%; 95% CI 39.7–89.2). In addition to genotype I (58.3%), genotype II (3/12; 16.7%; 95% CI 4.7–44.8), genotype III (1/12; 8.3%; 95% CI 1.5–35.4), and the mix of genotypes I and II (1/12; 8.3%; 95% CI 1.5–35.4) were identified in stray cats. Genotype I (1/4; 25.0%; 95% CI 4.6–69.9) (1/10; 10.0%; 95% CI 1.8–40.4) and the mix of genotypes I and II (1/4; 25.0%; 95% CI 4.6–69.9) (2/10; 20.0%; 95% CI 5.7–51.0) showed less distribution in household cats and cat owners than genotype II (Table 4).
The species identification PCR procedures of the ITS-2 region of the ribosomal DNA of T. canis, T. cati, and T. leonina showed 62 positive samples of T. cati in stray cats. Sequencing a 370-bp amplified fragment of four T. cati PCR-positive samples confirmed the results. In addition, the nucleotide sequencing analysis of four T. cati isolates revealed 100% homology with the corresponding fragment of the published ITS-2 sequences of T. cati retrieved from GenBank (MT341311 and KJ777157). Further phylogenetic analysis revealed four T. cati isolates in one cluster with the published ITS-2 sequences of T. cati retrieved from GenBank, with a 94% bootstrap value (Figure 4).
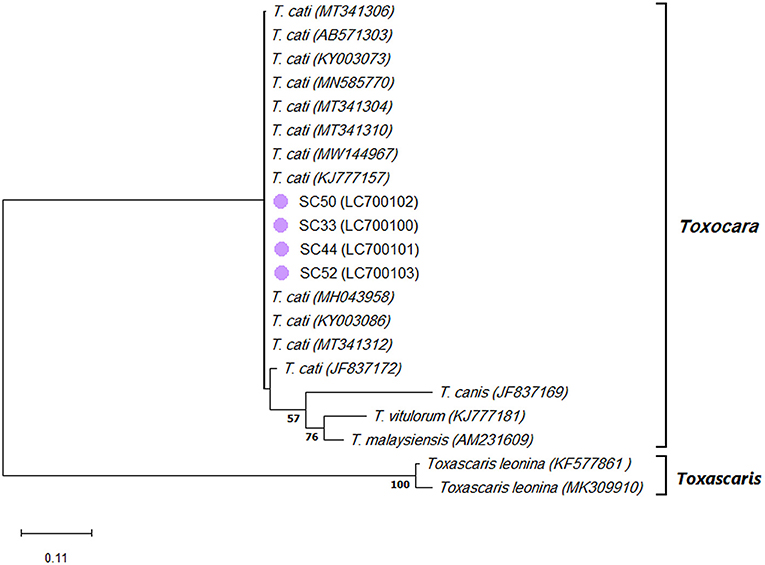
Figure 4. Phylogram of Toxocara spp. and Toxascaris leonina was inferred based on the nucleotide sequences of the ITS-2 region of the ribosomal DNA. The evolutionary relationship of Toxocara spp. and T. leonina was constructed by the Maximum Likelihood method and Kimura 2-parameter model, based on the nucleotide sequences of the ITS-2 region of the ribosomal DNA of Toxocara cati isolated from stray cats [SC] retrieved from this study (purple circles) compared with nucleotide sequences of Toxocara spp. and T. leonina from GenBank. Bootstrap values obtained from 1000 replicates are indicated on branches in percentage; only bootstrap values >50% are displayed. Evolutionary analyses were conducted in MEGA X.
Using the molecular analysis methods, we detected T. gondii and Toxocara spp. infections in the feces of stray and household cats and the blood of cat owners by amplifying the B1 gene of T. gondii and the ITS-2 region of the ribosomal DNA of Toxocara spp. Furthermore, by employing the multilocus genotyping of T. gondii based on BTUB, GRA6, SAG3, and APICO loci, we identified genotyping and genetic variability of T. gondii isolated from cats and humans in Tehran, a metropolitan area in Iran. We found a higher prevalence of T. gondii in cat owners than in cats, affected by the age and gender of humans and only the age of stray cats. Although we identified a high prevalence of T. cati infection in stray cats, we did not detect Toxocara spp. or T. leonina in household cats or their owners. We also revealed the predominance of genotype I of T. gondii in stray cats and genotype II of T. gondii in household cats and cat owners.
Although infected cats usually shed the oocysts once in their lives and for a short time (23, 24), infected humans carry bradyzoites of parasite for long-lasting periods inside their cells, and latent infection may persist. These biological behaviors of T. gondii can explain the higher prevalence of DNA parasites in the cat owner's buffy coat than in cats' feces. The reported prevalence of T. gondii in cats in different parts of the world is variable according to the diagnostic methods used and the variety in geographic, environmental, and diet of studied cats. For example, the prevalence of T. gondii in stray cats in Tehran (15.2%), compared to other molecular studies in Iran, was higher than the previous prevalence reported in Mashhad (4.5%) (25) and Ahvaz (7.2%)(26), and lower than the prevalence (24.1%) reported in Shiraz (27). Furthermore, compared to other molecular studies in the world, it is higher than the previous prevalence reported in South Korea (4.7% and 1.3%) (28, 29), the USA (2%) (30), and Malaysia (13%)(31), and lower than the prevalence reported in South Korea (17.5% and 30.6%)(32, 33) and Pakistan (34%)(34). In addition, the prevalence of T. gondii infection in household cats in the current study (18.2%) was higher than the prevalence reported in Switzerland (0.58%) (35), Poland (2.4%) (36), Malaysia (4%) (31), Thailand (4.7%) (37) and Kenya (7.8%) (38), and lower than reported from Italy (20.5%) (39). To the best of our knowledge, this is the first study performed on the molecular prevalence of T. gondii infection in cat owners worldwide. A systematic review and meta-analysis estimated that the seroprevalence of T. gondii infection in the general Iranian population was 39.3%, reported at 49% in Tehran (40), which is very close to our results (51.5%). Our previous study (18) confirms that detecting DNA circulating in the blood is comparable to the seropositive results.
The investigation of possible factors associated with T. gondii infection showed it was more frequent in stray cats under 1 year old (p = 0.001), similar to a study conducted in South Korea (28). However, Khodaverdi et al. (25) in Mashhad and Kwak et al. (29) in South Korea had not observed any statistically significant relationship between the age of cats and the prevalence of T. gondii infection. Moreover, a significant difference between cat owners' age and the prevalence rate was observed, comparable to the highest incidence previously reported in the age group over 40 years (40) and the correlation between increasing age and the prevalence of infection (41). It is assumed that through passing the years, exposure to microorganisms increases, whether through contaminated food and water, contact with infected cats, or other risk factors. The higher T. gondii infection in the women cat owners observed in our study was not confirmed by the earlier seroprevalence studies (40).
Multi-locus genotyping of BTUB, GRA6, SAG3, and APICO loci of Toxoplasma-positive isolates revealed genotype I in stray cats and genotype II in household cats and cats' owners were predominant. In addition, three genotypes of T. gondii and the mix of genotypes I and II were typed in the stray cats of Tehran, which demonstrated more variables than previous studies. For example, genotype I (one case), III (32 cases), and the mix of I and III (two cases) in Ahvaz, southwest of Iran, by PCR-RFLP of SAG2 locus (26), or genotype II in Mashhad (one case), northeastern Iran, by PCR-RFLP of SAG3 locus (25), and in South Korea (two isolates) (28) by sequencing SAG5D and SAG5E genes were reported. Therefore, compared to our study, genotyping the limited number of samples or typing with just one locus in the mentioned reports is the foremost possible cause for not detecting all genotypes. Furthermore, as reported before, conflicting findings in multilocus typing could be seen regarding the small sample size (42).
Despite the ubiquity of T. gondii infections, the presence of comprehensive multi-locus studies investigating the T. gondii genetic diversity in household cats and their cat owners are lacking. However, most studies in Iran have reported the T. gondii genotype of different hosts or soil isolates based on the analysis of one or two loci (43–50). In limited studies performed on household cats, in Switzerland, mixed infections with types I and II in the only positive sample, and in Germany, type II (83.8%) and type III (1.4%) have been reported in household cats (35, 51). We characterized the simultaneous T. gondii infection in only a household cat (C15) and its owner (H15). The mix-infection of genotypes I and II in the cat and the genotype II in the cat owner was typed. Although we cannot definitively and accurately explain this finding, it is assumed that the cat was infected due to hunting or contact with contaminated soil through its roaming behavior, according to the declaration of the cat owner. Therefore, it is also possible that the cat and its owner were infected from two different sources.
The species identification PCRs of Toxocara spp. and T. leonina revealed a high prevalence of T. cati infection (62/132; 47.0%; 95% CI 38.7–55.4) and no T. canis and T. leonina in stray cats. The ribosomal DNA of T. canis, T. cati, and T. leonina was not detected in the feces of household cats or the serum of cat owners. The high prevalence of T. cati infection (47.0%) was identified in stray cats by detecting the ribosomal DNA of T. cati in the feces. This finding increased the concern about the potential role of the cats as significant reservoirs for humans, especially children. Nevertheless, we did not find any cases of infection in the feces of household cats or the serum of cat owners. However, the reported prevalence of T. cati infection in cats has a variety of ranges depending on geographical regions and the detection methods. A relatively limited number of studies in different parts of Iran and the world have investigated the molecular prevalence of these nematodes in the feces of stray cats. The prevalence of T. cati in the current study was higher than in other studies performed on stray cats in the different cities of Iran (16, 52, 53) and Malaysia (54). In this study, we performed PCR to detect the DNA of these nematodes in the serum of cat owners and the feces of household cats for the first time, which was confirmed by not detecting the Toxocara IgG in the sera of cat owners. However, the anti-Toxocara antibodies were reported in the sera of residents of northeastern Iran (7.2%) (55) and northern Iran (23.5%) (56). None of the household cats or their owners were positive, which could be due to the lack of risk factors such as younger age and living in rural areas (56) or the result of attention to hygiene principles, routine anthelmintic therapy, and adequate veterinary care. Nevertheless, the high prevalence of T. cati infection in stray cats can cause contamination of the environment by excreting eggs that lead to infecting humans through soil or water. Therefore, the control and treatment of infection in cats are necessary to reduce the source of human disease and other paratenic hosts.
The main limitation of this study was collected samples from the sick or injured stray cats brought to veterinary centers and pet hospitals. Therefore, the results might not be a good representation of the entire stray cat population in the area, and this limitation should be considered in further studies. Another limitation was the relatively small sample size of household cats and their owners compared to the stray cats, which might be affected the prevalence and risk factors of infections. Nevertheless, finding many cat owners who didn't have other pets or had no close contact with other animals and were willing to cooperate in the study was difficult. Therefore, future studies with larger sample sizes are recommended to confirm the reported results in this study.
The high prevalence of T. cati infection (47%) in stray cats leads to considering the role of cats in human toxocariasis. Whereas the low prevalence of T. gondii in stray cats (15.2%) and household cats (18.2%) compared to their owners (51.5%) suggests a more subordinate role of direct contact with infected cats than consuming contaminated undercooked meat containing tissue cysts or contaminated food and water with sporulated oocysts in cases of human infections. The relatively high prevalence of Toxocara infection in cats necessitates attention to routine urban cat deworming programs and public health education in urban management to generally promote physical and mental health in the community and specifically live happily and healthy coexistence with these urban animals.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants and animal study were reviewed and approved by the Iranian Ministry of Health, Treatment, and Medical Education. The experimental protocols were reviewed by the Ethics Committee of Iran University of Medical Sciences (Approval No IR.IUMS.FMD.REC 1396.31834). The patients/participants provided their written informed consent to participate in this study.
PK: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, and original draft writing. SS-S methodology, validation, formal analysis, investigation, resources, and data curation. ARM: validation, review, and editing of the final manuscript. GN: methodology and formal analysis. ER: conceptualization, methodology, validation, formal analysis, resources, data curation, original draft writing, review and editing of the final manuscript, visualization, supervision, project administration, and funding acquisition. All authors contributed to the article and approved the submitted version.
The Iran University of Medical Sciences financially supported this study under grant number 96-03-30-31834 to ER.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to the cat owners who donated their blood samples and their cat feces samples for this study. We want to thank the authorities, veterinarians, and staff of veterinary clinics and pet hospitals for their help providing samples. This work represents a part of the Ph.D. dissertation of PK.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.927185/full#supplementary-material
APICO, apicoplast genetic marker; BTUB, Beta-tubulin; DLH, Domestic long hair; DSH, Domestic short hair; GRA6, granule dense 6; ITS-2, Internal transcribed spacer region 2; MEGA, molecular evolutionary genetic analysis; Kimura, 2-parameter; ML, maximum likelihood; NJ, neighbor-joining; nested PCR, nested polymerase chain reaction; PVPP, Polyvinylpolypyrrolidone; SAG3, Surface antigen 3; SNPs, single nucleotide polymorphisms.
1. Levy JK, Crawford PC. Humane strategies for controlling feral cat populations. J Am Vet Med Assoc. (2004) 225:1354–60. doi: 10.2460/javma.2004.225.1354
2. Kvac M, Hofmannova L, Ortega Y, Holubova N, Horcickova M, Kicia M, et al. Stray cats are more frequently infected with zoonotic protists than pet cats. Folia Parasitol. (2017) 64:034. doi: 10.14411/fp.2017.034
3. Smith NC, Goulart C, Hayward JA, Kupz A, Miller CM, van Dooren GG. Control of human toxoplasmosis. Int J Parasitol. (2021) 51:95–121. doi: 10.1016/j.ijpara.2020.11.001
4. Su C, Shwab EK, Zhou P, Zhu XQ, Dubey JP. Moving towards an integrated approach to molecular detection and identification of Toxoplasma gondii. Parasitology. (2010) 137:1–11. doi: 10.1017/S0031182009991065
5. Blader IJ, Coleman BI, Chen C-T, Gubbels M-J. Lytic cycle of Toxoplasma gondii: 15 years later. Annu Rev Microbiol. (2015) 69:463–85. doi: 10.1146/annurev-micro-091014-104100
6. Monteiro RM, Pena HF, Gennari SM, de Souza SO, Richtzenhain LJ, Soares RM. Differential diagnosis of oocysts of Hammondia-like organisms of dogs and cats by PCR-RFLP analysis of 70-kilodalton heat shock protein (HSP70) gene. Parasitol Res. (2008) 103:235–8. doi: 10.1007/s00436-008-0957-9
7. Robert-Gangneux F, Dardé M-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. (2012) 25:264–96. doi: 10.1128/CMR.00026-12
8. Herrmann DC, Maksimov P, Hotop A, Groß U, Däubener W, Liesenfeld O, et al. Genotyping of samples from German patients with ocular, cerebral and systemic toxoplasmosis reveals a predominance of Toxoplasma gondii type II. Int J Med Microbiol. (2014) 304:911–6. doi: 10.1016/j.ijmm.2014.06.008
9. Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: Correlation of parasite genotype with human disease. J Infect Dis. (1995) 172:1561–6. doi: 10.1093/infdis/172.6.1561
10. Sibley LD, Khan A, Ajioka JW, Rosenthal BM. Genetic diversity of Toxoplasma gondii in animals and humans. Philos Trans R Soc Lond B Biol Sci. (2009) 364:2749–61. doi: 10.1098/rstb.2009.0087
11. Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. (2010) 104:3–23. doi: 10.1179/136485910X12607012373957
12. Ma G, Holland CV, Wang T, Hofmann A, Fan C-K, Maizels RM, et al. Human toxocariasis. Lancet Infect Dis. (2018) 18:e14–24. doi: 10.1016/S1473-3099(17)30331-6
13. Mikaeili F, Mirhendi H, Hosseini M, Asgari Q, Kia EB. Toxocara nematodes in stray cats from Shiraz, southern Iran: intensity of infection and molecular identification of the isolates. Iran J Parasitol. (2013) 8:593–600.
14. Ozlati M, Spotin A, Shahbazi A, Mahami-Oskouei M, Hazratian T, Adibpor M, et al. Genetic variability and discrimination of low doses of Toxocara spp. from public areas soil inferred by loop-mediated isothermal amplification assay as a field-friendly molecular tool. Vet world. (2016) 9:1471. doi: 10.14202/vetworld.2016.1471-1477
15. Valizadeh M, Biderouni FT, Shahrokhi S, Ghanimatdan M, Nagahi A. Sequencing and phylogenetic analysis of mitochondrial cox1 and nad1 genes in Toxocara canis and Toxascaris leonina isolates from Iran. Bulg J Vet Med. (2021) 24:251–60. doi: 10.15547/bjvm.2274
16. Khademvatan S, Rahim F, Tavalla M, Abdizadeh R, Hashemitabar M. PCR-based molecular characterization of Toxocara spp. using feces of stray cats: A study from Southwest Iran. PLoS ONE. (2013) 8:e65293. doi: 10.1371/journal.pone.0065293
17. Coklin T, Farber J, Parrington L, Dixon B. Prevalence and molecular characterization of Giardia duodenalis and Cryptosporidium spp. in dairy cattle in Ontario, Canada. Vet Parasitol. (2007) 150:297–305. doi: 10.1016/j.vetpar.2007.09.014
18. Arshadi M, Akhlaghi L, Meamar AR, Alizadeh Ghavidel L, Nasiri K, Mahami-Oskouei M, et al. Sero-molecular detection, multi-locus genotyping, and clinical manifestations of ocular toxoplasmosis in patients in northwest Iran. Trans R Soc Trop Med Hyg. (2019) 113:195–202. doi: 10.1093/trstmh/try137
19. Schwab KJ, McDevitt JJ. Development of a PCR-enzyme immunoassay oligoprobe detection method for Toxoplasma gondii oocysts, incorporating PCR controls. Appl Environ Microbiol. (2003) 69:5819–25. doi: 10.1128/AEM.69.10.5819-5825.2003
20. Grigg ME, Ganatra J, Boothroyd JC, Margolis TP. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J Infect Dis. (2001) 184:633–9. doi: 10.1086/322800
21. Jacobs DE, Zhu X, Gasser RB, Chilton NB. PCR-based methods for identification of potentially zoonotic ascaridoid parasites of the dog, fox and cat. Acta Trop. (1997) 68:191–200. doi: 10.1016/S0001-706X(97)00093-4
22. Kumar S, Stecher G, Li M, Knyaz C, Tamura K, MEGA X. Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol. (2018) 35:1547–9. doi: 10.1093/molbev/msy096
23. Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol. (2008) 38:1257–78. doi: 10.1016/j.ijpara.2008.03.007
24. Elmore SA, Jones JL, Conrad PA, Patton S, Lindsay DS, Dubey JP. Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. (2010) 26:190–6. doi: 10.1016/j.pt.2010.01.009
25. Khodaverdi M, Razmi G. Prevalence and genotyping of Toxoplasma gondii in stray cats in Mashhad area, Iran. BMC Vet Res. (2019) 15:463. doi: 10.1186/s12917-019-2176-2
26. Tavalla M, Asgarian F, Kazemi F. Prevalence and genetic diversity of Toxoplasma gondii oocysts in cats of southwest of Iran. Infect Dis Health. (2017) 22:203–9. doi: 10.1016/j.idh.2017.08.003
27. Asgari Q, Mohammadpour I, Pirzad R, Kalantari M, Motazedian MH, Naderi S. Molecular and serological detection of Toxoplasma gondii in stray cats in Shiraz, South-central, Iran. Iran J Parasitol. (2018) 13:430–9.
28. Jung BK, Lee SE, Lim H, Cho J, Kim DG, Song H, et al. Toxoplasma gondii B1 gene detection in feces of stray cats around Seoul, Korea and genotype analysis of two laboratory-passaged isolates. Korean J Parasitol. (2015) 53:259–63. doi: 10.3347/kjp.2015.53.3.259
29. Kwak D, Seo M-G. Genetic analysis of zoonotic gastrointestinal protozoa and Microsporidia in shelter cats in South Korea. Pathogens. (2020) 9:894. doi: 10.3390/pathogens9110894
30. Lilly EL, Wortham CD. High prevalence of Toxoplasma gondii oocyst shedding in stray and pet cats (Felis catus) in Virginia, United States. Parasit Vectors. (2013) 6:266. doi: 10.1186/1756-3305-6-266
31. Nasiru Wana M, Mohd Moklas MA, Watanabe M, Zasmy Unyah N, Alhassan Abdullahi S, Ahmad Issa Alapid A, et al. Molecular detection and genetic diversity of Toxoplasma gondii oocysts in cat faeces from Klang Valley, Malaysia, using B1 and REP genes in 2018. Pathogens. (2020) 9:576. doi: 10.3390/pathogens9070576
32. Lee S-E, Kim N-H, Chae H-S, Cho S-H, Nam H-W, Lee W-J, et al. Prevalence of Toxoplasma gondii infection in feral cats in Seoul, Korea. J Parasitol. (2011) 97:153–5. doi: 10.1645/GE-2455.1
33. Lee S-E, Kim J-Y, Kim Y-A, Cho S-H, Ahn H-J, Woo H-M, et al. Prevalence of Toxoplasma gondii infection in stray and household cats in regions of Seoul, Korea. Korean J Parasitol. (2010) 48:267–70. doi: 10.3347/kjp.2010.48.3.267
34. Majid A, Ahmad N, Haleem S, Akbar Nu, Zareen S, Taib M, et al. Detection of toxoplasmosis in pets and stray cats through molecular and serological techniques in Khyber Pakhtunkhwa, Pakistan. BMC Vet Res. (2021) 17:357. doi: 10.1186/s12917-021-03064-9
35. Berger-Schoch AE, Herrmann DC, Schares G, Müller N, Bernet D, Gottstein B, et al. Prevalence and genotypes of Toxoplasma gondii in feline faeces (oocysts) and meat from sheep, cattle and pigs in Switzerland. Vet Parasitol. (2011) 177:290–7. doi: 10.1016/j.vetpar.2010.11.046
36. Sroka J, Karamon J, Dutkiewicz J, Wójcik Fatla A, Zajac V, Cencek T. Prevalence of Toxoplasma gondii infection in cats in southwestern Poland. Ann Agric Environ Med. (2018) 25:576–80. doi: 10.26444/aaem/94675
37. Chemoh W, Sawangjaroen N, Nissapatorn V, Sermwittayawong N. Molecular investigation on the occurrence of Toxoplasma gondii oocysts in cat feces using TOX-element and ITS-1 region targets. Vet J. (2016) 215:118–22. doi: 10.1016/j.tvjl.2016.05.018
38. Nyambura Njuguna A, Kagira JM, Muturi Karanja S, Ngotho M, Mutharia L, Wangari Maina N. Prevalence of Toxoplasma gondii and other gastrointestinal parasites in domestic cats from households in Thika Region, Kenya. Biomed Res Int. (2017) 2017:7615810. doi: 10.1155/2017/7615810
39. Veronesi F, Santoro A, Milardi GL, Diaferia M, Morganti G, Ranucci D, et al. Detection of Toxoplasma gondii in faeces of privately owned cats using two PCR assays targeting the B1 gene and the 529-bp repetitive element. Parasitol Res. (2017) 116:1063–9. doi: 10.1007/s00436-017-5388-z
40. Daryani A, Sarvi S, Aarabi M, Mizani A, Ahmadpour E, Shokri A, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: a systematic review and meta-analysis. Acta Trop. (2014) 137:185–94. doi: 10.1016/j.actatropica.2014.05.015
41. Wyman CP, Gale SD, Hedges-Muncy A, Erickson LD, Wilson E, Hedges DW. Association between Toxoplasma gondii seropositivity and memory function in nondemented older adults. Neurobiol Aging. (2017) 53:76–82. doi: 10.1016/j.neurobiolaging.2017.01.018
42. Ajzenberg D, Yera H, Marty P, Paris L, Dalle F, Menotti J, et al. Genotype of 88 Toxoplasma gondii isolates associated with toxoplasmosis in immunocompromised patients and correlation with clinical findings. J Infect Dis. (2009) 199:1155–67. doi: 10.1086/597477
43. Abdoli A, Arbabi M, Pirestani M, Mirzaghavami M, Ghaffarifar F, Dalimi A, et al. Molecular assessment of Neospora caninum and Toxoplasma gondii in hooded crows (Corvus cornix) in Tehran, Iran. Comp Immunol Microbiol Infect Dis. (2018) 57:69–73. doi: 10.1016/j.cimid.2018.06.008
44. Dalir Ghaffari A, Dalimi A. Molecular identification of Toxoplasma gondii in the native slaughtered cattle of Tehran Province, Iran. J Food Qual Hazards Control. (2019) 6:153–61. doi: 10.18502/jfqhc.6.4.1993
45. Tavalla M, Oormazdi H, Akhlaghi L, Shojaee S, Razmjou E, Hadighi R, et al. Genotyping of Toxoplasma gondii isolates from soil samples in Tehran, Iran. Iran J Parasitol. (2013) 8:227–33.
46. Zia-Ali N, Fazaeli A, Khoramizadeh M, Ajzenberg D, Dardé M, Keshavarz-Valian H. Isolation and molecular characterization of Toxoplasma gondii strains from different hosts in Iran. Parasitol Res. (2007) 101:111–5. doi: 10.1007/s00436-007-0461-7
47. Armand B, Solhjoo K, Kordshooli MS, Davami MH, Pourahmad M, Orfaee V. Toxoplasma gondii type I, predominant genotype isolated from sheep in South of Iran. Vet World. (2017) 10:386–92. doi: 10.14202/vetworld.2017.386-392
48. Arefkhah N, Pourabbas B, Asgari Q, Moshfe A, Mikaeili F, Nikbakht G, et al. Molecular genotyping and serological evaluation of Toxoplasma gondii in mothers and their spontaneous aborted fetuses in Southwest of Iran. Comp Immunol Microbiol Infect Dis. (2019) 66:101342. doi: 10.1016/j.cimid.2019.101342
49. Sharif M, Amouei A, Sarvi S, Mizani A, Aarabi M, Hosseini S-A, et al. Genetic diversity of Toxoplasma gondii isolates from ruminants: A systematic review. Int J Food Microbiol. (2017) 258:38–49. doi: 10.1016/j.ijfoodmicro.2017.07.007
50. Maani S, Rezanezhad H, Solhjoo K, Kalantari M, Erfanian S. Genetic characterization of Toxoplasma gondii isolates from human spontaneous aborted fetuses in Jahrom, southern Iran. Microb Pathog. (2021) 161:105217. doi: 10.1016/j.micpath.2021.105217
51. Herrmann D, Pantchev N, Vrhovec MG, Barutzki D, Wilking H, Fröhlich A, et al. Atypical Toxoplasma gondii genotypes identified in oocysts shed by cats in Germany. Int J Parasitol. (2010) 40:285–92. doi: 10.1016/j.ijpara.2009.08.001
52. Azimian H, Shokrani H, Fallahi S. Molecular evaluation of Toxocara species in stray cats using loop-mediated isothermal amplification (LAMP) technique as a rapid, sensitive and simple screening assay. Vet Med Sci. (2021) 7:647–53. doi: 10.1002/vms3.431
53. Torkan S, Ghandehari-Alavijeh M, Khamesipour F. Survey of the prevalence of Toxocara cati in stray cats in Isfahan city, Iran by PCR method. Trop biomed. (2017) 34:550–5.
54. Tun S, Ithoi I, Mahmud R, Samsudin NI, Kek Heng C, Ling LY. Detection of helminth eggs and identification of Hookworm species in stray cats, dogs and soil from Klang Valley, Malaysia. PLoS ONE. (2015) 10:e0142231. doi: 10.1371/journal.pone.0142231
55. Rezaiemanesh MR, Afzalaghaee M, Hamidi S, Eshaghzadeh A, Paydar M, Hejazi SH. Prevalence of toxocariasis and its related risk factors in humans, dogs and cats in northeastern Iran: a population-based study. Trans R Soc Trop Med Hyg. (2019) 113:399–409. doi: 10.1093/trstmh/trz011
Keywords: Toxoplasma gondii, Toxocara spp., zoonosis, human, cat, public health, Tehran, Iran
Citation: Karimi P, Shafaghi-Sisi S, Meamar AR, Nasiri G and Razmjou E (2022) Prevalence and Molecular Characterization of Toxoplasma gondii and Toxocara cati Among Stray and Household Cats and Cat Owners in Tehran, Iran. Front. Vet. Sci. 9:927185. doi: 10.3389/fvets.2022.927185
Received: 23 April 2022; Accepted: 25 May 2022;
Published: 22 June 2022.
Edited by:
Vikrant Sudan, Guru Angad Dev Veterinary and Animal Sciences University, IndiaReviewed by:
Veeranoot Nissapatorn, Walailak University, ThailandCopyright © 2022 Karimi, Shafaghi-Sisi, Meamar, Nasiri and Razmjou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elham Razmjou, cmF6bWpvdS5lQGl1bXMuYWMuaXI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.