- Hubei Key Laboratory of Animal Nutrition and Feed Science, Wuhan Polytechnic University, Wuhan, China
A total of 480 one-day-old AA broiler chicks were randomly allocated to one of four treatments in a 2 × 2 factorial to investigate the effects of tannic acid (TA) on growth performance, relative organ weight, antioxidant capacity, and intestinal health in broilers dietary exposed to aflatoxin B1 (AFB1). Treatments were as follows: (1) CON, control diet; (2) TA, CON + 250 mg/kg TA; (3) AFB1, CON + 500 μg/kg AFB1; and (4) TA+AFB1, CON + 250 mg/kg TA + 500 μg/kg AFB1. There were 10 replicate pens with 12 broilers per replicate. Dietary AFB1 challenge increased the feed conversion ratio during days 1 to 21 (P < 0.05). The TA in the diet did not show significant effects on the growth performance of broilers during the whole experiment period (P > 0.05). The liver and kidney relative weight was increased in the AF challenge groups compared with the CON (P < 0.05). The addition of TA could alleviate the relative weight increase of liver and kidney caused by AFB1 (P < 0.05). Broilers fed the AFB1 diets had lower activity of glutathione peroxidase, catalase, total superoxide dismutase, S-transferase, and total antioxidant capacity in plasma, liver and jejunum, and greater malondialdehyde content (P < 0.05). Dietary supplemented with 250 mg/kg TA increased the activities of antioxidative enzymes, and decreased malondialdehyde content (P < 0.05). In addition, AFB1 significantly reduced the villus height and crypt depth ratio in the ileum on day 42 (P < 0.05). In conclusion, supplementation with 250 mg/kg TA could partially protect the antioxidant capacity and prevent the enlargement of liver in broilers dietary challenged with 500 μg/kg AFB1.
Introduction
Aflatoxins are mainly produced by Aspergillus flavus and Aspergillus parasiticus, and widely exist in food and feed that are frequently caused health and economic problems in many countries (1). Among the 18 types of aflatoxin derivatives, aflatoxin B1 (AFB1) is the most common and toxic in the poultry feed industry (2). Poultry is extremely sensitive to AFB1, and long-term exposure to AFB1 may cause growth retardation, immunosuppression, hepatotoxic, and even death (3–5). Oxidative stress has been reported to play a significant role in the toxicity mechanism caused by AFB1 (6, 7). FDA (8) refines the maximum concentration of aflatoxin in poultry is 100 μg/kg of feed, whereas 500 μg/kg can be a practical testing concentration in feedstuff in the USA.
Chinese gallnut tannic acid (TA) belongs to the hydrolyzed tannin family, and is a polyphenolic compound of high molecular weight (500–3,000 Da), which can remove free radicals and prevent lipid oxidation (9). Because of the polyphenolic hydroxyl structure, the TA has various biological activities, such as antimicrobial, anti-inflammatory, anticancer, and immunomodulatory effects (10–12). Moreover, studies have shown that dietary supplementation with antioxidants, including plant extracts and tannins can protect broilers from AFB1-induced toxicity by enhancing the antioxidant capacity and immunity (6, 13–16). Nevertheless, it remains unclear whether dietary supplementation with TA could alleviate acute aflatoxicosis by improving the antioxidant capacity of broilers fed AFB1 contaminated diets.
Therefore, the aim of this study was to determine the effects of the TA on growth performance, antioxidative status, and intestinal histomorphology of broilers exposed to feed contaminated with 500 μg/kg AFB1.
Materials and methods
All animal procedures used in this study were performed in the experimental farm of Wuhan Polytechnic University, and were approved by the Institutional Animal Care and Use Committee of Wuhan Polytechnic University (Number: 20161121).
AFB1 and TA
The AFB1 (purity ≥98%, HPLC) was produced from Aspergillus flavus provided by Qingdao Pribolab Biological Engineering Company Limited (Shandong, China), and the AFB1 concentration in the feed was designed to 500 μg/kg in AFB1 treatments. Dietary AFB1 concentrations were confirmed by analysis (17). Briefly, feed samples were extracted with acetonitrile:water (86:14), and an aliquot of the extract was passed through a puriTox TC-M160 cleanup column (Trilogy Analytical Laboratory Inc., Washington, MO, USA) and suitably diluted with water before analysis using HPLC with Kobra cell postcolumn derivatization with fluorescence detection at 365 nm excitation and 440 nm emission.
The hydrolysable TA was extracted from Chinese gallnut by the Wufeng Chicheng Biotechnology Company Limited (Yichang, China), which contained ≥80% tannin, crude fiber <2.00%, ash <2.50%, and moisture <8.00%.
Dietary treatments and animal management
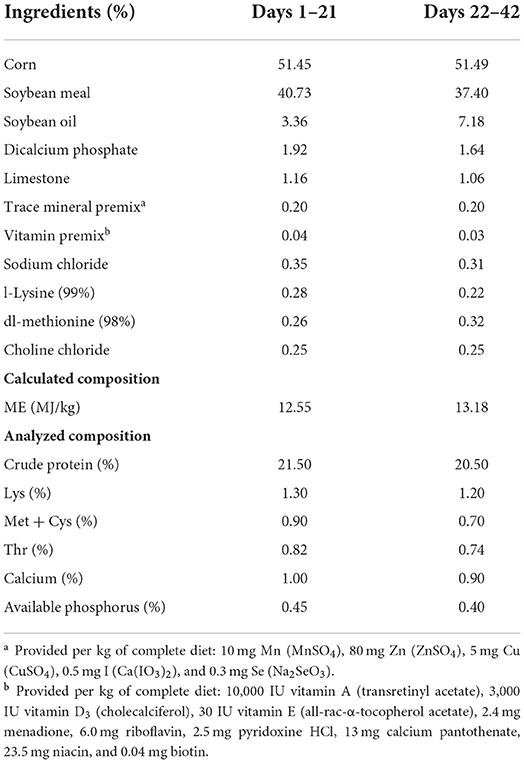
A 2 × 2 factorial complete randomized block design was employed and 480 one-day-old sex-mixed AA broilers were randomly assigned to 4 treatment groups, each with 10 replicates of 12 birds per pen. Experimental diets were as follows: (1) CON, basal diet; (2) TA, CON + 250 mg/kg A; (3) AFB1, CON + 500 μg/kg TA; and (4) TA+AFB1, CON + 250 mg/kg TA + 500 μg/kg AFB1. The basal diet was formulated to meet or exceed the nutrient requirements of AA broilers. Diets were fed in 2 phases: phase 1 (from days 1 to 21) and phase 2 (from days 22 to 42). The composition and nutrient levels of the basal diets are presented in Table 1.
All broiler chicks were reared in stainless steel pens (1.4 m × 1.4 m) in an environmentally controlled room at the Animal Research Center of Wuhan Polytechnic University and given ad libitum access to diets and water throughout the study. The room temperature was maintained at 33 ± 2°C for the first week and then gradually decreased to 24°C until the end of the experiment, and broilers were maintained on a 23 h constant light and 1 h darkness every day throughout the whole trial.
Growth performance
Broilers and feed were weighed on the beginning, days 21 and 42 of the trial, and calculated the average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR).
Sample collection
On days 21 and 42, two broilers from each replicate (20 broilers per group) were randomly selected and blood samples were aseptically collected from the wing vein into vacuum blood vessels. Plasma was obtained by centrifuging (3,000 × g for 15 min at 4°C) the whole blood and stored at −20°C for the assay of antioxidative parameters.
Then, the same broilers were weighed individually and euthanized by cervical dislocation. The liver, spleen, bursa of Fabricius, thymus, and kidney were removed cleaned of the adhering tissue by trained personnel and weighed. Relative organ weights were calculated as follows: Relative weight = (Organ weight)/(Final body weight) × 1,000. The small intestine was removed and gently cleaned with ice-cold saline. Intestinal segments (1–2 cm) taken from the mid-region of the duodenum, jejunum, and ileum were immediately fixed in 4% paraformaldehyde for the examination of morphological parameters. Additionally, the portion of liver and jejunum were sampled and stored at −80°C for analysis of antioxidant status.
Antioxidative status
Approximately 1 g of liver or jejunum was homogenized in 10 mL of ice-cold saline and centrifuged at 2,500 × g, 4°C for 10 min. The supernatants were collected for further analysis. The activities of glutathione peroxidase (GSH-Px), total superoxide dismutase (T-SOD), total antioxidant capacity (T-AOC), glutathione S-transferase (GST), catalase (CAT), and the content of malondialdehyde (MDA) in the plasma and supernatants were measured using purchased assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the instructions of the manufacturer (18).
Intestinal histomorphology
The intestinal histomorphology was measured as described by Guo et al. (19). Briefly, the fixed intestinal segments were embedded in paraffin. Consecutive sections (5 μm) were stained with hematoxylin and eosin and were observed for histomorphological examination. The measurements were performed with an Olympus optical microscope using ProgRes CapturePro software (Jenoptik, Jena, Germany). The villus height and crypt depth were measured from 10 randomly selected villi and associated crypts on each section at 40 × magnification. Villus height was measured from the tip of the villus to the crypt opening and crypt depth was measured from the base of the crypt to the level of the crypt opening. The villus height to crypt depth ratio (V/C) was then calculated from these measurements.
Statistical analyses
All experiment data were analyzed by a two-way ANOVA analysis using the GLM procedure of SPSS 26.0 software. In cases where the differences were significant, the means were compared by Duncan's multiple range test. The results are shown as mean and the standard error of mean (SEM). Significance was considered at P < 0.05, and 0.05 ≤ P < 0.10 was considered to have a trend of difference.
Results
Dietary analyses of AFB1
Biochemical tests indicated that the CON and TA diets were negative for AFB1 throughout the experiment. The analyzed concentration of AFB1 in AFB1 and AFB1+TA diets were 505.9 vs. 503.2 μg/kg during days 1 to 21, and 520.3 vs. 521.3 μg/kg during days 22–42, respectively.
Growth performance
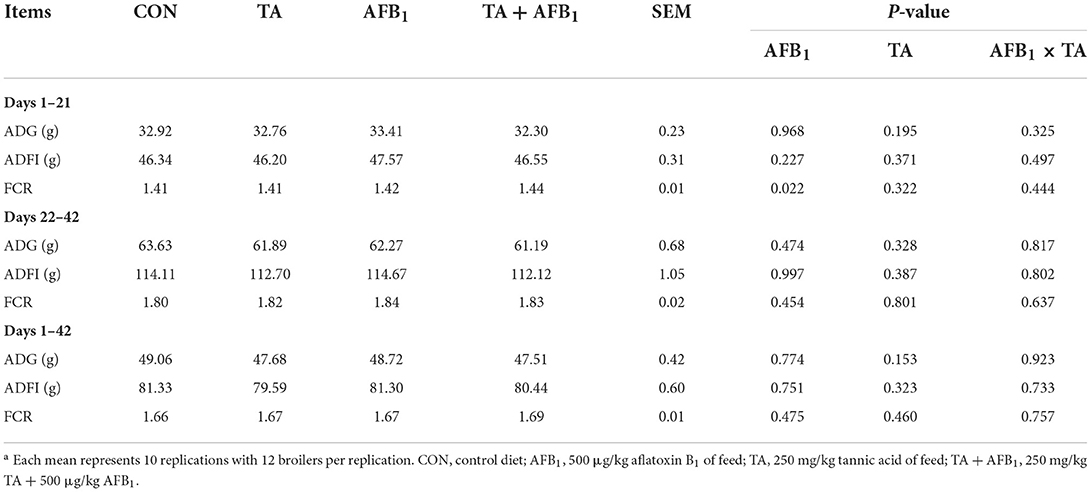
As shown in Table 2, AFB1 challenge increased the FCR during days 1–21 (P < 0.05). The addition of TA in the diet did not show significant effects on the ADG, ADFI, and FCR of broilers during the whole experiment period (P > 0.05). No interaction effect was observed between AFB1 and TA on the growth performance (P > 0.05).
Relative organ weight
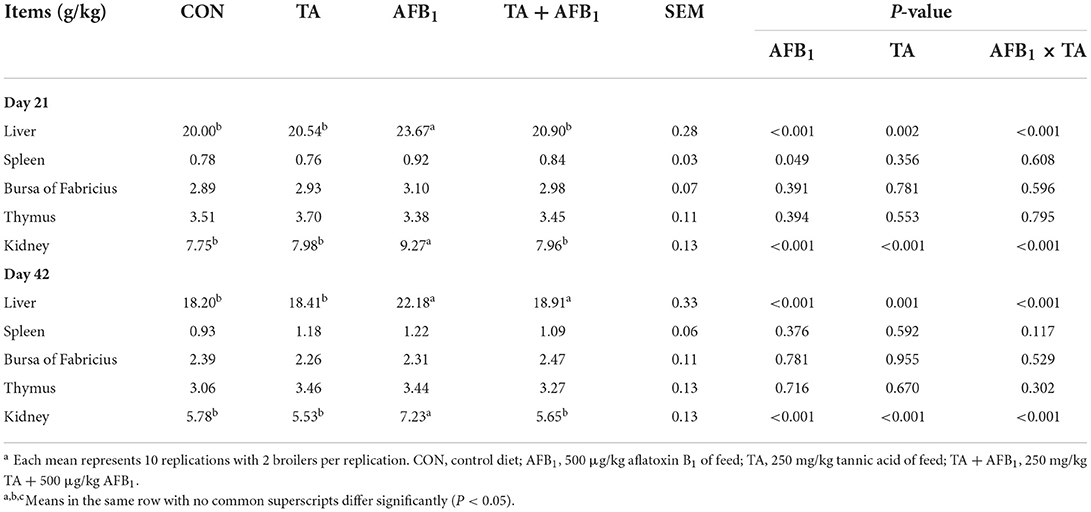
As shown in Table 3, on days 21 and 42, AFB1 and TA exhibited significant interactive effects on the relative weight of the liver and kidney in broilers (P < 0.05). The liver and kidney relative weight was increased in the AFB1 treatments compared with the CON (P < 0.05), while supplementation with TA into AFB1 contaminated diet decreased liver and kidney relative weight (P < 0.05). The relative weights of the spleen, bursa of Fabricius, and thymus were unaffected by AFB1 challenge and TA treatment on days 21 and 42 (P > 0.05).
Intestinal histomorphology
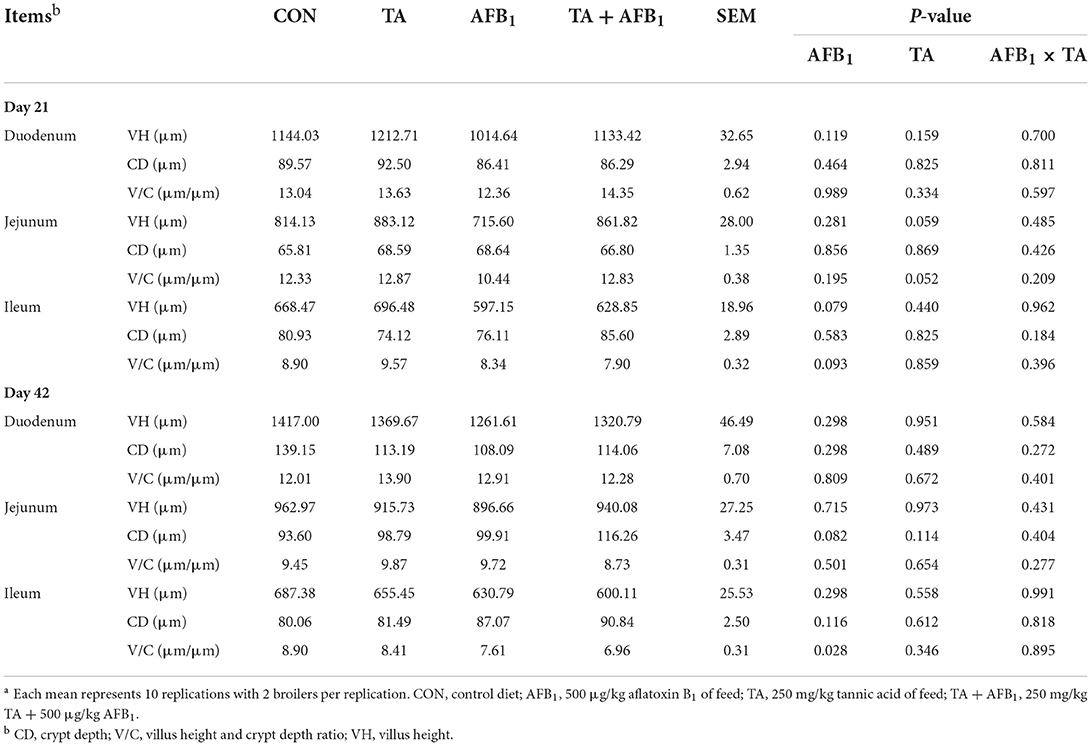
As presented in Table 4, on day 42, AFB1 challenge reduced the villus height and crypt depth ratio in the ileum (P < 0.05). The ileal villus height tended to decrease (P = 0.079), and the crypt depth of the jejunum tended to increase (P = 0.082) in AFB1 treatments compared with non-contaminated diets. The TA did not show significant effects on the intestinal histomorphology of broilers (P > 0.05). However, the villus height (P = 0.059) and villus height/crypt depth (P = 0.052) ratio were tended to increase in TA treatments. No interaction was found between AFB1 and TA in intestinal histomorphology (P > 0.05).
Antioxidant capacity
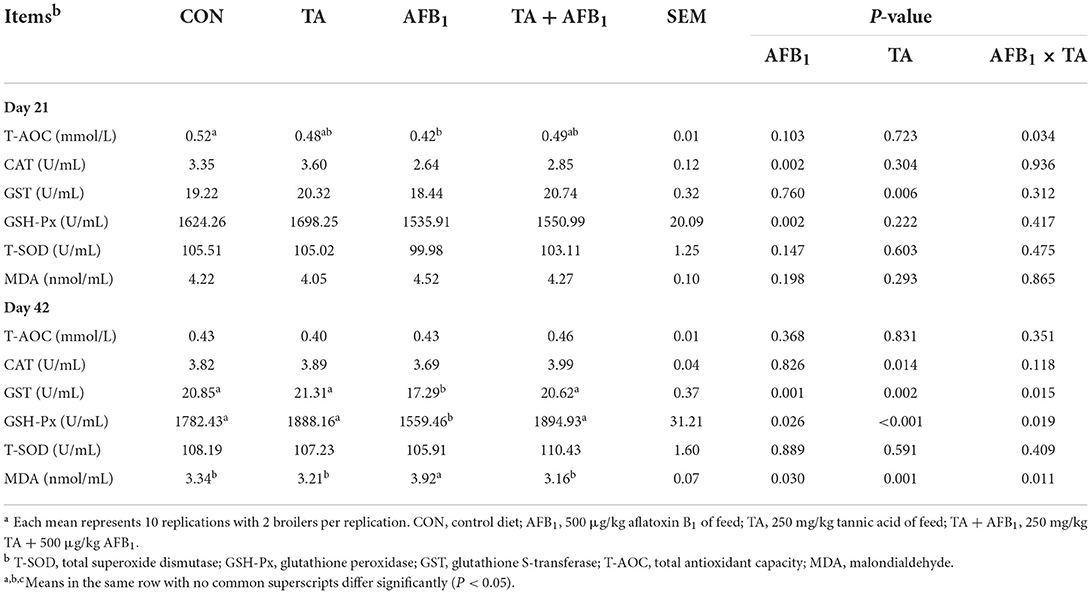
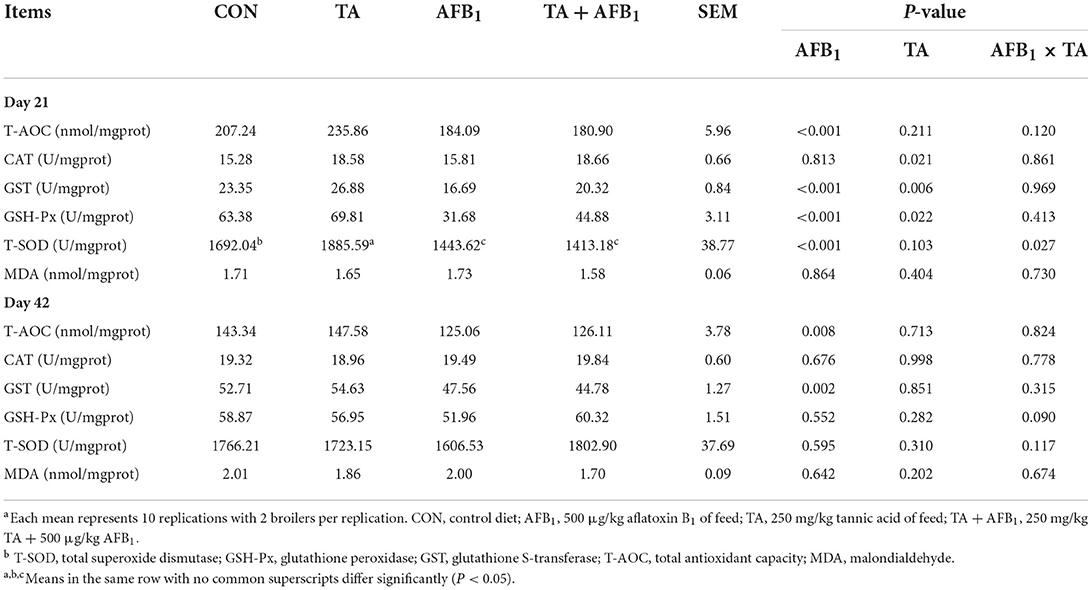
The results of the antioxidant capacity in the plasma are shown in Table 5, AFB1 challenge decreased plasma CAT and GSH-Px activities on day 21 (P < 0.05). Compared with the diet without TA, TA supplementation increased CAT activity in plasma on day 42 (P < 0.05). The AFB1 and TA exhibited interactive effects on the T-AOC, GST, GSH-Px, and MDA (P < 0.05). Compared with the CON, dietary expose to AFB1 decreased the T-AOC, GSH-Px, and GST activities on days 21 and 42, and increased the MDA content on day 42, respectively (P < 0.05). The addition of TA to AFB1 contaminated diet significantly improved the CAT, GSH-Px, and, GST activities, and decreased the MDA content on day 42 (P < 0.05).
As presented in Table 6, AFB1 challenge decreased the GST and T-AOC in the liver on days 21 and 42, as well as GSH-Px and T-SOD activity on day 21 (P < 0.05). Broilers fed the TA diet had greater hepatic CAT, GST, and GSH-Px activities on day 21 (P < 0.05). Furthermore, on day 21, AFB1 and TA showed interactive effects on the T-SOD in the liver (P < 0.05).
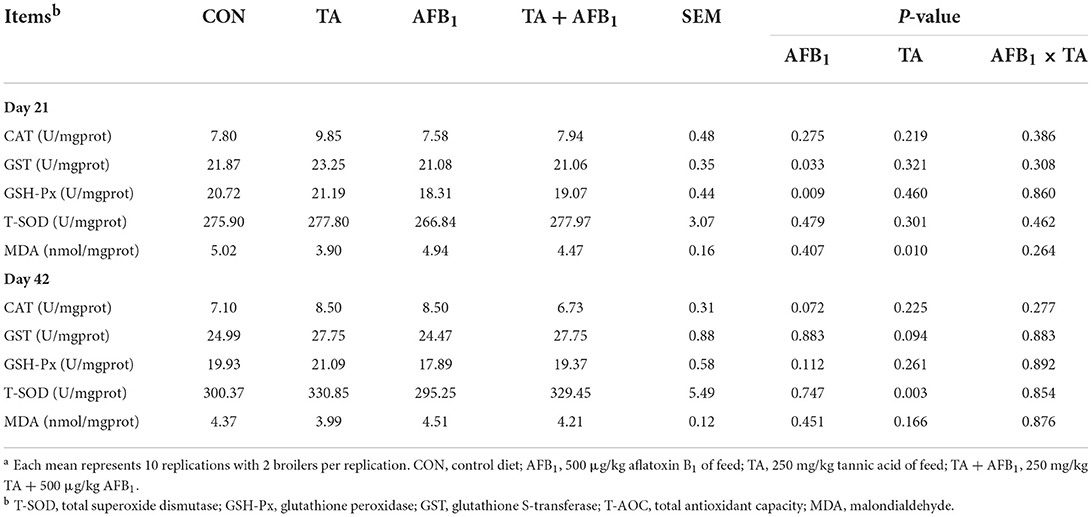
In Table 7, AFB1 challenge decreased the GST and GSH-Px activities in the jejunum on day 21 (P < 0.05). Dietary supplemented with TA increased the T-SOD activity in jejunum on day 42. The MDA content of jejunum was also decreased in the TA treatments compared with other treatments (P < 0.05).
Discussion
Dietary exposure to AFB1 can cause tremendous economic losses by reducing growth performance, feed efficiency, and increasing mortality in the poultry industry (20–24). In our study, we found that the administration of 500 μg/kg AFB1 diets increased FCR during days 1–21 in broilers. These results are in alignment with several studies, which demonstrated the detriment of broiler health and performance by feeding diets contaminated with 0.1–1 mg/kg AFB1 (25, 26). These adverse effects can be explained as AFB1 could inhibit protein synthesis and lipogenesis, reduce the activity of digestive enzymes, and change the energy metabolism of the cell (15, 27). We hypothesized that a commercially relevant concentration of AFB1 (500 μg/kg) during 42 days could decrease the growth rate in broilers. Unfortunately, the ADG and ADFI were not affected by the AFB1 challenge in the present study. Slizewska et al. (28) also reported that fed 1 mg/kg AFB1 of diet did not affect the ADG and ADFI of broilers. Likewise, Chen et al. (29) and Mesgar et al. (30) noted that feed intake, body weight gain, and feed efficiency were not affected by the 500 and 1,000 μg/kg of AFB1. Therefore, the toxic effects of AFB1 may be acute or chronic, influenced by the age, dose, diet composition, and duration of exposure (31).
In a previous study, we found that 250 and 500 mg/kg TA increased growth performance of broilers (32). In the contrary, supplementation with 250 mg/kg TA had no beneficial effect on the growth performance of broilers. Similar to the current results, Jamroz et al. (33) found that 250–500 mg/kg sweet chestnut tannin had no effect on performance, whereas 1,000 mg/kg TA reduced the final body weight in broilers. In addition, Choi et al. (34) reported that dietary supplementation of 500–5,000 mg/kg TA linearly decreased body weight of boilers infected with Eimeria Maxima. On the contrary, Liu et al. (35) found that 1,000 mg/kg chestnut tannins did not affect the body weight gain and feed intake in broilers. Cengiz et al. (36) also indicated that supplemented with 2,000 mg/kg chestnut tannin in broiler diets did not affect the performance. The dosage effect of TA on the growth performance of broilers seems to be unclear. However, it is reported that high dose of TA has negative effects on the growth of broilers, and biological effects are strongly dose-dependent (37, 38). Redondo et al. (39) hypothesized that the addition of excessive TA to the diet may increase the astringency and bitterness of the feed, thereby reducing the feed intake. Based on the different results, the inconsistency might be attributed to the source of tannic acid, administration dosage, diet composition, and age of the bird (40).
Aflatoxin has been known to mainly accumulated and metabolized in the liver and kidney after absorption, causing impairment of the liver and kidney (41, 42). In the present study, we observed that 500 μg/kg AFB1 caused a significant increase in the relative weight of liver and kidney, which is consistent with other studies (43–45). The enlargement of organ weight is attributed to disorders of lipid metabolism, and the inhibition of lipid transportation, leading to lipid deposition, which results in hepatomegaly (15, 46). Many studies describe the role of plant extract could ameliorate the adverse effect of AFB1 in broilers (47–49). In our previous study, we also found that the increase in liver and kidney relative weight in the AFB1 group was ameliorated by the supplementation of 250 and 500 mg/kg TA (32). Therefore, these results confirmed that TA has a protective effect on the liver and kidney damage caused by AFB1.
Intestinal villus height, crypt depth, and villus height/crypt depth ratio are important indexes to evaluate intestinal nutrient digestion and absorption capacity of poultry (50). These parameters especially the villus height/crypt depth ratio was positively related to the absorptive efficiency of the intestine (51). In the present study, intestinal histomorphology result revealed that dietary AFB1 exposure decreased the villus height and crypt depth ratio in the ileum of 42-day-old broilers. Similar to the results by Tavangar et al. (22), who reported that 1 mg/kg AFB1 decreased small intestine villus height and villus height to crypt depth ratio of broilers. These results showed that AFB1 could decrease the capacity of intestinal mucosa to digest and absorb nutrients by depressing intestinal development. Brus et al. (52) found that tannin extract could promote the proliferation of intestinal epithelial cells to promote intestinal development in vitro. Therefore, further studies need to be conducted to confirm the positive effect of TA on intestinal morphology in broilers.
It has been demonstrated that AFB1 could induce the production of reactive oxygen species (ROS) and oxidative stress, thereby inducing cell and DNA damage (53). The antioxidant system of organism can eliminate the adverse effects of ROS, and the GST, T-SOD, CAT, and GSH-Px are important endogenous antioxidant enzymes, which play a key role in scavenging free radicals and maintaining the intracellular redox equilibrium (15). In the present study, AFB1 significantly increased the concentrations of MDA and decreased the antioxidant enzyme activities of T-SOD, GSH-Px, GST, and CAT in the liver, jejunum, and the plasma of broilers when compared with the CON. These results are in consistent with previous studies, which demonstrated that different dosage of AFB1 decreased the activity of antioxidant enzymes, increased the lipid peroxidation, and inhibited the antioxidant capacity of broilers (14, 15, 54, 55). Recently, researchers have been interested in the usage of antioxidants to counter the toxic effects of aflatoxins (56). Our present results confirmed that 250 mg/kg TA could enhance antioxidative capacity, and alleviate the adverse effects of AFB1 on oxidative stress in the liver, jejunum, and plasma, which is similar to previous studies (57–59). Consequently, these results indicated that TA could play an important role in preventing the AFB1-induced oxidative damage in broilers.
Conclusion
In conclusion, supplementation with 250 mg/kg TA could alleviate the oxidative damage, and prevent the enlargement of liver in broilers dietary challenge with 500 μg/kg AFB1. Therefore, Chinese gallnut TA may be used as a feed additive in the prevention of aflatoxicosis and improve the health of poultry.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Wuhan Polytechnic University (Number: 20161121).
Author contributions
ZZ and BD conceived and designed the experiment. YX, JC, SG, SW, ZL, and LL performed the experiment. YX, ZL, and YQ analyzed the data. YX and ZZ wrote the manuscript. All authors read and approved the final manuscript.
Funding
This research was financially supported by the Key Projects of Hubei Province (No. 2022BBA0014) and the Research and Innovation Initiatives of Wuhan Polytechnic University (No. 2022RZ068).
Acknowledgments
We highly appreciate all other members of the Animal Nutrition and Intestinal Health Research Group of Wuhan Polytechnic University for providing assistance during the animal experiment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Negash D, A. review of aflatoxin: occurrence, prevention, and gaps in both food and feed safety. J Appl Microbiol Res. (2018) 1:35–43. doi: 10.15406/jnhfe.2018.08.00268
2. Mishra HN, Das C. A review on biological control and metabolism of aflatoxin. CRC Crit Rev Food Technol. (2003) 43:245–64. doi: 10.1080/10408690390826518
3. Hinton DM, Myers MJ, Raybourne RA, Francke-Carroll S, Sotomayor RE, Shaddock J, et al. Immunotoxicity of aflatoxin B1 in rats: effects on lymphocytes and the inflammatory response in a chronic intermittent dosing study. Toxicol Sci. (2003) 73:362–77. doi: 10.1093/toxsci/kfg074
4. Liu WC, Yang YY, Pushparaj K, Balasubramanian B. Evaluation of hepatic detoxification effects of Enteromorpha prolifera polysaccharides against aflatoxin B1 in broiler chickens. Antioxidants (Basel). (2022) 11:1757. doi: 10.3390/antiox11091757
5. Rawal S, Kim JE, Coulombe R. Aflatoxin B1 in poultry: Toxicology, metabolism and prevention. Res Vet Sci. (2010) 89:325–31. doi: 10.1016/j.rvsc.2010.04.011
6. Rajput SA, Sun L, Zhang NY, Khalil MM, Ling Z, Chong L, et al. Grape seed proanthocyanidin extract alleviates aflatoxinB1-induced immunotoxicity and oxidative stress via modulation of NF-κB and Nrf2 signaling pathways in broilers. Toxins. (2019) 11:23. doi: 10.3390/toxins11010023
7. Ren Y, Jin J, Zheng M, Yang Q, Xing F. Ethanol inhibits aflatoxin B1 biosynthesis in Aspergillus flavus by up-regulating oxidative stress-related genes. Front Microbiol. (2020) 10:2946. doi: 10.3389/fmicb.2019.02946
8. Food Drug Administration. Sec. 683, 100. Action Levels for Aflatoxins in Animal Food. (2019). Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-683100-action-levels-aflatoxins-animal-feeds (accessed March, 2019).
9. Gülçin I, Huyut Z, Elmastaş M, Aboul-Enein HY. Radical scavenging and antioxidant activity of tannic acid. Arabian J Chem. (2010) 3:43–53. doi: 10.1016/j.arabjc.2009.12.008
10. Liu S, Chen R, Hagedorn CH, Polyak SJ. Tannic acid inhibits hepatitis C virus entry into Huh7.5 cells. Plos ONE. (2015) 10:e0131358. doi: 10.1371/journal.pone.0131358
11. Orlowski P, Soliwoda K, Tomaszewska E, Bien K, Fruba A, Gniadek M, et al. Toxicity of tannic acid-modified silver nanoparticles in keratinocytes: potential for immunomodulatory applications. Toxicol In Vitro. (2016) 35:43–54. doi: 10.1016/j.tiv.2016.05.009
12. Soyocak A, Kurt H, Cosan DT, Saydam F, Gunes HV. Tannic acid exhibits anti-inflammatory effects on formalin-induced paw edema model of inflammation in rats. Hum Exp Toxicol. (2019) 38:096032711986415. doi: 10.1177/0960327119864154
13. Surai PF, Dvorska, J. E, Sparks, N. “Natural antioxidants and mycotoxins: theoretical considerations and practical applications”, in Poisonous Plants and Related Toxins. (2004). Wallingford UK: CABI Publishing, 494–503
14. Zhang NY, Qi M, Zhao L, Zhu MK, Guo J, Liu J, et al. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins. (2016) 8:327. doi: 10.3390/toxins8110327
15. Rajput SA, Sun L, Zhang N, Khalil MM, Gao X, Ling Z, et al. Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin B1. Toxins. (2017) 9:371. doi: 10.3390/toxins9110371
16. Hernandez C, Cadenillas L, Maghubi AE, Caceres I, Durrieu V, Mathieu C, et al. Mimosa tenuiflora aqueous extract: Role of condensed tannins in anti-aflatoxin B1 activity in Aspergillus flavus. Toxins. (2021) 13:391. doi: 10.3390/toxins13060391
17. Gowda NK, Ledoux DR, Rottinghaus GE, Bermudez AJ, Chen YC. Antioxidant efficacy of curcuminoids from turmeric (Curcuma longa L.) powder in broiler chickens fed diets containing aflatoxin B1. Br J Nutr. (2009) 102:1629–34. doi: 10.1017/S0007114509990869
18. Xiao YQ, Shao D, Sheng ZW, Wang Q, Shi SR. A mixture of daidzein and Chinese herbs increases egg production and eggshell strength as well as blood plasma Ca, P, antioxidative enzymes, and luteinizing hormone levels in postpeak, brown laying hens. Poult Sci. (2019) 98:3298–303. doi: 10.3382/ps/pez178
19. Guo S, Cheng Q, Li Y, Duan R, Hou Y, Yi D, et al. Effects of dietary coated-oleum cinnamomi supplementation on the immunity and intestinal integrity of broiler chickens. Anim Sci J. (2018) 89:1581–90. doi: 10.1111/asj.13094
20. Ortatatli M, Oguz H, Hatipoglu F, Karaman M. Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res Vet Sci. (2005) 78:61–8. doi: 10.1016/j.rvsc.2004.06.006
21. Solis-Cruz B, Hernandez-Patlan D, Petrone V, Pontin K, Latorre J, Beyssac E, et al. Evaluation of cellulosic polymers and curcumin to reduce aflatoxin B1 toxic effects on performance, biochemical, and immunological parameters of broiler chickens. Toxins. (2019) 11:121. doi: 10.3390/toxins11020121
22. Tavangar P, Gharahveysi S, Rezaeipour V, Irani M. Efficacy of phytobiotic and toxin binder feed additives individually or in combination on the growth performance, blood biochemical parameters, intestinal morphology, and microbial population in broiler chickens exposed to aflatoxin B1. Trop Anim Health Prod. (2021) 53:1–10. doi: 10.1007/s11250-021-02778-0
23. Wan XL, Li N, Chen YJ, Chen XS, Yang Z, Xu L, et al. Protective effects of lycopene on mitochondrial oxidative injury and dysfunction in the liver of aflatoxin B1-exposed broilers. Poult Sci. (2021) 100:101441. doi: 10.1016/j.psj.2021.101441
24. Alharthi AS, Al Sulaiman AR, Aljumaah RS, Alabdullatif AA, Elolimy AA, Alqhtani AH, et al. Protective effect of date pits on growth performance, carcass traits, blood Indices, intestinal morphology, nutrient digestibility, and hepatic aflatoxin residues of aflatoxin B1-exposed broilers. Agriculture. (2022) 12:476. doi: 10.3390/agriculture12040476
25. Denli M, Blandon JC, Guynot ME, Salado S, Perez JF. Effects of dietary AflaDetox on performance, serum biochemistry, histopathological changes, and aflatoxin residues in broilers exposed to aflatoxin B1. Poult Sci. (2009) 88:1444–51. doi: 10.3382/ps.2008-00341
26. Zuo RY, Chang J, Yin QQ, Wang P, Yang YR, Wang X, et al. Effect of the combined probiotics with aflatoxin B1-degrading enzyme on aflatoxin detoxification, broiler production performance and hepatic enzyme gene expression. Food Chem Toxicol. (2013) 59:470–5. doi: 10.1016/j.fct.2013.06.044
27. Bagherzadeh KF, Karimi TMA, Allameh A, Shariatmadari F. A novel aflatoxin-binding Bacillus probiotic: Performance, serum biochemistry, and immunological parameters in Japanese quail. Poult Sci. (2012) 91:1846–53. doi: 10.3382/ps.2011-01830
28. Slizewska K, Cukrowska B, Smulikowska S, Cielecka-Kuszyk J. The effect of probiotic supplementation on performance and the histopathological changes in liver and kidneys in broiler chickens fed diets with aflatoxin B1. Toxins. (2019) 11:112. doi: 10.3390/toxins11020112
29. Chen X, Horn N, Applegate TJ. Efficiency of hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of graded levels of aflatoxin B1 in broiler chicks. Poult Sci. (2014) 93:2037–47. doi: 10.3382/ps.2014-03984
30. Mesgar A, Aghdam SH, Bailey CA, Ebrahimnezhad Y, Mohan A. Effect of dietary L-Threonine and toxin binder on performance, blood parameters, and immune response of broilers exposed to aflatoxin B1. Toxins. (2022) 14:192. doi: 10.3390/toxins14030192
31. Dohnal V, Wu Q, Kuča K. Metabolism of aflatoxins: key enzymes and interindividual as well as interspecies differences. Arch Toxicol. (2014) 88:1635–44. doi: 10.1007/s00204-014-1312-9
32. Zhang ZF, Xi Y, Wang ST, Zheng LY, Qi Y, Guo SS, et al. Effects of Chinese gallnut tannic acid on growth performance, blood parameters, antioxidative status, intestinal histomorphology, and cecal microbial shedding in broilers challenged with aflatoxin B1. J Anim Sci. (2022) 100:1–8. doi: 10.1093/jas/skac099
33. Jamroz D, Wiliczkiewicz A, Skorupińska J, Orda J, Kuryszko J, Tschirch H, et al. Effect of sweet chestnut tannin (SCT) on the performance, microbial status of intestine and histological characteristics of intestine wall in chickens. Br Poult Sci. (2009) 50:687–99. doi: 10.1080/00071660903191059
34. Choi J, Tompkins YH, Teng PY, Gogal Jr RM, Kim WK. Effects of tannic acid supplementation on growth performance, oocyst shedding, and gut health of in broilers Infected with Eimeria Maxima. Animals. (2022) 12:1378. doi: 10.3390/ani12111378
35. Liu HS, Mahfuz SU, Wu D, Shang QH, Piao XS. Effect of chestnut wood extract on performance, meat quality, antioxidant status, immune function, and cholesterol metabolism in broilers-sciencedirect. Poult Sci. (2020) 99:4484–95. doi: 10.1016/j.psj.2020.05.053
36. Cengiz Ö, Köksal BH, Tatli O, Sevim Ö, Ahsan U, Bilgili SF, et al. Effect of dietary tannic acid supplementation in corn-or barley-based diets on growth performance, intestinal viscosity, litter quality, and incidence and severity of footpad dermatitis in broiler chickens. Livest Sci. (2017) 202:52–7. doi: 10.1016/j.livsci.2017.05.016
37. Aar P, Molist F, Klis J. The central role of intestinal health on the effect of feed additives on feed intake in swine and poultry. Anim Feed Sci Technol. (2017) 233:64–75. doi: 10.1016/j.anifeedsci.2016.07.019
38. Choi J, Yadav S, Wang J, Lorentz BJ, Lourenco JM, Callaway TR, et al. Effects of supplemental tannic acid on growth performance, gut health, microbiota, and fat accumulation and optimal dosages of tannic acid in broilers. Front Physiol. (2022) 13:912797. doi: 10.3389/fphys.2022.912797
39. Redondo LM, Chacana PA, Dominguez JE, Fernandez ME. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front Microbiol. (2014) 5:118. doi: 10.3389/fmicb.2014.00118
40. Choi J, Marshall B, Ko H, Shi H, Singh AK, Thippareddi H, et al. Antimicrobial and immunomodulatory effects of tannic acid supplementation in broilers infected with Salmonella Typhimurium. Poult Sci. (2022). 101:102111. doi: 10.1016/j.psj.2022.102111
41. Huff WE, Doerr JA. Synergism between aflatoxin and ochratoxin A in broiler chickens. Poult Sci. (1981) 60:550–5. doi: 10.3382/ps.0600550
42. Li S, Muhammad I, Yu H, Sun X, Zhang X. Detection of aflatoxin adducts as potential markers and the role of curcumin in alleviating AFB1-induced liver damage in chickens. Ecotox Environ Saf. (2019) 176:137–45. doi: 10.1016/j.ecoenv.2019.03.089
43. Shi YH, Xu ZR, Feng JL, Wang CZ. Efficacy of modified montmorillonite nanocomposite to reduce the toxicity of aflatoxin in broiler chicks. Anim Feed Sci Technol. (2006) 129:138–48. doi: 10.1016/j.anifeedsci.2005.12.006
44. Gowda NK, Ledoux DR, Rottinghaus GE, Bermudez AJ, Chen YC. Efficacy of turmeric (Curcuma longa), containing a known level of curcumin, and a hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of aflatoxin in broiler chicks. Poult Sci. (2008) 87:1125–30. doi: 10.3382/ps.2007-00313
45. Zhao L, Deng J, Xu ZJ, Zhang WP, Khalil MM, Karrow NA, et al. Mitigation of aflatoxin B1 hepatoxicity by dietary Hedyotis diffusa is associated with activation of NRF2/ARE signaling in chicks. Antioxidants. (2021) 10:878. doi: 10.3390/antiox10060878
46. Tung HT, Donaldson WE, Hamilton PB. Altered lipid transport during aflatoxicosis. Toxicol Appl Pharmacol. (1972) 22:97–104. doi: 10.1016/0041-008X(72)90229-3
47. Yin HB, Chen CH, Kollanoor-Johny A, Darre MJ, Venkitanarayanan K. Controlling Aspergillus flavus and Aspergillus parasiticus growth and aflatoxin production in poultry feed using carvacrol and trans-cinnamaldehyde. Poult Sci. (2015) 94:2183–90. doi: 10.3382/ps/pev207
48. Makki OF, Omidi A, Ansari Nik H, Hasheminejad SA, Senjedak HSM. Antiaflatoxin B1 effects of shirazi thyme (Zataria multiflora) in broilers, evaluation of performance and liver histopathology. Vet Sci Dev. (2016) 6:36–40. doi: 10.4081/vsd.2016.6090
49. Nazarizadeh H, Hosseini MS, Pourreza J. Effect of plant extracts derived from thyme and chamomile on the growth performance, gut morphology and immune system of broilers fed aflatoxin B1 and ochratoxin A contaminated diets. Ital J Anim Sci. (2019) 18:1073–81. doi: 10.1080/1828051X.2019.1615851
50. Jiang M, Fang J, Peng X, Cui H, Yu Z. Effect of aflatoxin B1 on IgA+ cell number and immunoglobulin mRNA expression in the intestine of broilers. Immunopharmacol Immunotoxicol. (2015) 37:450–7. doi: 10.3109/08923973.2015.1081933
51. Liu HW, Li K, Zhao JS, Deng W. Effects of chestnut tannins on intestinal morphology, barrier function, pro-inflammatory cytokine expression, microflora and antioxidant capacity in heat-stressed broilers. J Anim Physiol Anim Nutr. (2018) 102:717–26. doi: 10.1111/jpn.12839
52. Brus M, Gradišnik L, Trapecar M, Škorjanc D, FrangeŽ R. Beneficial effects of water-soluble chestnut (Castanea sativa Mill.) tannin extract on chicken small intestinal epithelial cell culture. Poult Sci. (2018) 97:1271–82. doi: 10.3382/ps/pex424
53. Choi KC, Chung WT, Kwon JK, Yu JY, Jang YS, Park SM, et al. Inhibitory effects of quercetin on aflatoxin B1-induced hepatic damage in mice. Food Chem Toxicol. (2010) 48:2747–53. doi: 10.1016/j.fct.2010.07.001
54. Cheng P, Ishfaq M, Yu H, Yang Y, Li S, Li X, et al. Curcumin ameliorates duodenal toxicity of AFB1 in chicken through inducing P-glycoprotein and downregulating cytochrome P450 enzymes. Poult Sci. (2020) 99:7035–45. doi: 10.1016/j.psj.2020.09.055
55. Sarker MT, Wan X, Yang H, Wang Z. Dietary lycopene supplementation could alleviate aflatoxin B1 induced intestinal damage through improving immune function and anti-oxidant capacity in broilers. Animals. (2021) 11:3165. doi: 10.3390/ani11113165
56. Umaya SR, Vijayalakshmi YC, Sejian V. Exploration of plant products and phytochemicals against aflatoxin toxicity in broiler chicken production: present status. Toxicon. (2021) 200:55–68. doi: 10.1016/j.toxicon.2021.06.017
57. Dong S, Li H, Casco L, Xiong Y, Guo KJ, Zoccarato I, et al. Antioxidative activity of the polyphenols from the involucres of Castanea mollissima blume and their mitigating effects on heat stress. Poult Sci. (2015) 94:1096–104. doi: 10.3382/ps/pev101
58. Farahat MH, Abdallah FM, Ali HA, Hernandez-Santana A. Effect of dietary supplementation of grape seed extract on the growth performance, lipid profile, antioxidant status and immune response of broiler chickens. Animal. (2017) 11:771–7. doi: 10.1017/S1751731116002251
Keywords: Aflatoxin B1, antioxidant capacity, broiler, growth performance, intestinal health, tannic acid
Citation: Xi Y, Chen J, Guo S, Wang S, Liu Z, Zheng L, Qi Y, Xu P, Li L, Zhang Z and Ding B (2022) Effects of tannic acid on growth performance, relative organ weight, antioxidative status, and intestinal histomorphology in broilers exposed to aflatoxin B1. Front. Vet. Sci. 9:1037046. doi: 10.3389/fvets.2022.1037046
Received: 05 September 2022; Accepted: 03 October 2022;
Published: 21 October 2022.
Edited by:
Wen-Chao Liu, Guangdong Ocean University, ChinaReviewed by:
Xi Wang, Southwest Minzu University, ChinaMuhammad Saeed, Northwest A&F University, China
Dafei Yin, Shenyang Agricultural University, China
Copyright © 2022 Xi, Chen, Guo, Wang, Liu, Zheng, Qi, Xu, Li, Zhang and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengfan Zhang, emhhbmc4NTA4MjBAaG90bWFpbC5jb20=; Binying Ding, ZGJ5aW5nNzQ3MUAxMjYuY29t
†These authors have contributed equally to this work
 Yu Xi
Yu Xi Jing Chen†
Jing Chen† Zhengfan Zhang
Zhengfan Zhang