
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Food. Sci. Technol. , 18 April 2024
Sec. Food Packaging and Preservation
Volume 4 - 2024 | https://doi.org/10.3389/frfst.2024.1376415
 Raquel A. Fernandes1,2,3*
Raquel A. Fernandes1,2,3* Sandro Lopes1
Sandro Lopes1 Nuno Ferreira1
Nuno Ferreira1 Jorge Santos1,2,3
Jorge Santos1,2,3 Jorge M. Martins2,3,4
Jorge M. Martins2,3,4 Luisa H. Carvalho2,3,4*
Luisa H. Carvalho2,3,4*The valorization of bioeconomy by-products holds immense significance in achieving sustainability goals and fostering resource efficiency, not only to reduce the amount of waste generated but also to significantly decrease the carbon footprint associated with several industrial fields, by promoting circular economy. The wine industry is not only one of the major contributors for world’s economy but also a great producer of by-products, with no valorization process associated. Grape stalks are a great source of chemical molecules, as polyphenols, that can be applied in the production of bio-adhesives. In the present work, grape stalk particles were used as raw material to obtain a highly rich extract with bonding properties through solid/liquid extraction. Different extraction agents were tested (water, NaOH, and Na2CO3) at varied concentrations (0.1%, 0.5%, and 1.0%, respectively). Additionally, extracts were chemically characterized, and the bonding capacity was also evaluated. Using NaOH 1.0%, an extraction efficiency of 8.9% and a total content of polyphenols of 1.4% were achieved. Moreover, all extracts presented an interesting bonding capacity (>1.0 MPa) by hot-press at 120°C for 120 s. The composite panel produced using grape stalk adhesive and vine strain particles fulfills the requirements for type P1 particleboards in terms of internal bond strength, reaching 0.66 MPa. The mechanical and wettability properties of composite material allow foreseeing a promising application in the food packaging industry.
The wine industry is one of the major contributors to the world economy of Portugal. According to the International Organization of Vine and Wine (OIV) in 2023, Portugal occupies the 11th place in the world ranking of wine production, with ca. 6.8 million hL (Santos et al., 2022a). In the winemaking process, significant quantities of by-products are generated, both liquid, as washing waters, and solid, namely, stalk (5%–7%), pomace (20%–25%), and lees (2%–6%) (Prozil et al., 2012; Baptista et al., 2023). In fact, for producing 100 L of wine, ca. 31 kg of solid and 50–140 L of liquid by-products are generated (Prozil et al., 2012; Baptista et al., 2023). One of the biggest issues associated with winemaking residues is the environmental impact of improper disposal. Traditional methods, such as landfilling or open-field burning, contribute to greenhouse gas emissions and soil degradation (Pujol et al., 2013; Filippi et al., 2021; Troilo et al., 2021; Atatoprak et al., 2022). The organic matter present in these types of by-products can also contribute to the environmental degradation due to the leaching of chemicals into the soil and water, thus posing a threat to ecosystems and human health (Pujol et al., 2013; Filippi et al., 2021; Troilo et al., 2021; Atatoprak et al., 2022).
To address these concerns, the valorization of winery residues offers a sustainable solution with great potential. In the last years, efforts have been made by academic and industrial researchers to develop and improve technologies to valorize winery by-products for food, health, energy, and, also, particleboard (PB) manufacturing (Botelho et al., 2018; Gao et al., 2021; Contreras M del et al., 2022; Ju et al., 2022; Rodrigues et al., 2022; Li et al., 2023). In the case of PB manufacturing, wine residues were used as raw material with traditional resin as binders (Ntalos and Grigoriou, 2002; Yeniocak et al., 2014; Wronka and Kowaluk, 2019). However, recent work reported by this research group showed the viability of using wine residues to obtain extracts with applicability in bio-adhesive formulation, which can be an interesting alternative to the use of synthetic binders (Santos et al., 2022a). On the other hand, by incorporating winery residues into the composition of particleboards, manufacturers can enhance the material’s properties, such as strength and durability, while simultaneously reducing the reliance on conventional wood fibers (Ntalos and Grigoriou, 2002; Yeniocak et al., 2014).
One of the emergent applications of wood-based materials is the packaging industry, once the conventional materials adopted, such as plastics, metals, and glass, have created tremendous environmental concerns (Mujtaba et al., 2022; Wang et al., 2022). These materials, derived from finite fossil fuel resources, not only contribute significantly to carbon emissions during production but also pose persistent challenges in end-of-life management (Mujtaba et al., 2022; Wang et al., 2022). The proliferation of non-biodegradable plastics has resulted in extensive pollution of terrestrial and aquatic ecosystems, with long-lasting environmental consequences. Additionally, the energy-intensive processes involved in manufacturing traditional packaging materials further exacerbate the industry’s carbon footprint (Mujtaba et al., 2022; Wang et al., 2022). Considering these challenges, a paradigm shift toward sustainable packaging materials is imperative to mitigate the ecological impact of the packaging industry. Wood has been used for packaging purposes for centuries not only to pack but also to transport, handle, preserve, present, and add value to many food products and sectors (Debeaufort, 2021). In fact, 9%–12% of overall production of packaging material is occupied by wood-based products due to the lower price, sustainability, and recyclability of this raw material (Debeaufort, 2021). However, the existing solutions are based on the use of virgin wood, with high potential to apply in other value-added industries. Therefore, the use of lignocellulosic by-products as raw material for the development of food packaging solutions seems to be a promising route to efficient and eco-friendly products (Sánchez-Safont et al., 2018; Qasim et al., 2021; Santos et al., 2021; Bascón-Villegas et al., 2022; Santos et al., 2024). Some works have been performed on the development of composite materials using lignocellulosic particles with other polymers, such as PHB and polyethylene glycol (Sánchez-Safont et al., 2018; Bascón-Villegas et al., 2022). Nevertheless, the development of solutions that are composed by lignocellulosic residues has been slightly explored, with only two reports, by our research group, on the use of poplar peeling and cardoon stalk to the production of composite materials (Santos et al., 2021; Santos et al., 2024). However, the bio-adhesives produced in both cases were obtained by mixing the polyphenolic extracts with citric acid, a cross-linking agent that enhances bonding performance.
In this way, the present work proves the use of a 100% bio-adhesive, obtained only from grape stalks, without the addition of cross-linkers or hardeners, to produce composite materials, using winery by-products (grape stalk and vine strains) as raw material. The impact of the extraction agent and its concentration on the efficiency of the process was studied. The chemical characterization of all extracts was also performed, as well as the evaluation of their bonding properties. Lastly, particleboards using the best performing bio-adhesive and grape stalk/vine strains particles were produced, and the physical–mechanical evaluation was accessed according to the standard methods for PB (density - EN 323, moisture content - EN 322, internal bond strength (IB) - EN 319, bending strength, BS - EN 310, and thickness swelling, TS - EN317). Additionally, the wettability properties were also studied. To the best of our knowledge, it was the first time that a particleboard 100% based on winery by-products was produced, with the requirements of EN 312 being fulfilled in terms of internal bond strength to be characterized as type P1 (general purpose boards for use in dry conditions).
Grape stalk and vine strains (Vitis vinifera L.) were collected from Quinta da Alameda in the center region of Portugal (Nelas, Viseu) and dried at 40 °C in an oven until reaching equilibrium moisture for further processing. Raw materials were grounded at 1,500 rpm using a 10-mm square grid in a cutting mill (Retsch SN 300) and then sieved using a vibrating sieve shaker (Retsch AS 200 control) with an amplitude of 2 mm for 2 min to select the fraction of particles with a size between 500 μm and 2 mm. Sodium hydroxide (97%), sodium carbonate (97%), gallic acid (anhydrous), sodium nitrite (anhydrous), and aluminum chloride (anhydrous) were supplied by Sigma-Aldrich and used as received. Folin–Ciocalteu reagent (VWR chemicals) was purchased from VWR and used as received. (+)-Catechin hydrate (>97.0%) and 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH, >97.0%) were provided by TCI and used as received.
The extraction of polyphenols was carried out at 70 °C in a 3-L jacketed borosilicate reactor. The extractive agent (aqueous NaOH and aqueous Na2CO3) with a certain concentration (0.1%, 0.5%, and 1.0% w/v) was pumped into the reactor with a flow rate of 20 mL min−1 under recirculating mode, and a solid/liquid ratio of 1/5 (w/w) was kept in all cases. Distilled water was also used as an extractive agent for comparison purposes. After 1 h of extraction, the suspension was filtered through a nylon membrane (125 µm), and the liquid extract was chemically characterized (pH, solid content, FTIR, ABES, and extraction yield). In order to improve the stability, the liquid extract was dried through the spray drying process at 180 °C, using a spray drying system (SD-06, LabPlant, United Kingdom). The liquid was fed at a flow rate of 50 mL min−1, and compressed air (a flow rate of 35 m3 h−1) was used for dispersion. Table 1 presents the codification attributed to all experiments, according to extraction conditions.
All the experiments were evaluated in terms of extraction efficiency (EE) according to Eq. 1:
The determination of the total phenolic content of liquid extracts (obtained immediately after the extraction and without any post-processing) was performed using the Folin–Ciocalteu method, as described elsewhere (Rodrigues et al., 2023). For this, 0.25 mL of the liquid extract was diluted in 15 mL of distilled water and then mixed with 1.25 mL of the Folin–Ciocalteu reagent (previously diluted in water 1:10 v:v). The mixture was homogenized and left in the dark for 8 min. After this period, 3.75 mL of the sodium carbonate solution (75 g L−1) and 4.75 mL of distilled water were added. The mixture was homogenized and left in the dark for 2 h. A blank sample was also prepared using distilled water. The absorbance of all samples was measured at 760 nm using a UV-vis Peak Instruments T-9100 spectrophotometer. Gallic acid was used as standard, and the phenolic content was expressed as gallic acid equivalent (GAE) per 100 g of raw material (on dried basis). The analyses were done in triplicate, and the mean value was calculated.
The total flavonoid content was performed through aluminum chloride colorimetric assay (Rodrigues et al., 2023). For this, 100 µL of the liquid extract was added to 4 mL of distilled water. Then, 0.30 mL of sodium nitrite (5% w/v) was mixed, and the solution was homogenized. After 5 min, 0.30 mL of aluminum chlorite (10% w/v) was added, and the mixture was left at room temperature for over 6 min. Upon this period, 2 mL of aqueous NaOH (1 M) and 3.30 mL of distilled water were added. After homogenization, the absorbance was measured at 510 nm in a UV-vis Peak Instruments T-9100 spectrophotometer. Catechin was used as the standard, and the total flavonoid content was expressed in terms of catechin equivalents (EC) per 100 g of raw material (on a dry basis). The analyses were done in triplicate, and the mean value was calculated.
The antioxidant activity of liquid extracts was quantified through the free radical scavenging activity of 1,1-diphenyl-2-picrylhydrazyl (DPPH•) (Rodrigues et al., 2023). In this measurement, 3 mL of the DPPH• methanolic solution (0.60 mM) was mixed with 100 µL of the liquid extract and left in dark at room temperature for 1 h. After the reaction, the absorbance was measured at 517 nm using a UV-vis Peak Instruments T-9100 spectrophotometer. The antioxidant activity (AA) was calculated according to Eq. 1:
where Ab is the absorbance of blank (distilled water) and As is the absorbance of the sample. The analyses were done in triplicate, and the mean value was calculated
FTIR spectra were recorded on a VERTEX 70 FTIR spectrometer (BRUKER, Biller-ica, MA, United States) in the transmittance mode and equipped with a high-sensitivity DLaTGS detector at room temperature. Dried samples (raw materials and polyphenolic extracts) were measured in the ATR mode with no pre-treatment, using an A225/Q PLATINUM ATR diamond crystal with a single-reflection accessory. The spectra were recorded from 4000 to 400 cm−1 with a resolution of 4 cm−1. All spectra were recorded and processed with OPUS 7.0 software.
The bonding capacity of adhesives was evaluated using an automated bonding evaluation system (ABES) (Adhesive Evaluation Systems, United States) at 120 °C and different pressing times (10, 30, 60, 120, and 180 s), as described elsewhere (Santos et al., 2022a). Before testing, wood veneer samples (Fagus sylvatica L., with 0.7 mm of thickness) were conditioned for 1 week at 20 °C and 53% of relative humidity. After this period, probes were cut into 117 mm × 20-mm strips using a pneumatically driven sample cutting device specific for this type of preparation.
For the test, two probes were along the fiber direction glued using 10 mg of adhesive with a 100 m2 overlap. After the chosen pressing time, probes were pulled, and the maximum shear strength was determined. Measurements were done in triplicate for each pressing time, and the results were averaged.
A bio-adhesive was produced by concentrating the polyphenolic extract up to 20% of solid content, using a rotary evaporator. The bio-adhesive was used in the manufacturing of particleboards (PBs), using grape stalk and vine strain particles (2 mm–500 µm) as raw material. Therefore, PBs with 250 × 250 mm2 (8 mm of thickness) were produced through hot-pressing with 10 wt% of bio-adhesive and a target density of 850 kg m−3. The mixture was hot pressed at 160 °C during 10 min at a fixed thickness of 8 mm. The panels were denoted GS or VS, taking into account the raw material used for the production of PBs (grape stalk–GS or vine strains–VS).
The physical–mechanical properties of obtained composite panels were evaluated according to the European standards in terms of density (EN 323:2010), moisture content (EN 322:2010), internal bond strength (IB; EN 319:2010), bending strength (BS; EN 310:2010), and thickness swelling (TS; EN 317:2010). Composite panels were classified according to the standard EN 312:2010.
The wetting behavior of composite panels was determined with the Mobile Surface Analyzer (MSA) One-Click SFE (Kruss, GmbH, Germany) equipped with a video measuring system, high-resolution camera, and a high-performance digitizing adapter that enables instantaneous and frequent registration. Advance software (Kruss, GmbH, Germany) was used for data collection. Water (polar) and diiodomethane (non-polar) were used as probe liquids. A measure of 2.0 μL drop of each liquid was placed on the sample surface for each measurement. Three test drops of each sample were performed for all liquids. The right and left contact angles between each droplet and the sample surface were collected at intervals of 1 s during the first 10 s and intervals of 10 s for a total duration of 60 s, and then, the average of the contact angles was automatically calculated to determine the surface free energy, according to the Owens, Wendt, Rabel, and Kaelble (OWRK) method.
All determinations were conducted in triplicate, and the data are presented as the means ± standard deviations. The impact of extractive agents (NaOH and Na2CO3) and their concentrations (0.1, 0.5% and 1.0% w/v) on the extraction efficiency and chemical properties of the obtained extracts (TPC, TFC, and AA) were statistically evaluated (p < 0.05) by two-way analysis of variance (ANOVA) using OriginPro 8.5.2 software and Tukey test for differences of means. Similarly, the variation in wetting properties (contact angle) was also statistically evaluated through two-way ANOVA and the Tukey test. Regarding the comparison of physical–mechanical properties and surface free energy of particleboards, statistical analysis was performed by one-way analysis of variance (ANOVA) at p < 0.05 using OriginPro 8.5.2 software.
Grape stalk (GS) and vine strain (VS) particles were characterized using FTIR analysis, and the obtained spectra are presented in Figure 1. Due to the lignocellulosic nature of GS and VS, cellulose, hemicellulose, and lignin are the main components (c.a. 70%–90%), and for that reason, the normalization of the intensity values of both FTIR spectra was done using the band of the C−O, C−C, and C−C−O stretch vibration at 1028 ± 5 cm−1.
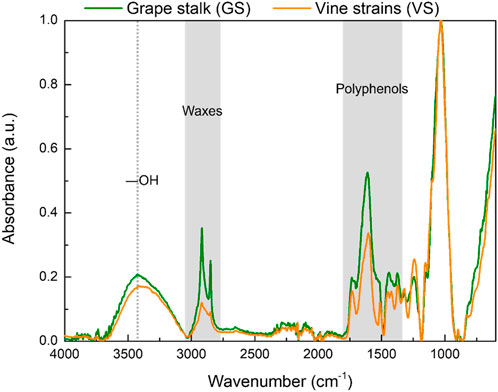
Figure 1. FTIR spectra of grape stalks (GS) and vine strains (VS). Highlighted zones refer to relevant bands of both materials.
As observed, GS and VS exhibit similar absorption spectra (Figure 1). The broad band at c.a. 3,400 cm−1 is attributed to −OH stretching (Prozil et al., 2012; Pujol et al., 2013; Prozil et al., 2014; Santos et al., 2022a). At c.a. 2,935 cm−1 and 2,873 cm−1 is identified two sharp peaks associated with the asymmetric and symmetric stretch of −CH2, respectively (Prozil et al., 2012; Pujol et al., 2013; Prozil et al., 2014; Santos et al., 2022a). An increase in the intensity of both peaks in the case of GS particles was observed, which indicates a high content of waxes and oils (Prozil et al., 2012; Pujol et al., 2013; Prozil et al., 2014; Santos et al., 2022a). Additionally, the region attributed to the presence of polyphenols (from 1300 cm−1 to 1800 cm−1) is also enhanced in the case of GS particles (Figure 1), corroborating its high potential for the recovery of phenolic compounds (Troilo et al., 2021; Jesus et al., 2022; Rodrigues et al., 2023).
The recovery of polyphenolic compounds from grape stalks is a widely studied topic, due to the significant amount of lignin and tannins in its composition (Pujol et al., 2013; Prozil et al., 2014; Rodrigues et al., 2022). Additionally, alkaline extraction is a well-known method for phenolics and lignin isolation from lignocellulosic biomass, with several operational advantages, such as low toxicity, safety, and interesting yield (Cavali et al., 2020; Oriez et al., 2020; Deniz, 2023). Grape stalk particles were used for alkaline extraction to obtain a phenolic-rich liquid extract showing potential as an adhesive. In order to optimize the process, two extractive agents were studied, namely, sodium hydroxide (NaOH) and sodium carbonate (Na2CO3). Moreover, the impact of the concentration of both agents was also evaluated, using aqueous solutions with 0.1%, 0.5%, and 1.0% w/v. Distilled water was also used as the extractive agent for comparison purposes. For each experiment that was determined, the extraction efficiency (EE) and the final extracts were chemically characterized in terms of total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity (AA), and the results are presented in Table 2.
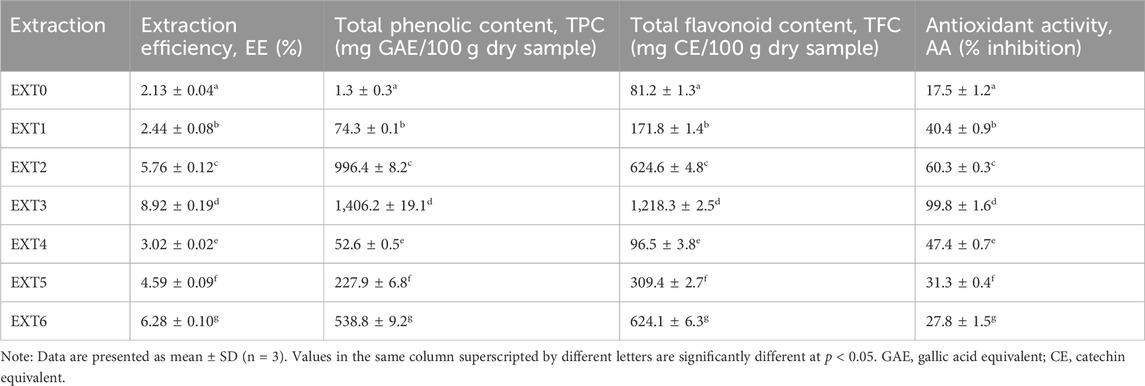
Table 2. Extraction efficiency (EE), total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity (AA) of the obtained extracts.
As it can be observed, the extractive agent had a significative effect (p < 0.05) on the EE of the process (Table 2). The use of NaOH or Na2CO3 as extraction agents allowed improving the efficiency in comparison with water alone, increasing from 2.13% (EXT0, Table 2) to 2.44% and 3.02% of EE, respectively (EXT1 and EXT4, Table 2). However, a sharp variation was noticed in the chemical properties of the extracts. The use of NaOH (EXT1, Table 2) and Na2CO3 (EXT4, Table 2) as extraction agents allowed obtaining extracts with a higher content of phenols and flavonoids than the extract obtained using water (EXT0, Table 2). Furthermore, the antioxidant capacity of the extracts was also significantly increased (p < 0.05) with the change in the extraction agent, from c.a. 18% (EXT0, Table 2) to more than 40% (EXT1 and EXT4, Table 2). According to the literature, several phenolic compounds present in lignocellulosic biomass, as grape stalks, are highly soluble under alkaline conditions, which allow their isolation and recovery (Cavali et al., 2020; Oriez et al., 2020). Therefore, the use of aqueous solutions of weak and strong basis, such as Na2CO3 and NaOH, as a solvent in the extraction process allow to obtain highly rich mixtures of polyphenols, such as tannins, flavan-3-ols, hydroxycinnamic acids, monomeric and oligomeric flavonols, and stilbenes (Cavali et al., 2020; Oriez et al., 2020; Troilo et al., 2021).
By tuning the concentration of extractive agents, it was possible to significantly improve (p < 0.05) the extraction efficiency. With 0.5% and 1.0% of NaOH, EE was improved to 5.76% (EXT2, Table 2) and 8.92% (EXT3, Table 2), respectively. In the case of Na2CO3, efficiency was 1.5 and 2 times higher with 0.5% (EXT5, Table 2) and 1.0% (EXT6, Table 2), respectively, when compared to the result obtained with Na2CO3 0.1% (EXT4, Table 2). In terms of chemical composition, both phenolic and flavonoid contents follow the same tendency, with a great increase in NaOH or Na2CO3 concentrations (Table 2). On the other hand, the variation in the antioxidant capacity of the extracts presents a quite different behavior depending on the extractive agent used. In the case of extractions with NaOH, increasing the concentration improves the extraction of phenolic compounds and flavonoids, which led to an increase in the AA of the extracts (EXT1-EXT3, Table 2). However, in the case of Na2CO3, the antioxidant potential decreased with the increase in the phenol derivatives and flavonoid content (EXT4-6, Table 2), which may indicate an antagonist effect between the extracted polyphenols. According to the literature, when two or more antioxidant compounds are present in the extract, an antagonist effect may occur, hindering the total antioxidant activity of the extract (Meyer et al., 1998; Pinelo et al., 2004; Hajimehdipoor et al., 2014; Skroza et al., 2022; Uduwana et al., 2023). This effect may be caused by chemical interactions between phenolic compounds, which can produce weaker antioxidant species, forming complexes and adducts, promote polymerization reactions, and neutralize oxidant radicals (Meyer et al., 1998; Pinelo et al., 2004; Hajimehdipoor et al., 2014; Skroza et al., 2022; Uduwana et al., 2023). For example, Meyer et al. (1998) proved the antagonist effect on antioxidant activity of catechin when combined with quercetin, cyanidin, and caffeic and ellagic acids, due to the chemical interaction between their functional groups and, consequently, to the reduced availability of hydroxyl groups for oxidation (Meyer et al., 1998). Furthermore, Uduwana et al. (2023) observed the same effect by combining green tea with bee honey and Citrus limonum extracts (Uduwana et al., 2023). Hajimehdipoor et al. (2014) concluded that ternary combinations of quercetin, rosmarinic acid, rutin, gallic acid, and caffeic acid also exhibit antagonist effect regarding antioxidant activity (Hajimehdipoor et al., 2014).
The impact of extraction agents (water, NaOH, and Na2CO3) on the chemical composition of the extracts (EXT0, EXT3, and EXT6) was evaluated by FTIR-ATR, and the obtained spectra are presented in Figure 2A. The normalization of the intensity values was done using the band of the aromatic skeletal vibration at (1600 ± 5) cm−1.
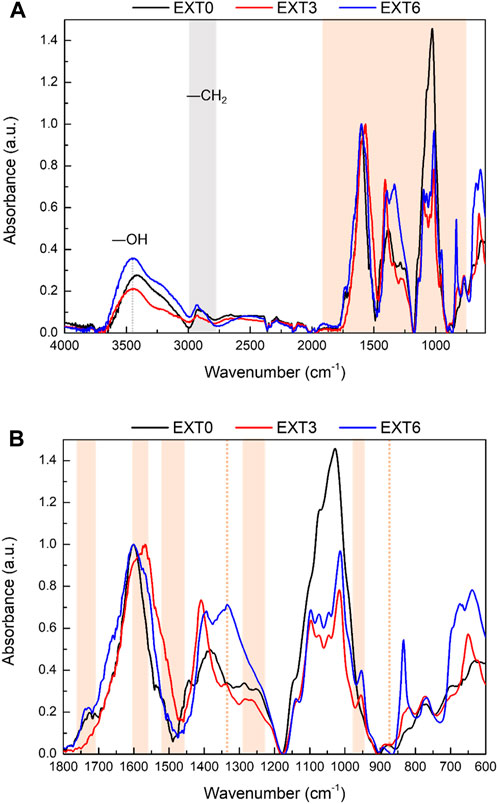
Figure 2. (A) FTIR spectra of EXT0, EXT3, and EXT6 and (B) a magnification of the region from 600 to 1800 cm−1. Highlighted zones refer to bands/peaks characteristic of the produced extracts.
As expected, grape stalk extracts are rich in flavonoids and stilbenes (Anastasiadi et al., 2012), and regardless of the extraction conditions used, all extracts showed the presence of the pattern bands due to the presence of these polyphenolic compounds (Figure 2A). However, the intensity of these bands is low in the spectrum of the extract obtained using only water as an extraction agent (EXT0). This confirms the results obtained for the characterization of phenolic and flavonoid content (Table 2). The low amount of extractable polyphenolic compounds in the extract obtained only with water is due to the fact that most of the water-extractable compounds were previously extracted in the winemaking process.
Regarding the extracts obtained using alkaline extraction agents (NaOH and Na2CO3), it was interesting to see that the alkaline agent used impacted the compounds present in the extracts. The spectra of the extract obtained with NaOH (EXT3, Figure 2B) show higher amount of stilbenes in the extract due to the intensity on its characteristics bands: a double peak at c.a. 1600 cm−1 and c.a. 1568 cm−1, corresponding to C−C aromatic double bond stretching and C−C oleophilic stretching, peaks at c.a. 1516 cm−1 and c.a. 1463 cm−1 due to benzene skeleton vibrations, at c.a. 987 cm−1 and c.a. 956 cm−1 due to the bending vibration of C=C−H (Ricci et al., 2015; Santos et al., 2022a; Santos et al., 2022b).
Furthermore, the spectrum of the extract obtained with Na2CO3 (EXT 6) shows a high proportion of tannins in the extract, based on the intensity of the bands at 1334 cm−1, 338 cm−1, and 1283 cm−1 due to the C−O−H of primary and tertiary alcohol bending vibrations; 1235 cm−1 of cyclic ether C−O−C stretching vibration; 1028 cm−1 of aromatic cycle bending in a plane vibration, characteristic of the presence of condensed tannins, and the bands at 1728 cm−1 due to the vibrations of the C=O groups (Pujol et al., 2013; Santos et al., 2022a; Santos et al., 2022b; Čabalová et al., 2023), at 1240 cm−1 due to the stretching vibration of the C–O groups; and at 865 cm−1, due to the aromatic C–H out-of-plane bending vibrations, characteristic of the presence of hydrolyzable tannins (Grasel et al., 2016).
The bonding properties of all extracts were evaluated through ABES. Therefore, the extracts were previously concentrated through a rotary evaporator until reached a solid content of 15%. Figure 3 presents the shear strength of each extract after hot-pressing at 120°C and different press times.
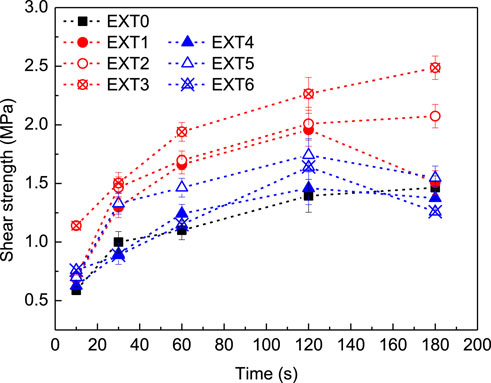
Figure 3. ABES shear strength of all extracts after hot-pressing at different pressing times (10, 30, 60, 120, and 180 s) and 120°C.
All extracts proved to have adhesive properties, reaching a shear strength over 1.0 MPa after 60 s of pressing time at 120 °C (Figure 3). However, significant differences were noted regarding the maximum of performance (Figure 3). In the case of EXT0, the maximum of shear strength was 1.5 MPa and was achieved only after 180 s of pressing (Figure 3). Using an extractive agent (NaOH or Na2CO3), the bonding capacity of extracts was significantly improved (Figure 3). Regarding Na2CO3 (EXT4-6, Figure 3), the best bonding performance was registered after 120 s (regardless of concentration), which means 33% less pressing time required to complete cure at 120°C. Additionally, higher amounts of Na2CO3 (0.5% and 1.0%) slightly increased the maxima of shear strength to 1.6 MPa and 1.7 MPa (EXT5 and EXT6, respectively; Figure 3). On the same way, NaOH extracts’ (EX1-3) also performed better than EXT0, not only by reducing the pressing time (in the case of EXT1) but also increasing the maximum of shear strength to values over 2.0 MPa (EXT2 and EXT3, Figure 3). These findings are closely related to the chemical composition of the extracts, particularly with the phenolic content. As it was previously discussed, hot water extraction has low efficiency to recover phenolics and flavonoids from grape stalks when compared to alkaline extraction agents (Table 2). In the literature, it is referred that the liquid extraction of lignocellulosic biomass, as grape stalk, generates a mixture of long- and short-chain polymers that can be successfully used as bio-adhesives in several industries (Muranaka et al., 2017; Ferreira-Santos et al., 2020; Sun et al., 2022; Zhang et al., 2022; Li et al., 2023; Šernek and Žigon, 2023). The bonding potential is commonly associated with the presence of phenolic compounds, and a high amount of polyphenols usually results in an enhanced bonding capacity of the extracts (Friedman and Jürgens, 2000; Benito-González et al., 2020; Oriez et al., 2020; Troilo et al., 2021; Rodrigues et al., 2023). Therefore, NaOH extracts (EXT1-3; Figure 3) exhibit improved adhesive properties when compared to Na2CO3 (EXT4-6, Figure 3) and water (EXT0, Figure 3) extracts possibly due to the higher TPC and TFC (Table 2).
The use of grape stalk as raw material for the production of bio-adhesives to apply in the particleboard industry was evaluated. In this way, EXT3, the best performing extract in terms of bonding capacity, was selected as adhesive, and two types of particles were used as lignocellulosic matrix, namely, grape stalk and vine strains. The objective was to evaluate a circular economy concept, with grape stalks being used for both applications (adhesive production and fiber) and also to study the effect of using other types of winery residue (vine strains) to improve physical–mechanical properties of the composite panels. Vine strains have a high content of cellulose (Benito-González et al., 2020; Senila et al., 2020; Jesus et al., 2022), which provides to this by-product increased resistance against tension forces when compared to grape stalk. In this way, EXT3 was previously concentrated to reach a solid content of 20% before producing the panels. The composite panels were hot-pressed at 160 °C for 10 min at a fixed thickness of 8 mm, with 10 wt% of adhesive. Figure 4 presents the obtained composite panels, using grape stalk (GS_P, Figure 4A) and vine strain (VS_P, Figure 4B) particles.

Figure 4. Samples of the composite panels produced using (A) grape stalk particles (GS_P) and (B) vine strains particles (VS_P) as the matrix and EXT3 as bio-adhesive.
Physical–mechanical performance of both materials was evaluated in terms of density (D), moisture content (MC), internal bond strength (IB), bending strength (IB), and thickness swelling (TS), and the results are presented in Table 3.
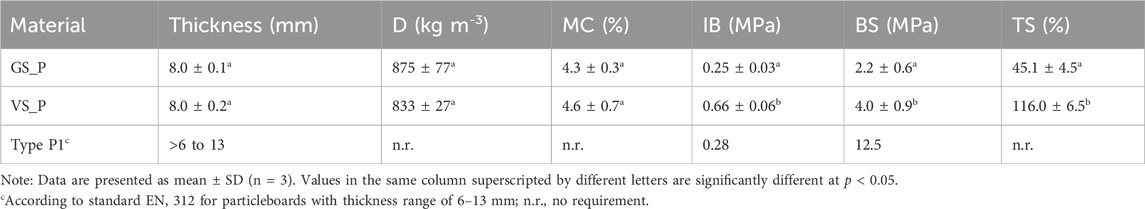
Table 3. Density (D), moisture content (MC), internal bond strength (IB), bending strength (BS), and thickness swelling (TS) of the composite panels produced using EXT3 as adhesive and grape stalks (GS) and vine strain (VS) particles.
As expected, both composite materials present similar (p < 0.05) thickness and density (c.a. 8.5 mm and 850 kg m−3, Table 3). Moreover, the moisture content was also very close in both cases (p < 0.05), with values between 4% and 5% (Table 3). Nevertheless, mechanical properties are substantially different, depending on the type of particles used. In the case of GS_P, IB was 0.25 MPa and BS was 2.2 MPa (Table 3). Using VS particles, the mechanical properties were significantly improved (p < 0.05), with an IB of 0.66 MPa and BS of 4.0 MPa (Table 3). As previously mentioned, vine strains have a higher content of cellulose than grape stalk, providing it higher resistance. Additionally, the mixture of grape stalk particles has a lower fraction of material with a size between 1 and 2 mm than the vine strain mixture, once that GS particles are less dense, and for that reason, the grinding process generates a higher amount of small particles (between 500 μm and 1 mm), which may negatively affect mechanical properties. Although BS is significantly lower regarding to the requirements of type P1 particleboards according to EN 312 (Table 3), the value of IB was 2.3 times higher (Table 3), which proves the potential application of VS_P. Regarding TS, the results showed that GS_P has much higher resistance to swelling than VS_P (Table 3), which can be attributed to the higher oleophilic characteristic of GS particles, as already proved by FTIR analyses (Figure 1), providing the composite material a lower tendency to absorb water and, consequently, to swell. Additionally, the lower content of cellulose in grape stalk particles (when compared to vine strains particles) also promotes the hydrophobic characteristic of GS_P, contributing to a lower TS (Prozil et al., 2012; Benito-González et al., 2020; Senila et al., 2020).
Taking into account the packaging purpose of these composite materials, both panels were characterized in terms of wettability, and the results are presented in Table 4.

Table 4. Contact angle (using water and diiodomethane) and surface free energy of grape stalk (GS_P) and vine strain (VS_P) composite panels.
The contact angles were very similar (p < 0.05) when water was used as test liquid (c.a. 50°, Table 4), revealing an equivalent hydrophilicity of the surface of both composite materials. However, when diiodomethane was used (Table 4), GS_P registered a higher contact angle (c.a. 70°, Table 4) than VS_P (c.a. 56°, Table 4), which may indicate a lower tendency to the penetration of oils and fats (Candan et al., 2012; Wang et al., 2017; Danish et al., 2019). Although surface free energy seems to corroborate this tendency, with VS_P reaching the highest value (c.a. 52 mJ m−2, Table 4), the difference was not statistically significant (p < 0.05). In this way, GS_P seems to be a valuable alternative to consider in packaging solutions, not only due to the 100% bio-character but also to its mechanical and wettability properties that provide resistance to mechanical stress caused by handling and transportation and also reduce chemical migration of compounds from package to food.
Grape stalk extracts were successfully applied as raw material for the production of a bio-adhesive. Two different extractive agents were evaluated, namely, NaOH and Na2CO3, as well as the effect of its concentration on the extraction efficiency. The extracts were characterized in terms of composition (total phenolic and flavonoid contents, antioxidant activity, and FTIR) and bonding capacity (ABES).
The extraction efficiency was improved using NaOH as the extractive agent, when compared to water and Na2CO3, with noticeable differences in the chemical composition of the respective extracts. According to FTIR analysis, NaOH was more efficient in the recovery of stilbenes, while the extracts obtained using Na2CO3 exhibit a higher fraction of tannins. Moreover, increasing the concentration of the extractive agents promoted an effective increase in the solubilization of polyphenols, as phenolics and flavonoids, and consequently, improved the efficiency of the process. Regarding adhesive properties, NaOH extracts also exhibit improved capacity by pressing at 120°C.
A composite material, 100% derived from waste from the wine industry, was developed by mixing the best performing extract with vine strains/grape stalk particles. The physical–mechanical performance of the composite panels was deeply influenced by the type of lignocellulosic particles chosen. Vine strain particles allowed to develop a composite material with enhanced resistance in terms of mechanical properties (internal bon and bending strength) when compared to the composite material produced using grape stalk particles. However, the use of grape stalk particles allowed obtaining a lower thickness swelling, which can be related to its higher content of waxes. Additionally, wettability properties demonstrate a lower tendency of grape stalk panel to be penetrated by oils and fats, which has a great relevance in terms of chemical contamination between food and package.
Considering food packaging applications, composite materials obtained from winery residues may be a valuable alternative to explore in order to increase the sustainable characteristic of this industry, without compromising resistance, functionality, and quality.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
RF: conceptualization, investigation, methodology, and writing–original draft. SL: methodology and writing–original draft. NF: methodology and writing–original draft. JS: conceptualization, formal analysis, and writing–review and editing. JM: conceptualization, project administration, and writing–review and editing. LC: conceptualization, formal analysis, project administration, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The authors gratefully acknowledge the funding by: LEPABE, UIDB/00511/2020 (DOI: 10.54499/UIDB/00511/2020) and UIDP/00511/2020 (DOI: 10.54499/UIDP/00511/2020), ALiCE, LA/P/0045/2020 (DOI: 10.54499/LA/P/0045/2020), supported by national funds through FCT/MCTES (PIDDAC), and Project “INOVC+: Smart Innovation Ecosystem of Centro Region of Portugal” (project number CENTRO-01-0246-FEDER-000044), partially supported by the FEDER (Fundo Europeu de Desenvolvimento Regional), through the Regional Operational Programme of Centre (CENTRO 2020) of the Portugal 2020 Partnership Agreement.
The authors acknowledge Quinta da Alameda (Nelas, Portugal) and MOVECHO Company (Nelas, Portugal) for providing raw materials and technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frfst.2024.1376415/full#supplementary-material
Anastasiadi, M., Pratsinis, H., Kletsas, D., Skaltsounis, A. L., and Haroutounian, S. A. (2012). Grape stem extracts: polyphenolic content and assessment of their in vitro antioxidant properties. LWT - Food Sci. Technol. 48 (2), 316–322. doi:10.1016/j.lwt.2012.04.006
Atatoprak, T., Amorim, M. M., Ribeiro, T., Pintado, M., and Madureira, A. R. (2022). Grape stalk valorization for fermentation purposes. Food Chem. Mol. Sci. 4, 100067. doi:10.1016/j.fochms.2021.100067
Baptista, S. L., Romaní, A., Cunha, J. T., and Domingues, L. (2023). Multi-feedstock biorefinery concept: valorization of winery wastes by engineered yeast. J. Environ. Manage 326, 116623. doi:10.1016/j.jenvman.2022.116623
Bascón-Villegas, I., Pereira, M., Espinosa, E., Sánchez-Gutiérrez, M., Rodríguez, A., and Pérez-Rodríguez, F. (2022). A new eco-friendly packaging system incorporating lignocellulose nanofibres from agri-food residues applied to fresh-cut lettuce. J. Clean. Prod., 372. doi:10.1016/j.jclepro.2022.133597
Benito-González, I., Jaén-Cano, C. M., López-Rubio, A., Martínez-Abad, A., and Martínez-Sanz, M. (2020). Valorisation of vine shoots for the development of cellulose-based biocomposite films with improved performance and bioactivity. Int. J. Biol. Macromol. 165, 1540–1551. doi:10.1016/j.ijbiomac.2020.09.240
Botelho, R. V., Bennemann, G. D., Torres, Y. R., and Sato, A. J. (2018). in Potential for use of the residues of the wine industry in human nutrition and as agricultural input. Editors A. M. Jordão, and F. Cosme (Rijeka: IntechOpen). Ch. 15.
Čabalová, I., Krilek, J., Kačík, F., Lagaňa, R., and Jurczyková, T. (2023). Valorization of wood-based waste from grapevine. Forests 14 (3), 442. doi:10.3390/f14030442
Candan, Z., Büyüksarı, U., Korkut, S., Unsal, O., and Çakıcıer, N. (2012). Wettability and surface roughness of thermally modified plywood panels. Ind. Crops Prod. 36 (1), 434–436. doi:10.1016/j.indcrop.2011.10.010
Cavali, M., Soccol, C. R., Tavares, D., Zevallos Torres, L. A., Oliveira de Andrade Tanobe, V., Zandoná Filho, A., et al. (2020). Effect of sequential acid-alkaline treatment on physical and chemical characteristics of lignin and cellulose from pine (Pinus spp.) residual sawdust. Bioresour. Technol. 316, 123884. doi:10.1016/j.biortech.2020.123884
Contreras M del, M., Romero-García, J. M., López-Linares, J. C., Romero, I., and Castro, E. (2022). Residues from grapevine and wine production as feedstock for a biorefinery. Food Bioprod. Process. 134, 56–79. doi:10.1016/j.fbp.2022.05.005
Danish, M., Nadhari, WNAW, Ahmad, T., Hashim, R., Sulaiman, O., Ahmad, M., et al. (2019). Surface measurement of binderless bio-composite particleboard through contact angle and fractal surfaces. Measurement 140, 365–372. doi:10.1016/j.measurement.2019.03.049
Debeaufort, F. (2021). Wood-based packaging. Packag. Mater. Process. Food, Pharm. Cosmet., 1–18. doi:10.1002/9781119825081.ch1
Deniz, I. (2023). Lignin recovery using alkaline pretreatment of walnut shells. Chem. Eng. Technol. 46 (9), 1911–1914. doi:10.1002/ceat.202200586
Ferreira-Santos, P., Zanuso, E., Genisheva, Z., Rocha, C. M. R., and Teixeira, J. A. (2020). Green and sustainable valorization of bioactive phenolic compounds from pinus by-products. Molecules 25 (12), 2931. doi:10.3390/molecules25122931
Filippi, K., Georgaka, N., Alexandri, M., Papapostolou, H., and Koutinas, A. (2021). Valorisation of grape stalks and pomace for the production of bio-based succinic acid by Actinobacillus succinogenes. Ind. Crops Prod. 15, 168. doi:10.1016/j.indcrop.2021.113578
Friedman, M., and Jürgens, H. S. (2000). Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 48 (6), 2101–2110. doi:10.1021/jf990489j
Gao, Z., Lang, X., Chen, S., and Zhao, C. (2021). Mini-review on the synthesis of lignin-based phenolic resin. Energy Fuels 35, 18385–18395. American Chemical Society. doi:10.1021/acs.energyfuels.1c03177
Grasel, F. dos S., Ferrão, M. F., and Wolf, C. R. (2016). Development of methodology for identification the nature of the polyphenolic extracts by FTIR associated with multivariate analysis. Spectrochim. Acta A Mol. Biomol. Spectrosc. 153, 94–101. doi:10.1016/j.saa.2015.08.020
Hajimehdipoor, H., Shahrestani, R., and Shekarchi, M. (2014). Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharmacogn. (RJP) 1. Available at: http://rjpharmacognosy.ir.
Jesus, M., Romaní, A., Mata, F., and Domingues, L. (2022). Current options in the valorisation of vine pruning residue for the production of biofuels, biopolymers, antioxidants, and bio-composites following the concept of biorefinery: a review. Antioxidants, Bio-Composites Follow. Concept Biorefinery A Rev. 14, 1640. Polymers. MDPI. doi:10.3390/polym14091640
Ju, J., Jin, S., Kim, S., Choi, J. H., Lee, H. A., Son, D., et al. (2022). Addressing the shortcomings of polyphenol-derived adhesives: achievement of long shelf life for effective hemostasis. ACS Appl. Mater Interfaces 14 (22), 25115–25125. doi:10.1021/acsami.2c03930
Li, W., Sun, H., Wang, G., Sui, W., Dai, L., and Si, C. (2023). Lignin as a green and multifunctional alternative to phenol for resin synthesis. Green Chem. 25, 2241–2261. Royal Society of Chemistry. doi:10.1039/d2gc04319j
Meyer, A. S., Heinonen, M., and Frankel, E. N. (1998). Antioxidant interactions of catechin, cyanidin, caffeic acid, quercetin, and ellagic acid on human LDL oxidation. Food Chem. 61 (1), 71–75. doi:10.1016/s0308-8146(97)00100-3
Mujtaba, M., Lipponen, J., Ojanen, M., Puttonen, S., and Vaittinen, H. (2022). Trends and challenges in the development of bio-based barrier coating materials for paper/cardboard food packaging; a review. Sci. Total Environ. 851 (June), 158328. doi:10.1016/j.scitotenv.2022.158328
Muranaka, Y., Nakagawa, H., Hasegawa, I., Maki, T., Hosokawa, J., Ikuta, J., et al. (2017). Lignin-based resin production from lignocellulosic biomass combining acidic saccharification and acetone-water treatment. Chem. Eng. J. 308, 754–759. doi:10.1016/j.cej.2016.09.117
Ntalos, G. A., and Grigoriou, A. H. (2002). Characterization and utilisation of vine prunings as a wood substitute for particleboard production. Ind. Crops Prod. 16 (1), 59–68. doi:10.1016/s0926-6690(02)00008-0
Oriez, V., Peydecastaing, J., and Pontalier, P. Y. (2020). Lignocellulosic biomass mild alkaline fractionation and resulting extract purification processes: conditions, yields, and purities. Clean. Technol. 2 (1), 91–115. doi:10.3390/cleantechnol2010007
Pinelo, M., Manzocco, L., Nuñez, M. J., and Nicoli, M. C. (2004). Interaction among phenols in food fortification: negative synergism on antioxidant capacity. J. Agric. Food Chem. 52 (5), 1177–1180. doi:10.1021/jf0350515
Prozil, S. O., Evtuguin, D. V., and Lopes, L. P. C. (2012). Chemical composition of grape stalks of Vitis vinifera L. from red grape pomaces. Ind. Crops Prod. 35 (1), 178–184. doi:10.1016/j.indcrop.2011.06.035
Prozil, S. O., Evtuguin, D. V., Silva, A. M. S., and Lopes, L. P. C. (2014). Structural characterization of lignin from grape stalks (Vitis vinifera L.). J. Agric. Food Chem. 62 (24), 5420–5428. doi:10.1021/jf502267s
Pujol, D., Liu, C., Fiol, N., Olivella, M. À., Gominho, J., Villaescusa, I., et al. (2013). Chemical characterization of different granulometric fractions of grape stalks waste. Ind. Crops Prod. 50, 494–500. doi:10.1016/j.indcrop.2013.07.051
Qasim, U., Osman, A. I., Al-Muhtaseb, A. H., Farrell, C., Al-Abri, M., Ali, M., et al. (2021). Renewable cellulosic nanocomposites for food packaging to avoid fossil fuel plastic pollution: a review. Environ. Chem. Lett. 19, 613–641. Springer Science and Business Media Deutschland GmbH. doi:10.1007/s10311-020-01090-x
Ricci, A., Parpinello, G. P., Olejar, K. J., Kilmartin, P. A., and Versari, A. (2015). Attenuated total reflection mid-infrared (ATR-MIR) spectroscopy and chemometrics for the identification and classification of commercial tannins. Appl. Spectrosc. 69 (11), 1243–1250. doi:10.1366/15-07957
Rodrigues, R. P., Gando-Ferreira, L. M., and Quina, M. J. (2022). Increasing value of winery residues through integrated biorefinery processes: a review. Molecules 27 (15), 4709. doi:10.3390/molecules27154709
Rodrigues, R. P., Sousa, A. M., Gando-Ferreira, L. M., and Quina, M. J. (2023). Grape pomace as a natural source of phenolic compounds: solvent screening and extraction optimization. Molecules 28 (6), 2715. doi:10.3390/molecules28062715
Sánchez-Safont, E. L., Aldureid, A., Lagarón, J. M., Gámez-Pérez, J., and Cabedo, L. (2018). Biocomposites of different lignocellulosic wastes for sustainable food packaging applications. Compos B Eng. 145, 215–225. doi:10.1016/j.compositesb.2018.03.037
Santos, J., Escobar-Avello, D., Magalhães, P., Magalhães, F. D., Martins, J. M., González-Álvarez, J., et al. (2022b). High-value compounds obtained from grape canes (Vitis vinifera L.) by steam pressure alkali extraction. Food Bioprod. Process. 133, 153–167. doi:10.1016/j.fbp.2022.04.003
Santos, J., Fernandes, R. A., Ferreira, N., Ferreira, I., Vieira, C., Magalhães, F. D., et al. (2024). New particleboards for food-packaging from poplar peeling by-products using a circular economy approach. Int. J. Adhes. Adhes. 129, 103563. doi:10.1016/j.ijadhadh.2023.103563
Santos, J., Pereira, J., Escobar-Avello, D., Ferreira, I., Vieira, C., Magalhães, F. D., et al. (2022a). Grape canes (Vitis vinifera L.) applications on packaging and particleboard industry: new bioadhesive based on grape extracts and citric acid. Polym. (Basel) 14 (6), 1137. doi:10.3390/polym14061137
Santos, J., Pereira, J., Ferra, J., Magalhães, F. D., Martins, J. M., and Carvalho, L. H. (2021). New cardoon (Cynara cardunculus L.) particleboards using cardoon leaf extract and citric acid as bio-adhesive. Mater. Circ. Econ. 3 (1), 14. doi:10.1007/s42824-021-00027-1
Senila, L., Kovacs, E., Scurtu, D. A., Cadar, O., Becze, A., Senila, M., et al. (2020). Bioethanol production from vineyard waste by autohydrolysis pretreatment and chlorite delignification via simultaneous saccharification and fermentation. Molecules 25, 2606. doi:10.3390/molecules25112606
Šernek, M., and Žigon, J. (2023). Curing and adhesive bond strength development in naturally-based adhesives. Biobased Adhesives Sources, Charact. Appl. doi:10.1002/9781394175406.ch7
Skroza, D., Šimat, V., Vrdoljak, L., Jolić, N., Skelin, A., Čagalj, M., et al. (2022). Investigation of antioxidant synergisms and antagonisms among phenolic acids in the model matrices using FRAP and ORAC methods. Antioxidants 11 (9), 1784. doi:10.3390/antiox11091784
Sun, Y., Pang, H., Li, Z., Kang, H., and Zhang, S. (2022). Polyphenols induced in situ organic-inorganic crosslinking/mineralization strategy for constructing eco-friendly soy adhesive with high waterproof bonding strength. Compos B Eng., 242. doi:10.1016/j.compositesb.2022.110027
Troilo, M., Difonzo, G., Paradiso, V. M., Summo, C., and Caponio, F. (2021). Bioactive compounds from vine shoots, grape stalks, and wine lees: their potential use in agro-food chains. Foods 10, 342. MDPI AG. doi:10.3390/foods10020342
Uduwana, S., Abeynayake, N., and Wickramasinghe, I. (2023). Synergistic, antagonistic, and additive effects on the resultant antioxidant activity in infusions of green tea with bee honey and Citrus limonum extract as additives. J. Agric. Food Res. 12, 100571. doi:10.1016/j.jafr.2023.100571
Wang, L., Elahi, E., Zhou, Y., Wang, L., and Zhang, S. (2022). A review of packaging materials’ consumption regulation and pollution control. Sustain. Switz. 14 (23), 15866–15916. doi:10.3390/su142315866
Wang, X., Wang, F., Yu, Z., Zhang, Y., Qi, C., and Du, L. (2017). Surface free energy and dynamic wettability of wood simultaneously treated with acidic dye and flame retardant. J. Wood Sci. 63 (3), 271–280. doi:10.1007/s10086-017-1621-8
Wronka, A., and Kowaluk, G. (2019). Selected properties of particleboard made of raspberry Rubus idaeus L. lignocellulosic particles. Ann. WULS, For. Wood Technol. 105 (105), 113–124. doi:10.5604/01.3001.0013.7727
Yeniocak, M., Göktaş, O., Erdil, Y. Z., Özen, E., and Alma, M. H. (2014). Investigating the use of vine pruning stalks (Vitis vinifera L. CV sultani) as raw material for particleboard manufacturing. Wood Res. 59 (1), 167–176.
Keywords: grape stalk, bio-adhesives, polyphenols, alkaline extraction, mechanical properties
Citation: Fernandes RA, Lopes S, Ferreira N, Santos J, Martins JM and Carvalho LH (2024) Binderless particleboards obtained 100% from winery by-products for the packaging industry. Front. Food. Sci. Technol. 4:1376415. doi: 10.3389/frfst.2024.1376415
Received: 25 January 2024; Accepted: 29 March 2024;
Published: 18 April 2024.
Edited by:
Aris E. Giannakas, University of Patras, GreeceReviewed by:
Ricardo Gómez García, Universidade Católica Portuguesa, PortugalCopyright © 2024 Fernandes, Lopes, Ferreira, Santos, Martins and Carvalho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raquel A. Fernandes, cmFxdWVsLmZlcm5hbmRlc0BhcmNwLnB0; Luisa H. Carvalho, bGhjYXJ2YWxob0Blc3Rndi5pcHYucHQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.