
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Hematol. , 07 February 2025
Sec. Hematopoiesis and Stem Cells
Volume 4 - 2025 | https://doi.org/10.3389/frhem.2025.1525132
This article is part of the Research Topic Niche Contributions to Clonal Expansion View all 3 articles
Hematopoietic stem cells (HSCs) undergo a functional decline during aging. The intrinsic characteristics of aged HSCs have been well-described and include a strong myeloid bias, an increase in total number, and a decrease in functionality during transplantation. The impact of the aged bone marrow microenvironment, or niche, on HSCs is less well understood. It is critical to understand the changing condition of the niche during aging, and its ability to support HSCs, as this could reveal the very signals and mechanisms needed to improve HSC fitness. Furthermore, heterochronic transplantation provides an approach to test the influence of an aged recipient niche on young donor HSCs, and conversely, of a young recipient niche on aged donor HSCs. Importantly, these experiments demonstrated that donor HSC engraftment is reduced if the recipient niche is aged, and conversely, the young niche can rejuvenate aged donor HSCs. Here we will focus on the interactions between aged HSCs and their microenvironment. We will highlight current controversies, research gaps, and future directions.
The aged hematopoietic compartment is characterized by skewed differentiation towards myeloid lineages and decline in normal cellular functions. These aging-associated abnormalities occur in the primitive HSCs, as well as in terminally differentiated immune cells (1, 2). Aged HSCs undergo phenotypic expansion but show reduced reconstitution and self-renewal capabilities upon stress (3–5). The proportion of myeloid-biased HSCs (my-HSCs) is increased during aging, leading to decreased lymphopoiesis, primarily in B cells, and diminished adaptive immunity; this is concomitant with increased myelopoiesis and incidence of myeloid malignancies (6–8). The essential role of my-HSCs in driving aged hematopoietic phenotypes is supported by a recent report, showing that antibody-mediated depletion of my-HSCs in aged mice rejuvenates the hematopoietic compartment and restores some features of youthful immunity (9).
Reciprocal transplants have shown that aged HSCs and progenitors transplanted into young recipients can partly reverse the aging phenotype (10–13). Conversely, young HSCs and progenitors can adopt an aged phenotype when transplanted into aged recipients (14). This provides strong evidence that the bone marrow (BM) microenvironment has a significant impact on HSCs throughout the lifespan. There is still controversy related to both the changes in niche cell numbers and their spatial distribution in the BM during aging that will be discussed in further detail below (15–21).
Aged HSCs exhibit distinct physical properties and molecular hallmarks. They can be sufficiently distinguished from young HSCs using deep machine-learning techniques based solely on their morphology (22). When transplanted, aged HSCs lodge further from the endosteum after homing (23, 24). In addition, aged HSCs display molecular hallmarks in comparison to young HSCs, including elevated small Rho GTPase Cdc42, loss of protein polarity (23), and altered epigenetic architecture (1). Although it remains unclear how these molecular alterations contribute to dysregulated HSC functions, targeting elevated Cdc42 via its specific inhibitor is shown to rejuvenate aged HSCs (23).
Megakaryocytes are an important component of the HSC niche and are thought to regulate HSC quiescence by secreting various factors, including CXCL4 (25–27). Analysis of the spatial relationship between HSCs and megakaryocytes has shown that HSCs are significantly closer to megakaryocytes in the niche, further supporting a functional relationship between the cell types (20, 25). Multiple studies have shown that megakaryocytes and megakaryocyte progenitors (MkP) expand in aged BM (14–17, 20). One hypothesis is that an increased distance between HSCs and megakaryocytes during aging contributes to loss of quiescence. However, there is still not a consensus on whether the distance between HSCs and megakaryocytes significantly changes during aging; some approaches show an increase (15, 17), while others do not (20).
Downregulation of DNA repair pathways cause early onset of aging-like phenotypes in mouse and human (28–30), suggesting that DNA damage and accumulation of DNA damage contribute to aged HSC phenotypes, for example, increased incidence of Clonal Hematopoiesis of Indeterminate Potential (CHIP) (31). CHIP refers to the expansion of peripheral blood cells derived from HSCs with at least one somatic driver mutation in healthy elderly individuals (32–34). CHIP is strongly linked to aging and confers an increased risk for blood cancers, non-hematological diseases (e.g., cardiovascular disease), and all-cause mortality (32–35). There is an approximately 2-3-fold increase in mutation frequency in aged HSCs (36, 37). However, such a linear increase in the frequency over time does not correlate with the exponential increase in CHIP and myeloid leukemia seen in the elderly. Mathematical modeling of HSC aging based on evolutionary theories further suggests that accumulation of DNA damage in HSCs is insufficient to alter HSC fitness (38, 39). Rather, these models suggest that extrinsic mechanisms in aged BM microenvironment are the major selective driving force for aging-associated CHIP and myeloid leukemia. This hypothesis is supported by the known roles of different BM microenvironment cell types in regulating adult HSC functions as detailed below.
Clonal hematopoiesis is associated with aging and leukemia initiation, and therefore must be studied in the context of the aged niche. Using a pool of transduced donor hematopoietic progenitor cells, Vas et al. found that transplantation into aged recipients reduced clonality compared to young recipients (40). This study also found that transplant of hematopoietic progenitor cells into an aged microenvironment produced the characteristic increase in myeloid and decrease in lymphoid cell output associated with aged HSCs.
HSCs and terminally differentiated immune cells exhibit functional decline during aging, as well as significant changes in lineage output (i.e, reduction or expansion of certain subsets). Aged immune cells are the main contributor to “inflammaging”, which refers to unresolved BM microenvironment and systemic inflammation in the absence of pathogens, through secreting inflammatory cytokines (41, 42). One example is IL-1 produced by myeloid cells that increases during aging, creating a vicious cycle of Tet2+/− clonal expansion that contributes to CHIP via increased HSPC proliferation (31). Similarly, chronic inflammation induced with IL-1β injections in young mice can recapitulate aspects of hematopoietic aging (19, 43). Another example is Ccl5 (RANTES) that is enriched in the aged microenvironment (12). Exposure of young HSCs to Ccl5 induced the same myeloid bias observed in aged HSCs. Interestingly, in Ccl5 knockout (KO) mice there was an increase in lymphocytes, suggesting that Ccl5 is required for steady-state balance of lymphoid and myeloid lineages. As evidence that the microenvironment has the potential to ameliorate aged HSCs, transplant of aged HSCs into Ccl5 KO recipients helped balance lineage output, with significantly fewer myeloid and more B cells being produced. Mechanistically, Ccl5 activates the mTOR pathway that has a critical role in the aging process.
The development of single cell RNA-Seq (scRNA-seq) technology has provided a comprehensive view of all immune cell types during aging, validating and further expanding our perspective on aged immunity (2). Since most of these studies were performed on immune cells harvested from peripheral tissues instead of BM, how the microenvironment impacts immunity during aging remains largely unknown.
Diminished phagocytosis by macrophages, neutrophils, and dendritic cells, and their reduced efferocytosis to engulf apoptotic cells, have been described in aged mice and humans (44–46). Consistent with the increased proportion of my-HSCs during aging, there is a gradual expansion of circulating myeloid cell populations, mainly monocytes and neutrophils, relative to lymphoid cell populations. Although significant changes occur in tissue-resident macrophages during aging, analysis of circulating monocytes (i.e., macrophage precursors) reveals an expansion of non-classical monocytes without significant transcriptomic alterations in young vs old healthy humans (2). By contrast, changes in short-lived neutrophils are observed in aged mouse BM, with significant expansion of the IL-1β-expressing subset of neutrophils (47, 48), suggesting a role for the aged BM microenvironment in age-associated neutrophil dysregulation. The expansion of pro-inflammatory aged neutrophils can be ameliorated by systemic dietary intervention, such as NAD(+) augmentation with nicotinamide riboside (49), providing a metabolic preventative approach.
The age-associated decrease in lymphopoiesis is primarily reflected in a reduced B cell compartment. Despite their decreased number, a progressive increase in B cell clonality is seen in aging mice, which is attributed to a cluster of plasma B cells (50). In addition, the aged B cell compartment shows altered B cell composition and function, such as increased incidence of monoclonal gammopathy of undetermined significance in mice and humans that has been associated with pre-malignant multiple myeloma (51), and expansion of “age-associated B cells” in mice (52, 53). These age-associated B cells are distinct from the conventional naïve and memory B cells and are thought to arise in response to damage-associated molecular patterns, such as debris and chromatin from apoptotic cells, via the TLR7/TLR9 axis. These age-associated B cells secret IL-4 and IL-10 on activation (52), further contributing to inflammaging.
As essential players in anti-infection and anti-cancer immunity, T cells, including both CD4+ and CD8+ T cells, undergo aging-associated changes in both mice and humans (2). It was proposed that T cell aging is represented by two-tier molecular hallmarks (54). The primary hallmarks include thymic involution, mitochondrial dysfunction, profound genetic and epigenetic alterations, and loss of proteostasis. The secondary hallmarks include reduction of the TCR repertoire, naïve-memory imbalance, T cell senescence, and lack of effector plasticity. Together, these age-associated changes in T cells lead to immunodeficiency and inflammaging. Therefore, the aged adaptive immune system is characterized by T cell dysfunction that is responsible for elevated susceptibility to infection and cancer, as well as increased autoimmunity. Recent evidence indicates that age-associated intrinsic alterations in CD4+ T cells are sufficient to reduce humoral responses in young mice (55) and accelerate organism-wide aging phenotypes (56, 57). Since T cell development and mature T cells mainly stay outside the BM, it is conceivable that the impact of an aged BM microenvironment may be limited to BM-resident T cells. In addition to their contribution to inflammaging, BM-resident CD4+ Treg cells promote the survival and clonal advantage of aged HSCs through MHC II engagement and Connexin 43-mediated transfer of cAMP (58).
In summary, aged hematopoietic cells contribute significantly to increased BM inflammation, which in turn further exacerbates hematopoietic dysfunction.
The BM vasculature is heterogeneous, with vessels that vary in both function and location, and there has been ongoing debate about the primary niche for HSCs in the BM (59). In broad terms, these are classified as endosteal capillaries and central marrow sinusoids, with both regions having been considered as the true home of HSCs (20, 60–62). More specifically, small capillaries in endosteal regions of the metaphysis and trabecular bone of the diaphysis, called transition zone vessels (TZVs) (61), or Type H capillaries (CD31High, Emcn+ (63)), connect to arterioles and are surrounded by osteoprogenitors (61, 63, 64). Sinusoids or Type L vessels (CD31Low, Emcn+ (63)), are broad and fenestrated, found throughout the BM, and facilitate trafficking of hematopoietic cells into and out of the circulation (65).
The changes that occur in BMECs during aging are still being resolved, with different research groups presenting multiple views (Figure 1). Some have observed that endosteal ECs, like arterioles and TZVs, are reduced during aging, but overall EC volume and area occupancy are unchanged, leading to the conclusion that BM sinusoids are preserved upon aging (15). Other studies also found ECs near the endosteum of aged BM were reduced and central marrow sinusoids were unchanged, although small capillaries throughout the BM are increased (16, 18). In contrast to these studies, Wu et al. recently showed that BM sinusoids are more abundant in aged BM, and the number of arterioles is unchanged (20). In a study of middle-aged female mice, histological analysis of bones showed no change in the number of sinusoids and arterioles, however, fluorescence-activated cell sorting (FACS), followed by scRNA-seq and analysis, showed a slightly higher percentage of arterioles and lower percentage of sinusoids (66). It has been difficult to reliably quantify aged BMECs and mesenchymal stromal cells (MSCs) by FACS, presumably because the cells become more fragile (13, 19, 21, 66, 67). These studies have used different protocols, types of bones, imaging techniques, and FACS to quantify niche cells in the aged BM, and these methods must be comprehensively compared before a consensus can be reached. Ultimately, it must be determined if and how the changing proportions and spatial distribution of BMECs during aging directly impacts HSC function.
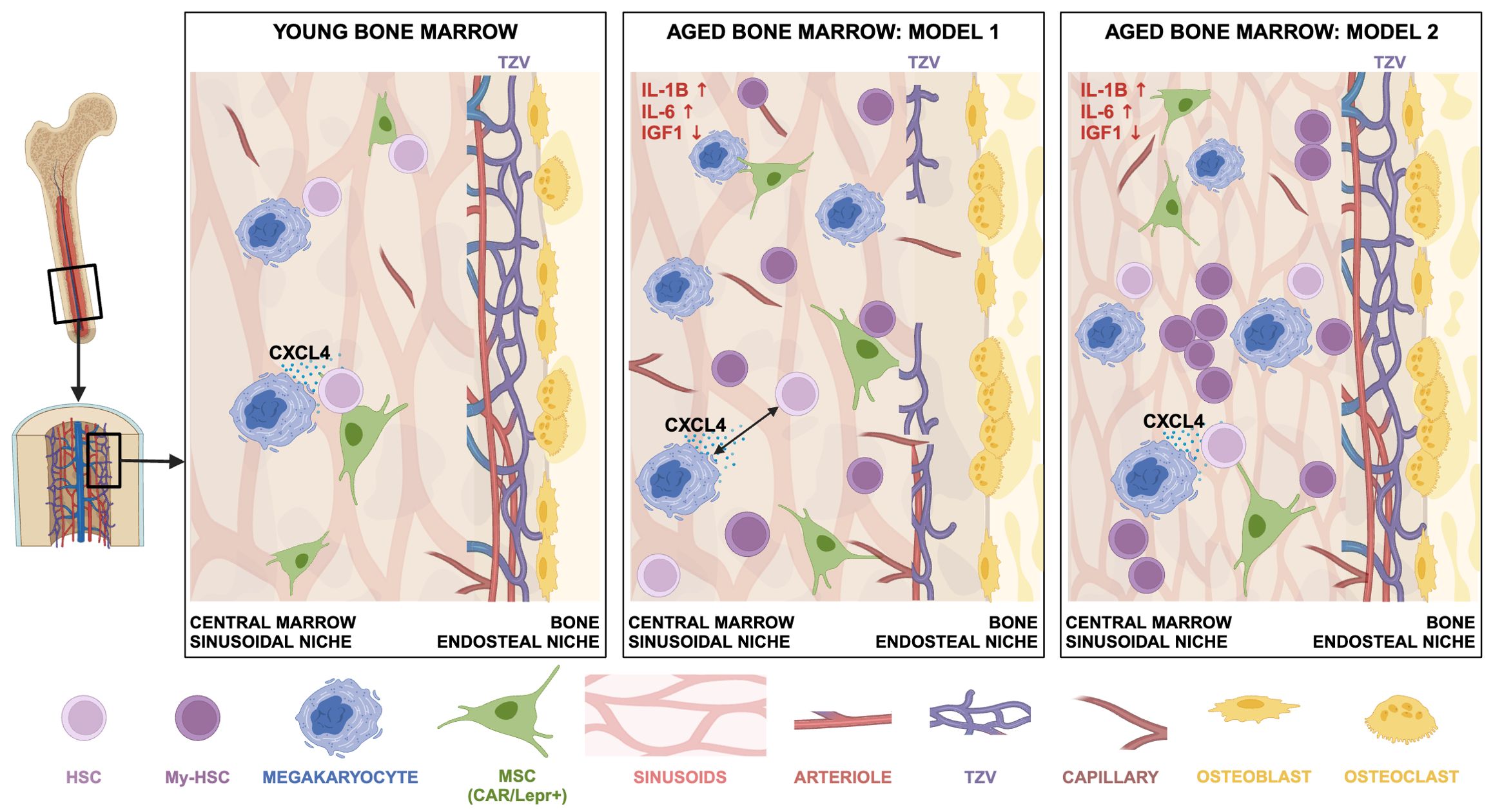
Figure 1. Different views of the changing bone marrow microenvironment during aging. Sinusoidal vessels and MSCs (CXCL12-abundant reticular (CAR)/Lepr+ cells) are abundant throughout the BM and are therefore always close to an HSC. Megakaryocytes promote HSC quiescence by producing CXCL4 (25). There is a consensus that during aging: 1) HSC and megakaryocyte numbers increase; 2) there is an increase in myeloid-biased HSCs (My-HSC); 3) inflammatory cytokines increase (e.g., IL-1β and IL-6), and IGF1 levels decrease; 4) osteoclasts increase and osteoblasts decrease, contributing to bone loss. Aged BM Model 1: The endosteal niche is compromised, with decreased numbers of TZVs and arterioles; it follows there are fewer HSCs near the endosteum. Sinusoids are largely unchanged in the central marrow, but capillaries are increased. There is greater distance between HSCs and megakaryocytes (15–19). Aged BM Model 2: The endosteal niche is intact and there is no change in arteriole numbers. Sinusoids in the central marrow are more abundant and shorter. There is no change in distances between HSCs and the endosteum, sinusoids, arterioles, or megakaryocytes. HSCs and progenitors tend to cluster closer together (20). Not shown: For clarity, many cell types, such as myeloid cells, have been excluded. Nestin-GFP+ MSCs, other MSC subtypes, and TH+ sympathetic nerve fibers are not shown because a consensus has not been reached on the abundance of these cell types during aging. Created in BioRender. Tamplin, O (2025). https://BioRender.com/l27j847.
Importantly, it may be functional changes and the supportive capacity of aged BMECs, such as cytokine production, that is more relevant than cell number or spatial relationships between HSCs and niche cell types. The reduced function of vasculature during aging has been well-described and reviewed elsewhere (68). Aged blood vessels become dilated, leaky, and have overall poor function. Reduced vascular endothelial growth factor (VEGF) signaling during aging, and associated capillary loss, may underlie the aging phenotype in many organ systems (69). Aged ECs have significantly lower levels of KITLG (aka SCF) and CXCL12 (aka SDF-1) (13, 18, 70). The AKT/mTOR axis specifically in ECs is required for maintaining HSC function (71). While mTOR inhibition is widely accepted as rejuvenating and promoting longevity (72), in BMECs reducing mTOR signaling negatively impacts HSC function (71). Aged ECs are sufficient to induce aging phenotypes in young HSCs (13), and similarly, chronic activation of inflammatory pathways in BMECs of young mice recreates the aging-associated myeloid-biased differentiation of HSCs (73). Blocking activated inflammatory pathways in BMECs can rescue HSC function (73), and likewise, young ECs have the capacity to restore some function in aged HSCs (13). Interestingly, young ECs can provide radioprotection for transplant recipients when co-infused with HSCs (13). Activation of Notch signaling in aged BMECs can restore some of the HSC support function, as the number of arterioles, capillaries, and phenotypic HSCs increased, but the number of functional HSCs did not increase, as determined by limiting dilution transplantation (18). Together, these findings suggest there is therapeutic potential in rejuvenating the aged niche to restore HSC function during aging.
The bone marrow receives a generous supply of nerves that enter the cavity with the vasculature that carry nutrients into the BM. Imaging and tracing studies revealed that the BM is largely comprised of sympathetic and sensory nerve fibers (17, 74–76). Many nerve fibers in the BM are tightly associated with arterioles, with very few nerve terminals located in the hematopoietic parenchyma and sinus walls. Sympathetic nerves are known to regulate various functions of HSCs at steady state and disease progression mainly via stromal cells, mediated by neurotransmitter noradrenaline binding to adrenergic receptors (17, 75–77). A recent study revealed that nociceptive nerves regulate HSC mobilization via the secretion of calcitonin gene-related peptide (CGRP) that acts directly on HSCs via CGRP receptor (74).
Neuropathy is common in elderly people. Consistent with this notion, one study revealed a significant reduction of bone marrow sympathetic innervation in old compared to young femurs (17). This study also indicated that surgical denervation or deletion of Adrb3 in young mice induces dramatic remodeling of the HSC niche and leads to premature aging-like changes in HSCs. Notably, they showed that supplementation of an ADRβ3 agonist, BRL37344, in old mice significantly rejuvenates the in vivo function of aged HSCs. This study highlights a potential novel approach for niche-targeted stem cell rejuvenation therapy. Similarly, neuropathy is also found in a mouse model of an aged-related blood disease, myeloproliferative neoplasm (MPN), induced by a mutant form of Janus kinase 2 (JAK2V617F) (77). Treatment with the same ADRβ3 agonist BRL37344 blocks myeloid expansion and disease progression. However, studies regarding the neural alterations with age and their contributions to HSC aging remain controversial. A conflicting study using whole-mount imaging of skulls and thick tibial sections did not find reduced sympathetic nerve fibers in the aged BM, and instead actually found increased sympathetic innervation (78). Whether these discrepancies result from the use of different bones and methodologies will require further investigation. In the latter study, they found that increased bone marrow adrenergic innervation promotes myeloid expansion through activating ADRβ2 (16). Interestingly, this study revealed that ADRβ3 exhibits opposite regulation of myelopoiesis as compared with ADRβ2. Lack of ADRβ3 accelerates HSC aging, and chronic treatment with an ADRβ3 agonist BRL37344 reduces HSC expansion and restores their myeloid skewing. The situation is further complicated by a phase II clinical trial that treated JAK2-V617F-positive patients with the sympathomimetic agonist mirabegron that yielded a slight overall hematologic improvement in a subset of patients, but didn’t reduce the JAK2-V617F allele burden (79). This raised the possibility that modulation of only one adrenergic signaling pathway is insufficient, and other alternative mechanisms may compensate. Further studies of other adrenergic signaling pathways are needed to clarify the neural contributions to the bone marrow niche and HSCs with age.
BM perivascular MSCs wrap around the blood vessels and represent an important cellular component in the HSC niche. MSCs have the potential to self-renew and differentiate into bone, fat and cartilage, and are highly enriched in niche factor expression, such as CXCL12 and SCF. However, BM MSCs are a very heterogenous cell population (80, 81), and it remains unresolved how the overall number of MSCs changes during aging. Some studies suggested a decline in MSC number in old individuals (82, 83), or no significant changes (84, 85), whereas other studies revealed an increase and/or decrease in different subsets of MSCs (17, 19). These discrepancies may be explained by different markers used to define MSCs, or different processing methodologies. However, despite these differences, common functional dysregulation of aged MSCs has been described. Importantly, when aged skeletal stem cell-derived stroma (i.e., bone, cartilage, and mesenchymal lineages, but not fat) is used for co-culture with young HSCs, it has the effect of producing age-related myeloid skewing of hematopoietic output (86, 87).
First, MSCs form colony-forming unit-fibroblasts (CFU-F) in vitro, and aged MSCs showed reduced CFU-F activity and reduced expression of HSC niche factors, including CXCL12, SCF, and ANGPT1. IGF1 produced by MSCs declines during aging and has a significant contribution to the HSC aging phenotype (11, 66). This dysregulation of aged MSCs could be rejuvenated by activating adrenergic signaling. Another common feature of MSCs from old individuals is their reduced osteoblast differentiation and increased bias toward adipocyte differentiation, with old bones showing an increase in the adipogenic marker PPARγ (88, 89). In old mice the adipogenic potential of Sca-1+ MSCs was unchanged but the osteogenic potential of Sca-1- MSCs was reduced (88). Loss of trabecular bone was also observed in old bones. Accumulation of marrow adipose tissue (MAT) was pronounced in old mice after being fed a high fat diet. Importantly, accumulation of adipocytes in the bone marrow contributes to age-related impairment of hematopoiesis. This age-related adipogenic skewing contributes to loss of osteoblasts, and increased BM adiposity, leading to a change in overall BM cellularity and bone density. The balance between adipo-osteogenic differentiation is regulated by critical signaling pathways (Extracellular matrix-Integrin, Wnt, Notch, BMP, Hedgehogs, and FGFs) and key transcription factors, such as PPARγ and C/EBPs for adipogenesis, and Runx2 and Osterix for osteogenesis (90). Recent studies also revealed microRNAs, circular and long RNAs as additional regulators in controlling the adipo-osteogenic balance (91–93). Adipocytes were considered to be negative regulators of hematopoiesis (94), however, growing evidence suggests they are involved in HSC regeneration (95, 96). Adipocytes are much less abundant in mouse bones compared to human bones that have increased adiposity during aging that correlates with increased adjacent myeloid cells and CD34+ stem and progenitor cells (97). An accumulation of osteoclasts from macrophages was observed with aging. The disruption of the balance between bone-forming osteoblast and bone-resorbing osteoclast leads to an imbalance in bone remodeling and often contributes to bone loss associated with osteoporosis (98). This is consistent with age- and menopause-induced bone loss seen in clinic (99).
Aging is characterized by increased inflammation, which is accompanied by cellular senescence. Consistent with other aging tissues, there is a strong inflammatory signature that emerges in MSCs and the aging stroma (16, 100). The stroma of middle-aged telomerase knockout mice (Terc-/-) had a dramatic increase in G-CSF levels and was less able to support HSCs (101). Recent studies have identified bone marrow stromal cells as sensors of age-associated changes and as a source of IL-1β to drive the proinflammatory nature of the bone marrow niche and HSC aging (19). These studies showed that blocking IL-1 signaling could rejuvenate hematopoietic aging and indicated that targeting IL-1 is a novel strategy to improve blood production during aging. The accumulation of BM adipocytes and increased fatty bone marrow and inflammatory signals during aging, specifically IL-6, can promote clonal hematopoiesis (102). Some studies found that BM MSCs underwent senescence in vitro along with aging, including increased DNA damage response and upregulation of senescence associated genes, p16(INK4a), p53, and p21. However, further studies are needed to investigate the senescence-associated phenotypes in bone marrow MSCs in vivo.
There are clear sex-related differences in hematopoiesis during aging (103–106). Our understanding of this has been complicated because studies have used, for example, only males (107), only females (66), or males and females (108). Sex-related differences in hormone levels, such as estrogen, increase HSC proliferation in females (109). Follicle-stimulating hormone (FSH) is higher in middle-aged and old female mice (104). Sex-related differences have also been found between male and female MSCs, with females having a lower CFU-F capacity (110). Female mice were more responsive to VEGF alleviation of aging phenotypes than males (69). The changes in sex hormones that occur during aging contribute to adipocyte accumulation in the BM (111, 112). Interestingly, the increase in HSC number that is associated with aging occurs in middle age (60-70 weeks) for female mice and at old age (85-90 weeks) for males (104). Although these middle-aged female mice had the aging hallmark of increased HSC frequency, they did not have the inflammatory signatures of old mice. These data suggest mouse studies must be carefully designed to consider if males and females will be grouped together, and how middle aged versus old will be defined. These factors could add to the already high degree of variability associated with aging phenotypes that may be partially resolved with larger sample sizes. To gain a more consistent understanding of changes in hematopoiesis across the lifespan of mouse models, not only the precise age, type of bone, and experimental methods must be considered, but also the sex.
A barrier to progress in this field is, of course, the time and cost required to age different mutants and transgenic lines. Although there are colonies of aged wild-type mice that are available to researchers, a shift in focus to middle-aged mice will make aging studies more accessible, as the wait time to reach study age could be reduced by 6 months (66).
There is ongoing debate about the importance of HSC location and distance between niche cells in the microenvironment (59). This is further complicated by the changes observed in both HSC and niche cell populations over the lifespan. An alternative perspective is that the spatial relationships between HSCs and niche cells may not be the most significant factor that impacts HSC regulation and function. Stated another way, perhaps the changes in distance between HSCs and niche cells during aging, at least those that do not require direct contact, such as Notch and Integrin, do not translate into functional changes. For example, during embryonic development, the effect of SHH and BMP morphogen gradients during patterning of the neural tube can extend up to ~100 microns, or many cell diameters (113).
There are also additional layers of spatial information present in the BM microenvironment, such as local oxygen tension and metabolites that are higher near the endosteum (114, 115). Furthermore, significant systemic changes are measurable in the BM fluid during aging that broadly indicate an inflammatory state (19, 66). Parabiosis has shown young blood-borne factors can rejuvenate old mice, just as old blood can accelerate aging of young mice (116). These studies show the exciting potential to reverse some of the effects of aging in HSCs and the BM microenvironment.
XG: Writing – original draft, Writing – review & editing. JZ: Writing – original draft, Writing – review & editing. OJT: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. XG was supported by the ASH Fellow-to-Faculty Scholar Award and the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (K01DK137045). JZ was supported by the National Cancer Institute (R01CA152108) and the National Institute of Aging (R01AG081469). OJT was supported by the NIH National Heart, Lung, and Blood Institute (R01HL174965, R01HL142998, R56HL142998), an American Society of Hematology Bridge Grant Award, and the Department of Cell and Regenerative Biology (University of Wisconsin-Madison). This work was also supported in part by the National Cancer Institute, and NIH grant P30 CA014520 (to the University of Wisconsin Carbone Cancer Center).
We would like to acknowledge the important contributions of our colleagues that we were not able to cite in this review because of space constraints.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Guidi N, Geiger H. Rejuvenation of aged hematopoietic stem cells. Semin Hematol. (2017) 54:51–5. doi: 10.1053/j.seminhematol.2016.10.005
2. Mogilenko DA, Shchukina I, Artyomov MN. Immune ageing at single-cell resolution. Nat Rev Immunol. (2022) 22:484–98. doi: 10.1038/s41577-021-00646-4
3. Chambers SM, Shaw CA, Gatza C, Fisk CJ, Donehower LA, Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. (2007) 5:e201. doi: 10.1371/journal.pbio.0050201
4. Beerman I, Maloney WJ, Weissmann IL, Rossi DJ. Stem cells and the aging hematopoietic system. Curr Opin Immunol. (2010) 22:500–6. doi: 10.1016/j.coi.2010.06.007
5. Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, et al. Clearance of senescent cells by abt263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. (2016) 22:78–83. doi: 10.1038/nm.4010
6. Morrison SJ, Wandycz AM, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. (1996) 2:1011–6. doi: 10.1038/nm0996-1011
7. Miller JP, Allman D. Linking age-related defects in B lymphopoiesis to the aging of hematopoietic stem cells. Semin Immunol. (2005) 17:321–9. doi: 10.1016/j.smim.2005.05.003
8. Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. (2008) 132:681–96. doi: 10.1016/j.cell.2008.01.036
9. Ross JB, Myers LM, Noh JJ, Collins MM, Carmody AB, Messer RJ, et al. Depleting myeloid-biased haematopoietic stem cells rejuvenates aged immunity. Nature. (2024) 628:162–70. doi: 10.1038/s41586-024-07238-x
10. Donnini A, Re F, Orlando F, Provinciali M. Intrinsic and microenvironmental defects are involved in the age-related changes of lin - C-kit+ Hematopoietic progenitor cells. Rejuvenation Res. (2007) 10:459–72. doi: 10.1089/rej.2006.0524
11. Young K, Eudy E, Bell R, Loberg MA, Stearns T, Sharma D, et al. Decline in igf1 in the bone marrow microenvironment initiates hematopoietic stem cell aging. Cell Stem Cell. (2021) 28:1473–82.e7. doi: 10.1016/j.stem.2021.03.017
12. Ergen AV, Boles NC, Goodell MA. Rantes/ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood. (2012) 119:2500–9. doi: 10.1182/blood-2011-11-391730
13. Poulos MG, Ramalingam P, Gutkin MC, Llanos P, Gilleran K, Rabbany SY, et al. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J Clin Invest. (2017) 127:4163–78. doi: 10.1172/JCI93940
14. Poscablo DM, Worthington AK, Smith-Berdan S, Forsberg EC. Megakaryocyte progenitor cell function is enhanced upon aging despite the functional decline of aged hematopoietic stem cells. Stem Cell Rep. (2021) 16:1598–613. doi: 10.1016/j.stemcr.2021.04.016
15. Sacma M, Pospiech J, Bogeska R, de Back W, Mallm JP, Sakk V, et al. Haematopoietic stem cells in perisinusoidal niches are protected from ageing. Nat Cell Biol. (2019) 21:1309–20. doi: 10.1038/s41556-019-0418-y
16. Ho YH, Del Toro R, Rivera-Torres J, Rak J, Korn C, Garcia-Garcia A, et al. Remodeling of bone marrow hematopoietic stem cell niches promotes myeloid cell expansion during premature or physiological aging. Cell Stem Cell. (2019) 25:407–18.e6. doi: 10.1016/j.stem.2019.06.007
17. Maryanovich M, Zahalka AH, Pierce H, Pinho S, Nakahara F, Asada N, et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med. (2018) 24:782–91. doi: 10.1038/s41591-018-0030-x
18. Kusumbe AP, Ramasamy SK, Itkin T, Mäe MA, Langen UH, Betsholtz C, et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature. (2016) 532:380–4. doi: 10.1038/nature17638
19. Mitchell CA, Verovskaya EV, Calero-Nieto FJ, Olson OC, Swann JW, Wang X, et al. Stromal niche inflammation mediated by IL-1 signalling is a targetable driver of haematopoietic ageing. Nat Cell Biol. (2023) 25:30–41. doi: 10.1038/s41556-022-01053-0
20. Wu Q, Zhang J, Kumar S, Shen S, Kincaid M, Johnson CB, et al. Resilient anatomy and local plasticity of naive and stress haematopoiesis. Nature. (2024) 627:839–46. doi: 10.1038/s41586-024-07186-6
21. Prisby RD, Ramsey MW, Behnke BJ, Dominguez JM 2nd, Donato AJ, Allen MR, et al. Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and no bioavailability in rats. J Bone Miner Res. (2007) 22:1280–8. doi: 10.1359/jbmr.070415
22. Wang S, Han J, Huang J, Islam K, Shi Y, Zhou Y, et al. Deep learning-based predictive classification of functional subpopulations of hematopoietic stem cells and multipotent progenitors. Stem Cell Res Ther. (2024) 15:74. doi: 10.1186/s13287-024-03682-8
23. Florian MC, Dörr K, Niebel A, Daria D, Schrezenmeier H, Rojewski M, et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell. (2012) 10:520–30. doi: 10.1016/j.stem.2012.04.007
24. Köhler A, Schmithorst V, Filippi M-D, Ryan MA, Daria D, Gunzer M, et al. Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood. (2009) 114:290–8. doi: 10.1182/blood-2008-12-195644
25. Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, et al. Megakaryocytes regulate hematopoietic stem cell quiescence through cxcl4 secretion. Nat Med. (2014) 20:1315–20. doi: 10.1038/nm.3707
26. Nakamura-Ishizu A, Takubo K, Kobayashi H, Suzuki-Inoue K, Suda T. Clec-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. J Exp Med. (2015) 212:2133–46. doi: 10.1084/jem.20150057
27. Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. (2014) 20:1321–6. doi: 10.1038/nm.3706
28. Vermeulen W, Bergmann E, Auriol J, Rademakers S, Frit P, Appeldoorn E, et al. Sublimiting concentration of tfiih transcription/DNA repair factor causes ttd-a trichothiodystrophy disorder. Nat Genet. (2000) 26:307–13. doi: 10.1038/81603
29. Lehmann AR. DNA repair-deficient diseases, xeroderma pigmentosum, cockayne syndrome and trichothiodystrophy. Biochimie. (2003) 85:1101–11. doi: 10.1016/j.biochi.2003.09.010
30. Prasher JM, Lalai AS, Heijmans-Antonissen C, Ploemacher RE, Hoeijmakers JH, Touw IP, et al. Reduced hematopoietic reserves in DNA interstrand crosslink repair-deficient ercc1-/- mice. EMBO J. (2005) 24:861–71. doi: 10.1038/sj.emboj.7600542
31. Caiado F, Kovtonyuk LV, Gonullu NG, Fullin J, Boettcher S, Manz MG. Aging drives Tet2+/- clonal hematopoiesis via IL-1 signaling. Blood. (2023) 141:886–903. doi: 10.1182/blood.2022016835
32. Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. (2014) 371:2477–87. doi: 10.1056/NEJMoa1409405
33. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. (2014) 371:2488–98. doi: 10.1056/NEJMoa1408617
34. Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, et al. Age-related mutations associated with clonal hematopoietic expansion and Malignancies. Nat Med. (2014) 20:1472–8. doi: 10.1038/nm.3733
35. Jan M, Ebert BL, Jaiswal S. Clonal hematopoiesis. Semin Hematol. (2017) 54:43–50. doi: 10.1053/j.seminhematol.2016.10.002
36. Vijg J, Busuttil RA, Bahar R, Dolle ME. Aging and genome maintenance. Ann N Y Acad Sci. (2005) 1055:35–47. doi: 10.1196/annals.1323.007
37. Moehrle BM, Nattamai K, Brown A, Florian MC, Ryan M, Vogel M, et al. Stem cell-specific mechanisms ensure genomic fidelity within hscs and upon aging of hscs. Cell Rep. (2015) 13:2412–24. doi: 10.1016/j.celrep.2015.11.030
38. Moehrle BM, Geiger H. Aging of hematopoietic stem cells: DNA damage and mutations? Exp Hematol. (2016) 44:895–901. doi: 10.1016/j.exphem.2016.06.253
39. Rozhok AI, Salstrom JL, DeGregori J. Stochastic modeling reveals an evolutionary mechanism underlying elevated rates of childhood leukemia. Proc Natl Acad Sci U.S.A. (2016) 113:1050–5. doi: 10.1073/pnas.1509333113
40. Vas V, Senger K, Dörr K, Niebel A, Geiger H. Aging of the microenvironment influences clonality in hematopoiesis. PLoS One. (2012) 7:e42080. doi: 10.1371/journal.pone.0042080
41. Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. (2007) 128:92–105. doi: 10.1016/j.mad.2006.11.016
42. Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. (2018) 14:576–90. doi: 10.1038/s41574-018-0059-4
43. Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang S, et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol. (2016) 18:607–18. doi: 10.1038/ncb3346
44. Butcher SK, Chahal H, Nayak L, Sinclair A, Henriquez NV, Sapey E, et al. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and cd16 expression in elderly humans. J Leukoc Biol. (2001) 70:881–6. doi: 10.1189/jlb.70.6.881
45. Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin Exp Immunol. (2008) 152:448–55. doi: 10.1111/j.1365-2249.2008.03658.x
46. Chougnet CA, Thacker RI, Shehata HM, Hennies CM, Lehn MA, Lages CS, et al. Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J Immunol. (2015) 195:2624–32. doi: 10.4049/jimmunol.1501006
47. Lu RJ, Taylor S, Contrepois K, Kim M, Bravo JI, Ellenberger M, et al. Multi-omic profiling of primary mouse neutrophils predicts a pattern of sex and age-related functional regulation. Nat Aging. (2021) 1:715–33. doi: 10.1038/s43587-021-00086-8
48. Van Avondt K, Strecker J-K, Tulotta C, Minnerup J, Schulz C, Soehnlein O. Neutrophils in aging and aging-related pathologies. Immunol Rev. (2023) 314:357–75. doi: 10.1111/imr.13153
49. Zong L, Tanaka-Yano M, Park B, Yanai H, Turhan FT, Croteau DL, et al. Nad(+) augmentation with nicotinamide riboside improves lymphoid potential of atm(-/-) and old mice hscs. NPJ Aging Mech Dis. (2021) 7:25. doi: 10.1038/s41514-021-00078-3
50. Tabula Muris C. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature. (2020) 583:590–5. doi: 10.1038/s41586-020-2496-1
51. Therneau TM, Kyle RA, Melton LJ 3rd, Larson DR, Benson JT, Colby CL, et al. Incidence of monoclonal gammopathy of undetermined significance and estimation of duration before first clinical recognition. Mayo Clin Proc. (2012) 87:1071–9. doi: 10.1016/j.mayocp.2012.06.014
52. Hao Y, O'Neill P, Naradikian MS, Scholz JL, Cancro MP. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood. (2011) 118:1294–304. doi: 10.1182/blood-2011-01-330530
53. Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, et al. Toll-like receptor 7 (Tlr7)-driven accumulation of a novel cd11c(+) B-cell population is important for the development of autoimmunity. Blood. (2011) 118:1305–15. doi: 10.1182/blood-2011-01-331462
54. Mittelbrunn M, Kroemer G. Hallmarks of T cell aging. Nat Immunol. (2021) 22:687–98. doi: 10.1038/s41590-021-00927-z
55. Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in cd4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. (2004) 200:1613–22. doi: 10.1084/jem.20041395
56. Desdin-Mico G, Soto-Heredero G, Aranda JF, Oller J, Carrasco E, Gabande-Rodriguez E, et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science. (2020) 368:1371–6. doi: 10.1126/science.aax0860
57. Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. (2018) 9:5435. doi: 10.1038/s41467-018-07825-3
58. Liao W, Liu C, Yang K, Chen J, Wu Y, Zhang S, et al. Aged hematopoietic stem cells entrap regulatory T cells to create a prosurvival microenvironment. Cell Mol Immunol. (2023) 20:1216–31. doi: 10.1038/s41423-023-01072-3
59. Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. (2014) 505:327–34. doi: 10.1038/nature12984
60. Chen JY, Miyanishi M, Wang SK, Yamazaki S, Sinha R, Kao KS, et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature. (2016) 530:223–7. doi: 10.1038/nature16943
61. Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN, et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. (2015) 526:126–30. doi: 10.1038/nature15250
62. Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. (2013) 502:637–43. doi: 10.1038/nature12612
63. Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. (2014) 507:323–8. doi: 10.1038/nature13145
64. Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial notch activity promotes angiogenesis and osteogenesis in bone. Nature. (2014) 507:376–80. doi: 10.1038/nature13146
65. Itkin T, Gur-Cohen S, Spencer JA, Schajnovitz A, Ramasamy SK, Kusumbe AP, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. (2016) 532:323–8. doi: 10.1038/nature17624
66. Young KA, Telpoukhovskaia MA, Hofmann J, Mistry JJ, Kokkaliaris KD, Trowbridge JJ. Variation in mesenchymal kitl/scf and igf1 expression in middle age underlies steady-state hematopoietic stem cell aging. Blood. (2024) 144:378–91. doi: 10.1182/blood.2024024275
67. Gomariz A, Helbling PM, Isringhausen S, Suessbier U, Becker A, Boss A, et al. Quantitative spatial analysis of haematopoiesis-regulating stromal cells in the bone marrow microenvironment by 3d microscopy. Nat Commun. (2018) 9:2532. doi: 10.1038/s41467-018-04770-z
68. Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. (2018) 123:849–67. doi: 10.1161/CIRCRESAHA.118.311378
69. Grunewald M, Kumar S, Sharife H, Volinsky E, Gileles-Hillel A, Licht T, et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science. (2021) 373:eabc8479. doi: 10.1126/science.abc8479
70. Tuljapurkar SR, McGuire TR, Brusnahan SK, Jackson JD, Garvin KL, Kessinger MA, et al. Changes in human bone marrow fat content associated with changes in hematopoietic stem cell numbers and cytokine levels with aging. J Anat. (2011) 219:574–81. doi: 10.1111/j.1469-7580.2011.01423.x
71. Ramalingam P, Poulos MG, Gutkin MC, Katsnelson L, Freire AG, Lazzari E, et al. Endothelial mTOR maintains hematopoiesis during aging. J Exp Med. (2020) 217(6):e20191212. doi: 10.1084/jem.20191212
72. Mannick JB, Lamming DW. Targeting the biology of aging with mtor inhibitors. Nat Aging. (2023) 3:642–60. doi: 10.1038/s43587-023-00416-y
73. Ramalingam P, Poulos MG, Lazzari E, Gutkin MC, Lopez D, Kloss CC, et al. Chronic activation of endothelial mapk disrupts hematopoiesis via nfkb dependent inflammatory stress reversible by scgf. Nat Commun. (2020) 11:666. doi: 10.1038/s41467-020-14478-8
74. Gao X, Zhang D, Xu C, Li H, Caron KM, Frenette PS. Nociceptive nerves regulate haematopoietic stem cell mobilization. Nature. (2021) 589:591–6. doi: 10.1038/s41586-020-03057-y
75. Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. (2006) 124:407–21. doi: 10.1016/j.cell.2005.10.041
76. Mendez-, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. (2008) 452:442–7. doi: 10.1038/nature06685
77. Lucas D, Scheiermann C, Chow A, Kunisaki Y, Bruns I, Barrick C, et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat Med. (2013) 19:695–703. doi: 10.1038/nm.3155
78. Chartier SR, Mitchell SAT, Majuta LA, Mantyh PW. The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience. (2018) 387:178–90. doi: 10.1016/j.neuroscience.2018.01.047
79. Drexler B, Passweg JR, Tzankov A, Bigler M, Theocharides AP, Cantoni N, et al. The sympathomimetic agonist mirabegron did not lower jak2-V617f allele burden, but restored nestin-positive cells and reduced reticulin fibrosis in patients with myeloproliferative neoplasms: results of phase ii study sakk 33/14. Haematologica. (2019) 104:710–6. doi: 10.3324/haematol.2018.200014
80. Baryawno N, Przybylski D, Kowalczyk MS, Kfoury Y, Severe N, Gustafsson K, et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell. (2019) 177:1915–32.e16. doi: 10.1016/j.cell.2019.04.040
81. Tikhonova AN, Dolgalev I, Hu H, Sivaraj KK, Hoxha E, Cuesta-Dominguez A, et al. The bone marrow microenvironment at single-cell resolution. Nature. (2019) 569:222–8. doi: 10.1038/s41586-019-1104-8
82. Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. (2008) 129:163–73. doi: 10.1016/j.mad.2007.12.002
83. Ganguly P, El-Jawhari JJ, Burska AN, Ponchel F, Giannoudis PV, Jones EA. The analysis of in vivo aging in human bone marrow mesenchymal stromal cells using colony-forming unit-fibroblast assay and the cd45(Low)Cd271(+) phenotype. Stem Cells Int. (2019) 2019:5197983. doi: 10.1155/2019/5197983
84. Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One. (2009) 4:e5846. doi: 10.1371/journal.pone.0005846
85. Meza-León B, Gratzinger D, Aguilar-Navarro AG, Juárez-Aguilar FG, Rebel VI, Torlakovic E, et al. Human, mouse, and dog bone marrow show similar mesenchymal stromal cells within a distinctive microenvironment. Exp Hematol. (2021) 100:41–51. doi: 10.1016/j.exphem.2021.06.006
86. Chan CKF, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, et al. Identification and specification of the mouse skeletal stem cell. Cell. (2015) 160:285–98. doi: 10.1016/j.cell.2014.12.002
87. Ambrosi TH, Marecic O, McArdle A, Sinha R, Gulati GS, Tong X, et al. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature. (2021) 597:256–62. doi: 10.1038/s41586-021-03795-7
88. Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. (2017) 20:771–84.e6. doi: 10.1016/j.stem.2017.02.009
89. Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of ppar-gamma2 transcription factor and tgf-beta/bmp signaling pathways. Aging Cell. (2004) 3:379–89. doi: 10.1111/j.1474-9728.2004.00127.x
90. Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. (2016) 23:1128–39. doi: 10.1038/cdd.2015.168
91. Saferding V, Hofmann M, Brunner JS, Niederreiter B, Timmen M, Magilnick N, et al. Microrna-146a controls age-related bone loss. Aging Cell. (2020) 19:e13244. doi: 10.1111/acel.13244
92. Li CJ, Xiao Y, Yang M, Su T, Sun X, Guo Q, et al. Long noncoding rna bmncr regulates mesenchymal stem cell fate during skeletal aging. J Clin Invest. (2018) 128:5251–66. doi: 10.1172/JCI99044
93. Huang HB, Luo HT, Wei NN, Liu ML, He F, Yang W, et al. Integrative analysis reveals a lineage-specific circular rna landscape for adipo-osteogenesis of human mesenchymal stem cells. Stem Cell Res Ther. (2022) 13:106. doi: 10.1186/s13287-022-02792-5
94. Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. (2009) 460:259–63. doi: 10.1038/nature08099
95. Zhou BO, Yu H, Yue R, Zhao Z, Rios JJ, Naveiras O, et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting scf. Nat Cell Biol. (2017) 19:891–903. doi: 10.1038/ncb3570
96. Wilson A, Fu H, Schiffrin M, Winkler C, Koufany M, Jouzeau J-Y, et al. Lack of adipocytes alters hematopoiesis in lipodystrophic mice. Front Immunol. (2018) 9:2573. doi: 10.3389/fimmu.2018.02573
97. Aguilar-Navarro AG, Meza-León B, Gratzinger D, Juárez-Aguilar FG, Chang Q, Ornatsky O, et al. Human aging alters the spatial organization between cd34+ Hematopoietic cells and adipocytes in bone marrow. Stem Cell Rep. (2020) 15:317–25. doi: 10.1016/j.stemcr.2020.06.011
98. Zhang H, Liesveld JL, Calvi LM, Lipe BC, Xing L, Becker MW, et al. The roles of bone remodeling in normal hematopoiesis and age-related hematological Malignancies. Bone Res. (2023) 11:15. doi: 10.1038/s41413-023-00249-w
99. Møller AMJ, Delaissé J-M, Olesen JB, Madsen JS, Canto LM, Bechmann T, et al. Aging and menopause reprogram osteoclast precursors for aggressive bone resorption. Bone Res. (2020) 8:27. doi: 10.1038/s41413-020-0102-7
100. Helbling PM, Piñeiro-Yáñez E, Gerosa R, Boettcher S, Al-Shahrour F, Manz MG, et al. Global transcriptomic profiling of the bone marrow stromal microenvironment during postnatal development, aging, and inflammation. Cell Rep. (2019) 29:3313–30.e4. doi: 10.1016/j.celrep.2019.11.004
101. Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, et al. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med. (2007) 13:742–7. doi: 10.1038/nm1578
102. Zioni N, Bercovich AA, Chapal-Ilani N, Bacharach T, Rappoport N, Solomon A, et al. Inflammatory signals from fatty bone marrow support dnmt3a driven clonal hematopoiesis. Nat Commun. (2023) 14:2070. doi: 10.1038/s41467-023-36906-1
103. Porcher L, Bruckmeier S, Burbano SD, Finnell JE, Gorny N, Klett J, et al. Aging triggers an upregulation of a multitude of cytokines in the male and especially the female rodent hippocampus but more discrete changes in other brain regions. J Neuroinflamm. (2021) 18:219. doi: 10.1186/s12974-021-02252-6
104. So EY, Jeong EM, Wu KQ, Dubielecka PM, Reginato AM, Quesenberry PJ, et al. Sexual dimorphism in aging hematopoiesis: an earlier decline of hematopoietic stem and progenitor cells in male than female mice. Aging (Albany NY). (2020) 12:25939–55. doi: 10.18632/aging.202167
105. Márquez EJ, Chung CH, Marches R, Rossi RJ, Nehar-Belaid D, Eroglu A, et al. Sexual-dimorphism in human immune system aging. Nat Commun. (2020) 11:751. doi: 10.1038/s41467-020-14396-9
106. Bacharach T, Kaushansky N, Shlush LI. Age-related micro-environmental changes as drivers of clonal hematopoiesis. Curr Opin Hematol. (2024) 31:53–7. doi: 10.1097/moh.0000000000000798
107. Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ. Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell. (2014) 15:37–50. doi: 10.1016/j.stem.2014.04.016
108. Zaro BW, Noh JJ, Mascetti VL, Demeter J, George B, Zukowska M, et al. Proteomic analysis of young and old mouse hematopoietic stem cells and their progenitors reveals post-transcriptional regulation in stem cells. eLife. (2020) 9:e62210. doi: 10.7554/eLife.62210
109. Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, et al. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. (2014) 505:555–8. doi: 10.1038/nature12932
110. Selle M, Koch JD, Ongsiek A, Ulbrich L, Ye W, Jiang Z, et al. Influence of age on stem cells depends on the sex of the bone marrow donor. J Cell Mol Med. (2022) 26:1594–605. doi: 10.1111/jcmm.17201
111. Elbaz A, Rivas D, Duque G. Effect of estrogens on bone marrow adipogenesis and sirt1 in aging C57bl/6j mice. Biogerontology. (2009) 10:747–55. doi: 10.1007/s10522-009-9221-7
112. Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporosis Int. (2008) 19:1323–30. doi: 10.1007/s00198-008-0574-6
113. Vetter R, Iber D. Precision of morphogen gradients in neural tube development. Nat Commun. (2022) 13:1145. doi: 10.1038/s41467-022-28834-3
114. Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. (2014) 508:269–73. doi: 10.1038/nature13034
115. Shao L, Elujoba-Bridenstine A, Zink KE, Sanchez LM, Cox BJ, Pollok KE, et al. The neurotransmitter receptor gabbr1 regulates proliferation and function of hematopoietic stem and progenitor cells. Blood. (2021) 137:775–87. doi: 10.1182/blood.2019004415
Keywords: aging, hematopoietic stem cells, niche, inflammation, microenvironment, immune function, endothelial cells, mesenchymal stromal cells
Citation: Gao X, Zhang J and Tamplin OJ (2025) The aging hematopoietic stem cell niche: a mini review. Front. Hematol. 4:1525132. doi: 10.3389/frhem.2025.1525132
Received: 08 November 2024; Accepted: 02 January 2025;
Published: 07 February 2025.
Edited by:
Tomer Itkin, Tel Aviv University, IsraelReviewed by:
Bianca Nowlan, The University of Queensland, AustraliaCopyright © 2025 Gao, Zhang and Tamplin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Zhang, emhhbmdAb25jb2xvZ3kud2lzYy5lZHU=; Xin Gao, eGdhbzM3QHdpc2MuZWR1; Owen J. Tamplin, dGFtcGxpbkB3aXNjLmVkdQ==
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.