
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 14 December 2016
Sec. Veterinary Regenerative Medicine
Volume 3 - 2016 | https://doi.org/10.3389/fvets.2016.00112
Objective: To evaluate outcomes in 36 dogs with a partial cranial cruciate ligament (CCL) tear treated with autologous bone marrow aspirate concentrate (BMAC) or adipose-derived progenitor cells (ADPC) with platelet-rich plasma (PRP) combination.
Materials and methods: Medical records of client-owned dogs diagnosed with an early partial (≤50%) tear of the craniomedial band of the CCL that was treated with BMAC–PRP or ADPC–PRP were reviewed from 2010 to 2015. Signalment, medical history, physical and orthopedic examination, objective temporospatial gait analyses, radiographs, day 0 and day 90 diagnostic arthroscopy findings, treatment, and outcome were among the data collected. A functional owner questionnaire, including the validated Helsinki chronic pain index (HCPI), was sent to owners whose dog was known to not have had a tibial plateau leveling osteotomy (TPLO). Statistical analysis was performed on data, where significance was established at p < 0.05.
Results: Stifle arthroscopy findings at 90 days posttreatment were available on 13 of the 36 dogs. In nine dogs, a fully intact CCL with marked neovascularization and a normal fiber pattern was found with all previous regions of disruption healed. One dog revealed significant improvement and received an additional injection. The remaining three dogs had a >50% CCL tear, and a TPLO was performed. Four additional dogs were known to have had a TPLO performed elsewhere. Baseline and day 90 posttreatment objective gait analyses were available on 11 of the 36 dogs. A significant difference was found between the treated limb total pressure index percent (TPI%) at day 0 and day 90 (p = 0.0124), and between the treated limb and contralateral limb TPI% at day 0 (p = 0.0003). No significant difference was found between the treated limb and contralateral limb TPI% at day 90 (p = 0.7466). Twelve questionnaires were returned, of which eight were performance/sporting dogs. Seven of the eight had returned to sport; the remaining dog had just begun a return to sport conditioning program 6 months posttreatment. All 12 respondents believed that their dog had an excellent or very good quality of life and rated their dog’s procedural outcome as excellent or good.
Conclusion: The use of BMAC–PRP and ADPC–PRP shows promise for the treatment of early partial CCL tears in dogs. Further studies are needed and should be randomized, blinded, and controlled.
The cranial cruciate ligament (CCL) is necessary to stabilize the stifle joint in dogs; however, its rupture is one of the most common causes of hind limb lameness and the most common stifle joint diseases in dogs (1–3). Surgical intervention is recommended to correct the instability, restore the stifle to function, and delay the onset of osteoarthritis. Although no specific surgical procedure has been established as the gold standard in veterinary medicine, intracapsular, extracapsular, and osteotomy techniques are often used (4–9). Short- and long-term complications can occur, including the progression of osteoarthritis (6, 10–19). While these techniques are typically utilized for an unstable stifle, or a complete tear, questions still remain as to how to proceed with an early partial tear where a functional, stable stifle remains. Although an early partial tear is likely to progress to a complete tear if left untreated due to the degenerative cascade of effects that occurs, the benefits of surgery may not outweigh the risks. Therefore, an alternative treatment option could be considered.
Mesenchymal stem cell (MSC) therapy is a more recent cell-based technique that is being utilized in numerous orthopedic conditions. Multipotent progenitor cells and MSCs are two types of adult stem cells used for tissue regeneration and their trophic effects (20). Found from various sources, including bone marrow and adipose tissue, MSCs and progenitor cells have the potential to differentiate into multiple cell lineages including bone, cartilage, and connective tissue such as ligaments (21, 22). Several studies report that MSCs are an optimal source for ligament regeneration due to their high proliferation and collagen production potential and ability to quickly differentiate into ligament fibroblasts (23, 24).
Numerous animal studies have been conducted to evaluate the regeneration potential of MSCs, which have revealed that MSCs result in cell engraftment in the CCL, meniscus, and cartilage when injected intra-articularly (25, 26). Another study found that intra-articularly injected MSCs can accelerate the healing of partial anterior cruciate ligament (ACL) tears in rats when evaluated biomechanically and histologically (27). In this study, in treatment group at 2 and 4 weeks after partial transection of the ACL, the transected area was covered with healing tissues in which GFP-positive cells were detected (27). The treatment group also had better histologic scores at 2 and 4 weeks and significantly higher ultimate failure load than the control group 4 weeks following transection (27). Similar results were found in a recent study on rats where 1 week following partial transection of the ACL, they received an intra-articular injection of either fresh bone marrow cells (BMC) or cultured MSCs that were labeled (28). At 4 weeks following injection, the cells were not only located within the ACL but the ACL also appeared both histologically normal and with more mature spindle cells (28). This study also showed that the tensile strength in the BMC group reached near normal levels at 4 weeks after injection and significantly higher levels of transforming growth factor-β1 in the ACL and knee joint fluid in the BMC group when compared to the control group at 4 weeks after injection (28). A recent study was performed on dogs diagnosed with a unilateral unstable CCL rupture that was surgically stabilized, which had a contralateral, stable partial CCL (29). At the time of surgical stabilization of the unstable stifle, bone marrow-derived mesenchymal stem cells (BM-MSCs) were collected, culture expanded, and subsequently injected intravenously and intra-articularly into the contralateral, stable stifle with a partial CCL. Inflammatory markers within synovium and serum were monitored for 8 weeks and showed that both systemic and synovial markers had decreased at 8 weeks postinjection. Thus, this study concluded that intra-articular injection of autologous BM-MSCs had profound effects on the correlation and conditional dependencies of inflammatory markers, which could ameliorate the risk of a second CCL rupture (29). Human clinical studies show similar potential, where patients have shown improvement in objective measures of ACL integrity and subjective outcomes after bone marrow MSC injection of grade 1 through three ACL tears (30). Safety for clinical use has been verified with no neoplastic complications (31).
Platelet-rich plasma (PRP) provides growth factors to enhance and promote MSC engraftment, providing a synergistic effect. Growth factors improve cell proliferation, promote an anabolic phase of cells, and stimulate MSC differentiation into fibroblasts (32–35). Additionally, PRP provides a three-dimensional scaffold for MSCs to facilitate differentiation and cell survival during delivery (36). A recent study showed that several intra-articular injections of PRP alone indicated evidence of CCL repair and remodeling in dogs (37). These findings promote the use of PRP in combination with MSCs to aid in ligament healing.
The purpose of this study was to evaluate the use of autologous bone marrow aspirate concentrate (BMAC) or adipose-derived progenitor cells (ADPC) with PRP combination and a recommended rehabilitation therapy program for the treatment of early partial CCL tears in dogs. Assessing outcomes in dogs has not been previously reported.
Medical records of client-owned dogs were reviewed for cases diagnosed arthroscopically with an early partial (≤50%) tear of the craniomedial band of the CCL that was treated with BMSC–PRP or ADPC–PRP between January 2010 and December 2015 at Veterinary Orthopedic & Sports Medicine Group (VOSM). Data collected included signalment, medical history, physical and orthopedic examination findings, objective temporospatial gait analysis findings, diagnostic radiography results, arthroscopy findings, treatments, and outcome. Patients with concomitant stifle joint problems, or orthopedic or neurologic conditions affecting the hind limbs, were excluded from analysis.
Dogs were assessed at baseline and 30, 60, and 90 days posttreatment with an orthopedic and neurologic examination. Degree of lameness, presence of stifle effusion, presence of cranial drawer and cranial tibial thrust in flexion and extension, and McMurray test result were among the findings recorded (38). Additional abnormal orthopedic or neurologic findings were also recorded.
Objective gait analysis was performed at baseline and 90 days posttreatment in a quiet room using a temporospatial pressure sensing walkway. The walkway system was equipped with an 8.23 m × 0.85 m portable mat with 29,952 encapsulated sensors (GaitFour Pressure Sensing Walkway, platinum version, CIR Systems Inc., Havertown, PA, USA). The active dimensions of the mat were 8.04 m × 0.61 m. A 1.25 m × 0.85 m section of inactive mat was placed at each end of the walkway system to provide transition surfaces when entering and exiting the system. The mat was calibrated by the manufacturer. The walkway system interfaced with a computer and software program for processing and storing raw data recorded from quadruped gait analysis (GaitFour, platinum version, CIR Systems Inc., Havertown, PA, USA). One camera was positioned at a height of 50 cm at the end of the walkway system to record movement (Logitech mega pixel web camera, Logitech, Freemont, CA, USA). Digital video files of each pass across the walkway system were automatically linked to the data files for footfall verification.
Each dog was familiarized with the room prior to walking on the mat and was handled by the same examiner. Dogs were made to walk on the mat until they appeared relaxed, approximately four to six passes per dog. Three passes were recorded at both a walk and a trot, where a pass was defined as a dog moving along the length of the walkway in one direction for four to six gait cycles. A satisfactory pass consisted of minimal head turning, a walk velocity of 0.9–1.2 m/s or trot velocity of 1.8–2.6 m/s, and less than two standard deviations in gait cycle velocity differences. The software program identified the paw print of each footfall and analyzed multiple variables for each pass. The data calculated for each pass provided the total pressure index percent (TPI%) (sum of peak pressure values recorded from each activated sensor by a paw during mat contact/total sum of peak pressure values for all feet × 100). The TPI% of the treated limb was compared to the TPI% of the contralateral limb at baseline and at 90 days posttreatment. The TPI% of the treated limb at day 0 was also compared to the treated limb TPI% at 90 days posttreatment. Dogs diagnosed with bilateral partial CCL tears were excluded from this analysis.
Routine lateral and craniocaudal radiographs were performed of both stifles in all patients at baseline and evaluated for evidence of stifle osteoarthritis by a board-certified veterinary surgeon (39).
A standard parapatellar portal was utilized for arthroscopic evaluation of the stifle. The stifle was clipped and aseptically prepared and a 22-gauge needle was placed into the lateral compartment. Synovial fluid was aspirated using a 5 ml syringe to ensure that the needle was in the joint. Approximately 10 ml of sterile saline solution was injected into the stifle to distend the joint. A 15 blade was used to create medial and lateral parapatellar stab incisions to allow insertion of the cannula and trochar into the stifle. A 1.9 or 2.7 mm arthroscope was inserted into the cannula, depending on the size of the dog. The stifle joint was explored arthroscopically and assessed all structures, including the craniomedial and caudolateral band of the CCL, the caudal cruciate ligament, and medial and lateral menisci. Cartilage breakdown was scored using the Outerbridge classification (40). The fat pad was not removed with a shaver, but rather an L-shaped probe (Graduated Black Probe 2.5 mm tip length, Arthrex Vet Systems, Naples, FL, USA) was inserted and used to move the fat pad for direct observation of the CCL. The torn portion (percent) of the CCL was measured using the standardized L probe. If ≤50% of the craniomedial band of the CCL was determined to be damaged, a BMAC–PRP or ADPC–PRP injection was to be performed. If a >50% tear was observed, a tibial plateau leveling osteotomy (TPLO) was performed. The arthroscopic incisions were closed routinely.
The dog was placed in lateral recumbency, and the hip was clipped and aseptically prepared. The vaclock syringe and bone marrow aspiration needle were primed with 20 ml of heparin 1,000 U/ml, leaving 5 ml in the vaclock syringe. A 15 blade was used to create a 0.5 cm stab incision at the point of the proximal femur and an 11 gauge × 110 mm bone marrow aspiration needle was inserted into the greater trochanter. Approximately 30 ml of bone marrow was obtained. The incision was closed routinely.
The jugular vein was clipped and aseptically prepared. An 18-gauge butterfly needle was used to collect 50 ml of blood into a 60 ml syringe that contained 10 ml of anticoagulant citrate dextrose solution A (ACD-A).
Bone marrow was processed using a validated system according to manufacturer’s protocols (BMC Stem Cell Kit, Companion Regenerative Therapies, Newark, DE, USA). Briefly, the 150–170-µm filter, 60-ml syringe, and concentrating device were primed with heparin 1,000 U/ml. The vaclock syringe containing the bone marrow was attached to the filter and injected through the filter to the empty 60 ml syringe. The bone marrow was then injected into the concentrating device, placed into the centrifuge (Executive Series Centrifuge II, Companion Regenerative Therapies, Newark, DE, USA), and spun for 10 min at 3,600 RPM. After centrifugation, a 60-ml syringe was used to aspirate the plasma layer. A 12-ml syringe was then used to aspirate 3 ml of BMAC. Approximately 30,000 MSCs were present in the initial 30-ml bone marrow sample (41).
Whole blood was processed using a validated system according to manufacturer’s protocols (PurePRP® Kit, Companion Regenerative Therapies, Newark, DE, USA). Briefly, the syringe containing the blood and ACD-A was injected into the first concentrating device, placed into the centrifuge (Executive Series Centrifuge II, Companion Regenerative Therapies, Newark, DE, USA), and spun for 1 min at 3,600 RPM. After centrifugation, a 60-ml syringe was used to aspirate the platelet plasma suspension layer and was then injected into the second concentrating device. The concentrating device was placed into the centrifuge and spun for 5 min at 3,800 RPM. After centrifugation, a 60-ml syringe was used to aspirate the plasma until the aspirating disc reached the bottom of the tube. A 12-ml syringe was used to inject 10 ml of air into the device to allow for re-suspension of the platelets at the base of the device. The device was swirled to re-suspend the platelets into the remaining plasma. The 12-ml syringe was then used to aspirate 4 ml of PRP.
To confirm the PRP product composition, a baseline blood and PRP product WBC differential, RBC concentration, and platelet concentration were obtained on all dogs using an in-house hematology analyzer that had been calibrated according to manufacturer standards (Idexx LaserCyte Hematology Analyzer, Idexx Laboratories, Westbrook, ME, USA). The PRP obtained a sixfold to sevenfold increase in platelets, 85% reduction in neutrophils, and 95% reduction in red blood cells from whole blood. Platelet, neutrophil, and red blood cell counts were verified prior to use.
The abdomen was clipped and aseptically prepared. A 4-cm incision was made in midline in the cranial abdomen, and a 2-cm incision was made through the linea. The falciform ligament was identified. Approximately 20 g of falciform adipose tissue was placed into a sterile container with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with fetal bovine serum, penicillin, and streptomycin. The incision was closed routinely.
The jugular vein was clipped and aseptically prepared. An 18-gauge butterfly needle was used to collect 25 ml of blood into a 30 ml syringe that contained 5 ml of citrate phosphate dextrose adenine anticoagulant.
Adipose and blood samples were shipped in validated shipping boxes at 4°C to the Virginia Tech Marion DuPont Scott Equine Medical Center Regenerative Medicine Service for processing. ADPC and ACS were processed using their proprietary protocols. Briefly, adipose was mechanically and enzymatically separated to release ADPCs. The cells were then washed in phosphate-buffered saline (PBS), and nucleated cells were counted and cultured in stem cell media at 37°C in a 5% CO2 incubator with 95% humidity. Cells were washed in PBS once at 70% confluency, trypsinized, and suspended in PRP. Approximately 5 million ADPCs were present in the sample, which were verified prior to suspension. PRP was prepared via centrifugation to obtain a fourfold increase in platelets and 80% reduction in white blood cells from whole blood. Platelet and white blood cells counts were verified prior to use. The ADPC–PRP was shipped to VOSM in validated shipping boxes at 4°C for subsequent injection.
Bone marrow aspirate concentrate and PRP were drawn into an empty syringe in a 1:1 ratio via a three-way stopcock. The stifle was clipped (if ADPC–PRP) and aseptically prepared. A 22-gauge needle was placed into the lateral compartment of the stifle and synovial fluid was aspirated using a 5-ml syringe to ensure the needle was in the joint. Approximately 2–4 ml of BMAC–PRP or 1–2 ml of ADPC–PRP was injected intra-articularly into the stifle; volume depended on the size of the dog. If the dog was below 15 kg in weight, they received 2 ml of BMAC–PRP or 1 ml of ADPC–PRP. If the dog was above 15 kg in weight, they received 4 ml of BMAC–PRP or 2 ml of ADPC–PRP. All dogs were placed into a soft-padded bandage for 12–24 h. Once the soft-padded bandage was removed, dogs were placed into a custom stifle orthotic that had been previously molded and constructed (Animal Orthocare, LLC, Chantilly, VA, USA) if the owner was concerned about their ability to confine and control their dog or if increased laxity had been noted on palpation at the initial examination. All owners were instructed to discontinue NSAIDs, corticosteroids, or anti-inflammatory supplements at least 2 weeks prior to injection. Owners were given the option to choose between BMAC–PRP and ADPC–PRP. The option to bank culture-expanded ADPCs and blood at the Virginia Tech Marion DuPont Scott Equine Medical Center was conveyed to the owner, while BMAC would not typically be banked. BMAC–PRP treatment would be performed the same day as stifle arthroscopy, while ADPC–PRP treatment would occur approximately 2 weeks after being sent for culture, which may play into the decision for owners that traveled a long distance to VOSM. Following the collection procedure and/or injection, patients were placed on tramadol (3–4 mg/kg by mouth every 8 h) or codeine (1–2 mg/kg by mouth every 8 h) for 3 days. The cost of each treatment was equal.
All patients were instructed to enroll in formal rehabilitation therapy following treatment consisting of once weekly manual/massage therapy, class IIIb low-level laser therapy for the affected stifle (5 J/cm2) and a twice daily at home exercise program for the first 8 weeks. NSAIDs, corticosteroids, anti-inflammatory supplements, class IV low level laser therapy, therapeutic ultrasound, hydrotherapy, and TENS/NMES were not permitted for the first 8 weeks posttreatment as their effect on stem cell and PRP therapy is still not fully known.
An owner questionnaire, including the validated Helsinki chronic pain index (HCPI), was e-mailed to all owners whose dog was known to not have had surgical repair following treatment. The questionnaire inquired about duration of lameness following treatment, if their performance/sporting dog returned to sport, and if so at what level compared to prior to injury. To assess the dog’s current well-being, owners were asked to rate their dog’s quality of life in the last 7 days (excellent, very good, good, fair, poor) and were asked their opinion of their dog’s procedural outcome (excellent, good, fair, poor). Possible chronic pain was assessed with the validated HCPI that contained questions on the dog’s mood, lameness, and willingness to move, play, and jump. The HCPI contained 11 questions whose answers were later given a value (0–4), giving a total index score range of 0–44. Individual question scores of 0 and 1 indicate normal behavior and movement, where scores of 2, 3, and 4 indicate pain with increasing severity. Therefore, healthy dogs usually have an HCPI between 0 and 11, and dogs with chronic pain will have a score of 12–44. The HCPI has been validated, reliability tested, and responsiveness tested (42, 43) (Table 1).
Data analyses were performed using a statistical software program (GraphPad Prism version 6.00, GraphPad Software, La Jolla, CA, USA). Gait analysis data were analyzed using a paired t-test that accounts for correlations between paired observations. A paired t-test will result in increased statistical power to detect a treatment effect when compared to an independent t-test. Outcome versus percent tear contingency data were analyzed using the Chi-square test for trend. Outcome versus use of a custom stifle orthotic contingency data were analyzed using the Fisher’s exact test. Outcome versus type of treatment (BMAC–PRP or ADPC–PRP) received contingency data were analyzed using a Fisher’s exact test. All the results were considered significant at p < 0.05.
In accordance with AAALAC International Rules of Accreditation, this study was performed with the approval of the VOSM Research Committee and with owner’s consent. All clients volunteered their dog for the study and provided written consent as required by Veterinary Orthopedic and Sports Medicine Group for every study participant. Medical records of 36 dogs diagnosed with an early partial (≤50%) CCL tear that were treated with BMSC–PRP or ADPC–PRP were reviewed. Ages ranged from 1 year to 9.5 years (mean 4.8 years, median 4.7 years), with 18 dogs being female (6 intact) and 18 dogs being male (12 intact). Body weights ranged from 4.8 to 60.5 kg (mean 25.9 kg, median 22.6 kg). Breeds included 10 (27.8%) Border Collies, 6 (16.7%) Labrador Retrievers, 3 (8.3%) Australian Shepherds, 2 (5.6%) Golden Retrievers, 2 (5.6%) Pembroke Welsh Corgis, 2 (5.6%) Shelties, and 1 (2.8%) of each of the following: Akbash, Basset Hound, Bull Terrier, Dutch Shepherd, German Shorthaired Pointer, Giant Schnauzer, Gordon Setter, Irish Setter, Keeshond, Mixed Breed, and Portuguese Water Dog. The majority of dogs were performance/sporting dogs [26 (72%) versus 10 (28%)] that were a companion dog only (Table 2). Duration of lameness ranged from 3 days to 4 years. Nineteen cases received BMAC–PRP for treatment, while 17 cases received ADPC–PRP. A custom stifle orthotic was used posttreatment in 14 dogs to prevent damage to the CCL during the recovery period.
Baseline orthopedic examination findings were available in 30 of the 36 dogs. A weight-bearing lameness and shortened stride was noted in 29 dogs, while 1 dog had a shortened stride only. On palpation, very mild effusion was present in the affected limb of 1 dog, mild effusion was present in 11 dogs, moderate effusion in 17 dogs, and 1 dog had no effusion. McMurray test for meniscal evaluation was negative in 29 dogs, while 1 dog was positive. Mild cranial drawer and tibial thrust were noted in flexion only in the affected limb in 23 dogs. Palpable evidence of stifle instability was absent in seven dogs.
Baseline radiograph findings were available in 30 of the 36 dogs. Very mild joint effusion was noted in the affected limb of 3 dogs, mild effusion in 12 dogs, and moderate effusion in 14 dogs. One dog had no effusion present on radiographs. Osteoarthritis was also present on radiographs in the affected limb of five dogs. Joint effusion was noted in the contralateral limb in 15 dogs, with osteoarthritis being present in 1 (39).
Both baseline and day 90 posttreatment objective gait analyses were available on 11 of the 36 dogs. Mean baseline TPI% was 17.7% (median 17.9%, range 13.1–22.8%) in the affected limb and 21.7% (median 21.5%, range 18.8–24.3%) in the unaffected limb. At 90 days posttreatment, mean TPI% was 20.1% (median 20.2%, range 16.9–22.8%) in the treated limb and 19.9% (median 19.2%, range 17.3–22.9%) in the contralateral limb. The TPI% of the treated limb was compared to the TPI% of the contralateral limb at baseline and at 90 days posttreatment. The TPI% of the treated limb at day 0 was also compared to the TPI% of the treated limb at 90 days posttreatment. A statistically significant difference was found between the treated limb TPI% at day 0 and day 90 (Figure 1; p = 0.0124), and between the treated limb and contralateral limb TPI% at day 0 (Figure 2; p = 0.0003). No significant difference was found between the treated limb and contralateral limb TPI% at day 90 (Figure 3; p = 0.7466).
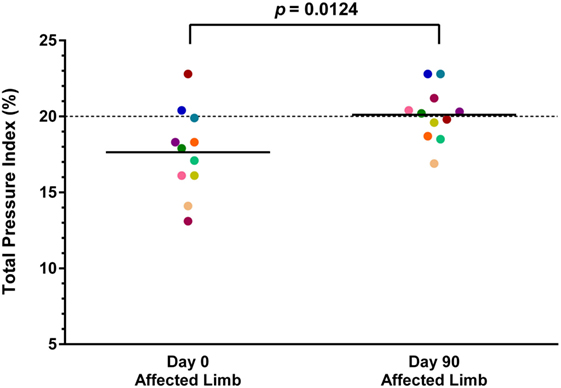
Figure 1. A statistically significant difference was found between the treated limb total pressure index percent (TPI%) at day 0 and day 90 (p = 0.0124) (solid line, mean; dotted line, normal TPI%).
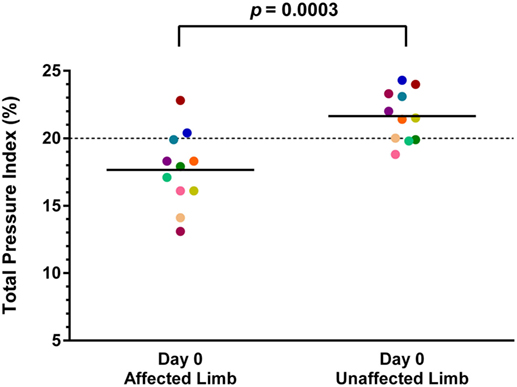
Figure 2. A statistically significant difference was found between the treated limb and contralateral limb total pressure index percent (TPI%) at day 0 (p = 0.0003) (solid line, mean; dotted line, normal TPI%).
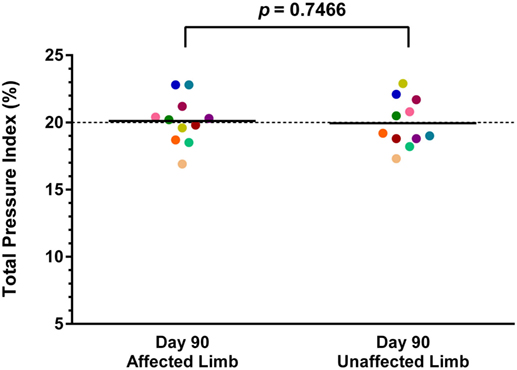
Figure 3. No significant difference was found between the treated limb and contralateral limb total pressure index percent (TPI%) at day 90 (p = 0.7466) (solid line, mean; dotted line, normal TPI%).
Baseline diagnostic stifle arthroscopy findings were available on all 36 dogs. Diagnosis was confirmed and the CCL was found to be partially torn unilaterally in 35 dogs (13 left, 22 right), and bilaterally in 1 dog. The dog with bilateral partial CCL tears was omitted from the study, and the other 35 dogs were included in this study. A percent tear of the craniomedial band of the CCL was recorded for each dog. The high end was used to calculate descriptive statistics where a range was reported. Mild fraying of the craniomedial band of the CCL was reported in 4 of the 35 dogs. The percent tear of the craniomedial band of the CCL ranged from 5 to 50% (mean 20.8%, median 20%) in the remaining 31 dogs (Figure 4). The meniscus was found to be intact in 34 dogs, whereas 1 dog was found to have a bucket handle tear of the caudal pole of the medial meniscus, and a partial meniscectomy of the medial meniscus was performed. Degenerative joint disease (DJD) findings were recorded in 31 of the 35 dogs. Very mild DJD was reported in 2 dogs, mild DJD was reported in 4 dogs, moderate DJD in 2 dogs, and no DJD was found in the remaining 23 dogs.
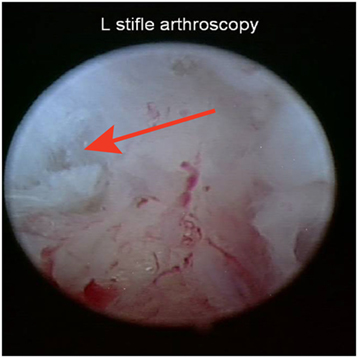
Figure 4. Representative image of an early partial tear of the craniomedial band of the cranial cruciate ligament at the baseline stifle arthroscopy.
Stifle arthroscopy findings at 90 days posttreatment were available on 13 of the 35 dogs. In 9 of the 13 dogs, a fully intact CCL with marked neovascularization and a normal fiber pattern was found with all previous regions of disruption healed. It appeared that the blood supply to the region of CCL injury was coming from the genicular artery (Figure 5). One dog was found to have significantly improved by way of an intact CCL with minor fraying, compared to a 25% tear of the craniomedial band of the CCL at baseline, and received an additional injection. The dog was found to have normal range of motion and no discomfort, tibial thrust, or lameness 90 days after the second injection. The remaining three dogs were found to have a >50% tear (either progressive since baseline or completely ruptured), and a TPLO was performed at VOSM. Four additional dogs were reported by the owner to have had a TPLO performed outside of VOSM.
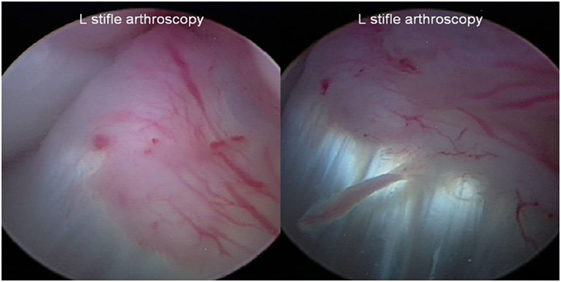
Figure 5. Representative images of a fully intact cranial cruciate ligament with marked neovascularization and a normal fiber pattern with all previous regions of disruption healed at the day 90 posttreatment stifle arthroscopy.
Questionnaires were sent to all owners whose dog was known to not have had surgical repair following treatment (29 dogs). Questionnaires were returned for 12 dogs (41%), 6 of which had received ADPC–PRP and 6 of which had received BMAC–PRP. Follow-up time between surgery and the questionnaire ranged from 0.5 to 2.9 years (mean 1.8 years, median 1.9 years). HCPI ranged from 0 to 17 (mean 4.4, median 1, standard deviation 6.3). Owners were asked how long their dog experienced lameness following treatment; the answers ranged from 0 to 12 months (mean 2.4 months, median 1.3 months). All 12 respondents believed their dog had an excellent (10) or very good (2) quality of life in the last 7 days and rated their dog’s procedural outcome as excellent (11) or good (1). Of the 12 questionnaires returned, 8 were reported to be performance/sporting dogs. According to the owner, seven of the eight dogs had returned to sport; the remaining dog had just begun a return to sport conditioning program 6 months posttreatment. Of the seven dogs that returned to sport, five dogs were reported to be competing at the same level compared to prior to injury, and two dogs were competing at a greater level compared to prior to injury (including faster times, higher jump height, and dropping bars less). According to the previously established VOSM Return to Agility Grading Scale (Table 3), of the four dogs that competed in agility, two dogs (50%) were grade 4 and two dogs (50%) were grade 5. Of the seven dogs that returned to sport, it was found that the greatest amount of time a dog had been competing since their procedure was 2.5 years.
The seven reported failures were found to have had a 50% (1), 25% (1), 20% (1), and 10% (4) tear at baseline. There was no association found between outcome and the percent tear reported at baseline (p = 0.5545). A custom stifle orthotic was used posttreatment in 14 dogs, with 4 of the reported failures having used an orthotic, and 3 failures not having used an orthotic. There was no association found between outcome and the use of an orthotic posttreatment (p = 0.5545). There were four reported failures that received ADPC–PRP treatment and three failures that received BMAC–PRP treatment. There was no association found between outcome and the treatment type received (p = 0.6843). All seven dogs that failed went on to have a TPLO procedure.
In this study, we investigated the use of autologous BMAC–PRP or ADPC–PRP combination for the treatment of early partial CCL tears in dogs and found that both provide promising potential for clinical use, which was supported by objective gait analysis, diagnostic arthroscopy, and validated functional questionnaire. Our results support the findings of previous studies in which MSCs engrafted to the CCL (27, 28) and when intra-articularly injected have been found to accelerate the healing of partial ACL tears in rats (30, 31). It is unknown if the MSCs used in our study engrafted in order to regenerate the CCL. Furthermore, our results demonstrate the clinical relevancy of these previous findings in dogs and provide further evidence to support clinical findings found in a previously reported case series in humans (32). Additionally, the questionnaire revealed that the greatest amount of time a dog had been competing since their procedure was 2.5 years, demonstrating that the CCL is able to withstand the continued rigor of sport.
The main limitations of this study are related to the nature of a retrospective study, including variation in treatments and rehabilitation therapy, loss to follow-up, and incomplete documentation. Most owners and patients whose data were collected for this study traveled from out of state for diagnosis and treatment, several being from across the country, thereby potentially contributing to the loss to follow-up and lack of follow-up findings. This element may also have played a role in the treatment type chosen by the owner. A greater return in questionnaires would be beneficial, thus positively or negatively affecting the outcomes associated with the survey, including the HCPI, the owner’s opinions on outcome, and the rate of dogs that returned to sport. Additionally, owner compliance to rehabilitation therapy recommendations was unknown. No associations that may provide a possible cause or correlation to treatment outcome could be deduced. Owner compliance to the strict at home confinement restrictions and guidelines given posttreatment are unknown and therefore may play a factor. Unfortunately, the exact stem cell numbers injected are unknown, which is a limitation of this study. However, previous validation work shows that there are approximately 5 million ADPC versus 30,000 BMAC (44). In spite of the large discrepancy in cell numbers between the ADPC and BMAC, it was determined that neither treatment was superior to the other in terms of outcome. Therefore, it appears that both treatment types possess the ability to regenerate the CCL; however, the cell number required still remains unknown. Additionally, difference in platelet concentration between the two systems used could affect outcome, and it is unclear what effect this had on outcomes for this study. Finally, because our standard of care is to utilize a stem cell and PRP combination, it is still unknown if stem cells or PRP alone could produce the same results.
In conclusion, our study provides promise to the use of autologous BMAC–PRP or ADPC–PRP combination as an alternative treatment option. However, further studies are needed to determine if they are reproducible in a well-populated randomized, blinded, and controlled trial, reporting data from BMAC and ADPC separately. Cell counts and biomarker assays should be included in future studies. Clinical findings and a validated functional questionnaire should be documented before and after treatment.
This was a retrospective study and only gathered data from previously performed treatments on client-owned dogs. In accordance with AAALAC International Rules of Accreditation, this study was performed with the approval of the VOSM Research Committee and with owner consent. All clients volunteered their dog for the study and provided written consent as required by Veterinary Orthopedic and Sports Medicine Group for every study participant. All clients volunteered their dog for the study and provided written consent as required by Veterinary Orthopedic and Sports Medicine Group for every study participant.
All the authors contributed to the conception or design of the work, drafting and revising, and final approval of the version to be published.
Dr. SC is a consultant for Companion Regenerative Therapies. The authors report no other conflicts of interest.
CCL, cranial cruciate ligament; TPLO, tibial plateau leveling osteotomy; TPI%, total pressure index percent; ACL, anterior cruciate ligament; MSC, mesenchymal stem cell; BMAC, bone marrow aspirate concentrate; ADPC, adipose-derived progenitor cells; PRP, platelet-rich plasma; BM-MSC, bone marrow-derived mesenchymal stem cells.
1. Piermattei DL, Flo GL, DeCamp CE. Chapter 18 – the stifle joint. In: Piermattei DL, Flo GL, DeCamp CE, editors. Brinker, Piermattei, and Flo’s Handbook of Small Animal Orthopedics and Fracture Repair. Philadelphia, PA: Saunders (2006). p. 562–632.
2. Johnson JA, Austin C, Breur GJ. Incidence of canine appendicular musculoskeletal disorders in 16 veterinary teaching hospitals from 1980 through 1989. Vet Comp Orthop Traumatol (1994) 7:56–69.
3. Korvick DL, Pijanowski GJ, Schaeffer DJ. Three-dimensional kinematics of the intact and cranial cruciate ligament-deficient stifle of dogs. J Biomech (1994) 27:77–87. doi: 10.1016/0021-9290(94)90034-5
4. Bodderker J, Druen S, Meyer-Lindenberg A, Fehr M, Nolte I, Wefstaedt P. Computer-assisted gait analysis of the dog: comparison of two surgical techniques for the ruptured cranial cruciate ligament. Vet Comp Orthop Traumatol (2012) 25:11–21. doi:10.3415/VCOT-10-02-0025
5. Slocum B, Slocum TD. Tibial plateau leveling osteotomy for repair of cranial cruciate ligament rupture in the canine. Vet Clin North Am Small Anim Pract (1993) 23:777–95. doi:10.1016/S0195-5616(93)50082-7
6. Molsa SH, Hyytiainen HK, Hielm-Bjorkman AK, Laitinen-Vapaavuori OM. Long-term functional outcome after surgical repair of cranial cruciate ligament disease in dogs. BMC Vet Res (2014) 10:266. doi:10.1186/s12917-014-0266-8
7. Nelson SA, Krotscheck U, Rawlinson J, Todhunter RJ, Zhang Z, Mohammed H. Long-term functional outcome of tibial plateau leveling osteotomy versus extracapsular repair in a heterogeneous population of dogs. Vet Surg (2013) 42:38–50. doi:10.1111/j.1532-950X.2012.01052.x
8. Cook JL, Luther JK, Beetem J, Karnes J, Cook CR. Clinical comparison of a novel extracapsular stabilization procedure and tibial plateau leveling osteotomy for treatment of cranial cruciate ligament deficiency in dogs. Vet Surg (2010) 39:315–23. doi:10.1111/j.1532-950X.2010.00658.x
9. Christopher SA, Beetem J, Cook JL. Comparison of long-term outcomes associated with three surgical techniques for treatment of cranial cruciate ligament disease in dogs. Vet Surg (2013) 42:329–34. doi:10.1111/j.1532-950X.2013.12001.x
10. DeLuke AM, Allen DA, Wilson ER, Lineberger JA, Lehenbauer TW, Fabiani M, et al. Comparison of radiographic osteoarthritis scores in dogs less than 24 months or greater than 24 months following tibial plateau leveling osteotomy. Can Vet J (2012) 53:1095–9.
11. Ledecky V, Hluchy M, Freilichman R, Hornak S, Knazovicky D. Clinical comparison and short-term radiographic evaluation of tight rope and lateral suture procedures for dogs after cranial cruciate ligament rupture. Vet Med (2014) 59:502–5.
12. Wolf RE, Scavelli TD, Hoelzler MG, Fulcher RP, Bastian RP. Surgical and postoperative complications associated with tibial tuberosity advancement for cranial cruciate ligament rupture in dogs: 458 cases (2007-2009). J Am Vet Med Assoc (2012) 240:1481–7. doi:10.2460/javma.240.12.1481
13. Molsa SH, Hielm-Bjorkman AK, Laitinen-Vapaavuori OM. Use of an owner questionnaire to evaluate long-term surgical outcome and chronic pain after cranial cruciate ligament repair in dogs: 253 cases (2004-2006). J Am Vet Med Assoc (2013) 243:689–95. doi:10.2460/javma.243.5.689
14. Au KK, Gordon-Evans WJ, Dunning D, O’Dell-Anderson KJ, Knap KE, Griffon D, et al. Comparison of short- and long-term function and radiographic osteoarthrosis in dogs after postoperative physical rehabilitation and tibial plateau leveling osteotomy or lateral fabellar suture stabilization. Vet Surg (2010) 39:173–80. doi:10.1111/j.1532-950X.2009.00628.x
15. Vasseur PB, Berry CR. Progression of stifle osteoarthrosis following reconstruction of the cranial cruciate ligament in 21 dogs. J Am Anim Hosp Assoc (1992) 28:129–36.
16. Rayward RM, Thomson DG, Davies JV, Innes JF, Whitelock RG. Progression of osteoarthritis following TPLO surgery: a prospective radiographic study of 40 dogs. J Small Anim Pract (2004) 45:92–7. doi:10.1111/j.1748-5827.2004.tb00209.x
17. Innes JF, Costello M, Barr FJ, Rudorf H, Barr AR. Radiographic progression of osteoarthritis of the canine stifle joint: a prospective study. Vet Radiol Ultrasound (2004) 45:143–8. doi:10.1111/j.1740-8261.2004.04024.x
18. Lazar TP, Berry CR, deHaan JJ, Peck JN, Correa M. Long-term radiographic comparison of tibial plateau leveling osteotomy versus extracapsular stabilization for cranial cruciate ligament rupture in the dog. Vet Surg (2005) 34:133–41. doi:10.1111/j.1532-950X.2005.00021.x
19. Lineberger JA, Allen DA, Wilson ER, Tobias TA, Shaiken LG, Shiroma J, et al. Comparison of radiographic arthritic changes associated with two variations of tibial plateau leveling osteotomy. A retrospective clinical study. Vet Comp Orthop Traumatol (2005) 18:13–7.
20. Jacobs SA, Roobrouck VD, Verfaillie CM, Van Gool SW. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol (2013) 91:32–9. doi:10.1038/icb.2012.64
21. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy (2006) 8:315–7. doi:10.1080/14653240600855905
22. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem (2006) 99:1285–97. doi:10.1002/jcb.20904
23. Van Eijk F, Saris DBF, Riesle J, Willems WJ, van Blitterswijk CA, Verbout AJ, et al. Tissue engineering of ligaments: a comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblast as cell source. Tissue Eng (2004) 10:893–903. doi:10.1089/1076327041348428
24. Chen J, Altman GH, Karageorgiou V, Horan R, Collette A, Volloch V, et al. Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J Biomed Mater Res A (2003) 67:559–70. doi:10.1002/jbm.a.10120
25. Agung M, Ochi M, Yanada S, Adachi N, Izuta Y, Yamasaki T, et al. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc (2006) 14:1307–14. doi:10.1007/s00167-006-0124-8
26. Linon E, Spreng D, Rytz U, Forterre S. Engraftment of autologous bone marrow cells into the injured cranial cruciate ligament in dogs. Vet J (2014) 202:448–54. doi:10.1016/j.tvjl.2014
27. Kanaya A, Deie M, Adachi N, Nishimori M, Yanada S, Ochi M. Intra-articular injection of mesenchymal stem cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy (2007) 23:610–7. doi:10.1016/j.arthro.2007.01.013
28. Oe K, Kushida T, Okamoto N, Umeda M, Nakamura T, Ikehara S, et al. New strategies for anterior cruciate ligament partial rupture using bone marrow transplantation in rats. Stem Cells Dev (2011) 20:671–9. doi:10.1089/scd.2010.0182
29. Muir P, Hans EC, Racette M, Volstad N, Sample SJ, Heaton C, et al. Autologous bone marrow-derived mesenchymal stem cells modulate molecular markers of inflammation in dogs with cranial cruciate ligament rupture. PLoS One (2016) 11(8):e0159095. doi:10.137/journal.pone.0159095
30. Centeno CJ, Pitts J, Al-Sayegh H, Freeman MD. Anterior cruciate ligament tears treated with percutaneous injection of autologous bone marrow nucleated cells: a case series. J Pain Res (2015) 8:437–47. doi:10.2147/JPR.S86244
31. Centeno CJ, Schultz JR, Cheever M, Freeman M, Faulkner M, Robinson S, et al. Safety and complications reporting update on the re-implantation of culture-expanded mesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther (2011) 6:368–78. doi:10.2174/157488810790442796
32. Chen L, Dong SW, Liu JP, Tao X, Tang KL, Xu JZ. Synergy of tendon stem cells and platelet-rich plasma in tendon healing. J Orthop Res (2012) 30:991–7. doi:10.1002/jor.22033
33. Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med (2003) 33:381–94. doi:10.2165/00007256-200333050-00004
34. Zhang J, Wang JH. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med (2010) 38:2477–86. doi:10.1177/0363546510376750
35. Smith JJ, Ross MW, Smith RKW. Anabolic effects of acellular bone marrow, platelet rich plasma, and serum on equine suspensory ligament fibroblasts in vitro. Vet Comp Orthop Traumatol (2006) 19:43–7.
36. Richardson LE, Dudhia J, Clegg PD, Smith R. Stem cells in veterinary medicine – attempts at regenerating equine tendon after injury. Trends Biotechnol (2011) 25:409–16. doi:10.1016/j.tibtech.2007.07.009
37. Cook JL, Smith PA, Bozynski CC, Kuroki K, Cook CR, Stoker AM, et al. Multiple injections of leukoreduced platelet rich plasma reduce pain and functional impairment in a canine model of ACL and meniscal deficiency. J Orthop Res (2016) 34:607–15. doi:10.1002/jor.23054
38. Corea JR, Moussa M, Al Othman A. McMurray’s test tested. Knee Surg Sports Traumatol Athrosc (1994) 2:70–2. doi:10.1007/BF01476474
39. Gordon WJ, Conzemius MG, Riedesel E, Besancon MF, Evans R, Wilke V, et al. The relationship between limb function and radiographic osteoarthritis in dogs with stifle osteoarthritis. Vet Surg (2003) 32:451–4. doi:10.1053/jvet.2003.50051
40. Schulz KS. What’s new in elbow arthroscopy. Proceedings of the 13th Annual American College of Veterinary Surgeons Symposium. Washington, DC (2003).
41. Canapp SO, Carr BJ, Huang R, Elmasri H, Cox C. Proceedings of the 6th Annual PRP & Regenerative Medicine Symposium & Cadaver Lab. Las Vegas, NV: The Orthobiologic Institute (2015).
42. Hielm-Bjorkman AK, Kuusela E, Liman A, Markkola A, Saarto E, Huttunen P, et al. Evaluation of methods for assessment of pain associated with chronic osteoarthritis in dogs. J Am Vet Med Assoc (2003) 222:1552–8. doi:10.2460/javma.2003.222.1552
43. Hielm-Bjorkman AK, Rita H, Tulamo RM. Psychometric testing of the Helsinki chronic pain index by completion of a questionnaire in Finnish by owners of dogs with chronic signs of pain caused by osteoarthritis. Am J Vet Res (2009) 70:727–34. doi:10.2460/ajvr.70.6.727
Keywords: bone marrow aspirate concentrate, adipose-derived progenitor cells, platelet-rich plasma, cranial cruciate ligament tear, anterior cruciate ligament tear, mesenchymal stem cells, regenerative therapy
Citation: Canapp SO Jr, Leasure CS, Cox C, Ibrahim V and Carr BJ (2016) Partial Cranial Cruciate Ligament Tears Treated with Stem Cell and Platelet-Rich Plasma Combination Therapy in 36 Dogs: A Retrospective Study. Front. Vet. Sci. 3:112. doi: 10.3389/fvets.2016.00112
Received: 07 June 2016; Accepted: 28 November 2016;
Published: 14 December 2016
Edited by:
Fausto Cremonesi, University of Milan, ItalyCopyright: © 2016 Canapp, Leasure, Cox, Ibrahim and Carr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sherman O. Canapp Jr., scanapp@vosm.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.