- 1Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2China International Neuroscience Institute (China-INI), Beijing, China
Objective: Arteriovenous fistulas (AVFs) in the craniocervical junction (CCJ) region are a rare occurrence with special clinical manifestations. This study retrospectively reviewed patients with CCJ AVFs treated at our neurosurgical center, aiming to enhance the understanding of CCJ AVFs.
Methods: A total of 113 patients with CCJ AVFs treated at our neurosurgical center between January 2013 and December 2020 were enrolled. They were grouped as patients with CCJ AVFs with spinal arterial feeders (n = 20) and patients with CCJ AVF without spinal arterial feeders (n = 93). Clinical presentation, angiographic characteristics, intraoperative findings, and treatment outcomes were analyzed.
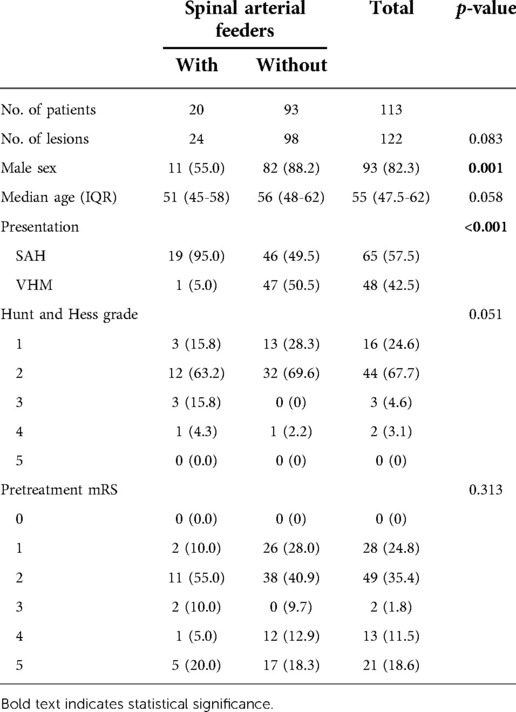
Results: The patients’ median age was 55 years (IQR 47.5–62 years). The proportion of males in the group without spinal arterial feeders was significantly higher (p = 0.001). Subarachnoid hemorrhage (SAH) was the most common clinical presentation, especially in the group with spinal arterial feeders (p < 0.001). There were significant differences in AVF type, fistula location, and direction of the venous drainage between the two groups (p < 0.001). Intervention embolization combined with microsurgery was more common in treating AVFs with spinal arterial feeders (p = 0.006). Spinal arterial feeders did not affect the outcome (p = 0.275).
Conclusions: SAH was the most common presentation of CCJ AVFs in this study. Microsurgery and interventional embolization were optional treatment strategies. The angioarchitecture of CCJ AVFs was essential for selecting treatment strategies.
Introduction
The craniocervical junction (CCJ), which includes the occipital bone and condyles, the atlas, and the axis, has unique and complex vascular and bone anatomy (1, 2). Arteriovenous fistulas (AVFs) in this region have complex anatomy and treatment. CCJ AVFs are rare vascular malformations found in less than 2% of patients with intracranial or spinal AVFs. In addition, this region of AVFs is quite different from other regions of spinal AVFs in terms of clinical characteristics, angioarchitecture, treatment modalities, and outcomes (3–6). In this study, we retrospectively reviewed 113 consecutive patients with CCJ AVFs treated at our neurosurgical center between 2013 and 2020 and divided these patients into two groups for subgroup analysis, i.e., those with and without spinal arterial feeders.
Materials and methods
Patients and follow-up
The study was approved by the medical ethics committee of our hospital. Informed consent was obtained from all patients. The data supporting the findings of this study were available from the corresponding author upon reasonable request.
This retrospective study included 113 patients with CCJ AVFs who were treated at our institution between January 2013 and December 2020. We collected patients’ clinical and radiological data, including baseline patient characteristics (age, sex, presentation), treatment strategies, outcomes [mRS (7) scores before and after treatment], and angiography files in the DICOM data format. SAHs were confirmed by computed tomography (CT) scanning of the head. For patients with Subarachnoid hemorrhage (SAH), we used the Hunt and Hess grading system (8). Venous hypertensive myelopathy (VHM) was defined as spinal cord edema and neurological dysfunction due to increased spinal venous pressure, which could present with motor and sensory dysfunction. AVFs were confirmed by DSA. Treatment strategies included microsurgery and embolization alone or a combination of the two. Intraoperative or postoperative angiography and intraoperative indocyanine green (ICG) angiography were used to confirm the obliteration of the fistula.
According to DSA and intraoperative sighting, each patient was diagnosed by two surgeons (neurosurgeons and neurointerventionalists with 20 years of experience in the field). Hiramatsu's (6) classification method was used with few modifications. In our definition, the location of the arteriovenous shunt was the standard of classification. Dural AVF (DAVF) is defined as the arteriovenous shunt located on the dural mater; radicular AVF (RAVF) is defined as the arteriovenous shunt located on the spinal nerve roots; epidural AVF (EDAVF) is defined as the arteriovenous shunt located outside the dura mater; perimedullary AVF (PAVF) is defined as the arteriovenous shunt located on the surface of the spinal cord.
Follow-up was performed by clinical examination or telephone interview. A total of 102 (90.3%) patients were followed up for at least 3 months, and 96 (85.0%) patients have been followed until now.
Statistical analyses
SPSS 24.0 software (IBM Corp., Armonk, New York, USA) was used for statistical analysis. The χ2 test and the Fisher exact test were used to compare categorical variables of two groups, different clinical presentation groups, and different treatment groups, and the Wilcoxon signed-rank test was used to compare outcomes (mRS scores). Baseline patient characteristics, angiographic findings, and AVF types were assessed for association with SAH. Results are presented as relative risk with 95% CI. The potential risk factors with p < 0.10 on univariate analysis were included as confounders in the multivariate logistic regression model for multivariate analysis to determine whether or not they were risk factors for SAH. The variables of the final model were selected by the forward conditional method. Results are presented as odds ratios with 95% CI. Statistical analyses were two-sided, with a p < 0.05 considered statistically significant.
Results
Clinical characteristics
The clinical characteristics data of two groups of patients are listed in Table 1. There were 93 (82.3%) male CCJ AVF patients, and the proportion of males in the group without spinal arterial feeders was higher (p = 0.001). The median age at presentation of overall patients was 55 years [interquartile range (IQR), 47.5–62 years]. SAH was the most common clinical presentation, especially in the group with spinal arterial feeders (p < 0.001). For patients with SAH, 63 (96.9%) patients presented with mild neurological dysfunction (Hunt and Hess grade 1–3). There was no significant difference in mRS scores between the two groups before the operation (p = 0.313).
Angioarchitecture
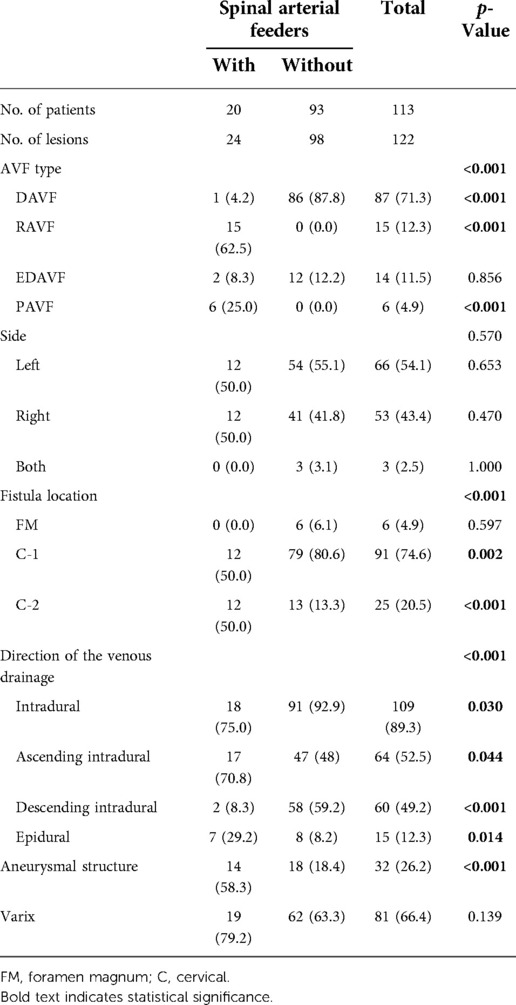
As shown in Table 2, there were 122 fistulas in 113 patients. A total of 104 (92.0%) patients had a single fistula, and 9 (8.0%) patients had dual fistulas. Among the 24 fistulas with spinal arterial feeders, 20 (83.3%) fistulas were fed by the anterior spinal artery (ASA) and 9 (37.5%) fistulas were fed by the lateral spinal artery (LSA). Overall, the most common CCJ AVF type was DAVF, with 87 fistulas (71.3%). The proportion of CCJ AVF type was significantly different among the two groups (p < 0.001). For CCJ AVFs with spinal arterial feeders, the most common CCJ AVF type was RAVF, with 15 (62.5%) fistulas. In contrast, there was no RAVF or PAVF in CCJ AVFs without spinal arterial feeders.
On the side of the lesion, CCJ AVFs with spinal arterial feeders were equally distributed on the left and right, while CCJ AVFs without spinal arterial feeders were more distributed on the left, and three (3.1%) fistulas were fed by both vertebral arteries. CCJ AVFs with spinal arterial feeders showed no tendency in fistula location, while most of the CCJ AVFs without spinal arterial feeders were located at the C-1 level, with 79 (80.6%) fistulas, and 6 (6.1%) fistulas were located at the level of the foramen magnum. There were differences in the location of the fistula between the two groups (p < 0.001).
There were 109 (89.3%) fistulas with intradural drainage and 15 (12.3%) fistulas with epidural drainage. The draining veins of CCJ AVFs without spinal arterial feeders were more inclined to drain to intradural, especially descending intradural (p = 0.030 and p < 0.001, respectively). Aneurysmal structures occurred at 32 (26.2%) fistulas, and CCJ AVFs with spinal arterial feeders seemed more likely to form aneurysmal structures (p < 0.001). Varices of draining veins occurred at 81 (66.4%) fistulas.
Treatment and outcomes
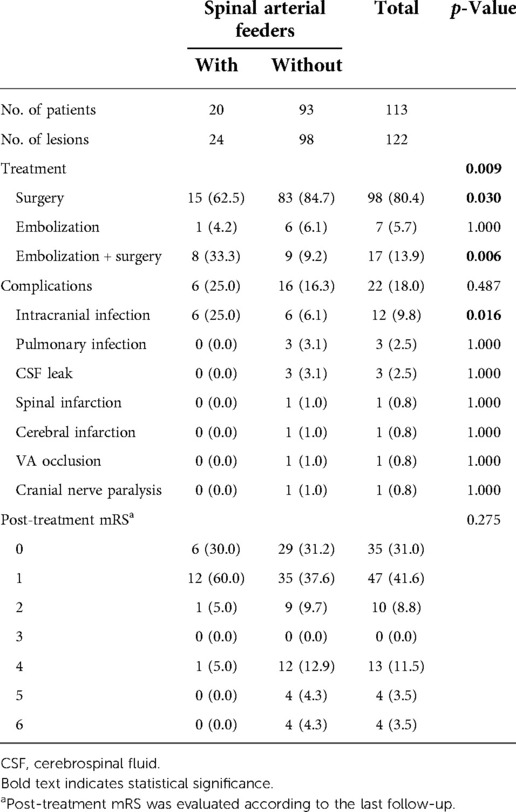
Treatment and outcome information is listed in Table 3. Microsurgery was the most common treatment strategy, as 98 (80.4%) fistulas were cured only by microsurgery. Interventional embolization and microsurgery were used to treat 17 (13.9%) fistulas (Figure 1). There were also differences in the choice of treatment strategies between the two groups (p = 0.009). Microsurgery was more common in the treatment of CCJ AVFs without spinal arterial feeders (p = 0.030) (Figure 2), and interventional embolization combined with microsurgery was more common in the treatment of CCJ AVFs with spinal arterial feeders (p = 0.006). All patients were evaluated by angiography or intraoperative indocyanine green angiography for AVFs disconnected.
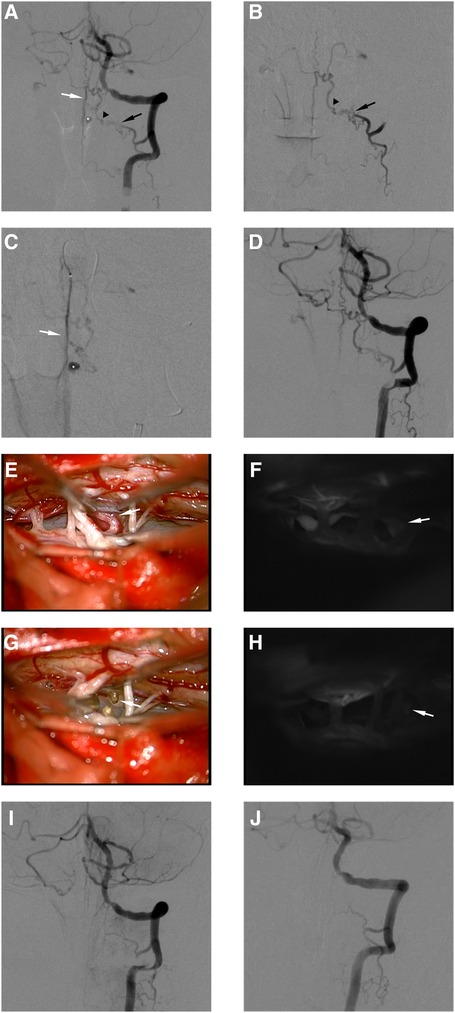
Figure 1. This female RAVF patient presented with SAH and was treated with interventional embolization and microsurgery. Preoperative angiography of left VA (A). Superselective angiography of RA (B). Superselective angiography of ASA (C). The AVF was fed by left C-2 RA (black arrows in A,B) and ASA (white arrows in A,C), and the feeder drained into the intradural vein (black arrowhead in A). The draining vein formed an aneurysmal structure (white asterisks in A,C). Postembolization angiography of left VA (D). We blocked blood supply to the aneurysmal structure by placing a coil in ASA. The intraoperative image showed that the draining vein was closely related to the nerve root (E). Indocyanine green angiography showed that the draining vein drains into the cephalic side (F). The same position of the draining vein was marked (white arrows in E,F). The intraoperative image showed that the draining vein and nerve root were disconnected (G). Indocyanine green angiography showed the disconnection of the AVF (H). The same position of the disconnected draining vein was marked (white arrows in G,H). Postoperative angiography 5 days after microsurgery of left VA (I). Postoperative angiography 3 months after microsurgery of left VA (J).
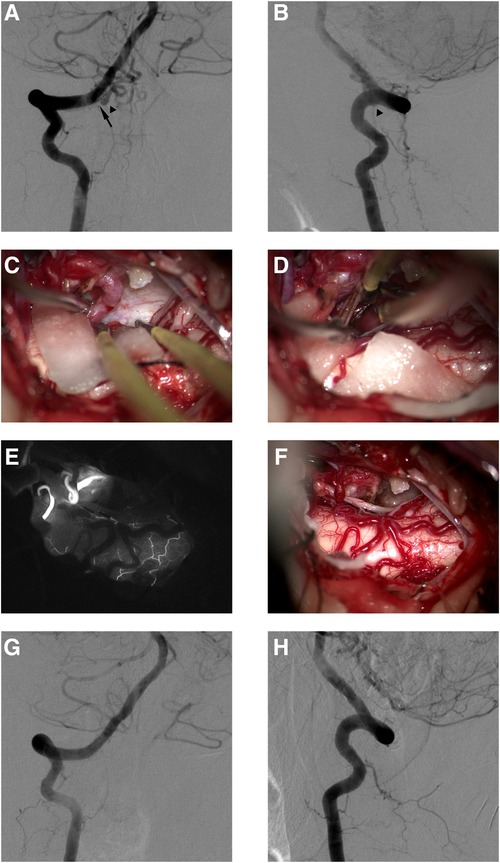
Figure 2. This female DAVF patient presented with VHM and was treated with microsurgery. Preoperative anteroposterior angiography of right VA (A). Preoperative lateral angiography of right RA (B). The AVF was fed by the right C-1 dural branch of VA (black arrow in A), and the feeder drained into the intradural vein (black arrowheads in A,B). The intraoperative image showed that the draining vein drains into the cephalic side (C). The intraoperative image showed that the draining vein was disconnected under the occlusion of the aneurysm clip (D). Intraoperative image (E) and indocyanine green angiography (F) in the same perspective showed the disconnection of the AVF. Postoperative anteroposterior angiography 4 days after microsurgery of right VA (G). Postoperative lateral angiography 4 days after microsurgery of right VA (H).
The incidence of postoperative complications was 18.0%, and there was no significant difference between the two groups. The most common complications were infection, and all infected patients recovered well. Cerebrospinal fluid (CSF) leak occurred in three (2.5%) patients. Spinal infarction occurred in one (0.8%) patient; during microsurgery, AVFs ruptured and bled again. After strict hemostasis, spinal cord infarction occurred, and the patient's lower limb muscle strength decreased. Cerebral infarction occurred in one (0.8%) patient; this patient developed cerebral vasospasm and cerebral infarction on the 8th day after microsurgery, later underwent decompressive craniectomy, and died after 1 month. In one (0.8%) patient, the VA was occluded on the side of the lesion due to treatment requirements, and the patient suffered from intermittent dizziness after occlusion. Cranial nerve paralysis occurred in one (0.8%) patient who presented with numbness of the tongue root.
The median mRS scores of pretreatment and post-treatment were 2 and 1. The mRS scores improved after treatment (p < 0.001). After treatment, 92 (81.4%) patients had good outcomes (mRS scores 0–2). Spinal arterial feeders did not affect the outcome (p = 0.275).
Risk factors associated with SAH
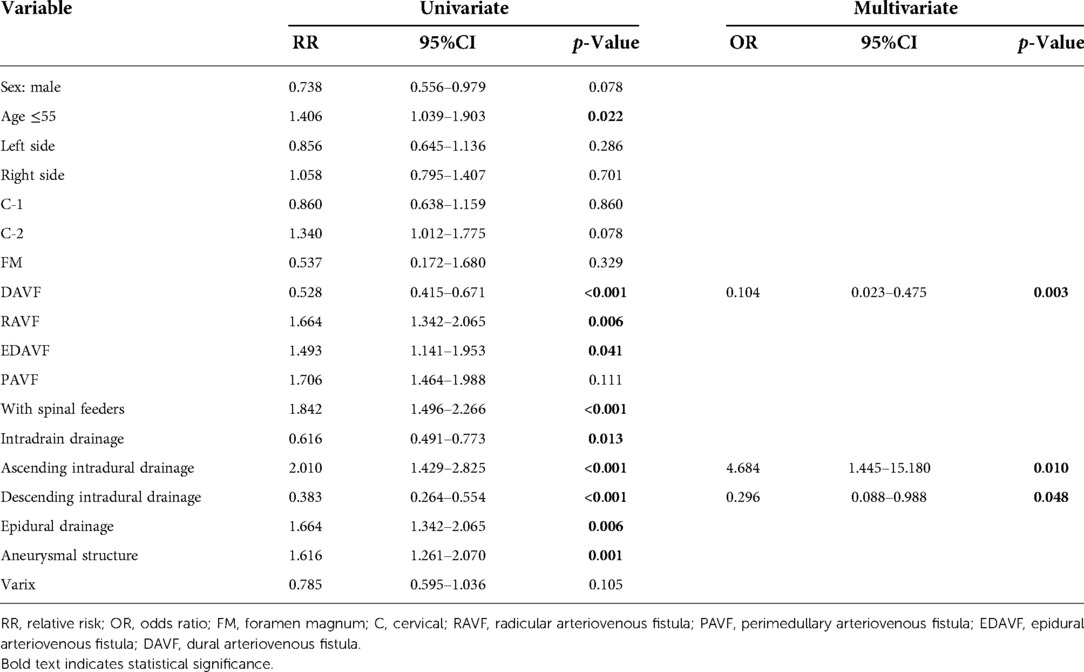
As shown in Table 4, we used univariate and multivariate analyses to identify risk factors associated with SAH. In univariate analysis, risk factors included age ≤55, RAVF, EDAVF, with spinal feeders, ascending intradural drainage, epidural drainage, and aneurysmal structure. After using the forward conditional method in in multivariate analysis, DAVF [odds ratio (OR) 0.104, 95%CI: 0.023–0.475, p = 0.003], ascending intradural drainage (OR: 4.684, 95%CI: 1.445–15.180, p = 0.010), and descending intradural drainage (OR: 0.296, 95%CI: 0.088–0.988, p = 0.048) were significantly associated with SAH.
Discussion
In the previous study, we carefully explored the classification and treatment strategies of CCJ AVFs with spinal arterial feeders using our single-center data (9). In the present study, we divided the CCJ AVFs into two groups according to whether they were with or without spinal arterial feeders to explore the differences between the two groups in clinical presentation and treatment strategies. In addition, we tried to find the risk factors for SAH, hoping to help our colleagues understand and treat CCJ AVFs through the experience of our center.
Clinical characteristics and angioarchitecture
In the previous systematic review, CCJ AVFs often occurred in middle age, and the male-to-female ratio was about 3:1 (5, 10). In the present study, the proportion of male patients was higher than previously reported in the group without spinal arterial feeders and total patients, while in the group with spinal arterial feeders, the proportion of male and female patients was almost equal.
Previous studies have shown that VHM and SAH are the most common clinical presentation of CCJ AVF patients (6, 11, 12). The proportion of the two clinical presentations in the group without spinal arterial feeders was equal. In the group with spinal arterial feeders, the incidence of SAH was significantly higher than that of VHM, which prompted us to explore the risk factors for SAH. In early studies, the direction of venous drainage was often considered a factor of different clinical presentations (5, 10, 13, 14). With a better understanding of the angioarchitecture of CCJ AVFs, aneurysmal structure, arterial feeder of ASA, and even different AVF types (e.g., RAVF) were considered as high-risk factors for SAH (6, 15). Our univariate analysis further confirmed that these were risk factors for SAH. In multivariate analysis, ascending intradural drainage was the most relevant risk factor for SAH. Compared to normal venous flow, the ascending intradural drainage could be regarded as high flow reflux, while descending intradural drainage only increased flow, which should explain the high probability of SAH in CCJ AVFs with ascending intradural drainage. However, with spinal feeders was not considered as a risk factor in multivariate analysis. More studies were needed to explore whether there was correlation between spinal feeders and SAH.
Treatment and outcomes
According to the experience of previous studies, microsurgery and interventional embolization were optional treatment strategies for CCJ AVFs. Nevertheless, like AVFs in other parts of the spinal cord, microsurgery was favored for CCJ AVFs, and it usually resulted in better outcomes (5, 11, 16–19). According to the treatment experience of our center, the angioarchitecture of CCJ AVFs was the key to our choice of treatment strategies.
Simple microsurgical treatment was more appropriate for lesions without spinal feeders and those mainly located in the dorsal or lateral position. According to the dorsal or lateral position of the lesion, the posterior median approach and the far lateral approach were selected. If the lesion only had a single draining vein, we preferred to electrocoagulate and disconnect the draining vein. In treating AVF with multiple arterial feeders and drainage varices, test occlusion and intraoperative ICG fluorescence angiography were necessary to determine the location of the shunt and abnormal blood vessels (11, 20, 21). Compared with interventional embolization, which might lead to false embolism and cause brainstem infarction, microsurgery was often safer and more reliable. For microsurgery in the CCJ region, our center also routinely used intraoperative neurophysiological monitoring to help preserve neurophysiological function. After drainage veins were disconnected, it was necessary to perform ICG fluorescence angiography or intraoperative DSA to ensure that the lesion was completely removed. If intraoperative angiography could not be performed, it was necessary to perform postoperative DSA in time.
For lesions with spinal feeders or those mainly located in the ventral position, the architecture was more complex and often formed aneurysmal structures. In Takai's study, for lesions with an aneurysmal structure or varix, if only the AVF was treated, the aneurysmal structure or varix might spontaneously shrink (22), which might provide a new way for the treatment of such complex CCJ AVF. However, in the absence of additional evidence, we preferred disconnecting the drainage veins and handling the aneurysmal structure. During microsurgery, it is almost impossible to clip the ventral aneurysm or disconnect the ventral drainage vein. Therefore, for these CCJ AVFs, we often performed interventional embolization of abnormal ventral vessels first, as it was relatively safe to embolize near the fistula from the ASA. However, if we chose to embolize from radicular artery, glue might enter the ASA or LSA through radiculopial artery or radiculomedullary artery and cause ischemic events (22–24). Nevertheless, because ASA was very thin and at an acute angle with the vertebral artery, it was often difficult to put the microcatheter in place. For this reason, one patient only received microwire electrocoagulation during interventional therapy. Due to the complex vascular anatomy in this region, adhesive glue was safer and more reliable than nonadhesive glue, and we preferred n-BCA or Glubran 2 (GEM Srl, Viareggio, Italy) to Onyx (Medtronic, CA, USA). In a meta-analysis of outcomes following surgical vs. endovascular treatment of spinal DAVF, Onyx was associated with significantly higher odds of initial failure or late recurrence than n-BCA (OR: 3.87, 95%CI: 1.73–8.68, I2: 0%, p < 0.001) (25). Similarly, n-BCA was also most commonly used in the research of Yoo (26); yet, ischemic events occurred in six (46.2%) patients. Therefore, the prevention of ischemic events during embolization was an important step.
In the present study, the probability of postoperative complications was 18.0% (22/122), and the most common complications were infections. The incidence of intracranial infection was higher in the group with spinal arterial feeders (p = 0.016), which might be related to the long microsurgery time due to the complex angioarchitecture. Notably, in the group with spinal arterial feeders, no patients had infarction-related complications, which might be related to more patients in this group undergoing both interventional embolization and microsurgery. In a recent multicenter study, the complications’ overall incidence was 26% (25/97). Ischemic complications were the most common complications, and the associated risk factors were interventional embolization (OR: 4.3, 95%CI: 1.1–16, p = 0.030) and spinal arterial feeders (OR: 3.8, 95%CI: 1.03–14, p = 0.045) (19, 22). In treating CCJ AVFs with complex angioarchitecture, it was necessary to carefully identify the blood vessels to avoid ischemic events, especially during interventional embolization.
In previous studies, younger age (p = 0.043) and hemorrhagic presentation (p = 0.002) were significant predictors of better outcomes (5). Also, the type of CCJ AVF was a factor affecting the outcome. mRS scores could improve significantly (p = 0.0389) in patients with DAVF (6). A recent study showed that poor outcomes were associated with a pretreatment mRS score ≥3 (OR: 13, 95%CI: 2.7–62, p = 0.001) and complications (OR: 5.8, 95%CI: 1.3–26, p = 0.020), while spinal arterial feeders was not a significant factor (p = 0.64) (19). In the present study, spinal arterial feeders had a greater influence on the clinical presentation and the choice of treatment strategies and had no significant impact on the outcomes.
Study limitations
The present study also has several limitations. First, this was a retrospective single-center study. The experience of our center may not necessarily apply to other centers. Second, this study mainly used mRS scores for outcome ranks, which may not be sufficient to reflect subtle changes. Third, incorrect mRS scores also may occur, especially during telephone interviews. Thus, prospective multicenter studies should be conducted to further confirm these findings and guide clinical treatment.
Conclusions
This study divided CCJ AVFs into two groups according to whether they were with or without spinal arterial feeders. SAH was the most common presentation of CCJ AVFs. Microsurgery and interventional embolization were optional treatment strategies. The angioarchitecture of CCJ AVF was essential to our choice of treatment strategies. As microsurgery was often safer and more reliable, it could be the first treatment choice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the medical ethics committee of Xuanwu Hospital of Capital Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZS wrote the manuscript and arranged ideas; YM revised the manuscript and designed writing ideas; YW followed up with patients and did the statistics; CH analyzed imaging data; GL, PZ, TH, LS, and PH guided the operation and controlled the operation steps; MY analyzed imaging data and performed idea supervision; and HZ performed manuscript and idea supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (award number 82101460).
Acknowledgments
We thank Kun Yang for his support to the statistical part of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rhoton AL Jr. The foramen magnum. Neurosurgery. (2000) 47(3 Suppl):S155–93. doi: 10.1097/00006123-200009001-00017
2. Martin MD, Bruner HJ, Maiman DJ. Anatomic and biomechanical considerations of the craniovertebral junction. Neurosurgery. (2010) 66(3 Suppl):2–6. doi: 10.1227/01.NEU.0000365830.10052.87
3. Jellema K, Tijssen CC, van Gijn J. Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain. (2006) 129(Pt 12):3150–64. doi: 10.1093/brain/awl220
4. Hiramatsu M, Sugiu K, Hishikawa T, Haruma J, Tokunaga K, Date I, et al. Epidemiology of dural arteriovenous fistula in Japan: analysis of Japanese registry of neuroendovascular therapy (JR-NET2). Neurol Med Chir (Tokyo). (2014) 54(1):63–71. doi: 10.2176/nmc.st.2013-0172
5. Wang JY, Molenda J, Bydon A, Colby GP, Coon AL, Tamargo RJ, et al. Natural history and treatment of craniocervical junction dural arteriovenous fistulas. J Clin Neurosci. (2015) 22(11):1701–7. doi: 10.1016/j.jocn.2015.05.014
6. Hiramatsu M, Sugiu K, Ishiguro T, Kiyosue H, Sato K, Takai K, et al. Angioarchitecture of arteriovenous fistulas at the craniocervical junction: a multicenter cohort study of 54 patients. J Neurosurg. (2018) 128(6):1839–49. doi: 10.3171/2017.3.JNS163048
7. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. (1988) 19(5):604–7. doi: 10.1161/01.str.19.5.604
8. Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. (1968) 28(1):14–20. doi: 10.3171/jns.1968.28.1.0014
9. Song Z, Ma Y, Hong T, Zhang H. Arteriovenous fistulas fed by spinal arterial feeders at the craniocervical junction region. Oper Neurosurg (Hagerstown). (2022) 23(6):472–81. doi: 10.1227/ons.0000000000000390
10. Zhao J, Xu F, Ren J, Manjila S, Bambakidis NC. Dural arteriovenous fistulas at the craniocervical junction: a systematic review. J Neurointerv Surg. (2016) 8(6):648–53. doi: 10.1136/neurintsurg-2015-011775
11. Zhong W, Zhang J, Shen J, Su W, Wang D, Zhang P, et al. Dural arteriovenous fistulas at the craniocervical junction: a series case report. World Neurosurg. (2019) 122:e700–12. doi: 10.1016/j.wneu.2018.10.124
12. Goto Y, Hino A, Shigeomi Y, Oka H. Surgical management for craniocervical junction arteriovenous Fistula targeting the intradural feeder. World Neurosurg. (2020) 144:e685–92. doi: 10.1016/j.wneu.2020.09.041
13. Aviv RI, Shad A, Tomlinson G, Niemann D, Teddy PJ, Molyneux AJ, et al. Cervical dural arteriovenous fistulae manifesting as subarachnoid hemorrhage: report of two cases and literature review. AJNR Am J Neuroradiol. (2004) 25(5):854–8.15140735
14. Kai Y, Hamada J-i, Morioka M, Yano S, Mizuno T, Kuratsu J-i. Arteriovenous fistulas at the cervicomedullary junction presenting with subarachnoid hemorrhage: six case reports with special reference to the angiographic pattern of venous drainage. AJNR Am J Neuroradiol. (2005) 26(8):1949–54.16155140
15. Onda K, Yoshida Y, Watanabe K, Arai H, Okada H, Terada T. High cervical arteriovenous fistulas fed by dural and spinal arteries and draining into a single medullary vein: report of 3 cases. J Neurosurg Spine. (2014) 20(3):256–64. doi: 10.3171/2013.11.SPINE13402
16. Fujimoto S, Takai K, Nakatomi H, Kin T, Saito N. Three-dimensional angioarchitecture and microsurgical treatment of arteriovenous fistulas at the craniocervical junction. J Clin Neurosci. (2018) 53:140–6. doi: 10.1016/j.jocn.2018.04.065
17. Firsching R, Kohl J, Skalej M, Beuing O. Resolution of brainstem edema after neurosurgical occlusion of dural arteriovenous fistulas of the craniocervical junction: report of three cases and review. J Neurol Surg A Cent Eur Neurosurg. (2020) 81(1):80–5. doi: 10.1055/s-0039-1688560
18. Day AL, Turkmani AH, Chen PR. Spinal arteriovenous fistulae: surgical management. Handb Clin Neurol. (2017) 143:189–98. doi: 10.1016/b978-0-444-63640-9.00018-7
19. Takai K, Endo T, Seki T, Inoue T, Koyanagi I, Mitsuhara T. Neurosurgical versus endovascular treatment of craniocervical junction arteriovenous fistulas: a multicenter cohort study of 97 patients. J Neurosurg. (2021) 137(2):373–80. doi: 10.3171/2021.10.Jns212205
20. Sorenson TJ, La Pira B, Hughes J, Lanzino G. How I do it: surgical ligation of craniocervical junction dural AV fistulas. Acta Neurochir (Wien). (2017) 159(8):1489–92. doi: 10.1007/s00701-017-3200-6
21. Kanemaru K, Yoshioka H, Hashimoto K, Murayama H, Ogiwara M, Yagi T, et al. Efficacy of intraarterial fluorescence video angiography in surgery for dural and perimedullary arteriovenous fistula at craniocervical junction. World Neurosurg. (2019) 126:e573–9. doi: 10.1016/j.wneu.2019.02.097
22. Takai K, Endo T, Seki T, Inoue T, Koyanagi I, Mitsuhara T. Ischemic complications in the neurosurgical and endovascular treatments of craniocervical junction arteriovenous fistulas: a multicenter study. J Neurosurg. (2022) 137(6):1776–85. doi: 10.3171/2022.3.Jns22341
23. Sato K, Endo T, Niizuma K, Fujimura M, Inoue T, Shimizu H, et al. Concurrent dural and perimedullary arteriovenous fistulas at the craniocervical junction: case series with special reference to angioarchitecture. J Neurosurg. (2013) 118(2):451–9. doi: 10.3171/2012.10.JNS121028
24. Umana GE, Scalia G, Chaurasia B, Fricia M, Passanisi M, Graziano F, et al. Perimedullary arteriovenous fistulas of the craniovertebral junction: a systematic review. J Craniovertebr Junction Spine. (2020) 11(3):157–62. doi: 10.4103/jcvjs.JCVJS_106_20
25. Goyal A, Cesare J, Lu VM, Alvi MA, Kerezoudis P, Brinjikji W, et al. Outcomes following surgical versus endovascular treatment of spinal dural arteriovenous fistula: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. (2019) 90(10):1139–46. doi: 10.1136/jnnp-2019-320648
Keywords: arteriovenous fistula, arteriovenous shunt, craniocervical junction, spinal vascular malformation, subarachnoid hemorrhage
Citation: Song Z, Ma Y, Wang Y, He C, Li G, Zhang P, Hong T, Sun L, Hu P, Ye M and Zhang H (2023) Arteriovenous fistulas in the craniocervical junction region: With vs. without spinal arterial feeders. Front. Surg. 9:1076549. doi: 10.3389/fsurg.2022.1076549
Received: 21 October 2022; Accepted: 21 November 2022;
Published: 6 January 2023.
Edited by:
Albert Sufianov, I.M. Sechenov First Moscow State Medical University, RussiaReviewed by:
Jorge Marcelo Mura, Instituto de Neurocirugía, ChileHidehito Kimura, Kobe University, Japan
© 2023 Song, Ma, Wang, He, Li, Zhang, Hong, Sun, Hu, Ye and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongqi Zhang eHd6aGFuZ2hxQDE2My5jb20= Ming Ye MTM5MTEwMDY1NTFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Abbreviations: AVF, arteriovenous fistula; CCJ, craniocervical junction; SAH, subarachnoid hemorrhage; VHM, venous hypertensive myelopathy; RAVF, radicular arteriovenous fistula; PAVF, perimedullary arteriovenous fistula; EDAVF, epidural arteriovenous fistula; DAVF, dural arteriovenous fistula; RA, radiculomedullary artery; ASA, anterior spinal artery; LSA, lateral spinal artery
 Zihao Song
Zihao Song Yongjie Ma
Yongjie Ma Yinqing Wang
Yinqing Wang Chuan He
Chuan He Guilin Li
Guilin Li Peng Zhang1,2
Peng Zhang1,2 Peng Hu
Peng Hu Ming Ye
Ming Ye Hongqi Zhang
Hongqi Zhang