
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Food. Sci. Technol. , 09 May 2023
Sec. Food Biotechnology
Volume 3 - 2023 | https://doi.org/10.3389/frfst.2023.1190063
This article is part of the Research Topic Post-Pandemic Foods: Development, Processing, Acceptance and Quality View all 5 articles
Yarrowia lipolytica is a non-conventional non-pathogenic, generally regarded as safe yeast. It has been isolated from a wide variety of places, from foodstuffs like beer, cheese and sausages to beetle guts and human mouths. It is strictly aerobic and Crabtree-negative. Y. lipolytica harbours various biochemical and physiological traits that make it relevant for biotechnological and food-related applications. Until recently, the application and effect of Y. lipolytica on lipid-containing foodstuff, that is, meat and dairy, have been researched and discussed meticulously. The yeasts’ potential as a synthesiser of several high-value food ingredients, such as organic acids, aromas, and emulsifiers from a range of diverse substrates, from ethanol to olive oil waste, is of interest in a biorefinery context. Interestingly the use of Y. lipolytica as a starter culture in foodstuffs beyond meat and dairy is minimal, despite its ability to synthesise interesting aromas and organic acids that could increase the organoleptic quality of fermented beverages. Besides the indulgence factor, Y. lipolytica synthesises a wide range of functional and bioactive compounds that can act as active ingredients in functional beverages, adding to its potential in producing novel beverages.
Yarrowia lipolytica is a dimorphic, non-conventional, strictly aerobic yeast. The yeast was already isolated and identified in 1928 and has been known under several different names until 1972 when David Yarrow reclassified it into its current genus. In acknowledgement of David Yarrow’s work, it was later renamed Yarrowia. The name of the species, lipolytica, comes from the yeast’s ability to hydrolyse lipids. It has since been studied extensively (Yarrow, 1972; Barth and Gaillardin, 1997; Coelho et al., 2010; Nicaud, 2012; Darvishi Harzevili, 2014; Groenewald et al., 2014; Sutherland et al., 2014; Zinjarde, 2014; Madzak, 2018; Bankar et al., 2020; Fickers et al., 2020; Madzak, 2021). Y. lipolytica has been used as a model organism for non-conventional yeast due to its considerable physiological differences from other model yeasts, e.g., Saccharomyces cerevisiae (Nicaud, 2012; Sutherland et al., 2014).
Y. lipolytica is most often found in substrates containing hydrophobic carbon sources, e.g., oil-polluted soil and marine environments and in as exotic a place as termite gut. The yeast survives such environments due to efficient degradation pathways of diverse substrates (Zinjarde and Pant, 2000; Fickers et al., 2005; Fukuda, 2013; Shukla et al., 2018; Gálvez-López et al., 2019; Madzak, 2021). Y. lipolytica utilise some sugars (fructose, glucose and lactose), lipids, proteins, alcohols, sugar alcohols and some organic acids (see Figure 1) (Mansour et al., 2008; Beopoulos et al., 2010; Arslan et al., 2016; Spagnuolo et al., 2018; Madzak, 2021). It tolerates extremely diverse environments; low temperatures, hypersaline conditions, and acidic and alkaline pH. In a food context, this means that Y. lipolytica has mostly been isolated from meat and dairy products, such as cheeses, yoghurt, kefir, sausages and poultry (Barth and Gaillardin, 1997; Nicaud, 2012; Zinjarde, 2014; Madzak, 2018; Zieniuk and Fabiszewska, 2018). In this context, Y. lipolytica has been regarded both as a spoilage and a desired organism (Zinjarde, 2014). However, it has also been isolated from traditional Belgian sour beer, where it has been suggested to play a specific role in sour beer production (Spitaels et al., 2014; Spitaels et al., 2015). To our knowledge, this finding has not been explored further. Y. lipolytica seems also to have been isolated from soft drinks, juices, wine, must and cider (Deák and Beuchat, 1987; Groenewald et al., 2014).
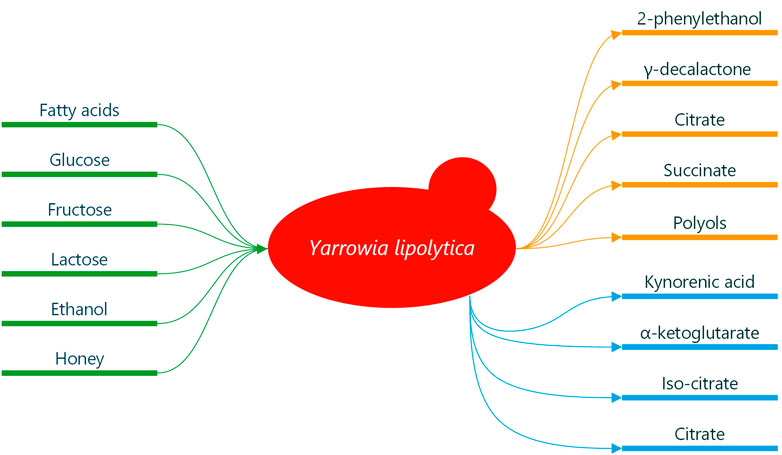
FIGURE 1. Compounds relevant in a fermented beverage setting synthesised by Y. lipolytica. Green indicates carbon sources which can derive from relevant substrates. Orange indicates compounds of interest for flavour quality in beverages. Blue indicates compounds of relevance in functional beverages.
Y. lipolytica is generally regarded as a non-pathogen and is reported as such as it does not cause disease in healthy people, and most strains of Y. lipolytica are unable to grow at temperatures higher than 32°C (Lelieveld et al., 1996; Nicaud, 2012; Groenewald et al., 2014). Only within the last decade has Y. lipolytica been considered a part of the natural human mycobiota, primarily found in adults’ mouths and respiratory tracts (Desnos-Ollivier et al., 2020). Furthermore, no reports of the direct production of toxic compounds by Y. lipolytica have been made (Groenewald et al., 2014). The yeast is, however, occasionally seen as an organism causing opportunistic biofilm infections in mainly immunocompromised individuals, meaning that it acts similarly to other non-pathogen yeast such as S. cerevisiae (Holzschu et al., 1979; Groenewald et al., 2014). The infections are easily treated; in some cases, they can even disappear without treatment (Groenewald et al., 2014). Despite this, Y. lipolytica has been classified as a Biosafety Level 1 in most European countries and by the Public Health Services (Washington DC, United States). Multiple industrial production applications of Y. lipolytica have been labelled as “Generally Regarded as Safe” (GRAS), e.g., in citrate, erythritol, and β-carotene production. Thus, the yeast itself has gained a GRAS status. Furthermore, Y. lipolytica has been labelled as a “recommended biological agent for production purposes” by the European Food and Feed Cultures Association (EFFCA) and as a “microorganism with a documented use in food” by the International Dairy Federation (IDF) (Groenewald et al., 2014; Desnos-Ollivier et al., 2020; Madzak, 2021).
Most focus on the use of Y. lipolytica has, until today, been from a biotech point of view. Particular emphasis has been on optimising the production of one or more metabolites of particular interest, and Y. lipolyticas’ ability to secrete a myriad of interesting compounds has been thoroughly tested and optimised both in wild strains and in engineered strains, e.g., citrate, iso-citrate, 2-phenylethanol, γ-decalactone, sugar alcohols, Single Cell Oils (SCO), and Single Cell Proteins (SCP) (Anastassiadis et al., 2002; Finogenova et al., 2002; Papanikolaou et al., 2002; Anastassiadis et al., 2007; Iucci et al., 2007; Patrignani et al., 2007; Mansour et al., 2008; Bankar et al., 2009; Beopoulos et al., 2009; Celińska et al., 2013; 2015; Groenewald et al., 2014; Sibirny et al., 2014; Zinjarde, 2014; Braga and Belo, 2015; Kamzolova and Morgunov, 2017 Sarris et al., 2017; Kamzolova et al., 2018; Madzak, 2018; Carsanba et al., 2020; Fickers et al., 2020; Małajowicz et al., 2020 Kamzolova and Morgunov, 2021; Kothari et al., 2022). Only non-engineered strains will be discussed in the current review due to general consumer reluctance to food produced using engineered strains (Curtis et al., 2004).
Outside of a biotechnological context, the use of Y. lipolytica in food has mainly been on meat and dairy products due to the extreme lipolytic and proteolytic activity of the yeast. It has been suggested in multiple articles that Y. lipolytica can be used as a starter for the production of both cheese and dried fermented sausages, with very diverse applications, e.g., faster ripening, improved textural and flavour quality, and bioprotection of the products (Freitas et al., 1999; Wyder et al., 1999; van den Tempel and Jakobsen, 2000; Addis et al., 2001; Gardini et al., 2001; Lourens-Hattingh and Viljoen, 2002; Ferreira and Viljoen, 2003; Żarowska et al., 2004; Lanciotti et al., 2005; Larpin et al., 2006; Iucci et al., 2007; Patrignani et al., 2007; Mansour et al., 2008; Patrignani et al., 2011a; Patrignani et al., 2011b; Büyükta, 2013; Szoltysik et al., 2013; Zinjarde, 2014; Centeno et al., 2017). Y. lipolytica has furthermore been suggested to possess probiotic traits (Rai et al., 2019; Agarbati et al., 2021a; Agarbati et al., 2021b) and has the potential for use in the production of functional food (Rai et al., 2019; Jach and Malm, 2022). More scarcely alternative suggestions for uses for Y. lipolytica as a starter have been seen, e.g., for the fermentation of green coffee and palm kernels for improving flavour profiles (Lee et al., 2017; Zhang et al., 2019). In common for all the abovementioned applications, they apply surface fermentation of a solid medium. This is most probably due to the obligate aerobic nature of Y. lipolytica (Barth and Gaillardin, 1997; Patrignani et al., 2007; Bankar et al., 2009; Gori et al., 2013; Sutherland et al., 2014). To the best of our knowledge, only one patent application has been filed (by CSK Food Enrichment BV) on a starter culture containing Y. lipolytica and Kluyveromyces lactis for application in cheese production (Meijer et al., 2020). We have been unable to find any commercial starter cultures containing Y. lipolytica for a meat-related application. This limits the commercial application of Y. lipolytica as a starter in food production. Even more limited is the application and knowledge of Y. lipolytica in fermented beverages, despite its potential.
The use of yeast as a probiotic organism and in the production of functional food is still in its early stages in commercial large scale production, but it has received significant attention in recent years (Kumura et al., 2004; Fleet and Balia, 2006; Martirosyan and Leem, 2019; Rai et al., 2019; Sadeghi et al., 2022). Thus, exploring the potential for Y. lipolytica in that context could prove relevant and interesting. As earlier mentioned, Y. lipolytica has been isolated from kefir (Rohm and Lehner, 1990; Lu et al., 2014; Kalamaki and Angelidis, 2017) and traditionally fermented Belgian sour beer (Spitaels et al., 2014). However, the knowledge about the functionality of Y. lipolytica in these products is minimal.
Agarbati et al. (2021a) tested several yeasts with probiotic traits to produce kefir in a co-fermentation with a commercial strain of the lactic acid bacterium Lactobacillus casei. They found that, among other yeasts, Y. lipolytica showed promising results as a co-fermenter for producing kefir on an industrial scale. However, they also emphasised the importance of further research to determine scalability, and whether Y. lipolytica can be used as a probiotic organism in a safe manner (Agarbati et al., 2021a).
Gutiérrez et al. (2018) performed a vast screening experiment testing the performance and production of pleasant aroma compounds by non-conventional yeasts in three different media; glucose wort, grape juice and apple juice. It was concluded that out of multiple yeast species tested, Y. lipolytica was one of the most promising (Gutiérrez et al., 2018).
Lastly, the performance of a strain of Y. lipolytica has been evaluated in brewers’ wort under different temperatures and aeration conditions. It was found that Y. lipolytica can grow in a brewers’ wort containing iso-α-acid, and the only sugars consumed were glucose and fructose (Sørensen et al., 2022). Thus, Y. lipolytica could be considered maltose-negative (Flores et al., 2000; Coelho et al., 2010; Spagnuolo et al., 2018). Under high aeration conditions, 75%–80% of the total amino acids were consumed, and the interesting aroma compound 2-phenylethanol was produced (Sørensen et al., 2022).
One potential challenge when using Y. lipolytica as a starter is its dimorphic nature. Y. lipolytica can morph into a filamentous hyphae form, which is highly undesirable in liquid fermentations due to upscaling problems derived from increased viscosity (Magdouli et al., 2018; Timoumi et al., 2018). A long list of external factors (e.g., nutrients, pH, and temperature) act as stressors and can induce the hyphae growth form (Timoumi et al., 2018). This issue could putatively be solved by immobilising the cells, which have been applied to Y. lipolytica and other yeasts before with good results (Fang and Zhang, 2008; Nedovic et al., 2009), by thorough selection work, searching for better production strains, or by applying omics tools to increase the understanding of the phenomenon.
In the following, we will review the potential for Y. lipolytica as a starter for producing functional fermented beverages and beverages with improved organoleptic qualities. It will be reviewed in fruit, grain, honey and dairy-based contexts.
Continuous demand from consumers for “natural” and “healthy” food and beverages, preferably with official health claims, makes the use of Y. lipolytica as a starter interesting. First it is Crabtree negative (Dashko et al., 2014), thus not produce alcohol, meaning it will not impose regulatory issues, secondly it can synthesise several compounds already used as additives in food and beverages to obtain organoleptic pleasing and stable products. Using Y. lipolytica might make it possible to make a “natural” “clean-label” product without additives but with the same qualities.
Y. lipolytica is a producer of 2-phenylethanol, an important aroma compound in the food and cosmetic industries. Its aroma is described as fresh and rose-like (Hua and Xu, 2011; Celińska et al., 2013; Celińska et al., 2015). Celińska et al. (2015) showed that Y. lipolytica synthesises 2-phenylethanol in a medium with glucose as a carbon source, and Sørensen et al. (2022) have shown that it can also be produced in a brewers’ wort where both glucose and fructose were consumed (Celińska et al., 2015; Sørensen et al., 2022). This opens up the use of Y. lipolytica as an in situ producer of 2-phenylethanol as part of the fermentation of glucose-containing substrates, e.g., brewers’ wort, glucose wort and various fruit juices (see Figure 1).
Furthermore, Y. lipolytica can synthesise lactones from fatty acids used as a fruity aroma compound in multiple industries. γ-decalactone is the most interesting of these and the one with the most focus in the literature. It is described as pleasantly oily-peach-like (Braga and Belo, 2016; Małajowicz et al., 2020). It has been found that the main component of castor oil, ricinoleic acid, a C18-hydroxylated fatty acid, is one of the most promising substrates for optimised production of γ-decalactone (Braga and Belo, 2013; Braga and Belo, 2015; Braga and Belo, 2016; Małajowicz et al., 2020; Kothari et al., 2022). More than 400 fatty acids are found in milk, with the diversity originating from which animal it is derived from and what feed is given (Barłowska and Litwińczuk, 2009; Markiewicz-Kęszycka et al., 2013; Djordjevic et al., 2019). With this diversity, it is likely possible to obtain milk with a suitable fatty acid content which can support the synthesis of γ-decalactone by Y. lipolytica when used as a starter in milk (see Figure 1).
Both abovementioned aroma compounds are interesting for beverage applications and could be of interest if produced naturally during fermentation. This, however, needs further research with optimisation, up-scaling, and product consistency in mind.
Citrate (CA), iso-citrate (ICA), succinate and α-ketoglutarate are all intermediates of the tricarboxylic acid cycle (Stern, 1957; Vickery, 1962; Aurich et al., 2012). CA and ICA have similar physico-chemical properties (Kamzolova and Morgunov, 2019). Y. lipolytica is well known for overproduction of both when under nitrogen limitation (Antonucci et al., 2001; Cavallo et al., 2017; Kamzolova and Morgunov, 2017; Madzak, 2021). Synthesis of CA and ICA by Y. lipolytica has been seen on substrates with various carbon sources, such as glucose, glycerol, ethanol, lactose, n-alkenes, lipids and fatty acids (Beopoulos et al., 2009; Kamzolova et al., 2013; Arslan et al., 2016; Kamzolova and Morgunov, 2017; Kamzolova and Morgunov, 2019; Bankar et al., 2020; Madzak, 2021; Jach and Malm, 2022; Park and Ledesma-Amaro, 2023). Depending on the strain of Y. lipolytica and medium, the proportions of CA to ICA vary significantly. More CA than ICA is produced when grown on glucose and glycerol (Förster et al., 2007; Moeller et al., 2007; Rywińska et al., 2010; Morgunov et al., 2013; Otto et al., 2013; Kamzolova et al., 2015; Kamzolova and Morgunov, 2017), where identical amounts or more ICA than CA is produced when grown in a medium with plant lipids as C-source (Aurich et al., 2012; Kamzolova and Morgunov, 2017; Kamzolova et al., 2020).
CA is a common additive used in both food and beverages. It contributes to a pleasant tartness, helps lower pH, and can work as a flavour enhancer (Merritt and Bouchard, 1979; Soccol et al., 2006; Kirimura et al., 2011; Quitmann et al., 2014; Cavallo et al., 2017; Fickers et al., 2020). From a functional viewpoint, CA and its derivates can act as synergists, thus aiding primary antioxidant stability (Pokorny et al., 2001; Quitmann et al., 2014), inhibiting the growth of bacteria directly and by chelating essential divalent cations (Hirshfield et al., 2003; Shi et al., 2022), protect against oxidative deterioration in flavour and colour in food and beverages (Cavallo et al., 2017; Kamzolova et al., 2020). Lastly, CA can act as a chelating agent in food and beverages enhancing product stability by forming stable water-soluble complexes with free metal ions in the product, thus avoiding reactions between metal ions and other compounds that can cause precipitation, loss of nutritional qualities and off-flavours to form (Nauta, 1991). Applying Y. lipolytica as a starter for an in situ production of CA seems highly relevant in multiple fermented beverages. For instance, in the production of a novel type of sour beer, where lactic acid is typically the primary souring agent (Domizio et al., 2016; Osburn et al., 2018; Ciosek et al., 2020), the amount of CA likely produced could yield a balanced and palatable liquid. Additionally, the production of 2-phenylethanol could be envisioned, and the resulting fermented beverage would have a pleasant tartness with a rose-like aroma (see Figure 1).
ICA has, for many years, primarily been used as a biochemical reagent for the analysis of enzymes (Finogenova et al., 2005; Kamzolova and Morgunov, 2019). To the best of our knowledge, the organoleptic qualities of ICA have never been reported. It has, within the last 2 decades, gained interest as an agent for treatments of anaemia caused by iron deficiency (Finogenova et al., 2005), a powerful antioxidant in cells of infusoria under oxygen-infused stress (Kamzolova et al., 2018), aid in relieving neuro toxication induced by lead and molybdenum salts (Morgunov et al., 2019), and lastly, it can be used to help resorption of blood clots (Finogenova et al., 2005). It is likely that if Y. lipolytica is used as a starter for producing a fermented beverage, then a considerable amount of ICA could be produced (see Figure 1). The fermentation with Y. lipolytica could ultimately result in a functional beverage due to all the functional characteristics of ICA.
Other organic acids of interest synthesised by Y. lipolytica in beverage-like substrates include succinate, α-ketoglutarate and kynurenic acid (see Figure 1) (Chernyavskaya et al., 2000; Kamzolova et al., 2009; Otto et al., 2013; Wróbel-Kwiatkowska et al., 2020a; Wróbel-Kwiatkowska et al., 2020b). Succinate also presents itself with multiple uses. It is used in the food industry as a flavour enhancer, acidifier and antimicrobial agent (Otto et al., 2013; Quitmann et al., 2014). It is also used in medicine for multiple purposes, e.g., as an anti-hypoxic, antistress and immunoactive compound (Kamzolova et al., 2009). The health benefits of α-ketoglutarate have been extensively reviewed, including antioxidative qualities and potential benefits on wound healing and muscle growth (Gyanwali et al., 2022 and references herein). Furthermore, it has been shown to extend the lifespan of worms, insects and mammals as well as delay the decline in fertility as an effect of age in humans (Wu et al., 2016; Su et al., 2019; Asadi Shahmirzadi et al., 2020; Rhoads and Anderson, 2020; Zhang et al., 2021). Kynurenic acid is also regarded as an interesting compound for its health benefits. It exhibits antioxidative, anti-inflammatory, and anticonvulsant properties. It has furthermore been shown to inhibit colon cancer and has abilities to protect the human brain (Han et al., 2010; Walczak et al., 2014; Walczak et al., 2020). Wróbel-Kwiatkowska et al. have shown that a strain of Y. lipolytica could synthesise kynurenic acid in submerged fermentation in a medium containing fructose and tryptophan or honey (Wróbel-Kwiatkowska et al., 2020a; Wróbel-Kwiatkowska et al., 2020b). The possibility of using beverages to administer kynurenic acid to humans has been shown (Turska et al., 2018). This makes the use of Y. lipolytica for producing a functional fermented beverage containing kynurenic acid highly relevant. Such a beverage could be based on fruit juices containing fructose, liquids containing honey, or a combination. Further research into the production of this type of fermented beverage is necessary.
Wild type strains of Y. lipolytica has been shown to produce polyols like mannitol, arabitol and erythritol in medias contining glucose (see Figure 1) (Ghezelbash et al., 2012; Workman et al., 2013; Papanikolaou et al., 2017). Polyols are interestin in a beverage setting and are commonly used as an ingredient in food for their ability to act as flavour enhancers, low calorie sweeteners, as well as being suitable for consumption by diabetics (Grembecka, 2015; Rzechonek et al., 2018; Jach and Malm, 2022).
The ability of Y. lipolyticas to consume most of the available amino acids in both cheese like media and brewers’ wort (Mansour et al., 2008; Sørensen et al., 2022) makes it highly interesting when looking at the potential shelf life of a fermented beverage made with it. In, e.g., a beer context, low amino acid concentration prolongs flavour stability simultaneously with a decrease in potential bioactivity (Jones and Pierce, 1964; Ferreira and Guido, 2018). This, combined with the previously mentioned abilities of several of the organic acids to acidify and lower pH and the antimicrobial, chelating and antioxidative properties of CA and ICA, will most likely yield extremely stable fermented beverages, both in a chemical and microbiological context.
Y, lipolytica possesses many traits that could prove highly valuable for producing novel fermented beverages. It is “safe-to-use” in both a food and biotech context. It can synthesise and excrete multiple compounds of interest, e.g., the aroma compounds 2-phenylethanol and γ-decalactone, a selection of interesting organic acids that encompasses properties for either enhanced flavour, functional effect in humans, acts as a preservative or a combination of these. Y. lipolytica has furthermore been suggested to have probiotic properties. It can assimilate large amounts of amino acids, which, in combination with metabolites with preserving qualities, can result in a very shelf-stable product. Along with its lack of ethanol production in both aerobic and anaerobic fermentations, these traits make further experimentation with Y. lipolytica in an alcohol free fermented beverage setting highly relevant.
Conceptualisation, ABS, JH, and NA, methodology, ABS, JH, and NA, software, ABS investigation, ABS, resources, ABS and NA, data curation, ABS, writing—original draft preparation, ABS, writing—review and editing, ABS, JH, and NA, visualisation, ABS, supervision, ABS, JH, and NA, project administration, ABS funding acquisition, ABS and NA. All authors contributed to the article and approved the submitted version.
This research was funded by Innovation Fund Denmark, grant number 7038-00197B.
The authors would like to thank Lene Kierkegaard for reading the manuscript and supplying highly relevant comments.
ABS and JH were employed by Carlsberg A/S, Carlsberg Research Laboratory, Brewing Science and Technology.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Addis, E., Fleet, G. H., Cox, J. M., Kolak, D., and Leung, T. (2001). The growth, properties and interactions of yeasts and bacteria associated with the maturation of Camembert and blue-veined cheeses. Int. J. Food Microbiol. 69, 25–36. doi:10.1016/S0168-1605(01)00569-4
Agarbati, A., Ciani, M., Canonico, L., Galli, E., and Comitini, F. (2021a). Exploitation of yeasts with probiotic traits for kefir production: Effectiveness of the microbial consortium. Fermentation 8, 9. doi:10.3390/fermentation8010009
Agarbati, A., Marini, E., Galli, E., Canonico, L., Ciani, M., and Comitini, F. (2021b). Characterization of wild yeasts isolated from artisan dairies in the Marche region, Italy, for selection of promising functional starters. LWT - Food Sci. Technol. 139, 110531. doi:10.1016/j.lwt.2020.110531
Anastassiadis, S., Aivasidis, A., and Wandrey, C. (2002). Citric acid production by Candida strains under intracellular nitrogen limitation. Appl. Biochem. Microbiol. 60, 81–87. doi:10.1007/s00253-002-1098-1
Anastassiadis, S., Kamzolova, S., and Rehm, H. (2007). Comparative study of the effect of iron on citrate-producing yeast growing on different substrates. Avgi/Sohos 57002.
Antonucci, S., Bravi, M., Bubbico, R., Di Michele, A., and Verdone, N. (2001). Selectivity in citric acid production by Yarrowia lipolytica. Enzyme Microb. Technol. 28, 189–195. doi:10.1016/s0141-0229(00)00288-x
Arslan, N. P., Aydogan, M. N., and Taskin, M. (2016). Citric acid production from partly deproteinized whey under non-sterile culture conditions using immobilized cells of lactose—Positive and cold-adapted Yarrowia lipolytica B9. J. Biotechnol. 231, 32–39. doi:10.1016/j.jbiotec.2016.05.033
Asadi Shahmirzadi, A., Edgar, D., Liao, C.-Y., Hsu, Y.-M., Lucanic, M., Asadi Shahmirzadi, A., et al. (2020). Alpha-ketoglutarate, an endogenous metabolite, extends lifespan and compresses morbidity in aging mice. Cell Metab. 32, 447–456. doi:10.1016/j.cmet.2020.08.004
Aurich, A., Specht, R., Müller, R. A., Stottmeister, U., Yovkova, V., Otto, C., et al. (2012). Microbiologically produced carboxylic acids used as building blocks in organic synthesis. Subcell. Biochem. 64, 391–423. doi:10.1007/978-94-007-5055-5_19
Bankar, A. V., Kumar, A. R., and Zinjarde, S. S. (2009). Environmental and industrial applications of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 84, 847–865. doi:10.1007/s00253-009-2156-8
Bankar, A., Jadhav, L., and Phalke, V. (2020). “Metagenomic insights of Yarrowia lipolytica in food industry,” in Metagenomic systems biology, 159–183. doi:10.1007/978-981-15-8562-3_8
Barłowska, J., and Litwińczuk, Z. (2009). Nutritional and pro-health properties of milk fat. Med. Weter. 65, 171–174.
Barth, G., and Gaillardin, C. (1997). Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiol. Rev. 19, 219–237. doi:10.1111/j.1574-6976.1997.tb00299.x
Beopoulos, A., Chardot, T., and Nicaud, J.-M. (2009). Yarrowia lipolytica: A model and a tool to understand the mechanisms implicated in lipid accumulation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 91, 692–696. doi:10.1016/j.biochi.2009.02.004
Beopoulos, A., Desfougeres, T., Sabirova, J., Zinjarde, S., Neuvéglise, C., and Nicaud, J. M. (2010). “The hydrocarbon-degrading oleaginous yeast Yarrowia lipolytica,” in Handbook of hydrocarbon and lipid microbiology, 2111–2121. doi:10.1007/978-3-540-77587-4_152
Braga, A., and Belo, I. (2013). Immobilization of Yarrowia lipolytica for aroma production from Castor oil. Appl. Biochem. Biotechnol. 169, 2202–2211. doi:10.1007/s12010-013-0131-4
Braga, A., and Belo, I. (2015). Production of γ-decalactone by Yarrowia lipolytica: Insights into experimental conditions and operating mode optimization. J. Chem. Technol. Biotechnol. 90, 559–565. doi:10.1002/jctb.4349
Braga, A., and Belo, I. (2016). Biotechnological production of γ-decalactone, a peach like aroma, by Yarrowia lipolytica. World J. Microbiol. Biotechnol. 32, 169. doi:10.1007/s11274-016-2116-2
Büyükta, D. (2013). “Effect of Yarrowia lipolytica on the aroma characteristics of Turkish fermented sausages (Sucuk),” in 59th International Congress of Meat Science and Technology, Izmir, Turkey, 18-23rd August 2013.
Carsanba, E., Papanikolaou, S., Fickers, P., and Erten, H. (2020). Lipids by Yarrowia lipolytica strains cultivated on glucose in batch cultures. Microorganisms 8, 1054. doi:10.3390/microorganisms8071054
Cavallo, E., Charreau, H., Cerrutti, P., and Foresti, M. L. (2017). Yarrowia lipolytica: A model yeast for citric acid production. FEMS Yeast Res. 17, fox084. doi:10.1093/femsyr/fox084
Celińska, E., Kubiak, P., Białas, W., Dziadas, M., and Grajek, W. (2013). Yarrowia lipolytica: The novel and promising 2-phenylethanol producer. J. Ind. Microbiol. Biotechnol. 40, 389–392. doi:10.1007/s10295-013-1240-3
Celińska, E., Olkowicz, M., and Grajek, W. (2015). L-Phenylalanine catabolism and 2-phenylethanol synthesis in Yarrowia lipolytica--mapping molecular identities through whole-proteome quantitative mass spectrometry analysis. FEMS Yeast Res. 15, fov041. doi:10.1093/femsyr/fov041
Centeno, J. A., Garabal, J. I., Docampo, F., Lorenzo, J. M., and Carballo, J. (2017). Recovering traditional raw-milk Tetilla cheese flavour and sensory attributes by using Kocuria varians and Yarrowia lipolytica adjunct cultures. Int. J. Food Microbiol. 251, 33–40. doi:10.1016/j.ijfoodmicro.2017.03.014
Chernyavskaya, O. G., Shishkanova, N. V., Il’chenko, A. P., and Finogenova, T. V. (2000). Synthesis of alpha-ketoglutaric acid by Yarrowia lipolytica yeast grown on ethanol. Appl. Microbiol. Biotechnol. 53, 152–158. doi:10.1007/s002530050002
Ciosek, A., Rusiecka, I., and Poreda, A. (2020). Sour beer production: Impact of pitching sequence of yeast and lactic acid bacteria. J. Inst. Brew. 126, 53–58. doi:10.1002/jib.590
Coelho, M., Amaral, P., and Belo, I. (2010). “Yarrowia lipolytica: An industrial workhorse,” in Current research, technology and education topics in applied microbiology and microbial biotechnology, 930–944.
Curtis, K., Mccluskey, J., and Wahl, T. (2004). Consumer acceptance of genetically modified food products in the developing world. AgBioForum 7, 69–74.
Darvishi Harzevili, F. (2014). Biotechnological applications of the yeast Yarrowia lipolyticaCham: Springer International Publishing, 17–74. doi:10.1007/978-3-319-06437-6
Dashko, S., Zhou, N., Compagno, C., and Piškur, J. (2014). Why, when, and how did yeast evolve alcoholic fermentation? FEMS Yeast Res. 14, 826–832. doi:10.1111/1567-1364.12161
Deák, T., and Beuchat, L. R. (1987). Identification of foodborne yeasts. J. Food Prot. 50, 243–264. doi:10.4315/0362-028X-50.3.243
Desnos-Ollivier, M., Letscher-Bru, V., Neuvéglise, C., and Dromer, F. (2020). Yarrowia lipolytica causes sporadic cases and local outbreaks of infections and colonisation. Mycoses 63, 737–745. doi:10.1111/myc.13095
Djordjevic, J., Ledina, T., Baltic, M. Z., Trbovic, D., Babic, M., and Bulajic, S. (2019). Fatty acid profile of milk. IOP Conf. Ser. Earth Environ. Sci. 333, 012057. doi:10.1088/1755-1315/333/1/012057
Domizio, P., House, J., Joseph, C. M., Bisson, L., and Bamforth, C. (2016). Lachancea thermotolerans as an alternative yeast for the production of beer: Lachancea thermotolerans in beer fermentation. J. Inst. Brew. 122, 599–604. doi:10.1002/jib.362
Fang, L., and Zhang, W. G. (2008). γ-Decalactone production by immobilized Yarrowia lipolyticacells. Biotechnol 5.
Ferreira, I. M., and Guido, L. F. (2018). Impact of wort amino acids on beer flavour: A review. Fermentation 4, 23. doi:10.3390/fermentation4020023
Ferreira, A. D., and Viljoen, B. C. (2003). Yeasts as adjunct starters in matured Cheddar cheese. Int. J. Food Microbiol. 86, 131–140. doi:10.1016/S0168-1605(03)00252-6
Fickers, P., Benetti, P., Wache, Y., Marty, A., Mauersberger, S., Smit, M., et al. (2005). Hydrophobic substrate utilisation by the yeast Yarrowia lipolytica, and its potential applications. FEMS Yeast Res. 5, 527–543. doi:10.1016/j.femsyr.2004.09.004
Fickers, P., Cheng, H., and Sze Ki Lin, C. (2020). Sugar alcohols and organic acids synthesis in Yarrowia lipolytica: Where are we? Microorganisms 8, 574. doi:10.3390/microorganisms8040574
Finogenova, T., Kamzolova, S., Dedyukhina, E., Shishkanova, N., Il’chenko, A., Morgunov, I., et al. (2002). Biosynthesis of citric and isocitric acids from ethanol by mutant Yarrowia lipolytica N 1 under continuous cultivation. Appl. Microbiol. Biotechnol. 59, 493–500. doi:10.1007/s00253-002-1022-8
Finogenova, T. V., Morgunov, I. G., Kamzolova, S. V., and Chernyavskaya, O. G. (2005). Organic acid production by the yeast Yarrowia lipolytica: A review of prospects. Appl. Biochem. Microbiol. 41, 418–425. doi:10.1007/s10438-005-0076-7
Fleet, G. H., and Balia, R. (2006). “The public health and probiotic significance of yeasts in foods and beverages,” in Yeasts in food and beverages (Berlin, Heidelberg, New York: Springer-Verlag), 381–397.
Flores, C. L., Rodríguez, C., Petit, T., and Gancedo, C. (2000). Carbohydrate and energy-yielding metabolism in non-conventional yeasts. FEMS Microbiol. Rev. 24, 507–529. doi:10.1111/j.1574-6976.2000.tb00553.x
Förster, A., Jacobs, K., Juretzek, T., Mauersberger, S., and Barth, G. (2007). Overexpression of the ICL1 gene changes the product ratio of citric acid production by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 77, 861–869. doi:10.1007/s00253-007-1205-4
Freitas, A. C., Pintado, A. E., Pintado, M. E., and Malcata, F. X. (1999). Role of dominant microflora of Picante cheese on proteolysis and lipolysis. Int. Dairy J. 9, 593–603. doi:10.1016/S0958-6946(99)00129-6
Fukuda, R. (2013). Metabolism of hydrophobic carbon sources and regulation of it in n-alkane-assimilating yeast Yarrowia lipolytica. Biosci. Biotechnol. Biochem. 77, 1149–1154. doi:10.1271/bbb.130164
Gálvez-López, D., Chávez-Meléndez, B., Vázquez-Ovando, A., and Rosas-Quijano, R. (2019). The metabolism and genetic regulation of lipids in the oleaginous yeast Yarrowia lipolytica. Braz J. Microbiol. 50, 23–31. doi:10.1007/s42770-018-0004-7
Gardini, F., Suzzi, G., Lombardi, A., Galgano, F., Crudele, M. A., Andrighetto, C., et al. (2001). A survey of yeasts in traditional sausages of southern Italy. FEMS Yeast Res. 1, 161–167. doi:10.1111/j.1567-1364.2001.tb00027.x
Ghezelbash, G. R., Nahvi, I., and Rabbani, M. (2012). Study of polyols production by Yarrowia lipolytica in batch culture and optimization of growth conditions for maximum production. Jundishapur J. Microbiol. 5, 546–549. doi:10.5812/jjm.3524
Gori, K., Ryssel, M., Arneborg, N., and Jespersen, L. (2013). Isolation and identification of the microbiota of Danish farmhouse and industrially produced surface-ripened cheeses. Microb. Ecol. 65, 602–615. doi:10.1007/s00248-012-0138-3
Groenewald, M., Boekhout, T., Neuvéglise, C., Gaillardin, C., Van Dijck, P. W. M., and Wyss, M. (2014). Yarrowia lipolytica: Safety assessment of an oleaginous yeast with a great industrial potential. Crit. Rev. Microbiol. 40, 187–206. doi:10.3109/1040841x.2013.770386
Gutiérrez, A., Boekhout, T., Gojkovic, Z., and Katz, M. (2018). Evaluation of non-Saccharomyces yeasts in the fermentation of wine, beer and cider for the development of new beverages. J. Inst. Brew. 124, 389–402. doi:10.1002/jib.512
Gyanwali, B., Lim, Z. X., Soh, J., Lim, C., Guan, S. P., Goh, J., et al. (2022). Alpha-Ketoglutarate dietary supplementation to improve health in humans. Trends Endocrinol. Metab. 33, 136–146. doi:10.1016/j.tem.2021.11.003
Han, Q., Cai, T., Tagle, D. A., and Li, J. (2010). Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol. Life Sci. 67, 353–368. doi:10.1007/s00018-009-0166-4
Hirshfield, I. N., Terzulli, S., and O’Byrne, C. (2003). Weak organic acids: A panoply of effects on bacteria. Sci. Prog. 86, 245–269. doi:10.3184/003685003783238626
Holzschu, D. L., Chandler, F. W., Ajello, L., and Ahearn, D. G. (1979). Evaluation of industrial yeasts for pathogenicity. Sabouraudia 17, 71–78. doi:10.1080/00362177985380091
Hua, D., and Xu, P. (2011). Recent advances in biotechnological production of 2-phenylethanol. Biotechnol. Adv. 29, 654–660. doi:10.1016/j.biotechadv.2011.05.001
Iucci, L., Patrignani, F., Belletti, N., Ndagijimana, M., Elisabetta Guerzoni, M., Gardini, F., et al. (2007). Role of surface-inoculated Debaryomyces hansenii and Yarrowia lipolytica strains in dried fermented sausage manufacture. Part 2: Evaluation of their effects on sensory quality and biogenic amine content. Meat Sci. 75, 669–675. doi:10.1016/j.meatsci.2006.09.016
Jach, M. E., and Malm, A. (2022). Yarrowia lipolytica as an alternative and valuable source of nutritional and bioactive compounds for humans. Molecules 27, 2300. doi:10.3390/molecules27072300
Jones, M., and Pierce, J. S. (1964). Absorbtion of amino acids from wort by yeasts. J. Inst. Brew. 70, 307–315. doi:10.1002/j.2050-0416.1964.tb01996.x
Kalamaki, M. S., and Angelidis, A. S. (2017). Isolation and molecular identification of yeasts in Greek kefir. Int. J. Dairy Technol. 70, 261–268. doi:10.1111/1471-0307.12329
Kamzolova, S. V., and Morgunov, I. G. (2017). Metabolic peculiarities of the citric acid overproduction from glucose in yeasts Yarrowia lipolytica. Bioresour. Technol. 243, 433–440. doi:10.1016/j.biortech.2017.06.146
Kamzolova, S. V., and Morgunov, I. G. (2019). Microbial production of (2R,3S)-isocitric acid: State of the arts and prospects. Appl. Microbiol. Biotechnol. 103, 9321–9333. doi:10.1007/s00253-019-10207-4
Kamzolova, S. V., and Morgunov, I. G. (2021). Effect of metabolic regulators and aeration on isocitric acid synthesis by Yarrowia lipolytica grown on ester-aldehyde fraction. Fermentation 7, 283. doi:10.3390/fermentation7040283
Kamzolova, S., Vinokurova, N., Shemshura, O., Bekmakhanova, N., Lunina, J., Samoylenko, V., et al. (2009). The production of succinic acid by yeast Yarrowia lipolytica through a two-step process. Appl. Microbiol. Biotechnol. 98, 7959–7969. doi:10.1007/s00253-014-5887-0
Kamzolova, S. V., Dedyukhina, E. G., Samoilenko, V. A., Lunina, J. N., Puntus, I. F., Allayarov, R. L., et al. (2013). Isocitric acid production from rapeseed oil by Yarrowia lipolytica yeast. Appl. Microbiol. Biotechnol. 97, 9133–9144. doi:10.1007/s00253-013-5182-5
Kamzolova, S. V., Vinokurova, N. G., Lunina, J. N., Zelenkova, N. F., and Morgunov, I. G. (2015). Production of technical-grade sodium citrate from glycerol-containing biodiesel waste by Yarrowia lipolytica. Bioresour. Technol. 193, 250–255. doi:10.1016/j.biortech.2015.06.092
Kamzolova, S. V., Shamin, R. V., Stepanova, N. N., Morgunov, G. I., Lunina, J. N., Allayarov, R. K., et al. (2018). Fermentation conditions and media optimization for isocitric acid production from ethanol by Yarrowia lipolytica. Biomed. Res. Int. 2018, e2543210. doi:10.1155/2018/2543210
Kamzolova, S., Samoylenko, V., Lunina, J., and Morgunov, I. (2020). Effects of medium components on isocitric acid production by Yarrowia lipolytica yeast. Fermentation 6, 112. doi:10.3390/fermentation6040112
Kirimura, K., Honda, Y., and Hattori, T. (2011). “Citric acid,” in Industrial biotechnology and commodity products (Elsevier Inc.), 135–142. doi:10.1016/B978-0-08-088504-9.00169-0
Kothari, S., Vadgama, R., Bhat, K., Lali, A., and Anil, A. (2022). Process optimization for production and purification of γ-decalactone from ricinoleic acid using Yarrowia lipolytica NCIM 3590. Biocatal. Agric. Biotechnol. 39, 102285. doi:10.1016/j.bcab.2022.102285
Kumura, H., Tanoue, Y., Tsukahara, M., Tanaka, T., and Shimazaki, K. (2004). Screening of dairy yeast strains for probiotic applications. J. Dairy Sci. 87, 4050–4056. doi:10.3168/jds.S0022-0302(04)73546-8
Lanciotti, R., Vannini, L., Lopez, C. C., Gobbetti, M., and Guerzoni, M. E. (2005). Evaluation of the ability of Yarrowia lipolytica to impart strain-dependent characteristics to cheese when used as a ripening adjunct. Int. J. Dairy Technol. 58, 89–99. doi:10.1111/j.1471-0307.2005.00197.x
Larpin, S., Mondoloni, C., Goerges, S., Vernoux, J.-P., Guéguen, M., and Desmasures, N. (2006). Geotrichum candidum dominates in yeast population dynamics in Livarot, a French red-smear cheese. FEMS Yeast Res. 6, 1243–1253. doi:10.1111/j.1567-1364.2006.00127.x
Lee, L. W., Tay, G. Y., Cheong, M. W., Curran, P., Yu, B., and Liu, S. Q. (2017). Modulation of the volatile and non-volatile profiles of coffee fermented with Yarrowia lipolytica: I. Green coffee. LWT 77, 225–232. doi:10.1016/j.lwt.2016.11.047
Lelieveld, H. L. M., Boon, B., Bennett, A., Brunius, G., Cantley, M., Chmiel, A., et al. (1996). Safe biotechnology. 7. Classification of microorganisms on the basis of hazard. Working party "safety in biotechnology" of the European federation biotechnology. Appl. Microbiol. Biotechnol. 45, 723–729. doi:10.1007/s002530050754
Lourens-Hattingh, A., and Viljoen, B. C. (2002). Survival of dairy-associated yeasts in yoghurt and yoghurt-related products. Food Microbiol. 19, 597–604. doi:10.1006/fmic.2002.0515
Lu, M., Wang, X., Sun, G., Qin, B., Xiao, J., Yan, S., et al. (2014). Fine structure of Tibetan kefir grains and their yeast distribution, diversity, and shift. PLOS ONE 9, e101387. doi:10.1371/journal.pone.0101387
Madzak, C. (2018). Engineering Yarrowia lipolytica for use in biotechnological applications: A review of major achievements and recent innovations. Mol. Biotechnol. 60, 621–635. doi:10.1007/s12033-018-0093-4
Madzak, C. (2021). Yarrowia lipolytica strains and their biotechnological applications: How natural biodiversity and metabolic engineering could contribute to cell factories improvement. J. Fungi 7, 548. doi:10.3390/jof7070548
Magdouli, S., Brar, S. K., and Blais, J. F. (2018). Morphology and rheological behaviour of Yarrowia lipolytica: Impact of dissolved oxygen level on cell growth and lipid composition. Process Biochem. 65, 1–10. doi:10.1016/j.procbio.2017.10.021
Małajowicz, J., Nowak, D., Fabiszewska, A., and Iuliano, A. (2020). Comparison of gamma-decalactone biosynthesis by yeast Yarrowia lipolytica MTLY40-2p and W29 in batch-cultures. Biotechnol. Biotechnol. Equip. 34, 330–340. doi:10.1080/13102818.2020.1749528
Mansour, S., Beckerich, J. M., and Bonnarme, P. (2008). Lactate and amino acid catabolism in the cheese-ripening yeast Yarrowia lipolytica. Appl. Environ. Microbiol. 74, 6505–6512. doi:10.1128/aem.01519-08
Markiewicz-Kęszycka, M., Czyżak-Runowska, G., Lipińska, P., and Wójtowski, J. (2013). Fatty acid profile of milk - a review. Bull. Vet. Inst. Pulawy 57, 135–139. doi:10.2478/bvip-2013-0026
Martirosyan, D. M., and Leem, C. (2019). The bioactive compounds of probiotic foods/supplements and their application in managing mental disorders. Bioact. Compd. Health Dis. 2, 206–220. doi:10.31989/bchd.v2i10.431
Meijer, W. C., Brandsma, J. B., and Hafkamp, A. A. G. (2020). Cheese having sheep-like and/or goaty flavour attributes. Available at: https://patents.google.com/patent/US20200390120A1/en (Accessed January 27, 2023).
Moeller, L., Strehlitz, B., Aurich, A., Zehnsdorf, A., and Bley, T. (2007). Optimization of citric acid production from glucose by Yarrowia lipolytica. Eng. Life Sci. 7, 504–511. doi:10.1002/elsc.200620207
Morgunov, I. G., Kamzolova, S. V., and Lunina, J. N. (2013). The citric acid production from raw glycerol by Yarrowia lipolytica yeast and its regulation. Appl. Microbiol. Biotechnol. 97, 7387–7397. doi:10.1007/s00253-013-5054-z
Morgunov, I. G., Kamzolova, S. V., Karpukhina, O. V., Bokieva, S. B., and Inozemtsev, A. N. (2019). Biosynthesis of isocitric acid in repeated-batch culture and testing of its stress-protective activity. Appl. Microbiol. Biotechnol. 103, 3549–3558. doi:10.1007/s00253-019-09729-8
Nauta, T. (1991). “Chelating agents,” in Food additives user’s handbook (Oxford: Springer), 273–279.
Nedovic, V., Manojlovic, V., Leskosek-Cukalovic, I., Bugarski, B., and Willaert, R. (2009). “State of the art in immobilized/encapsulated cell technology in fermentation processes,” in Food engineering interfaces. Editors J. M. Aguilera, R. Simpson, J. Welti-chanes, D. Bermudez-aguirre, and G. Barbosa-canovas (Springer Science+Business Media), 119–146.
Osburn, K., Amaral, J., Metcalf, S. R., Nickens, D. M., Rogers, C. M., Sausen, C., et al. (2018). Primary souring: A novel bacteria-free method for sour beer production. Food Microbiol. 70, 76–84. doi:10.1016/j.fm.2017.09.007
Otto, C., Holz, M., and Barth, G. (2013). “Production of organic acids by Yarrowia lipolytica,” in Yarrowia lipolytica: Biotechnological applications microbiology monographs. Editor G. Barth (Berlin, Heidelberg: Springer), 137–149. doi:10.1007/978-3-642-38583-4_5
Papanikolaou, S., Chavlot, I., Komaitis, M., Marc, I., and Aggelis, G. (2002). Single cell oil production by Yarrowia lipolytica growing on an industrial derivative of animal fat in batch cultures. Appl. Microbiol. Biotechnol. 58, 308–312. doi:10.1007/s00253-001-0897-0
Papanikolaou, S., Kampisopoulou, E., Blanchard, F., Rondags, E., Gardeli, C., Koutinas, A. A., et al. (2017). Production of secondary metabolites through glycerol fermentation under carbon-excess conditions by the yeasts Yarrowia lipolytica and Rhodosporidium toruloides. Eur. J. Lipid Sci. Technol. 119, 1600507. doi:10.1002/ejlt.201600507
Park, Y.-K., and Ledesma-Amaro, R. (2023). What makes Yarrowia lipolytica well suited for industry? Trends Biotechnol. 41, 242–254. doi:10.1016/j.tibtech.2022.07.006
Patrignani, F., Iucci, L., Vallicelli, M., Guerzoni, M. E., Gardini, F., and Lanciotti, R. (2007). Role of surface-inoculated Debaryomyces hansenii and Yarrowia lipolytica strains in dried fermented sausage manufacture. Part 1: Evaluation of their effects on microbial evolution, lipolytic and proteolytic patterns. Meat Sci. 75, 676–686. doi:10.1016/j.meatsci.2006.09.017
Patrignani, F., Vannini, L., Gardini, F., Guerzoni, M. E., and Lanciotti, R. (2011a). Variability of the lipolytic activity and volatile molecules production by a strain of Yarrowia lipolytica in pork fat and its dependence on environmental conditions. Meat Sci. 89, 21–26. doi:10.1016/j.meatsci.2011.03.015
Patrignani, F., Vannini, L., Gardini, F., Guerzoni, M. E., and Lanciotti, R. (2011b). Variability of the lipolytic activity in Yarrowia lipolytica strains in pork fat. Meat Sci. 88, 689–693. doi:10.1016/j.meatsci.2011.02.030
Pokorny, J., Yanishlieva, N., and Gordon, M. (2001). Antioxidants in food. 1st ed. Florida: CRC Press.
Quitmann, H., Fan, R., and Czermak, P. (2014). Acidic organic compounds in beverage, food, and feed production. Adv. Biochem. Eng. Biotechnol. 143, 91–141. doi:10.1007/10_2013_262
Rai, A. K., Pandey, A., and Sahoo, D. (2019). Biotechnological potential of yeasts in functional food industry. Trends Food Sci. Technol. 83, 129–137. doi:10.1016/j.tifs.2018.11.016
Rhoads, T. W., and Anderson, R. M. (2020). Alpha-ketoglutarate, the metabolite that regulates aging in mice. Cell Metab. 32, 323–325. doi:10.1016/j.cmet.2020.08.009
Rohm, H., and Lehner, M. (1990). Characteristics properties of Austrian kefir. Ernährung 14, 571–574.
Rywińska, A., Rymowicz, W., Żarowska, B., and Skrzypiński, A. (2010). Comparison of citric acid production from glycerol and glucose by different strains of Yarrowia lipolytica. World J. Microbiol. Biotechnol. 26, 1217–1224. doi:10.1007/s11274-009-0291-0
Rzechonek, D. A., Dobrowolski, A., Rymowicz, W., and Mirończuk, A. M. (2018). Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 38, 620–633. doi:10.1080/07388551.2017.1380598
Sadeghi, A., Ebrahimi, M., Shahryari, S., Kharazmi, M. S., and Jafari, S. M. (2022). Food applications of probiotic yeasts; focusing on their techno-functional, postbiotic and protective capabilities. Trends Food Sci. Technol. 128, 278–295. doi:10.1016/j.tifs.2022.08.018
Sarris, D., Stoforos, N. G., Mallouchos, A., Kookos, I. K., Koutinas, A. A., Aggelis, G., et al. (2017). Production of added-value metabolites by Yarrowia lipolytica growing in olive mill wastewater-based media under aseptic and non-aseptic conditions. Eng. Life Sci. 17, 695–709. doi:10.1002/elsc.201600225
Shi, Y., Pu, D., Zhou, X., and Zhang, Y. (2022). Recent progress in the study of taste characteristics and the nutrition and health properties of organic acids in foods. Foods 11, 3408. doi:10.3390/foods11213408
Shukla, S. P., Plata, C., Reichelt, M., Steiger, S., Heckel, D. G., Kaltenpoth, M., et al. (2018). Microbiome-assisted carrion preservation aids larval development in a burying beetle. Proc. Natl. Acad. Sci. U. S. A. 115, 11274–11279. doi:10.1073/pnas.1812808115
Sibirny, A., Madzak, C., and Fickers, P. (2014). “Genetic engineering of nonconventional yeasts for the production of valuable compounds,” in Microbial biotechnology: Progress and trends (Boca Raton, FL, USA: CRC Press), 63–111. doi:10.1201/9781351228701-9
Soccol, C. R., Vendenberghe, L. P. S., Rodrigues, C., and Pandey, A. (2006). New perspectives for citric acid production and application. Food Technol. Biotechnol. 44, 141–149.
Sørensen, A. B., Petersen, M. A., Garde, A., and Arneborg, N. (2022). The consumption of amino acids and production of volatile aroma compounds by Yarrowia lipolytica in brewers’ wort. Fermentation 8, 579. doi:10.3390/fermentation8110579
Spagnuolo, M., Shabbir Hussain, M., Gambill, L., and Blenner, M. (2018). Alternative substrate metabolism in Yarrowia lipolytica. Front. Microbiol. 9, 1077. doi:10.3389/fmicb.2018.01077
Spitaels, F., Wieme, A. D., Janssens, M., Aerts, M., Daniel, H.-M., Van Landschoot, A., et al. (2014). The microbial diversity of traditional spontaneously fermented lambic beer. PLoS One 9, e95384. doi:10.1371/journal.pone.0095384
Spitaels, F., Wieme, A. D., Janssens, M., Aerts, M., Van Landschoot, A., De Vuyst, L., et al. (2015). The microbial diversity of an industrially produced lambic beer shares members of a traditionally produced one and reveals a core microbiota for lambic beer fermentation. Food Microbiol. 49, 23–32. doi:10.1016/j.fm.2015.01.008
Stern, J. R. (1957). “[69] Assay of tricarboxylic acids,” in Methods in enzymology (Academic Press), 425–431. doi:10.1016/S0076-6879(57)03409-6
Su, Y., Wang, T., Wu, N., Li, D., Fan, X., Xu, Z., et al. (2019). Alpha-ketoglutarate extends Drosophila lifespan by inhibiting mTOR and activating AMPK. Aging 11, 4183–4197. doi:10.18632/aging.102045
Sutherland, J. B., Cornelison, C., and Crow, S. A. (2014). “Yarrowia lipolytica (Candida lipolytica),” in Encyclopedia of food microbiology. Editors C. A. Batt, and M. L. Tortorello, 374–378.
Szoltysik, M., Dabrowska, A., Babij, K., Pokora, M., Zambrowicz, A., Polomska, X., et al. (2013). Biochemical and microbiological changes in cheese inoculated with Yarrowia lipolytica yeast. Żywność Nauka Technol. Jakość 20. doi:10.15193/zntj/2013/89/049-064
Timoumi, A., Guillouet, S. E., Molina-Jouve, C., Fillaudeau, L., and Gorret, N. (2018). Impacts of environmental conditions on product formation and morphology of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 102, 3831–3848. doi:10.1007/s00253-018-8870-3
Turska, M., Pelak, J., Turski, M. P., Kocki, T., Dukowski, P., Plech, T., et al. (2018). Fate and distribution of kynurenic acid administered as beverage. Pharmacol. Rep. 70, 1089–1096. doi:10.1016/j.pharep.2018.05.011
van den Tempel, T., and Jakobsen, M. (2000). The technological characteristics of Debaryomyces hansenii and Yarrowia lipolytica and their potential as starter cultures for production of Danablu. Int. Dairy J. 10, 263–270. doi:10.1016/S0958-6946(00)00053-4
Vickery, H. B. (1962). A suggested new nomenclature for the isomers of isocitric acid. J. Biol. Chem. 237, 1739–1741. doi:10.1016/s0021-9258(19)73928-3
Walczak, K., Turski, W. A., and Rajtar, G. (2014). Kynurenic acid inhibits colon cancer proliferation in vitro: Effects on signaling pathways. Amino Acids 46, 2393–2401. doi:10.1007/s00726-014-1790-3
Walczak, K., Wnorowski, A., Turski, W. A., and Plech, T. (2020). Kynurenic acid and cancer: Facts and controversies. Cell Mol. Life Sci. 77, 1531–1550. doi:10.1007/s00018-019-03332-w
Workman, M., Holt, P., and Thykaer, J. (2013). Comparing cellular performance of Yarrowia lipolytica during growth on glucose and glycerol in submerged cultivations. Amb. Express 3, 58. doi:10.1186/2191-0855-3-58
Wróbel-Kwiatkowska, M., Turski, W., Juszczyk, P., Kita, A., and Rymowicz, W. (2020a). Improved production of kynurenic acid by Yarrowia lipolytica in media containing different honeys. Sustainability 12, 9424. doi:10.3390/su12229424
Wróbel-Kwiatkowska, M., Turski, W., Kocki, T., Rakicka-Pustułka, M., and Rymowicz, W. (2020b). An efficient method for production of kynurenic acid by Yarrowia lipolytica. Yeast 37, 541–547. doi:10.1002/yea.3469
Wu, N., Yang, M., Gaur, U., Xu, H., Yao, Y., and Li, D. (2016). Alpha-Ketoglutarate: Physiological functions and applications. Biomol. Ther. 24, 1–8. doi:10.4062/biomolther.2015.078
Wyder, M.-T., Bachmann, H.-P., and Puhan, Z. (1999). Role of selected yeasts in cheese ripening: An evaluation in foil wrapped raclette cheese. LWT - Food Sci. Technol. 32, 333–343. doi:10.1006/fstl.1999.0555
Yarrow, D. (1972). Four new combinations in yeasts. Antonie Leeuwenhoek 38, 357–360. doi:10.1007/bf02328105
Żarowska, B., Wojtatowicz, M., Polomska, X., Juszczyk, P., and Chrzanowska, J. (2004). Factors affecting killer activity of some yeast species occurring in Rokpol cheese. Folia Microbiol. 49, 713–717. doi:10.1007/BF02931554
Zhang, W., Zhao, F., Zhao, F., Yang, T., and Liu, S. (2019). Solid-state fermentation of palm kernels by Yarrowia lipolytica modulates the aroma of palm kernel oil. Sci. Rep. 9, 2538. doi:10.1038/s41598-019-39252-9
Zhang, Z., He, C., Gao, Y., Zhang, L., Song, Y., Zhu, T., et al. (2021). α-ketoglutarate delays age-related fertility decline in mammals. Aging Cell 20, e13291. doi:10.1111/acel.13291
Zieniuk, B., and Fabiszewska, A. (2018). Yarrowia lipolytica: A beneficious yeast in biotechnology as a rare opportunistic fungal pathogen: A minireview. World J. Microbiol. Biotechnol. 35, 10. doi:10.1007/s11274-018-2583-8
Zinjarde, S. S., and Pant, A. (2000). Crude oil degradation by free and immobilized cells of Yarrowia lipolytica NCIM 3589. J. Environ. Sci. Health A 35, 755–763. doi:10.1080/10934520009377000
Keywords: Yarrowia lipolytica, fermented beverages, beer, non-conventional yeast, functional beverages, GRAS, novel fermented beverages
Citation: Sørensen AB, Harholt J and Arneborg N (2023) Application of Yarrowia lipolytica in fermented beverages. Front. Food. Sci. Technol. 3:1190063. doi: 10.3389/frfst.2023.1190063
Received: 20 March 2023; Accepted: 24 April 2023;
Published: 09 May 2023.
Edited by:
Thaddeus Chukwuemeka Ezeji, The Ohio State University, United StatesReviewed by:
Afroditi Chatzifragkou, University of Reading, United KingdomCopyright © 2023 Sørensen, Harholt and Arneborg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anders Bagger Sørensen, YW5kZXJzLmJhZ2dlci5zb3JlbnNlbkBjYXJsc2JlcmcuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.