- 1Department of Urology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
- 2Department of Urology, Baoji Central Hospital, Baoji, Shaanxi, China
Zinner’s syndrome (ZS) is a rare congenital malformation due to abnormal development of the urogenital tract. It is characterized by the triad of unilateral renal agenesis, ipsilateral seminal vesicle cyst and ipsilateral ejaculatory duct obstruction. Cases are rarely reported in China since the incidence of the disease is low. Symptoms also vary widely among patients and its etiology is unclear. In this article, we described two patients with totally different cinicopathological and genetic features based on exon sequencing.
Introduction
Zinner’s syndrome is a triad of mesonephric duct anomalies comprising unilateral renal agenesis, seminal vesicle cyst, and ejaculatory duct obstruction. It is a rare urogenital congenital developmental anomaly that occurs in early embryogenesis and affects the distal portion of the Wolffian duct (1). The syndrome usually presents mainly in the second and third decade of life (after the beginning of sexual activity) and is accompanied by prostatitis, painful ejaculation, hematospermia, perineal pain or discomfort, and sometimes infertility (2, 3). Due to the non-specificity of clinical symptoms, there are difficulties in the diagnosis of the disease, which also cause obstacles to the selection of correct treatment measures for Zinner’s syndrome. Therefore, accumulating experience in diagnosis and treatment of the disease is beneficial to the patient group. This paper introduces two patients with Zinner’s syndrome, one of whom was found to have abnormal development of one side of the kidney during the prenatal examination, while the other had no additional special symptoms except the abnormality indicated by the imaging examination, was married and had children already. This article reviewed the patients’ medical history, main clinical manifestations, treatment process, various tests, pathological data and imaging findings. We also performed the analysis of the exon sequencing of these two patients to look for the genetic mutation associated with this disease.
Case description
Case 1
A 17-year-old male patient was admitted with a complaint of right renal dysplasia for 17 years and perineal pain for 1 week. No obvious lesions were found, as the patient’s external genitalia developed normally, and the bilateral scrotum was symmetrical. There was perineal distending pain accompanied by dysuria and painful urination, but the pain did not worsen when compressed. Transrectal ultrasonography showed that an oval cystic anechoic area of approximately 36*25 mm in size with a thin and smooth cyst wall could be detected at the base of the prostate, and the sonographer thought it could be an ejaculatory duct cyst. For further evaluation, the MRI scan results suggested that the right ureter was dilated with an end opening in the urethral prostatic part and that the right seminal vesicle gland was dilated with a seminal vesicle gland cyst. The same-day chest CT showed that the shadow of the right kidney was small and the left kidney was enlarged. The imaging data are shown in Figure 1. Taking a medical history revealed that the ultrasound examination of the patient’s mother during pregnancy indicated that the fetus was suspected of having a left solitary kidney. After that, the patient had a regular physical examination every year, except for dysplasia of the right kidney, and he had no other special uncomfortable symptoms. The patient first developed swelling and pain of the right testis with symptoms of the urinary system 2 years prior, and transrectal aspiration of pus was performed during the follow-up treatment. To further evaluate renal function, we performed renal dynamic imaging plus GFR measurement. The results showed that the right kidney could not be displayed, but the left renal blood perfusion and parenchyma function were approximately normal, GFR: L= 95.93 ml/min.
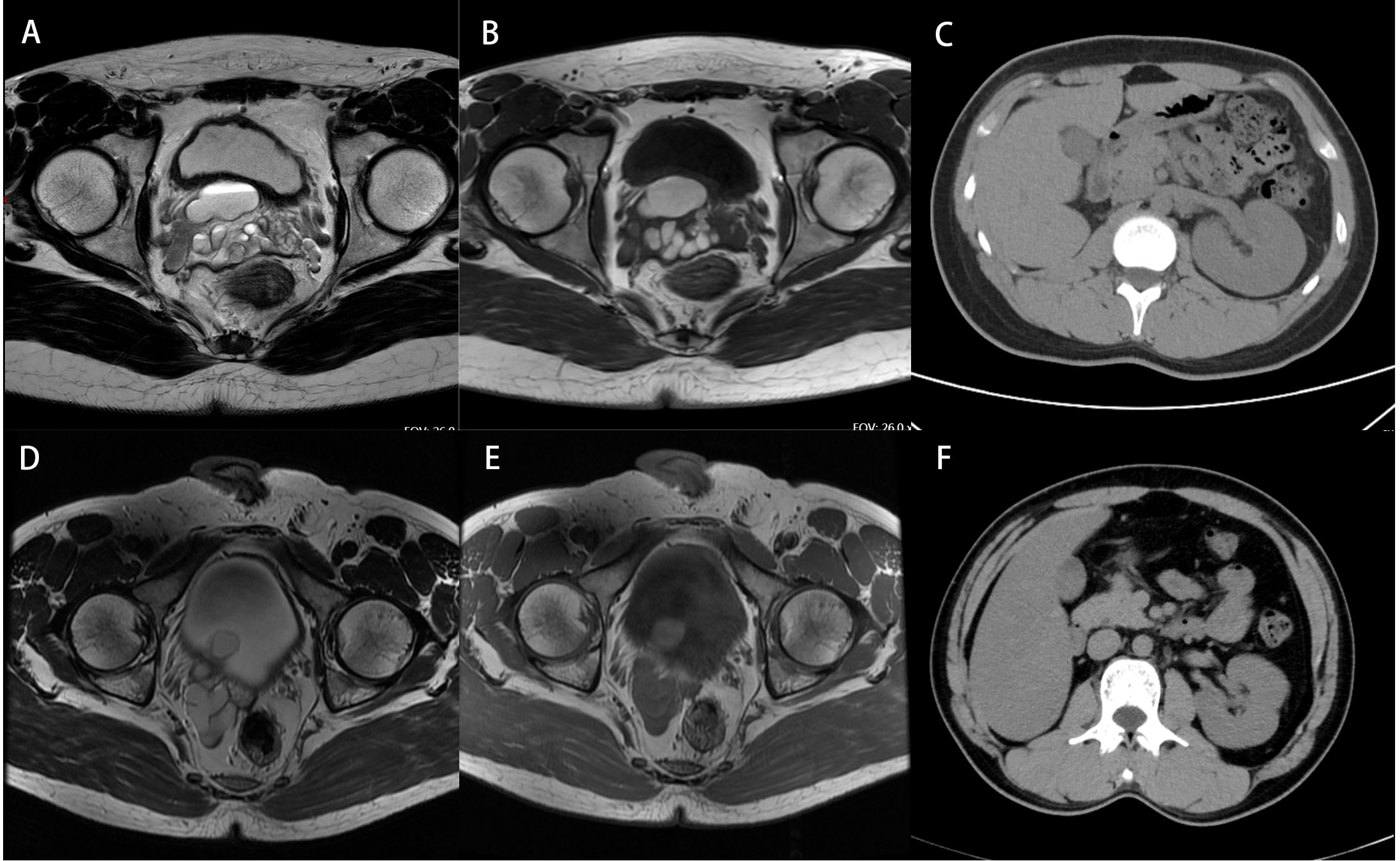
Figure 1 Pathological images of surgically removed glandular tissue of (A–C) case 1 and (D–F) case 2.
After discussing the patient’s history, clinical manifestations and imaging findings, the diagnosis of Zinner’s syndrome was made in our hospital, and robot-assisted laparoscopic resection of the right kidney and ureter plus right seminal vesicle resection was selected as the operation method. The operation went smoothly with little intraoperative blood loss. The condition of the patient was stable, and he was discharged on the 3rd postoperative day. Figure 2 shows the tissue resected during the operation, and the pathological images are shown in Figure 3. No postoperative complications have yet been reported.

Figure 3 Imaging data of patients. (A, B) MRI of case 1 shows a right seminal vesicle cyst. (C) CT of case 1 shows a small right kidney and an enlarged left kidney outline. (D, E) MRI of case 2 shows a right seminal vesicle cyst combined with the right ectopic ureter and lower ureteral calculi. (F) CT of case 2 shows the absence of the right kidney.
Case 2
A 32-year-old male patient was admitted to the hospital with a six-month complaint of a right seminal vesicle cyst found by physical examination. Physical examination after admission showed that the right testis was not palpated, while the external genitalia developed normally, and tenderness was not present in the perineum. The patient himself has never felt any discomfort symptoms to date. Following the medical history, we determined that the patient had a right orchiectomy for cryptorchidism 22 years prior. He was seen at the outpatient department of our hospital which revealed a right seminal vesicle cyst. Repeated urinary tract ultrasound showed atrophy of the right kidney whose location was in the pelvis and that the inner diameter of the right seminal vesicle was larger than that of the left seminal vesicle. To evaluate renal function, we performed renal dynamic imaging plus GFR measurement. The results showed that the left renal blood perfusion and parenchymal function were generally normal, GFR: L= 77.96 ml/min, while the right kidney could not be displayed, and a mass shadow with no radionuclide uptake distribution, which had a density equivalent to that of soft tissue, could be seen in the right seminal vesicle. Furthermore, the pelvic MRI scan showed that the right seminal vesicle cyst was combined with the right ectopic ureter and lower ureteral calculi, and only the left testis and spermatic cord were seen in the photo.
The diagnosis of Zinner’s syndrome was made based on the patient’s medical history, clinical manifestations and imaging findings. Then, we selected laparoscopic complete resection of the right kidney and ureter and resection of the right seminal vesicle cyst as the surgical procedure for this patient. The whole operation was successful, with approximately 50 ml of intraoperative blood loss in total. The patient recovered well and was discharged 6 days after the operation. No postoperative complications have been reported to date.
Discussion
Zinner’s syndrome is a rare congenital disorder of the male urinary system, and the relevant literature suggests that the incidence rate of the population is only 0.0046% (3). In order to further evaluate the diagnosis and treatment of Zinner’s syndrome in China This paper summarizes 14 individual cases of Zinner’s syndrome reported in China in recent 10 years, as shown in Table 1.
It is currently believed that the disease is caused by abnormal growth of the distal Wolffian duct during the 4th and 13th weeks of gestation. This abnormal development results in the failure of the ureteral bud to migrate and combine with the metanephros to form the kidney, and the end of the mesonephric duct is also deformed (16). During the 3rd and 8th week of gestation, paired mesonephric ducts and paramesonephric ducts are present in both male and female embryos. The ureteral buds arise from the dorsal aspect of the distal mesonephric ducts at the 4th week of gestation and migrate in a dorsocranial fashion to meet and induce differentiation of the metanephric blastema, which will form the definitive adult kidney. In males, under the influence of testosterone and Mullerian inhibitory factor, the paramesonephric ducts regress and the caudal part of mesonephric ducts differentiates into hemitrigone, bladder neck, urethra up to the external sphincter, seminal vesicle, vas deferens, ejaculatory ducts, and epididymis. Maldevelopment of the distal mesonephric duct results in absence or malposition of the ureteral budding, and therefore, ipsilateral renal agenesis or dysplasia. Also, it may lead to malformation of mesonephric duct derivatives (17, 18). However, no studies have focused on the genetic changes in patients with Zinner’s syndrome. As a congenital developmental deformity, is there some certain genes closely related to the onset of the disease? In order to explore this problem, we conducted whole exome sequencing on these two patients to obtain the tested results of single nucleotide variants (SNVs) and insertions or deletions (InDels) that met the quality control requirements. Software Annovar (19) was used to annotate the results in terms of location, frequency, gene function, toxicity and so on. Then filter out the mutations which the frequency of the 1000 Genomes Project database > 0.01 and the ExAC (Exome Aggregation Consortium) database in east Asia > 0.01 in the crowd, eliminate the individual diversity and remain possibly pathogenic rare mutations. After that, variation in the exon region or splicing site region is retained, and the synonym mutation is removed to obtain which have an impact on gene expression products. Finally, the mutation that be predicted had an impact on protein structure or function by more than two tools in CADD (20), SIFT (21), Polyphen2 (22) and MutationTaster2 (23) were selected. After the above steps, the potentially meaningful mutation sites were finally screened respectively. Considering that Zinner’s syndrome is a congenital developmental disorder, germ line/developmental related mutation were further selected from the above screened and presented in the Supplementary Tables 1–4. The germ line/developmental related mutations detected in both cases are listed in Table 2. It is worth noting that the BMP4, RPGRIP1L SEC63, SETD2 as genes associated with urinary system morphogenesis or development, are mutated in both cases.
The syndrome is mainly clinically manifested as seminal vesicle cyst, ejaculatory duct obstruction, and ipsilateral renal agenesia (24, 25). The corresponding symptoms of Zinner’s syndrome are atypical, including ejaculation disturbance, local pain, bladder irritation, epididymitis or prostatitis and infertility (1, 26). According to cases reported in China in recent 10 years, discomfort during urination, such as pollakisuria, urgent and painful urination, is the most common symptom, followed by pain in the perineum or lower abdomen, and other non-specific urinary system symptoms such as infertility, dry ejaculation, blood spermia and digestive system discomfort also account for a certain proportion. In addition to patients with corresponding clinical symptoms, there are nearly 1/5 patients without any discomforts. Of the two patients reported in this paper, the younger one had obvious symptoms of perineal and lower abdominal pain and urination discomfort, while the other did not have any noticeable discomfort symptoms, but only reported abnormalities in the results of physical examination.
On imaging, almost every patient with Zinner’s syndrome will have unilateral renal absence and seminal vesicle cyst, which is also the most important and diagnostic imaging manifestations of Zinner’s syndrome. Nearly half of patients have abnormal ureteral dilatation that may be manifested as an X-ray mass in the pelvic region. Generally, in the imaging examination, patients with unilateral absence of kidney and abnormal mass in the ipsilateral pelvis should consider the diagnosis of Zinner’s syndrome.
From the summary and analysis results, the clinical symptoms and imaging findings of Zinner’s syndrome are atypical, which is one of the reasons for misdiagnosis. Therefore, for patients with above atypical urinary symptoms and corresponding imaging findings, attention should be paid to the diagnosis of Zinner’s syndrome. However, there were patients with no specific symptoms other than imaging abnormalities in case 2 and three patients in Table 1. For this kind of patients, imaging examination is the most important diagnostic method. A small number of patients have cryptorchidism in addition to the above clinical symptoms and imaging findings, such as case 2. Is cryptorchidism also related to the congenital dysplasia of the disease? The diagnosis of Zinner’s syndrome is mainly based on the results of clinical symptoms and imaging examinations. Common examinations include transrectal or transabdominal ultrasonography, CT scan, and magnetic resonance imaging (27, 28); sometimes cystoscopy can also find abnormal manifestations. A cystic change of approximately 2*2 cm in size was seen in the right ureteral orifice through cystoscopy during bladder examination of the second patient in this paper. Therefore, care should be taken to avoid misdiagnosis when cystoscopy suggests abnormalities in patients with urologic symptoms.
In terms of disease treatment for patients with obvious symptoms of discomfort, the main principle of Zinner’s syndrome is still symptomatic treatment to reduce the symptoms caused by the mass effect of seminal vesicle cysts (24), including more conservative treatments such as cyst aspiration and sclerotherapy, as well as radical resection (28, 29). However, the results of the reported cases in recent 10 years show that cyst aspiration and sclerotherapy have a greater chance of recurrence than excisional surgery. Cyst aspiration and sclerotherapy has the advantages of convenient operation and less trauma, but the treatment is not thorough and the recurrence rate remains higher; although the recurrence rate after open radical resection is low, because of the deep position of seminal vesicle gland, there is a higher risk of injury to bladder, rectum and other organs during the operation, and the postoperative recovery time will also be prolonged. With the development of minimally invasive techniques, especially robot-assisted surgery, in recent years, the high risk of structural damage to the bladder neck, external sphincter, and rectum that may be caused by open surgery has been significantly reduced (30). In case 1 of this article, the parents of the patient considered that he was young and had not yet married or had children, so they chose robot-assisted laparoscopic surgery with more detailed dissection of the deep pelvic location as well as less surgical trauma.
Conclusion
As a rare disease with a low incidence, Zinner’s syndrome has very different clinicopathological manifestations, and in most cases, the symptoms are nonspecific. These factors lead to the clinical misdiagnosis and neglect of the disease. Therefore, in the process of diagnosis and treatment, patients with specific imaging manifestations should be aware that their disease may be Zinner’s syndrome to avoid misdiagnosis and mistreatment. Although this study filled the gap in the genetic research of Zinner’s syndrome to a certain extent, the number of included cases was too small to obtain any statistical significance. The sample number of cases needed to be further expanded.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
RD: Writing – original draft. KW: Writing – review & editing. FJ: Writing – review & editing. JF: Writing – review & editing. DH: Writing – review & editing. LL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Clinical Application Research Grant of the First Affiliated Hospital of Xi’an Jiaotong University.
Acknowledgments
The authors appreciate Prof. Xinyang Wang and Ke Xu from the Department of Urology, the First Affiliated Hospital of Xi’an Jiaotong University for helping with this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fruro.2023.1257368/full#supplementary-material
References
1. Mehra S, Ranjan R, Garga UC. Zinner syndrome-a rare developmental anomaly of the mesonephric duct diagnosed on magnetic resonance imaging. Radiol Case Rep (2016) 11:313–7. doi: 10.1016/j.radcr.2016.04.002
2. Jiang XS, Wang HJ, Lin JH, Guo Y, Sun CH, Lin L, et al. Zinner's syndrome: clinical features and imaging diagnosis. Asian J andrology (2018) 20:316–7. doi: 10.4103/1008-682x.210295
3. Sheih CP, Hung CS, Wei CF, Lin CY. Cystic dilatations within the pelvis in patients with ipsilateral renal agenesis or dysplasia. J Urol (1990) 144:324–7. doi: 10.1016/s0022-5347(17)39444-2
4. Liao Y, Pan S, Liu G, Hu Y, Li F. Ultrasound diagnosis and treatmentof zinner syndrome: one case report. J Med Imaging (2021) 31:2155–6.
5. Cao J, Zhu S, Bai Z, Wang Z, Wen X, He J, et al. Zinner syndrome: A case report and review of the literature. Natl J Andrology (2017) 23:452–3.
6. Yu M ZL, Wu Z, Hu Y, Jin L. Zinner syndrome: a case report. J Contemp Urologic Reprod Oncol (2016) 8:367–8.
7. Lu C HX, Yang G, Yang J. Zinner syndrome: 2 cases report. J Modern Urol (2019) 24:502–3. doi: 10.3969/j.issn.1009-8291.2019.06.021
8. Wang M, Li B, Liu X, Zhang Y, Luo Y, Wu G, et al. Diagnosis and minimally invasive treatment of zinner syndrome (Report of two cases and literature review). J Clin Urol (2015) 30:1118–21. doi: 10.13201/j.issn.1001-1420.2015.12.017
9. Wang Z JW, Li Z, Jiang G. New clinical diagnosis and treatment of zinner syndrome: a case report. J Clin Urol (2018) 33:420–2. doi: 10.13201/j.issn.1001-1420.2018.05.022
10. Ni D WC, Qi W, Ma L, Zhang Y. Zinner syndrome: A case report and literatures review. Chin J Endourology (2022) 16:173–6. doi: 10.3877/cma.j.issn.1674-3253.2022.02.017
11. Liao Y, Cao Y, Xin Y, Wang Y. Adult intraperitoneal cryptorchidism with zinner syndrome: a case report. J Modern Urol (2023) 28:365–6. doi: 10.3969/j.issn.1009-8291.2023.04.020
12. Tan W KH, Zhou S, Mao Z, Yang K, Li T. Zinner syndrome easily misdiagnosed: a case report. Chin J Endourology (2021) 15:442–3. doi: 10.3877/cma.j.issn.1674-3253.2021.05.019
13. Zhang S, Yang W, Cui Z, Lin M. Congenital seminal vesicle cyst complicated with ipsilateral renal agenesis: a case report and literature review. J Modern Urol (2015) 21:212–21. doi: 10.3969/j.issn.1009-8291.2016.03.016
14. Juho YC, Wu ST, Tang SH, Cha TL, Meng E. An unexpected clinical feature of zinner's syndrome - a case report. Urol Case Rep (2015) 3:149–51. doi: 10.1016/j.eucr.2015.06.015
15. Liu Z, Miao C, Zhuang X, Xing J. Zinner syndrome: Cases report and review of the literature. Asian J Surg (2021) 44:523–4. doi: 10.1016/j.asjsur.2020.12.004
16. van den Ouden D, Blom JH, Bangma C, de Spiegeleer AH. Diagnosis and management of seminal vesicle cysts associated with ipsilateral renal agenesis: a pooled analysis of 52 cases. Eur Urol (1998) 33:433–40. doi: 10.1159/000019632
17. Lin CC, Sheu JC, Tsai PS, Lee MD, Lin TH, Tsai JD. Zinner syndrome in children: clinical presentation, imaging findings, diagnosis, and outcome. Pediatr Nephrol (Berlin Germany) (2022) 37:3075–84. doi: 10.1007/s00467-022-05516-2
18. Passos I, Britto RL. Diagnosis and treatment of müllerian malformations. Taiwan J Obstet Gynecol (2020) 59:183–8. doi: 10.1016/j.tjog.2020.01.003
19. Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res (2010) 38:e164. doi: 10.1093/nar/gkq603
20. Kircher M, Witten DM, Jain P, O'Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet (2014) 46:310–5. doi: 10.1038/ng.2892
21. Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res (2003) 31:3812–4. doi: 10.1093/nar/gkg509
22. Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet (2013) 76(1):452 54. doi: 10.1002/0471142905.hg0720s76
23. Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods (2014) 11:361–2. doi: 10.1038/nmeth.2890
24. Pereira BJ, Sousa L, Azinhais P, Conceição P, Borges R, Leão R, et al. Zinner's syndrome: an up-to-date review of the literature based on a clinical case. Andrologia (2009) 41:322–30. doi: 10.1111/j.1439-0272.2009.00939.x
25. Ibrahimi A, Hosni A, Ziani I, Laamrani FZ, El Sayegh H, Jroundi L, et al. Zinner's syndrome: A rare diagnosis of dysuria based on imaging. Case Rep Urol (2020) 2020:8826664. doi: 10.1155/2020/8826664
26. King BF, Hattery RR, Lieber MM, Berquist TH, Williamson B Jr, Hartman GW. Congenital cystic disease of the seminal vesicle. Radiology (1991) 178:207–11. doi: 10.1148/radiology.178.1.1984306
27. Trigaux JP, Van Beers B, Delchambre F. Male genital tract malformations associated with ipsilateral renal agenesis: sonographic findings. J Clin ultrasound JCU (1991) 19:3–10. doi: 10.1002/jcu.1870190103
28. Di Paola V, Gigli R, Totaro A, Manfredi R. Zinner syndrome: two cases and review of the literature. BMJ Case Rep (2021) 14:74 5. doi: 10.1136/bcr-2021-243002
29. Sridhar AN, Zacharakis E, Dudderidge T, Kelly JD, Nathan S. Robot-assisted management of Zinner's syndrome: report of seminal vesicle sparing technique and review of literature. J Robot Surg (2014) 8:185–7. doi: 10.1007/s11701-013-0430-3
Keywords: Zinner’s syndrome, seminal vesicle cyst, unilateral renal dysplasia, genetic mutation, exon sequencing
Citation: Dai R, Jiang F, Fan J, He D, Li L and Wu K (2023) Clinicopathological and genetic features of Zinner’s syndrome: two case reports and review of the literature. Front. Urol. 3:1257368. doi: 10.3389/fruro.2023.1257368
Received: 15 August 2023; Accepted: 28 September 2023;
Published: 19 October 2023.
Edited by:
Ramadan Saleh, Sohag University, EgyptReviewed by:
Hussein Kandil, Fakih IVF Fertility Center, United Arab EmiratesTuncay Toprak, Fatih Sultan Mehmet Education and Research Hospital, Türkiye
Copyright © 2023 Dai, Jiang, Fan, He, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaijie Wu, Nzk5MDQyMDg1QHFxLmNvbQ==
 Ruijie Dai
Ruijie Dai Fan Jiang
Fan Jiang Junjie Fan
Junjie Fan Dalin He
Dalin He Lei Li
Lei Li Kaijie Wu
Kaijie Wu