- 1College of Animal Science and Technology, Guangxi University, Nanning, China
- 2Guangxi Zhuang Autonomous Region Engineering Research Center of Veterinary Biologics, Guangxi University, Nanning, China
Background: Fasciola gigantica, a tropical liver fluke, infects buffalo in Asian and African countries, causing significant economic losses and posing public health threats. The diagnostic of buffalo fascioliasis caused by F. gigantica is vital in fascioliasis control and preventation. The 22nd gel filtration chromatography fraction of F. gigantica Excretory-Secretory Products (FgESP), namely Fasciola 22 (F22), which was used as a diagnostic antigen in indirect ELISA, has demonstrated great potential for fascioliasis diagnosing. In the absence of rapid diagnostic methods, the use of a colloidal gold immunochromatographic strip based on F22 was applied to detect F. gigantica infection in buffalo.
Methods: In the present study, the 22nd gel filtration chromatography fraction of FgESP (F22) was used as an antigen to establish the colloidal gold-based immunochromatographic strip (ICS). The nitrocellulose membrane was incubated with F22 at the test line (T line) and goat anti-mouse secondary antibody at the control line (C line). The mouse anti-buffalo secondary antibody 2G7 conjugated to colloidal gold particles was used as the detection system for line visualization. The strip was assembled and developed by optimizing reaction conditions. The sensitivity, specificity, stability, and early diagnostic value of the strip were evaluated employing buffalo-derived sera.
Results: An immunochromatographic strip for the rapid detection of antibodies against F. gigantica-FgICS was developed. The strip demonstrated high sensitivity and specificity. Sensitivity tests confirmed positive results even when the positive reference serum was diluted 4,096 times. Except for one Schistosoma japonicum-positive serum that tested positive via FgICS, specificity tests confirmed no cross-reactivity with other positive sera of Schistosoma japonicum and Babesia bovis. The strip remained stable after storage at 4°C for up to 3 months. In infected buffalo, antibodies could be detected as early as 14–21 days post-infection. The detection of 17 positive sera yielded an 82.4% positive rate via FgICS vs. a 100.0% positive rate via ELISA based on FgESP. For FgICS, the 95% confidence interval of sensitivity was 84.8–95.4%, while specificity was 4.2–14.7%.
Conclusion: The immunochromatographic strip FgICS developed in this study provides a simple and rapid method of F. gigantica antibody detection and infected buffalo monitoring in the field.
Introduction
Infection with Fasciola species, including Fasciola hepatica and Fasciola gigantica has been reported in a wide variety of mammalian species globally, including human, cattle, buffalo, and sheep, causing tremendous economic loss (1, 2). The epidemic of F. hepatica is mainly prevalent in temperate regions, while the epidemic of F. gigantica is mainly prevalent in tropical and subtropical regions (3). The World Health Organization estimates that 2.4 million people in more than 70 countries have been infected with Fasciola, and several millions people are at risk of infection (4). Compared with human, fasciolasis ruminants such as cattle, sheep and buffalo are also common globally. A report in Australia detected that the infection rate of Fasciola in sheep and cattle was 52.2 and 26.5%, respectively. In Asia, the infection rate of cattle was 0.7–69.2%, and that of goats was 0.0–47.0%, which suggested that Fasciola infection cause enormous economic losses to husbandry in various regions.
Buffalo are economically important animals in Asian and African countries, producing meat and milk but also serving as working animals in rural areas. In 2021, a survey, in which dissection was used for examining F. gigantica adult, suggested that all the flukes were F. gigantica, and no Fasciola hepatica or the intermediate form was found in Nanning, South of the China (5). Thus, F. gigantica infection in buffalo cannot be ignored. The development and improvement of diagnostic methods represent crucial countermeasures in preventing losses caused by F. gigantica. Given the low sensitivity and time-consuming process of coprological examination, serological examination is generally used in fascioliasis diagnosis (6, 7).
At present, serological analysis for F. gigantica mainly focuses on ELISA, which can be conducted through the detection of circulating antibodies or antigens (8). With ELISA, specific anti-F. gigantica antibodies can be detected as early as 2 weeks post-infection (pi) in buffalo, which is before circulating antigens can be detected (8). Diagnostic antigens, including FgESP, FgF22, rFgCatL1, and rFgSAP-2, have been widely used in recent years for the establishment of ELISAs, with all producing the desired effect (9–12). However, their labor-intensive and time-consuming nature, along with the professional personnel and special laboratory materials and equipment required for their use, render ELISA unsuitable for use in the field. Hence, a convenient and rapid test, such as an immunochromatographic strip, is needed for in-field diagnosis of F. gigantica infection in buffalo. This study pioneered a colloidal gold immunochromatographic strip based on F22 to detect antibodies against F. gigantica in buffalo. The specificity, sensitivity, and stability of this testing method were evaluated by F. gigantica-positive and -negative sera. Results were independently validated via both ELISA and the strip test.
Materials and methods
Sera collection
The animal study, including sera collection were approved by the Ethics Committee of the School of Animal Science and Technology, Guangxi University. The animals used in this study were handled in accordance with good animal practices as required by the Animal Ethics Procedures and Guidelines of the People's Republic of China.
All sera employed in this study were buffalo-derived. Reference sera, including F. gigantica-positive and -negative sera, the positive serum was collected from buffaloes artificially infected with 200 metacercariaes 4 weeks post-infection, and the negative serum was provided by Guangxi Buffalo Research Institute, Chinese Academy of Agricultural Sciences. Sera positive for Schistosoma japonicum and Babesia bovis were kindly provided by the Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences and the Lanzhou Veterinary Research Institute. A total of 17 F. gigantica positive sera were used for detection, including 11 fluke-positive sera and 6 experiment-positive sera. Fluke-positive sera were collected from a slaughterhouse in Nanning City, Guangxi Zhuang Autonomous Region. Six experimentally-infected sera were collected from buffaloes artificially infected with 250 metacercariaes. Samples of F. gigantica-infected serum (250 metacercaria infected) 0–14 weeks pi were collected weekly, and buffaloes were tested Fasciola infection-negative before the infection. Adult flukes of Fasciola gigantica were found in liver and bile duct of the infected buffaloes after autopsy and eggs were also found in the feces of the artificially infected buffaloes, which confirmed that the sera from these buffaloes were positive. The Buffalo Institute of Guangxi Zhuang Autonomous Region kindly provided 325 untested sera, of which 100 were selected randomly for further diagnosis. These buffaloes provided with sera were treated by adding Niclofolan (5 mg/kg) to the feed grain, once in March and once in October every year.
Preparation of antigen F22
Fasciola gigantica flukes were collected and Excretory-Secretory Product (FgESP) then collected. Flukes were washed with 37°C pre-warmed PBS 3 to 4 times and 0.01 M PBS (filtered via 0.22 μm filter) was added into the petri dish containing washed flukes (2 flukes/mL), which was then incubated at 37°C for 2 h. The culture broth was centrifuged at 3,000 g for 30 min and the supernatant collected after filtration with a 0.45 μm filter (Millipore, USA).
F22 was prepared as described by Jin (11). Briefly, the concentration of FgESP was adjusted to 10 mg/mL after lyophilization for subsequent chromatography. The column (GE Healthcare, USA) was equilibrated with 0.01 M PBS and 5 mL of FgESP was loaded. The protein was eluted with 0.01M PBS, the volume of each component was 2 mL, and the 22nd component was collected. A bicinchoninic acid (BCA) Protein Assay Kit (TIANGEN BIOTECH, Beijing, China) was then applied to determine protein concentration.
Preparation of 40 nm colloidal gold
Colloidal gold was prepared according to published methods (13). Briefly, 1 mL of 1% trisodium citrate (w/v) was quickly added to 100 mL of 0.01% HAuCl4 solution (w/v), heated to a slight boil, and stirred constantly for 15 min. As the solution naturally cooled to room temperature (RT), the pH was adjusted to 7.3 using 0.01 M potassium carbonate.
Preparation and labeling of 2G7 colloidal gold
Mouse anti-buffalo secondary antibody 2G7 was prepared according to the Wu method (14). Briefly, to thaw the frozen hybridoma cell strain, vials were quickly warmed in a 37°C water bath and gently washed with 10 mL of pre-warmed DMEM medium. Broth containing hybridomas was centrifuged at 1,000 rpm and resuspended with complete medium containing 20% fetal bovine serum. Hybridoma cells were cultured in a 37°C germ-free incubator containing 5% CO2 for 24 h until they were adhered to the wall, with replacement of one half of the complete medium; these were then used to expand the culture and produce sufficient hybridoma cells. To obtain the monoclonal antibody 2G7, 0.2 mL hybridoma cells with the concentration 3 × 106/mL were intraperitoneally injected into a total of 10 mice of 8-week-old female BALB/c. One week after injection, the ascites fluid was collected and centrifuged at 12,000 rpm for 20 min; supernatant with high titer was then collected and stored at −80°C for later use.
Then 18 μL of 2G7 mouse anti-buffalo secondary antibody (1 mg/mL) was added to 1 mL of colloidal gold solution and shaken gently for 15 min, after which 0.1 mL 10% BSA (filtered via 0.45 μm filter) was added to block non-specific binding sites. The resulting solution was centrifuged at 12,000 rpm for 30 min and the resuspended pellet in 0.2 mL of resuspension buffer (0.05 M sodium borate, 5% BSA, 20% sucrose, 0.1% Tween-20) was sprayed onto glass fiber pads and dried at 37°C for 90 min.
Assembly of the immunochromatographic strip
Nitrocellulose membrane was incubated with F22 at the test line (T line) and incubated with goat anti-mouse secondary antibody at the control line (C line). Incubated membranes were then dried at 37°C for 30 min. The sample pad, conjugate pad, nitrocellulose membrane, and absorbent pad were subsequently assembled on a backing plate in the appropriate order. Then assembled plate was cut into 4 mm × 80 mm strips using a cutting machine.
Detection of sensitivity, specificity, and stability
For detection limit study of FgICS, the reference F. gigantica-positive serum was gradient diluted, including 1:4, 1:16, 1:64, 1:128, 1:256, 1:1,024, and 1:4,096 dilutions. These diluted sera were dispensed onto the sample pad with 100 μL of each test strip and the results observed after 10 min. The detection limit study of indirect ELISA based on FgESP were also performed.
For cross reactivity study, 10 sera positive for S. japonicum and 1 serum positive for B. bovis were tested with a dilution of 1:64, performed as described above. The cross reactivity study of indirect ELISA based on FgESP were also performed.
For stability detection, prepared test strips were sealed together with a desiccant and stored at 4°C. After being stored for 1 and 3 months, test strips of the same batch were applied to detect the reference F. gigantica-positive and F. gigantica-negative sera.
Early diagnosis effect evaluation
To evaluate the early diagnostic efficiency of the FgICS, sera of 3 F. gigantica-infected buffalo collected before the infection (week 0th) and 1–14 weeks pi weekly were tested. An aliquot of 100 μL of each serum with the serial dilution 1:64 was dispensed onto the sample pad, and the earliest week post-infection of each buffalo when antibodies could be detected was confirmed with a positive result via ICS. Indirect ELISA based on FgESP was also performed on these sera according to methods developed by Zhang et al. (15), with some modifications to make a comparison between these two methods.
Diagnosis of F. gigantica
The FgICS strips and indirect ELISA based on FgESP were used in parallel for the detection of F. gigantica antibodies in buffalo. Of the 117 sera administered for subsequent diagnosis, 17 were positive and 100 were untested. Both FgICS and ELISA were performed as described above.
The agreement of positive serum rates between FgICS and FgESP-ELISA was evaluated using Cohen's Kappa (k) statistic (SPSS version 26), with agreement considered almost perfect (0.8 < k < 1), substantial (0.6 < k < 0.8), moderate (0.4 < k < 0.6), fair (0.2 < k < 0.4), or slight (0 < k < 0.2).
Results
F22 preparation
As shown in Figure 1, four protein absorption peaks at UV280nm were successively generated from chromatography. F22 was located in the first peak (P1).
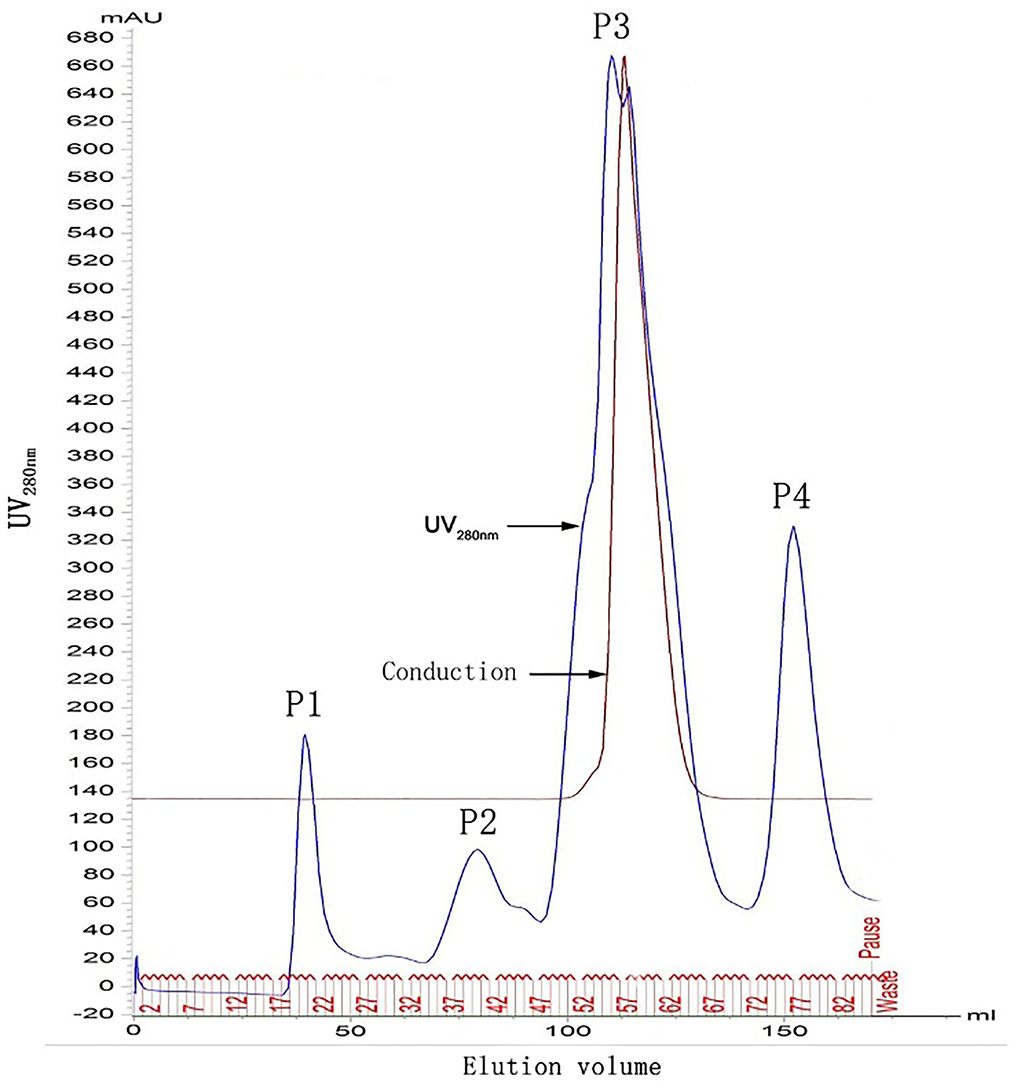
Figure 1. Chromatographic graphs of FgESP. P1, P2, P3, and P4 indicate 4 protein absorption peaks at UV280nm. F22 was located in the first peak (P1).
Sensitivity, specificity, and stability detection
The prepared strips were applied to detect different dilutions of reference sera. Positive sera provided a visible color at both the T line and C line of the strip even when diluted 4,096 times. It was observed that when the serum dilution factor is >1:16 or <1:1,024, the red color of the T-line becomes significantly lighter (Figure 2a). Negative sera appeared only at the C line, and the T line was invisible across all diluted gradients. These results indicate that the detection limit of the test strip was >1:4,096 (Figure 2a). While FgESP-ELISA can produce positive results when the reference serum is diluted 1,600 times (not shown).

Figure 2. Sensitivity, specificity, and stability detection of FgICS. (a) Strip sensitivity detection showed low detection limit of FgICS even at 1:4096. FgICS detection limit was evaluated via serial dilution of the positive and negative sera. 1–6 (positive): 1:4, 1:16, 1:64, 1:256, 1:1,024, 1:4,096; 7–12 (negative): 1:4, 1:16, 1:64, 1:256, 1:1,024, 1:4,096. The test line result can be judged by the naked eye. (b) Strip specificity tests showed no cross-reactivity except for one serum positive for Schistosoma japonicum. Buffalo sera positive for S. japonicum and B. bovis were used to determine strip test specificity. 1–10: S. japonicum-positive sera. 11: Babesia bovis-positive serum. (c) Strip was stable after storage for up to 3 months. 1–2: The stability of the FgICS after storage for 1 month; 3–4: The stability of the FgICS after storage for 3 months. While 1 and 3 were reference F. gigantica-positive serum, 2 and 4 were F. gigantica-negative serum.
Ten positive sera of S. japonicum and 1 positive serum of B. bovis were employed to evaluate the cross reactivity of the FgICS. All samples, except for one S. japonicum-positive serum (No. 7), yielded negative results (Figure 2b). For FgESP-ELISA, all samples, except for three S. japonicum-positive sera (No. 4, No. 5, and No. 7), yielded negative results (not shown).
The storage test using reference-positive and -negative sera showed that FgICS was still viable after storage at 4°C for 1 or 3 months (Figure 2c).
Early diagnosis effect evaluation
Sera of 3 buffalo experimentally infected with F. gigantica (A1, A2, and A3) were collected weekly from 0 to 14 weeks pi and tested using FgICS. Results showed that anti-F. gigantica antibodies could be detected within 2–4 weeks pi (Figure 3), just like the indirect ELISA based on FgESP (not shown).

Figure 3. Early diagnosis effect evaluation of FgICS showed antibodies could be detected within 2–4 weeks pi. Detection of sera from 3 experimentally infected buffalo A1 (a), A2 (b), and A3 (c) by FgICS. 1–15: sera collected weekly from 0 weeks before infection and 1–14 weeks pi.
Diagnosis of F. gigantica
For 17 F. gigantica positive sera, all 11 fluke-positive sera tested positive via FgESP-ELISA, while 2 tested negative via FgICS (No. 2 and No. 7). Of the 6 experimentally F. gigantica-infected sera, all tested positive via FgESP-ELISA, while 1 tested negative via FgICS (No. 3). These results yielded positive rates of 82.4% (14/17) and 100.0% (17/17) for FgICS and ELISA, respectively (Supplementary Table 1).
FgICS and FgESP-ELISA were also applied to 100 field sera from standardly raised buffalo. The results showed that they were all negative both in FgICS and FgESP-ELISA antibody detection. The false-positive rate was 0%, and an almost perfect agreement was confirmed between FgICS and FgESP-ELISA, with a high Cohen's kappa value (k = 0.869).
Discussion
Immunochromatographic strips represent a portable, rapid, and easy-to-conduct method for the seroepidemiological screening and have been widely applied in the diagnosis of parasitic infections, such as Babesia (16–18), Toxoplasma (19), Paragonimiasis Skrjabini (20) and Schistosoma japonica (21, 22). However, immunochromatographic strips in the diagnosis of F. gigantica did not established. Given that the antigen-antibody binding of ICS should be completed in a relatively short time (typically within 10 min), this method necessitates higher sensitivity of diagnostic antigens. For F. gigantica, the indirect ELISA was established based on several antigens (9–12, 23), including FgESP, rFgCatL1, FgF22, and rFgSAP-2. Among them, FgESP and FgF22 showed no cross-reactivity with cattle-derived Paramphistomum epiclitum-positive serum and buffalo-derived B. bovis-positive serum, indicating its low cross-reactivity. However, additional research that employed FgESP and FgCatL1 for the development of ICS yielded no promising results. F22, the optimal component screened out from FgESP after molecular sieve chromatography, has demonstrated significantly higher sensitivity than that of FgESP in the indirect ELISA (11) and was thus selected for ICS development. Here, an immunochromatographic strip based on F22 was established. It was observed that when the serum dilution factor is >1:16 or <1:1,024, the red color of the T-line becomes significantly lighter (Figure 2). Therefore, while detecting serum via FgICS, a serum dilution ratio between 1:16 and 1:1,024 is recommended to achieve credible results. Additionally, compared with the FgESP-ELISA, FgICS showed lower detection limit of serum. This lower detection limit makes FgICS viable for fascioliasis diagnosis. Regarding the specificity detection, due to the limitations of buffalo-derived serum, only 1 sample of B. bovis-positive serum was applied here, and undoubtedly, more than 3 sample would convincing. Besides, considering the distant relatives between F. gigantica and B. Bovis, trematode serum such as buffalo-derived P. epiclitum, which causes great loss in Asian buffalo breeding, should be applied in the following cross-reactivity detection.
Results suggested that the FgICS was developed successfully. Specifically, (i) it is sensitive enough both for early detection and for latent infections; (ii) it is specific enough to differentiate between F. gigantica and S. japonicum, though its specificity must be further verified due to limited serum species; and (iii), it showed an almost perfect agreement with FgESP-ELISA. Another advantage of FgICS is that it requires no special expertise or equipment. It is also a time-saving process, needing only 10 min to be completed. Furthermore, FgICS is stable during long storage. Thus, FgICS represents a suitable diagnostic tool for the rapid detection of F. gigantica infection under field conditions in buffalo and for the seroepidemiological screening of buffaloes from different area, which would in turn conduce to the prevention and control of this disease.
Conclusion
An immunochromatographic strip was developed and optimized. With relatively high sensitivity and specificity, FgICS represents a portable, reliable, and fast diagnostic tool of F. gigantica and was thus proposed as a powerful supplement to current diagnostic assays.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by Ethics Committee of the School of Animal Science and Technology, Guangxi University.
Author contributions
WZ conceived the project. WJ carried out laboratory work. WW prepared 2G7. JW, KH, and WJ wrote the manuscript. ZW and YG performed buffalo maintenance and serum collection. WD received the manuscript and contributed to the final submission. All authors read and approved the final manuscript.
Funding
This study was supported by the National Key Research and Development Program (Grant No. 2017YFD0501202-1) and the Science and Technology Major Project of Guangxi (Grant No. AA17204057).
Acknowledgments
The authors would like to thank the National Key Research and Development Program and the Science and Technology Major Project of Guangxi. The authors would also extend their gratitude to WW for the preparation of 2G7, Zhiqiang Fu of Shanghai Veterinary Research Institute, and Guiquan Guan of Lanzhou Veterinary Research Institute for providing buffalo ser positive for Schistosoma japonicum and Babesia bovis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2022.1004932/full#supplementary-material
References
1. Mehmood K, Zhang H, Sabir AJ, Abbas RZ, Ijaz M, Durrani AZ, et al. A review on epidemiology, global prevalence and economical losses of fasciolosis in ruminants. Microb Pathog. (2017) 109:253–62. doi: 10.1016/j.micpath.2017.06.006
2. Mar SL, David BR, Judit S, Javier GM. Fascioliasis and fasciolopsiasis: current knowledge and future trends. Res Vet Sci. (2020) 134:27–35. doi: 10.1016/j.rvsc.2020.10.011
3. Davies CN, Jan S. Fasciola species introgression: just a fluke or something more? Trends Parasitol. (2021) 37:25–34. doi: 10.1016/j.pt.2020.09.008
4. World Health Organization. Human Fascioliasis: Review Provides Fresh Perspectives on Infection and Control. (2020). Available online at: https://www.who.int/news/item/26-07-2018-human-fascioliasis-review-provides- fresh-perspectives-on-infection-and-control (accessed August 27, 2022).
5. Wu ZJ, Wang JH, Meng Z, Jin WK, He KX, Zhang WY, et al. Identification of Fasciola spp. based on ITS-2 reveals the Fasciola gigantica infection in buffaloes in Nanning city, South China. Vet Parasitol. (2021) 300:109585. doi: 10.1016/j.vetpar.2021.109585
6. Guobadia EE, Fagbemi BO. Detection of circulating Fasciola gigantica antigen in experimental and natural infections of sheep with fasciolosis. Vet Parasitol. (1996) 65:29–39. doi: 10.1016/0304-4017(96)00937-5
7. Marcilla A, Bargues MD, Mas-Coma S. A PCR-RFLP assay for the distinction between Fasciola hepatica and Fasciola gigantica. Mol Cell. Probes. (2002) 16:327–33. doi: 10.1006/mcpr.2002.0429
8. Mas-Coma S, Valero MA, Bargues MD. Fascioliasis. Adv Exp Med Biol. (2014) 766:77–114. doi: 10.1007/978-1-4939-0915-5_4
9. Andleeb A, Rohit L, Savita B, Arun A, Ajayta R, Jayanta C, et al. Serodiagnosis of Fasciola gigantica infection in buffaloes with native cathepsin-L proteases and recombinant cathepsin L1-D. Acta Parasitol. (2020) 65:413–21. doi: 10.2478/s11686-020-00177-3
10. Jin WK. Preparation of Fasciola gigantica diagnostic antigen and the establishment and application of immunological diagnostic method for bovine fascioliasis [dissertation/master's thesis]. Guangxi University, Nanning, China (2021).
11. Jin WK, Yuan XX, Wang JH, Hou LJ, Zheng MW, Wu ZJ, et al. Establishment of indirect ELISA for bovine fascioliasis diagnosis based on chromatographic component of Fasciola gigantica excretory-secretory products. China Anim Husbandry Vet Med. (2021) 48:9. doi: 10.16431/j.cnki.1671-7236.2021.08.034
12. Sugiyama T, Ichikawa-Seki M, Sato H, Kounosu A, Tanaka M, Maruyama H. Enzyme-linked immunosorbent assay (ELISA) using recombinant Fasciola cathepsin L1 for the diagnosis of human fasciolosis caused by Fasciola hepatica/gigantica hybrid type. Parasitol Int. (2021) 82:102311. doi: 10.1016/j.parint.2021.102311
13. Yang S, Yang J, Zhang G, Wang X, Qiao S, Zhao D, et al. Development of an immunochromatographic strip for the detection of antibodies against foot-and-mouth disease virus serotype O. J Virol Methods. (2010) 165:139–44. doi: 10.1016/j.jviromet.2010.01.001
14. Wu WD, Zhang WY, Hou LJ; Beijing Tianqizhixin Intellectual Property Agency Co. Ltd. Production and Application of IgG Monoclonal Antibody Against Buffalo. China patent CN109507421B. Beijing: State Intellectual Property Office of P.R.China (2022).
15. Zhang WY, Moreau E, Yang BZ, Li ZQ, Hope JC, Howard CJ, et al. Humoral and cellular immune responses to Fasciola gigantica experimental infection in buffaloes. Res Vet Sci. (2006) 80:299–307. doi: 10.1016/j.rvsc.2005.07.003
16. Huang X, Xuan X, Xu L, Zhang S, Yokoyama N, Suzuki N, et al. Development of an immunochromatographic test with recombinant EMA-2 for the rapid detection of antibodies against Babesia equi in Horses. J Clin Microbiol. (2004) 42:359–61. doi: 10.1128/JCM.42.1.359-361.2004
17. Kim C, Alhassan A, Verdida RA, Yokoyama N, Xuan XN, Fujisaki K, et al. Development of two immunochromatographic tests for the serodiagnosis of bovine babesiosis. Vet Parasitol. (2007) 148:137–43. doi: 10.1016/j.vetpar.2007.05.016
18. Kim C, Blanco C, Alhassan A, Iseki H, Yokoyama N, Xuan XN, et al. Development of a rapid immunochromatographic test for simultaneous serodiagnosis of bovine babesioses caused by Babesia bovis and Babesia bigemina. Am J Trop Med Hyg. (2008) 78:117. doi: 10.4269/ajtmh.2008.78.117
19. Wang YH, Li XR, Wang GX, Yin H, Cai XP, Fu BQ, et al. Development of an immunochromatographic strip for the rapid detection of Toxoplasma gondii circulating antigens. Parasitol Int. (2011) 60:105–07. doi: 10.1016/j.parint.2010.11.002
20. Wang Y, Wang LF, Zhang JW, Wang GX, Chen WB, Chen L, et al. Preparation of colloidal gold immunochromatographic strip for detection of Paragonimiasis skrjabini. PLoS ONE. (2014) 9:e92034. doi: 10.1371/journal.pone.0092034
21. Xu R, Feng JT, Hong Y, Zhao DY, Lin JJ, Lu K, et al. A novel colloidal gold immunochromatography assay strip for the diagnosis of schistosomiasis japonica in domestic animals. Infect Dis Poverty. (2017) 6:84. doi: 10.1186/s40249-017-0297-z
22. Manal K, Faten S, Zeinab D, Sara M, Shimaa A, Abeer B, et al. Development of new lateral-flow immunochromatographic strip using colloidal gold and mesoporous silica nanoparticles for rapid diagnosis of active schistosomiasis. Asian Pac J Trop Bio. (2019) 9:315–322. doi: 10.4103/2221-1691.262083
Keywords: buffalo, colloidal gold, diagnosis, Fasciola gigantica, immunochromatographic
Citation: Wang J, He K, Wu Z, Jin W, Wu W, Guo Y, Zhang W and Di W (2022) Development of a colloidal gold immunochromatographic strip for the rapid detection of antibodies against Fasciola gigantica in buffalo. Front. Vet. Sci. 9:1004932. doi: 10.3389/fvets.2022.1004932
Received: 27 July 2022; Accepted: 01 September 2022;
Published: 16 September 2022.
Edited by:
Azlin Mohd Yasin, National University of Malaysia, MalaysiaReviewed by:
Bahador Sarkari, Shiraz University of Medical Sciences, IranPiyanan Taweethavonsawat, Chulalongkorn University, Thailand
Copyright © 2022 Wang, He, Wu, Jin, Wu, Guo, Zhang and Di. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiyu Zhang, endlaXl1QGd4dS5lZHUuY24=; Wenda Di, ZGl3ZW5kYUBneHUuZWR1LmNu
†These authors have contributed equally to this work
 Jinhui Wang1,2†
Jinhui Wang1,2† Wenda Di
Wenda Di