- 1Department of Surgery, Shanghai Jiao Tong University Affiliated Sixth People's Hospital, Shanghai, China
- 2Department of Surgery, Heilongjiang Provincial Hospital Affiliated to Harbin Institute of Technology, Harbin, China
Background: Nodal status is a vital prognostic factor for ampullary adenocarcinoma. This study was designed to evaluate the clinical significance of the positive nodes in this disease.
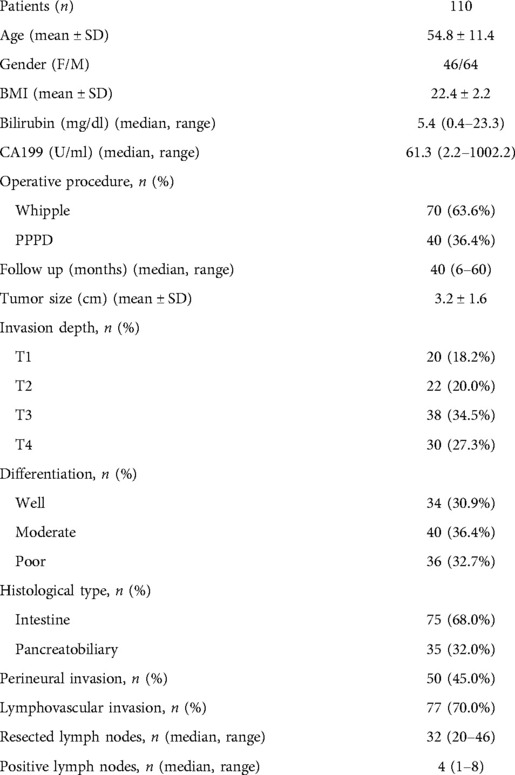
Methods: Data from 110 patients who underwent curative pancreatoduodenectomy for ampullary adenocarcinoma between January 2007 and December 2018 were retrospectively collected and analyzed.
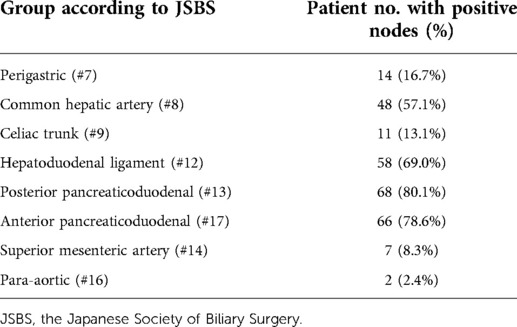
Results: The median number of lymph nodes per patient was 32 (20–46). Metastatic lymph nodes were found in 84 (76.4%) patients. In patients with positive nodules, the most commonly involved nodes were the #13 (80.1%) and #17 (78.6%) nodes, followed by #12 (69.0%) and #8 nodes (57.1%). Patients with 3–4 positive nodes among #13, #17, #12, and #8 had lower survival rates than those with 0 or 1–2 nodes.
Conclusion: Ampullary adenocarcinoma commonly spreads to #13, #17, #12, and #8 lymph nodes. These nodes affected the patients' survival rates dramatically.
Introduction
Ampullary adenocarcinoma is a rare type of cancer, accounting for 6% of periampullary neoplasms (1–4). Surgery gives rise to a better prognosis in ampullary adenocarcinoma patients than the one for pancreatic or biliary cancer (5–7). Variable factors such as resection margin, histologic type, invasion depth, perineural, and lymphatic invasion have been reported as predictors of a patient's prognosis. Because lymph node (LN) status is pivotal for ampullary adenocarcinoma (2, 8, 9), understanding the importance of lymphatic spread is critical for curative resection. Some studies reporting node metastasis have previously been published (9–11); however, due to the small study population in these studies (range 12–21), the importance of LN spread was not well known. Lymphadenectomy in patients with adenocarcinoma of the duodenal papilla increases surgical time, but not morbidity and mortality benefit, as well as hospital length of stay (12). However, the therapeutic benefit of the performance of a wider node dissection in patients with periampullary adenocarcinoma remains unclear (13). The purpose of this study was to identify the prognostic significance of positive nodes in ampullary adenocarcinoma.
Methods
Enrolled patients
The data of 121 ampullary adenocarcinoma patients who underwent operations from January 2007 to December 2018 in Shanghai Jiao Tong University Affiliated Sixth People's Hospital and Heilongjiang Provincial Hospital Affiliated to Harbin Institute of Technology were retrospectively collected based on a prospective database. Eleven patients were excluded due to R1 or R2 resection. The study population consisted of 70 patients who underwent the Whipple procedure and 40 patients who underwent the pylorus-preserving procedure (PPPD). All of the 110 patients underwent R0 resection. The demographics data, namely, age, gender, BMI, bilirubin, CA199, operative procedure, follow-up time, tumor size, invasion depth, differentiation, histological type, perineural invasion, lymphovascular invasion, resected lymph nodes, and positive lymph nodes, were collected and studied. The study was approved by the ethics committee of Shanghai Jiao Tong University. It was conducted following the principles of the Declaration of Helsinki. Each patient provided written informed consent.
Surgical procedure
Curative resection was achieved when a negative resected margin was found, and all metastatic LNs were completely removed. All patients underwent surgery with standard regional lymphadenectomy including the peripancreatic LNs, the common and proper hepatic artery LNs, the LNs in hepatoduodenal ligament, and the LNs in the right lateral area of SMV (superior mesenteric vessel). However, the aortocaval and para-aortic LNs were only removed when they were found to be enlarged or positive in imaging before operation.
Pathological examination
All adenocarcinomas originated from the ampulla of Vater. The histopathological examination of pancreaticoduodenectomy specimens was performed following the detailed standardized protocol (14). Tumor differentiation was ranked as well, moderate, and poor. The resected margin was evaluated. R0 resection was defined as the resected margins of the pancreas, common bile duct, duodenum, and retropancreatic tissues were free of cancer under the microscope. LNs were classified into groups and numbered following the General Rules for Surgical and Pathological Studies on Cancer of the Biliary Tract advised by the Japanese Society of Biliary Surgery (JSBS).
Statistical analysis
Continuous variables were expressed as median and range or mean ± standard deviation, and categorical variables were expressed as number and percentage. The χ2 test was performed for nominal data. Univariate analysis was performed using the χ2 test or Fisher's exact test for categorical variables. When the data did not follow normal distributions, the non-parametric Mann–Whitney U test was applied. The Kaplan–Meier survival rate was compared using the log-rank test. Significant factors identified in univariate analysis were then subjected to multivariate analysis and were analyzed using Cox proportional hazard regression analysis. Significance was considered when P < 0.05. SPSS 20 (SPSS, Chicago, IL, USA) was used for statistics.
Results
Patient demographics
The median follow-up time of the 110 patients was 40 (6–60) months (Table 1), and other clinical features are also shown in Table 1. There were 3,482 LNs resected from 110 patients were pathologically diagnosed. The median number of LNs per patient was 32 (20–46), 34 (20–42) for the Whipple procedure, and 27 (20–46) for the PPPD. Eighty-four (76.4%) patients had LN metastases. The median number of positive regional LNs was 4 (1–8).
Distribution of LNs
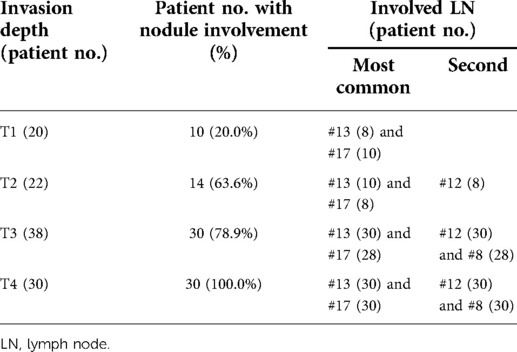
The highest incidence of positive LNs was identified in posterior and anterior pancreaticoduodenum (#13, #17) (80.1%, 78.6%), followed by the LNs of the hepatoduodenal ligament (#12) (69.0%) and the common hepatic artery (#8) (57.1%) (Table 2). There were six patients with #8 or #12 LNs involved but without #13 or #17 LNs involved. The relationship between positive LNs and tumor invasion depth is shown in Table 3. The ratios of involved LNs at T1, T2, T3, and T4 were 20.0%, 63.6%, 78.9%, and 100.0%, respectively. The most common positive LNs were #13 and #17 LNs in all T stages, and the second most common positive LNs were #12 in the T2 stage, and #12 and #8 in both T3 and T4 stages.
Survival analysis
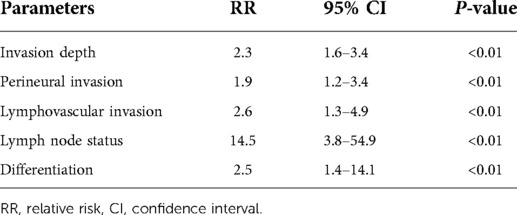
In univariate analysis, depth of invasion, LN status, tumor differentiation, perineural invasion, and lymphovascular invasion were strongly associated with the patients' 5-year overall survival rate after surgery (P < 0.01). A significant difference was found when the 5-year survival rate was compared between T1 + T2 and T3 + T4 tumors (75.3% vs. 36.4%, P < 0.01). Patients with negative LNs had a higher 5-year survival rate than those with positive LNs (71.3% vs. 42.3%, P < 0.01). There was a significant difference in the 5-year survival rates between well + moderate and poor differentiated tumors (64.3% vs. 44.5%, P < 0.01). Patients without perineural invasion or lymphovascular invasion had a higher 5-year survival rate than their counterparts (75.1% vs. 31.2%, P < 0.01 and 81.4% vs. 48.3%, P < 0.01) (Table 4). In the multivariate analysis, invasion depth (RR: 2.3, 95% CI: 1.6–3.4, P < 0.01), perineural invasion (RR: 1.9, 95% CI: 1.2–3.4, P < 0.01), lymphovascular invasion (RR: 2.6, 95% CI: 1.63–4.9, P < 0.01), LN status (RR: 14.5, 95% CI: 3.8–54.9, P < 0.01) and differentiation (RR: 2.5, 95% CI: 1.4–14.1, P < 0.01) were independently correlated with the patient's survival (Table 5).
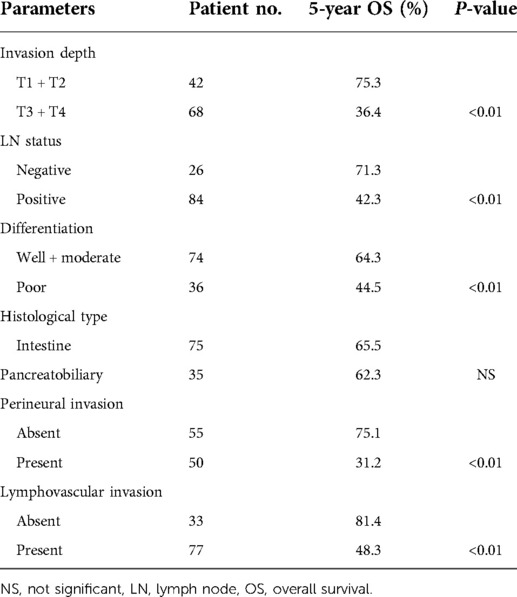
Table 4. Univariate analysis of pathological variables on survival in patients with ampullary adenocarcinoma.
Impact of the number of positive #13, #17, #12, and #8 LNs on patients' survival
Patients with 3–4 positive nodes among the #13, #17, #12, and #8 LNs had a lower survival rate than patients with 0 or 1–2 nodes (P < 0.01) (Figure 1). In the multivariate analysis of LNs (#13, #17, #12, and #8) on patients' survival, patients with 1–2 positive nodes had a higher risk of death than those with negative nodes (RR: 3.5, 95% CI: 2.3–5.8, P < 0.01), and patients with 3–4 positive nodes had a higher risk of death as compared with patients with negative nodes (RR: 13.5, 95% CI: 3.1–44.5, P < 0.01) (Table 6).
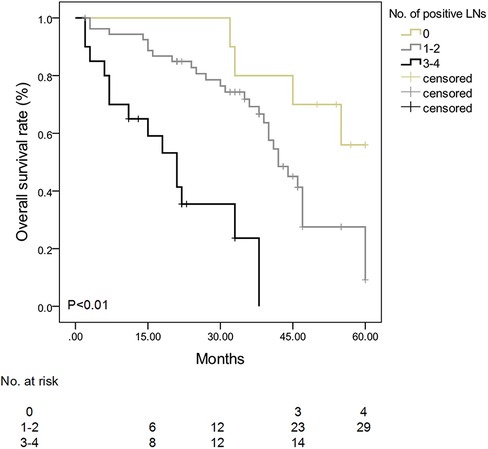
Figure 1. Survival curve stratified by the number of positive #13, #17, #12, and #8 lymph nodes in patients with ampullary adenocarcinoma. Patients with 3–4 positive nodes among #13, #17, #12, and #8 LNs had a lower survival rate than patients with 0 or 1–2 nodes (P < 0.01).
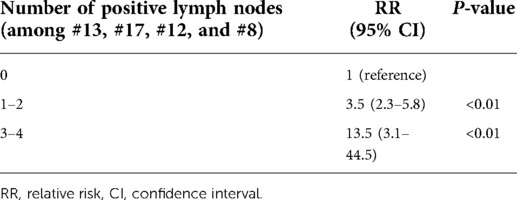
Table 6. Multivariate analysis of lymph node (#13, #17, #12, and #8) status on survival in patients with ampullary adenocarcinoma.
Discussion
The risk of LNs metastasis increases with tumor T stages as demonstrated by Lee et al. (6), Bronsert et al. (15, 16), and our study. Analysis based on the T stage seems to be more reasonable than other gross types. Delcore et al. found that tumor size was an independent factor for patient survival (17), but Berger et al. demonstrated that pancreatic invasion but not tumor size was the indicator for survival (18). Once the cancer cells penetrate the sphincter of Oddi, LNs are going to be involved. The invasion depth is a significant parameter for prognosis. Lee et al. found that the most commonly involved nodes were #13 LNs followed by #17 LNs. But no #12 and #8 LNs were involved. They suggested that the number of positive LNs, rather than their ratio and location, independently affected the patient's survival. They further found that the most common positive LNs were #13 and #17 LNs in the T1 stage. The #13 LNs were the most common positive LNs, and the #17 LNs were the second most common positive LNs in T2 to T4 stages. In this study, we found that the most common positive LNs were #13 and #17 LNs in all T stages, and the second most common positive LNs were #12 in the T2 stage and #12 and #8 in both T3 and T4 stages. The findings were quite different probably due to a relatively small patient number in Lee's study.
In the present study, the highest LN metastatic rates were seen in #13 and #17 LNs, followed by #12 and #8 LNs. The pattern of LN distribution found in our study is also quite different from other previous studies (10, 11). The #13 and #17 LNs were most frequently involved and had an increased incidence of involvement accompanied by T stages, suggesting that the #13 and #17 LNs were the initial and major metastatic locations. Kayahara et al. (10) suggested that the #13 LN was important for metastasis, and we also shared this opinion. Compared with the previous reports (9, 10, 17, 19), they showed that the occurrences of LN metastases to the perigastric region, common hepatic artery, and celiac trunk were rare. But we got the opposite result. We found that all T1 lesions had pancreaticoduodenal LNs (#13 and #17) metastasis while perigastric (#8 and #12) metastasis was exhibited only in T2, T3, and T4 tumors. This finding indicated that ampullary adenocarcinoma with pancreatic invasion seemed to be more like a pancreatic malignancy with a more extensive LN involvement (13).
Recently, the use of metastatic LN alone or the number of total LN in predicting prognosis may have a bias in the prognostic evaluation (18). The notion that the extensive regional LN dissection contributes to curative resection remains unproven. A randomized trial did not demonstrate the advantage of a more extensive regional node dissection (19). In this study, there is a significant difference in the survival between the numbers of positive #13, #17, #12, and #8 LNs, suggesting the advantage of regional LN dissection. Schwarz and Smith suggested that a pathological examination of a specimen should include at least 10 LNs (13). An inaccurate pathological process could miss positive LNs; therefore, the diagnosis from a skilled and experienced pathologist should be advocated. Hurtuk et al. reported a number of dissected LNs of 15 (2–38) (20). In our study, the median number of resected LN was 32 (20–46).
Due to the low prevalence of this disease, it is unlikely to conclude that the extended LN dissection will give rise to the patient's survival unless an adequate randomized trial with a sufficient sample size is introduced. However, we did find that the #13, #17, #12, and #8 LNs played a vital role in LN metastasis of ampullary adenocarcinoma which affected the patients' survival dramatically. It was necessary to perform a complete node resection in the areas of hepatoduodenal ligament and common hepatic artery in patients with ampullary adenocarcinoma, especially in T2, T3, and T4 lesions.
This study had some limitations concerning its retrospective nature. Although there were limitations, the new findings were remarkable and may contribute to this field in the following aspects. First, although the importance of LN metastasis on the prognosis of ampullary carcinoma was well established, this study demonstrated and confirmed the powerful importance of the analysis with a large number of LNs and more precisely described the pattern of LN distribution than previous reports. Second, according to the T stage, we described the spread pattern of LNs metastasis. This helps to decide the appropriate extension of LN dissection. Third, #13, #17, #12, and #8 LNs are so important that they need surgeon's attention during operation. The more LNs were dissected, the better prognosis would be achieved.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of Shanghai Jiao Tong University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
D-SH and Z-QZ were responsible for the conception and design of the study. Administrative support was provided by Z-YZ, JG, X-PW, and D-SH. Study materials or patients were provided by Z-YZ, JG, X-PW, and D-SH. Collection and assembly of data were carried out by all authors. Data analysis and interpretation were performed by Z-YZ, JG, and X-PW. Manuscript writing and final approval of the manuscript was performed by all authors.
Funding
Z-YZ is currently receiving a grant (81974379) from the National Natural Science Foundation of China. Z-QZ is currently receiving a grant (19ZR1438700) from the Natural Science Foundation of Shanghai and a grant (ZH2018QNB05) from the Interdisciplinary Program of Shanghai Jiao Tong University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hsu HP, Shan YS, Hsieh YH, Yang TM, Lin PW. Predictors of recurrence after pancreaticoduodenectomy in ampullary cancer: comparison between non-, early and late recurrence. J Formos Med Assoc. (2007) 106:432–43. doi: 10.1016/S0929-6646(09)60292-8
2. Pomianowska E, Westgaard A, Mathisen Ø, Clausen OP, Gladhaug IP. Prognostic relevance of number and ratio of metastatic lymph nodes in resected pancreatic, ampullary, and distal bile duct carcinomas. Ann Surg Oncol. (2013) 20:233–41. doi: 10.1245/s10434-012-2592-z
3. Askew J, Connor S. Review of the investigation and surgical management of resectable ampullary adenocarcinoma. HPB (Oxford). (2013) 15:829–38. doi: 10.1111/hpb.12038
4. Adsay V, Ohike N, Tajiri T, Kim GE, Krasinskas A, Balci S, et al. Ampullary region carcinomas: definition and site specific classification with delineation of four clinicopathologically and prognostically distinct subsets in an analysis of 249 cases. Am J Surg Pathol. (2012) 36:1592–608. doi: 10.1097/PAS.0b013e31826399d8
5. Robinson SM, Rahman A, Haugk B, French JJ, Manas DM, Jaques BC, et al. Metastatic lymph node ratio as an important prognostic factor in pancreatic ductal adenocarcinoma. Eur J Surg Oncol. (2012) 38:333–9. doi: 10.1016/j.ejso.2011.12.020
6. Lee JH, Lee KG, Ha TK, Jun YJ, Paik SS, Park HK, et al. Pattern analysis of lymph node metastasis and the prognostic importance of number of metastatic nodes in ampullary adenocarcinoma. Am Surg. (2011) 77:322–9. doi: 10.1177/000313481107700322
7. Shimizu Y, Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Miyazaki M. The morbidity, mortality, and prognostic factors for ampullary carcinoma and distal cholangiocarcinoma. Hepatogastroenterology. (2008) 55:699–703. PMID: 18613437
8. Lazaryan A, Kalmadi S, Almhanna K, Pelley R, Kim R. Predictors of clinical outcomes of resected ampullary adenocarcinoma: a single-institution experience. Eur J Surg Oncol. (2011) 37:791–7. doi: 10.1016/j.ejso.2011.06.008
9. Yoshida T, Matsumoto T, Shibata K, Yokoyama H, Morii Y, Sasaki A, et al. Patterns of lymph node metastasis in carcinoma of the ampulla of Vater. Hepatogastroenterology. (2000) 47:880–3. PMID: 10919052
10. Kayahara M, Nagakawa T, Ohta T, Kitagawa H, Miyazaki I. Surgical strategy for carcinoma of the papilla of Vater on the basis of lymphatic spread and mode of recurrence. Surgery. (1997) 121:611–17. doi: 10.1016/S0039-6060(97)90048-9
11. Shirai Y, Ohtani T, Tsukada K, Hatakeyama K. Patterns of lymphatic spread of carcinoma of the ampulla of Vater. Br J Surg. (1997) 84:1012–16. doi: 10.1002/bjs.1800840734
12. Uchiyama K, Yamamoto M, Yamaue H, Ariizumi S, Aoki T, Kokudo N, et al. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. (2011) 18:443–52. doi: 10.1007/s00534-010-0349-2
13. Schwarz RE, Smith DD. Lymph node dissection impact on staging and survival of extrahepatic cholangiocarcinomas, based on U.S. Population data. J Gastrointest Surg. (2007) 11:158–65. doi: 10.1007/s11605-006-0018-6
14. Verbeke CS, Leitch D, Menon KV, McMahon MJ, Guillou PJ, Anthoney A. Redefining the R1 resection in pancreatic cancer. Br J Surg. (2006) 93:1232–7. doi: 10.1002/bjs.5397
15. Bronsert P, Kohler I, Werner M, Makowiec F, Kuesters S, Hoeppner J, et al. Intestinal-type of differentiation predicts favourable overall survival: confirmatory clinicopathological analysis of 198 periampullary adenocarcinomas of pancreatic, biliary, ampullary and duodenal origin. BMC Cancer. (2013) 13:428. doi: 10.1186/1471-2407-13-428
16. Winter JM, Cameron JL, Olino K, Herman JM, de Jong MC, Hruban RH, et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J Gastrointest Surg. (2010) 14:379–87. doi: 10.1007/s11605-009-1080-7
17. Delcore R, Connor CS, Thomas JH, Friesen SR, Hermreck AS. Significance of tumor spread in adenocarcinoma of the ampulla of Vater. Am J Surg. (1989) 158:593–6. doi: 10.1016/0002-9610(89)90201-8
18. Berger AC, Watson JC, Ross EA, Hoffman JP. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. (2004) 70:235–40. PMID: 15055847
19. Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. (2002) 236:355–66. doi: 10.1097/00000658-200209000-00012
Keywords: ampullary adenocarcinoma, metastasis, lymph node, prognosis, survival
Citation: Zhang Z, Guan J, Wang X, Hao D and Zhou Z (2022) Analysis of lymph node spread and its prognostic significance in ampullary adenocarcinoma: A retrospective study. Front. Surg. 9:901615. doi: 10.3389/fsurg.2022.901615
Received: 22 March 2022; Accepted: 21 July 2022;
Published: 26 August 2022.
Edited by:
Mark Girgis, University of California, United StatesReviewed by:
Claude Bertrand, CHU UCL Namur Site Godinne, BelgiumJae Hoon Lee, University of Ulsan, South Korea
© 2022 Zhang, Guan, Wang, Hao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Di-Si Hao ZHNoYW8xOTY4QHNpbmEuY29t Zun-Qiang Zhou c3VyZ2Vvbl96aG91QDE2My5jb20=
†These two authors contributed equally to this work.
Speciality Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Zheng-Yun Zhang
Zheng-Yun Zhang Jiao Guan1,†
Jiao Guan1,† Xin-Ping Wang
Xin-Ping Wang Zun-Qiang Zhou
Zun-Qiang Zhou