
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Food. Sci. Technol. , 10 January 2024
Sec. Food Biotechnology
Volume 3 - 2023 | https://doi.org/10.3389/frfst.2023.1329037
This article is part of the Research Topic Enhancing Functional Dairy Foods: Bioactive Compounds in Fermented Matrices View all 3 articles
Fermentation can potentiate goat milk’s beneficial properties or generate new bioactive ingredients. In this narrative review, we summarize the current knowledge on the potential of fermented goat milk (FGM) products to improve different biomarkers of a modern epidemic: obesity and its comorbidities. In vitro studies have indicated functional properties of bioactive peptides or lipids obtained from FGM, showing potential to prevent Cardiovascular Disease development and anti-inflammatory activity. Probiotic strains derived from goat milk have prevented diet-induced obesity in animal models, and can represent a better techno-functional alternative to ferment this matrix than traditional starters. A small number of studies evaluated the functional properties of FGM in animal models of obesity. The observed in vitro effects of isolated compounds are generally in agreement with in vivo models’ findings. Overall, reports showed a positive impact on the prevention of body weight gain and several metabolic, inflammatory, and oxidative parameters. Nevertheless, evidence is limited and the mechanisms involved are fairly unknown. Comparisons between non-fermented and fermented products in vivo studies are lacking. Functional properties of FGM products aimed at improving the health of obese patients lack support from clinical trials. Considering the current evidence, the administration of complete FGM can be more beneficial to obese patients than the administration of its isolated compounds (lipids, peptides, or microorganisms). The addition of plant by-products with prebiotic or antioxidant properties is a promising strategy to enhance FGM functionality.
Goat milk-derived products are specially developed in Europe, as a regular component in Mediterranean diet, but represent a small fraction of commercialized dairy in other regions. Nevertheless, goat farmers are mainly located in developing countries of Asia, Africa, and Latin America (Sahlu and Goetsch, 2005). These ruminants can adapt to arid regions where cows cannot find suitable, natural forage resources. Therefore, they can be fed in marginal lands, and are usually raised by small farmers. These families are vital in every country’s agricultural system, as they maintain rural populations, can exploit, protect, and enhance natural resources, and provide a starting point for the development of new products or marketing systems (NIFA-USDA, 2023). Goat herds have a crucial impact on the economies of these families, for which an increasing demand and novel developments of its derived products can diminish rural poverty (Peacock, 2005). In contrast with intensive feeding practices, small farms are usually extensive systems where goats graze or browse local forage resources. The latter has proven to transfer higher amounts of bioactive compounds from vegetation to goat milk (Delgadillo-Puga and Cuchillo-Hilario, 2021).
Goat milk has multiple compositional differences compared to cow milk, generally regarded as health-promoting: lower allergenicity, easier digestibility, and higher content of short and medium-chain fatty acids (dos Santos et al., 2023). Nevertheless, its consumption is usually limited to its manufacturing origin, and the “goaty” flavor is undesirable for many consumers (Chen et al., 2023). Fermentation of goat milk or derived products, such as cheese, can potentiate its benefits or create new ones (Dubeuf, 2014). Fermentation is traditionally driven by the lactic acid bacteria (LAB) starters Streptococcus thermophilus and Lactobacillus bulgaricus, and can potentially reduce the “goaty” flavor, improve the consumers’ acceptability and carry beneficial probiotics. Nevertheless, fermented goat milk (FGM) products are scarcely addressed in scientific literature compared to other functional foods (Chen et al., 2023).
Functional foods can exert beneficial effects such as oxidative status, intestinal mucosal immunity, or microbiome balance. Furthermore, they can impact metabolic pathways involved in specific diseases. Obesity is considered a multifactorial, metabolic, and heterogeneous disease, where a chronic inflammatory state plays a pivotal role. It is defined as an abnormal or excessive fat accumulation that may impair health and is frequently associated with multiple comorbidities, such as type 2 Diabetes Mellitus (T2DM), steatotic liver disease (SLD), and cardiovascular diseases (CVDs) (Purnell, 2023). It constitutes a major public health threat for adults and children in most developed countries, and its prevalence is also rising in low- and middle-income countries (Endalifer and Diress, 2020). As the essential cause of obesity is a chronic energy imbalance related to dietary habits, functional foods present a high potential as a preventive strategy or therapeutic intervention. At present, there is no study revising the original research articles regarding the functionality of FGM products for obese individuals, analyzing the mechanisms involved. In this mini review, we will address the current knowledge on the effects of FGM products specifically to prevent and treat human obesity and its associated pathologies.
Compositional differences between FGM and bovine milk-fermented products have been recently reviewed (Chen et al., 2023). Moreno-Fernández et al. (2016) reported that FGM using conventional yogurt starters presented a fatty acid profile that is considered more health-promoting when compared with fermented cow milk: lauric, oleic, and linoleic acids are found in higher levels. In accordance, Marquez et al. (2022) reported that oleic acid content was increased by Lactobacillus delbrueckii CRL1447 fermentation of goat milk. Oleic and linoleic acid have insulin-sensitizing and lipid-lowering properties, reducing blood triglyceride and LDL cholesterol in animal models through their anti-inflammatory activity (Rehman et al., 2020; Ruze et al., 2023). Linoleic acid is generally regarded as health-promoting, although some controversy has arisen regarding its intake in Western diets (Mercola and D'Adamo, 2023). Furthermore, FGM reported by Moreno-Fernández et al. (2016) showed lower amounts of myristic and palmitic acids when compared to raw goat milk. These are fatty acids associated with higher CVD risk (Li et al., 2022). Apart from the inferences that can be derived from compositional analysis, few reports validate the functional properties of FGM through in vitro and in vivo studies. Figure 1 summarizes the information discussed in this study, showing the reported beneficial effects of FGM or its bioactive components on biomarkers of obesity and associated comorbidities.
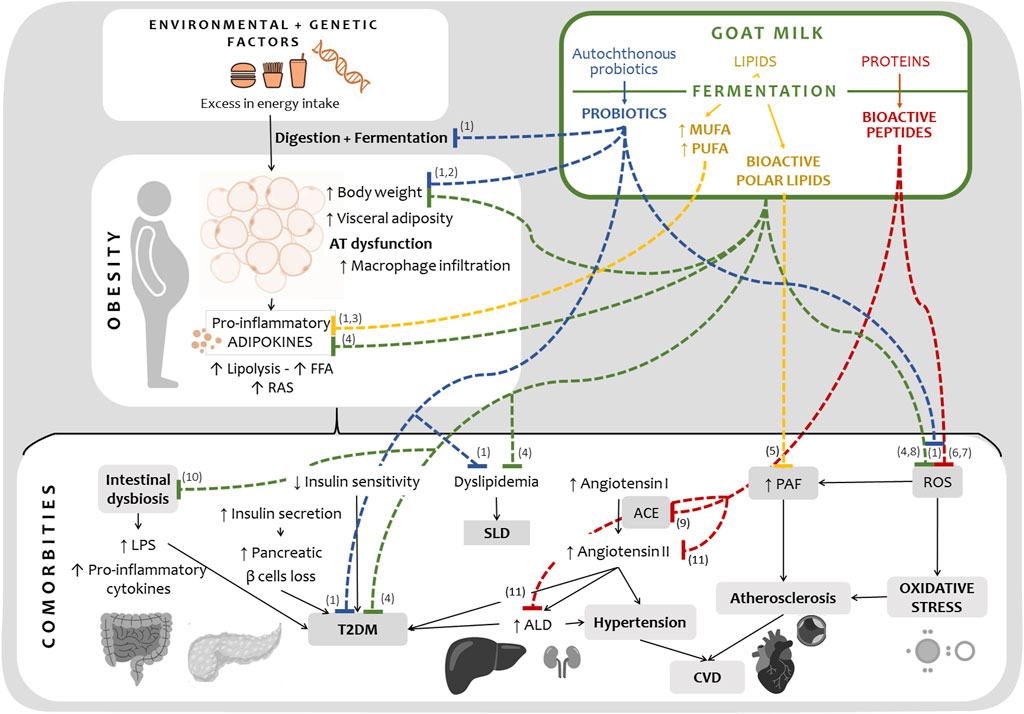
FIGURE 1. Current evidence on functional effects of fermented goat milk, or its isolated compounds, on obesity and associated comorbidities’ physiopathology. Dotted lines indicate inhibition of a specific enzyme or reduction of the disease’s biomarkers. Mechanisms and interactions in the physiopathology are simplified for illustration purposes. AT: Adipose tissue. FFA: free fatty acids. RAS: Local Renin-Angiotensin system. T2DM: Type 2 Diabetes Mellitus. SLD: Steatotic Liver Disease. ACE, angiotensin I-converting enzyme. ALD, Aldosterone. CVD, Cardiovascular Disease. PAF: Platelet-activating factor. ROS, Reactive oxygen species. References: 1 Marquez et al. (2022), Russo et al. (2020); 2 Fabersani et al. (2018); 3 Moreno-Fernández et al. (2016); 4 Altamimy et al. (2022); 5 Poutzalis et al. (2015); 6 Panchal et al. (2020); 7 Moreno-Montoro et al. (2017); 8 Mukdsi et al. (2013); 9 Parmar et al. (2017), Quirós et al. (2005), Gómez-Ruiz et al. (2006); 10 Neves Casarotti et al. (2020); 11 Lu et al. (2018).
Different scientific approaches are used to evaluate the functionality of a specific food. Regarding obesity and associated pathologies, cell culture techniques using human or animal adipocytes, hepatocytes, and immune cells allow the study of the influence of these foods or their particular ingredients on metabolic and inflammatory responses. These in vitro assays can provide meaningful information and mechanistic insights, although correspondence with in vivo effects is not guaranteed (Mirbagheri et al., 2019). Still, they provide an alternative that is more ethical, less expensive, and more or less effective in comparison with in vivo trials.
CVDs are the leading cause of mortality in people with obesity (Blüher, 2019). The increased risk of CVDs in people with obesity is mediated by established risk factors, such as dyslipidemia, atherosclerosis, T2DM, and hypertension (Khafagy and Dash, 2021). Certain components of FGM may have functional properties that improve CVD biomarkers. The anti-atherogenic properties of the lipid fraction of goat milk, yogurt, and cheese obtained through the fermentation by S. thermophilus and Lactobacillus bulgaricus were evaluated by Poutzalis et al. (2015). These authors reported that the total lipids and the polar lipids fractions of all three dairy products had inhibitory activities against platelet-activating factor (PAF)-induced platelet activation. PAF (1-O-alkyl-2-acetylsn-glyceryl-3-phosphocholine) is a crucial inflammatory phospholipid mediator implicated in atherogenesis (Demopoulos et al., 2003), activating leukocytes and their binding to endothelial cells (Nasopoulou et al., 2013). Higher activity was observed in fermented products, especially cheese. Therefore, the fermentation process increased the potential functionality, mainly in the polar lipid fraction, whereas no further analyses were performed to identify the mechanisms involved. Previous reports in cow’s milk and yogurt agree with these results (Antonopoulou et al., 1996).
Angiotensin I-converting enzyme (ACE)-inhibitory activity is one of the most studied functional characteristics of bovine and goat dairy products. This ACE-inhibition activity is attributed to casein-derived peptides, which were purified from Lacticaseibacillus casei NK9-FGM (Parmar et al., 2017), goat milk kefir (Quirós et al., 2005) and cheese (Gómez-Ruiz et al., 2006). Minervini et al. (2009) reported in vitro ACE-inhibitory activity in different FGM, demonstrating that it is a strain-dependent characteristic present in different LAB species. Enzymatic hydrolysis is frequently used to obtain these peptides (Lee et al., 2005; Geerlings et al., 2006). Qiao et al. (2022) identified an ACE-inhibitory peptide from goat milk casein enzymatic-hydrolysates, resistant to an in vitro digestion process. This purified peptide inhibited the excessive proliferation and migration induced by ACE on aortic vascular smooth cells in vitro. Furthermore, transcriptome sequencing analysis showed that several CVD-related pathways were differently expressed in peptide-treated cells, especially in genes involved in vascular remodeling. Considering that hypertension is a major risk factor leading to CVD, and that is frequently treated with ACE-inhibitory medicines, these goat milk peptides can be considered functional food ingredients for susceptible consumers.
Goat milk whey protein (WP) hydrolysates have also shown antihypertensive and antioxidant properties (Sakkas et al., 2022). Du et al. (2022) described the potential hypoglycemic activity of several WP hydrolysates, studying the in vitro inhibition of enzymes involved in glucose metabolism: dipeptidyl peptidase IV, α-glucosidase, and α-amylase.
Patients with obesity present higher oxidative stress than normal-weight individuals (Martínez-Martínez and Cachofeiro, 2022). Oxidative stress is a common pathway that links obesity with related diseases, such as atherosclerosis and T2DM. In this regard, Panchal et al. (2020) fermented goat milk using Lactobacillus fermentum M4 and observed the presence of peptides with antioxidant activity. Likewise, Moreno-Montoro et al. (2017) reported small (<3 kDa), non-basic bioactive peptides in fermented goat milk using traditional starters, showing high total antioxidant capacity and ACE-inhibitory activity.
Gut dysbiosis is associated with obesity, characterized by a predominance of Firmicutes (Clostridium, Prevotella, and Methanobrevibacter) and a deficiency in beneficial genera such as Bacteroides, Bifidobacterium, Lactobacillus, and Akkermansia (Amabebe et al., 2020). Administration of probiotics, prebiotics, and synbiotics can modulate this dysbiosis. The most used and most effective strains of probiotics are LAB, specifically the genus Lactobacillus and Bifidobacterium (Álvarez-Arraño and Martín-Peláez, 2021a). The effects of a probiotic are strain- and dose-specific (Green et al., 2020). Selected single-strain LAB administration to obese individuals has demonstrated clinical success in reducing body weight, body fat mass, and fat percentage (Álvarez-Arraño and Martín-Peláez, 2021b).
Increasing research is conducted towards the specific determination of anti-obesity properties of probiotic LAB: lipid-lowering, hypoglycemic, and modulation of the intestinal dysbiosis, for example,. LAB are also the main class of fermenters in the dairy industry, where autochthonous microorganisms are preferred to ferment each food matrix. The microbiota of goat dairy products is mainly comprised by Lactobacillus and Enterococcus species (Medina et al., 2011). Limosilactobacillus fermentum CRL1446, Lactiplantibacillus paraplantarum CRL1449, and CRL1472 strains isolated from goat milk and cheese were selected for their in vitro anti-obesity properties, such as inhibition of α-glucosidase, presence of bile salts hydrolase (BSH) activity, and cholesterol assimilation capacity (Marquez et al., 2022). The intestinal α-glucosidase enzyme is responsible for the hydrolysis of glycosidic bonds, releasing glucose and increasing postprandial blood glucose (Bajinka et al., 2023). BSH produces the deconjugation of primary bile acids, which in turn decreases cholesterol absorption by enterocytes and improves fecal excretion (Bourgin et al., 2021). These LAB strains also reduced triglyceride content in Caenorhabditis elegans when it was grown in the presence of these bacteria. Likewise, administration of these strains to mice fed a high-fat diet (HFD) decreased body weight gain compared with the placebo group, and ameliorated hyperglycemia and dyslipidemia. These LAB were added to an FGM, which was an appropriate vehicle for the potential probiotics for 21 days in cold storage. This FGM was fermented using an autochthonous starter, Lactobacillus delbrueckii subsp. indicus CRL1447, as probiotic strains’ acidifying capacity in milk was insufficient.
Intestinal microbiota modifications are usually studied in vivo in preclinical or clinical trials. Nevertheless, an in vitro study using the Simulator of Human Intestinal Microbial Ecosystem (SHIME) system was used to evaluate a low-fat goat milk supplemented with passion fruit by-product and fermented with Lacticaseibacillus casei Lc-1 and S. thermophilus TA040 (Neves Casarotti et al., 2020). The effect of this product on the microbiota of obese donors was considered beneficial, showing an increased abundance of Bifidobacterium and Lactobacillus, and decreased short-chain fatty acids-producer microorganisms (Prevotella, Megamonas, Bacteroides, and Succinivibrio genera). The inclusion of fruit by-products is intended to contribute with prebiotic compounds that can act synergistically with probiotics.
Therapeutic approaches for obesity were discovered, validated, and optimized in animal models. Murine animals are the standard for preclinical evaluations to study many human disorders, including obesity. HFD is intended to imitate the Western diet and induces fat accumulation in mice or rats. Although specific differences have been described, mice and rats with HFD-induced obesity constitute a generally accepted model to predict human weight loss interventions (Kleinert et al., 2018).
Non-fermented goat milk has been evaluated as a preventive strategy in obesity models. Delgadillo-Puga et al. (2020) demonstrated that the administration of goat milk had beneficial regulatory effects on blood lipid profile and redox status, increased glucose tolerance, and decreased inflammatory markers, liver steatosis, and body fat mass in mice fed a HFD. These effects were apparently due to increased insulin sensitivity and energy utilization. Kalyan et al. (2018) described similar results in rats, whether casein or fat fraction of goat milk was administered.
At present, FGM products are scarcely evaluated through in vivo obesity models. Altamimy et al. (2022) observed that the administration of naturally-fermented goat milk (an artisanal product named oggtt or Madheer) to rats fed a HFD had a hypoglycemic, hypolipidemic, antioxidant, and anti-inflammatory effect. Both a low or high dose of oggtt induced similar results. Similarly, Fabersani et al. (2018) reported that administration of goat milk yogurt decreased body weight gain and body mass index in HFD-fed rats. The addition of yacon flour (70 g/L yogurt) accentuated these effects, likely due to its prebiotic and phenolic compounds.
Mukdsi et al. (2013) evaluated the effects of the administration of goat milk cheese manufactured with the autochthonous strain L. fermentum CRL1446 on the oxidative status in mice. Lactobacillus fermentumCRL1446 presents feruloyl esterase (FE) activity, which allows the esterified ferulic acid to be hydrolyzed from the cell walls of vegetables. Ferulic acid and its intestinal metabolites present antioxidant activity. These authors observed that the administration of this cheese produced a 2-fold increase in intestinal FE activity, a 2-fold decrease in plasmatic thiobarbituric acid-reactive substances levels, and a 3-fold increase in glutathione reductase activity in normal mice. These effects were also observed when the probiotic strain CRL1446 was administered to HFD-fed mice (Russo et al., 2020; Marquez et al., 2022). Beneficial regulations in metabolic parameters, such as body weight gain, visceral fat accumulation, blood glucose metabolism, and lipid profile were also observed in these preventive in vivo trials.
At present, one clinical study evaluated the effects of FGM products on obesity or associated pathologies. Lu et al. (2018) administered a peptide product obtained from a non-specified FGM to 45 prehypertensive and overweight volunteers. A placebo-controlled clinical study assessed the antihypertensive effect of the product, offered as chewable tablets, during an 8-week administration, with a 2-week follow-up. The results showed that the supplementation significantly lowered blood pressure parameters by decreasing angiotensin II and aldosterone levels. Anthropometric parameters, lipid profile, ACE, and angiotensin I levels were unaffected. To the best of our knowledge, studies on the administration of whole FGM products to obese patients were not published.
This narrative review analyzed the reported effects of FGM products on the mechanisms involved in obesity development and its associated diseases. Both the lipid and the peptidic fractions of goat milk are modified during fermentation, which can imply the production of bioactive molecules. This relies on the specific starter strain/s. Metabolites (small peptides, bioactive lipids) and certain strains of the autochthonous microbiota of goat milk have demonstrated in vitro functional activities that are implicated in obesity physiopathology: anti-atherogenic, antihypertensive, lipid-lowering, hypoglycemic, antioxidant, gut microbiota regulation. Whole FGM products could be more efficient as a therapeutic strategy than the separated administration of its isolated compounds. Preclinical studies that effectively demonstrate the beneficial effects of FGM related to obesity, Cardiovascular Disease, and Type 2 Diabetes Mellitus are scarce. Therefore, evidence is limited and the mechanisms involved are fairly unknown. Up to date, there is only one clinical study on this matter, which reported a hypotensive effect of a FGM-peptide product in overweight individuals. Although current knowledge positively supports the hypothesis that FGM products present beneficial metabolic and microbiota-modulating activities, clinical studies with obese patients are lacking. Complete in vitro to clinical studies should be performed for each product developed.
EA: Conceptualization, Data curation, Investigation, Methodology, Software, Visualization, Writing–original draft, Writing–review and editing, Formal Analysis, Validation. AM: Investigation, Methodology, Software, Visualization, Writing–review and editing. MR: Investigation, Methodology, Visualization, Writing–review and editing. PG-C: Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing–review and editing. RM: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing–original draft, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by FONCyT, grant number Préstamo BID PICT-2018-2151, PICT-2021-CAT-II-00071, PICT-2020-03777, CONICET PIP 0406, CONICET PIP 0869, Universidad Nacional de Tucumán PIUNT A722.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Altamimy, K. M., Alshammari, G. M., Yagoub, A. E. A., Albekairi, N. A., Alshehri, S., Saleh, A., et al. (2022). Saudi traditional fermented goat milk protects against experimental non-alcoholic fatty liver disease by hypoglycaemic and antioxidant potentials. Fermentation 8, 735. doi:10.3390/fermentation8120735
Álvarez-Arraño, V., and Martín-Peláez, S. (2021a). Effects of probiotics and synbiotics on weight loss in subjects with overweight or obesity: a systematic review. Nutrients 13, 3627. doi:10.3390/nu13103627
Álvarez-Arraño, V., and Martín-Peláez, S. (2021b). Effects of probiotics and synbiotics on weight loss in subjects with overweight or obesity: a systematic review. Nutrients 13, 3627. doi:10.3390/nu13103627
Amabebe, E., Robert, F. O., Agbalalah, T., and Orubu, E. S. (2020). Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 123, 1127–1137. doi:10.1017/S0007114520000380
Antonopoulou, S., Semidalas, C. E., Koussissis, S., and Demopoulos, C. A. (1996). Platelet-activating factor (PAF) antagonists in foods: a study of lipids with PAF or anti-PAF-like activity in cow's milk and yogurt. J. Agric. Food Chem. 44, 3047–3051. doi:10.1021/jf950619y
Bajinka, O., Sylvain Dovi, K., Simbilyabo, L., Conteh, I., and Tan, Y. (2023). The predicted mechanisms and evidence of probiotics on type 2 diabetes mellitus (T2DM). Archives Physiology Biochem., 1–16. doi:10.1080/13813455.2022.2163260
Blüher, M. J. N. R. E. (2019). Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298. doi:10.1038/s41574-019-0176-8
Bourgin, M., Kriaa, A., Mkaouar, H., Mariaule, V., Jablaoui, A., Maguin, E., et al. (2021). Bile salt hydrolases: at the crossroads of microbiota and human health. Microorganisms 9, 1122. doi:10.3390/microorganisms9061122
Chen, L., Bagnicka, E., Chen, H., and Shu, G. (2023). Health potential of fermented goat dairy products: composition comparison with fermented cow milk, probiotics selection, health benefits and mechanisms. Food Funct. 14, 3423–3436. doi:10.1039/d3fo00413a
Delgadillo-Puga, C., and Cuchillo-Hilario, M. (2021). Reviewing the benefits of grazing/browsing semiarid rangeland feed resources and the transference of bioactivity and pro-healthy properties to goat milk and cheese: obesity, insulin resistance, inflammation and hepatic steatosis prevention. Anim. (Basel) 11, 2942. doi:10.3390/ani11102942
Delgadillo-Puga, C., Noriega, L. G., Morales-Romero, A. M., Nieto-Camacho, A., Granados-Portillo, O., Rodriguez-Lopez, L. A., et al. (2020). Goat's milk intake prevents obesity, hepatic steatosis and insulin resistance in mice fed A high-fat diet by reducing inflammatory markers and increasing energy expenditure and mitochondrial content in skeletal muscle. Int. J. Mol. Sci. 21, 5530. doi:10.3390/ijms21155530
Demopoulos, C. A., Karantonis, H. C., and Antonopoulou, S. (2003). Platelet activating factor—a molecular link between atherosclerosis theories. Eur. J. Lipid Sci. Technol. 105, 705–716. doi:10.1002/ejlt.200300845
Dos Santos, W. M., Guimarães Gomes, A. C., De Caldas Nobre, M. S., De Souza Pereira, Á. M., Dos Santos Pereira, E. V., Dos Santos, K. M. O., et al. (2023). Goat milk as a natural source of bioactive compounds and strategies to enhance the amount of these beneficial components. Int. Dairy J. 137, 105515. doi:10.1016/j.idairyj.2022.105515
Du, X., Jing, H., Wang, L., Huang, X., Wang, X., and Wang, H. J. L. (2022). Characterization of structure, physicochemical properties, and hypoglycemic activity of goat milk whey protein hydrolysate processed with different proteases. LWT 159, 113257. doi:10.1016/j.lwt.2022.113257
Dubeuf, J.-P. (2014). Science, technology, innovation and governance for the goat sectors. Small Ruminant Res. 121, 2–6. doi:10.1016/j.smallrumres.2014.05.016
Endalifer, M. L., and Diress, G. (2020). Epidemiology, predisposing factors, biomarkers, and prevention mechanism of obesity: a systematic review. J. Obes. 2020, 6134362. doi:10.1155/2020/6134362
Fabersani, E., Grande, M. V., Aráoz, M. V. C., Zannier, M. L., Sánchez, S. S., Grau, A., et al. (2018). Metabolic effects of goat milk yogurt supplemented with yacon flour in rats on high-fat diet. J. Funct. Foods 49, 447–457. doi:10.1016/j.jff.2018.08.042
Geerlings, A., Villar, I., Zarco, F. H., Sánchez, M., Vera, R., Gomez, A. Z., et al. (2006). Identification and characterization of novel angiotensin-converting enzyme inhibitors obtained from goat milk. J. dairy Sci. 89, 3326–3335. doi:10.3168/jds.S0022-0302(06)72369-4
Gómez-Ruiz, J. Á., Taborda, G., Amigo, L., Recio, I., and Ramos, M. (2006). Identification of ACE-inhibitory peptides in different Spanish cheeses by tandem mass spectrometry. Eur. Food Res. Technol. 223, 595–601. doi:10.1007/s00217-005-0238-0
Green, M., Arora, K., and Prakash, S. (2020). Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int. J. Mol. Sci. 21, 2890. doi:10.3390/ijms21082890
Kalyan, S., Meena, S., Kapila, S., Sowmya, K., and Kumar, R. (2018). Evaluation of goat milk fat and goat milk casein fraction for anti-hypercholesterolaemic and antioxidative properties in hypercholesterolaemic rats. Int. Dairy J. 84, 23–27. doi:10.1016/j.idairyj.2018.03.012
Khafagy, R., and Dash, S. J. F. I. C. M. (2021). Obesity and cardiovascular disease: the emerging role of inflammation. Front. Cardiovasc Med. 8, 768119. doi:10.3389/fcvm.2021.768119
Kleinert, M., Clemmensen, C., Hofmann, S. M., Moore, M. C., Renner, S., Woods, S. C., et al. (2018). Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 14, 140–162. doi:10.1038/nrendo.2017.161
Lee, K., Kim, S., Ryu, J., Shin, H., and Lim, J. (2005). Separation and purification of angiotensin converting enzyme inhibitory peptides derived from Goat`s milk casein hydrolysates. Asian-Australasian J. animal Sci. 18, 741–746. doi:10.5713/ajas.2005.741
Li, Z., Lei, H., Jiang, H., Fan, Y., Shi, J., Li, C., et al. (2022). Saturated fatty acid biomarkers and risk of cardiometabolic diseases: a meta-analysis of prospective studies. Front. Nutr. 9, 963471. doi:10.3389/fnut.2022.963471
Lu, T. M., Chiu, H. F., Lu, Y. Y., Han, Y. C., Shen, Y. C., Venkatakrishnan, K., et al. (2018). Efficacy of fermented goat milk on blood pressure in prehypertensive adults: a randomized, placebo-controlled, clinical trial. J. Food Biochem. 42, e12474. doi:10.1111/jfbc.12474
Marquez, A., Andrada, E., Russo, M., Bolondi, M. L., Fabersani, E., Medina, R., et al. (2022). Characterization of autochthonous lactobacilli from goat dairy products with probiotic potential for metabolic diseases. Heliyon 8, e10462. doi:10.1016/j.heliyon.2022.e10462
Martínez-Martínez, E., and Cachofeiro, V. (2022). Oxidative stress in obesity. Antioxidants 11, 639–641. doi:10.3390/antiox11040639
Medina, R. B., Oliszewski, R., Abeijón Mukdsi, M. C., Van Nieuwenhove, C. P., and González, S. N. (2011). Sheep and goat's dairy products from South America: microbiota and its metabolic activity. Small Ruminant Res. 101, 84–91. doi:10.1016/j.smallrumres.2011.09.028
Mercola, J., and D'adamo, C. R. (2023). Linoleic acid: a narrative review of the effects of increased intake in the standard American diet and associations with chronic disease. Nutrients 15, 3129. doi:10.3390/nu15143129
Minervini, F., Bilancia, M. T., Siragusa, S., Gobbetti, M., and Caponio, F. (2009). Fermented goats’ milk produced with selected multiple starters as a potentially functional food. Food Microbiol. 26, 559–564. doi:10.1016/j.fm.2009.03.008
Mirbagheri, M., Adibnia, V., Hughes, B. R., Waldman, S. D., Banquy, X., and Hwang, D. K. (2019). Advanced cell culture platforms: a growing quest for emulating natural tissues. Mater. Horizons 6, 45–71. doi:10.1039/c8mh00803e
Moreno-Fernández, J., Díaz-Castro, J., Alférez, M. J., Hijano, S., Nestares, T., and López-Aliaga, I. (2016). Production and chemical composition of two dehydrated fermented dairy products based on cow or goat milk. J. Dairy Res. 83, 81–88. doi:10.1017/S0022029915000722
Moreno-Montoro, M., Olalla-Herrera, M., Rufián-Henares, J. Á., Martínez, R. G., Miralles, B., Bergillos, T., et al. (2017). Antioxidant, ACE-inhibitory and antimicrobial activity of fermented goat milk: activity and physicochemical property relationship of the peptide components. Food Funct. 8, 2783–2791. doi:10.1039/c7fo00666g
Mukdsi, M. C. A., Haro, C., González, S. N., and Medina, R. B. (2013). Functional goat milk cheese with feruloyl esterase activity. J. Funct. Foods 5, 801–809. doi:10.1016/j.jff.2013.01.026
Nasopoulou, C., Gogaki, V., Panagopoulou, E., Demopoulos, C., and Zabetakis, I. J. A. S. J. (2013). Hen egg yolk lipid fractions with antiatherogenic properties. Anim. Sci. J. 84, 264–271. doi:10.1111/j.1740-0929.2012.01067.x
Neves Casarotti, S., Fernanda Borgonovi, T., De Mello Tieghi, T., Sivieri, K., and Lucia Barretto Penna, A. (2020). Probiotic low-fat fermented goat milk with passion fruit by-product: in vitro effect on obese individuals' microbiota and on metabolites production. Food Res. Int. 136, 109453. doi:10.1016/j.foodres.2020.109453
NIFA-USDA (2023). Importance of family and small farms United States department of agriculture official website: national institute of food and agriculture. Available at: https://www.nifa.usda.gov/topics/small-family-farms [Accessed 04-October-2023].
Panchal, G., Hati, S., and Sakure, A. J. L. (2020). Characterization and production of novel antioxidative peptides derived from fermented goat milk by L. fermentum. LWT 119, 108887. doi:10.1016/j.lwt.2019.108887
Parmar, H., Hati, S., and Sakure, A. (2017). In vitro and in silico analysis of novel ACE-inhibitory bioactive peptides derived from fermented goat milk. Int. J. Peptide Res. Ther. 24, 441–453. doi:10.1007/s10989-017-9630-4
Peacock, C. (2005). Goats—a pathway out of poverty. Small Ruminant Res. 60, 179–186. doi:10.1016/j.smallrumres.2005.06.011
Poutzalis, S., Anastasiadou, A., Nasopoulou, C., Megalemou, K., Sioriki, E., and Zabetakis, I. (2015). Evaluation of the in vitro anti-atherogenic activities of goat milk and goat dairy products. Dairy Sci. Technol. 96, 317–327. doi:10.1007/s13594-015-0266-x
Purnell, J. Q. (2023). “Definitions, classification, and epidemiology of obesity,” in Endotext (South Dartmouth (MA): MDText.com, Inc).
Qiao, Z., Wang, J., He, Z., Pan, L., Feng, K., Peng, X., et al. (2022). A novel angiotensin I-converting enzyme inhibitory peptide derived from goat milk casein hydrolysate modulates angiotensin II-stimulated effects on vascular smooth muscle cells. Front. Nutr. 9, 878768. doi:10.3389/fnut.2022.878768
Quirós, A., Hernández-Ledesma, B., Ramos, M., Amigo, L., and Recio, I. (2005). Angiotensin-converting enzyme inhibitory activity of peptides derived from caprine kefir. J. Dairy Sci. 88, 3480–3487. doi:10.3168/jds.S0022-0302(05)73032-0
Rehman, K., Haider, K., Jabeen, K., and Akash, M. S. H. (2020). Current perspectives of oleic acid: regulation of molecular pathways in mitochondrial and endothelial functioning against insulin resistance and diabetes. Rev. Endocr. Metabolic Disord. 21, 631–643. doi:10.1007/s11154-020-09549-6
Russo, M., Marquez, A., Herrera, H., Abeijon-Mukdsi, C., Saavedra, L., Hebert, E., et al. (2020). Oral administration of Lactobacillus fermentum CRL1446 improves biomarkers of metabolic syndrome in mice fed a high-fat diet supplemented with wheat bran. Food and Funct. 11, 3879–3894. doi:10.1039/d0fo00730g
Ruze, R., Liu, T., Zou, X., Song, J., Chen, Y., Xu, R., et al. (2023). Obesity and type 2 diabetes mellitus: connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 14, 1161521. doi:10.3389/fendo.2023.1161521
Sahlu, T., and Goetsch, A. L. (2005). A foresight on goat research. Small Ruminant Res. 60, 7–12. doi:10.1016/j.smallrumres.2005.06.002
Keywords: bioactive compounds, bioactive peptides, goat dairy products, cardiovascular disease, type 2 diabetes mellitus, probiotics
Citation: Andrada E, Marquez A, Russo M, Gauffin-Cano P and Medina R (2024) Fermented goat milk as a functional food for obesity prevention or treatment: a narrative review. Front. Food. Sci. Technol. 3:1329037. doi: 10.3389/frfst.2023.1329037
Received: 27 October 2023; Accepted: 29 December 2023;
Published: 10 January 2024.
Edited by:
Claudia Inés Vénica, CONICET Instituto de Lactología Industrial (INLAIN), ArgentinaReviewed by:
Miguel A. Ortega, University of Alcalá, SpainCopyright © 2024 Andrada, Marquez, Russo, Gauffin-Cano and Medina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roxana Medina, cm1lZGluYUBjZXJlbGEub3JnLmFy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.