- 1Faculty of Medicine and Health, St Vincent’s Clinical School, University of New South Wales, Sydney, NSW, Australia
- 2Department of Clinical Pharmacology, St Vincent’s Hospital, Sydney, NSW, Australia
- 3Sydney School of Public Health, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia
- 4Institute for Musculoskeletal Health, University of Sydney and Sydney Local Health District, Sydney, NSW, Australia
- 5Department of Cardiology, St Vincent's Hospital, Sydney, NSW, Australia
- 6Department of Rheumatology, St Vincent's Hospital, Sydney, NSW, Australia
- 7St Vincent’s Centre for Applied Medical Research, Sydney, NSW, Australia
Introduction: Gout may complicate solid organ transplantation with potentially serious consequences. An accurate prevalence of gout in this population is unknown.
Objectives: This study aimed to estimate the prevalence of gout in the heart and/or lung transplantation population through a systematic review and meta-analysis.
Methods: MEDLINE, Embase, PsycINFO, CENTRAL and Cochrane Library (inception to February 2022) were searched for studies that reported the prevalence and/or incidence of gout in heart and/or lung transplant recipients. Two authors extracted outcomes data. Data were pooled using a random effects model. Overall quality of evidence was assessed using GRADE. Primary outcomes were the prevalence of pre- or post-transplant gout expressed as a prevalence rate (95% CI). Secondary outcomes included risk factors for gout, adverse events, and therapeutic complications of gout treatment.
Results: Ten studies were included. Gout prevalence (PR) was 8% pre-transplant (PR = 0.08; 95% CI: 0.05–0.12; 4 studies n = 651) and 6% post-transplant (PR = 0.06; 95% CI: 0.06–0.06; 10 studies n = 45,298). Post-transplant gout prevalence in heart transplant recipients was almost three times higher than lung transplant recipients (PR = 0.16; 95% CI: 0.13–0.20 vs. PR = 0.06; 95% CI: 0.05–0.06 respectively). Patients with a pre-transplant history of gout had a higher risk of developing post-transplant gout than patients without (RR = 3.61; 95% CI: 2.19–5.95). Factors associated with gout and outcomes for heart and/or lung transplant recipients with gout were comprehensively reviewed from the included studies.
Conclusion: Gout is highly prevalent in heart and/or lung transplant patients. Pre-transplant gout is predictive of developing symptomatic post-transplant gout. This has significant implications for management of heart/lung transplant patients.
Systematic Review Registration: https://www.crd.york.ac.uk/, PROSPERO (CRD42020190632).
Introduction
Gout is an inflammatory arthritis caused by tissue deposition of monosodium urate. While this is a chronic process, gout may cause acute attacks, characterised by sudden onset pain, tenderness, swelling, erythema, and warmth of affected joints/tissues. Gout is a significant health issue due to its association with cardiovascular, renal and metabolic disease, and overall reduced life expectancy (1, 2). Gout carries a substantial global burden of disease with an estimated worldwide prevalence of up to 4% of the global population (3) and has progressively increased over time in some countries (4).
Solid organ transplant recipients have an increased risk of developing gout, which may be explained by both the underlying pathology and medications used to treat these conditions. For example, heart transplant recipients may have concurrent renal impairment, or develop hypoxia-induced uric acid synthesis (5–7), increasing their susceptibility to hyperuricaemia. Additionally, administering loop and thiazide diuretics to heart failure patients can decrease renal uric acid excretion, resulting in hyperuricemia and an increased susceptibility to pre-transplant gout (5–8). Immunosuppressive medications may drive hyperuricaemia themselves, or have significant interactions with pharmaceuticals used to treat acute gout or lower serum urate (9–16).
Given the risk factors outlined above, gout remains an important, yet underappreciated cause of morbidity in the heart/lung transplantation population. An accurate estimate of the burden of gout in this population is required to address this significant issue.
Objective
Although the current literature acknowledges the presence of gout in heart/lung transplant recipients, there remains a paucity of studies assessing the prevalence of gout in heart or lung transplant patients. Furthermore, risk factors and outcomes for gout in the heart/lung transplant cohort have not been assessed in depth. To date, this is the first systematic review and meta-analysis that has attempted to quantitate gout prevalence in this population.
Methods
The protocol was registered on PROSPERO on 5 June 2020 (receipt number: 190632). This review was conducted in accordance with PRISMA guidelines (17).
Design
Types of studies
Observational studies (e.g., cohort studies, case-control studies, cross-sectional studies) reporting on the incidence or prevalence of gout in individuals who had undergone heart, lung or heart and lung transplantation were included. There were no restrictions for language and translations were attempted for non-English published articles/data. As clinical diagnosis of gout has remained largely unchanged for many decades, there was no restriction on the year of publication.
Participants
Studies were eligible if they included patients who had undergone a heart transplant, lung transplant, or heart-lung transplant. Studies were excluded if they did not explicitly mention gout as a comorbidity, adverse event or an outcome.
Comparison
The “gout” group consisted of heart and/or lung transplant patients who had a gout flare before and/or after their transplant. The “no gout” group consisted of heart and/or lung transplant patients who never had a gout flare before or after transplantation.
Electronic searches
The search strategy was developed by [redacted] and edited by [redacted]. A search was performed in MEDLINE, Embase, PsycINFO, CENTRAL and Cochrane Library (all from inception to February 2022, without language restrictions) for eligible reports. Reference lists of relevant observational studies were screened. Search terms included “gout” AND “transplantation” OR “heart transplant” OR “lung transplant”.
Study selection
Four independent reviewers [redacted] screened titles and abstracts. Six reviewers [redacted] independently inspected the full manuscript of potentially eligible observational studies to determine eligibility.
Assessment of heterogeneity
Clinical heterogeneity was assessed by comparing participant characteristics, type and dosage of immunosuppressive medications, duration of follow-up, method of gout diagnosis, and the type and dosage of gout medications.
Overall quality of evidence rating
The Gradings of Recommendations Assessment, Development and Evaluation (GRADE) method was used for evaluating overall quality of evidence (18). Baseline quality of evidence was reported as “high” and downgraded a level for each of the four factors: limitations in study design, result inconsistency [wide variance of point estimates across studies or if statistical heterogeneity between trials was large (I2 > 50)] (19), result imprecision (wide confidence intervals, total sample size less than <300), and publication bias (assessed using funnel plot analysis/Egger's regression test for 10 or more studies). It was not necessary to downgrade for indirectness as this review encompassed a specific review question. Overall quality of evidence was rated as “high”, “moderate”, “low” or “very low”.
Data collection
Two reviewers (BC, CAS) extracted data using piloted extraction forms. Other investigators were also consulted (RD, LG, RP, EU). Non-English articles were translated. Information on outcomes data and study characteristics were collected.
Bias assessment
Two reviewers (BC, CAS) independently assessed the risk of bias. Cohort studies were assessed using the Newcastle-Ottawa Scale (NOS) for quality assessment of non-randomised studies (20). Studies with a score of >7 or higher were deemed to have a low risk of bias, studies with a score ≤6 were deemed to have a high risk of bias. Cross-sectional studies were assessed using an adapted version of Hoy et al.'s risk of bias tool for prevalence studies (21). Studies were classified as having low, moderate, or high risk of bias.
Outcome measures
The primary outcome was to assess gout prevalence in people undergoing heart and/or lung transplantation, pre- and post-transplant. These include gout flares, intercritical gout (i.e., between flares) and chronic gouty arthritis as defined by the European League Against Rheumatism (EULAR) (22).
The secondary outcomes were risk factors, adverse events, therapeutic complications and transplant-related mortality in heart and/or lung transplant recipients with gout.
Adverse events data included: interval between transplant and gout flare, sites of gout flare and tophi formation, duration of gout, complications of gout, infection and acute rejection episodes. Serum urate levels and renal function in the post- heart and/or lung transplant gout cohort were collected.
Subgroup analysis
A sub-group analysis compared gout prevalence in heart transplant patients with lung transplant patients. Patients with no history of pre-transplant gout were compared with patients with a history of pre-transplant gout. The pre-transplant prevalence of gout was compared with post-transplant prevalence of gout.
Data synthesis
The meta-analysis and subgroup analysis was carried out using Comprehensive Meta-Analysis random-effects model Version 3 (23). Prevalence ratios (PR) were expressed as the total number of transplant patients with gout over the total number of transplant patients. Results for dichotomous data were presented as risk ratio (RR) with 95% confidence intervals (CI). Results not able to be pooled are described descriptively.
Results
Study selection
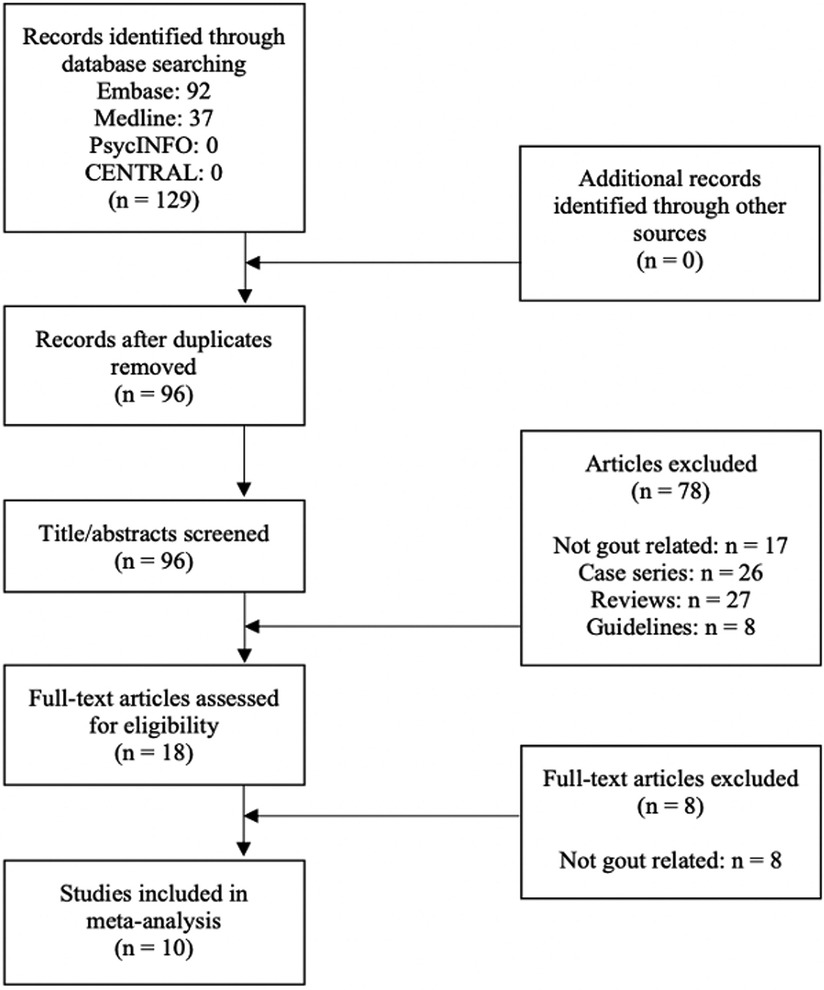
A total of 129 studies were identified from the searches. After duplicate articles were removed, 96 articles were included in title/abstract screening. Eighteen articles were deemed relevant for a full-text review, of which 10 articles met eligibility criteria (24–33). Reasons for exclusion of the eight studies after full-text review are described in Figure 1.
There were data of sufficient quality to perform a metaanalysis on gout prevalence and the association of premorbid gout with post-transplant gout flare (GRADE ratings in Supplementary Tables S3–S5).
Characteristics of included studies
Included studies are summarised in Table 1. Seven were retrospective cohort studies (24–27, 29–31), one was a prospective cohort study (28), and two were cross-sectional studies (32, 33).
Among the 5/10 studies that characterised age and/or gender, mean age ranged from 41 to 63 years and most patients were male. The follow-up period ranged from 1 to 15 years. Nine studies provided details of immunosuppressants administered to transplant recipients (24, 26–33) 7/10 studies explicitly reported their methods to identify gout (Table 1) (25, 26, 28, 30–33).
Risk of bias
All eight cohort studies were assessed to have a high risk of bias (Supplementary Table S1) (24–31). Both cross-sectional studies were assessed to have a low risk of bias (Supplementary Table S2) (32, 33).
Prevalence of pre-transplant gout in heart and/or lung transplant patients
There was low quality evidence from four studies (n = 651) that the pre-transplant prevalence of gout in heart and/or heart-lung transplant patients was 8% (PR = 0.08; 95% CI: 0.05–0.12) (Figure 2; Supplementary Table S3) (26–28, 31–33).
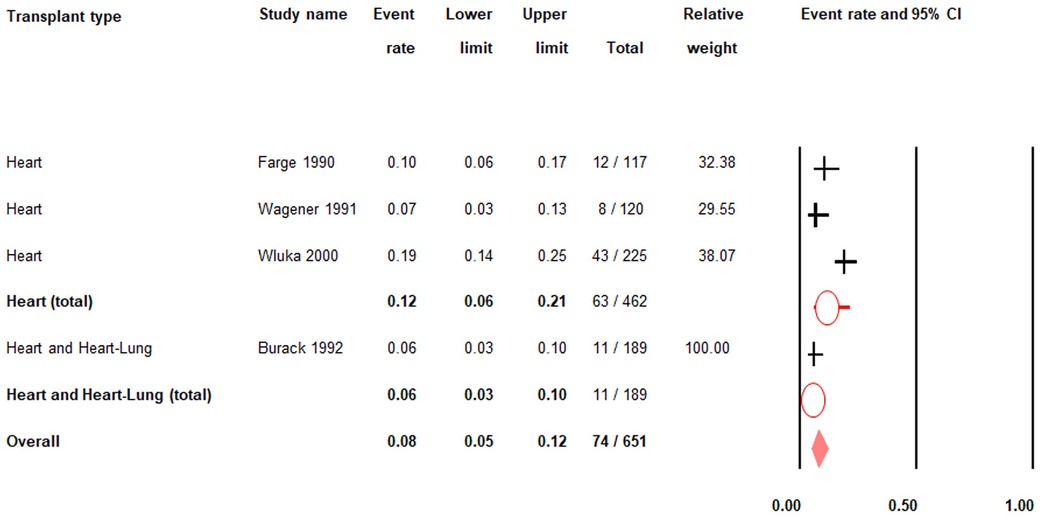
Figure 2. Pre-transplant prevalence of gout. 95% CI; |: prevalence rate; O: total prevalence rate for each transplant type; ♦: overall prevalence rate.
Among heart transplant patients only, there was very low quality evidence from three studies (n = 462) that the pre-transplant prevalence of gout was 12% (PR = 0.12; 95% CI: 0.06–0.21) (Figure 2; Supplementary Table S3) (27, 28, 31, 33).
There was moderate quality evidence from one study (n = 189) that the pre-transplant prevalence of gout in heart and heart-lung transplant patients was 6% (PR = 0.06; 95% CI: 0.03–0.10) (Figure 2; Supplementary Table S3) (26). There were no data on the pre-transplant prevalence of gout in patients who had lung transplants only.
Prevalence of post-transplant gout in heart and/or lung transplant patients
There was low quality evidence from ten studies (n = 45,298) that the post-transplant prevalence of gout was 6% (PR = 0.06; 95% CI: 0.06–0.06) (Figure 3; Supplementary Table S4) (24–33).
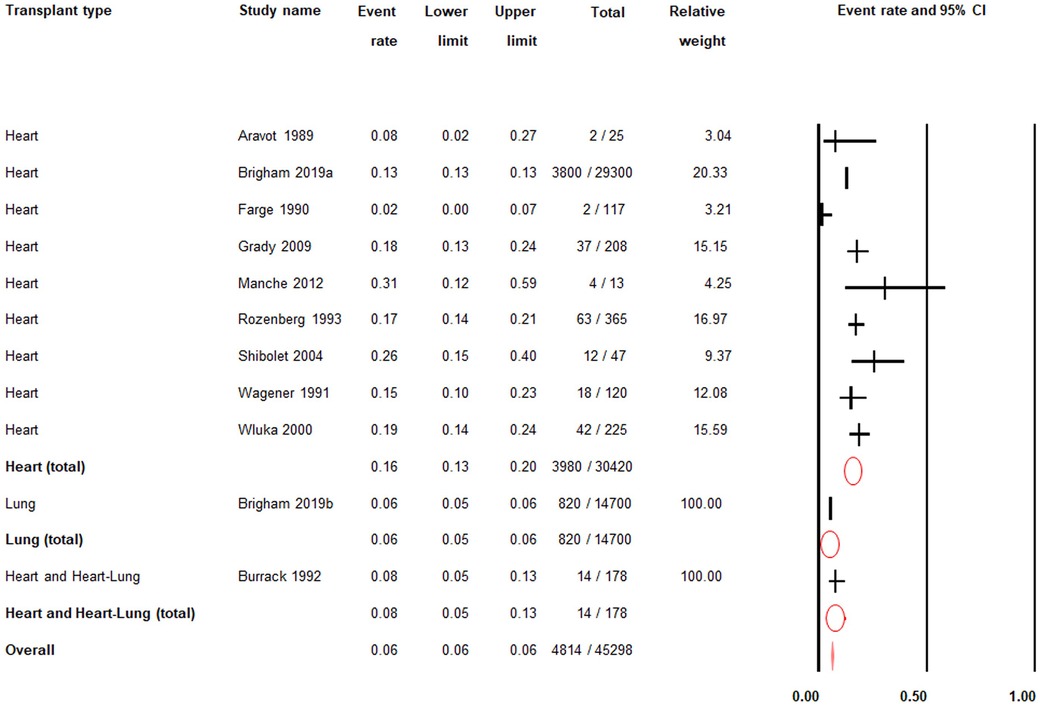
Figure 3. Post-transplant prevalence of gout. 95% CI; |: prevalence rate; O: total prevalence rate for each transplant type; ♦: overall prevalence rate.
Among heart transplant patients only, there was low quality evidence from nine studies (n = 30,420) that the post-transplant prevalence of gout was 16% (PR = 0.16; 95% CI: 0.13–0.20) (Figure 3; Supplementary Table S4).
Among lung transplant patients only, there was moderate quality evidence from one study (n = 14,700) that the post-transplant prevalence of gout was 6% (PR = 0.06; 95% CI: 0.05–0.06) (Figure 3; Supplementary Table S4).
Among heart and heart-lung transplant patients, there was moderate quality evidence from one study (n = 178) that the post-transplant prevalence of gout was 8% (PR = 0.08; 95% CI: 0.05–0.13) (Figure 3; Supplementary Table S4).
Relative risk of post-transplant gout
There was moderate quality evidence from two studies (n = 342) that the relative risk of experiencing a post-transplant gout flare was higher in patients who had a pre-transplant history of gout than patients who had no prior history of gout (RR = 3.61; 95% CI: 2.19–5.95) (Supplementary Figure S1; Supplementary Table S5) (27, 31).
Risk factors for gout development in transplant recipients
4/10 studies reported the mean ages of gout patients (26, 29, 31, 32). Two of these studies reported that patients with gout were significantly older than patients without gout, and a significantly higher prevalence of gout was seen in males compared to females (31, 32).
4/10 studies reported that diuretics were used more commonly among heart transplant recipients with gout compared to heart transplant recipients without gout (26, 30–32). One study reported statistically significant findings (31).
Characteristics of post-transplant populations with gout
Interval between operation and gout flare
3/10 studies reported the duration between transplant and gout flare (26, 27, 31). One study reported a mean of 17 months between transplantation and gout flare (range: 1–41) (26). Another study reported an interval of 6 months between operation and gout flare in recipients with pre-transplant gout, and 18 months in recipients with new-onset gout (27). A third study reported a mean of 25.9 months in patients with pre-transplant gout and 43.9 months in patients with new-onset gout (31).
None of the studies mentioned if any patient suffered from an inpatient gout flare, i.e., immediately post-transplant before their initial discharge from hospital.
Site of gout flares and tophi formation
2/10 studies reported the site of gout flares in post-transplant populations (26, 27). In both studies, the first metatarsophalangeal joint was most commonly affected. Other joints affected included the midtarsal, ankle, elbow, wrist, and small hand joints (26).
Tophi formation was reported in 4/10 studies (26, 27, 31, 32). In one study, tophi formation was seen in 6/14 (42.9%) of new-onset gout patients and 2/11 (18.2%) of those with recurrent gout. In three other studies, tophaceous gout was seen 7.9%–50% of patients (27, 31, 32).
Symptom duration
No study reported the frequency or duration of episodes of flare.
Articular complications
2/10 studies reported articular complications in post-transplant populations (26, 32). Bacterial infections of the joint, bursa, or tophi occurred after transplantation in 1/20 (5%) of recipients with recurrent gout, and 3/20 (15%) of recipients with new-onset gout (26). One patient required a surgical debridement because of a bacterial superinfection of a tophus in the olecranon bursa. 2/63 (3.2%) of patients with gout showed signs of osteoarticular damage (32).
Mortality
1/10 studies compared mortality rates between patients with gout and patients without gout (31). The mortality rate was 4/23 (17.4%) in patients with new-onset gout, 3/19 (15.8%) in patients with recurrent gout, and 45/159 (28.3%) in patients who never had gout.
Therapeutic complications of gout medications
3/10 studies reported changes to immunosuppressants directly because of gout medications (27, 29, 31). One study stated that azathioprine was progressively discontinued before allopurinol could be initiated (27). In a second study, azathioprine was switched to mycophenolate mofetil when allopurinol was introduced in a patient with gout (29). In another study, azathioprine was ceased with cyclophosphamide or mycophenolate substituted in 5/6 (83%) of those with pancytopenia, and in 9/18 (50%) without pancytopenia (31).
Hyperuricaemia
3/10 studies reported the mean serum urate levels in heart transplant recipients (26, 30, 32). The prevalence of hyperuricaemia among heart transplant recipients ranged from 72%–100%. Serum urate was reported to increase post-transplant; other factors associated with an elevated serum urate were cyclosporine use, diuretic use, and tophaceous gout (26, 32).
Discussion
While gout has been a recognised comorbidity in heart and/or lung transplantation for decades (34), this review is the first to characterise gout prevalence in heart and/or lung transplant patients in the literature.
The results of this study highlight the significant risk of gout in heart and lung transplant populations. This study reported low-quality evidence that the pre- and post-transplant prevalence of gout in heart and/or lung transplant patients was 8% and 6% respectively. In comparison, the estimated all-age prevalence of gout in western countries is between 0.5% and 5% of the general population (35). The increased prevalence of gout in the heart and lung transplant cohort likely reflects the pre-transplant disease state and medication use (e.g., cardiac failure and subsequent diuretic use) which increases susceptibility to hyperuricemia.
This study reported medium quality of evidence that the risk of post-transplant gout is greater in patients with pre-transplant gout compared to patients without pre-transplant gout. This augments the previous observation that flares of gout occur earlier post-transplant in patients with pre-existing gout (27, 31).
The risk factors for gout development in post-transplant populations are similar to the general population. Multiple studies reported that patients with gout were significantly older than patients without gout, and males were more likely to develop gout compared to females (27). Diuretics remain a key risk factor, and were used more commonly in heart transplant recipients with gout compared to recipients without gout (31, 32).
Treatment of gout may itself give rise to additional complications in this population. Calcineurin inhibitors (CNI) may contribute to hyperuricaemia and pose significant drug-drug interactions with agents used to treat gout flares, such as colchicine. The potentially serious interaction of azathioprine with xanthine oxidase inhibitors (e.g., allopurinol) is well-described. However, modern immunosuppressants may overcome some of these issues: for example, mycophenolate mofetil, which does not interact with allopurinol, is increasingly used in place of azathioprine. Nonetheless, azathioprine may still be used in certain clinical scenarios and as such clinicians must be aware of this significant interaction.
This study had several strengths. Firstly, it is the first meta-analysis to assess gout prevalence in the heart/lung transplant population. The study population was extracted from heterogenous clinical settings, and the characteristics of post-transplant recipients with gout were assessed in detail.
Limitations to this study include the large statistical heterogeneity between studies, which resulted in considerable variance in gout prevalence. While this study did not place limits on study age, the clinical diagnosis of gout has remained essentially unchanged over the study period and thus study age has limited impact on estimation of gout prevalence in this regard. Only one study mentioned gout prevalence in lung transplant recipients (26, 30–32), hindering a direct comparison between heart and lung transplant patients. There are limited data on the prevalence of pre-transplant gout: one study specifically excluded patients with pre-transplant gout (25), while another study excluded patients with pre- transplant gout when characterising patients with post-transplant gout (30). The true prevalence of gout may be confounded by the lack of standardisation in the diagnosis of gout. Finally, most of the selected studies did not perform multivariate analyses to assess the significance of potential risk factors such as age, race, gender, or comorbidities such as renal impairment.
To improve the quality of evidence of gout prevalence in these populations, future heart/lung transplantation studies would benefit from use of established gout diagnostic criteria (36), using a sufficient duration of follow up to capture incident gout (31), report gout incidence over regular time periods (e.g., monthly intervals post-transplantation) and capture gout attacks in the immediate post-transplantation period. Serum urate should be determined regularly pre-and post-transplant. Future studies should include gout as an outcome measure to allow tracking of gout prevalence over time, particularly as immunosuppressive treatment and other factors that influence hyperuricaemia change. For example, the prevalence of hyperuricaemia and gout may increase as transplant candidacy guidelines permit patients with renal dysfunction; furthermore, the background burden of gout appears to be increasing which may be mirrored in transplant populations (4, 37). There is also a paucity of data of the prevalence of gout in the combined heart-kidney, lung-kidney and thoracoabdominal triple organ transplant setting.
The prevalence of gout in heart/lung transplant populations as determined in this study is higher than that reported in the general population. In context of the increasing worldwide prevalence of gout, guidelines for managing gout in this population are paramount for the guidance of future practice. However, despite major advancements in gout therapy and guidelines published by the American College of Rheumatology (ACR) (38) to inform gout management, there are no specific guidelines on the management of gout in the setting of concurrent immunosuppressive therapy in heart and/or lung transplant patients. Notably, the International Society for Heart and Lung Transplantation (ISHLT) Guidelines for the Care of Heart Transplant Recipients recommends the use of anti-hyperuricaemic therapies for gout in heart transplant patients; the results of this study provide an accurate estimate of gout burden in this population to support this recommendation (39). Finally, awareness of gout prevalence and the potential pitfalls in gout management in this population would serve to improve patient outcomes and safety. Adoption of an anticipatory approach, or screening transplant patients for underlying hyperuricaemia or gout, may improve patient outcomes and would benefit from further study.
There is considerable pre- and post-transplant prevalence of gout in heart and lung transplantation recipients. Pre-existing gout increases the risk of a post-transplant gout flare. Addressing the factors that drive prevalence, as well as the management of gout, are significant areas of unmet need in the heart/lung transplantation population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
BC: Writing – original draft, Writing – review & editing. RD: Writing – original draft, Writing – review & editing. EU: Writing – original draft, Writing – review & editing. CA: Writing – original draft, Writing – review & editing. AK: Writing – original draft, Writing – review & editing. LG: Writing – original draft, Writing – review & editing. RP: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank Dr. Sophie Stocker for translation of a non-English article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frtra.2024.1356058/full#supplementary-material
Abbreviations
ACR, American College of Rheumatology; CI, confidence interval; EULAR, European League Against Rheumatism; GRADE, grading of recommendations, assessment, development and evaluations; NOS, Newcastle-Ottawa scale; NSAID, non-steroidal anti-inflammatory drug; PR, prevalence ratio; RR, risk ratio.
References
1. Robinson PC, Merriman TR, Herbison P, Highton J. Hospital admissions associated with gout and their comorbidities in New Zealand and England 1999–2009. Rheumatology (Oxford). (2013) 52:118–26. doi: 10.1093/rheumatology/kes253
2. Perez-Ruiz F, Martínez-Indart L, Carmona L, Herrero-Beites AM, Pijoan JI, Krishnan E. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis. (2014) 73:177–82. doi: 10.1136/annrheumdis-2012-202421
3. Singh JA, Gaffo A. Gout epidemiology and comorbidities. Semin Arthritis Rheum. (2020) 50(3, Supplement):S11–6. doi: 10.1016/j.semarthrit.2020.04.008
4. Pathmanathan K, Robinson PC, Hill CL, Keen HI. The prevalence of gout and hyperuricaemia in Australia: an updated systematic review. Semin Arthritis Rheum. (2021) 51:121–8. doi: 10.1016/j.semarthrit.2020.12.001
5. Bruderer S, Bodmer M, Jick SS, Meier CR. Use of diuretics and risk of incident gout: a population-based case-control study. Arthritis Rheumatol. (2014) 66:185–96. doi: 10.1002/art.38203
6. Reyes AJ. Cardiovascular drugs and serum uric acid. Cardiovasc Drugs Ther. (2003) 17:397–414. doi: 10.1023/B:CARD.0000015855.02485.e3
7. Hueskes BAA, Roovers EA, Mantel-Teeuwisse AK, Janssens HJEM, van de Lisdonk EH, Janssen M. Use of diuretics and the risk of gouty arthritis: a systematic review. Semin Arthritis Rheum. (2012) 41:879–89. doi: 10.1016/j.semarthrit.2011.11.008
8. El-Sheikh AAK, van den Heuvel JJMW, Koenderink JB, Russel FGM. Effect of hypouricaemic and hyperuricaemic drugs on the renal urate efflux transporter, multidrug resistance protein 4. Br J Pharmacol. (2008) 155:1066–75. doi: 10.1038/bjp.2008.343
9. English J, Evan A, Houghton DC, Bennett WM. Cyclosporine-induced acute renal dysfunction in the rat. Evidence of arteriolar vasoconstriction with preservation of tubular function. Transplantation. (1987) 44:135–41. doi: 10.1097/00007890-198707000-00027
10. Lanese DM, Conger JD. Effects of endothelin receptor antagonist on cyclosporine-induced vasoconstriction in isolated rat renal arterioles. J Clin Invest. (1993) 91:2144–9. doi: 10.1172/JCI116440
11. Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. (1984) 311:699–705. doi: 10.1056/NEJM198409133111103
12. Kanbay M, Akcay A, Huddam B, Usluogullari CA, Arat Z, Ozdemir FN, et al. Influence of cyclosporine and tacrolimus on serum uric acid levels in stable kidney transplant recipients. Transplant Proc. (2005) 37:3119–20. doi: 10.1016/j.transproceed.2005.08.042
13. Soubhia RMC, Mendes GEF, Zocoler Mendonça F, Baptista MAS, Cipullo JP, Burdmann EA. Tacrolimus and nonsteroidal anti-inflammatory drugs: an association to be avoided. Am J Nephrol. (2005) 25:327–34. doi: 10.1159/000086569
14. Speeg KV, Maldonado AL, Liaci J, Muirhead D. Effect of cyclosporine on colchicine secretion by the kidney multidrug transporter studied in vivo. J Pharmacol Exp Ther. (1992) 261:50–5. 1348538
15. Gearry RB, Day AS, Barclay ML, Leong RW, Sparrow MP. Azathioprine and allopurinol: a two-edged interaction. J Gastroenterol Hepatol. (2010) 25:653–5. doi: 10.1111/j.1440-1746.2010.06254.x
16. Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. (2003) 111:1133–45. doi: 10.1172/JCI16432
17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J. (2009) 339:b2700. doi: 10.1136/bmj.b2700
18. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. Br Med J. (2004) 328:1490–4. doi: 10.1136/bmj.328.7454.1490
19. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol. (2011) 64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017
20. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2019). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed May 16, 2022).
21. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. (2012) 65:934–9. doi: 10.1016/j.jclinepi.2011.11.014
22. Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castaneda J, et al. 2018 updated European league against rheumatism evidence-based recommendations for the diagnosis of gout. Ann Rheum Dis. (2020) 79:31–8. doi: 10.1136/annrheumdis-2019-215315
23. Comprehesnive Meta Analysis. Meta-analysis Manual V3. (2013). Available online at: https://www.meta-analysis.com/downloads/Meta-Analysis%20Manual%20V3.pdf (accessed May 16, 2022).
24. Aravot DJ, Banner NR, Khaghani A, Fitzgerald M, Radley-Smith R, Mitchell AG, et al. Cardiac transplantation in the seventh decade of life. Am J Cardiol. (1989) 63:90–3. doi: 10.1016/0002-9149(89)91082-5
25. Brigham MD, Milgroom A, Lenco MO, Tudor T, Kent JD, LaMoreaux B, et al. Prevalence of gout in the surviving United States solid organ transplantation population. Transplant Proc. (2019) 51:3449–55. doi: 10.1016/j.transproceed.2019.08.037
26. Burack DA, Griffith BP, Thompson ME, Kahl LE. Hyperuricemia and gout among heart transplant recipients receiving cyclosporine. Am J Med. (1992) 92:141–6. doi: 10.1016/0002-9343(92)90104-J
27. Farge D, Liote F, Guillemain R, Vulser C, Amrein C, Couetil JP, et al. Hyperuricemia and gouty arthritis in heart transplant recipients. Am J Med. (1990) 88:553. doi: 10.1016/0002-9343(90)90448-M
28. Grady KL, Wang E, Higgins R, Heroux A, Rybarczyk B, Young JB, et al. Symptom frequency and distress from 5 to 10 years after heart transplantation. J Hear Lung Transplant. (2009) 28:759–68. doi: 10.1016/j.healun.2009.04.020
29. Manche A. Early and late outcomes after heart transplantation in a low-volume transplant centre. Malta Med J. (2012) 24:30–4. Available online at: https://www.um.edu.mt/umms/mmj/PDF/353.pdf. Published 2021 (accessed May 16, 2022).
30. Shibolet O, Elinav E, Ilan Y, Safadi R, Ashur Y, Eid A, et al. Reduced incidence of hyperuricemia, gout, and renal failure following liver transplantation in comparison to heart transplantation: a long-term follow-up study. Transplantation. (2004) 77:1576–80. doi: 10.1097/01.TP.0000128357.49077.19
31. Wluka AE, Ryan PFJ, Miller AM, Richardson M, Bergin PJ, Page JL, et al. Post-cardiac transplantation gout: incidence of therapeutic complications. J Heart Lung Transplant. (2000) 19:951–6. doi: 10.1016/S1053-2498(00)00175-3
32. Rozenberg S, Frih L, Lang T, Koeger AC, Cabrol A, Gandjbackch I, et al. Rheumatologic manifestations in heart transplant recipients. A cross-sectional study of 365 patients. Rev Rhum Ed Fr. (1993) 60(1):10–5. Available online at: https://pubmed.ncbi.nlm.nih.gov/8242020/. Published 1993 (accessed May 16, 2022).8242020
33. Wagener P, Schulte D, Wagenbreth BH I. Rheumatological manifestation in patients after cardiac transplantation. Aktuelle Rheumatol. (1991) 16:48–51. doi: 10.1055/s-2008-1047380
34. Kahl LE, Thompson ME, Griffith BP. Gout in the heart transplant recipient: physiologic puzzle and therapeutic challenge. Am J Med. (1989) 87:289–94. doi: 10.1016/S0002-9343(89)80153-6
35. Kuo C-F, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. (2015) 11:649–62. doi: 10.1038/nrrheum.2015.91
36. Neogi T, Jansen TLTA, Dalbeth N, Fransen J, Schumacher HR, Berendsen D, et al. Gout classification criteria: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. (2015) 2015(74):1789–98. doi: 10.1136/annrheumdis-2015-208237
37. Zhang J, Jin C, Ma B, Sun H, Chen Y, Zhong Y, et al. Global, regional and national burdens of gout in the young population from 1990 to 2019: a population-based study. RMD Open. (2023) 9:e003025. doi: 10.1136/rmdopen-2023-003025
38. FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AM, et al. 2020 American college of rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). (2020) 72:744–60. doi: 10.1002/acr.24180
Keywords: gout, hyperuricaemia, heart transplant (HTx), lung transplant (LTx), heart/lung transplant, uric acid
Citation: Chui B, Day R, Umashankar E, Abdel Shaheed C, Keogh A, Girgis L and Penglase R (2024) Meta-analysis and systematic review of gout prevalence in the heart/lung transplantation population. Front. Transplant. 3:1356058. doi: 10.3389/frtra.2024.1356058
Received: 15 December 2023; Accepted: 15 April 2024;
Published: 20 May 2024.
Edited by:
Charles Hoopes, The University of Alabama at Birmingham, United StatesReviewed by:
Samy Riad, Mayo Clinic, United StatesStanley Wolfe, Allegheny General Hospital, United States
Letizia Corinna Morlacchi, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico di Milano, Italy
© 2024 Chui, Day, Umashankar, Abdel Shaheed, Keogh, Girgis and Penglase. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ross Penglase, cm9zcy5wZW5nbGFzZUBzdmhhLm9yZy5hdQ== @rosspenglase
†These authors have contributed equally to this work
‡ORCID Benedict Chui orcid.org/0000-0002-1670-5959
Richard Day orcid.org/0000-0002-6045-6937
Eshwar Umashankar orcid.org/0000-0003-3867-3510
Christina Abdel Shaheed orcid.org/0000-0003-1258-5125
Anne Keogh orcid.org/0000-0002-8788-603X
Laila Girgis orcid.org/0000-0001-7416-9448
Ross Penglase orcid.org/0000-0002-4061-4301
 Benedict Chui1,‡
Benedict Chui1,‡ Richard Day
Richard Day Christina Abdel Shaheed
Christina Abdel Shaheed Ross Penglase
Ross Penglase