
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg., 05 October 2021
Sec. Thoracic Surgery
Volume 8 - 2021 | https://doi.org/10.3389/fsurg.2021.747249
 Hsin-Ying Lee1
Hsin-Ying Lee1 Min-Shu Hsieh2
Min-Shu Hsieh2 Hsien-Chi Liao3
Hsien-Chi Liao3 Pei-Hsing Chen4
Pei-Hsing Chen4 Xu-Heng Chiang4
Xu-Heng Chiang4 Kuan-Chuan Tsou5
Kuan-Chuan Tsou5 Tung-Ming Tsai6
Tung-Ming Tsai6 Jen-Hao Chuang6
Jen-Hao Chuang6 Mong-Wei Lin7*
Mong-Wei Lin7* Hsao-Hsun Hsu7
Hsao-Hsun Hsu7 Jin-Shing Chen6,7
Jin-Shing Chen6,7Background: As the overall survival of patients with cancer continues to improve, the incidence of second primary malignancies seems to be increasing. Previous studies have shown controversial results regarding the survival of patients with primary lung cancer with previous extrapulmonary malignancies. This study aimed to determine the clinical picture and outcomes of this particular subgroup of patients.
Materials and Methods: We included 2,408 patients who underwent pulmonary resection for primary lung cancer at our institute between January 1, 2011 and December 30, 2017 in this retrospective study. Medical records were extracted and clinicopathological parameters and postoperative prognoses were compared between patients with lung cancer with and without previous extrapulmonary malignancies.
Results: There were 200 (8.3%) patients with previous extrapulmonary malignancies. Breast cancer (30.5%), gastrointestinal cancer (17%), and thyroid cancer (9%) were the most common previous extrapulmonary malignancies. Age, sex, a family history of lung cancer, and preoperative carcinoembryonic antigen levels were significantly different between the two groups. Patients with previous breast or thyroid cancer had significantly better overall survival than those without previous malignancies. Conversely, patients with other previous extrapulmonary malignancies had significantly poorer overall survival (p < 0.001). The interval between the two cancer diagnoses did not significantly correlate with clinical outcome.
Conclusion: Although overall survival was lower in patients with previous extrapulmonary malignancies, previous breast or thyroid cancer did not increase mortality. Our findings may help surgeons to predict prognosis in this subgroup of patients with primary lung cancer.
In recent decades, major improvements in cancer treatment, including molecular targeted and immune modulation therapies, have allowed patients to survive long enough to develop subsequent primary malignancies (1). Moreover, advancements in diagnostic approaches, including new imaging techniques and cancer biomarkers, have resulted in earlier and higher detection rates of multiple primary cancers (2, 3). Defined as cancers with more than one independent primary malignancy in the same or different organs, multiple primary cancers may develop in a synchronous or metachronous fashion (4). Although this phenomenon was first described by Billroth (5) in 1889, Cahan et al. (6) in 1969 were the first to report multiple primary cancers involving the lungs. Later, Hofmann et al. (7) demonstrated the incidence of second primary lung cancer at 1.6 per 100,000 population. More recently, Hu et al. (8) identified 178 (5.0%) patients with primary lung cancer undergoing surgery who had different types of previous extrapulmonary malignancies.
While there is no doubt that multiple primary malignancies involving the lungs can occur, the therapeutic strategies and survival outcomes of patients with lung cancer with a history of extrapulmonary malignancies remain controversial. An increased risk of synchronous lung cancer in patients with breast cancer has been reported, suggesting that genetic factors may increase the risk of multiple primary malignancies (9). Additionally, the widespread use of radiotherapy and common risk factors may contribute to an increased risk of second primary lung cancer in patients with a history of head and neck cancer (10–12).
In this study, we aimed to analyze the survival differences among patients with lung cancer with and without previous extrapulmonary malignancies. Additionally, we sought to identify potential risk factors for second primary lung cancer occurrence to develop strategies aimed at prevention and early diagnosis among high-risk individuals.
In this retrospective study, we obtained data from 2,737 patients with newly diagnosed primary lung cancer who underwent pulmonary resection performed by a single surgical team at the National Taiwan University Hospital between January 2011 and December 2017. A history of previous extrapulmonary malignancies was histologically confirmed from patients' medical records. Controversial cases with possible lung metastases were excluded to avoid any misinterpretation. Cases of atypical adenomatous hyperplasia and metachronous second lung cancers were also excluded. Furthermore, patients were excluded if they were not indicated for curative resection or if there was missing data on tumor size, lymph node metastasis, volume of blood loss, duration of hospital stay, and chest tube placement. The final cohort comprised 2,408 patients. Of these, 2,208 patients presented with no previous extrapulmonary malignancies, and 200 presented with previous extrapulmonary malignancies prior to developing primary lung cancer. The studies involving human participants were reviewed and approved by the Institutional Review Board of the National Taiwan University Hospital, Taipei, Taiwan (approval number: 20200411RIND). Written informed consent was waived owing to the retrospective nature of this study.
Basic characteristics, including age, sex, smoking status, Eastern Cooperative Oncology Group (ECOG) performance status, a family history of lung cancer, underlying comorbidities (diabetes mellitus, hypertension, heart disease, and end-stage renal disease), and preoperative findings (serum carcinoembryonic antigen [CEA] level and pulmonary function test) were retrieved from electronic medical records. An abnormal CEA level was defined as ≥5 ng/mL. The surgical method was classified as sublobar resection (wedge resection and segmentectomy) or extensive resection (lobectomy, bilobectomy, and pneumonectomy). The surgical method was determined at the surgeons' discretion and was approved during a weekly multidisciplinary lung cancer meeting.
Patients were monitored in the outpatient clinic using physical examinations, serum CEA measurements, and chest computed tomography (CT) every 6 months for the first 2 years. Thereafter, patients were monitored using similar methods every 6–12 months, according to the physicians' instructions. Brain magnetic resonance imaging/CT, positron emission tomography/bone scan, bronchoscopy, lymph node biopsy, chest ultrasonography, and other tests were performed whenever any symptoms or signs of tumor recurrence were observed.
Information on the tumor size, predominant histological type, degree of differentiation, visceral pleural invasion (VPI), lymphovascular invasion (LVI), pathological T and N stage, number of dissected lymph nodes, number of dissected lymph node stations, and resection margins were collected from pathological reports of preoperative biopsies, intraoperative frozen sections, or postoperative specimens. Malignancies were classified as second primaries when the histological features were distinct or confirmed to be different after immunohistochemical staining. Histopathological patterns were classified according to the 2015 World Health Organization criteria (13). Lung cancer, breast cancer, and thyroid cancer staging were determined based on the eighth edition of the American Joint Committee on Cancer Tumor-Node-Metastasis staging system (14–16).
The time interval between the diagnosis of the two cancers was defined as the time in years between the diagnosis of a previous extrapulmonary malignancy and that of primary lung cancer. When multiple extrapulmonary malignancies were present in one patient, the shortest time interval was adopted.
Extrapulmonary malignancies were divided into seven subgroups: breast cancer, gastrointestinal cancer, thyroid cancer, gynecological cancer, head and neck cancer, genitourinary cancer, and others. Gastrointestinal cancers included colorectal, gastric, pancreatic, gallbladder, and liver cancers; gynecological cancers included endometrial, cervical, and ovarian cancers; genitourinary cancers included renal, urothelial, bladder, and prostate cancers; and others included hematological, central nervous system, soft tissue, and salivary gland cancers. Patients were defined as having multiple previous extrapulmonary malignancies if they had cancer in more than one of these subgroups.
Descriptive statistics are reported as mean ± standard deviation for continuous data and as numbers (percentages) for categorical data. Student's t test was conducted for continuous variables, and the Chi-square test or Fisher's exact test was conducted for categorical variables, depending on the cell size. The Kaplan–Meier method was used to plot survival curves. Statistical comparisons between survival distributions were made using the log-rank test. Multivariate analysis using the Cox proportional hazards model was performed to identify factors associated with 5-year overall survival (OS). All p values were two-sided, and a p < 0.05 was considered statistically significant. The statistical software used for all analyses was SPSS for MAC (version 25.0; SPSS, Chicago, IL, USA).
The study cohort included 2,408 patients with primary lung cancer, of whom 200 (8.3%) had previous extrapulmonary malignancies, and 59 (2.4%) received neoadjuvant therapy. The mean follow-up time was 39.1 ± 23.4 months. Only age, sex, a family history of lung cancer, and preoperative CEA levels were different between the two groups. Patients with previous extrapulmonary malignancies were older (62.0 ± 11.3 vs. 60.3 ± 11.2 years; p = 0.045), predominantly female (71.0 vs. 63.3%; p = 0.030), less likely to have a family history of lung cancer (12.5 vs. 19.7%; p = 0.013), and more likely to have higher preoperative CEA levels (≥ 5 ng/mL: 17.0 vs. 11.0%; p = 0.020) than those with lung cancer only. Patient demographics and clinical characteristics are presented in Table 1.
No significant differences were observed in the pathological features of lung cancer between patients with and without previous extrapulmonary malignancies. The most common histological type in both groups was adenocarcinoma (lung cancer only: 91.5%; lung cancer with previous extrapulmonary malignancies: 88.5%). Among patients with lung cancer with previous extrapulmonary malignancies, 162 (81.0%) had tumors ≤ 3 cm (T1). Detailed information on the pathological features is listed in Table 2.
No significant differences in perioperative outcomes were observed between patients with and without previous extrapulmonary malignancies. Sublobar resection was slightly more common than extensive resection in both groups. This may be because most lung cancers were early-stage cancers. Of the 2,408 patients, only two (0.1%) died during the first 30 postoperative days. Other perioperative outcomes are presented in Table 3.
In the univariate analysis, many factors were significantly associated with poor OS, including older age, male sex, poor performance status (ECOG ≥ 1), smoking status, higher preoperative CEA levels (≥ 5 ng/mL), extensive resection, thoracotomy, non-adenocarcinoma histology, poorly differentiated tumors, VPI, LVI, positive resection margins, more advanced stage (stage II–IV), and type of primary malignancy other than breast or thyroid cancer. However, only poor performance status (ECOG ≥ 1), higher preoperative CEA levels (≥ 5 ng/mL), poorly differentiated tumors, more advanced stage (stage II–IV), and type of primary malignancy other than breast or thyroid cancer were independent factors significantly associated with poor OS in the multivariate analysis (Table 4).
There was a significant difference in OS between patients with and without previous extrapulmonary malignancies. The 5-year OS in patients with lung cancer only was 94.3%, whereas it was 88.8% in patients with previous extrapulmonary malignancies (p = 0.032). After excluding patients with previous breast or thyroid cancer, the difference in 5-year OS between patients with lung cancer only and those with previous extrapulmonary malignancies was even larger (94.3 vs. 81.1%; p < 0.001), as no patients with previous breast or thyroid cancer died during follow-up, and previous breast and thyroid cancers were independent factors associated with better OS (Figure 1).
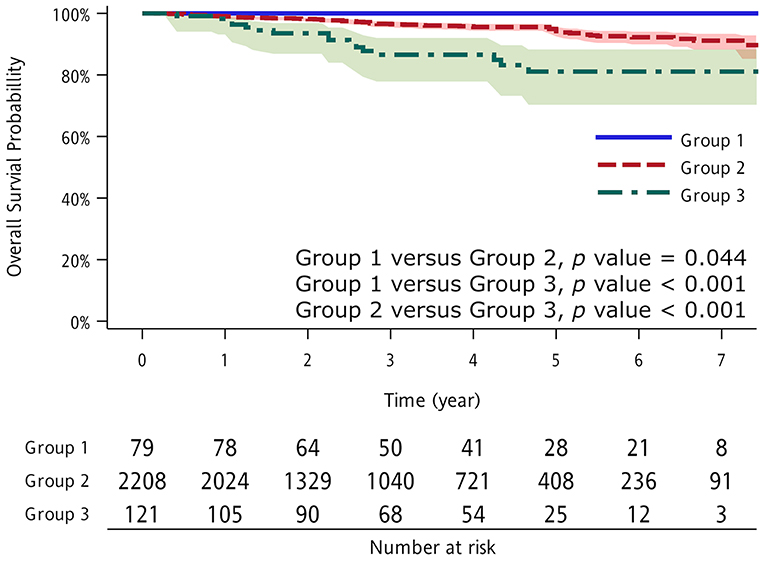
Figure 1. Kaplan–Meier overall survival (OS) curve according to study group. Group 1: thyroid and breast cancer as previous extrapulmonary malignancies; Group 2: no previous extrapulmonary malignancies; and Group 3: other cancers as previous extrapulmonary malignancies.
Among the 200 patients with lung cancer with previous extrapulmonary malignancies, breast, gastrointestinal, and thyroid cancers were the most frequent previous extrapulmonary malignancies. Of the 34 patients with gastrointestinal cancer, 20 had colorectal cancer, six had gastric cancer, five had liver cancer, one had pancreatic cancer, one had gallbladder cancer, and one had a gastrointestinal stromal tumor (Figure 2A). As stated above, previous breast or thyroid cancer was associated with improved survival compared to other previous extrapulmonary malignancies. There were only two patients with stage IV lung cancer and zero pneumonectomy or bilobectomy in this group. Furthermore, by analyzing the available data on the staging of previous extrapulmonary malignancies, majority of the patients present with early breast and thyroid cancer. Conversely, previous gastrointestinal, head and neck, or gynecological cancer was associated with poor survival, with hazard ratios of 3.162 (p = 0.025), 5.017 (p = 0.006), and 6.162 (p < 0.001), respectively (Supplementary Figure 1; Supplementary Tables 1, 3).
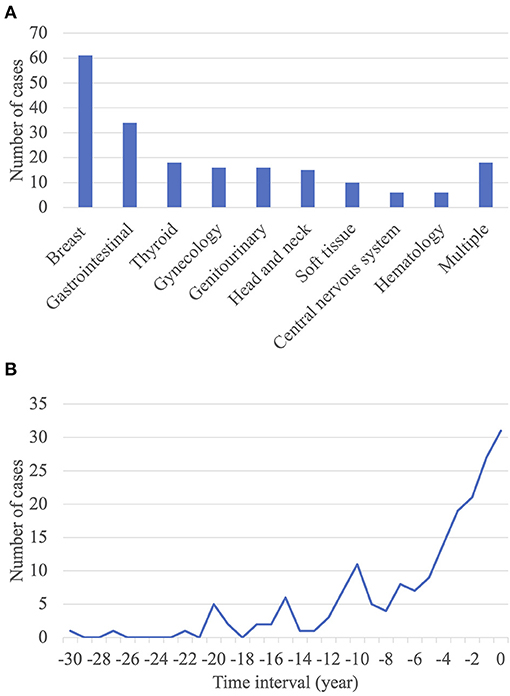
Figure 2. Characteristics of extrapulmonary malignancies. (A) Distribution of occurrence sites of previous extrapulmonary malignancies. (B) Time interval between the diagnosis of previous extrapulmonary malignancies and that of primary lung cancer.
The mean interval between the diagnosis of a previous extrapulmonary malignancy and that of primary lung cancer was 5.4 ± 5.8 years. Approximately half of the patients (n = 112; 56.0%) had an interval of ≤ 5 years (Figure 2B). There was no significant correlation between the interval between tumor diagnoses and OS (Supplementary Table 1).
Lung cancer is the leading cause of cancer mortality in the United States and worldwide (17). The outcomes of patients with lung cancer with previous extrapulmonary malignancies have received much attention in recent decades. However, the impact of previous malignancies on survival and prognosis is still not clearly defined. Our study showed that the incidence of previous extrapulmonary malignancies was 8.3% in patients with primary lung cancer. Several clinicopathological features, including age, sex, a family history of lung cancer, and preoperative CEA levels, were significantly different between the two groups. Furthermore, patients with previous breast or thyroid cancer had significantly better OS than those without previous malignancies. Conversely, patients with other previous extrapulmonary malignancies had significantly poorer OS.
The incidence of previous extrapulmonary malignancies in our study was similar to those reported previously (1–22%) (7, 8, 18–26). Corresponding to an increase in the incidence of multiple primary cancers, a surge of 5.7% in lung cancer as a second primary malignancy was also noted between 1988–1992 and 2011–2014 in the United States National Cancer Institute's Surveillance, Epidemiology, and End Results Program (19). Aside from common risk factors, such as cancer-promoting lifestyle factors and environmental interactions (27), genetic predisposition in the form of germline mutations (28, 29) and the subsequent carcinogenic effects of cancer treatment have been reported as potential mechanisms for the development of multiple primary cancers. Additional lung cancer risk was observed in patients with Hodgkin's lymphoma who were treated with radiotherapy (30, 31). A meta-analysis of 13 studies demonstrated that radiotherapy for breast cancer was significantly associated with a relative risk of 1.22 for second lung cancer (32), with an excess relative risk of 8.5% per Gray (33). Another well-established explanation for the growing incidence of multiple primary tumors is the prolonged survival rates of patients with cancer combined with increased concern over personal health and surveillance among cancer survivors (8).
Supplementary Table 2 reviews several studies that examined clinicopathological features in patients with lung cancer with previous extrapulmonary malignancies. Some reported that patients with previous extrapulmonary malignancies were older (18, 20, 23, 24). We found similar results; in our study, these patients were ~2 years older than those without previous extrapulmonary malignancies. This may be attributable to the longer exposure to carcinogens and greater genetic susceptibility in older individuals (34). These patients also had higher preoperative CEA levels. This marker underlies a distinct characteristic of extra- or intrapulmonary adenocarcinoma among patients with multiple primary malignancies (35). However, smoking habits between the two study subgroups did not differ, which agrees with the mathematical modeling of the Liverpool Lung Project (LLP). By showing a higher impact of cumulative non-smoking risk on lung cancer over smoking alone, additive effects of lung disease and environmental exposures should be recognized in the non-smoking population (36). Another significant feature that contradicted with accepted knowledge is the lower incidence of a family history of lung cancer. This may indicate a different etiology in this subgroup, corresponding with those of previous studies suggesting that the occurrence of multiple malignancies may be multifactorial in origin (20–23, 26). Further studies are warranted to investigate the hereditary contribution toward tumorigenesis in this subgroup.
Exposure to prior cancer treatment may lead to treatment intolerance in subsequent cancers. This affects the surgeons' selection of surgical methods and postoperative management. However, our results showed no differences in preoperative pulmonary reserve or intraoperative surgical margins in patients with and without previous extrapulmonary malignancies. Therefore, there was no need for extra consideration of surgical method selection, tumor approach, or preference for non-intubated airway management. Moreover, surgical blood loss, length of postoperative hospital stay, and chest tube duration did not differ between the two groups, indicating that no special care was needed. This result corresponds with those of several previous studies (23, 25).
Because of the undetermined interference caused by the previous cancer, whether to include patients with multiple primary malignancies in clinical trials remains controversial (37–40). Our results indicate that some previous extrapulmonary malignancies have a negative impact on survival. However, it is noteworthy that prior breast or thyroid cancer resulted in a better outcome. Higher performance status, lower CEA levels, well and moderately differentiated tumors, and early-stage cancers also served as favorable prognostic factors in the multivariate analysis. Broader inclusion of different subgroups of patients with lung cancer in future clinical trials is necessary to expand their authenticity and generalizability. The factors we identified may serve as the eligibility criteria to confirm equality between patients with and without previous extrapulmonary malignancies. Notably, in our study, the interval between the diagnoses of the two cancers was concentrated in the first 5 years and did not significantly impact survival trends. This calls into question the legitimacy of the current practice of excluding prior cancer within 5 years of enrollment and underscores the concerns of excluding specific groups of patients.
Breast cancer was the most common previous extrapulmonary malignancy reported by our and other studies (7, 8, 19, 20, 22, 24, 25). This may have accounted for the higher proportion of female patients in our group with lung cancer with previous extrapulmonary malignancies. According to the Taiwan Cancer Registry, breast and lung cancer have been among the top three most common cancers since 2003. The launch of a national screening program for breast cancer (41) and the recent advocation of low-dose CT (42) have been reported as factors contributing to this phenomenon, leading to greater discovery of both cancers.
Our results should be interpreted in light of several limitations. First, the study cohort only consisted of surgically treated patients. This may have underestimated the overall incidence of lung cancer in patients with previous extrapulmonary malignancies. Nevertheless, as the largest tertiary hospital in Taiwan, one-third of the early lung cancer resections in the country are performed at our institution, which provides an adequate number of samples. Second, as a single-center, retrospective study, our results may not be generalizable to the entire population. Although further studies are required in patients of different ethnic backgrounds, we reduce other possible bias by conducting our research over a short period of time and treat our patients under similar operative protocol. Third, because of the lack of data linkage between hospitals, we are incapable of knowing the status of previous malignancies, if they were evaluated and treated outside our hospital. However, by analyzing the existing documentation in the electronic medical record, we did make an effort to explore the additional relationships between previous cancer staging and lung cancer outcome.
In conclusion, lung cancer was more likely to develop in patients with previous breast, gastrointestinal, or thyroid cancer within 5 years after the first primary malignancy diagnosis. Although OS was lower in patients with previous extrapulmonary malignancies, previous breast or thyroid cancer did not increase mortality. Our findings may help surgeons to predict the prognosis in this subgroup of patients with primary lung cancer, and to arrange follow-up examinations more frequently.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
M-WL, H-HH, J-SC, and M-SH contributed to conception and design of the study. J-HC, H-YL, P-HC, and X-HC organized the database. K-CT, T-MT, and H-CL performed the statistical analysis. H-YL wrote the first draft of the manuscript. X-HC, P-HC, K-CT, and M-WL wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that this study received funding from the Ministry of Science and Technology, Taiwan [grant number: 107-2221-E-002-080-MY3], National Taiwan University Hospital, Taiwan [grant number: NTUH109-S4659] and Taiwan Lung Foundation. The funder had the following involvement with the study: English language editing and article processing fee. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The support of the staff of all departments and institutes of the National Taiwan University Hospital is gratefully acknowledged.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2021.747249/full#supplementary-material
1. Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. (2010) 10:760–74. doi: 10.1038/nrc2947
2. Demandante CG, Troyer DA, Miles TP. Multiple primary malignant neoplasms: case report and a comprehensive review of the literature. Am J Clin Oncol. (2003) 26:79–83. doi: 10.1097/00000421-200302000-00015
3. Rosso S, Terracini L, Ricceri F, Zanetti R. Multiple primary tumours: incidence estimation in the presence of competing risks. Popul Health Metr. (2009) 7:5. doi: 10.1186/1478-7954-7-5
4. Lin MW, Wu CT, Kuo SW, Chang YL, Yang PC. Clinicopathology and genetic profile of synchronous multiple small adenocarcinomas: implication for surgical treatment of an uncommon lung malignancy. Ann Surg Oncol. (2014) 21:2555–62. doi: 10.1245/s10434-014-3642-5
5. Billroth T. Die allgemeine chirurgische pathologie und therapie in 51 vorlesungen: ein handbuch fur studirende und aerzte. Berlin: Auflage (1889).
6. Cahan WG. Multiple primary cancers, one of which is lung. Surg Clin North Am. (1969) 49:323–35. doi: 10.1016/s0039-6109(16)38791-6
7. Hofmann HS, Neef H, Schmidt P. Primary lung cancer and extrapulmonary malignancy. Eur J Cardiothorac Surg. (2007) 32:653–8. doi: 10.1016/j.ejcts.2007.06.024
8. Hu XL, Xu ST, Wang XC, Hou DN, Bao C, Yang D, et al. Lung cancer patients with a previous extra-pulmonary malignancy should not be considered homogeneous: a clinicopathological analysis of 3530 surgical cases. Clin Transl Oncol. (2019) 21:348–54. doi: 10.1007/s12094-018-1933-1
9. Lin EP, Lin CH, Yang CY, Lu TP, Chang SN, Hsiao TH, et al. Population-based cohort study reveals distinct associations between female lung cancer and breast cancer in Taiwan. JCO Clin Cancer Inform. (2018) 2:1–14. doi: 10.1200/CCI.18.00065
10. Budnik J, DeNunzio NJ, Singh DP, Milano MT. Second primary non-small-cell lung cancer after head and neck cancer: a population-based study of clinical and pathologic characteristics and survival outcomes in 3597 patients. Clin Lung Cancer. (2020) 21:195–203. doi: 10.1016/j.cllc.2019.02.017
11. Dikshit RP, Boffetta P, Bouchardy C, Merletti F, Crosignani P, Cuchi T, et al. Risk factors for the development of second primary tumors among men after laryngeal and hypopharyngeal carcinoma. Cancer. (2005) 103:2326–33. doi: 10.1002/cncr.21051
12. Holland JM, Arsanjani A, Liem BJ, Hoffelt SC, Cohen JI, Stevens KR. Second malignancies in early stage laryngeal carcinoma patients treated with radiotherapy. J Laryngol Otol. (2002) 116:190–3. doi: 10.1258/0022215021910500
13. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. (2015) 10:1243–60. doi: 10.1097/JTO.0000000000000630
14. Rami-Porta R, Asamura H, Travis WD, Rusch VW. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. (2017) 67:138–55. doi: 10.3322/caac.21390
15. Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual: Updates to the AJCC Breast TNM Staging System: The 8th Edition. CA: A Cancer Journal for Clinicians. (2017) 67:290–303. doi: 10.3322/caac.21393
16. Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual: Differentiated and Anaplastic Thyroid Carcinoma: AJCC Staging Manual Eighth Edition Changes. CA Cancer J Clin. (2018) 68:55–63. doi: 10.3322/caac.21439
17. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
18. Donin NM, Kwan L, Lenis AT, Drakaki A, Chamie K. Second primary lung cancer in United States cancer survivors, 1992–2008. Cancer Causes Control. (2019) 30:465–75. doi: 10.1007/s10552-019-01161-7
19. Deng L, Harð*ardottír H, Song H, Xiao Z, Jiang C, Wang Q, et al. Mortality of lung cancer as a second primary malignancy: a population-based cohort study. Cancer Med. (2019) 8:3269–77. doi: 10.1002/cam4.2172
20. Shan S, She J, Xue ZQ, Su CX, Ren SX, Wu FY. Clinical characteristics and survival of lung cancer patients associated with multiple primary malignancies. PLoS ONE. (2017) 12:e0185485. doi: 10.1371/journal.pone.0185485
21. Lu MS, Chen MF, Huang YK, Liu HP, Tsai YH. Clinical outcome in lung cancer with a second malignancy: the time sequence matters. Med (Baltimore). (2016) 95:e5203. doi: 10.1097/MD.0000000000005203
22. Reinmuth N, Stumpf P, Stumpf A, Muley T, Kobinger S, Hoffmann H, et al. Characteristics of lung cancer after a previous malignancy. Respir Med. (2014) 108:910–7. doi: 10.1016/j.rmed.2014.02.015
23. Pagès PB, Mordant P, Grand B, Badia A, Foucault C, Dujon A, et al. History of multiple previous malignancies should not be a contraindication to the surgical resection of lung cancer. Ann Thorac Surg. (2013) 95:1000–5. doi: 10.1016/j.athoracsur.2012.11.072
24. Pagès PB, Mordant P, Cazes A, Grand B, Foucault C, Dujon A, et al. Prognosis of lung cancer resection in patients with previous extra-respiratory solid malignancies. Eur J Cardiothorac Surg. (2013) 44:534–8. doi: 10.1093/ejcts/ezt031
25. Quadrelli S, Lyons G, Colt H, Chimondeguy D, Silva C. Lung cancer as a second primary malignancy: increasing prevalence and its influence on survival. Ann Surg Oncol. (2009) 16:1033–8. doi: 10.1245/s10434-008-0296-1
26. Furák J, Troján I, Szöke T, Wolfárd A, Nagy E, Németh I, et al. Lung cancer as a second primary malignant tumor: prognostic values after surgical resection. Interact Cardiovasc Thorac Surg. (2008) 7:50–3. doi: 10.1510/icvts.2007.160846
28. Hisada M, Garber JE, Fung CY, Fraumeni JF Li FP. Multiple primary cancers in families with Li-Fraumeni syndrome. J Natl Cancer Inst. (1998) 90:606–11. doi: 10.1093/jnci/90.8.606
29. Cheung M, Kadariya Y, Talarchek J, Pei J, Ohar JA, Kayaleh OR. Germline BAP1 mutation in a family with high incidence of multiple primary cancers and a potential gene-environment interaction. Cancer Lett. (2015) 369:261–5. doi: 10.1016/j.canlet.2015.09.011
30. van Leeuwen FE, Somers R, Taal BG, van Heerde P, Coster B, Dozeman T, et al. Increased risk of lung cancer, non-Hodgkin's lymphoma, and leukemia following Hodgkin's disease. J Clin Oncol. (1989) 7:1046–58. doi: 10.1200/JCO.1989.7.8.1046
31. van Leeuwen FE, Klokman WJ, Hagenbeek A, Noyon R, van den Belt-Dusebout AW, van Kerkhoff EH, et al. Second cancer risk following Hodgkin's disease: a 20-year follow-up study. J Clin Oncol. (1994) 12:312–25. doi: 10.1200/JCO.1994.12.2.312
32. Grantzau T, Overgaard J. Risk of second non-breast cancer after radiotherapy for breast cancer: a systematic review and meta-analysis of 762,468 patients. Radiother Oncol. (2015) 114:56–65. doi: 10.1016/j.radonc.2014.10.004
33. Grantzau T, Thomsen MS, Væth M, Overgaard J. Risk of second primary lung cancer in women after radiotherapy for breast cancer. Radiother Oncol. (2014) 111:366–73. doi: 10.1016/j.radonc.2014.05.004
34. Aunan JR, Cho WC, Søreide K. The biology of aging and cancer: a brief overview of shared and divergent molecular hallmarks. Aging Dis. (2017) 8:628–42. doi: 10.14336/AD.2017.0103
35. Egan ML, Todd CW. Carcinoembryonic antigen: synthesis by a continuous line of adenocarcinoma cells. J Natl Cancer Inst. (1972) 49:887–9. doi: 10.1093/jnci/49.3.887
36. Bravo-Iñiguez CE, Fox SW, De Leon LE, Tarascio JN, Jaklitsch MT, Jacobson FL. Cumulative nonsmoking risk factors increase the probability of developing lung cancer. J Thorac Cardiovasc Surg. (2019) 158:1248–54.e1. doi: 10.1016/j.jtcvs.2019.04.098
37. Gerber DE, Laccetti AL, Xuan L, Halm EA, Pruitt SL. Impact of prior cancer on eligibility for lung cancer clinical trials. J Natl Cancer Inst. (2014) 106:dju302. doi: 10.1093/jnci/dju302
38. Laccetti AL, Pruitt SL, Xuan L, Halm EA, Gerber DE. Effect of prior cancer on outcomes in advanced lung cancer: implications for clinical trial eligibility and accrual. J Natl Cancer Inst. (2015) 107:djv002. doi: 10.1093/jnci/djv002
39. Pruitt SL, Laccetti AL, Xuan L, Halm EA, Gerber DE. Revisiting a longstanding clinical trial exclusion criterion: impact of prior cancer in early-stage lung cancer. Br J Cancer. (2017) 116:717–25. doi: 10.1038/bjc.2017.27
40. Laccetti AL, Pruitt SL, Xuan L, Halm EA, Gerber DE. Prior cancer does not adversely affect survival in locally advanced lung cancer: a national SEER-Medicare analysis. Lung Cancer. (2016) 98:106–13. doi: 10.1016/j.lungcan.2016.05.029
41. Health Promotion Administration. Ministry of Health and Welfare. Annual report of health promotion administration. Taiwan (2017).
Keywords: clinicopathological feature, extrapulmonary malignancy, overall survival, prognosis, breast cancer, lung resection
Citation: Lee H-Y, Hsieh M-S, Liao H-C, Chen P-H, Chiang X-H, Tsou K-C, Tsai T-M, Chuang J-H, Lin M-W, Hsu H-H and Chen J-S (2021) Previous Extrapulmonary Malignancies Impact Outcomes in Patients With Surgically Resected Lung Cancer. Front. Surg. 8:747249. doi: 10.3389/fsurg.2021.747249
Received: 26 July 2021; Accepted: 06 September 2021;
Published: 05 October 2021.
Edited by:
Hasan Fevzi Batirel, Marmara University, TurkeyReviewed by:
Zeynep Bilgi, Istanbul Medeniyet University Göztepe Prof Dr Süleyman Yalçin City Hospital, TurkeyCopyright © 2021 Lee, Hsieh, Liao, Chen, Chiang, Tsou, Tsai, Chuang, Lin, Hsu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mong-Wei Lin, mwlin@ntu.edu.tw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.