- 1Department of Zoology, Faculty of Biological Sciences, Kohat University of Science and Technology, Kohat, Pakistan
- 2Department of Botanical and Environmental Sciences, Faculty of Biological Sciences, Kohat University of Science and Technology, Kohat, Pakistan
- 3Department of Medical Laboratories Sciences, College of Applied Medical Sciences in Al-Quwayiyah, Shaqra University, Riyadh, Saudi Arabia
Haemonchus contortus is an infectious gastrointestinal nematode parasite of small ruminants. This study addresses the in vitro/in vivo anti-haemonchiasis potential, toxicological effects, and mechanism of action of nanoparticles. Online databases were used to search and retrieve the published literature (2000 to 2021). A total of 18 articles were selected and reviewed, out of which, 13 (72.2%) studies reported in vitro, 9 (50.0%) in vivo, and 4 (22.2%) both in vitro/in vivo efficacy of different nanoparticles. Mostly, organic nanoparticles (77.7%) were used including polymeric (85.7%) and lipid nanoparticles (14.3%). The highest efficacy, in vitro, of 100% resulted from using encapsulated bromelain against eggs, larvae, and adult worm mortality at 4, 2, and 1 mg/ml, respectively. While in vivo, encapsulated Eucalyptus staigeriana oil reduced worm burden by 83.75% and encapsulated Cymbopogon citratus nano-emulsion by 83.1%. Encapsulated bromelain, encapsulated Eucalyptus staigeriana oil, and encapsulated Cymbopogon citratus nano-emulsion were safe and non-toxic in vivo. Encapsulated bromelain damaged the cuticle, caused paralysis, and death. Nanoparticles could be a potential source for developing novel anthelmintic drugs to overcome the emerging issue of anthelmintic resistance in H. contortus. Studies on molecular effects, toxicological consequences, and different pharmacological targets of nanoparticles are required in future research.
Introduction
Haemonchus contortus is a highly infectious gastrointestinal parasitic nematode of small ruminants. The parasite causes acute anemia, hemorrhagic gastroenteritis, diarrhea, edema, stunted growth, and death of severely affected animals. H. contortus affects millions of ruminants annually, resulting in substantial economic losses due to decreased milk, meat, and wool production, loss of body weight, and cost of anthelmintic drugs (1, 2). The available anthelmintic agents such as imidazothiazole, benzimidazole, and ivermectin among others are becoming ineffective due to the rising issue of chemoresistance in helminths (3–7).
Helminth resistance to multiple anthelmintic drugs is increasing at an alarming speed and has raised great public health concerns (8). In the near future, it would be very difficult to control some of the parasites with prevailing anthelmintic drugs. Some studies reported that sheep nematode populations are highly resistant to oxfendazole (88%), levamisole (41%), and ivermectin (59%) in farm animals (9). Therefore, it is indispensable and timely to develop novel anthelmintics, which are suitable, environmentally friendly, cost effective, and potentially active.
Nanoparticles, owing to their small size, remarkable surface reactivity, and their biomedical applications, are becoming the leading candidates for the development of new anthelmintic drugs (10). They are able to cross membranes and generate reactive oxygen species (ROS), leading to great reactivity and finally death of infectious agents (11, 12). Recently, anthelmintic potential of nanoparticles is being constantly evaluated for controlling parasitic infections (13).
Nanoparticles are widely used in modern medicines, such as vaccines, diagnostic procedures, medical devices, drug delivery, imaging, and antimicrobial therapies (14). Several applications of nanomaterials as anthelmintics have been reported, including inorganic and organic nanoparticles (13, 15–29). Since the anthelminthic use of nanoparticles, we aimed to systematically address the in vitro/in vivo anti-haemonchiasis potential of nanoparticles, toxicological effects, and mechanism of action. This review will also help to highlight the existing gaps in nanoparticle research against H. contortus.
Methodology
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (30). No protocol was followed for conducting this systematic review. The PRISMA checklist is provided in the supporting information section (Supplementary Table S1).
Searching Criteria
Different databases, e.g., ScienceDirect, Google Scholar, Scopus, and PubMed were searched to find relevant published literature (2000 to 2021). Research articles published in the English language were gathered for this systematic review. Keywords such as nanoparticles, nanoparticles nematicidal activity, anthelmintic activity of nanomaterials, in vitro/in vivo activity of nanoparticles, and nanoparticles mechanism of toxicity/inhibition. “Nanoparticles AND anthelmintics OR nematicidal,” “Nanoparticles AND Haemonchus contortus,” “Anthelmintic AND Haemonchus contortus,” “anthelmintic in vitro OR in vivo.” The list of references of published articles was carefully observed, and related titles were searched and downloaded. Moreover, other related literature was also searched and included to discuss and support the findings of the current review.
Inclusion/Exclusion Criteria
The inclusion criteria were (a) nanoparticles tested in vitro/in vivo, (b) articles containing information on assay types, concentration and time exposure used, inhibition/efficacy of nanoparticles, and size of nanoparticles, and (c) original research articles published in English. However, articles dealing with (a) molecular, prevalence, and epidemiological aspects of H. contortus, (b) nanoparticles studies dealing with parasites other than H. contortus, (c) studies that tested chemicals/drugs other than nanoparticles, (d) plant extracts used against H. contortus, and (e) nanoparticles used as a candidate for vaccine were out of the scope and were excluded from this review.
Data Extraction
Endnote (Thomson Reuters, San Francisco, CA, USA) was used to compile the articles. The selected articles were carefully reviewed by the researchers to extract the relevant information including nanoparticle(s) name and size, biological species used, time of exposure, concentration used, inhibition/efficacy, toxicological and pharmacological effects, author(s) name, country of study, and year of publication. Figures and tables were formulated to arrange the extracted data. Moreover, Inkscape (0.92) (https://inkscape.org/) was also used as a drawing tool.
Quantitative Analysis
Jaccard Similarity Index
Jaccard similarity index (JI) was calculated to determine the similarity between the two sets of studies reported in this review. One set of the study is the “in vitro pharmacological validation of nanoparticles” and the other one is the “in vivo pharmacological validation of nanoparticles.” The following formula was used for JI similarity (31):
where “a” is the total number of nanoparticles used in vitro, “b” is the total number of nanoparticles used in vivo as anthelmintic against H. contortus, and “c” is the number of nanoparticles common to both in vitro and in vivo studies.
Results
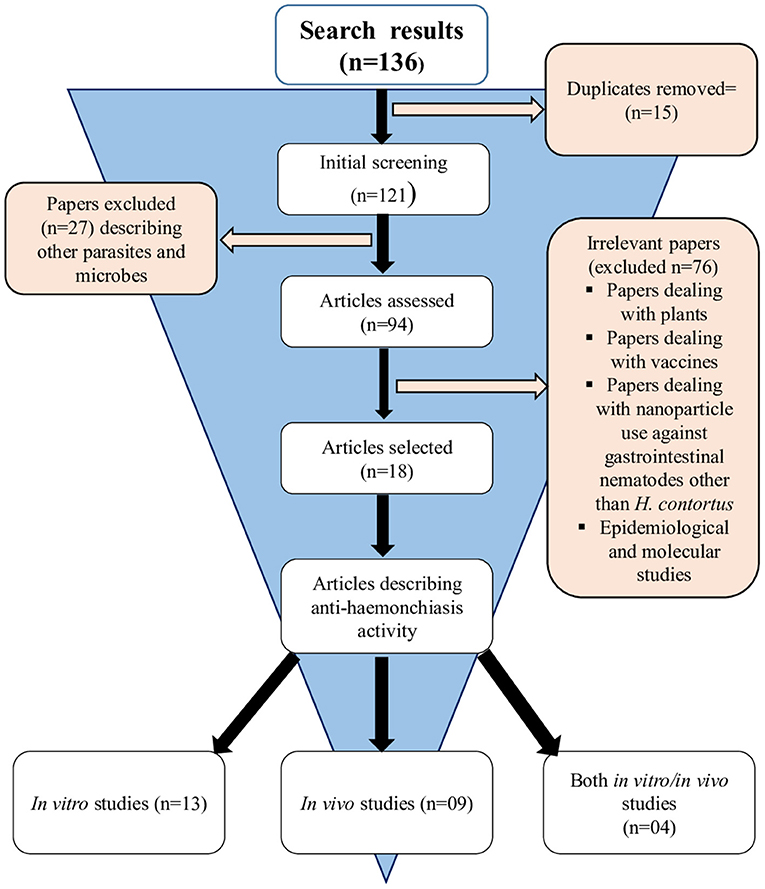
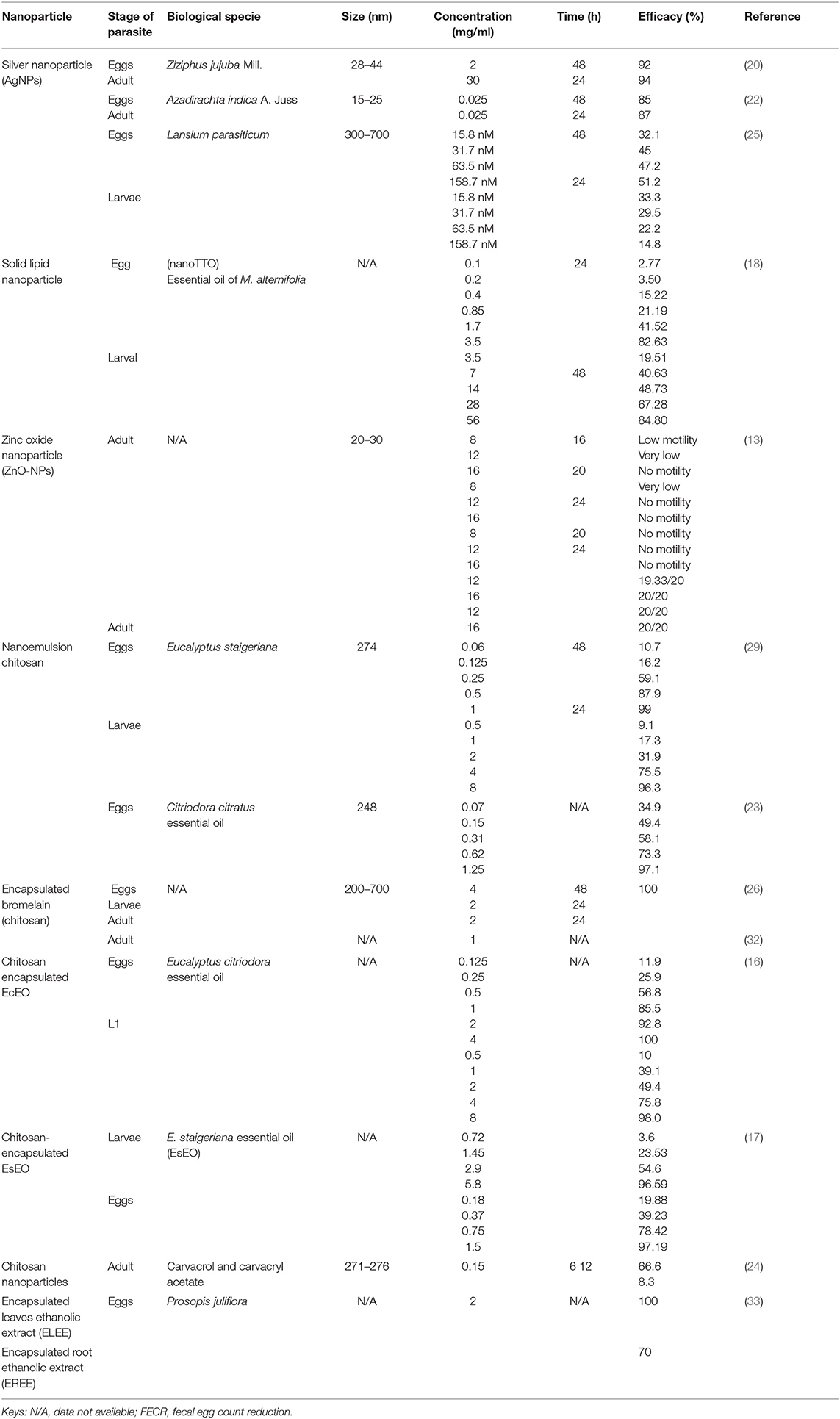
A total of 136 (n = 136) research articles were found and downloaded from online search databases. Eighteen (n = 18) articles were selected and thoroughly reviewed for this study. All the irrelevant and duplicate articles were removed (Figure 1). The quality of the selected articles was assessed, and the articles were summarized as author(s) name, nanoparticles used, biological species/compound used in combination with nanoparticles, source of nanoparticles, country name, release profile, reported quality control, as well as characterization (Table 1).
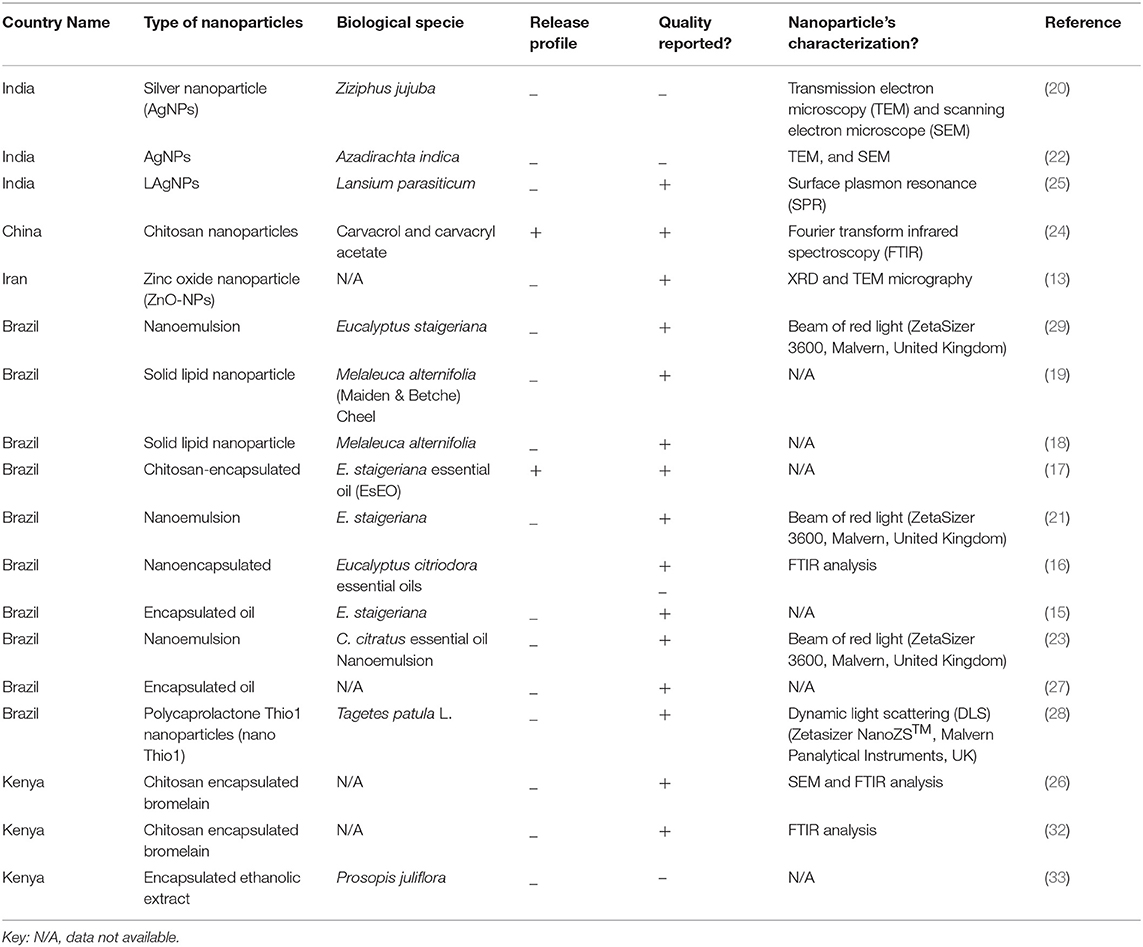
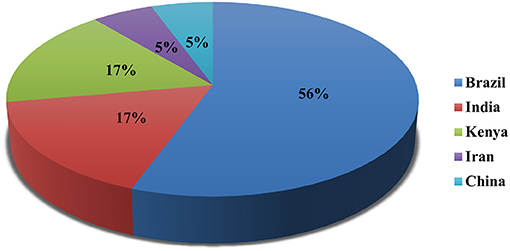
Out of 18 articles, 13 (72.2%) studies reported in vitro, 9 (50.0%) in vivo, and 4 (22.2%) reported both in vitro/in vivo efficacy of nanoparticles. In vitro studies were more than in vivo. Mostly, studies were carried out in Brazil (n = 10; 56.0%), Kenya, and India (n = 3; 17.0% each) (Figure 2). Most of the studies were reported in the year 2020 (n = 4) and 2017 (n = 3), followed by 2013 and 2016 (n = 2 each) (Figure 3). Mostly, organic nanoparticles (77.7%) were used including polymeric (85.7%) and lipid nanoparticles (14.3%). The remaining (22.2%) were non-organic nanoparticles comprised of metals (75.0%) and metal oxides (25.0%). Among metal and metal oxides nanoparticles, silver and zinc oxide were reported. The active substances were mainly encapsulated by using polycaprolactone and chitosan as a polymeric matrix. The release kinetics of chitosan encapsulated Eucalyptus staigeriana essential oil and chitosan nanoparticles loaded with carvacrol and carvacryl acetate were performed using dialysis membrane method. However, this important piece of information was found missing in most of the selected articles.
The effects of nanoparticles were evaluated using the egg hatching test (EHT), larval development test (LDT), adult worm mortality test (AWM), and adult worm motility test (AWM) in vitro, whereas in vivo efficacy was evaluated by using worm burden reduction and fecal egg count reduction tests (FECRT). Egg hatching test was the commonly used assay in vitro, while the worm burden reduction was common in vivo (Figure 4).
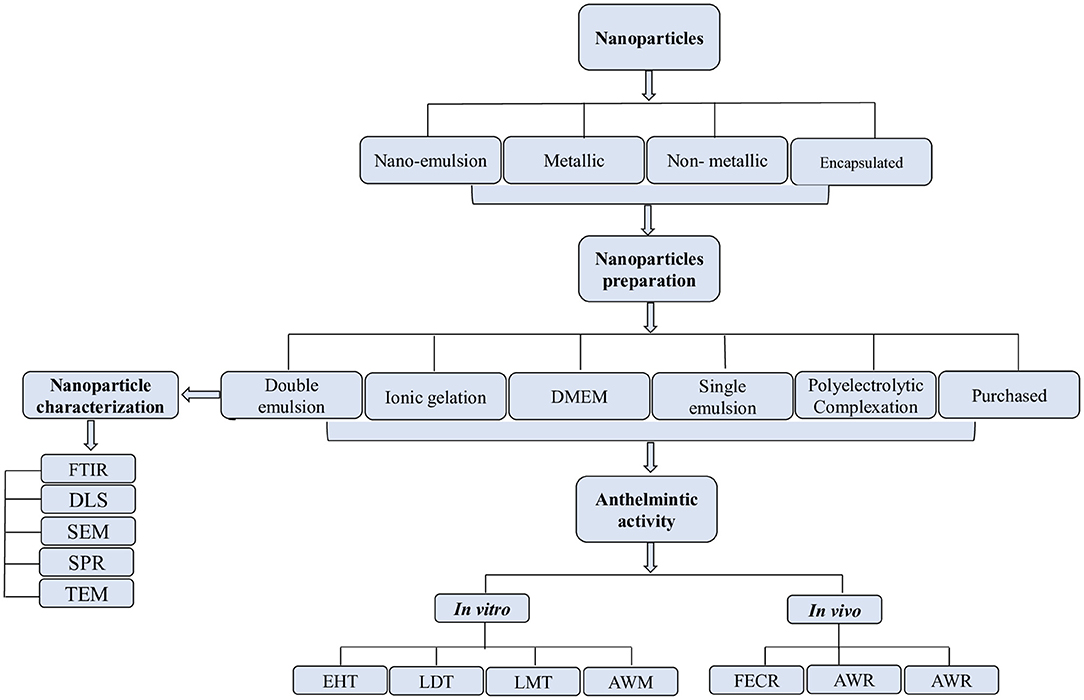
Figure 4. Process of selection, preparation, and characterization of nanoparticles for in vitro/in vivo anthelmintic activity.
The results exhibited that the doses used in the in vitro studies ranging from 0.025 to 56 and 0.20 to 500 mg/kg for in vivo. The most common exposure time against eggs hatching was 48 h, whereas it was 24 h for larvae and adults. The highest efficacy of 100% was a result of using encapsulated bromelain against eggs, larvae, and adult worm mortality at a concentration of 4, 2, and 1 mg/ml, respectively (Table 2). Encapsulated E. staigeriana oil reduced worm burden by 83.75% and encapsulated Cymbopogon citratus nano-emulsion by 83.1% in vivo (Table 3).
The double emulsion method was the frequently used (n = 4) technique for nanoparticle preparation than the ionic gelation method (n = 2), and Dulbecco's modified eagle medium (DMEM), polyelectrolytic complexation system, and single emulsion method (n = 1 each). The methods used for nanoparticle characterization were Fourier transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), x-ray diffraction (XRD), and surface plasmon resonance (SPR).
Comparative Analysis of Common Nanoparticles
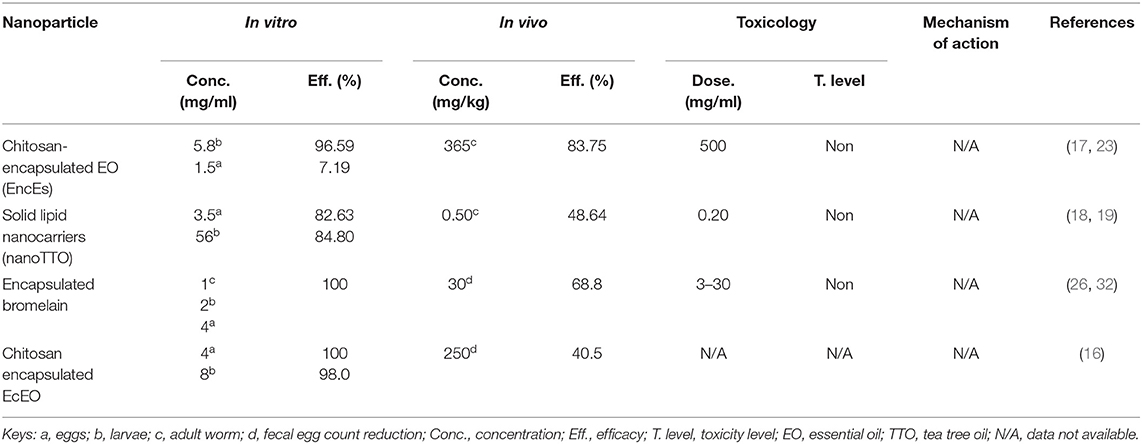
Nanoparticles evaluated for both in vitro and in vivo efficacies were compared to know their effectiveness against the parasite. Four nanoparticles were commonly evaluated for in vitro as well as in vivo efficacy (Table 4). Since minimum concentration used and maximum efficacy obtained, the encapsulated bromelain was highly effective (100%) in vitro; however, the in vivo efficacy was not satisfactory (68.8%) at the tested concentration. Similarly, the chitosan-encapsulated EO (EncEs) were potentially active against eggs and larvae in vitro (98.0 and 97.0%, respectively), while high activity (84.0%) was also reported in vivo with a relatively higher concentration. Moreover, the nanoparticles were more effective in vitro compared with in vivo.
Toxicity Evaluation
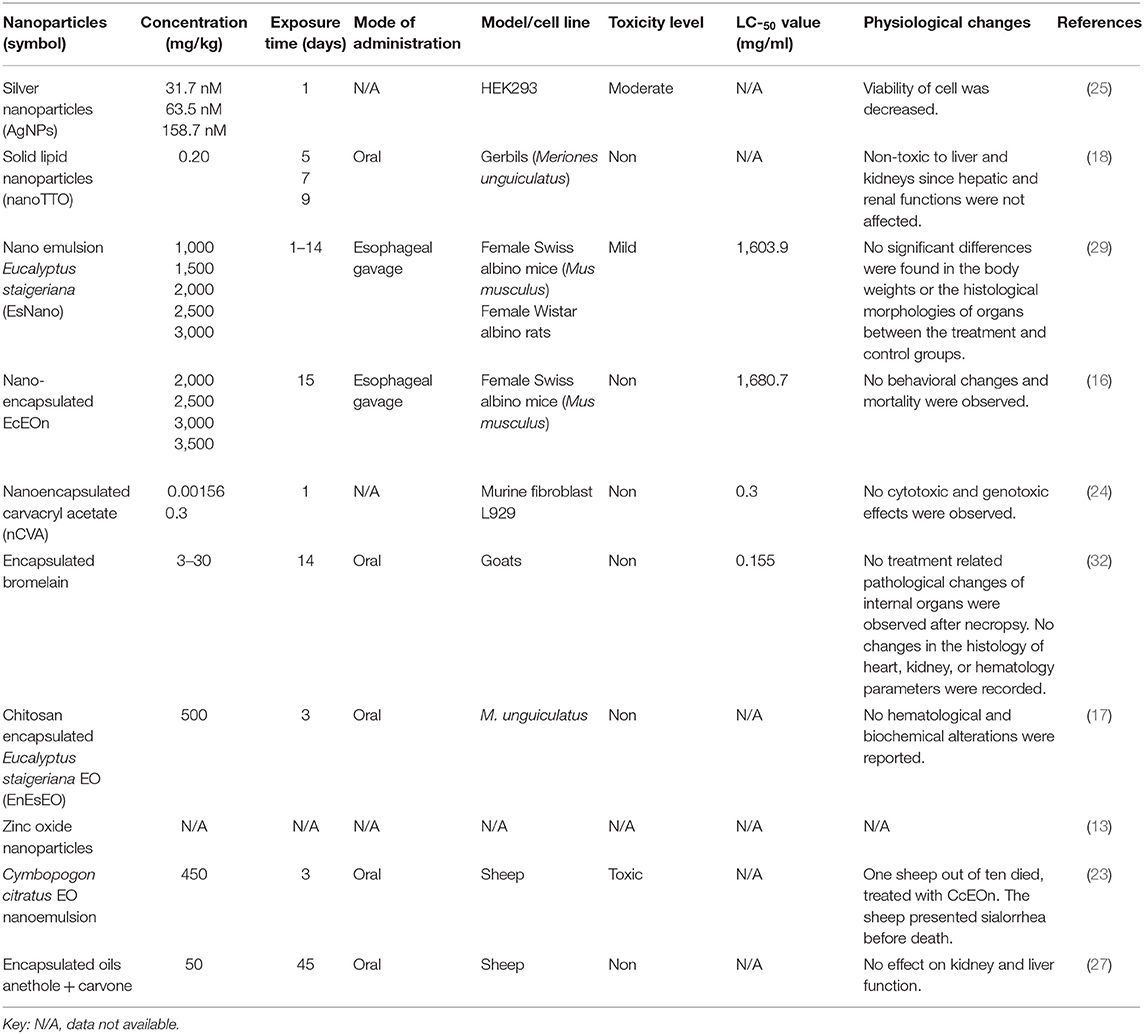
Toxicity and toxic doses of different nanoparticles were reviewed and reported (Table 5). Among the tested nanoparticles, nano-tea tree oil (TTO), EncEs, EcEOn, nanoencapsulated carvacryl acetate (nCVA), and encapsulated bromelain were reported as non-toxic at the tested concentrations, while AgNPs and EsNano were moderately and mildly toxic in HEK293 cell lines and female Swiss albino mice, respectively. Zinc oxide nanoparticles were not evaluated for their toxicity. Nanoparticles were either orally administered or through esophageal gavage. The LC50 for AgNPs, CcEOn, and nano-TTO was not calculated.
Jaccard Similarity Index
The two datasets, i.e., in vitro and in vivo use of nanoparticles was checked for their similarity by using JI similarity formula and 22.2% similarity was found.
Discussion
The main constraints of profitable products in the livestock sector are parasites and parasitic resistance to anthelmintic drugs around the world. To resolve the huge economic losses, it is important to improve the control of main parasitic diseases through alternative, less harmful, biodegradable, and ecologically safe anthelmintic strategies. Nanoparticles may reduce the risk of resistance of H. contortus to the anthelmintic drugs and overcome the resistance mechanisms adapted by the parasite, potentiating the drug target, and increasing bioavailability of the drug. The current systematic review assessed the in vitro/in vivo nematicidal potential and toxicological implications along with the mechanism of action of various nanoparticles against H. contortus. The results of this study will help to identify potential approaches to design new nanoparticulate drugs and ways to meet the current research limitations in prospective studies.
Nanoparticles were commonly evaluated in vitro, and only few studies had reported in vivo effectiveness of different nanoparticles. Previously, in vitro studies were mostly reported than in vivo and justify the current findings (34–36). In vitro studies are inexpensive and less time consuming, and anthelmintic effects at different life stages of the parasite can easily be studied (37). After initial screening, effective substance/product can further be evaluated for in vivo efficacy (38). In vivo studies are useful to know the host immune response to a particular anthelmintic agent, toxicological and pharmacological effects, and in vivo efficacy. However, in vivo studies are expensive and difficult to reproduce the results, required long experimental duration, and has lower precision (39). The research field is highly inundated with in vitro studies, and in vivo studies are insufficient; therefore, in vivo evaluation of nanoparticles would be of great importance in future studies (36).
Organic nanoparticles were among the frequently used nanoparticles. Nanoparticles can easily be produced in large quantities using different approaches and are highly biodegradable and biocompatible. These nanoparticles possess the capacity to solve, absorb, and encapsulate a drug in a polymer matrix and are excellent nanocarriers for the controlled and sustained release of drugs (40, 41). Among metal and metal oxides, AgNPs and ZnO were reported. AgNPs have profound antiparasitic and antibacterial activities. Antiparasitic activity of AgNPs was inhibition of metabolic activities and cell proliferation of Leishmania spp. promastigotes. Antiviral activities of AgNPs have also been demonstrated to stop viral replication process and prevent binding of virus particles to host cell receptors. These nanoparticles have promising efficacy as anticancer agents and could be a reason that they have attracted more attention as an anthelmintic agent (42–46). ZnO nanoparticles are widely used and important candidates for developing novel drugs due to their non-toxic, antiparasitic, antifungal, and antimicrobial effects. These can also be used for gene delivery and can cause death of cancerous cells without effecting normal healthy cells (47, 48).
Nanoparticles were encapsulated using a polymeric matrix, mainly chitosan and polycaprolactone, to improve the controlled drug release. Encapsulation of bioactive substances also improves the absorption and bioavailability by facilitating the diffusion through epithelium. The most common are aliphatic polyesters and their copolymers. Polycaprolactone (PCL) is a synthetic aliphatic polyester approved by the FDA and has some advantages: it is hydrophobic, biodegradable, biocompatible, and relatively inexpensive (49). In addition, due to its low toxicity, it is suitable for intravenous or oral administration (50, 51). Chitosan, a natural polymer obtained by the deacetylation of chitin, was the frequently used encapsulating matrix. The chitosan microsphere formulation for the controlled release of drugs improves their dissolution and bioavailability (52, 53). Hence, increases in efficient drug delivery may increase the overall effectiveness of the targeted drug/compound. Furthermore, its excellent biodegradability and non-toxic nature were the core reasons for which chitosan was selected as an encapsulating agent for evaluating anti-haemonchiasis nanoparticles (21).
The release kinetics of nanoparticles was the neglected aspect and barely studied in the reviewed articles. It is an important and critical aspect that helps to understand the dosage form behavior, and assess the safety and efficacy of a desired drug during the various stages of development. To maximize the effectiveness of nanoparticle targeting, drug release from nanoparticles needs to be slow enough to avoid substantial drug loss before the carrier reaches the site of action thereby reducing toxicity (54, 55). After nanoparticle accumulation at the target site, optimizing efficacy will require tunability of the drug release rate (56). Therefore, determination of product quality and performance becomes a crucial aspect during nanoparticulate dosage form development. When designed appropriately, an in vitro release profile can reveal fundamental information on the dosage form and its behavior, as well as provide details on the release mechanism and kinetics, enabling a rational and scientific approach to drug product development (57). Thus, the kinetics of drug release from nanoparticles should be an essential feature of their design and a property monitored for the quality control of nanoparticle formulations (58).
The frequently reported assay was egg hatching test (EHT) followed by larval development test (LDT). The possible reason for such an extensive use of these assays may be attributed to the fact that these tests take into account variations in the habits, behavior, and sensibility of these life forms of the parasite and permit the exposure of different potential pharmacological sites for future pharmacodynamics investigation (59). Moreover, APMT and AWMT were less likely to be used in in vitro studies for anthelmintic evaluation. It is because of the lack of a culture system yielding adults of this nematode parasite, which prevents a preliminary investigation of the efficacy of anthelmintics at this stage (60); hence, the in vitro tests using free-living stages of nematode parasites are considered as the best means of screening the anthelmintic activity of new substances/products (61).
The most widely utilized in vivo assessment of nanoparticles against H. contortus was the FECR test. The major benefit of this examination is that, regardless of their mode of operation, it can be carried out with all anthelmintics (59). Gerbils and sheep have been primarily used in in vivo experiments as animal models. Using sheep as a model can be explained by the fact that domestic animals are a valuable component of clinical studies for several purposes, including simple availability and management, accessibility to early examine diseased tissues as well as the models, and also require disease characteristics to be explored at an early level (62). However, a study also evaluated the efficacy of plants against intestinal nematode parasites by using mice as models and reported high anthelmintic efficacy (63). Rodent use as an animal model may have some drawbacks; rodents provide a completely different internal environment (habitat) to the nematodes than small ruminants; thus, the drug efficacy may be lower or higher based on the habitat and drug absorption site of the host (64). Rodents are monogastric, and sheep are polygastric animals, which can also alter the drug mechanism of distribution and biotransformation. However, efficacy test on rodents can help researchers deduce the prescriptions to be used on sheep and goats (63).
Toxicity
LAgNPs were moderately toxic when tested on HEK293 cell lines. The HEK293 cell viability decreased in a dose- and time-dependent manner. The lowest concentration produced no significant toxic effects; however, with an increase in concentration, i.e., 31.7, 63.5, and 158.7 nM, the observed decrease in the viability was 77.5 ± 5.06, 71.3 ± 9.8, and 62.1 ± 9.3%, respectively (25). Oral administration of CcEOn at 450 mg/kg concentration resulted in the death of 1 sheep out of 10. The sheep suffered from sialorrhea before death (23). However, the death of the sheep was not confirmed through necropsy and was assumed that the sheep may have aspirated the essential oil, and the wrong route of administration was attributed as the cause of death. Other studies also supported the non-toxic nature of CcEO at 1 and 800 mg/kg in rats and gerbils, respectively (65, 66) (Figure 5).
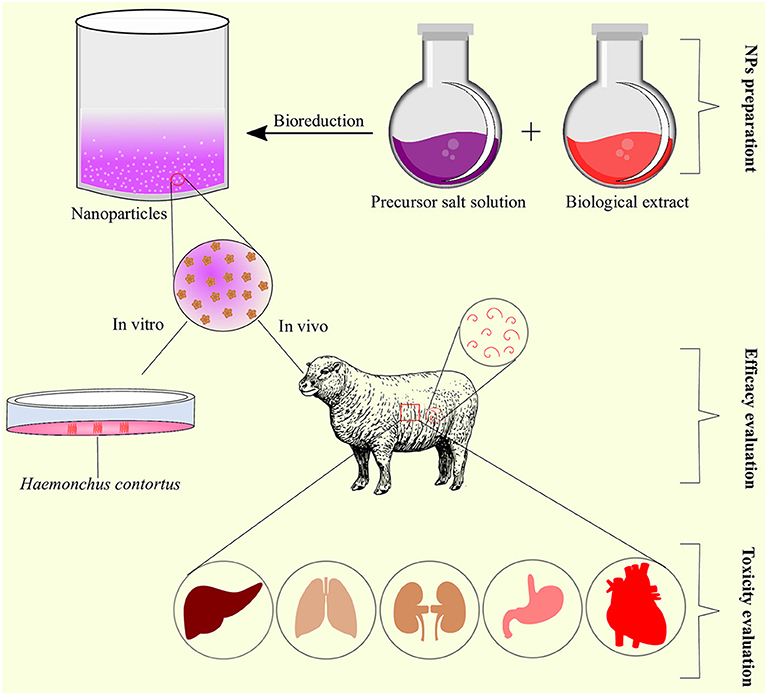
Figure 5. Schematic representation of nanoparticles preparation, efficacy, and toxicity evaluation as anthelmintic agents.
EsNano was found non-toxic after acute and subchronic toxicity evaluation in rats. The tested concentrations did not produce any change in the hematological parameters except a slight increase in the white blood cells (WBCs) after subchronic toxicity (29). There was no change in the body weight and histological morphologies of organs in treated rats (29). Nano encapsulated carvacryl acetate (nCVA) had no cytotoxic and genotoxic/mutagenic effects on murine fibroblast cell lines at the tested concentration. The non-toxic effects of nanoparticles were attributed to CVA and the biopolymers as they had no toxicity (24). Esophageal gavage administration of nanoencapsulated EcEO was safe, and no behavioral changes and mortality were recorded after acute toxicity evaluation (16). Similarly, nanoTTO produced non-significant differences in the hematological and serum biochemical profiles of the treated and untreated groups (18). Encapsulated bromelain was non-toxic as no pathological and histological changes were observed at concentrations ranging from 3 to 30 mg/kg after necropsy. The hematological parameters also remained unchanged at the same concentrations, confirming the non-toxic effect of bromelain in vivo (32). Encapsulated E. staigeriana essential oil (EnEsEO) was found non-toxic when orally administered to gerbils at 500 mg/kg concentration (17). However, the toxicity of ZnO-NPs was not evaluated and should be evaluated in future studies.
Mechanism of Action
The pharmacological activity of a drug depends on how it interacts with the targeted biomolecules, i.e., receptors (67). Pharmacological activity is an important phenomenon to know the precise target of the nanoparticle with anthelmintic efficacy against the parasite or other organism/pathogen under observation (36).
Exposure of H. contortus to LAgNPs produced morphological and physiological effects. Morphologically, LAgNPs caused complete distortion of the cuticle and shrank the body. Physiologically, levels of reactive oxygen and nitrogen species were significantly increased, which resulted in oxidative stress and caused physical damage to tissues of the worm (25). In response to oxidative stress, a sharp increase in stress-responsive activities of enzymes, like catalase, superoxide dismutase, and glutathione peroxidase activities, along with the concentration of glutathione, was observed in worm tissue, which indicated a LAgNPs-responsive alteration of metabolism (25). Moreover, AgNPs also depleted the levels of glycogen, lipids, and protein contents of H. contortus. Parasites produce energy from stored carbohydrates (glycogen) to perform major metabolic processes (20). Glycogen is the chief energy reserve in most of the nematodes that exist in environments of low oxygen tension (68). Lipids are the chief functional and structural components of nematode parasites. Plasma membranes and eggs contain lipids as an important energy source in the free-living stages, any depletion or damage to lipid constituents may lead to mortality of the parasite. Therefore, lipid biosynthesis inhibition could be a potential target to develop an effective anti-haemonchiasis drug (20).
Proteins, like enzymes, are very important for normal physiological functioning and to carry out key metabolic activities. Hence, reduced protein content would hamper the normal physiological activities of the worms and may be accounted for mortality at higher concentrations. Egg morphological alterations justify the disintegration and shrinkage of H. contortus larvae development (20). Some studies reported, a drastic decrease in 5′ nucleosidase, ATPase, alkaline, and acid phosphatases of intestinal cestodes treated with AuNPs (69). Encapsulated bromelain is highly effective against nematode parasites (70), and it was found that bromelain damages the cuticle of H. contortus leading to paralysis and death (71).
The ZnO-NPs completely paralyzed the parasites. These nanoparticles can adversely affect the antioxidant systems of H. contortus by inducing severe oxidative stress resulting in denaturation of the antioxidant enzymes. Various concentrations of ZnO-NPs imposed controversial alteration on the activities of the antioxidant enzymes including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px) (13). This increase in the oxidative stress and reactive oxygen species (ROS) can damage proteins, carbohydrates, lipids, and DNA of the parasite (72). Therefore, disruption of the antioxidant system of the parasite unables H. contortus to survive against the host generated free radicals.
Conclusion and Future Recommendations
Nanoparticles could be a potential source for developing novel anthelmintic drugs to overcome the emerging issue of anthelmintic resistance in H. contortus. Mostly, in vitro studies have reported the anthelmintic efficacy of nanoparticles. More studies are required to evaluate and describe the effects of nanoparticles on a molecular level, toxicological consequences, and different pharmacological targets along with exact mechanism of action using suitable animal models. Furthermore, the size of the nanoparticles was not determined in some of the studies, which is one of the crucial aspects of nanoparticles, and should be considered in future studies to provide more in-depth information of the nanoparticles under consideration.
Chitosan-encapsulated EO and encapsulated bromelain were highly effective both in vitro and in vivo with no observed toxic effects at the tested concentration. However, the release profile of mostly nano-encapsulated compound(s) was missing, and hence, the controlled and sustained drug release properties are unknown. These nanoparticles should further be evaluated and could be alternative sources of anti-haemonchiasis agents.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
RA and NA conceptualized the study, conducted the formal analysis, curated the data, and wrote the original draft. RA and SM formulated the methodology. RA sourced the software and provided supervision for the study. RA, SNK, AM, and SFA reviewed and edited the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.789977/full#supplementary-material
References
1. Wang C, Li F, Zhang Z, Yang X, Ahmad AA Li X, et al. Recent research progress in China on Haemonchus contortus. Front Microbiol. (2017) 8:1509. doi: 10.3389/fmicb.2017.01509
2. Tariq KA. A review of the epidemiology and control of gastrointestinal nematode infections of small ruminants. Proc Natl Acad Sci India B Biol Sci. (2015) 85:693–703. doi: 10.1007/s40011-014-0385-9
3. Easwaran C, Harikrishnan TJ, Raman M. Multiple anthelmintic resistance in gastrointestinal nematodes of sheep in Southern India. Vet Arh. (2009) 79:611–20.
4. Kaplan RM, Vidyashankar AN. An inconvenient truth: global worming and anthelmintic resistance. Vet Parasitol. (2012) 186:70–8. doi: 10.1016/j.vetpar.2011.11.048
5. Das G. Levamisole and fenbendazole resistance among gastrointestinal nematodes in goats at Jabalpur, Madhya Pradesh. J Vet Parasitol. (2015) 29:98–102.
6. Dixit AK, Das G, Dixit P, Singh AP, Kumbhakar NK, Sankar M, et al. An assessment of benzimidazole resistance against caprine nematodes in Central India. Trop Anim Health Prod. (2017) 49:1471–8. doi: 10.1007/s11250-017-1349-x
7. Furgasa W, Abunna F, Yimer L, Haile G. Review on anthelmintic resistance against gastrointestinal nematodes of small ruminants: its status and future perscpective in Ethiopia. J Vet Sci Ani Husb. (2018) 6:407.
8. Torres-Acosta JF. Mendoza-de-Gives P, Aguilar-Caballero AJ, Cuéllar-Ordaz JA. Anthelmintic resistance in sheep farms: update of the situation in the American continent. Vet Parasitol. (2012) 189:89–96. doi: 10.1016/j.vetpar.2012.03.037
9. Melo ACFL, Bevilaqua CML, Reis IF. Resistance to benzimidazoles anthelmintics in gastrointestinal nematodes of small ruminants from the Brazilian northeastern semiarid region. Braz Anim Sci. (2009) 10:294–300.
10. Adeyemi OS, Whiteley CG. Interaction of nanoparticles with arginine kinase from Trypanosoma brucei: kinetic and mechanistic evaluation. Int J Biol Macromol. (2013) 62:450–6. doi: 10.1016/j.ijbiomac.2013.09.008
11. Butkus MA, Labare MP, Starke JA, Moon K, Talbot M. Use of aqueous silver to enhance inactivation of coliphage MS-2 by UV disinfection. Appl Environ Microbiol. (2004) 70:2848–53. doi: 10.1128/AEM.70.5.2848-2853.2004
12. Bhardwaj R, Saudagar P, Dubey V. Nanobiosciences: a contemporary approach in antiparasitic drugs. Mol Cell Pharmacol. (2012) 4:97–103.
13. Esmaeilnejad B, Samiei A, Mirzaei Y, Farhang-Pajuh F. Assessment of oxidative/nitrosative stress biomarkers and DNA damage in Haemonchus contortus, following exposure to zinc oxide nanoparticles. Acta Parasitol. (2018) 63:563–71. doi: 10.1515/ap-2018-0065
14. Zhu X, Radovic-Moreno AF, Wu J, Langer R, Shi J. Nanomedicine in the management of microbial infection - overview and perspectives. Nano Today. (2014) 9:478–98. doi: 10.1016/j.nantod.2014.06.003
15. de Aquino Mesquita M, JB ESJ, Panassol AM, de Oliveira EF, Vasconcelos AL, de Paula HC, et al. Anthelmintic activity of Eucalyptus staigeriana encapsulated oil on sheep gastrointestinal nematodes. Parasitol Res. (2013) 112:3161–5. doi: 10.1007/s00436-013-3492-2
16. Ribeiro J, Ribeiro W, Camurça-Vasconcelos A, Macedo I, Santos J, Paula H, et al. Efficacy of free and nanoencapsulated Eucalyptus citriodora essential oils on sheep gastrointestinal nematodes and toxicity for mice. Vet Parasitol. (2014) 204:243–8. doi: 10.1016/j.vetpar.2014.05.026
17. Ribeiro WL, Macedo IT, dos Santos JM, de Oliveira EF, Camurça-Vasconcelos AL, de Paula HC, et al. Activity of chitosan-encapsulated Eucalyptus staigeriana essential oil on Haemonchus contortus. Exp Parasitol. (2013) 135:24–9. doi: 10.1016/j.exppara.2013.05.014
18. Grando T, De Sá M, Baldissera M, Oliveira C, De Souza M, Raffin R, et al. In vitro activity of essential oils of free and nanostructured Melaleuca alternifolia and of terpinen-4-ol on eggs and larvae of Haemonchus contortus. J Helminthol. (2016) 90:377–82. doi: 10.1017/S0022149X15000401
19. Grando TH, Baldissera MD, Gressler LT, de Sá MF, Bortoluzzi BN, Schafer AS, et al. Melaleuca alternifolia anthelmintic activity in gerbils experimentally infected by Haemonchus contortus. Exp Parasitol. (2016) 170:177–83. doi: 10.1016/j.exppara.2016.09.004
20. Preet S, Tomar RS. Anthelmintic effect of biofabricated silver nanoparticles using Ziziphus jujuba leaf extract on nutritional status of Haemonchus contortus. Small Rumin Res. (2017) 154:45–51. doi: 10.1016/j.smallrumres.2017.07.002
21. Ribeiro WL, Camurça-Vasconcelos AL, dos Santos JM, Macedo IT., Ribeiro JdC, Oliveira EFd, et al. The use of Eucalyptus staigeriana nanoemulsion for control of sheep haemonchosis. Braz J Vet Res. (2017) 37:221–6. doi: 10.1590/s0100-736x2017000300004
22. Tomar R, Preet S. Evaluation of anthelmintic activity of biologically synthesized silver nanoparticles against the gastrointestinal nematode, Haemonchus contortus. J Helminthol. (2017) 91:454. doi: 10.1017/S0022149X16000444
23. Macedo ITF, Oliveira LMB, André WPP, Araújo Filho JV, Santos J, Rondon FCM, et al. Anthelmintic effect of Cymbopogon citratus essential oil and its nanoemulsion on sheep gastrointestinal nematodes. Rev Bras Parasitol Vet. (2019) 28:522–7. doi: 10.1590/s1984-29612019065
24. André WP, Paiva JR, Cavalcante GS, Ribeiro WL., Araújo JVd, Cavalcanti BC, et al. Chitosan nanoparticles loaded with carvacrol and carvacryl acetate for improved anthelmintic activity. J Braz Chem Soc. (2020) 31:1614–22. doi: 10.21577/0103-5053.20200047
25. Goel V, Kaur P, Singla LD, Choudhury D. Biomedical evaluation of Lansium parasiticum extract-protected silver nanoparticles against Haemonchus contortus, a parasitic worm. Front Mol Biosci. (2020) 7:595646. doi: 10.3389/fmolb.2020.595646
26. Hunduza A, Kagira J, Maina N, Andala D, Cheruiyot K, Kahiro S. In vitro anthelmintic activity of chitosan encapsulated bromelain against eggs, larval and adult stages of Haemonchus contortus. J Appl Life Sci Int. (2020) 23:28–38. doi: 10.9734/jalsi/2020/v23i330151
27. Katiki LM, Araujo RC, Ziegelmeyer L, Gomes ACP, Gutmanis G, Rodrigues L, et al. Evaluation of encapsulated anethole and carvone in lambs artificially- and naturally-infected with Haemonchus contortus. Exp Parasitol. (2019) 197:36–42. doi: 10.1016/j.exppara.2019.01.002
28. Politi FAS, Bueno RV, Zeoly LA, Fantatto RR, Eloy JO, Chorilli M, et al. Anthelmintic activity of a nanoformulation based on thiophenes identified in Tagetes patula L. (Asteraceae) against the small ruminant nematode Haemonchus contortus. Acta Trop. (2021) 219:105920. doi: 10.1016/j.actatropica.2021.105920
29. Ribeiro WL, Camurça-Vasconcelos AL, Macedo IT, dos Santos JM, de Araújo-Filho JV, Ribeiro Jde C, et al. In vitro effects of Eucalyptus staigeriana nanoemulsion on Haemonchus contortus and toxicity in rodents. Vet Parasitol. (2015) 212:444–7. doi: 10.1016/j.vetpar.2015.07.019
30. Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint–preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. (2009) 89:873–80. doi: 10.1093/ptj/89.9.873
31. Kayani S, Ahmad M, Sultana S, Khan Shinwari Z, Zafar M, Yaseen G, et al. Ethnobotany of medicinal plants among the communities of Alpine and Sub-alpine regions of Pakistan. J Ethnopharmacol. (2015) 164:186–202. doi: 10.1016/j.jep.2015.02.004
32. Wasso S, Maina N, Kagira J. Toxicity and anthelmintic efficacy of chitosan encapsulated bromelain against gastrointestinal strongyles in Small East African goats in Kenya. Vet World. (2020) 13:177–83. doi: 10.14202/vetworld.2020.177-183
33. Kipyegon C, Helen KL, Patrick KG, Francis NK, Odhiambo RS, Jackson MK, et al. In vitro ovicidal activity of encapsulated ethanolic extract of Prosopis juliflora against Haemonchus contortus eggs. J Pharm Biol Sci. (2015) 10:18e22. doi: 10.9790/3008-10541822
34. Albalawi AE, Alanazi AD, Baharvand P, Sepahvand M, Mahmoudvand H. High potency of organic and inorganic nanoparticles to treat cystic echinococcosis: an evidence-based review. Nanomaterials (Basel). (2020) 10:2538. doi: 10.3390/nano10122538
35. Ali R, Khan S, Khan M, Adnan M, Ali I, Khan TA, et al. A systematic review of medicinal plants used against Echinococcus granulosus. PLoS One. (2020) 15:e0240456. doi: 10.1371/journal.pone.0240456
36. Ali R, Rooman M, Mussarat S, Norin S, Ali S, Adnan M, et al. A systematic review on comparative analysis, toxicology, and pharmacology of medicinal plants against Haemonchus contortus. Front Pharmacol. (2021) 12:644027. doi: 10.3389/fphar.2021.644027
37. Demeler J, Gill JH, von Samson-Himmelstjerna G, Sangster NC. The in vitro assay profile of macrocyclic lactone resistance in three species of sheep trichostrongyloids. Int J Parasitol Drugs Drug Resist. (2013) 3:109–18. doi: 10.1016/j.ijpddr.2013.04.002
38. Zips D, Thames HD, Baumann M. New anticancer agents: in vitro and in vivo evaluation. In Vivo. (2005) 19:1–7.
39. Lacey E, Redwin J, Gill J, Demargheriti V, Waller P. A larval development assay for the simultaneous detection of broad spectrum anthelmintic resistance. In: Resistance of Parasites to Antiparasitic Drugs Round Table Conference Held at the 7th International Congress of Parasitology. Paris: Merck (1990). p. 177–84.
40. Prabhu RH, Patravale VB, Joshi MD. Polymeric nanoparticles for targeted treatment in oncology: current insights. Int J Nanomedicine. (2015) 10:1001–18. doi: 10.2147/IJN.S56932
41. Bhatia D, Mittal A, Malik DK. Antimicrobial activity of PVP coated silver nanoparticles synthesized by Lysinibacillus varians. 3 Biotech. (2016) 6:196. doi: 10.1007/s13205-016-0514-7
42. Allahverdiyev AM, Abamor ES, Bagirova M, Ustundag CB, Kaya C, Kaya F, et al. Antileishmanial effect of silver nanoparticles and their enhanced antiparasitic activity under ultraviolet light. Int J Nanomedicine. (2011) 6:2705–14. doi: 10.2147/IJN.S23883
43. Galdiero S, Falanga A, Vitiello M, Cantisani M, Marra V, Galdiero M. Silver nanoparticles as potential antiviral agents. Molecules. (2011) 16:8894–918. doi: 10.3390/molecules16108894
44. Dos Santos CA, Seckler MM, Ingle AP, Gupta I, Galdiero S, Galdiero M, et al. Silver nanoparticles: therapeutical uses, toxicity, and safety issues. J Pharm Sci. (2014) 103:1931–44. doi: 10.1002/jps.24001
45. Gaafar M, Mady R, Diab R. Shalaby ThI. Chitosan and silver nanoparticles: promising anti-toxoplasma agents. Exp Parasitol. (2014) 143:30–8. doi: 10.1016/j.exppara.2014.05.005
46. Wei L, Lu J, Xu H, Patel A, Chen ZS, Chen G. Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discov Today. (2015) 20:595–601. doi: 10.1016/j.drudis.2014.11.014
47. Taylor E, Webster TJ. Reducing infections through nanotechnology and nanoparticles. Int J Nanomed. (2011) 6:1463–73. doi: 10.2147/IJN.S22021
48. Mirzaei H, Darroudi M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceramics Int. (2017) 43(1 Part B):907–14. doi: 10.1016/j.ceramint.2016.10.051
49. Sinha VR, Bansal K, Kaushik R, Kumria R, Trehan A. Poly-ϵ-caprolactone microspheres and nanospheres: an overview. Int J Pharm. (2004) 278:1–23. doi: 10.1016/j.ijpharm.2004.01.044
50. Chen J, Huang C, Chen Z. Study on the biocompatibility and toxicology of biomaterials-poly(epsilon-caprolactone). J Biomed Eng. (2000) 17:380–2.
51. Hernán Pérez de, la Ossa D, Ligresti A, Gil-Alegre ME, Aberturas MR, Molpeceres J, Di Marzo V, et al. Poly-ε-caprolactone microspheres as a drug delivery system for cannabinoid administration: development, characterization and in vitro evaluation of their antitumoral efficacy. J Control Release. (2012) 161:927–32. doi: 10.1016/j.jconrel.2012.05.003
52. Jayakumar R, Nwe N, Tokura S, Tamura H. Sulfated chitin and chitosan as novel biomaterials. Int J Biol Macromol. (2007) 40:175–81. doi: 10.1016/j.ijbiomac.2006.06.021
53. Dash M, Chiellini F, Ottenbrite RM, Chiellini E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci. (2011) 36:981–1014. doi: 10.1016/j.progpolymsci.2011.02.001
54. Loew S, Fahr A, May S. Modeling the release kinetics of poorly water-soluble drug molecules from liposomal nanocarriers. J Drug Deliv. (2011) 2011:376548. doi: 10.1155/2011/376548
55. Zeng L, An L, Wu X. Modeling drug-carrier interaction in the drug release from nanocarriers. J Drug Deliv. (2011) 2011:370308. doi: 10.1155/2011/370308
56. Johnston MJ, Semple SC, Klimuk SK, Edwards K, Eisenhardt ML, Leng EC, et al. Therapeutically optimized rates of drug release can be achieved by varying the drug-to-lipid ratio in liposomal vincristine formulations. Biochim Biophys Acta. (2006) 1758:55–64. doi: 10.1016/j.bbamem.2006.01.009
57. D'Souza S A. Review of in vitro drug release test methods for nano-sized dosage forms. Adv Pharm. (2014) 2014:304757. doi: 10.1155/2014/304757
58. Modi S, Anderson BD. Determination of drug release kinetics from nanoparticles: overcoming pitfalls of the dynamic dialysis method. Mol Pharm. (2013) 10:3076–89. doi: 10.1021/mp400154a
59. Demeler J, Kleinschmidt N, Küttler U, Koopmann R, von Samson-Himmelstjerna G. Evaluation of the egg hatch assay and the larval migration inhibition assay to detect anthelmintic resistance in cattle parasitic nematodes on farms. Parasitol Int. (2012) 61:614–8. doi: 10.1016/j.parint.2012.06.003
60. Geary TG, Sangster NC, Thompson DP. Frontiers in anthelmintic pharmacology. Vet Parasitol. (1999) 84:275–95. doi: 10.1016/S0304-4017(99)00042-4
61. Asase A, Oteng-Yeboah AA, Odamtten GT, Simmonds MS. Ethnobotanical study of some Ghanaian anti-malarial plants. J Ethnopharmacol. (2005) 99:273–9. doi: 10.1016/j.jep.2005.02.020
62. Sadia S, Tariq A, Shaheen S, Malik K, Ahmad M, Qureshi H, et al. Ethnopharmacological profile of anti-arthritic plants of Asia-a systematic review. J Herb Med. (2018) 13:8–25. doi: 10.1016/j.hermed.2018.08.003
63. Camurça-Vasconcelos A, Bevilaqua C, Morais S, Maciel M, Costa C, Macedo I, et al. Anthelmintic activity of Croton zehntneri and Lippia sidoides essential oils. Vet Parasitol. (2007) 148:288–94. doi: 10.1016/j.vetpar.2007.06.012
64. Hennessy D. Modifying the formulation or delivery mechanism to increase the activity of anthelmintic compounds. Vet Parasitol. (1997) 72:367–90. doi: 10.1016/S0304-4017(97)00106-4
65. Fandohan P, Gnonlonfin B, Laleye A, Gbenou JD, Darboux R, Moudachirou M. Toxicity and gastric tolerance of essential oils from Cymbopogon citratus, Ocimum gratissimum and Ocimum basilicum in Wistar rats. Food Chem Toxicol. (2008) 46:2493–7. doi: 10.1016/j.fct.2008.04.006
66. Macedo IT, Oliveira LM, Ribeiro WL, Santos JM, Silva K, Araújo Filho JV, et al. Anthelmintic activity of Cymbopogon citratus against Haemonchus contortus. Rev Bras Parasitol Vet. (2015) 24:268–75. doi: 10.1590/S1984-29612015059
67. Roy J. 9 - Pharmacological concepts and drugs. In: Roy J, editor. An Introduction to Pharmaceutical Sciences. Sawston: Woodhead Publishing (2011). p. 205–30. doi: 10.1533/9781908818041.205
68. Sood M. Histochemical, biochemical and immunological studies in Haemonchus contortus (Nematoda: Trichostrongyloidea)– an Indian perspective. J Parasit Dis. (2006) 30:4–15.
69. Kar PK, Murmu S, Saha S, Tandon V, Acharya K. Anthelmintic efficacy of gold nanoparticles derived from a phytopathogenic fungus, Nigrospora oryzae. PLoS One. (2014) 9:e84693. doi: 10.1371/journal.pone.0084693
70. Domingues LF, Giglioti R, Feitosa KA, Fantatto RR, Rabelo MD, de Sena Oliveira MC, et al. In vitro and in vivo evaluation of the activity of pineapple (Ananas comosus) on Haemonchus contortus in Santa Inês sheep. Vet Parasitol. (2013) 197:263–70. doi: 10.1016/j.vetpar.2013.04.031
71. Stepek G, Lowe AE, Buttle DJ, Duce IR, Behnke JM. In vitro and in vivo anthelmintic efficacy of plant cysteine proteinases against the rodent gastrointestinal nematode, Trichuris muris. Parasitology. (2006) 132(Pt 5):681–9. doi: 10.1017/S003118200500973X
Keywords: Haemonchus contortus, nanoparticles, anthelmintic, gastrointestinal nematode, toxicity, anthelmintic resistance
Citation: Ali R, Ahmad N, Mussarat S, Majid A, Alnomasy SF and Khan SN (2021) Nanoparticles as Alternatives for the Control of Haemonchus contortus: A Systematic Approach to Unveil New Anti-haemonchiasis Agents. Front. Vet. Sci. 8:789977. doi: 10.3389/fvets.2021.789977
Received: 05 October 2021; Accepted: 10 November 2021;
Published: 13 December 2021.
Edited by:
Wesley Lyeverton Correia Ribeiro, Federal University of Ceara, BrazilReviewed by:
Filippe Elias De Freitas Soares, Universidade Federal de Lavras, BrazilWeibson Paz Pinheiro André, Centro Universitário Leão Sampaio (Unileão), Brazil
Copyright © 2021 Ali, Ahmad, Mussarat, Majid, Alnomasy and Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rehman Ali, cmVobWFuYWxpNzY4MEBnbWFpbC5jb20=; Sultan F. Alnomasy, cy5hbG5vbWFzeUBzdS5lZHUuc2E=; Shahid Niaz Khan, c2hhaGlkQGt1c3QuZWR1LnBr
†These authors have contributed equally to this work and share first authorship
 Rehman Ali
Rehman Ali Nisar Ahmad
Nisar Ahmad Sakina Mussarat
Sakina Mussarat Abdul Majid
Abdul Majid Sultan F. Alnomasy3*
Sultan F. Alnomasy3* Shahid Niaz Khan
Shahid Niaz Khan