- Department of Biomedical Science, Ontario Veterinary College, University of Guelph, Guelph, ON, Canada
Fetal bovine serum (FBS) remains widely used as a supplement in cell culture media used in the isolation and expansion of mesenchymal stromal cells (MSC) despite longstanding practical, clinical, and ethical concerns over its use. As a result, research on alternative culture media supplement solutions that conserve crucial MSC characteristics has become increasingly relevant. Species-specific supplements and serum-free media such as platelet lysate or chemically defined media have been assessed for their effect in MSC cultures regarding proliferation, differentiation, and immunomodulatory capacity. While none of the alternatives offer a complete solution in replacing traditional FBS supplemented media for culturing MSCs for all species, short-term or transitional use of FBS-free media can perform equally well and could address some of the concerns over the use of FBS.
Introduction
The field of stem cell research has gained significant traction due to the therapeutic potential of stem cells in human and veterinary medicine (1). Mesenchymal stromal cells (MSC) are a common type of multipotent cell used in experimental therapy for their capacity to differentiate into tissues such as cartilage and connective tissue and to modulate immune cell function (2, 3). These non-specialized cells can be obtained from many sources in the body and possess an extensive capacity to proliferate and self-renew making them the focus of potential treatments for many diseases including cardiovascular disease, spinal cord injuries, bone fractures, and autoimmune diseases (4, 5). However, the proliferation of these cells to useful therapeutic doses requires extensive culture in media which, often in the veterinary field, contains fetal bovine serum (FBS).
FBS is currently the standard culture supplement used for the expansion of domestic animal MSCs and most other types of cells grown in culture (6). It has been an integral part of general cell culture practices for more than 50 years due to its exceptional composition of factors required for both animal and human cell growth and proliferation (7). FBS also possesses very low levels of immunoglobulins relative to serum from mature cows, which reduces risk of provoking an immune response (8). However, FBS remains a concern to researchers and clinicians alike due to high rates of product variability, clinical risks of adverse reactions due to bovine proteins or disease, and ethical considerations over its production (9). Research into FBS alternatives for culturing MSC is therefore a very active area of investigation. In this review, the main concerns associated with culturing MSCs in FBS and the current state of FBS alternatives are discussed with a focus on work done in domestic animals.
FBS in MSC Culture
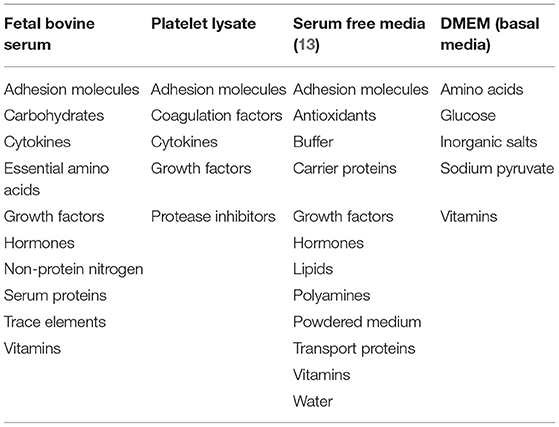
Over 50 years ago, it was observed that FBS improved cell survival in culture with little understanding of how (10). Price and Gregory (11) identified key components of FBS including nutrients, hormones, transport proteins, growth factors, and attachment factors (Table 1), some combination of which support the attachment and growth of MSCs (12).
A critical property of MSCs for their isolation, identification, and culture is their adherence to plastic (14, 15). While there is yet to be a definitive factor in serum that promotes attachment of cells to plastic (15), it has been proposed that fibronectin and vitronectin both may contribute (16). A study of human MSCs showed that culture surfaces pre-treated with FBS promoted better attachment and proliferation than untreated culture surfaces (17). While in equine MSCs isolated from cord blood showed no difference in FBS coating on the culture flask on proliferation, while in bone marrow MSCs (BM-MSCs) fibronectin was required for cellular adhesion (18, 19). Yet, equine adipose derived MSCs (AD-MSCs) have been successfully grown without coating of the flask (20). In addition, studies growing MSCs in the absence of serum indicate that FBS supplementation is required for initial attachment during the isolation stage (15, 21).
The contribution to cell proliferation of various hormones, nutrients, growth factors, and other components in FBS is poorly understood. Some have suggested that hormones were the main avenue by which FBS supported cell growth (21). Through work done by Hayashi and Sato (21), FBS was replaced by supplementing media with T3, thyrotropin releasing hormone, transferrin, and parathyroid hormone, which successfully maintained the growth of a rat pituitary cell line for 10 days. This culture condition produced cellular proliferation at a rate of 60–100% that of FBS cultured cells, suggesting that these components of FBS are critical in supporting cell growth (21).
Glucose is one of the better understood molecules in MSC survival, and it serves as an example of why proper supplementation is key to MSC function. Nuschke et al. (22) found that human BM-MSCs rely heavily on glucose, as this key nutrient is rapidly depleted, while other nutrients, such as L-glutamine, pyruvate, and amino acids important for the growth of other cell types, are used sparingly (22). Glucose supply is important across all MSC source species and tissues since they possess very limited intracellular glucose storage or ATP reserve. Therefore, as little as 3–5 days of glucose deprivation in culture induces rapid cell death (22–25). However, high glucose concentrations have been associated with reduced proliferation, increased apoptosis, lower cell viability, and altered differentiation potential (26–28).
Growth factors are also important for MSC proliferation. Devireddy et al. found fibroblast growth factor (FGF), platelet derived growth factor (PDGF), transforming growth factor-beta 1 (TGF-ß1), and epidermal growth factor (EGF) all beneficial in supporting canine MSC growth (13). Proliferation of human BM-MSCs was greatly enhanced by TGF-ß and bFGF supplementation in the absence of serum, while TGF-ß alone slowed proliferation (15). Similarly, TGF-ß supplemented media in the absence of serum alone resulted in fewer canine AD-MSCs, but proliferation improved when additional growth factors like bFGF or PDGF were added (13). In equine cells, TGF-ß addition to FBS media increased proliferation rates when compared to plain FBS-supplemented media, likely due to serum providing other growth factors like PDGF and bFGF (29). These results illustrate the complex influences of growth factors required for cellular growth. More work is needed to identify the specific factors that FBS provides for MSC culture across different species.
Challenges With FBS in MSC Culture
Variability
It is well-known among researchers that much variability occurs between batches of FBS (9). Hence, FBS batch testing is common practice within labs, which is both time-consuming and expensive (9). An early study assessed various batches of FBS from a single supplier, finding wide variation in component concentrations, which in turn impacted cell proliferation between batches (11). Variability had been noted even at its earliest use in cell culture as Puck et al. observed not all FBS batches promoted cell growth equally (10). They noted anecdotally that higher quality batches of serum came in the summer and fall months, and batches in the winter were less effective at growing human skin cells (10). Later studies began to assess which media variations impact cell growth. Certain factors, such as high growth hormone and low endotoxin level, were associated with improved growth of human lymphoma and primate kidney cell lines with other factors being equal (11). These influential factors varied widely between the batches tested (endotoxin 0.000–10 ng/mL, growth hormone 18.7–51.6 ng/mL) (11). Although these studies were not done on MSCs, they are indicative of the variability that occurs among FBS batches.
The quality of FBS has also been shown to differ based on collection and processing methods (30). It was found that FBS collected by research protocols had a consistently improved cell growth compared to commercially available FBS (30). They noted large variations in FBS's ability to support cell growth in the commercially produced FBS (30). More recently, commercial FBS was compared to FBS produced at the Avicenna Research Institute, and it was found that the two sera were very similar in physiochemical properties as well as hemoglobin and endotoxin level (31). When assessing its ability to support cell culture it was found that the viability significantly differed in 3 out of 10 cell lines when comparing commercial FBS to Avicenna produced FBS (31). This emphasizes the variability that can occur in FBS production. Gstraunthaler et al. (32) stated that variability is driven by poor regulations on FBS production. What begins as a practical problem for the successful in vitro culture of MSCs can turn into commercial and clinical problems in that FBS batch-to-batch variability may also lead to an inconsistent cellular product over the long-term.
Immune Response
The most important clinical concern in using FBS lies in its potential to provoke adverse reactions from bovine xenoproteins or disease from viral or prion contamination following injection of FBS-grown MSCs (8). MSCs have been shown to incorporate FBS proteins, which may lead to an expression of antibodies post-injection (33). Two bovine xenoprotiens that have been identified to be able to incorporate into mammalian cells include N-glycolylneuraminic acid (Neu5Gc) and bovine apolipoprotein B-100 (33, 34). The incorporation of Neu5Gc has been shown to greatly reduced the therapeutic potential of MSC due to Neu5Gc antibodies targeting injected MSCs (33). Neu5Gc is naturally produced in most animals, therefore the reaction to this molecule is largely a human concern rather than a veterinary one (33). Yet, there are conflicting reports about whether European dog breeds produce Neu5Gc (35, 36). Neu5Gc is not the only immunogenic contaminant of concern.
When children with osteogenesis imperfecta were injected with BM-MSCs, it was found that one of the six patients experienced an immune response against their MSC injection (37). This patient had developed antibodies against FBS with a 150-fold increase in these antibodies after their second injection suggesting that multiple injections can exacerbate immune responses (37). Another study looked at hematopoietic stem cell transplantation and immune response against the MSCs used and found that patients did not develop antibodies against MSCs, but anti-FBS antibodies were present (38). The expression of anti-FBS antibodies varied in the patients, consistent with healthy individuals (38). Additionally, Spees et al. observed that rat BM-MSCs cultured in typical 20% FBS media, had a humoral immune response present after repeated injection (3). Human BM-MSCs cultured in these conditions had 7 to 30 mg of bovine protein contamination per 100 million MSCs (3). Researchers transferred the human MSCs to media containing 10% adult human serum including growth factors for 5–10 days, resulting in elimination of 99.99% of FBS contamination from the BM-MSCs (3). When done in rat cells using adult rat serum with growth hormones, it eliminated the immune response previously observed with cells grown in FBS (3).
In an equine study, 89% of horses had anti-BSA antibodies prior to MSC administration most likely due to previous vaccinations, which may have also used FBS (39). It was observed that after administration of allogeneic adipose and bone marrow derived MSCs cultured in 10% FBS, there were no significant changes in their anti-BSA antibody titers (39). There was however the development of anti-MSC antibodies observed, but there was no correlation between the horses' level of anti-MSC antibodies and adverse reactions to the treatment, despite multiple MSC injections (39). Horses were also tested for hypersensitivity reactions after previous injection of MSCs cultured in FBS (40). Horses were intradermally injected with FBS, and this produced a significantly greater immune response than injection with saline (40).
A recent study sought to delineate the difference in immune response of horses injected with BM-MSC that were grown in FBS verses autologous and allogenic bone marrow supernatant (41). This work is one of few that focuses on the immune response and clinical outcome of equine BM-MSCs cultured in FBS. There were no differences in antibodies against FBS between the FBS and bone marrow supplemented group, but cells cultured without FBS showed less edema at the site of injection and more MSCs present in the synovium where they were injected (41). They found greater cell death of MSCs cultured in FBS and attributed this to the antibodies against FBS that were already present in the horse, likely due to previous vaccinations (41).
MSCs themselves are not immunogenic and are known to modulate the immune system through paracrine factors (42). These immunomodulatory functions of MSCs are well-established, but it is unclear the role FBS plays in promoting or detracting from MSCs' immunomodulatory function (43). In antimicrobial research, it was observed that lower concentrations of FBS increased equine BM-MSCs' antimicrobial function (44). They attributed this to FBS's ability to bind, and as a result inactivate, antimicrobial peptides (44, 45). Work by Tang et al. has shown that antimicrobial peptides, TP4-A12I, A15I, become inactivated when bound to the albumin and α1-antitrypsin complex in fetal but not adult serum (45). More work needs to be done to determine the effect that FBS has on MSC immunomodulatory function.
Despite these concerns, it should be clearly stated that there are both human and equine MSC clinical products produced in FBS-media that have received market approval in Europe, Canada, New Zealand, Japan, and South Korea (46, 47). The two veterinary stem cell products currently approved by the European Medicines Agency, HorStem (EquiCord) and Arti-Cell Forte (Boehringer Ingelheim), are both composed of allogeneic equine MSCs grown in FBS. Arti-Cell Forte is grown in serum-free medium for the last 72 h prior to clinical use, which may reduce the bovine protein content significantly based on previous studies done showing 95% reduction in 48 h of alternate culture (3, 48).
Contamination
FBS has been cited as a source of viral and prion contamination of cell culture, which can cause infection in patients being treated with MSCs (49–51). Parviviruses, pestiviruses, prions, and bovine viral diarrhea virus (BVDV) are of great concern and therefore diligently screened for (51, 52). BVDV is of particular concern as a study found that the virus is still infectious after heating for 30 min at 56°C, a common sterilization method (53) and can infect non-bovine ungulate species (54). Currently, FBS is filtered and tested for viral contaminants through the Code of Federal Regulations Title 9 (9CFR) guidelines, which require that FBS be tested for 7 specific viruses (50, 55). However, researchers have analyzed commercial FBS batches and determined that the rate of contamination is much higher than what is being reported suggesting that current screening methods are not adequate (49, 56–58).
Mycoplasma is a common form of contamination for cell culture that can arise from various different sources including human contamination and contaminated animal supplements (59). In the 1960–1970s, animal products were a major source of contamination, although presently FBS is rarely the source of mycoplasma contamination (59). This has been shown by a more recent study assessing mycoplasma content in Columbian produced FBS where they found no contamination of mycoplasma (60). A recent study assessed gamma irradiated FBS produced in Argentina for mycoplasma and found that 14% of FBS samples contained mycoplasma DNA out of 124 samples (61). When cells were cultured in these FBS batches, none of the cells tested positive for mycoplasma infection (61). This indicates that, although mycoplasmas were present in certain FBS batches, gamma irradiation did a successful job of inactivating them and preventing infection of cell culture (61).
Alternatives to FBS in MSC Culture
Species-Specific Serum
When looking for replacements for FBS supplementation in cell culture, the first logical option is to use a species-specific serum. Yet, little research in the veterinary field has been done on the topic. When utilizing different sources of serum, it is important to considered differences in composition. Varying effects have been reported on the effect of horse serum compared to FBS on various cell types (62–66) However, while some comparative analysis of components has shown differences between horse and bovine sera (63), more comprehensive characterization is still needed. In the human field, adult serum has been used to expand MSCs in culture and have shown mixed results with more long-term studies needed (3, 67–69). A study done in human BM-MSC found that using allogenic serum for expansion resulted in cell cycle arrest and death when compared to autologous serum after a single passage (68). With autologous serum, umbilical cord MSC (CB-MSC) proliferated at a rate that was comparable to cells grown with FBS supplementation (69). This was extended over 20 passages with no changes in morphology (69). Moreover, BM-MSC and CB-MSC cultured in 10% adult serum show a similar ability to differentiate and immunomodulate when compared to FBS culture (68, 69).
A challenge with species-specific serum is the quantity of blood required to expand large numbers of MSCs (70). Horses have been shown to tolerate up to 25% of their blood volume being drawn (71). In dogs, a study suggests that there is increased risk in removing more than 15% of a sedated dog's blood volume (72). While also taking animal size into account, this would create a greater challenge in obtaining substantial amounts of canine blood when compared to equine. Recent studies have looked at the impact of different serum sources on equine BM-MSC growth (73, 74). One study found that BM-MSCs cultured in species-specific serum had similar viability and morphology with FBS-grown cells having a shorter population doubling time (74). The other study compared autologous horse serum and standard horse serum to FBS and found that autologous serum was comparable to FBS where standard horse serum decreased BM-MSC proliferation (73). Chondrogenic differentiation in autologous serum showed similar outcomes in equine serum, though cells grown in FBS showed greater bactericidal activity compared to equine serum (74). These researchers also detected no difference between the autologous and allogenic equine serum used, which differs from some of the work done with human MSCs (68, 74). It should be noted Eydt et al. failed to culture cells from 2 out of 12 donors used in autologous serum raising some concern about its reliability as a supplement (73). More work is needed in this area to determine how species-specific serum can impact all therapeutic functions of MSCs.
Serum Free Alternatives
Platelet Lysate
Platelet lysate (PL) was originally tested as an alternative culture additive due to its crucial role in hemostasis and growth in mammalian species (75). Mammalian blood plasma contains cell growth factors such as platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and epidermal growth factor (EGF) (75). PL is prepared from the serum of pooled allogenic or autologous blood (76). This eliminates the risk of cross-species contamination, and pooled allogenic PL is inexpensive to produce and use in large animal cell cultures (76–78).
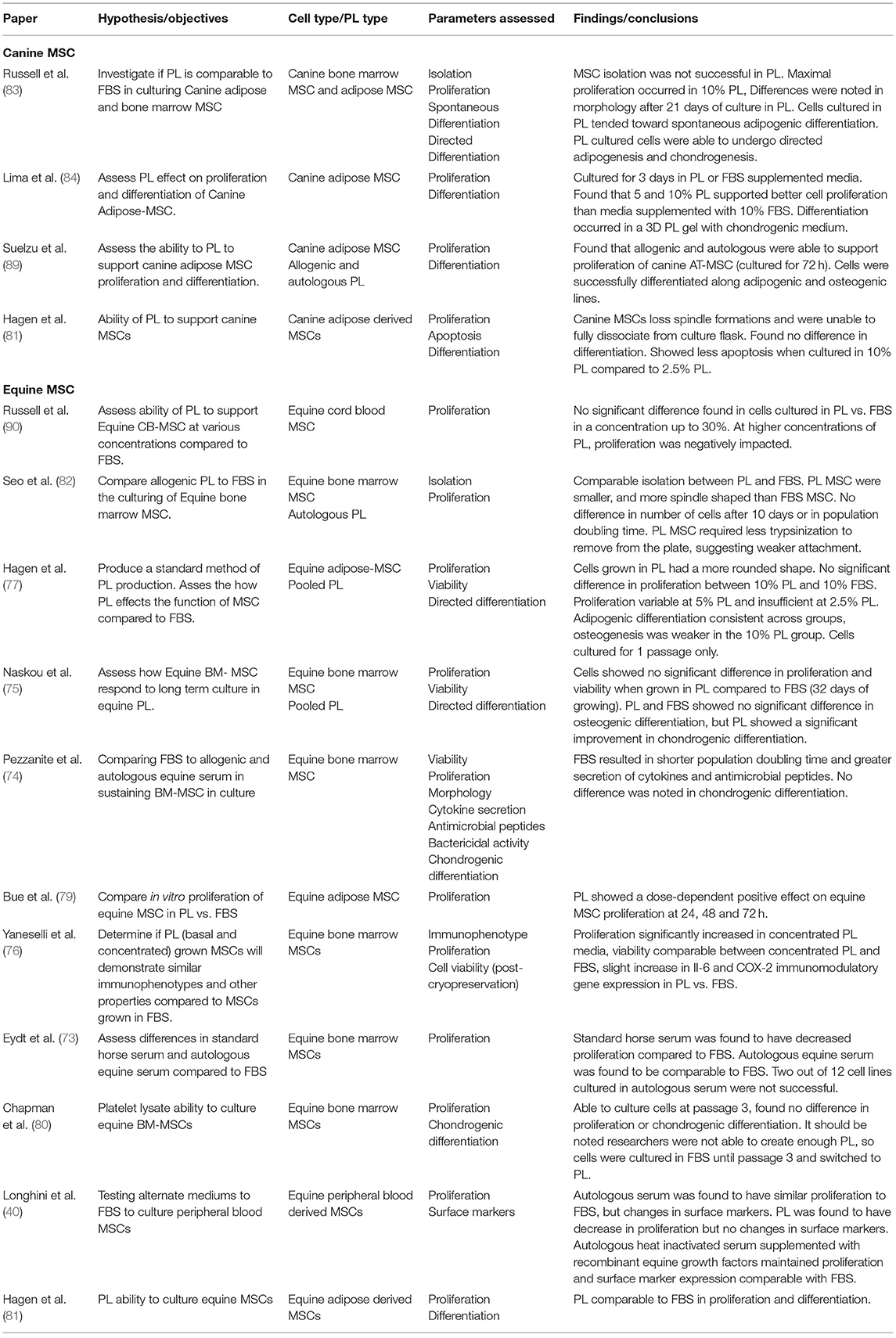
Studies have shown comparable proliferation rates of equine MSCs derived from adipose tissue, cord blood, and bone marrow in equine PL (ePL) when compared to FBS (76, 78–82). Some studies found a clear dose-dependent positive effect on the proliferation rate of equine MSC with increased concentrations of ePL (76, 78, 79). However, the dose-dependent effect of PL on MSC proliferation may be limited, and there may be an optimal threshold for ePL concentration as we have previously reported (78). ePL concentrations higher than 30% showed a detrimental effect on CB-MSC proliferation rates compared to equal concentrations of FBS (78). One study assessed chondrogenic differentiation in equine BM-MSCs cultured in ePL and found no difference between ePL and FBS cultured cells (80), while another study in equine peripheral blood derived MSCs found a decrease in proliferation in cells cultured in 15% PL compared to FBS (40). In canine AD-MSCs, Hagen et al. found that when cultured in PL, the cells lost their spindle-like formations and were unable to be dissociated from the culture flask (81). In our canine studies, we found that PL induced spontaneous adipogenic differentiation with lipid droplets appearing in as few as 4 days in BM-MSC and AD-MSCs (83). Another group of researchers did not observe the same spontaneous adipogenic induction from canine PL, but only cultured AD-MSCs for 3 days (84). Directed differentiation of canine and equine MSCs has been shown to be maintained in cells cultured in PL when compared to FBS (75, 82–84). Immunomodulatory function of MSCs also needs to be preserved in PL culture. Both Naskou et al. and Yaneselli et al. demonstrated that equine BM-MSC immunomodulatory properties were comparable between those cultured in PL and those in FBS (75, 76).
It is important to note that heparin is often added to PL culture to prevent gel formation in cell cultures (85). As heparin is usually porcine- or bovine-source, the resulting cell product will not be free of xenoproteins. If a xeno-free product is desired, mechanical or chemical depletion of fibrinogen in PL should be considered as an alternative (85, 86).
Chemically Defined Media
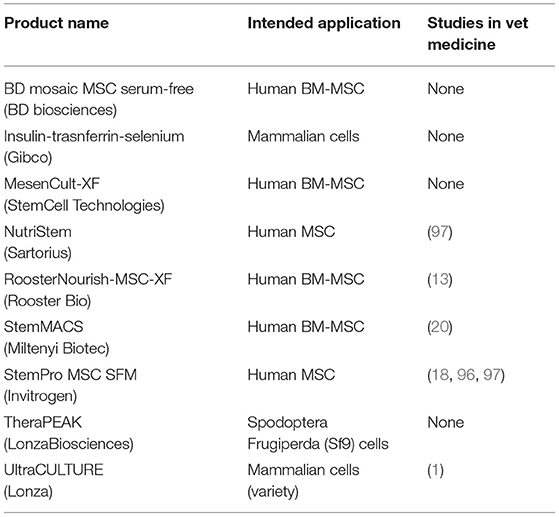
A defined serum-free media (SFM) represents an ideal formulation for consistency across all cell cultures, experiments, and labs (87). It provides standardized components of the media while mitigating issues of contamination, variability, and ethics (88). There are many chemically defined or semi-defined SFM products that are currently commercially available for cell culture (Table 2). However, these SFM are optimized for human stem cell culture. Researchers have been investigating if these SFM can be used with veterinary animal cell cultures (18, 91, 92).
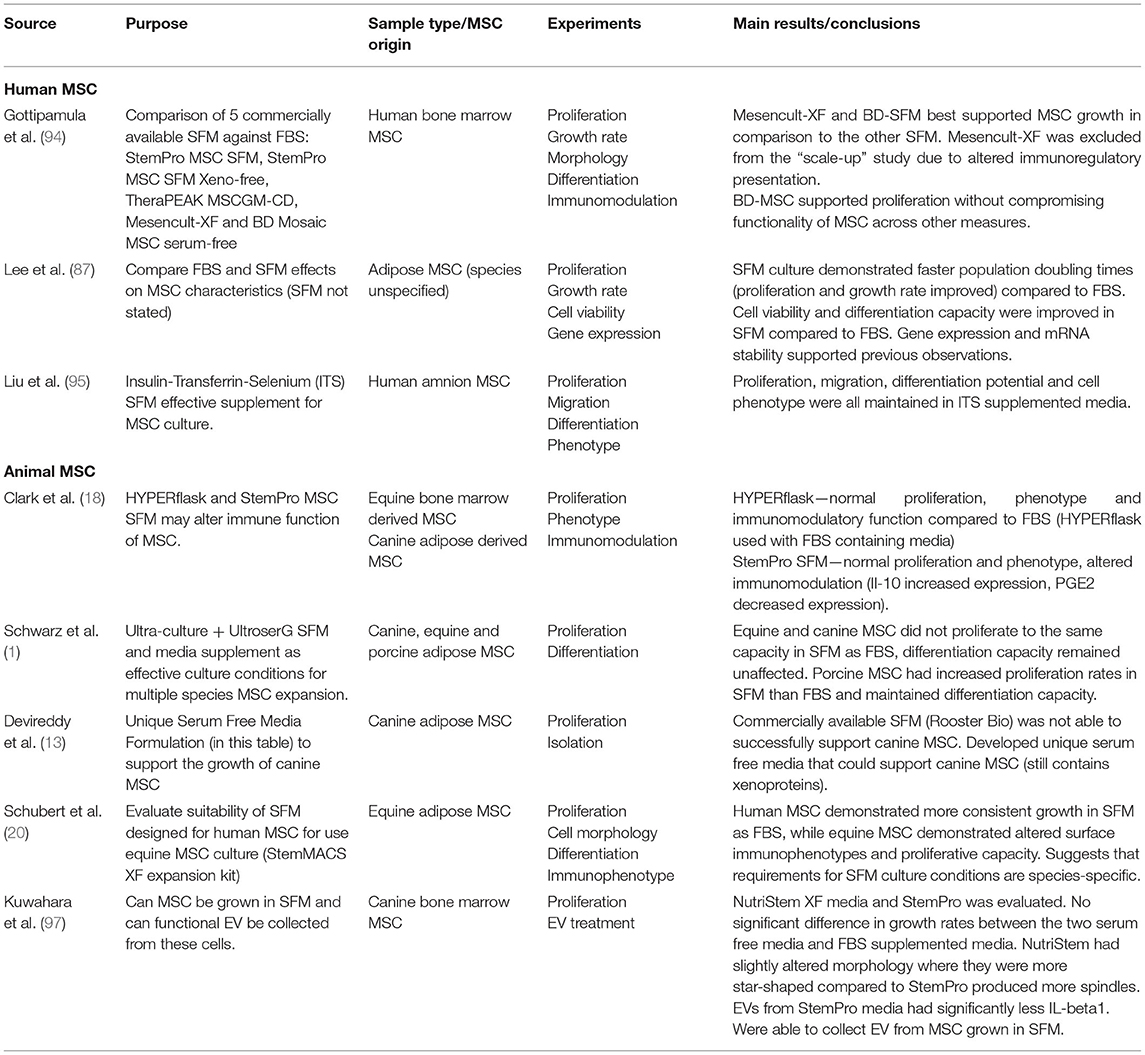
The challenge with developing SFM is obtaining definable components that can support cell growth and function. Proliferation is one of the standard tests used to evaluate the efficacy of SFM culture (93). StemPro MSC SFM (Invitrogen), StemPro MSC SFM Xeno-free (Invitrogen), TheraPEAK MSC growth medium (LonzaBiosciences), MesenCult-XF (StemCell Technologies), BD-Mosaic hMSC SF (BD Biosciences), and Insulin-Transferrin-Selenium (ITS) (Gibco) (Table 3) all demonstrated slower doubling times for human BM-MSC, AD-MSC and MSCs derived from amniotic tissue compared to FBS (18, 87, 94, 95). In the veterinary field, Ultra-culture and UltroserG SFM demonstrated significantly reduced proliferative capacity in canine and equine AD-MSC, but significantly increased proliferation in porcine AD-MSC emphasizing the challenge of species-specific differences (1). Another group of researchers found no difference in proliferation of equine BM-MSC and canine AD-MSC grown in StemPro SFM when compared to FBS (18). Work done in our lab found that using StemPro SFM in equine CB-MSC culture resulted in slower proliferation compared to FBS (96). These differences could be attributed to tissue source variation. Finally, utilizing StemMACS MSC expansion kit, researchers were able to isolate and culture equine AD-MSCs with no difference in proliferation compared FBS cultured cells (20). Yet, these cells displayed altered morphology when visually assessed, and altered surface marker expression when compared to control cells (20).
Osteogenic and chondrogenic differentiation potential of BM-MSC, AD-MSC, and amnion derived MSCs after culture in SFM, BD Mosaic hMSC SF, ITS, and StemPro SFM was comparable to cells cultured in FBS (87, 94, 95). Canine, equine, and porcine MSC osteogenic and chondrogenic differentiation potential was evaluated after culture in Ultra-culture and UltroserG SFM, and both were found to support capacities equal to FBS-cultured AD-MSCs (1). There is limited research evaluating veterinary animal MSC expansion in BD Mosaic hMSC SF, ITS, and StemPro SFM, which could be an area of interest moving forward.
Looking at immunomodulatory properties of mammalian MSC, StemPro MSC SFM produce altered immunomodulatory function of equine and canine BM-MSCs when compared to FBS-containing medium (18). It was observed that both equine BM-MSCs and canine AD-MSCs maintained their ability to inhibit T-cell proliferation, although MSC mediated IL-10 secretion was significantly increased and PGE2 secretion was significantly decreased in SFM (18).
In addition to those commercially available, researchers have attempted to formulate their own SFM using a species-specific approach. Devireddy et al. (13) formulated a SFM (Tables 1, 4) targeting canine AD-MSCs. While this medium did support proliferation of canine AD-MSCs, a challenge with this medium is that it is not completely free of xenogeneic proteins in that BSA was included, which may pose a risk of immune reaction in the recipient (13). More research needs to be performed to confirm the effects of different SFM formulae on MSCs from different species and tissue sources.
FBS Reduction
With the shortcomings of serum alternatives, it seems that complete replacement of FBS in MSC culture may not be feasible at present. It may prove beneficial to investigate ways that serum can be reduced until a better solution is found.
There is evidence to suggest that reducing use of FBS in MSC culture may be sufficient to overcome some of the major concerns related to its use. Currently, FBS is still required to isolate and establish MSC cell cultures. This results in the cells being switched from FBS to an alternative media after they are well-established (74). Clark et al.s' used commercially available SFM, but required the culture flasks to be coated in bovine fibronectin, a potentially xenogeneic substance (18). A short-term transition period just prior to clinical use may be advisable to produce a xenogeneic-free cellular product. This was shown in human BM-MSCs where media was switched from containing fluorescently labeled FBS to adult human serum for a 42-h culture period, after which signal from the FBS was reduced 10,000-fold (3). In a separate study, switching BM-MSCs from FBS medium to SFM found a 95% reduction in intracellular labeled FBS after 48 h (48). Similarly, there was a significant decrease in incorporated Neu5Gc from FBS when BM-MSCs were switched to media containing adult human serum for 1 week (33). In equine BM-MSCs, cells that were initially cultured in FBS then switched to equine serum for 72 h were found to be comparable to cells cultured only in FBS supplemented medium (74). Reducing FBS concentrations to 1–2% has also been shown to improve BM-MSCs' antimicrobial function with a similar trend seen with equine serum (44, 74). While some caution should be observed that a stress response may be caused due to the abrupt change in culture conditions, a short period of serum reduction or substitution may be a viable option to reduce risk of adverse outcomes from FBS.
Conclusion
Overall, FBS is effective when it comes to promoting the establishment and expansion of MSCs in vitro. Yet, there are enough concerns associated with its use in cell therapy to merit a search for alternatives. Based on the current state of the literature, there does not seem to be a clear replacement for FBS for all aspects of its current use. Researchers have experimented with species-specific serum, serum-free, and serum-reduced media, but none provide a perfect replacement option. In the veterinary space, PL or a defined SFM may hold the most promise. Short-term PL culture appears to support proliferation, differentiation capacity, and immune function comparable to FBS culture. To minimize risk, using FBS-free media for a short-term transition culture period preceding clinical administration may be prudent.
Author Contributions
CP conceptualized the study. CP, KM, JR, JD, KR, and TK outlined the paper collaboratively. CP, KM, JR, and JD reviewed the literature and wrote the report collaboratively. KR and TK supervised the work and edited the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was partial supported by Mitacs Accelerate (Grant #460950) and the Department of Biomedical Sciences, Ontario Veterinary College.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schwarz C, Leicht U, Rothe C, Drosse I, Luibl V, Röcken M, et al. Effects of different media on proliferation and differentiation capacity of canine, equine and porcine adipose derived stem cells. Res Vet Sci. (2012) 93:457–62. doi: 10.1016/j.rvsc.2011.08.010
3. Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ, et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. (2004) 9:747–56. doi: 10.1016/j.ymthe.2004.02.012
4. Dazzi F, Horwood NJ. Potential of mesenchymal stem cell therapy. Curr Opin Oncol. (2007) 19:650–5. doi: 10.1097/CCO.0b013e3282f0e116
5. Musiał-Wysocka A, Kot M, Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. (2019) 28:801–12. doi: 10.1177/0963689719837897
6. Taylor SE, Clegg PD. Collection and propagation methods for mesenchymal stromal cells. Vet Clin N Am Equine Pract. (2011) 27:263–74. doi: 10.1016/j.cveq.2011.05.003
7. Gstraunthaler G, Lindl T, van der Valk J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology. (2013) 65:791–3. doi: 10.1007/s10616-013-9633-8
8. Hemeda H, Giebel B, Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy. (2014) 16:170–80. doi: 10.1016/j.jcyt.2013.11.004
9. van der Valk J, Gstraunthaler G. Fetal bovine serum (FBS)—a pain in the dish? Altern Lab Anim. (2017) 45:329–32. doi: 10.1177/026119291704500611
10. Puck TT, Cieriuta SJ, Robinson A. Genetics of somatic mammalian cells. III Long-term cultivation of euploid cells from human and animal subjects. J Exp Med. (1958) 108:945–56. doi: 10.1084/jem.108.6.945
11. Price PJ, Gregory EA. Relationship between in vitro growth promotion and biophysical and biochemical properties of the serum supplement. In Vitro. (1982) 18:576–84. doi: 10.1007/BF02810081
12. Fang C-Y, Wu C-C, Fang C-L, Chen W-Y, Chen C-L. Long-term growth comparison studies of FBS and FBS alternatives in six head and neck cell lines. PLoS ONE. (2017) 12:e0178960. doi: 10.1371/journal.pone.0178960
13. Devireddy LR, Myers M, Screven R, Liu Z, Boxer L. A serum-free medium formulation efficiently supports isolation and propagation of canine adipose-derived mesenchymal stem/stromal cells. PLoS ONE. (2019) 14:e0210250. doi: 10.1371/journal.pone.0210250
14. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. (2006) 8:315–7. doi: 10.1080/14653240600855905
15. Jung S, Sen A, Rosenberg L, Behie LA. Identification of growth and attachment factors for the serum-free isolation and expansion of human mesenchymal stromal cells. Cytotherapy. (2010) 12:637–57. doi: 10.3109/14653249.2010.495113
16. Hayman E, Pierschbacher M, Suzuki S, Rouslahti E. Vitronectin-A Major Cell Attachment-Promoting Protein in Fetal Bovine Serum (1985) 160:245–58.
17. Nakao M, Inanaga D, Nagase K, Kanazawa H. Characteristic differences of cell sheets composed of mesenchymal stem cells with different tissue origins. Reg Ther. (2019) 11:34–40. doi: 10.1016/j.reth.2019.01.002
18. Clark KC, Kol A, Shahbenderian S, Granick JL, Walker NJ, Borjesson DL. Canine and equine mesenchymal stem cells grown in serum free media have altered immunophenotype. Stem Cell Rev Rep. (2016) 12:245–56. doi: 10.1007/s12015-015-9638-0
19. De Schauwer C, Meyer E, Cornillie P, De Vliegher S, van de Walle GR, Hoogewijs M, et al. Optimization of the isolation, culture, and characterization of equine umbilical cord blood mesenchymal stromal cells. Tissue Eng Part C Methods. (2011) 17:1061–70. doi: 10.1089/ten.tec.2011.0052
20. Schubert S, Brehm W, Hillmann A, Burk J. Serum-free human MSC medium supports consistency in human but not in equine adipose-derived multipotent mesenchymal stromal cell culture. Cytometry Part A. (2018) 93:60–72. doi: 10.1002/cyto.a.23240
21. Hayashi I, Sato GH. Replacement of serum by hormones permits growth of cells in a defined medium. Nature. (1976) 259:132–4. doi: 10.1038/259132a0
22. Nuschke A, Rodrigues M, Wells AW, Sylakowski K, Wells A. Mesenchymal stem cells/multipotent stromal cells (MSCs) are glycolytic and thus glucose is a limiting factor of in vitro models of MSC starvation. Stem Cell Res Ther. (2016) 7:179. doi: 10.1186/s13287-016-0436-7
23. Moya A, Paquet J, Deschepper M, Larochette N, Oudina K, Denoeud C, et al. Human mesenchymal stem cell failure to adapt to glucose shortage and rapidly use intracellular energy reserves through glycolysis explains poor cell survival after implantation. Stem Cells. (2018) 36:363–76. doi: 10.1002/stem.2763
24. Mylotte LA, Duffy AM, Murphy M, O'Brien T, Samali A, Barry F, et al. Metabolic flexibility permits mesenchymal stem cell survival in an ischemic environment. Stem Cells. (2008) 26:1325–36. doi: 10.1634/stemcells.2007-1072
25. Potier E, Ferreira E, Meunier A, Sedel L, Logeart-Avramoglou D, Petite H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. (2007) 13:1325–31. doi: 10.1089/ten.2006.0325
26. Cramer C, Freisinger E, Jones RK, Slakey DP, Dupin CL, Newsome ER, et al. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev. (2010) 19:1875–85. doi: 10.1089/scd.2010.0009
27. Liu Y, Li Y, Nan L, Wang F, Zhou S, Wang J, et al. The effect of high glucose on the biological characteristics of nucleus pulposus-derived mesenchymal stem cells. Cell Biochem Funct. (2020) 38:130–40. doi: 10.1002/cbf.3441
28. Zafarvahedian E, Roohi A, Sepand MR, Ostad SN, Ghahremani MH. Effect of metformin and celecoxib on cytotoxicity and release of GDF-15 from human mesenchymal stem cells in high glucose condition. Cell Biochem Funct. (2017) 35:407–13. doi: 10.1002/cbf.3289
29. Berglund AK, Fisher MB, Cameron KA, Poole EJ, Schnabel LV. Transforming growth factor-β2 downregulates major histocompatibility complex (MHC) I and MHC II surface expression on equine bone marrow-derived mesenchymal stem cells without altering other phenotypic cell surface markers. Front Vet Sci. (2017) 4:84. doi: 10.3389/fvets.2017.00084
30. Boone CW, Mantel N, Caruso TD, Kazam E, Stevenson RE. Quality control studies on fetal bovine serum used in tissue culture. In Vitro. (1971) 7:174–89. doi: 10.1007/BF02617963
31. Vojgani Y, Shirazi A, Zarei S, Yeganeh O, Jeddi-Tehrani M. Comparison of efficacies of fetal bovine sera from different suppliers in cell culture experiments. Comp Clin Path. (2018) 27:519–27. doi: 10.1007/s00580-017-2622-0
32. Gstraunthaler G, Lindl T, Van Der Valk J. A severe case of fraudulent blending of fetal bovine serum strengthens the case for serum-free cell and tissue culture applications. Altern Lab Anim. (2014) 42:207–9. doi: 10.1177/026119291404200308
33. Heiskanen A, Satomaa T, Tiitinen S, Laitinen A, Mannelin S, Impola U, et al. N-glycolylneuraminic acid xenoantigen contamination of human embryonic and mesenchymal stem cells is substantially reversible. Stem Cells. (2007) 25:197–202. doi: 10.1634/stemcells.2006-0444
34. Sakamoto N, Tsuji K, Muul LM, Lawler AM, Petricoin EF, Candotti F, et al. Bovine apolipoprotein B-100 is a dominant immunogen in therapeutic cell populations cultured in fetal calf serum in mice and humans. Blood. (2007) 110:501–8. doi: 10.1182/blood-2007-01-066522
35. Jahan M, Thomson PC, Wynn PC, Wang B. The non-human glycan, N-glycolylneuraminic acid (Neu5Gc), is not expressed in all organs and skeletal muscles of nine animal species. Food Chem. (2021) 343:128439. doi: 10.1016/j.foodchem.2020.128439
36. Yasue S, Handa S, Miyagawa S, Inoue J, Hasegawa A, Yamakawa T. Difference in form of sialic acid in red blood cell glycolipids of different breeds of dogs. J Biochem. (1978) 83:1101–7. doi: 10.1093/oxfordjournals.jbchem.a131999
37. Horwitz EM, Gordon PL, Koo WKK, Marx JC, Neel MD, McNall RY, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. (2002) 99:8932–7. doi: 10.1073/pnas.132252399
38. Sundin M, Örvell C, Rasmusson I, Sundberg B, Ringdén O, Le Blanc K. Mesenchymal stem cells are susceptible to human herpesviruses, but viral DNA cannot be detected in the healthy seropositive individual. Bone Marrow Transplant. (2006) 37:1051–9. doi: 10.1038/sj.bmt.1705368
39. Owens SD, Kol A, Walker NJ, Borjesson DL. Allogeneic mesenchymal stem cell treatment induces specific alloantibodies in horses. Stem Cells Int. (2016) 2016:1–8. doi: 10.1155/2016/5830103
40. Longhini ALF, Salazar TE, Vieira C, Trinh T, Duan Y, Pay LM, et al. Peripheral blood-derived mesenchymal stem cells demonstrate immunomodulatory potential for therapeutic use in horses. PLoS ONE. (2019) 14:e0212642. doi: 10.1371/journal.pone.0212642
41. Rowland AL, Burns ME, Levine GJ, Watts AE. Preparation technique affects recipient immune targeting of autologous mesenchymal stem cells. Front Vet Sci. (2021) 8:724041. doi: 10.3389/fvets.2021.724041
42. Uder C, Brückner S, Winkler S, Tautenhahn H-M, Christ B. Mammalian MSC from selected species: features and applications: cross-species MSC. Cytometry Part A. (2018) 93:32–49. doi: 10.1002/cyto.a.23239
43. Silva-Carvalho AÉ, Neves FAR, Saldanha-Araujo F. The immunosuppressive mechanisms of mesenchymal stem cells are differentially regulated by platelet poor plasma and fetal bovine serum supplemented media. Int Immunopharmacol. (2020) 79:106172. doi: 10.1016/j.intimp.2019.106172
44. Pezzanite LM, Chow L, Johnson V, Griffenhagen GM, Goodrich L, Dow S. Toll-like receptor activation of equine mesenchymal stromal cells to enhance antibacterial activity and immunomodulatory cytokine secretion. Vet Surg. (2021) 50:858–71. doi: 10.1111/vsu.13628
45. Tang W-H, Wang C-F, Liao Y-D. Fetal bovine serum albumin inhibits antimicrobial peptide activity and binds drug only in complex with α1-antitrypsin. Sci Rep. (2021) 11:1267. doi: 10.1038/s41598-020-80540-6
46. Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. (2020) 6:eaba.6884. doi: 10.1126/sciadv.aba6884
47. Zygmuntowicz A, Burmańczuk A, Markiewicz W. Selected biological medicinal products and their veterinary use. Animals. (2020) 10:2343. doi: 10.3390/ani10122343
48. Joswig A-J, Mitchell A, Cummings KJ, Levine GJ, Gregory CA, Smith R, et al. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res Ther. (2017) 8:42. doi: 10.1186/s13287-017-0503-8
49. Gagnieur L, Cheval J, Gratigny M, Hébert C, Muth E, Dumarest M, et al. Unbiased analysis by high throughput sequencing of the viral diversity in fetal bovine serum and trypsin used in cell culture. Biologicals. (2014) 42:145–52. doi: 10.1016/j.biologicals.2014.02.002
50. Marcus-Sekura C, Richardson JC, Harston RK, Sane N, Sheets RL. Evaluation of the human host range of bovine and porcine viruses that may contaminate bovine serum and porcine trypsin used in the manufacture of biological products. Biologicals. (2011) 39:359–69. doi: 10.1016/j.biologicals.2011.08.003
51. Nuttall P, Luther P, Stott E. Viral contamination of bovine foetal serum and cell cultures. Nature. (1977) 266:835–7. doi: 10.1038/266835a0
52. Shih DTB, Burnouf T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. N Biotechnol. (2015) 32:199–211. doi: 10.1016/j.nbt.2014.06.001
53. Nagayama K, Oguma K, Sentsui H. Survey on vertical infection of bovine viral diarrhea virus from fetal bovine sera in the field. J Vet Med Sci. (2015) 77:1531–4. doi: 10.1292/jvms.14-0556
54. Evans CA, Reichel MP. Non-bovine species and the risk to effective control of bovine viral diarrhoea (BVD) in cattle. Pathogens. (2021) 10:1263. doi: 10.3390/pathogens10101263
55. Hawkes PW. Fetal bovine serum: geographic origin and regulatory relevance of viral contamination. Bioresour Bioproc. (2015) 2:34–34. doi: 10.1186/s40643-015-0063-7
56. Kniazeff AJ, Wopschall LJ, Hopps HE, Morris CS. Betection of bovine viruses in fetal bovine serum used in cell culture. In Vitro. (1975) 11:400–3. doi: 10.1007/BF02616377
57. Kozasa T, Aoki H, Nakajima N, Fukusho A, Ishimaru M, Nakamura S. Methods to select suitable fetal bovine serum for use in quality control assays for the detection of adventitious viruses from biological products. Biologicals. (2011) 39:242–8. doi: 10.1016/j.biologicals.2011.06.001
58. Sadeghi M, Kapusinszky B, Yugo DM, Phan TG, Deng X, Kanevsky I, et al. Virome of US bovine calf serum. Biologicals. (2017) 46:64–7. doi: 10.1016/j.biologicals.2016.12.009
59. Nikfarjam L, Farzaneh P. Prevention and detection of mycoplasma contamination in cell culture. Cell J. (2012) 13:203–12.
60. Camacho CPC, Mármol LE, Carrillo EF, Medina SJM, Gutiérrez FAA. Detección de siete virus y de Mycoplasma en suero fetal bovino por PCR en tiempo real. (2011) 4:585–97
61. Pecora A, Pérez López J, Jordán MJ, Franco LN, Politzki R, Ruiz V, et al. Analysis of irradiated Argentinean fetal bovine serum for adventitious agents. J Vet Diagn Investig. (2020) 32:892–7. doi: 10.1177/1040638720951556
62. Ahmed YA, Tatarczuch L, Pagel CN, Davies HM, Mirams M, Mackie EJ. Hypertrophy and physiological death of equine chondrocytes in vitro. Equine Vet J. (2007) 39:546–52. doi: 10.2746/042516407X223699
63. Fernyhough ME, Hausman GJ, Dodson MV. Progeny from dedifferentiated bovine adipocytes display protracted adipogenesis. cells Tissues Organs. (2008) 188:359–72. doi: 10.1159/000134007
64. Franke J, Abs V, Zizzadoro C, Abraham G. Comparative study of the effects of fetal bovine serum versus horse serum on growth and differentiation of primary equine bronchial fibroblasts. BMC Vet Res. (2014) 10:119. doi: 10.1186/1746-6148-10-119
65. Hornsby PJ, Sturek M, Harris SE, Simonian MH. Serum and growth factor requirements for proliferation of human adrenocortical cells in culture: comparison with bovine adrenocortical cells. In Vitro. (1983) 19:863–9. doi: 10.1007/BF02618166
66. Wu H, Ren Y, Li S, Wang W, Yuan J, Guo X, et al. In vitro culture and induced differentiation of sheep skeletal muscle satellite cells. Cell Biol Int. (2012) 36:579–87. doi: 10.1042/CBI20110487
67. Popov A, Scotchford C, Grant D, Sottile V. Impact of serum source on human mesenchymal stem cell osteogenic differentiation in culture. Int J Mol Sci. (2019) 20:5051. doi: 10.3390/ijms20205051
68. Shahdadfar A, Frønsdal K, Haug T, Reinholt FP, Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. (2005) 23:1357–66. doi: 10.1634/stemcells.2005-0094
69. Thaweesapphithak S, Tantrawatpan C, Kheolamai P, Tantikanlayaporn D, Roytrakul S, Manochantr S. Human serum enhances the proliferative capacity and immunomodulatory property of MSCs derived from human placenta and umbilical cord. Stem Cell Res Ther. (2019) 10:79. doi: 10.1186/s13287-019-1175-3
70. Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Cell culture medium composition and translational adult bone marrow-derived stem cell research. Stem Cells. (2006) 24:1409–10. doi: 10.1634/stemcells.2005-0654
71. Malikides N, Hodgson JL, Rose RJ, Hodgson DR. Cardiovascular, haematological and biochemical responses after large volume blood collection in horses. Vet J. (2001) 162:44–55. doi: 10.1053/tvjl.2001.0583
72. Ferreira RRF, Gopegui RR, Matos AJFD. Volume-dependent hemodynamic effects of blood collection in canine donors—Evaluation of 13% and 15% of total blood volume depletion. Anais Da Academia Brasileira de Ciências. (2015) 87:381–8. doi: 10.1590/0001-3765201520140210
73. Eydt C, Geburek F, Schröck C, Hambruch N, Rohn K, Pfarrer C, et al. Sternal bone marrow derived equine multipotent mesenchymal stromal cells (MSCs): investigations considering the sampling site and the use of different culture media. Vet Med Sci. (2016) 2:200–10. doi: 10.1002/vms3.36
74. Pezzanite L, Chow L, Griffenhagen G, Dow S, Goodrich L. Impact of three different serum sources on functional properties of equine mesenchymal stromal cells. Front Vet Sci. (2021) 8:e634064. doi: 10.3389/fvets.2021.634064
75. Naskou MC, Sumner SM, Chocallo A, Kemelmakher H, Thoresen M, Copland I, et al. Platelet lysate as a novel serum-free media supplement for the culture of equine bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. (2018) 9:75. doi: 10.1186/s13287-018-0823-3
76. Yaneselli K, Barrachina L, Remacha AR, Algorta A, Vitoria A, Cequier A, et al. Effect of allogeneic platelet lysate on equine bone marrow derived mesenchymal stem cell characteristics, including immunogenic and immunomodulatory gene expression profile. Vet Immunol Immunopathol. (2019) 217:109944. doi: 10.1016/j.vetimm.2019.109944
77. Hagen A, Lehmann H, Aurich S, Bauer N, Melzer M, Moellerberndt J, et al. Scalable production of equine platelet lysate for multipotent mesenchymal stromal cell culture. Front Bioeng Biotechnol. (2021) 8:613621. doi: 10.3389/fbioe.2020.613621
78. Russell KA, Koch TG. Equine platelet lysate as an alternative to fetal bovine serum in equine mesenchymal stromal cell culture – too much of a good thing? Equine Vet J. (2016) 48:261–4. doi: 10.1111/evj.12440
79. Bue MD, Riccò S, Conti V, Merli E, Ramoni R, Grolli S. Platelet lysate promotes in vitro proliferation of equine mesenchymal stem cells and tenocytes. Vet Res Commun. (2007) 31:289–92. doi: 10.1007/s11259-007-0099-z
80. Chapman H-S, Gale AL, Dodson ME, Linardi RL, Ortved KF. Autologous platelet lysate does not enhance chondrogenic differentiation of equine bone marrow-derived mesenchymal stromal cells despite increased TGF-β1 concentration. Stem Cells Dev. (2020) 29:144–55. doi: 10.1089/scd.2019.0239
81. Hagen A, Holland H, Brandt V-P, Doll CU, Häußler TC, Melzer M, et al. Platelet lysate for mesenchymal stromal cell culture in the canine and equine species: analogous but not the same. Animals. (2022) 12:189. doi: 10.3390/ani12020189
82. Seo J, Tsuzuki N, Haneda S, Yamada K, Furuoka H, Tabata Y, et al. Comparison of allogeneic platelet lysate and fetal bovine serum for in vitro expansion of equine bone marrow-derived mesenchymal stem cells. Res Vet Sci. (2013) 95:693–8. doi: 10.1016/j.rvsc.2013.04.024
83. Russell KA, Gibson TWG, Chong A, Co C, Koch TG. Canine platelet lysate is inferior to fetal bovine serum for the isolation and propagation of canine adipose tissue- and bone marrow-derived mesenchymal stromal cells. PLoS ONE. (2015) 10:e0136621. doi: 10.1371/journal.pone.0136621
84. Lima VP, Tobin GC, de Jesus Pereira MR, Silveira MD, Witz MI, Nardi NB. Chondrogenic effect of liquid and gelled platelet lysate on canine adipose-derived mesenchymal stromal cells. Res Vet Sci. (2019) 124:393–8. doi: 10.1016/j.rvsc.2019.04.022
85. Laner-Plamberger S, Lener T, Schmid D, Streif DA, Salzer T, Öller M, et al. Mechanical fibrinogen-depletion supports heparin-free mesenchymal stem cell propagation in human platelet lysate. J Transl Med. (2015) 13:354. doi: 10.1186/s12967-015-0717-4
86. Naskou MC, Sumner S, Berezny A, Copland IB, Peroni JF. Fibrinogen-depleted equine platelet lysate affects the characteristics and functionality of mesenchymal stem cells. Stem Cells Dev. (2019) 28:1572–80. doi: 10.1089/scd.2019.0070
87. Lee J, Kang M, Choi J, Chun Y, Jang J, Yang Y, et al. Comparative analysis of FBS containing media and serum free chemically defined media for adipose derived stem cells production. Cytotherapy. (2019) 21(Suppl. 5):S82. doi: 10.1016/j.jcyt.2019.03.497
88. Kinzebach S, Bieback K. Expansion of mesenchymal stem/stromal cells under xenogenic-free culture conditions. In: Weyand B, Dominici M, Hass R, Jacobs R, Kasper C, editors. Mesenchymal Stem Cells—Basics and Clinical Application I. Berlin; Heidelberg: Springer (2013). p. 33–57.
89. Suelzu CM, Conti V, Khalidy Y, Montagna S, Strusi G, Di Lecce R, et al. Xenobiotic-free medium guarantees expansion of adipose tissue-derived canine mesenchymal stem cells both in 3D fibrin-based matrices and in 2D plastic surface cultures. Cells. (2020) 9:2578. doi: 10.3390/cells9122578
90. Russell KA, Chow NHC, Dukoff D, Gibson TWG, LaMarre J, Betts DH, et al. Characterization and immunomodulatory effects of canine adipose tissue- and bone marrow-derived mesenchymal stromal cells. PLoS ONE. (2016) 11:e0167442. doi: 10.1371/journal.pone.0167442
91. Carmelo JG, Fernandes-Platzgummer A, Diogo MM, da Silva CL, Cabral JMS. A xeno-free microcarrier-based stirred culture system for the scalable expansion of human mesenchymal stem/stromal cells isolated from bone marrow and adipose tissue. Biotechnol J. (2015) 10:1235–47. doi: 10.1002/biot.201400586
92. dos Santos F, Campbell A, Fernandes-Platzgummer A, Andrade PZ, Gimble JM, Wen Y, et al. A xenogeneic-free bioreactor system for the clinical-scale expansion of human mesenchymal stem/stromal cells. Biotechnol Bioeng. (2014) 111:1116–27. doi: 10.1002/bit.25187
93. Gottipamula S, Muttigi MS, Kolkundkar U, Seetharam RN. Serum-free media for the production of human mesenchymal stromal cells: a review. Cell Prolif. (2013) 46:608–27. doi: 10.1111/cpr.12063
94. Gottipamula S, Muttigi MS, Chaansa S, Ashwin KM, Priya N, Kolkundkar U, et al. Large-scale expansion of pre-isolated bone marrow mesenchymal stromal cells in serum-free conditions. J Tissue Eng Regen Med. (2016) 10:108–19. doi: 10.1002/term.1713
95. Liu X, Zhang T, Wang R, Shi P, Pan B, Pang X. Insulin-transferrin-selenium as a novel serum-free media supplement for the culture of human amnion mesenchymal stem cells. Ann Clin Lab Sci. (2019) 49:63–71.
96. Oji S. Effect of Serum Serum-Free Supplementation on Equine Mesenchymal Stromal Cells During In-Vitro Culture by [University of Guelph]. (2020). Available online at: https://hdl.handle.net/10214/23702 (accessed January 2021).
Keywords: mesenchymal stromal cell, fetal bovine serum, platelet lysate, serum-free media, serum reduction, species specific serum
Citation: Pilgrim CR, McCahill KA, Rops JG, Dufour JM, Russell KA and Koch TG (2022) A Review of Fetal Bovine Serum in the Culture of Mesenchymal Stromal Cells and Potential Alternatives for Veterinary Medicine. Front. Vet. Sci. 9:859025. doi: 10.3389/fvets.2022.859025
Received: 20 January 2022; Accepted: 08 April 2022;
Published: 03 May 2022.
Edited by:
Lauren Virginia Schnabel, North Carolina State University, United StatesReviewed by:
Joaquim Vives, Banc de Sang i Teixits, SpainTaralyn M. McCarrel, University of Florida, United States
Catharina De Schauwer, Ghent University, Belgium
Copyright © 2022 Pilgrim, McCahill, Rops, Dufour, Russell and Koch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas G. Koch, dGtvY2hAdW9ndWVscGguY2E=
†These authors have contributed equally to this work and share first authorship
 Cara R. Pilgrim
Cara R. Pilgrim Kiera A. McCahill
Kiera A. McCahill Jenna G. Rops†
Jenna G. Rops† Jaustin M. Dufour
Jaustin M. Dufour Keith A. Russell
Keith A. Russell Thomas G. Koch
Thomas G. Koch