- 1Department of Plant Sciences, North Dakota State University, Fargo, ND, United States
- 2Department of Plant Pathology, North Dakota State University, Fargo, ND, United States
- 3Department of Agricultural and Biosystems Engineering, North Dakota State University, Fargo, ND, United States
Plant-based bioenergy by-products such as corn distillers’ dried grains with solubles (DDGS) are widely utilized as animal feed sources and feed ingredients due to their balanced nutritional profile and animal health protective functional qualities. Bioprocessing of this bioenergy by-product using beneficial lactic acid bacteria (LAB)-based fermentation strategy to improve animal-health targeted functional qualities has wider relevance for animal feed applications. In this study, liquid extracts of corn DDGS were fermented with Lactiplantibacillus plantarum and Lactobacillus helveticus. The unfermented and fermented extracts were then analyzed (at 0, 24, 48 and 72-h) for their total soluble phenolic content (TSP), phenolic profile, antioxidant activity via ABTS and DPPH radical scavenging activity, and antimicrobial activity against the gut pathogen Helicobacter pylori using in vitro assay models. Statistical differences in antioxidant activity and phenolic content were observed among the unfermented and fermented extracts. The major phenolic compounds detected in corn DDGS were gallic, dihydroxybenzoic, p-coumaric, caffeic and ferulic acid, and catechin. Antimicrobial activity against H. pylori was observed for the unfermented extracts and the antimicrobial activity was attributed to the growth of a corn DDGS-endemic culture. The culture was isolated, sequenced, and identified as Bacillus amyloliquefaciens. Results of this study indicated that processing strategies of by-products such as LAB- based fermentation of corn DDGS could affect its bioactive-linked functional qualities due to microbial interaction with the phytochemicals. Furthermore, plant by-products can serve as novel sources of beneficial microflora that have relevance in wider agriculture, food safety, and therapeutic applications.
1 Introduction
Distillers dried grains with solubles (DDGS) is a corn bioenergy by-product from the dry grinding process during ethanol production. In 2021, over 15 billion gallons of ethanol were produced from corn in the United States with 44 million metric tons of DDGS being generated in the process (U.S. Grains Council, 2022). Corn DDGS is commonly used as a livestock feed in the dairy and beef cattle industry and used as an animal feed and ingredient source for poultry, swine, sheep, goat, and horses, as well as in aquaculture diets for fish and shrimp (U.S. Grains Council, 2018). The nutrient composition and digestibility of DDGS can vary among corn sources with ash, fiber, fat, lysine, tryptophan, and phosphorus content having high variation among the different DDGS sources (U.S. Grains Council, 2018). Feed rations supplemented with corn DDGS were found to alter the intestinal microbiota of the broiler chickens, and DDGS was negatively correlated with the genera Faecalibacterium and Streptococcus while Turicibacter was positively associated with corn DDGS supplemented broiler feed (Abudabos et al., 2017). Egg-laying hens fed on a diet supplemented with corn DDGS resulted in the egg yolk having a lower proportion of saturated fatty acids and a higher proportion of unsaturated fatty acids, while DDGS supplemented up to 10% in the diet had no adverse effect on egg-laying performance (Jiang et al., 2013). Recent studies have looked at replacing or supplementing soybean meal with corn DDGS as animal feed for poultry, swine, and cattle, with the goal of improving the nutritional qualities of the feed (Paine et al., 2018; Rho et al., 2018; Ajao et al., 2022; Chesini et al., 2022; Ding et al., 2022). Apart being used as animal feed sources, corn DDGS can potentially be utilized as a value-added functional material for wood composites due to their physical and chemical attributes (Liaw et al., 2019).
There is an increasing interest in microbial fermentation of corn DDGS as a value-added strategy to improve its role as an animal feed. The carbohydrate component of corn DDGS can be converted to other compounds including succinic acid, acetone, butanol, ethanol, and lactic acid (Iram et al., 2020). In addition to improving the nutritional value through microbial fermentation, corn DDGS is a rich source of phytochemical compounds that may provide antioxidant and wider animal health benefits in addition to the macronutrient and micronutrient composition (Shin et al., 2018). Protein hydrolysates of corn DDGS were found to have potential use as naturally derived antioxidants in food, pet food, and feed systems with good protection against lipid oxidation, which is relevant for the improvement of stability of the product during storage (Hu et al., 2020). In our previous studies, we have utilized LAB based fermentation of food substrates, like hulled emmer and pear juice to improve the health-protective health benefits in terms of its in vitro antioxidant and antihyperglycemic activity (Ankolekar et al., 2012; Christopher et al., 2021). However, LAB fermentation of corn-DDGS as substrate source targeting improvement of animal feed quality and antimicrobial property has not been explored previously. The scope and utilization of LAB-based fermentation of corn-DDGS to improve animal feed qualities targeting wider animal health and antimicrobial benefits is novel and investigated for the first time.
Apart from the nutritional and health benefits of corn DDGS which can be utilized in animal feed source to improve animal health, the native microflora associated with such by-products often have diverse biotechnological applications due to the production of useful enzymes or proteins. Therefore, it is important to screen and bioprocess bioactive enriched corn DDGS to improve their antioxidant and animal health-relevant properties. Additionally, isolating and characterizing the associated microflora of corn DDGS for potential biotechnological applications in agricultural, industrial, or pharmaceutical industries has wider relevance. Isolation and identification of novel microflora from corn-DDGS and its subsequent utilization in antimicrobial feed and agricultural applications has diverse value-added benefits. Therefore, the aim of this study was to advance the biotransformation of corn DDGS using LAB-based fermentation strategy to improve the phenolic phytochemical-linked functional qualities such as antioxidant activity and antimicrobial property against the gut pathogen Helicobacter pylori. Targeting H. pylori as a model antibacterial screening is based on the rationale that it shares similar environmental or growth conditions with bacterial pathogens such as Campylobacter jejuni, which is a major poultry-related food-borne pathogen, and hence can be subsequently targeted in poultry food safety applications. Furthermore, an unknown microbial culture isolated from unfermented corn DDGS was identified and the antimicrobial activity against H. pylori was characterized.
2 Materials and methods
2.1 Preparation of the corn DDGS extracts
The corn DDGS sample was obtained from the Tharaldson ethanol plant (Casselton, North Dakota, USA) and was extracted in duplicate based on the cold-water extraction protocol as described in a previous study (Christopher et al., 2018). For this study, the corn DDGS sample was blended with cold distilled water in a 1:4 ratio using a waring blender set at medium speed for 5 min. The extracts were then centrifuged at 8,500 rpm for 20 min and the supernatant was collected and re-centrifuged at 8,500 rpm for 15 min. The extracts were then pasteurized in a water bath set at 70°C for 15 min, after which the extracts were immediately cooled down using an ice bath, followed by storage at 4°C prior to advancing LAB fermentation.
2.2 Bacterial strains used
Lactiplantibacillus plantarum (ATCC 8014), Lactobacillus helveticus (ATCC 15009), and Helicobacter pylori (ATCC 43579) strains were used in this study. The L. plantarum and L. helveticus cultures were stored as frozen stocks in MRS broth (Difco) containing 20% glycerol as the cryoprotectant. The H. pylori culture was stored as frozen stocks in H. pylori special peptone broth (HPSP) containing 10 g L−1 special peptone (Oxoid, Basingstoke, UK), 5 g L−1 sodium chloride (Fisher Scientific, MA, USA), 5 g L−1 yeast extract (Difco), and 5 g L−1 beef extract (Difco), with 20% glycerol as the cryoprotectant. The MRS and HPSP agar plates were prepared by the addition of 15 g L−1 of granulated agar (Difco) to the respective broths. All media were autoclaved prior to use. For the revival of frozen bacterial stocks, 100 μL of thawed L. plantarum, L. helveticus, and H. pylori stocks were inoculated in 10 mL of the respective MRS or HPSP broth and incubated at 37°C for 24 h. Then 100 μL of the 24- h culture was re-inoculated into 10 mL sterile MRS or HPSP broth and incubated at 37°C for another 24 h. The revived cultures were used in the respective fermentation and in-vitro antimicrobial analysis.
2.3 Fermentation of the corn DDGS extracts
Fermentation of the corn DDGS extracts was done based on the protocol as described earlier (Ankolekar et al., 2012). The frozen stocks of L. plantarum and L. helveticus were revived in MRS broth and 10 mL of the revived cultures were added to the respective sterile flasks, each containing 90 mL of the pasteurized corn DDGS extracts. For the unfermented extract or control, 10 mL of sterile MRS broth was added to the extract instead of the cultures. The extracts were then incubated in duplicate at 37°C for 72 h and samples were collected at the 0, 24, 48, and 72-h fermentation timepoints for in vitro analysis. The growth of L. plantarum and L. helveticus was measured at each time point by serially diluting the fermented extracts followed by plating onto MRS agar plates. The plates were then incubated anaerobically at 37°C for 48 h in BBL GasPak jars (Becton, Dickinson & Co.) containing the BD GasPak EZ anaerobe container system sachets (Becton, Dickinson & Co.), after which the number of colonies were counted and expressed in log CFU mL−1. The samples collected from the unfermented and fermented extracts at the 0, 24, 48 and 72-h timepoints were centrifuged at 8,500 rm for 15 min, after which the supernatant was collected and the pH of one of the duplicates was adjusted close to neutral using 1 M NaOH, while a corresponding amount of water was added to the other duplicate (without pH adjustment) in order maintain equal volume. The unfermented and fermented extracts (with and without pH adjustment) were analyzed for their TSP content and antioxidant activity at the 0, 24, 48, and 72-h time points of fermentation. The samples of the unfermented and fermented extracts (with and without pH adjustment) at each time point were also filter-sterilized using sterile .22 µm syringe filters (Millipore Corp, MA, USA) and then stored at −20°C for later analysis of the phenolic profile and antimicrobial activity.
2.4 Total soluble phenolic (TSP) content
The TSP content of the unfermented and fermented corn DDGS extracts were determined using the Folin-Ciocalteu method based on the protocol as described previously (Shetty et al., 1995). To determine TSP content, .5 mL aliquots of the unfermented and fermented extracts were taken into the respective glass tubes, after which 1 mL of 95% ethanol, .5 mL of 50% (v/v) Folin-Ciocalteu reagent, and 1 mL of 5% sodium carbonate were added sequentially to the extracts. The tubes were then mixed using a vortex machine and incubated for 1 h under dark condition. The absorbance values of corn-DDGS fermented and unfermented extracts were then measured at 725 nm with a UV-visible spectrophotometer (Genesys 10S UV-VIS spectrophotometer, Thermo Scientific, NY, USA). Using a standard curve of different concentrations of gallic acid in 95% ethanol, the absorbance values of the extracts were converted into the TSP content, which was expressed in milligram gallic acid equivalents per Gram dry weight (mg GAE g−1 DW).
2.5 Phenolic profile characterization using high-performance liquid chromatography
Phenolic profile of the corn DDGS samples was characterized using high-performance liquid chromatography (HPLC) method, in which 5 μL of the unfermented and fermented corn DDGS extracts were injected using an Agilent ALS 1200 auto-extractor into an Agilent 1,260 series (Agilent Technologies, Palo Alto, CA) HPLC equipped with a D1100 CE diode array detector. A gradient elution with two solvents, 10 mM phosphoric acid (pH 2.5) and 100% methanol, were used. The methanol concentration was increased to 60% for the first 8 min, then to 100% over the next 7 min, then decreased to 0% for the next 3 min and was maintained for 7 min with a total run time of 25 min per injected sample run. The analytical column used was Agilent Zorbax SB-C18, 250–4.6 mm i.d., with packing material of 5 μm particle size at a flow rate of .7 mL min−1 at room temperature. The absorbance values were recorded at 214 nm, 230 nm, 260 nm, and 306 nm and the chromatogram was integrated using Agilent Chem station enhanced integrator. Pure standards of p-coumaric acid, gallic acid, dihydroxybenzoic acid, caffeic acid, ferulic acid and catechin in 100% methanol were used to calibrate the respective standard curves and retention times. The phenolic compounds detected in the extracts were expressed in micro Gram per Gram dry weight (µg g−1).
2.6 Antioxidant activity
The antioxidant activity of the unfermented and fermented corn DDGS extracts was measured by their scavenging activity against the free radicals 2, 2-Dipheny-1-Picryl hydrazyl (DPPH) (D9132-5G, Sigma-Aldrich), and 2, 2-Azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) (A1888-5G, Sigma-Aldrich) respectively. The DPPH scavenging assay was based on the protocol as described previously (Kwon et al., 2006) in which .25 mL of the extract was added to 1.25 mL of 60 mM DPPH (prepared in 95% ethanol) while the control had .25 mL of 95% ethanol instead of the extract. After 5 min of incubation, the extracts and their corresponding controls were centrifuged at 13,000 rpm for 1 min and the absorbance values of the supernatant was measured at 517 nm using a UV-visible spectrophotometer (Genesys 10S UV-VIS spectrophotometer, Thermo Scientific, NY). The ABTS scavenging assay was based on the protocol as described previously (Re et al., 1999) in which .05 mL of the extract was added to 1 mL of ABTS (prepared in 95% ethanol) while the control had .05 mL of 95% ethanol instead of the extract. After 2 min of incubation, the extracts and their corresponding controls were centrifuged at 13,000 rpm for 1 min and the absorbance values of the supernatant was measured at 734 nm using a UV-visible spectrophotometer (Genesys 10S UV-VIS spectrophotometer, Thermo Scientific, NY). The absorbance values from the DPPH and ABTS radical scavenging assays were used to calculate the percentage of antioxidant activity of the extracts using the following formula:
2.7 Antimicrobial activity
The in vitro antimicrobial activity of the unfermented and fermented corn DDGS extracts against H. pylori was measured using the agar diffusion method based on the protocol described previously (Ranilla et al., 2017). The frozen H. pylori culture was revived and streaked onto HPSP agar plates with the help of sterile cotton swabs. Then sterile 12.7 mm paper discs (BBL Taxo, Becton, Dickinson & Co.) were placed on the HPSP agar plates and 100 µL of the filter sterilized extracts from the 0, 24, 48, and 72-h fermentation time points were added to their respective paper discs with each plate having a control disc of sterile water. The plates were incubated at 37°C for 48 h in BBL GasPak jars (Becton, Dickinson & Co.) containing BD GasPak Campy container system sachets (Becton, Dickinson & Co.) to help maintain a microaerophilic environment. After incubation, the plates were examined for any zones of inhibition (no growth) around the discs, and the diameter of the zones of inhibition was expressed in millimeters. To determine if the antimicrobial activity was due to the bacterial or fungal growth present on the surface of the pasteurized corn DDGS extracts, the corn DDGS extracts were untreated, pasteurized (70°C for 15 min), or autoclaved (121°C for 20 min) respectively, followed by incubation at 37°C for 72 h. At the 0, 24, 48, and 72-h time points of incubation, samples were collected and centrifuged at 8,500 rpm for 15 min, after which the supernatant was collected and filter-sterilized for analysis of in vitro antimicrobial activity against H. pylori.
2.8 Isolation of a microorganism from pasteurized corn DDGS extract
The pasteurized corn DDGS extracts were incubated at 37°C for up to 72 h under aerobic conditions. After 24 h of incubation, the microorganism growth present on the surface of the extracts was streaked onto nutrient agar plates (Difco, Becton Dickinson & Co.) using an inoculation loop, and the plates were incubated at 37°C for 24 h. The isolated colonies were then sub-cultured in 10 mL nutrient broth (Difco, Becton Dickinson & Co.) for another 24 h at 37°C after which the culture broth was serially diluted, and the higher dilutions were streaked onto nutrient agar plates and incubated for another 24 h at 37°C. The plates containing the isolated colonies were used as the stock plates. The isolated colonies from the stock plates were inoculated in nutrient broth and incubated at 37°C for a period of 72 h. At the 0, 24, 48, and 72-h incubation time points, samples of the culture broth were collected aseptically and centrifuged at 8,500 rpm for 15 min. The cell free supernatant (CFS) containing the nutrient broth without the culture was collected and filter-sterilized using .20 µm syringe filters (Millipore Corp, MA, USA), followed by storage at −20°C for later analysis of in vitro antimicrobial activity against H. pylori.
2.9 Characterization of antimicrobial activity
The effect of pH and temperature on the antimicrobial activity of the CFS collected at 0, 24, 48, and 72-h time points of incubation were analyzed. To determine the effect of pH on antimicrobial activity, the CFS was adjusted to acidic (pH 3.0), neutral (pH7.0), or alkaline (pH 9.0) pH respectively using 1N HCl or 1N NaOH, while CFS without pH adjustment (native pH) was used as control treatment. To determine the effect of temperature, the CFS was autoclaved at 121°C for 20, 30, and 45 min respectively, while the CFS without heat treatment was used as control. The pH-adjusted and heat-treated CFS along with their corresponding controls were filter-sterilized and stored at −20°C for later analysis of in vitro antimicrobial activity against H. pylori. The antimicrobial activity of CFS collected at 0, 24, 48, and 72-h time points of incubation was also compared to Nisin, which is an antibacterial peptide. Nisin was prepared to a working concentration of 1000 IU mL−1 in .02 N HCl and was filter sterilized and stored at −20°C prior to analysis in the in vitro antimicrobial assay.
2.10 PCR with bacterial and fungal-specific primers
Under aseptic conditions, a loopful of the culture isolated from pasteurized corn DDGS extracts was used in DNA extraction with the Qiagen DNeasy Plant Mini Kit (Qiagen, Cat. # 69106), and the quality of extracted DNA was determined with a UV-Vis spectrophotometer (NanoDrop™ 2000; ThermoFisher). To determine if the growth isolated from pasteurized corn-DDGS extract (unfermented) was prokaryotic or eukaryotic, conventional PCR (C1000 Touch thermal cycler, BioRad) was performed using bacterial-specific 16s rRNA universal primers and fungal-specific ITS4/5 primers. The PCR was performed in 50 µL reaction volumes containing 1X Green GoTaq reaction buffer with 1.5 mM MgCl2 (Promega, Madison, WI), .2 mM of each dNTP, .4 µM of each primer, 1.25u of GoTaq DNA polymerase (Promega, Madison, WI), .5 µg of template DNA, and nuclease-free water. For the ITS4/5 primers, the following thermocycler parameters were used: initial denaturation at 95°C for 3 min; 31 cycles of 94°C for 90 s, 60°C for 90 s, and 72°C for 2 min, followed by a final extension at 72°C for 10 min. For the 16s rRNA primers, the following thermocycler parameters were used: initial denaturation at 95°C for 10 min; 36 cycles of 95°C for 30 s, 55°C for 1 min, and 72°C for 2 min, followed by a final extension at 72°C for 5 min. For both PCR runs, DNA isolated from Colletotrichum coccodes and Streptomyces scabies, both laboratory isolates, were used as the respective fungal and bacterial positive controls while sterile water was used as the negative control. The PCR product was purified using a PCR clean up kit (MidSci IBI Gel Extraction Kit, item #ASDNARNAKIT6) and was sent to MCLAB (www.mclab.com/DNA-Sequencing-Services.html) for sequencing. The sequence data was analyzed using a consensus tool (https://www.geneious.com/) and the resulting consensus sequence was compared to the nucleotide database using the Nucleotide BLAST tool at NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
2.11 PCR with Bacillus specific primer and construction of the phylogenetic tree
The DNA extracted from the bacterial isolate was subjected to conventional PCR using the Bacillus specific forward primer BacF (5′- GGGAAACCGGGGCTAATACCGGAT- 3′) and the universal bacterial 16S rDNA reverse primer R1378 (5′-CGGTGTGTACAAGGCCCGGGAACG-3′) as described earlier (Garbeva et al., 2003). The PCR was performed in 50 µL reaction volumes containing 1X Green GoTaq reaction buffer with 1.5 mM MgCl2 (Promega, Madison, WI), .2 mM of each dNTP, .4 µM of each primer, 1.25u of GoTaq DNA polymerase (Promega, Madison, WI), .5 µg of template DNA, and nuclease-free water. The thermocycler parameters used were as follows: initial denaturation at 95°C for 15 min; forty cycles of 95°C for 60 s, 63°C for 30 s, and 72°C for 60 s, followed by a final extension at 72°C for 8 min. DNA isolated from Bacillus subtilis (ATCC 6051) and Helicobacter pylori (ATCC 43579) were used as the respective positive and negative controls. The PCR product was purified and sent to Eurofins (https://www.eurofins.com/genomic-services/our-services/custom-dna-sequencing/) for sequencing. From the resulting sequence data, a consensus sequence was generated using MultAlign (http://multalin.toulouse.inra.fr/multalin/). A pairwise sequence alignment of the forward sequence with a reverse complement of the reverse sequence was done using the Smith-Waterman algorithm, and the missing base pairs were filled with the help of complementary base-pairing from the aligned sequences. This step was done to ensure that the final 16s rRNA sequence had the best base coverage. The final consensus sequence was submitted to the GenBank (https://submit.ncbi.nlm.nih.gov/) and the accession number ON553411 was assigned to the 16S rRNA sequence (https://submit.ncbi.nlm.nih.gov/subs/?search=SUB11502195). Nucleotide BLAST tool at NCBI was used to compare ON553411 against the database with the taxonomy ID - 1,386 (Bacillus species) in the organism category. A phylogenetic analysis of ON553411 was done with MEGA X software (Kumar et al., 2018) in which the sequence was first aligned using MUSCLE algorithm followed by phylogenetic analysis via the neighbor-joining, maximum-parsimony, and maximum-likelihood method, each performed with 100 bootstrap replications.
2.12 Statistical analysis
Fermentation, phenolic profile characterization, and antioxidant and antimicrobial assays were repeated two times with repeat extractions and duplicate samples. The means and standard error were calculated from 12 n) data points using Microsoft Excel XP software. The analysis of covariance was determined using Statistical Analysis Software (SAS version 9.4, SAS Institute, Cary, NC). Statistical mean separation among extracts, fermentation time points, and extract × fermentation time point interactions were determined using Tukey’s test at .05 probability level.
3 Results and discussion
3.1 Total soluble phenolic content (TSP) and phenolic profile
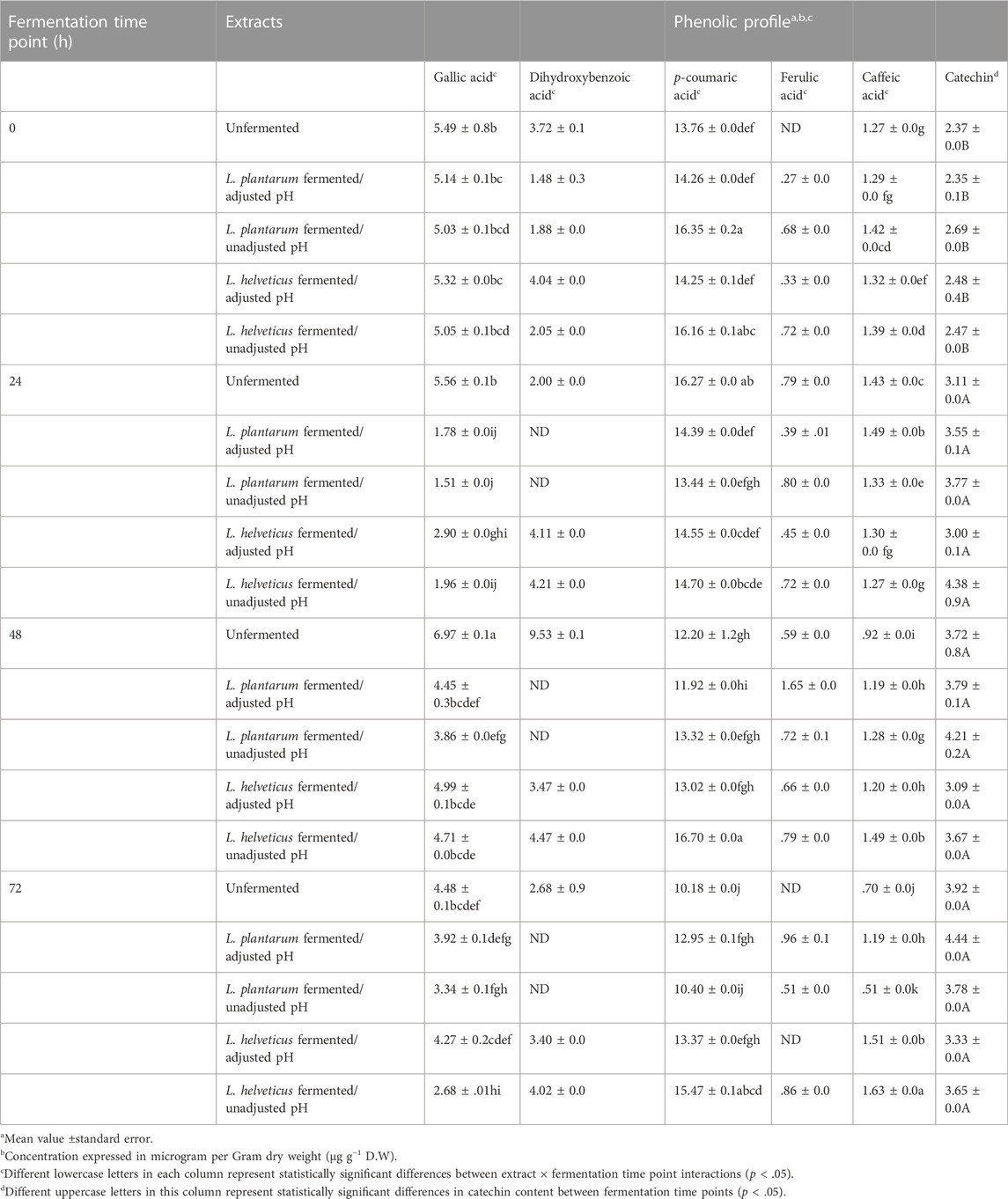
The TSP content of the corn DDGS extracts (unfermented and fermented) ranged from 2.49 to 2.85 mg GAE g−1 DW (Figure 1). Statistical differences in TSP content were observed among main effects of extracts and fermentation time points individually (p < .05), but not among extract × fermentation time point interactions. Overall, unfermented extracts had higher TSP content and mean TSP content increased after 24 h of fermentation. The stability of TSP in unfermented and fermented corn DDGS after 24 h has relevance in utilizing them in value-added feed applications targeting phytochemical-linked functional benefits. In our previous studies, we have targeted improvement of phenolic stability in food substrates like wheat and pear juice using LAB based fermentation (Ankolekar et al., 2012; Christopher et al., 2021). In the current study, similar fermentation strategy was used to enhance stability and bioavailability of phenolic bioactive in animal feed substrate, such as corn-DDGS.
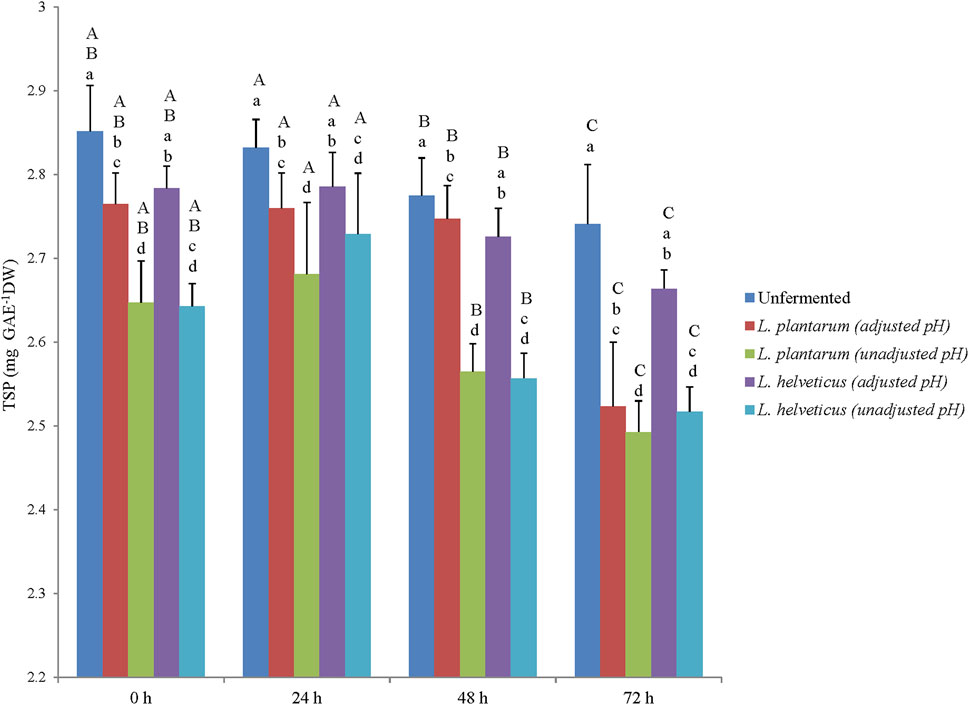
FIGURE 1. Total soluble phenolic content (mg GAE g−1) of the unfermented and fermented corn DDGS extracts. Different lowercase letters represent statistical differences in TSP content among the extracts (p < .05). Different uppercase letters represent statistical differences in TSP content among the fermentation time points (p < .05).
Individual phenolic compounds detected through HPLC analysis of the unfermented and fermented corn DDGS were gallic acid, dihydroxybenzoic acid, p-coumaric acid, ferulic acid, caffeic acid, and catechin, with their respective concentrations ranging from 1.51 to 6.97, 1.48 to 9.53, 10.18 to 16.70, .27 to 1.65, .51 to 1.63, and 2.35–4.44 μg g−1 (Table 1). In a previous study, corn-DDGS samples collected from different ethanol production plants in the US were analyzed for their phytochemical content and the study found that corn DDGS had a higher content of tocopherols, tocotrienols, lutein and ferulic acid (free and bound) when compared to yellow corn (Shin et al., 2018). The same study found the free ferulic acid content to be 3 times higher in the corn DDGS samples when compared to yellow corn (Shin et al., 2018). In another study, corn DDGS samples from three different ethanol production plants were found to have the phenolic compounds-vanillic, caffeic, p-coumaric, ferulic, and sinapic acids, with ferulic and p-coumaric acid accounting for 80% of the total phenolic acid content (Luthria et al., 2012). Also, the same study found the total phenolic acid content to be 3 times higher in the corn DDGS samples when compared to the yellow corn samples (Luthria et al., 2012).
In the current study, p-coumaric acid was the dominant phenolic acid while ferulic acid was present in smaller quantities (Table 1). Dihydroxybenzoic acid was detected in the unfermented and L. helveticus fermented extracts and not in the L. plantarum fermented extracts, thereby indicating possible microbial metabolism of dihydroxybenzoic acid by L. plantarum. Also, gallic acid was higher in the unfermented extracts when compared to the fermented extracts at the 24 and 48-h time points, thereby indicating possible microbial metabolism of gallic acid by L. helveticus and L. plantarum. Furthermore, the low pH generated during fermentation due to growth of LAB can also affect the stability of phenolic compounds, as a pH close to neutral favors a better stability of these water-soluble phenolics (Friedman and Jürgens, 2000). These results indicate that fermentation of corn DDGS with LAB did not drastically alter the TSP content, however the profile and concentration of individual phenolic compounds can be altered depending upon the type of LAB used for fermentation and total duration of fermentation. The result of this study has wider relevance to utilize LAB based fermentation for improving animal feed quality and subsequently enhancing animal health benefits of corn-DDGS, and can also be explored for other animal feed applications.
3.2 Antioxidant activity
The ABTS and DPPH radical scavenging activity of corn DDGS extracts (unfermented and fermented) ranged from 74% to 83.9% and 57.3%–81.8% respectively (Figures 2, 3). Statistical differences in ABTS and DPPH radical scavenging activity were observed among extracts, fermentation time points, and extract × fermentation time point interactions (p < .05). Overall, the unfermented and fermented corn DDGS extracts had higher ABTS scavenging activity when compared to DPPH scavenging activity. This difference in activity between two free radical scavenging assays is due to the chemical nature of the radicals in which DPPH being a more stable radical, requires stronger antioxidant activity when compared to ABTS. In a previous study, corn DDGS samples collected from different ethanol production plants in the US were analyzed for their antioxidant activity via DPPH radical scavenging activity, and the activity was found to vary greatly among the corn DDGS samples, with activity being 3 times higher in the corn DDGS samples when compared to yellow corn (Shin et al., 2018). Similarly in another study, the antioxidant activity of corn DDGS samples from three different ethanol production plants was found to be 2.5 times higher when compared to yellow corn (Luthria et al., 2012). Protein hydrolysates and peptides from corn by-products display antioxidant activity which can be used to improve the shelf-life of animal feed (Hu et al., 2020; Sharma et al., 2022). In the current study, LAB fermentation resulted in an improvement in antioxidant activity (based on DPPH results) after 24 h fermentation. The results of the current study indicated that fermentation of corn DDGS, specifically for 24 h can be a potential strategy to improve the stability of phenolic phytochemicals and associated antioxidant activity targeting wider animal feed and animal health benefits. In future, other animal feed sources like soybean or canola meal can be targeted for LAB based fermentation to potentially improve animal health relevant functional qualities of feed or feed ingredients.
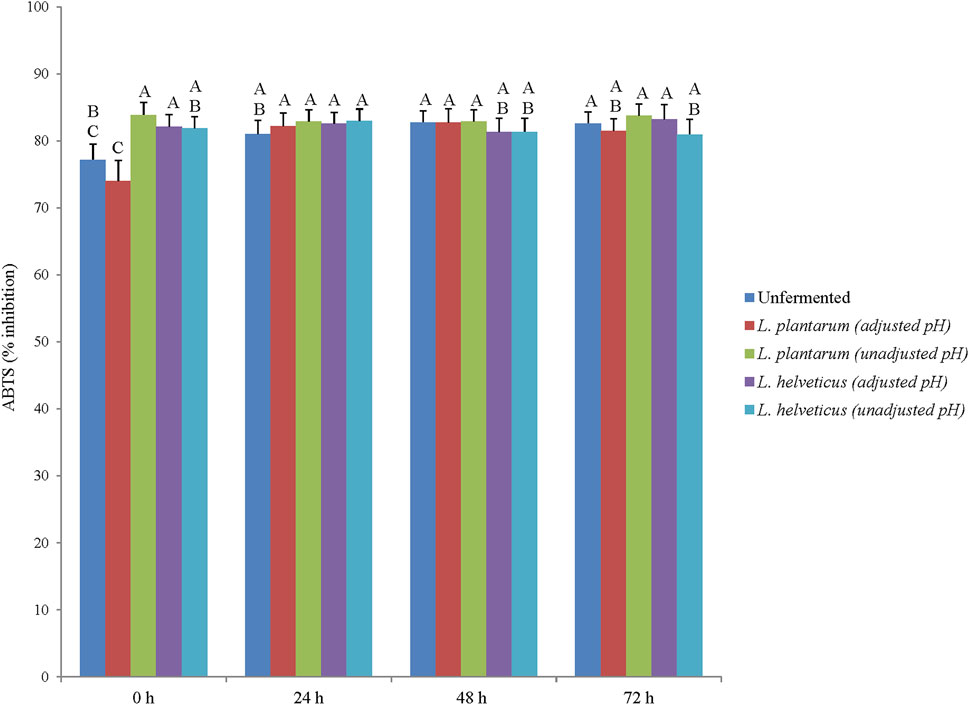
FIGURE 2. 2, 2-Azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) scavenging activity (% inhibition). Different letter represents statistical differences in ABTS scavenging activity among the extract × fermentation time point interactions (p < .05).
FIGURE 3. 2, 2-Dipheny-1-Picryl hydrazyl (DPPH) scavenging activity (% inhibition). Different letter represents statistical differences in DPPH scavenging activity among the extract × fermentation time point interactions (p < .05).
3.3 Antimicrobial activity
Antimicrobial activity against H. pylori was observed only among the unfermented extracts at the 48 and 72 h fermentation time points, while no antimicrobial activity was observed for the extracts fermented with L. plantarum and L. helveticus (with and without pH adjustment) (Figures 4, 5). The zones of inhibition for the unfermented extracts ranged from 2 to 4 mm and 11–12 mm at the 48 and 72 h fermentation time points respectively.
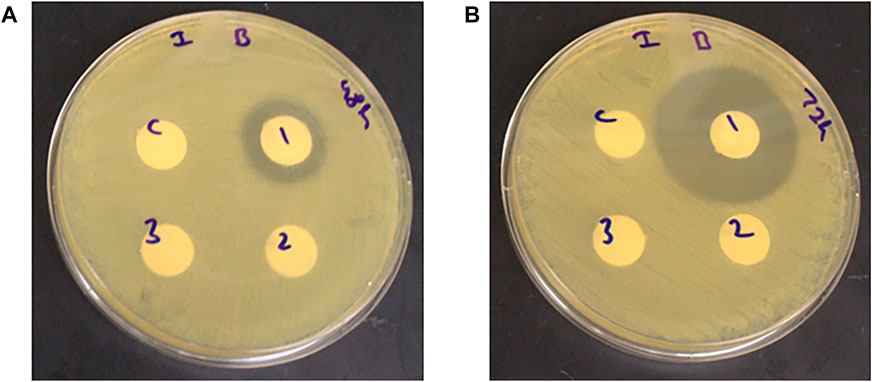
FIGURE 4. Antimicrobial activity of unfermented and L. plantarum fermented (with and without pH adjustment) corn-DDGS extracts against H. pylori at (A) 48-and (B) 72-h fermentation time points. C- control (sterile water), one- unfermented extract, 2-fermented extract (adjusted pH), and three- fermented extract (unadjusted pH). Zone of inhibition measured in millimeters (mm).
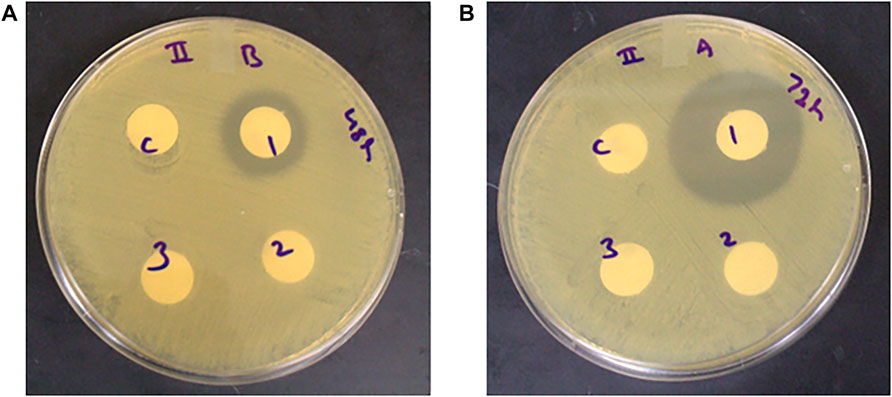
FIGURE 5. Antimicrobial activity of unfermented and L. heveticus fermented (with and without pH adjustment) corn DDGS extracts against H. pylori at (A) 48-and (B) 72-h fermentation time points. C- control (sterile water), one- unfermented extract, two- fermented extract (adjusted pH), and three- fermented extract (unadjusted pH). Zone of inhibition measured in millimeters (mm).
Growth of an unknown microorganism was observed at the surface of the unfermented pasteurized corn DDGS extracts after 24 h of incubation at 37°C. To confirm if the antimicrobial activity was due to the microorganism growth, the corn DDGS extracts were untreated, pasteurized, or autoclaved, followed by incubation at 37°C for 72 h. A microbial lawn appeared at the surface of the pasteurized corn DDGS extracts after 24 h of incubation, while some turbidity was observed for the untreated extracts which could be due to microbial growth, and no turbidity or growth was observed in the autoclaved extracts (Figure 6).

FIGURE 6. Presence of microorganism growth at the surface of pasteurized corn-DDGS extracts. I- untreated extract, II- pasteurized extract (70°C for 15 min), and III- autoclaved extract (121°C for 20 min).
Antimicrobial activity was observed only for the pasteurized extract at the 48 and 72 h incubation time points, with zones of inhibition ranging from 6 to 10 mm and 7–14 mm, respectively (Figure 7). These results indicated that the pasteurization of the corn DDGS extract was responsible for the growth of the microorganism endemic to the corn DDGS sample, and pasteurization led to the potential activation of bacterial spores, which later was confirmed belonging to spore producing genus of Bacillus. Furthermore, the microorganism growth which was present only in the pasteurized corn DDGS extracts was responsible for exhibiting antimicrobial activity against H. pylori.
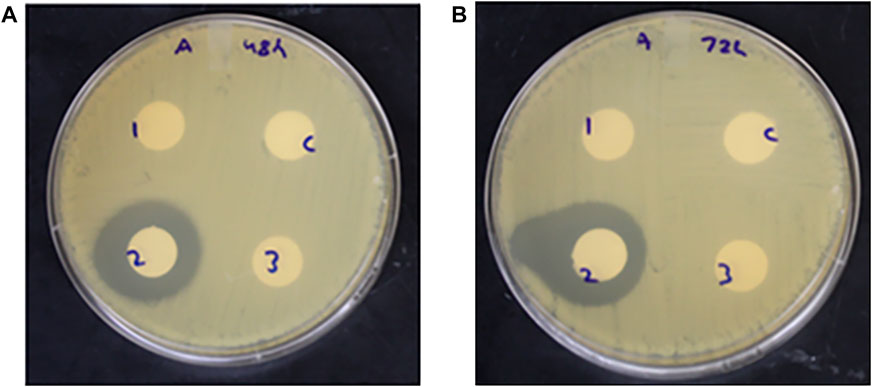
FIGURE 7. Antimicrobial activity of untreated, pasteurized, and autoclaved corn DDGS extracts against H. pylori at the (A) 48-h and (B) 72-h incubation time points. C- control (sterile water), one- untreated extract, two- pasteurized extract, and three- autoclaved extract. Zone of inhibition measured in millimeters (mm).
3.4 Characterization of antimicrobial activity
The antimicrobial activity was affected by pH and high temperature. With regard to the effect of pH, at the 48-h incubation time point, no antimicrobial activity was observed for CFS adjusted to acidic pH (pH 3.0) while the activity was unaffected at neutral (pH 7.0) and alkaline (pH 9.0) when compared to the control (native pH 5.0) (Figure 8). At the 72-h incubation time point, mild antimicrobial activity was observed at acidic pH (pH 3.0) while the activity was unaffected at neutral and alkaline pH (Figure 5).

FIGURE 8. Antimicrobial activity of pH-adjusted cell free supernatant (CFS) against H. pylori at (A) 48- h and (B) 72-h incubation time points. C- control (native pH 5.0), one- acidic (pH 3.0), two- neutral (pH 7.0), and three- alkaline (pH 9.0). Zone of inhibition measured in millimeters (mm).
In terms of the effect of temperature, at the 48-h incubation time point, no antimicrobial activity was observed for CFS treated at 121°C for 20, 30, and 45 min respectively when compared to the control (unadjusted pH) (Figure 9). However, at the 72-h incubation time point, mild antimicrobial activity was observed for CFS treated at 121°C for 20, 30, and 45 min respectively when compared to the control (untreated) (Figure 9).
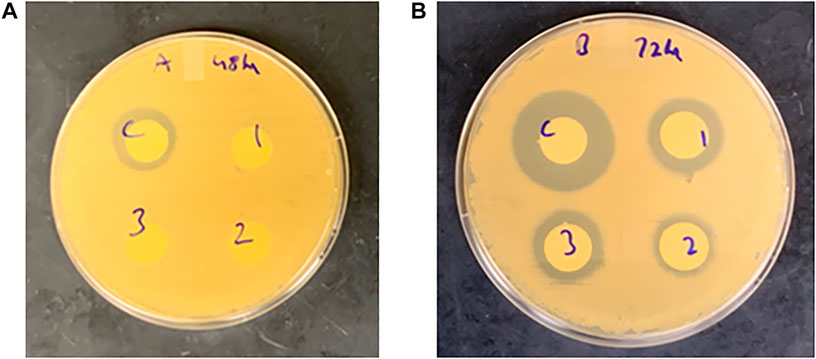
FIGURE 9. Antimicrobial activity of heat-treated (121°C) cell free supernatant (CFS) against H. pylori at (A) 48-h and (B) 72-h incubation time points. C- control (untreated), 1–20 min, 2–30 min, and 3–45 min. Zone of inhibition measured in millimeters (mm).
The sensitivity of antimicrobial activity to pH and high temperature indicated the possibility of extracellular antimicrobial peptides produced by unknown bacterial/fungal growth are potentially responsible for antimicrobial activity against H. pylori. Furthermore, the mild antimicrobial activity observed for heat-treated CFS at the 72-h incubation time point indicated that sufficient concentration of these antimicrobials was produced to show antimicrobial activity even at a high temperature, thereby indicating that these antimicrobials are generally heat stable. Finally, the CFS at the 48 and 72-h incubation time points had similar antimicrobial activity when compared to Nisin (Figure 10), thereby indicating that these potential peptides would have relevant applications for developing natural antibiotics with potential antimicrobial activity against other bacterial pathogens. Further in future studies more detailed functional characterization to confirm the peptide nature of antimicrobial is essential.
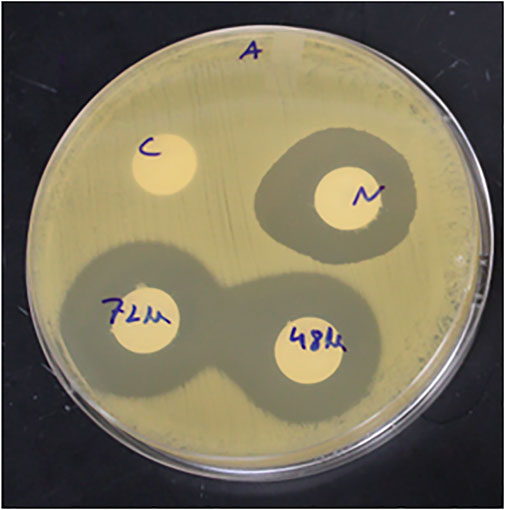
FIGURE 10. Antimicrobial activity of cell free supernatant (CFS) and Nisin against H. pylori at 48-h and 72-h incubation time points. C- control (sterile water), N- Nisin (1000 IU/mL), 48 h- and 72 h-incubation time point.
3.5 Isolation and identification of microorganism growth
The isolated microbial growth on the stock plates appeared as dry wrinkled colonies on the nutrient agar plate which is the colony morphology often associated with certain Bacillus spp., (Figure 11) (Koneman et al., 1997). The ability of Bacillus spp., to form a biofilm or lawn at the surface of the liquid medium (Figure 6) has also been reported in a previous study (Lu et al., 2018). Species belonging to Bacillus are rod-shaped, endospore-forming, Gram-positive bacteria that are abundant in the soil and can produce structurally diverse antimicrobial peptides that exhibit a wide spectrum of antibiotic activity (Sumi et al., 2015). The formation of endospores enables Bacillus spp., to survive long periods of adverse environmental conditions and the germination of endospores can occur due to pressure, chemical treatment, or sublethal heat treatment (Cronin and Wilkinson, 2008; Luu et al., 2015). In the current study, the pasteurization of the corn DDGS extracts at 70°C allowed endospores to survive which then germinated under normal cellular growth conditions resulting in the growth of the Bacillus isolate, while the untreated and autoclaved (121°C) extracts did not show any growth of the isolate (Figure 6). The absence of the microbial lawn in the untreated extract can be attributed to the non-germination of endospores due to no sublethal heat treatment, while autoclaving the extracts at 121°C could have potentially denatured the endospores. Furthermore, during the fermentation experiment, the absence of the microbial lawn in the fermented extracts can be attributed to the low pH generated by growth of LAB which can potentially inhibit the growth of the Bacillus isolate.
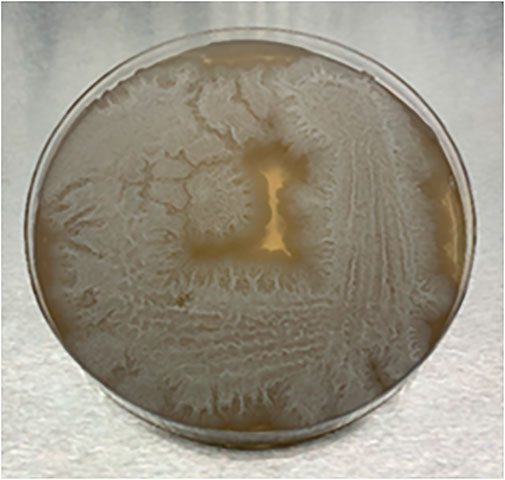
FIGURE 11. Colony morphology of unknown microbial growth isolated from pasteurized corn-DDGS extracts.
3.6 PCR identification of microorganism growth and phylogenetic analysis
To identify whether the growth was bacterial or fungal, the isolated culture was subjected to PCR using bacterial-specific 16s rRNA primers and fungal-specific ITS4/5 primers. PCR with 16s rRNA primers gave an amplified product while no product was observed with the fungal-specific primers, therefore indicating that the isolated growth was bacterial (Figure 12). Sequence analysis showed a close match with Bacillus spp (99.51%–99.75%). However, a higher degree of specificity could not be determined with the universal 16s rRNA primers. To improve the accuracy of the bacterial identification, PCR with Bacillus-specific 16S rRNA primers was performed (Figure 13), and sequence analysis of the PCR product (956 bp) (consensus sequence assigned as ON553411) showed a close match (99.9%) with Bacillus amyloliquefaciens, B. velezensis, B. subtilis, and B. siamensis. To determine the closest match among these candidates, a phylogenetic tree was constructed using the neighbor joining method with 100 bootstrap replications and ON553411 was found to share a cluster with B. amyloliquefaciens strain B6 16S rRNA gene partial sequence (MN908674.1) (Figure 14).
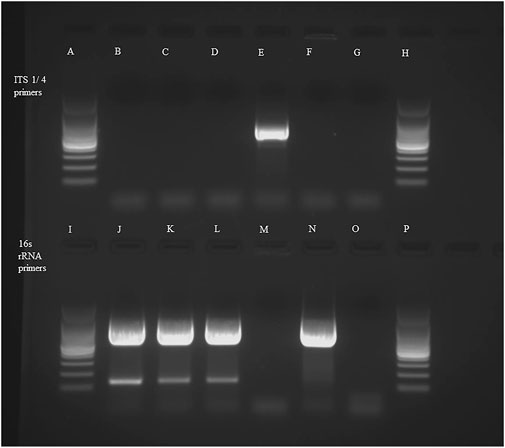
FIGURE 12. PCR with fungal-specific ITS 1/4 primers: (A)- 100 bp ladder, (B)- DNA isolate (first replicate), (C)- DNA isolate (second replicate), (D)- DNA isolate (third replicate), (E)-fungal control (Colletotrichum coccodes), (F)- bacterial control (Streptomyces scabies), (G)-negative control (water), and (H)- 100 bp ladder. PCR with bacterial-specific 16s rRNA primers: (I)- 100 bp ladder, (J)- DNA isolate (first replicate), (K)- DNA isolate (second replicate), (L)- DNA isolate (third replicate), (M)-fungal control (C. coccodes), (N)- bacterial control (S. scabies), (O)- negative control (water), (P)- 100 bp ladder.
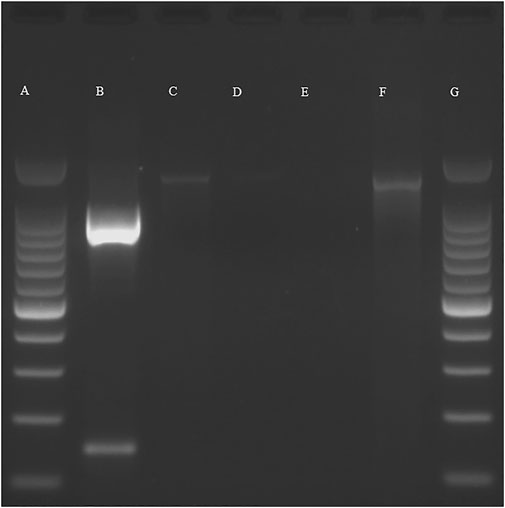
FIGURE 13. PCR with Bacillus-specific primers: (A)- 100 bp ladder, (B)- 16s rRNA PCR product, (C)- positive control (B. subtilis), (D)-negative control (H. pylori), (E)-negative control (water), (F)- DNA isolate (single replicate), (G)- 100 bp ladder.
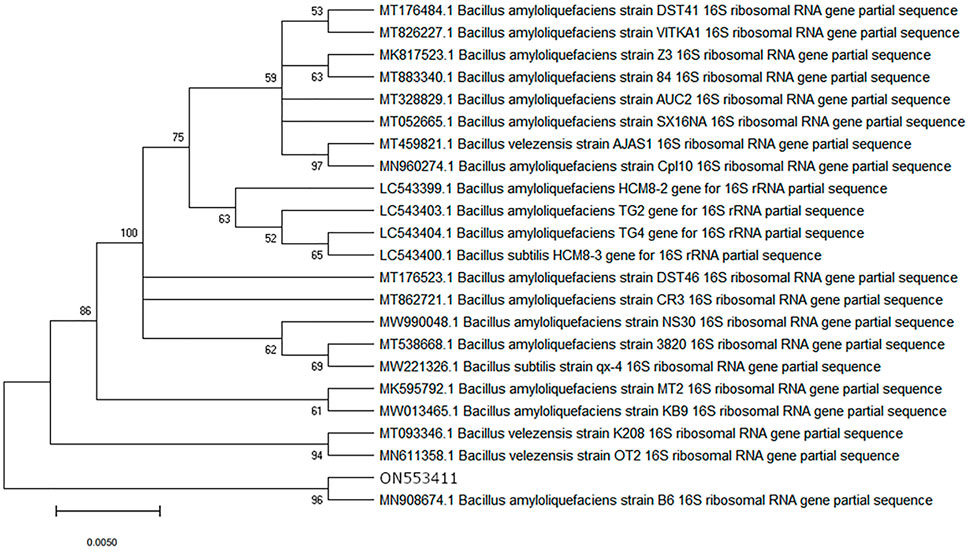
FIGURE 14. Phylogenetic analysis of ON553411 sequence based on 16SrRNA gene sequences of Bacillus strains. Neighbor joining method was used with 100 bootstrap replications and genetic distances were computed by Kimura’s two-parameter model. Only bootstrap percentages above 50% are shown. Bars, .005 substitutions per nucleotide position.
Although PCR based methods using 16S rRNA sequencing are used to discriminate between Bacillus spp., a high degree of sequence similarity can make it difficult to accurately discriminate between the species. In a previous study, a Bacillus strain isolated from soil shared a high sequence similarity (95%–99%) with 16S rRNA and gyrB sequences of B. amyloliquefaciens and B. velezensis (Wang, Lee, Tai & Kuo, 2008). In the same study, a phylogenetic analysis of the 16S rRNA sequences showed that the Bacillus strain isolated from the soil was in the same cluster as other Bacillus species including B. velezensis, B. amyloliquefaciens, and B. vallismortis, thereby making it difficult to accurately identify the isolated strain (Wang et al., 2008). Similarly in another study, sequence analysis of the primer annealing sites revealed no clear-cut differences in the variable region (V 1) of the 16S rRNA and gyrB gene among the B. cereus and B. thuringiensis strains that were tested (Chen & Tsen, 2002). These studies indicated that identification of Bacillus spp., can be challenging due to high sequence similarity, especially among strains of B. amyloliquefaciens and B. velezensis. Apart from the production of antimicrobial peptides against H. pylori, the corn DDGS isolate which was identified as a B.amyloliquefaciens strain could also have potential antimicrobial activity against other human bacterial pathogens, in addition to other relevant biotechnological applications. Indeed, B. amyloliquefaciens and other Bacillus species can act as plant growth promoters, biocontrol agents, probiotics, bioremediation agents, as well as producers of commercial enzymes and antibiotics (Raddadi et al., 2012; Zhang et al., 2020; Ngalimat et al., 2021).
4 Conclusion
Plant-based by-products such as corn DDGS from biotechnological or industrial processing often have further applications as animal feed due to their suitable calorific content and potential phytochemical-linked health-protective benefits. Furthermore, bio-transformative strategies such as fermentation with LAB can promote value-added functional qualities to the animal feed in terms of altering or improving these associated health protective benefits such as antioxidant properties of the feed. Lactic acid bacteria based fermentation strategy is widely used in food substrates to improve shelf-life and human health targeted qualities food and beverages. However, in this study, LAB-based fermentation strategy was explored for the first time to improve phenolic bioactive-linked animal health benefits of animal feed substrate like corn-DDGS. The results of this study demonstrated that LAB-based fermentation altered the phytochemical content and composition, as well as the antioxidant-associated health protective benefits of corn DDGS. In addition to plant-based by-products being utilized as animal feed, these by-products can also serve as a source for novel native microflora that have suitable biotechnological, industrial, or pharmaceutical applications. In this study, a microbe with antimicrobial activity against H. pylori was isolated from corn DDGS and identified as B. amyloliquefaciens. Further studies are essential for in depth analysis of antimicrobial activity of this corn DDGS isolate against other gut and foodborne related pathogens such as Salmonella, Listeria or E. coli. In addition, the production of other useful peptides or enzymes from the corn DDGS isolate with relevant biotechnological applications should also be analyzed. Hence corn DDGS is a suitable substrate for fermentation strategies aimed at improving the phenolic-linked health-protective benefits in animal feed, as well as a potential source for novel microorganisms that have biotechnological, industrial, or pharmaceutical applications. Isolation and identification of such beneficial microorganism (B. amyloliquefaciens) from corn DDGS also has major contribution in the field of agriculture, animal feed and health, and potential application in synthetic antibiotic replacement strategies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/- ON553411.
Author contributions
AC, DS, and KS contributed to the conceptualization and design of the study. AC, JO, and JM performed the experimentation and data analysis. AC, DS, and KS edited and reviewed the final draft of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frfst.2023.1075789/full#supplementary-material
References
Abudabos, A. M., Al-Atiyat, R. M., Albatshan, H. A., Aljassim, R., Aljumaah, M. R., Alkhulaifi, M. M., et al. (2017). Effects of concentration of corn distillers dried grains with solubles and enzyme supplementation on cecal microbiota and performance in broiler chickens. Appl. Microbiol. Biotechnol. 101 (18), 7017–7026. doi:10.1007/s00253-017-8448-5
Ajao, A. M., White, D., Kim, W. K., and Olukosi, O. A. (2022). Partial replacement of soybean meal with canola meal or corn DDGS in low-protein diets supplemented with crystalline amino acids—effect on growth performance, whole-body composition, and litter characteristics. Animals 12 (19), 2662. doi:10.3390/ani12192662
Ankolekar, C., Pinto, M., Greene, D., and Shetty, K. (2012). In vitro bioassay based screening of antihyperglycemia and antihypertensive activities of Lactobacillus acidophilus fermented pear juice. Innov. Food Sci. Emerg. Technol. 13, 221–230. doi:10.1016/j.ifset.2011.10.008
Chen, M. L., and Tsen, H. Y. (2002). Discrimination of Bacillus cereus and Bacillus thuringiensis with 16S rRNA and gyrB gene based PCR primers and sequencing of their annealing sites. J. Appl. Microbiol. 92 (5), 912–919. doi:10.1046/j.1365-2672.2002.01606.x
Chesini, R. G., Takiya, C. S., Dias, M. S., Silva, T. B., Nunes, A. T., Grigoletto, N. T., et al. (2022). Dietary replacement of soybean meal with heat-treated soybean meal or high-protein corn distillers grains on nutrient digestibility and milk composition in mid-lactation cows. J. Dairy Sci. 106, 233–244. doi:10.3168/jds.2022-21904
Christopher, A., Sarkar, D., and Shetty, K. (2021). Improving phenolic-linked antioxidant, antihyperglycemic and antibacterial properties of emmer and conventional wheat using beneficial lactic acid bacteria. Appl. Microbiol. 1 (2), 270–288. doi:10.3390/applmicrobiol1020020
Christopher, A., Sarkar, D., Zwinger, S., and Shetty, K. (2018). Ethnic food perspective of North Dakota common emmer wheat and relevance for health benefits targeting type 2 diabetes. J. Ethn. Foods 5 (1), 66–74. doi:10.1016/j.jef.2018.01.002
Cronin, U. P., and Wilkinson, M. G. (2008). Bacillus cereus endospores exhibit a heterogeneous response to heat treatment and low-temperature storage. Food Microbiol. 25 (2), 235–243. doi:10.1016/j.fm.2007.11.004
Ding, X. M., Qi, Y. Y., Zhang, K. Y., Tian, G., Bai, S. P., Wang, J. P., et al. (2022). Corn distiller's dried grains with solubles as an alternative ingredient to corn and soybean meal in Pekin duck diets based on its predicted AME and the evaluated standardized ileal digestibility of amino acids. Poult. Sci. 101, 101974. doi:10.1016/j.psj.2022.101974
Friedman, M., and Jürgens, H. S. (2000). Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 48 (6), 2101–2110. doi:10.1021/jf990489j
Garbeva, P., Van Veen, J. A., and Van Elsas, J. D. (2003). Predominant Bacillus spp. in agricultural soil under different management regimes detected via PCR-DGGE. Microb. Ecol. 45 (3), 302–316. doi:10.1007/s00248-002-2034-8
Hu, R., Dunmire, K. M., Truelock, C. N., Paulk, C. B., Aldrich, G., and Li, Y. (2020). Antioxidant performances of corn gluten meal and DDGS protein hydrolysates in food, pet food, and feed systems. J. Agric. Food Res. 2, 100030. doi:10.1016/j.jafr.2020.100030
Iram, A., Cekmecelioglu, D., and Demirci, A. (2020). Distillers’ dried grains with solubles (DDGS) and its potential as fermentation feedstock. Appl. Microbiol. Biotechnol. 104 (14), 6115–6128. doi:10.1007/s00253-020-10682-0
Jiang, W., Zhang, L., and Shan, A. (2013). The effect of vitamin E on laying performance and egg quality in laying hens fed corn dried distillers grains with solubles. Poult. Sci. 92 (11), 2956–2964. doi:10.3382/ps.2013-03228
E. W. Koneman, S. D. Allen, W. M. Janda, P. C. Schreckenberger, and W. C. WinnJr (Editors) (1997). Color atlas and textbook of diagnostic microbiology 5th ed. (New York, NY: Lippincott-Raven Publishers).
Kwon, Y. I. I., Vattem, D. A., and Shetty, K. (2006). Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 15 (1), 107–118.
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35 (6), 1547. doi:10.1093/molbev/msy096
Liaw, J. D., Bajwa, D. S., Shojaeiarani, J., and Bajwa, S. G. (2019). Corn distiller’s dried grains with solubles (DDGS)-A value added functional material for wood composites. Ind. Crops Prod. 139, 111525. doi:10.1016/j.indcrop.2019.111525
Lu, Z., Guo, W., and Liu, C. (2018). Isolation, identification, and characterization of novel Bacillus subtilis. J. Vet. Med. Sci. 80 (3), 427–433. doi:10.1292/jvms.16-0572
Luthria, D. L., Liu, K., and Memon, A. A. (2012). Phenolic acids and antioxidant capacity of distillers dried grains with solubles (DDGS) as compared with corn. J. Am. Oil Chem. Soc. 89 (7), 1297–1304. doi:10.1007/s11746-012-2025-y
Luu, S., Cruz-Mora, J., Setlow, B., Feeherry, F. E., Doona, C. J., and Setlow, P. (2015). The effects of heat activation on Bacillus spore germination, with nutrients or under high pressure, with or without various germination proteins. Appl. Environ. Microbiol. 81 (8), 2927–2938. doi:10.1128/AEM.00193-15
Ngalimat, M. S., Yahaya, R. S. R., Baharudin, M. M. A. A., Yaminudin, S. M., Karim, M., Ahmad, S. A., et al. (2021). A review on the biotechnological applications of the operational group Bacillus amyloliquefaciens. Microorganisms 9 (3), 614. doi:10.3390/microorganisms9030614
Paine, J. M., Jones, C. K., Lattimer, J., and Crane, A. R. (2018). Impact of including distillers’ dried grains with solubles at expense of soybean meal on Boer-influenced goat growth performance. Transl. Anim. Sci. 2 (1), S93. doi:10.1093/tas/txy074
Raddadi, N., Crotti, E., Rolli, E., Marasco, R., Fava, F., and Daffonchio, D. (2012). in The most important Bacillus species in Biotechnology” in Bacillus thuringiensis Biotechnology. Editor E. Sansinenea (Dordrecht: Springer), 329–345.
Ranilla, L, G., Christopher, A., Sarkar, D., Shetty, K., Chirinos, R., and Campos, D. (2017). Phenolic composition and evaluation of the antimicrobial activity of free and bound phenolic fractions from a Peruvian purple corn (Zea mays L.) accession. J. Food Sci. 82 (12), 2968–2976. doi:10.1111/1750-3841.13973
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., and Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26 (9-10), 1231–1237. doi:10.1016/S0891-5849(98)00315-3
Rho, Y., Wey, D., Zhu, C., Kiarie, E., Moran, K., van Heugten, E., et al. (2018). Growth performance, gastrointestinal and digestibility responses in growing pigs when fed corn–soybean meal-based diets with corn DDGS treated with fiber degrading enzymes with or without liquid fermentation. J. Anim. Sci. 96 (12), 5188–5197. doi:10.1093/jas/sky369
Sharma, S., Pradhan, R., Manickavasagan, A., Thimmanagari, M., Saha, D., Singh, S. S., et al. (2022). Production of antioxidative protein hydrolysates from corn distillers solubles: Process optimization, antioxidant activity evaluation, and peptide analysis. Ind. Crops Prod. 184, 115107. doi:10.1016/j.indcrop.2022.115107
Shetty, K., Curtis, O. F., Levin, R. E., Witkowsky, R., and Ang, W. (1995). Prevention of vitrification aßociated with in vitro shoot culture of oregano. (Origanum vulgare) by Pseudomonas spp. J. Plant Physiol. 147 (3-4), 447–451. doi:10.1016/S0176-1617(11)82181-4
Shin, E. C., Shurson, G. C., and Gallaher, D. D. (2018). Antioxidant capacity and phytochemical content of 16 sources of corn distillers dried grains with solubles (DDGS). Anim. Nutr. 4 (4), 435–441. doi:10.1016/j.aninu.2018.07.003
Sumi, C. D., Yang, B. W., Yeo, I. C., and Hahm, Y. T. (2015). Antimicrobial peptides of the genus Bacillus: A new era for antibiotics. Can. J. Microbiol. 61 (2), 93–103. doi:10.1139/cjm-2014-0613
U.S. Grains Council (2022). Production and exports. Available at: https://grains.org/buying-selling/ddgs/(Accessed October 15, 2022).
U.S. Grains Council (2018). User handbook. Available at: https://grains.org/buying-selling/ddgs/user-handbook/(Accessed October 15, 2022).
Wang, L. T., Lee, F. L., Tai, C. J., and Kuo, H. P. (2008). Bacillus velezensis is a later heterotypic synonym of Bacillus amyloliquefaciens. Int. J. Syst. Evol. Microbiol. 58 (3), 671–675. doi:10.1099/ijs.0.65191-0
Keywords: antioxidant, antimicrobial, corn DDGS, fermentation, lactic acid bacteria
Citation: Christopher A, Ostrander J, Mathew J, Sarkar D and Shetty K (2023) Corn distiller’s dried grains with solubles as a target for fermentation to improve bioactive functionality for animal feed and as a source for a novel microorganism with antibacterial activity. Front. Food. Sci. Technol. 3:1075789. doi: 10.3389/frfst.2023.1075789
Received: 20 October 2022; Accepted: 02 January 2023;
Published: 17 January 2023.
Edited by:
Jailane de Souza Aquino, Federal University of Paraíba, BrazilReviewed by:
Jonas Viškelis, Lithuanian Research Centre for Agriculture and Forestry, LithuaniaSohail, Humboldt University of Berlin, Germany
Abdeslam Asehraou, Mohamed Premier University, Morocco
Copyright © 2023 Christopher, Ostrander, Mathew, Sarkar and Shetty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kalidas Shetty, a2FsaWRhcy5zaGV0dHlAbmRzdS5lZHU=
 Ashish Christopher
Ashish Christopher Jesse Ostrander2
Jesse Ostrander2 Dipayan Sarkar
Dipayan Sarkar Kalidas Shetty
Kalidas Shetty