- 1Breast Unit, Department of Surgical Science, Policlinico Tor Vergata University, Rome, Italy
- 2Anatomic Pathology, Department of Experimental Medicine, Policlinico Tor Vergata University, Rome, Italy
- 3Department of Cardiothoracic Anesthesia, Tor Vergata University Hospital, Rome, Italy
- 4Department of Diagnostic Imaging and Interventional Radiology, Molecular Imaging and Radiotherapy, Policlinico Tor Vergata University, Rome, Italy
- 5Section of Gynecology and Obstetrics, Academic Department of Biomedicine and Prevention, University Tor Vergata, Rome, Italy
- 6Division of Thoracic Surgery, Department of Surgical Science, Policlinico Tor Vergata University, Rome, Italy
- 7Plastic Surgery, Great Hormond Hospital for Children NHS Foundation Trust, London, United Kingdom
Breast reconstruction plays a fundamental role in the therapeutic process of breast cancer treatment and breast implants represents the leading breast reconstruction strategy. Breast Implant Associated-Anaplastic Large Cell Lymphoma (BIA-ALCL), locoregional recurrence in the skin flap, and skin flap necrosis are well-known complications following mastectomy and immediate breast reconstruction (IBR). We report a case of locoregional cancer recurrence in the mastectomy flap mimicking BIA-ALCL, in a patient who underwent 6 breast procedures in four facilities across 15 years including immediate breast reconstruction with macrotextured breast implants. Despite the rate and onset of the disease, clinicians should be aware of BIA-ALCL. Due to the risk of false negative results of fine needle aspiration, clinical suspicion of BIA-ALCL should drive clinicians' choices, aside from cytological results. In the present case, surgical capsulectomy of the abnormal periprosthesic tissue revealed locoregional recurrence.
Introduction
Breast reconstruction attained a fundamental role in the therapeutic process of women facing breast cancer on the path to restore the female body image and quality of life (1–3). Regardless of stage and age (4, 5), Breast implants represent the primary breast reconstruction strategy (81.2%) (6).
Despite its popularity, textured breast implants were associated with the onset of Breast Implant Associated-Anaplastic Large Cell Lymphoma (BIA-ALCL), a rare form of T-cell Lymphoma (7). In this perspective, macrotextured breast implants were retired from the market in 2019 due to the risk of the BIA-ALCL (8, 9). BIA-ALCL clinical presentation is represented by unilateral late peri-implant cold seroma containing malignant cells, or less commonly as a mass attached to the breast implants with or without regional lymph node involvement (7). With a median onset of 8 years from implants introduction, BIA-ALCL incidence is difficult to calculate due to the unknown number of women with breast implants worldwide (10).
Another complication of mastectomy and immediate breast reconstruction (IBR) is represented by locoregional recurrence in the skin flap (11). Diversely, skin flap necrosis is a well-known complication after IBR, occurring in 5–30% of cases (12). Meticulous surgical technique is required in order to reduce the risk of this complication (12). Achieving the optimal balance between oncologic outcome and skin necrosis requires a resection radical as possible without compromising the skin flap viability (13).
Herein, we report a case of locoregional breast cancer recurrence in the mastectomy flap mimicking BIA-ALCL, in a patient who underwent prophylactic mastectomy with immediate reconstruction with macrotextured breast implants. The Institutional Review Board PTV: Policlinico Tor Vergata University waived the need for a formal approval for the clinical reports. The work was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from the patient for publication of this case report and the accompanying images. This work is reported by following the CARE guidelines.
Case Report
A 51-years-old G2P1 smoker (31 packs-year) post-menopausal woman was admitted as an outpatient to our facilities with a breast cancer diagnosis in the skin flap of previous mastectomy. Family history was positive for breast cancer (mother) and brain cancer (brother). Past medical history enlisted previous diagnoses of depression under medical treatment and hysterectomy in 1989 due to miscarriage.
Breast history reported a previous lumpectomy for a benign phyllodes tumor in 2006 in another facility. Subsequently, in 2011, in a second different facility and during postoperative follow-up, the patient underwent fine-needle aspiration which was classified as suspicious for malignancy (C4). Wire-guided lumpectomy demonstrated Flat Epithelial Atypia, adenosis, apocrine metaplasia, and ductal papillomatosis.
At the end of the same year, due to the increase of broad microcalcification in the left breast and positive family history, the patient underwent a bilateral prophylactic Nipple Sparing Mastectomy (NSM) with implants of macrotexured Allergan 410 MF 420 g breast implants. Both breast specimens revealed the absence of cancer. Postoperative course reported severe nipple-areola necrosis with full-thickness skin necrosis that required two-time surgical revision and left breast implant substitution. Then, due to the poor aesthetic result, the patient decided to undergo a bilateral prosthesis removal in a third facility and refused further breast reconstruction.
Following the breast implants removal, the patient continued postoperative follow-up. Eventually, in January 2020, the patient went through outpatient evaluation after detecting evidence of left breast mass in the retroareolar residual tissue. Ultrasound guided biopsy revealed the presence of an Invasive ductal carcinoma. After surgical and psychiatric outpatient evaluation, the patient agreed to undergo a bilateral reconstruction. The patient underwernt a left breast lumpectomy plus sentinel lymph node biopsy, a right retroareolar breast tissue remnant removal and a bilateral positioning of breast tissue expander with methylene blue. The left breast mass revealed an invasive ductal carcinoma G1 with a low grade ductal carcinoma in situ. Estrogen receptor (ER) positive 95%, Progesterone receptor (PR) positive 95%, Ki67 10%, and c-Erb-B2 negative score (HER2 score) 0. Sentinel lymph node biopsy revealed four lymph nodes, two of which were proven micrometastatic and 1 with isolated tumor cells. The right breast remnants didn't reveal any breast lesion. The postoperative course was regular and no breast modified Clavien-Dindo ≥2 complications were reported (14).
However, during postoperative follow up 6 months after the operation, the patient reported left breast discomfort, swelling, and green urinary output as seen in a breast implant rupture. Preoperative fine needle aspiration of the seroma didn't demonstrate any atypical cell. Prior surgery, magnetic resonance imaging was not performed due to the previous breast tissue expander insertion. Patient was admitted to the hospital for a left breast tissue expander substitution on July 3rd,2020.
Surgical exploration revealed periprothesic breast tissue thickening and yellow citrine seroma without rupture of breast implant tissue expander, mimicking BIA-ALCL symptoms. During the surgical procedure, periprothesic tissue specimen and periprosthetic fluid effusion were collected and sent to cytological and microbiological evaluation. The periprostetic fluid and periprothesic didn't reveal bacterial colonization, nor the presence of atypical cell (Figure 1). The periprothesic breast tissue revealed several homolateral infiltrative breast cancer recurrence nidus in the fibroadipose tissue, as shown in Figure 2.
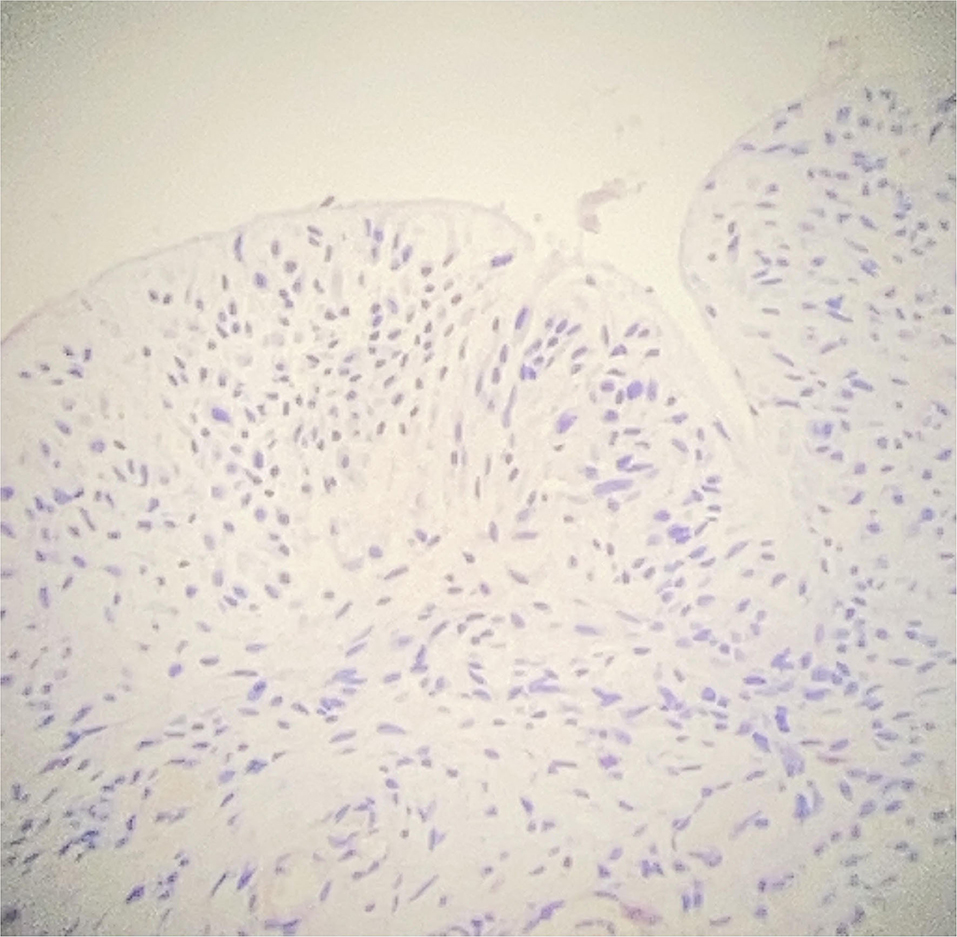
Figure 1. Immunohistochemical (IHC) study of periprothestic staining. CD30 negative staining on periprosthetic capsule (original magnification 10×).
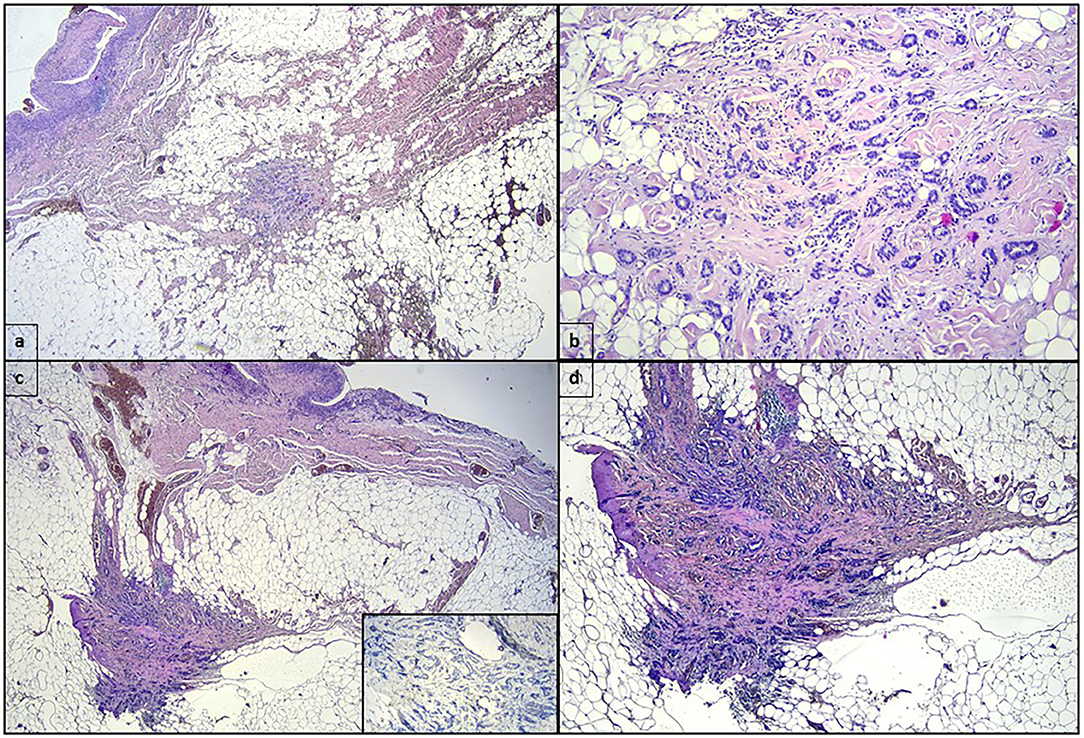
Figure 2. (a) One mm-wide invasive ductal carcinoma in the fibroadipose tissue surrounding the periprosthetic capsule, whose inner surface can be recognized in the upper left of the panel (hematoxylin eosin, original magnification 2×). (b) Higher magnification highlights the invasive ductal carcinoma shown in (a) (hematoxylin eosin, original magnification 10×). (c) Another section of the fibroadipose tissue surrounding the periprosthetic capsule showing a second focus of invasive ductal carcinoma 2 millimeters-wide. The insert shows the absence of myoepithelial layer in the ductal structures (hematoxylin eosin, original magnification 2×; immunostaining for p63, clone Leica, 10× in the insert). (d) Higher magnification of invasive ductal carcinoma showed in (c) (hematoxylin eosin, original magnification 4×).
Discussion
The postoperative onset of BIA-ALCL is an emerging problem in breast reconstructive surgery (7, 15). Breast cancer patients may experience increased levels of anxiety and or depression (16, 17). Media coverage could have given rise to anxiety in breast cancer patients who are planned to undergo implant-based reconstruction (15). Common clinical symptoms include abrupt late cold seroma or periprothestic mass after several years (7–10) from breast implant insertion (7, 9) with a higher reported rate of occurrence in patients with macrotexured implants (8). With a median onset of 8 years from implants introduction, BIA-ALCL cases has been reported even after macrotextured breast implant substitution with smooth prosthesis (7). Despite the low incidence of the disease, in our clinical case, positive clinical history for macrotexured Allergan 410 MF 420 g breast implant 9 years previously and early intraoperative abnormal thickness of periprothesic tissue were consistent with BIA-ALCL diagnosis.
BIA-ALCL current management consists of breast ultrasound and magnetic resonance imaging (10). Ultrasound represents the first choice for evaluating effusion volumes, breast mass dimensions and for obtaining a large amount of fluid (at least 10 mL, but ideally 50 ml) (18, 19). Magnetic resonance imaging provides useful information as the presence of a mass or axillary lymphadenopathy (18). In our clinical case, magnetic resonance imaging was not performed due to device manufacturers recommendation (20). Despite the increased awareness due to media coverage in recent years (15), current imaging appears suboptimal in BIA-ALCL detection (18) and requires assessment in a tertiary breast cancer facility (21, 22). Correct evaluation of late seroma is challenging (19, 23). In literature at least a case of BIA-ALCL with negative aspiration cytology is reported in literature (23). Conversely, up to 0.8% patients with macrotextured breast implants could develop seroma during the follow up period (24), and it was calculated that <5% of delayed seromas may represent BIA-ALCL (23).
However, an additional complication of mastectomy plus IBR, as seen in our patient, is represented by locoregional recurrence of breast cancer (11). Clinicians should aspire to maintain the rate of locoregional recurrence below 5%, utilizing post-mastectomy radiotherapy (PMRT) (25).
Lymphatic spread, metastasis caused by tumor seeding, and incomplete tumor removal are described in the literature as a cause of locoregional recurrence following mastectomy (26). However, another cause of locoregional recurrence may be linked to residual breast tissue in the superficial margins, which could potentially promote metachronous breast cancer (27). Breast carcinoma in residual breast tissue following prophylactic bilateral procedure is described in the literature and the personal risk appears to be linked with familial and personal history, as recorded in our patient (26, 28). Moreover, several cases of Nipple discharge in women with previous NSM with or without pregnancy are reported in the literature supporting this theory (29, 30).
In our clinical case, several different breast surgical procedures in different facilities could have partially affected the risk of locoregional recurrence. Kesson et al. reported a reduction of 18% in breast cancer mortality at 5 years and 11% in all-cause mortality at 5 years when patients were treated by a multidisciplinary team (31). The modern approach of breast treatment and follow up is headed by the knowledge of breast cancer etiology (32, 33) and the subsequent development of systemic tailored strategy in a multidisciplinary setting (4, 31).
In this particular clinical case, the patient underwent six breast procedures in four facilities across 15 years and in our opinion, depression may have played a pivotal role in the decision making process. Depression is a well-known risk factor for poor medication-taking behavior, adherence to treatment and reduced survivorship among breast cancer patients (34). To avoid any detrimental effect on the clinical outcome, we routinely offer a preoperative psychological evaluation for any breast cancer patient. In addition to reduced patient mortality, treatment in a centralized tertiary facility reduces the number of unnecessary interventions (35), reduces the hospitalization of patients (36–38), and provides a lower rate of complications during reconstruction surgery (1) or innovative approach to reduce the impact of surgery (36, 39). In our opinion, tertiary breast cancer facility could produce a higher medical standard for patients and could provide a better medical treatment for breast pathology.
Conclusion
The postoperative onset of BIA-ALCL is an emerging problem in breast reconstructive surgery (7, 15). Despite the low incidence and late onset of the disease, clinicians should be aware of this rare entity. Due to the risk of false negative results of fine needle aspiration, surgeons should carefully perform surgical exploration to avoid under treatment and misdiagnosis. Clinical suspicion of BIA-ALCL should drive clinicians' choices, aside from cytological results. In the present case, surgical capsulectomy of the abnormal periprosthesic tissue revealed locoregional recurrence. Psychiatric disorder represents a risk factor for adherence to treatment and reduced survivorship. Psychological assessment should be routinely provided in tertiary breast cancer facilities.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
MM and MP prepared the manuscript. MC, FS, AD, and CD acquired the data. EG, FS, and LA performed histological examination. VB, FT, and AS performed data analysis. AF, CP, TP, and MP performed interpretation of external imaging. RM, GV, and OB performed review of the literature. All authors contributed to the article and approved the submitted version.
Funding
This study was found with the non-conditional contribution of the Italian Ministry of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely acknowledge all the health care workers involved in the COVID-19 pandemic.
References
1. Bielli A, Bernardini R, Varvaras D, Rossi P, Di Blasi G, Petrella G, et al. Characterization of a new decellularized bovine pericardial biological mesh: structural and mechanical properties. J Mech Behav Biomed Mater. (2018) 78:420–6. doi: 10.1016/j.jmbbm.2017.12.003
2. Buonomo OC, Varvaras D, Montuori M, Vanni G, Venditti D, Elia S, et al. One-stage immediate implant-based breast reconstruction, using biological matrices after conservative mastectomies: preliminary experience of the University Hospital of Tor Vergata, Rome. Chir. (2015) 28:221–6.
3. Galimberti V, Vicini E, Corso G, Morigi C, Fontana S, Sacchini V, et al. Nipple-sparing and skin-sparing mastectomy: review of aims, oncological safety and contraindications. Breast. (2017) 34:S82–S4. doi: 10.1016/j.breast.2017.06.034
4. Buonomo OC, Grasso A, Pistolese CA, Anemona L, Portarena I, Meucci R, et al. Evaluation of concordance between histopathological, radiological and biomolecular variables in breast cancer neoadjuvant treatment. Anticancer Res. (2020) 40:281–6. doi: 10.21873/anticanres.13950
5. Sada A, Day CN, Hoskin TL, Degnim AC, Habermann EB, Hieken TJ. Mastectomy and immediate breast reconstruction in the elderly: trends and outcomes. Surgery. (2019) 166:709–14. doi: 10.1016/j.surg.2019.05.055
6. American Society of Plastic surgery. 2018 Plastic Surgery Statistics Report. (2018). Available online at: www.PlasticSurgery.org (Accessed April 2, 2020).
7. Mempin M, Hu H, Chowdhury D, Deva A, Vickery K. The A, Bm and c's of silicone breast implants: anaplastic large cell lymphoma, biofilm and capsular contracture. Mater (Basel, Switzerland). (2018) 11:2393. doi: 10.3390/ma11122393
8. De Boer M, Van Leeuwen FE, Hauptmann M, Overbeek LIH, De Boer JP, Hijmering NJ, et al. Breast implants and the risk of anaplastic large-cell lymphoma in the breast. JAMA Oncol. (2018) 4:335–341. doi: 10.1001/jamaoncol.2017.4510
9. Groth KA, Graf R. Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) and the textured breast implant crisis. Aesthetic Plast Surg. (2019) 44:1–12. doi: 10.1007/s00266-019-01521-3
10. Marra A, Viale G, Pileri SA, Pravettoni G, Viale G, De Lorenzi F, et al. Breast implant-associated anaplastic large cell lymphoma: a comprehensive review. Cancer Treat Rev. (2020) 84:101963. doi: 10.1016/j.ctrv.2020.101963
11. Abe O, Abe R, Enomoto K, Kikuchi K, Koyama H, Masuda H, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. (2005) 366:2087–106. doi: 10.1016/S0140-6736(05)67887-7
12. Robertson SA, Jeevaratnam JA, Agrawal A, Cutress RI. Mastectomy skin flap necrosis: challenges and solutions. Breast Cancer Targets Ther. (2017) 9:141–52. doi: 10.2147/BCTT.S81712
13. Marta GN, Poortmans P, de Barros AC, Filassi JR, Freitas-Junior R, Audisio RA, et al. Reply to: mastectomy skin flap thickness. Eur J Surg Oncol. (2018) 44:1119–20. doi: 10.1016/j.ejso.2018.04.009
14. Panhofer P, Ferenc V, Schütz M, Gleiss A, Dubsky P, Jakesz R, et al. Standardization of morbidity assessment in breast cancer surgery using the Clavien Dindo Classification. Int J Surg. (2014) 12:334–9. doi: 10.1016/j.ijsu.2014.01.012
15. O'Neill AC, Zhong T, Hofer SOP. Implications of breast implant-associated anaplastic large cell lymphoma (bia-alcl) for breast cancer reconstruction: an update for surgical oncologists. Ann Surg Oncol. (2017) 24:3174–179. doi: 10.1245/s10434-017-6014-0
16. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin North Am. (2017) 101:1099–113. doi: 10.1016/j.mcna.2017.06.005
17. Vanni G, Materazzo M, Pellicciaro M, Ingallinella S, Rho M, Santori F, et al. Breast cancer and COVID - 19: the effect of fear on patients' decision-making process. In vivo (Brooklyn). (2020) 34:1651–9. doi: 10.21873/invivo.11957
18. Adrada BE, Miranda RN, Gaiane ∙, Rauch M, Arribas E, Kanagal-Shamanna R, et al. Breast implant-associated anaplastic large cell lymphoma: sensitivity, specificity, and findings of imaging studies in 44 patients. Breast Cancer Res Treat. (2014) 147:1–4. doi: 10.1007/s10549-014-3034-3
19. Di Napoli A, Pepe G, Giarnieri E, Cippitelli C, Bonifacino A, Mattei M, et al. Cytological diagnostic features of late breast implant seromas: from reactive to anaplastic large cell lymphoma. PLoS ONE. (2017) 12:e0181097. doi: 10.1371/journal.pone.0181097
20. Marano AA, Henderson PW, Prince MR, Dashnaw SM, Rohde CH. Effect of MRI on breast tissue expanders and recommendations for safe use. J Plast Reconstr Aesthetic Surg. (2017) 70:1702–7. doi: 10.1016/j.bjps.2017.07.012
21. Ministero della Salute Direzione Generale Dei Dispositivi Medici e del Servizio Farmaceutico. Protesi mammarie testurizzate e Linfoma Anaplastico a Grande Cellule. Roma: Ulteriori indicazioni del Ministero della Salute.
22. Cardoso MJ, Wyld L, Rubio IT, Leidenius M, Curigliano G, Cutuli B, et al. EUSOMA position regarding breast implant associated anaplastic large cell lymphoma (BIA-ALCL) and the use of textured implants. Breast. (2019) 44:90–3. doi: 10.1016/j.breast.2019.01.011
23. Ghosh T, Duncavage E, Mehta-Shah N, McGuire PA, Tenenbaum M, Myckatyn TM. A cautionary tale and update on breast implant–associated anaplastic large cell lymphoma (BIA-ALCL). Aesthetic Surg J. (2020) 40:1288–300. doi: 10.1093/asj/sjz377
24. Spear SL, Murphy DK, Barbara S. Natrelle round silicone breast implants: core study results at 10 years. Plast Reconstr Surg. (2014) 133:1354. doi: 10.1097/PRS.0000000000000021
25. Hehr T, Baumann R, Budach W, Duma MN, Dunst J, Feyer P, et al. Radiotherapy after skin-sparing mastectomy with immediate breast reconstruction in intermediate-risk breast cancer: Indication and technical considerations. Strahlentherapie und Onkol. (2019) 195:949–63. doi: 10.1007/s00066-019-01507-9
26. Griepsma M, De Roy Van Zuidewijn DBW, Grond AJK, Siesling S, Groen H, De Bock GH. Residual breast tissue after mastectomy: how often and where is it located? Ann Surg Oncol. (2014) 21:1260–6. doi: 10.1245/s10434-013-3383-x
27. Cao D, Tsangaris TN, Kouprina N, Wu LS-F, Balch CM, Vang R, et al. The superficial margin of the skin-sparing mastectomy for breast carcinoma: factors predicting involvement and efficacy of additional margin sampling. Ann Surg Oncol. (2008) 15:1330–40. doi: 10.1245/s10434-007-9795-8
28. Willemsen HW, Kaas R, Peterse JH, Rutgers EJT. Breast carcinoma in residual breast tissue after prophylactic bilateral subcutaneous mastectomy. Eur J Surg Oncol. (1998) 24:331–2. doi: 10.1016/S0748-7983(98)80018-8
29. Orzalesi L, Aldrovandi S, Calabrese C, Casella D, Brancato B, Cataliotti L. Nipple discharge after nipple-sparing mastectomy: should the areola complex always be removed? Clin Breast Cancer. (2011) 11:270–2. doi: 10.1016/j.clbc.2011.02.003
30. Tang R, Kelly BN, Smith BL, Lanahan CR, Brown CL, Gadd MA, et al. Nipple discharge after nipple-sparing mastectomy with and without associated pregnancy. Clin Breast Cancer. (2019) 19:e534–e9. doi: 10.1016/j.clbc.2019.03.003
31. Kesson EM, Allardice GM, George WD, Burns HJG, Morrison DS. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13 722 women. BMJ. (2012) 344: doi: 10.1136/bmj.e2718
32. Buonomo OC, Caredda E, Portarena I, Vanni G, Orlandi A, Bagni C, et al. New insights into the metastatic behavior after breast cancer surgery, according to well-established clinicopathological variables and molecular subtypes. PLoS ONE. (2017) 12:e0184680. doi: 10.1371/journal.pone.0184680
33. Sihto H, Lundin J, Lundin M, Lehtimäki T, Ristimäki A, Holli K, et al. Breast cancer biological subtypes and protein expression predict for the preferential distant metastasis sites: a nationwide cohort study. (2011). doi: 10.1186/bcr2944
34. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. (2000) 160:2101–7. doi: 10.1001/archinte.160.14.2101
35. Pistolese CA, Lamacchia F, Tosti D, Anemona L, Ricci F, Censi M, et al. Reducing the number of unnecessary percutaneous biopsies: the role of second opinion by expert breast center radiologists. Anticancer Res. (2020) 40:939–50. doi: 10.21873/anticanres.14027
36. Vanni G, Materazzo M, Perretta T, Meucci R, Anemona L, Buonomo C, et al. Impact of awake breast cancer surgery on postoperative lymphocyte responses. In Vivo (Brooklyn). (2019) 33:1879–1884. doi: 10.21873/invivo.11681
37. Vanni G, Pellicciaro M, Materazzo M, Dauri M, D'angelillo RM, Buonomo C, et al. Awake breast cancer surgery: strategy in the beginning of COVID-19 emergency. Breast Cancer. (2020) 28:137–44. doi: 10.1007/s12282-020-01137-5
38. Buonomo O, Granai A, Felici A, Piccirillo R, De Liguori Carino N, Guadagni F, et al. Day-surgical management of ductal carcinoma in situ (dcis) of the breast using wide local excision with sentinel node biopsy. Tumori J. (2002) 88:S48–S9. doi: 10.1177/030089160208800342
Keywords: breast implant associated-anaplastic large cell lymphoma, breast cancer, locoregional recurrence, macro textured breast implants, residual breast tissue, case report, breast seroma, immediate breast reconstruction
Citation: Materazzo M, Vanni G, Pellicciaro M, Anemona L, Servadei F, Giacobbi E, Farinaccio A, Pistolese CA, Perretta T, Chiocchi M, Bruno V, Tacconi F, Sadri A, De Majo A, Di Pasquali C, Meucci R, Santori F, Cotesta M and Buonomo OC (2021) Case Report: Early Breast Cancer Recurrence Mimicking BIA-ALCL in a Patient With Multiple Breast Procedures. Front. Surg. 8:606864. doi: 10.3389/fsurg.2021.606864
Received: 15 September 2020; Accepted: 18 February 2021;
Published: 09 March 2021.
Edited by:
Charles Malata, Anglia Ruskin University, United KingdomReviewed by:
Marzia Salgarello, Catholic University of the Sacred Heart, ItalyBenedetto Ielpo, Hospital del Mar, Spain
Copyright © 2021 Materazzo, Vanni, Pellicciaro, Anemona, Servadei, Giacobbi, Farinaccio, Pistolese, Perretta, Chiocchi, Bruno, Tacconi, Sadri, De Majo, Di Pasquali, Meucci, Santori, Cotesta and Buonomo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Pellicciaro, bWFyY29wZWxsNjJAZ21haWwuY29t
 Marco Materazzo
Marco Materazzo Gianluca Vanni
Gianluca Vanni Marco Pellicciaro
Marco Pellicciaro Lucia Anemona2
Lucia Anemona2 Erika Giacobbi
Erika Giacobbi Andrea Farinaccio
Andrea Farinaccio Amir Sadri
Amir Sadri Camilla Di Pasquali
Camilla Di Pasquali Oreste Claudio Buonomo
Oreste Claudio Buonomo