- 1Fisheries Ecology and Conservation Laboratory, Harbor Branch Oceanographic Institute, Florida Atlantic University, Fort Pierce, FL, United States
- 2School of Biological, Environmental and Earth Sciences, The University of Southern Mississippi, Hattiesburg, MS, United States
- 3Marine Resources Section, Department of Environment and Natural Resources, St. George's, Bermuda
- 4Natural History Museum, Bermuda Aquarium, Museum and Zoo, Flatts, Bermuda
- 5National Marine Fisheries Service, Southeast Fisheries Science Center, Mississippi Laboratories, Pascagoula, MS, United States
Cownose rays (Family Rhinopteridae) are highly migratory pelagic rays that are generally restricted to continental shelves. Despite 100's of years of natural history records, cownose rays have never been reported in Bermuda, an atoll-like coral reef ecosystem that is separated from the continental mainland United States by ~1,000 km. Here we compile evidence that the Atlantic cownose ray (Rhinoptera bonasus) has recently established in Bermuda, supported by both morphological and genetic data. Potential ecological and inter-specific competition concerns are presented as well as probable physical mechanisms that facilitated this recent and presumed range expansion.
1 Introduction
Situated in the northwest region of the Sargasso Sea, Bermuda comprises a group of oceanic islands and surrounding reefs that approximate an atoll formation (1). The islands are isolated from the North American continent (~1,000 km from Cape Hatteras, North Carolina) and are considered part of the tropical northwestern Atlantic ecoregion (2). However, at 32°N latitude, Bermuda supports the northernmost coral reef ecosystem in the Atlantic and is more accurately considered subtropical, characterized by significantly lower faunal and algal diversity than analogous ecosystems in the Greater Caribbean (3).
Research on elasmobranchs (i.e., sharks and batoids) in Bermuda has been modest, with a few studies of the sixgill shark (Hexanchus griseus) (4, 5), satellite tracking of the tiger shark (Galeocerdo cuvier) (6, 7), and the only research on batoids coming from a single species, the whitespotted eagle ray (Aetobatus narinari) (8–11). The whitespotted eagle ray has long been considered the sole inshore ray species in Bermuda (12); however, reports of large dasyatid rays (e.g., Bathytosia centroura) are emerging [(13); iNaturalist].
Cownose rays (family Rhinopteridae) are highly migratory pelagic stingrays that occur worldwide in tropical and temperate seas (14, 15). Currently, eight species have been identified in the sole genus Rhinoptera, including three in the North Atlantic basin—R. bonasus and R. brasiliensis [western Atlantic species that are both Vulnerable on the International Union for Conservation of Nature (IUCN) Red List of Threatened Species (16, 17)], and R. marginata [a Critically Endangered eastern Atlantic species (18)]. To date, distribution patterns of these species have been limited to the continental shelf margins (16–18) with no reports from oceanic islands (Figure 1). Here, we report on the first observations of cownose rays in Bermuda, whose arrival is estimated to have occurred as early as 2012, with observations of the species continuing to be sustained through today.
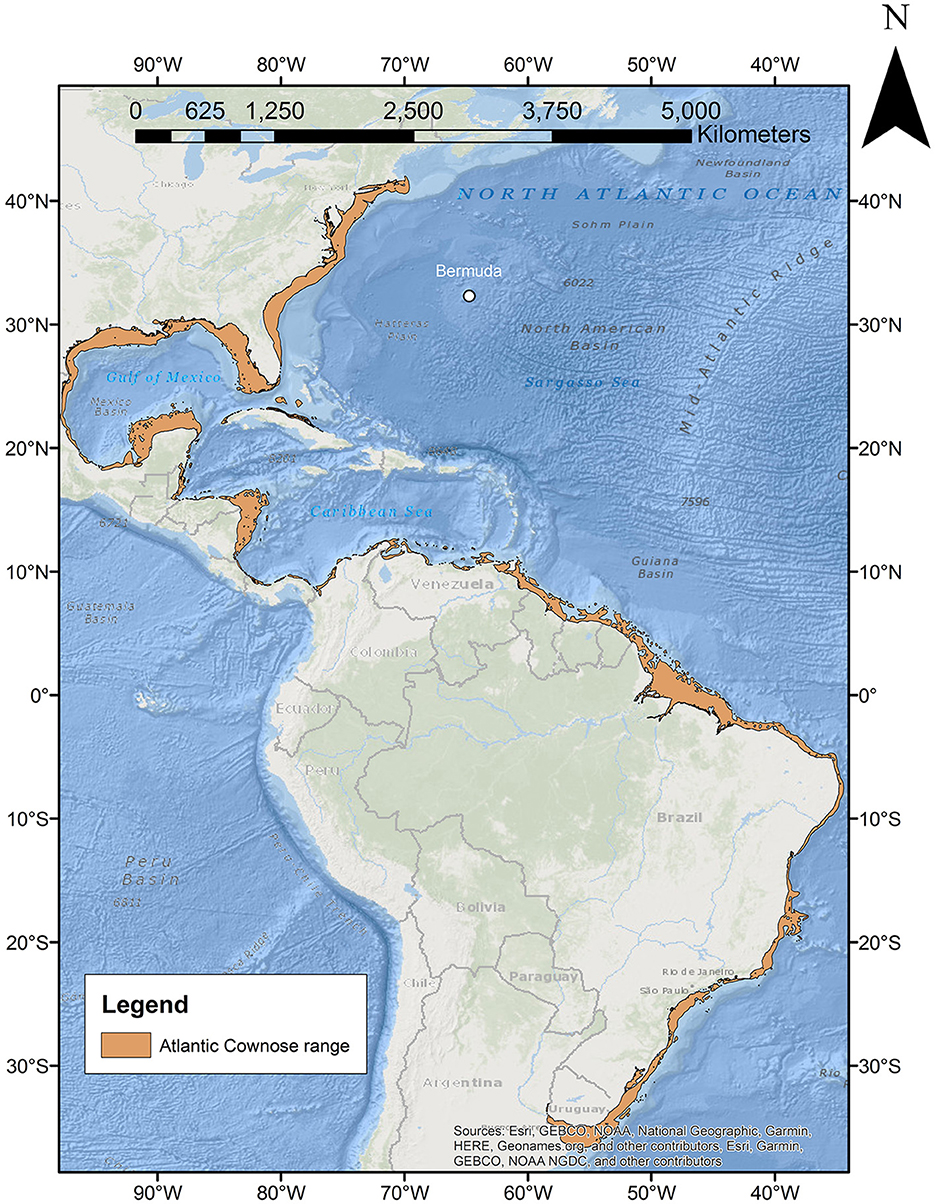
Figure 1. Map of current R. bonasus distribution (orange polygons) as per Carlson et al. (16) in relation to Bermuda (white circle).
2 Methods
We compiled recent information on cownose rays from Bermuda using multiple data sources: (1) informal, personal communications with Fisheries Officers and staff at the Bermuda Aquarium, Museum, and Zoo and Bermuda Institute of Ocean Sciences; (2) photographs contributed to Fisheries Officers by local citizen-scientists; and (3) recent on-water observations and collections conducted by the authors.
2.1 On-water observations and collections
Visual surveys were conducted by scientists in October 2022, July 2023, and October 2023. Surveys followed the methods of Ajemian et al. (8), targeting whitespotted eagle rays. Briefly, 2–3 individuals stood at the bow of the vessel while navigating across Harrington Sound and Flatts Inlet at slow speeds (< 4 kt). When a ray was encountered, the position and time was recorded onto a handheld GPS unit (Garmin GPSMAP 78sc). Additional observations such as estimated group sizes and behaviors were also recorded.
Opportunistic collections of cownose rays by hand, seine, or entanglement nets were conducted by the Bermuda Aquarium, Museum, and Zoo in 2021 to acquire specimens for public display (n = 2) as well as by the authors herein during a single incidental encounter in October 2022 (n = 3). All rays were measured for disk width, weighed, and photographed. Additionally, to assign preliminary species identifications, tooth series counts were taken from the dorsal dental plate of the latter three individuals, following Jones et al. (19).
2.2 Genetic species identifications
Fin clips were collected and preserved in 70% ethanol from four cownose rays; one from an animal caught in 2021 that remains in captivity and three during the October 2022 surveys. Genomic DNA was extracted from ~10–20 mg of each fin clip tissue with a Qiagen DNeasyTM DNA extraction kit (Qiagen, USA), using the manufacturer's protocol, except that tissue samples were digested for ~12 h. A 442-base pair (bp) portion of the mitochondrial (mtDNA) NADH dehydrogenase subunit 2 (ND2) gene was amplified using a forward primer (5′-GAACCCYTTAATCCTCTYCATC-3′) by McDowell and Fisher (unpubl. data) and a reverse primer (5′-GGATTGATAGTACGCCTATGG-3′) by Weber et al. (20). Polymerase Chain Reaction (PCR) reactions and cycling conditions followed the methods described in Weber et al. (20). PCR products were cleaned using 3 μL of ExoSAP-IT (ThermoFisher) and sequenced by Eurofins Genomics, LLC (Louisville, KY) on an Applied Biosystems™ 3730XL DNA Analyzer with BigDye™ Terminator 3.1 sequencing chemistry (Applied Biosystems™). Forward and reverse sequences were aligned to create a consensus sequence for each individual in CodonCode v. 6.0.2 (CodonCode Corporation, Dedham, USA). Species identifications were verified via a NCBI Blast search that compared resultant haplotypes to known mtDNA ND2 haplotypes for cownose rays in the north Atlantic basin.
3 Results
3.1 Occurrence and distribution
Local entities recollect cownose ray presence in Bermuda as early as 2012, and they have since been observed across most of the island's protected coastal waters but not on the exposed South Shore (Figure 2, C.T. Flook, Bermuda Institute of Ocean Sciences, personal communication, October 2023). However, the first available photographic evidence of cownose rays was obtained from an encounter on 12 May 2016 by a citizen scientist. These photographs were taken at Mill Creek in the parish of Pembroke, and although only two individuals were photographed swimming together (Figure 3), school sizes of up to 12 individuals were reported from the adjacent Great Sound within months of that sighting (M. Jones, personal communication, May 2016). Recent on-water surveys revealed 14 individual sightings of cownose rays in the Flatt's Inlet—Harrington Sound region of Bermuda between October 2022 and 2023 (Figure 2). Sightings included group sizes ranging from 1 to 12 individuals, with the largest groups observed in Flatt's Inlet and smaller groups (1–2 individuals) in the southern and western portions of Harrington Sound.
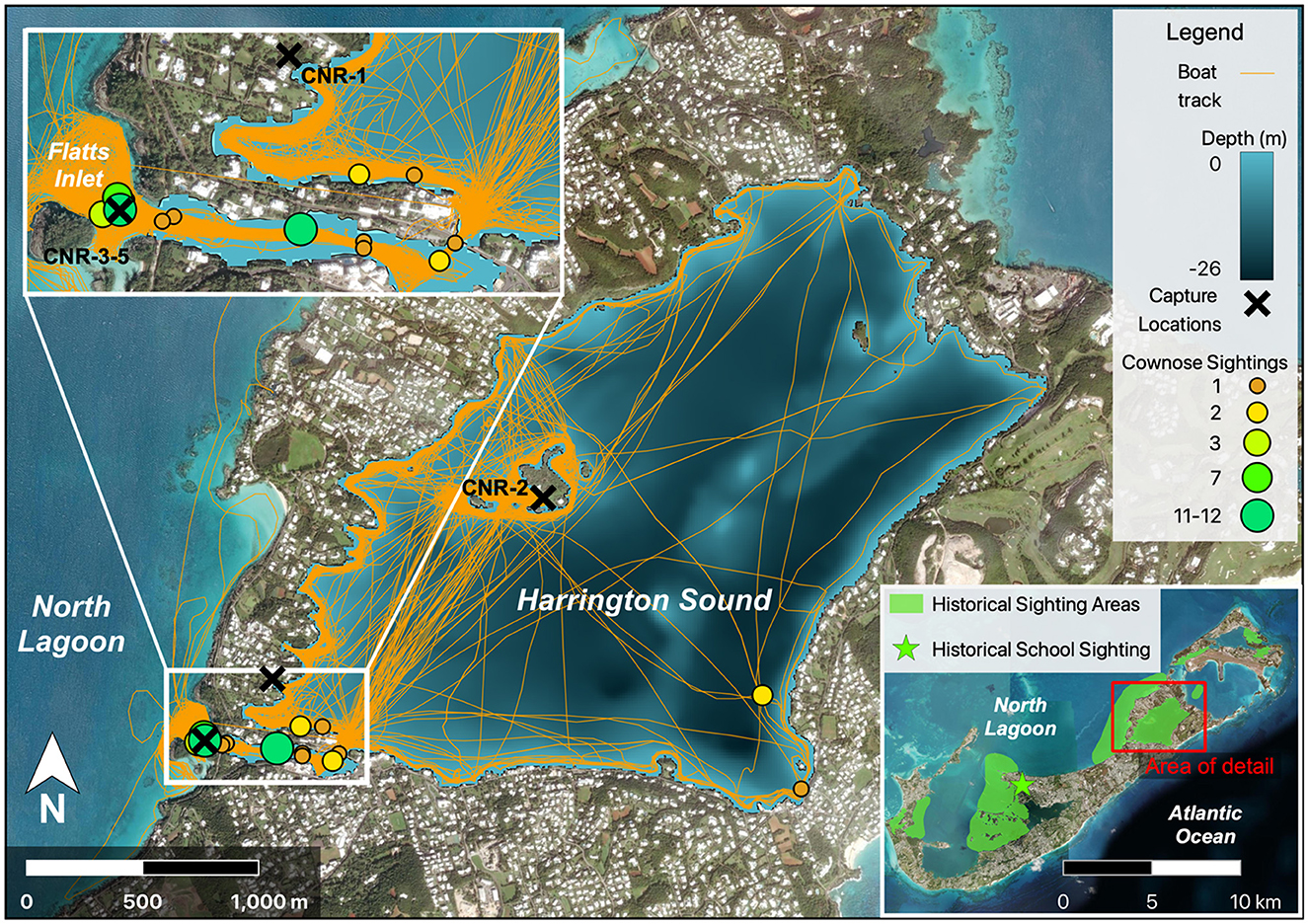
Figure 2. Map of cownose ray sightings across Bermuda. Inset map (bottom right) represents the overview of Bermuda and all regions where cownose rays have been reported (green polygon), with the green star marking Mill Creek where photographs were obtained from 2016 (Figure 3). Red box bounds the Harrington Sound and Flatts Inlet region, which is expanded in detail on the left (top-left) and displays sighting locations and estimated school sizes from 2021 to 2023, overlain atop vessel tracks (orange polylines) and bathymetry of Harrington Sound. Black “X” marks capture location of rays collected in 2021 and 2022.
3.2 Collections
A total of five individual cownose rays were collected between 2021 and 2022. All individuals collected were females, and thus maturity state could not be assessed based on external anatomy. The first two individuals (CNR-1 and CNR-2) were captured as sole individuals in Harrington Sound while the three individuals collected in 18-Oct 2022 (CNR-3,4, and 5) were part of a school size estimated at 12 individuals in Flatts Inlet (Figure 3).
3.3 Species identifications
Tooth series counts for the three individuals collected in October 2022 were all 7, suggesting the captured species was R. bonasus (Table 1). Genetic analysis for the fin clips from four cownose rays revealed two mtDNA ND2 haplotypes that matched known haplotypes for R. bonasus, with only a single bp mutation between the two haplotypes. The haplotype for the single animal caught in 2011 (CNR-2) matched 100% with RBON16 (GenBank accession no. MT410202), whereas the haplotypes for the three individuals caught in the October 2022 surveys (CNR-3-5) were 100% similar to RBON1 (GenBank accession no. MT410194) [see Weber et al. (20)].

Table 1. Biological and genetic characteristics of the five individual cownose rays collected and analyzed in the study.
4 Discussion
4.1 Species identification
The combination of morphological and genetic data support the species identification of R. bonasus. Tooth column counts do not match identifications for congeneric species in the Atlantic, R. brasiliensis or R. marginata, both of which are reported to have 13 series (21). The two mtDNA ND2 haplotypes from the four individuals were a 100% match to known R. bonasus haplotypes. RBON1, found in three individuals, is a common haplotype previously found in individuals from the east coast of the U.S. and the northeastern Gulf of Mexico [see Weber et al. (20)]. RBON16, seen here in one individual, was previously only found in a single individual from the east coast of the U.S. (20). These haplotypes are 5.66–5.88% different to known R. brasiliensis mtDNA ND2 haplotypes [see Weber et al. (20)], making it unlikely the Bermuda specimens are R. brasiliensis. While the possibility that these individuals are hybrids cannot be ruled out since mtDNA is maternally inherited, hybridization of cownose rays in the wild has not been documented.
4.2 Range expansion of the Atlantic cownose ray
Research on R. bonasus occurrence and migratory patterns in the Greater Caribbean region (including the Caribbean Sea, Gulf of Mexico, Bahamas, Bermuda, and the Florida peninsula) is sparse outside of continental U.S. and Mexican waters. To date, compiled data suggest the species broadly extends across the Atlantic coastlines of the South and Central American continents (16). As such, the recent finding of cownose rays in Bermuda, after hundreds of years of natural history records, suggests this is a novel species range expansion to this atoll-like system.
It is currently unknown whether R. bonasus in Bermuda is resident or seasonally migratory. Based on thermal conditions alone, cownose rays can likely reside in Bermuda for extended periods. Outmigration of cownose rays along the Atlantic coast (i.e., southbound departure from the Chesapeake Bay in fall) is triggered by changes in thermal conditions; in particular, as temperatures fall below 20°C (22, 23). In Bermuda, wintertime temperatures can approach 15°C within shallow inshore bays (1), which could force rays to become migratory. However, since those conditions do not last for long periods, it is possible rays simply move to warmer waters in the North Lagoon, similar to the pattern observed in the northern Gulf of Mexico (9, 24), or are resident, as reported in parts of south Florida (25, 26). Along the Atlantic coast of the U.S., northward migration cues for females and males depend on different factors: sea surface temperature for females, and day of year for males (22). While all collections of cownose rays in Bermuda to date are of females, the acquisition of small, immature rays (e.g., CNR-3) suggests pupping may have recently occurred here. Moreover, there have been reports of behaviors approximating copulation (C. Aming, personal communication, July 2023), including close-following and biting of pectoral fins as described by McCallister et al. (10), suggesting that male rays are also present in the area. Should cownose rays continue to survive in Bermuda waters, the species' low fecundity (1 pup/year) will limit its capacity for rapid population growth (16). However, a more accurate assessment of the current population size is sorely needed.
4.3 Potential mechanisms of dispersion
While Bermuda is (at its closest) 960 km from Cape Hatteras, North Carolina, and thus within the migration distances (< 1,000 km) recorded for cownose rays along the continental U.S. (27, 28), movements over deep ocean basins (i.e., off the continental shelf) appear uncommon [max recorded depth ~50 m (27)]. Though capable of extensive migrations (100's of km) in the Gulf of Mexico (29), the native and related pelagic ray species A. narinari does not appear to leave Bermuda waters seasonally (8, 9), although movements into deeper waters are likely in the winter (11). The tiger shark, on the other hand, regularly migrates between Bermuda, Bahamas, and the Turks and Caicos Islands (6), Lemon (Negaprion brevirostris) and blacktip (Carcharhinus limbatus) sharks are capable of similar migrations over deep waters and have also been recorded to connect waters of the U.S. Virgin Islands and Florida Atlantic coast (30). As a benthic mesopredator, such an offshore migration would be energetically expensive and potentially risky for cownose rays, suggesting that physical (i.e., abiotic) forcing facilitated the species' dispersion.
Oceanography may have played a major role in this founder dispersal event for R. bonasus, as oceanic currents significantly influence the trajectory of migrating marine animals (31, 32). Often interacting with oceanography are atmospheric conditions such as windage and extreme weather events (e.g., storms), which have been shown to trigger abnormal migratory behaviors in other large marine taxa such as loggerhead sea turtles (Caretta caretta) (33). Interestingly, during the time period preceding the expansion of cownose rays to Bermuda (i.e., winter 2010), the North Atlantic Ocean experienced a pronounced southward shift in westerly winds that were also unusually strong and influenced current dynamics in the region (34). This transition, fueled by anomalously negative North Atlantic Oscillation Indices in winter 2009–2010, December 2010, and March 2013, narrowed a normally larger convergence zone in the Sargasso Sea and facilitated unprecedented advection of floating Sargassum seaweed toward the eastern Atlantic (34), including Bermuda (S.R. Smith, pers. obs). This climatological anomaly and associated oceanographic changes may have played a similar role in shifting pelagic cownose rays eastward from their established range. Indeed, a single cownose ray that was satellite tagged in Chesapeake Bay in October 2009 and moved southward off the Carolinas in November was apparently geolocated in the Gulf Stream at the end of its track in January 2010 (27), suggesting potential temporal alignment with these conditions. This movement pattern was considered unusual for rays tagged in Chesapeake Bay, which are otherwise located off central Florida's Atlantic coast during wintertime (27, 28). Unfortunately, information on other tagged individuals was not available from this same period, restricting our interpretation of this basin-scale event as the sole mechanism behind cownose ray dispersion.
Extensive tropical storm activity also occurred between Bermuda and the continental U.S. in the years leading up to the first purported cownose ray sightings (http://coast.noaa.gov/hurricanes), including storms that could have interacted with southward migrating cownose rays in fall (e.g., Hurricane Sandy, October 2012) and displaced these animals offshore into the Gulf Stream. Similar events may have also played a role in re-distributing other benthic ray species recently sighted in Bermuda such as B. centroura (13), although this stingray is known to inhabit much deeper waters (35) and could possibly have gone unnoticed until recently. In summary, it is possible that multiple oceanographic changes and weather events facilitated cownose ray arrival to Bermuda.
4.4 Ecological considerations
Cownose rays have likely been in Bermuda for a decade already. This presents an interesting set of potential interactions with an otherwise stable subtropical marine ecosystem. One concern is related to the locally protected A. narinari, which is a native resident of Bermuda (12). Rays of the genera Rhinoptera and Aetobatus overlap in habitat and trophic niche elsewhere in subtropical to warm temperate waters around the globe. Due to the presence of mollusks in both species' diet, fishermen in the Mexican Gulf have expressed concern over competition between the two species and even suspected cownose rays negatively impact the more valuable eagle rays, which are fished locally (36). However, recent dietary data suggest the two species exhibit only modest niche overlap (37). A recent isotopic study suggested Aetobatus is less trophically constrained than Rhinoptera, possibly due to its larger size, gape, and buccal morphology, allowing it to exploit a wider range of prey (38). In Florida's Indian River Lagoon, the two genera occupy similar spaces, although visitation patterns to the same habitats are asynchronous (26). Additionally, cownose rays appear more associated with estuaries and can tolerate considerably lower salinities than A. narinari (24, 39), although these conditions are uncommon in Bermuda. Mill Creek, where the first confirmed (via photographs) encounter of R. bonasus occurred, is the only true estuary in Bermuda (40) and is lined with red and black mangroves and adjoining marsh land (41). Thus, while cownose rays have clearly expanded to other parts of Bermuda, this affinity for areas influenced by freshwater may partition them away from habitats occupied by eagle rays, and limit competition between the species.
4.5 Research needs
Several basic questions regarding cownose rays need to be addressed to understand their potential to interact with native species and resources, as well as how the species may fare in Bermuda in the future. The first is a question of how many cownose rays are actually present in Bermuda. At the moment, it remains unclear as to whether there is a single group of cownose rays that is repeatedly re-sighted in various locations, or whether the species is more broadly distributed across inshore sounds and harbors. This information could be gleaned using systematic aerial surveys, which has been successful elsewhere in documenting cownose ray distribution and extent (24, 42–44). Additionally, monitoring of size-class data may help to determine the level of reproductive success of the established population. Next, dietary information should be obtained from cownose rays to identify the prey resources that the species is interacting with, and whether any of these are shared with the protected A. narinari. Lastly, more research should be conducted into the potential mechanisms that facilitated the arrival of cownose rays to Bermuda, as this could reveal whether additional introductions of this species (and others) are possible in the future.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee, Florida Atlantic University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CH: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. LC: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. JP: Conceptualization, Data curation, Investigation, Project administration, Resources, Writing – original draft, Writing – review & editing. SS: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. CJ: Conceptualization, Data curation, Methodology, Resources, Writing – original draft, Writing – review & editing. NP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was partially supported by a National Science Foundation grant to MA (OCE #2143655). Genetic analysis was supported by the University of Southern Mississippi and the Mississippi INBRE, funded by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number: P20GM103476.
Acknowledgments
The authors would like to thank the following individuals from BAMZ for field and logistical support: E. Andrew, C. Aming, K. Cooper, B. Outerbridge, and I. Walker. Special thanks are due to several individuals who provided reports, including C. Flook, as well as folks from FAU Harbor Branch such as M. McCallister, T. J. Ostendorf, and B. C. DeGroot.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The first author declared that he was an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Coates KA, Fourqurean JW, Kenworthy WJ, Logan A, Manuel SA, Smith SR. Introduction to Bermuda: geology, oceanography and climate. In: Sheppard CRC, , editor. Coral Reefs of the United Kingdom Overseas Territories. Coral Reefs of the World 4. Dordrecht: Springer Netherlands (2013). p. 115–33.
2. Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson MAX, et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience. (2007) 57:573–83. doi: 10.1641/B570707
3. Locke JM, Coates KA, Bilewitch JP, Holland LP, Pitt JM, Smith SR, et al. Biogeography, biodiversity and connectivity of Bermuda's reefs. In:Sheppard CRC, , editor. Coral reefs of the United Kingdom Overseas Territories. Coral Reefs of the World 4. Dordrecht: Springer Netherlands (2013). p. 153–72.
4. Clark E, Kristof E. Deep-sea elasmobranchs observed from submersibles off Bermuda, Grand Cayman, Freeport, Bahamas. In:Pratt HL, Gruber SH, Taniuchi T, , editors. Elasmobranchs as Living Resources: Advances in the Biology, Ecology Systematics, and the Status of the Fisheries. Silver Spring, MD: NOAA Tech Rep NMFS (1990). p. 269–84.
5. Carey FG, Clark E. Depth telemetry from the sixgill shark, Hexanchus griseus, at Bermuda. Environ Biol Fishes. (1995) 42:7–14. doi: 10.1007/BF00002345
6. Lea JS, Wetherbee BM, Queiroz N, Burnie N, Aming C, Sousa LL, et al. Repeated, long-distance migrations by a philopatric predator targeting highly contrasting ecosystems. Sci Rep. (2015) 5:11202. doi: 10.1038/srep11202
7. Lea JS, Wetherbee BM, Sousa LL, Aming C, Burnie N, Humphries NE, et al. Ontogenetic partial migration is associated with environmental drivers and influences fisheries interactions in a marine predator. ICES J Mar Sci. (2018) 75:1383–92. doi: 10.1093/icesjms/fsx238
8. Ajemian MJ, Powers SP, Murdoch TJ. Estimating the potential impacts of large mesopredators on benthic resources: integrative assessment of spotted eagle ray foraging ecology in Bermuda. PLoS ONE. (2012) 7:e40227. doi: 10.1371/journal.pone.0040227
9. Ajemian MJ, Powers SP. Towed-float satellite telemetry tracks large-scale movement and habitat connectivity of myliobatid stingrays. Environ Biol Fishes. (2014) 97:1067–81. doi: 10.1007/s10641-014-0296-x
10. McCallister M, Mandelman J, Bonfil R, Danylchuk A, Sales M, Ajemian M. First observation of mating behavior in three species of pelagic myliobatiform rays in the wild. Environ Biol Fishes. (2020) 103:163–73. doi: 10.1007/s10641-019-00943-x
11. Brewster LR, Cahill BV, Burton MN, Dougan C, Herr JS, Norton LI, et al. First insights into the vertical habitat use of the whitespotted eagle ray Aetobatus narinari revealed by pop-up satellite archival tags. J Fish Biol. (2021) 98:89–101. doi: 10.1111/jfb.14560
12. Smith-Vaniz WF, Collette BB, Luckhurst BE. Fishes of Bermuda: history, zoogeography, annotated checklist, and identification keys. Am Soc Ichthy Herp Spec Publ. (1999) 4:424.
14. Bigelow HB, Schroeder WC. Fishes of the Gulf of Maine (No. 592). Washington, DC: US Government Printing Office (1953).
15. McEachran JD, De Carvalho MR, Carpenter KE. Batoid Fishes. The Living Marine Resources of the Western Central Atlantic. (2002). p. 507–89.
16. Carlson J, Charvet P, Avalos C, Blanco-Parra MP, Briones Bell-lloch A, Cardenosa D, et al. Rhinoptera bonasus. The IUCN Red List of Threatened Species 2020: e.T60128A3088381. (2020). doi: 10.2305/IUCN.UK.2020-3.RLTS.T60128A3088381.en
17. Carlson J, Charvet P, Avalos C, Blanco-Parra MP, Briones Bell-lloch A, Cardenosa D, et al. Rhinoptera brasiliensis. The IUCN Red List of Threatened Species 2020: e.T44595A2997621. (2020). doi: 10.2305/IUCN.UK.2020-3.RLTS.T44595A2997621.en
18. Jabado RW, Chartrain E, De Bruyne G, Derrick D, Dia M, Diop M, et al. Rhinoptera marginata. The IUCN Red List of Threatened Species 2021: e.T161463A49318282. (2021). doi: 10.2305/IUCN.UK.2021-2.RLTS.T161463A49318282.en
19. Jones CM, Hoffmayer ER, Hendon JM, Quattro JM, Lewandowski J, Roberts MA, et al. Morphological conservation of rays in the genus Rhinoptera (Elasmobranchii, Rhinopteridae) conceals the occurrence of a large batoid, Rhinoptera brasiliensis Müller, in the northern Gulf of Mexico. Zootaxa. (2017) 4286:499–514. doi: 10.11646/zootaxa.4286.4.3
20. Weber HK, Jones CM, Ajemian MJ, McCallister MP, Winner BL, Poulakis GR, et al. Genetic evidence supports a range extension for the Brazilian cownose ray Rhinoptera brasiliensis in the western North Atlantic. J Fish Biol. (2021) 98:577–82. doi: 10.1111/jfb.14582
21. Last P, Naylor G, Séret B, White W, de Carvalho M, Stehmann M. Rays of the World. Clayton, VIC: CSIRO Publishing (2016).
22. Bangley CW, Edwards ML, Mueller C, Fisher RA, Aguilar R, Heggie K, et al. Environmental associations of cownose ray (Rhinoptera bonasus) seasonal presence along the US Atlantic Coast. Ecosphere. (2021) 12:e03743. doi: 10.1002/ecs2.3743
23. Smith JW, Merriner JV. Age and growth, movements and distribution of the cownose ray, Rhinoptera bonasus, in Chesapeake Bay. Estuaries. (1987) 10:153–64. doi: 10.2307/1352180
24. Ajemian MJ, Powers SP. Seasonality and ontogenetic habitat partitioning of cownose rays in the northern Gulf of Mexico. Estuar Coasts. (2016) 39:1234–48. doi: 10.1007/s12237-015-0052-2
25. Collins AB, Heupel MR, Motta PJ. Residence and movement patterns of cownose rays Rhinoptera bonasus within a south-west Florida estuary. J Fish Biol. (2007) 71:1159–78. doi: 10.1111/j.1095-8649.2007.01590.x
26. Cahill BV, DeGroot BC, Brewster LR, Lombardo SM, Bangley CW, Ogburn MB, et al. Visitation patterns of two ray mesopredators at shellfish aquaculture leases in the Indian River Lagoon, Florida. PLoS ONE. (2023) 18:e0285390. doi: 10.1371/journal.pone.0285390
27. Omori KL, Fisher RA. Summer and fall movement of cownose ray, Rhinoptera bonasus, along the east coast of United States observed with pop-up satellite tags. Environ Biol Fishes. (2017) 100:1435–49. doi: 10.1007/s10641-017-0654-6
28. Ogburn MB, Bangley CW, Aguilar R, Fisher RA, Curran MC, Webb SF, et al. Migratory connectivity and philopatry of cownose rays Rhinoptera bonasus along the Atlantic coast, USA. Mar Ecol Prog Ser. (2018) 602:197–211. doi: 10.3354/meps12686
29. DeGroot BC, Bassos-Hull K, Wilkinson KA, Lowerre-Barbieri S, Poulakis GR, Ajemian MJ. Variable migration patterns of whitespotted eagle rays Aetobatus narinari along Florida's coastlines. Mar Biol. (2021) 168:1–21. doi: 10.1007/s00227-021-03821-2
30. Legare B, DeAngelis B, Skomal G. After the nursery: regional and broad-scale movements of sharks tagged in the Caribbean. Mar Ecol. (2020) 41:e12608. doi: 10.1111/maec.12608
31. Hays GC. Ocean currents and marine life. Curr Biol. (2017) 27:R431–510. doi: 10.1016/j.cub.2017.01.044
33. Crowe LM, Hatch JM, Patel SH, Smolowitz RJ, Haas HL. Riders on the storm: loggerhead sea turtles detect and respond to a major hurricane in the Northwest Atlantic Ocean. Mov Ecol. (2020) 8:1–13. doi: 10.1186/s40462-020-00218-6
34. Johns EM, Lumpkin R, Putman NF, Smith RH, Muller-Karger FE, Rueda-Roa DT, et al. The establishment of a pelagic Sargassum population in the tropical Atlantic: biological consequences of a basin-scale long distance dispersal event. Progr Oceanogr. (2020) 182:102269. doi: 10.1016/j.pocean.2020.102269
35. Carlson J, Charvet P, Avalos C, Briones Bell-lloch A, Cardenosa D, Espinoza E, et al. Bathytoshia centroura. The IUCN Red List of Threatened Species 2020: e.T104065040A3122808. (2020). doi: 10.2305/IUCN.UK.2020-3.RLTS.T104065040A3122808.en
36. Cuevas-Zimbrón E, Pérez-Jiménez JC, Méndez-Loeza I. Spatial and seasonal variation in a target fishery for spotted eagle ray Aetobatus narinari in the southern Gulf of Mexico. Fish Sci. (2011) 77:723–30. doi: 10.1007/s12562-011-0389-9
37. Serrano-Flores F, Torres-Rojas YE, Ajemian MJ, Mendoza-Carranza M, Pérez-Jiménez JC. Advances in the study of the trophic niche of batoids with distribution in Mexican waters. Mar. Ecol. (2021) 42:e12687. doi: 10.1111/maec.12687
38. Chan AJ, Raoult V, Jaine FR, Peddemors VM, Broadhurst MK, Williamson JE. Trophic niche of Australian cownose rays (Rhinoptera neglecta) and whitespotted eagle rays (Aetobatus ocellatus) along the east coast of Australia. J Fish Biol. (2022) 100:970–8. doi: 10.1111/jfb.15028
39. Roskar G, McCallister MP, Schaefer AM, Ajemian MJ. Elasmobranch community dynamics in Florida's southern Indian River Lagoon. Estuar Coast. (2021) 44:801–17. doi: 10.1007/s12237-020-00804-2
40. Lyons WB, Armstrong PB, Gaudette HE. Trace metal concentrations and fluxes in Bermuda sediments. Mar Pollut Bull. (1983) 14:65–8. doi: 10.1016/0025-326X(83)90194-7
41. Thomas ML. Mangrove swamps in Bermuda. Atoll Res Bull. (1993) 386:1–17. doi: 10.5479/si.00775630.386.1
42. Blaylock RA. Distribution and abundance of the cownose ray, Rhinoptera bonasus, in lower Chesapeake Bay. Estuaries. (1993) 16:255–63. doi: 10.2307/1352498
43. Goodman MA, Conn PB, Fitzpatrick E. Seasonal occurrence of cownose rays (Rhinoptera bonasus) in North Carolina's estuarine and coastal waters. Estuar Coasts. (2011) 34:640–51. doi: 10.1007/s12237-010-9355-5
Keywords: elasmobranch, Bermuda, Atlantic, cownose, batoid, ray, range expansion, migration
Citation: Ajemian MJ, Hampton CM, Coleman LM, Pitt JM, Smith SR, Jones CM and Phillips NM (2024) Recent expansion of the Atlantic cownose ray (Rhinoptera bonasus) into Bermudian waters. Front. Fish Sci. 2:1394011. doi: 10.3389/frish.2024.1394011
Received: 29 February 2024; Accepted: 18 April 2024;
Published: 17 May 2024.
Edited by:
Julia L. Y. Spaet, University of Cambridge, United KingdomReviewed by:
Andrés Javier Jaureguizar, Comisión de Investigaciones Científicas, ArgentinaManuel Mendoza-Carranza, The South Border College (ECOSUR), Mexico
Copyright © 2024 Ajemian, Hampton, Coleman, Pitt, Smith, Jones and Phillips. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew J. Ajemian, bWFqZW1pYW5AZmF1LmVkdQ==
 Matthew J. Ajemian
Matthew J. Ajemian Cecilia M. Hampton1
Cecilia M. Hampton1 Lauren M. Coleman
Lauren M. Coleman Joanna M. Pitt
Joanna M. Pitt Struan R. Smith
Struan R. Smith Christian M. Jones
Christian M. Jones