- 1School of Physical Education, Shandong University, Jinan, China
- 2Department of Physical Education Teaching and Research, Guangdong Dance and Drama College, Foshan, China
Introduction: To investigate the causal associations between accelerometer-based physical activity (PA), sedentary behavior (SB), and seven common geriatric syndromes (GSs) (frailty, falls, delirium, urinary incontinence, dysphagia, hearing loss, and visual impairment) by Mendelian randomization (MR) analysis.
Methods: Instrumental variables from a genome-wide association study were used for MR analysis. The exposure factors were three PA phenotypes (average acceleration, overall activity, and moderate-intensity activity) and one SB phenotype (SB). The outcome variables were seven common GSs. The inverse variance weighted (IVW) method was utilized for the primary MR analysis. Additionally, sensitivity, pleiotropy, and heterogeneity analyses were subsequently conducted to assess the robustness of the present study’s findings.
Results: According to the primary MR results obtained using the IVW method, genetically predicted PA (average acceleration) decreased the risk of two GSs (frailty, p = 0.01; dysphagia, p = 0.03). Similarly, overall activity decreased the risk of two GSs (frailty, p = 0.01; delirium, p = 0.03), and moderate-intensity activity reduced the risk of three GSs (urinary incontinence, p = 0.04; hearing loss, p = 0.02; visual impairment, p = 0.01). Furthermore, SB was causally correlated with a greater risk for three GSs (frailty, p = 0.03; fall, p = 0.01; dysphagia, p = 0.04).
Conclusion: This study provided evidence that accelerometer-based PA may be causally associated with a lower risk of GSs, while SB may increase the risk of GSs.
1 Introduction
The term geriatric syndromes (GSs) refers to a group of medical conditions in the older adult, such as frailty, falls, delirium, and incontinence, associated with age increase (1, 2). Due to the complex pathogenesis involving dysregulated metabolism, immune system decline, and musculoskeletal dysfunction, the incidence of GSs has shown an upward trend consistently in recent years (3). According to recent epidemiological advancements, GSs affect approximately 10–33% of adults aged 65 and above worldwide, with more than 40% experiencing two or more symptoms simultaneously (4, 5). These conditions lead to long-term disability, emotional distress, and social isolation, all of which diminish the quality of life for the older adult and impose an economic burden on the whole society (6, 7). As reported, healthcare costs associated with GSs have been estimated to reach $164 billion annually in the US and over $182 billion in 18 European nations combined (7, 8). Therefore, GSs pose not only a medical issue but also a significant challenge to public health and socio-economic factors.
Physical activity (PA) has gained increasing attention in recent decades due to its lower cost and higher adherence than traditional strategies like medication, long-term nursing, and hospitalization (9, 10). There is strong evidence that PA was associated with a risk reduction in the incidence of GSs. A previous Longitudinal cohort study found that maintaining a regular frequency of PA is associated with frailty among European community-dwelling older adult (11). Another cross-sectional study conducted in Japan discovered a correlation between increased leisure-time PA and a reduced incidence of dysphagia, suggesting the potential protective role of PA on GSs (12). Conversely, insalubrious lifestyles such as sedentary behavior (SB) in older adults hurt muscle strength (13), bone health (14), and cognitive function (15). Several cohort studies investigating lifestyle factors and risk of GSs reported that SB was independently and positively associated with frailty and falls (16, 17). As two modifiable lifestyles, both PA and SB are strongly associated with several GSs. However, existing observational studies cannot eliminate the potential for reverse causation and confounding factors, hindering the establishment of causal relationships. Previous studies on PA and SB have primarily used self-reported activity measures instead of directly measuring overall mean acceleration with a wrist-worn accelerometer (18). This reliance on self-validated measures introduces the potential for information bias (19, 20). Therefore, the causal relationship between objectively measured PA, SB, and GSs remains uncertain.
Mendelian randomization (MR) study is a statistical method to infer potential causal relationships between exposure and outcome (21). Although MR methods have limitations, such as genetic variants only explaining a portion of the exposure variability and the possibility of unrecognized confounders still existing. An advantage of MR studies is their ability to strengthen result justification by minimizing the impact of confounding factors on result accuracy. Simultaneously, MR studies offer more robust evidence to ascertain the causal relationship between exposure and outcome (22). Therefore, this study used MR to investigate the causal relationship between accelerometer-based PA, SB, and GSs. These findings can potentially contribute novel strategies for preventing, diagnosing, and treating GSs.
2 Materials and methods
2.1 Study design
This study used two-sample MR to analyze the causal relationship between PA, SB, and GSs. A valid MR analysis must be supported by three key assumptions (23): (1) the selected genetic variants as instrumental variables (IVs) are robustly correlations with the exposure (24); (2) there were no unmeasured confounders for associations between genetic variants and outcomes (25); (3) the genetic variants affect the outcome only through their effect on the exposure of interest, that is, there is no horizontal pleiotropy between genetic variants and outcome (26). The overall design was shown in Figure 1.
For this MR study, we prioritized using IVs from Genome-Wide Association Studies (GWAS) databases over those from single-nation databases. This approach was chosen due to several advantages associated with GWAS databases (27): (1) GWAS databases include genetic data from a wide range of populations globally, providing a more comprehensive selection of SNPs. This ensures that our study captures a representative sample of genetic variations; (2) GWAS typically involve large-scale sample collections, resulting in higher statistical power and more reliable findings than smaller, single-nation studies; (3) the inclusion of diverse populations in GWAS helps to minimize regional and ethnic differences, enhancing the validity of our causal inferences.
In addition, we followed the Strengthening the Reporting of Observational Studies in Epidemiology–Mendelian Randomization (STROBE-MR) reporting guidelines in this study (28). All studies contributing data to these analyses obtained institutional review board approval from the respective countries, aligning with the Declaration of Helsinki (29). The present study did not need additional ethical approval since the original studies have received appropriate ethics and institutional review board approval.
2.2 Data sources
Three types of accelerometer-based PA and one type of accelerometer-based SB were included in our study as exposures, including acceleration average (AccAve), overall activity, moderate-intensity physical activity (MPA), and SB. GWAS for AccAve came from a recent study on PA among 377,234 participants from the UK Biobank (30). It required participants to wear an Axivity AX3 accelerometer for at least 72 h in a week, data <72 h or not completed every hour of the 24-h cycle, and other outliers were excluded (31). Overall activity, MPA, and SB were from another GWAS on PA and SB measured using wrist-worn accelerometers (N = 91,105); participants were asked to wear activity trackers over 7 days (32). Details of PA, SB, and accelerometers are in Supplementary Table S1.
Drawing from the GSs literature, this study focused on seven common GSs as outcomes, including frailty (4), urinary incontinence (2), dysphagia (33), delirium, fall (1), hearing loss and visual impairment (34). The source of GWAS for exposures and outcomes were shown in Table 1, and detailed information on each of the GSs is shown in Supplementary Table S2.
It is important to note that this part of the study was time-consuming for both the human subjects and the researchers due to the need for extended wear time and rigorous data collection protocols.
2.3 Genetic instrumental variable selection
Inclusion criteria: (1) single nucleotide polymorphisms (SNPs) with complete genetic significance, ensuring independence and high correlation between exposure factors and outcome variables, were chosen as instrumental variables (35). In previous MR studies, accelerometer-based GWAS of PA identified only 2 independent genome-wide significant SNPs with 5–14% heritability of PA (19). Heritability estimates indicate that SNPs, not currently identified as genome-wide significant, may play a role in the variation of PA. Consequently, SNPs were chosen to achieve genome-wide significance (p < 5e−06). This approach of adjusting statistical thresholds for genetic tools has been employed in earlier MR studies where physical activity served as an exposure (19); (2) A criterion of F > 10 was employed to define a strong association. To evaluate the strength of the instrumental variables (IVs), the F statistic of an individual SNP was computed. If F > 10, it indicates a negligible possibility of weak instrumental variable bias (36); (3) to exclude the influence of gene pleiotropy on the results, the linkage disequilibrium coefficient r2 was set to 0.001, and the width of the linkage disequilibrium region was set to 10,000 kb, to ensure the independence of each SNP (21, 37).
Exclusion criteria: given the close association of various confounding factors with the pathogenesis of sarcopenia, SNPs were scrutinized against the PhenoScanner database1 to identify potential violations of independence and exclusivity assumptions. SNPs closely linked to the geriatric syndrome were then excluded. After screening based on inclusion and exclusion criteria, the detailed information on SNPs used as instrumental variables in the MR analyses is shown in Supplementary Tables S3–S6.
2.4 Statistical analysis
In this paper, we used the following four different methods to estimate the causal relationship between PA, SB, and GSs: (1) we used the inverse variance weighted (IVW) method as the main analysis, which is essentially a meta-analysis method. IVW assesses causality by meta-analyzing the Wald ratios for each of the included SNP (37, 38); (2) we used MR-Egger to detect several violations of the assumptions of the standard instrumental variables and provide estimates of effects that are unaffected by these violations. MR-Egger also provides a sensitivity analysis for the robustness of the results of MR studies (23); (3) we also used the weighted median approach because this method yields reliable estimates of causal effects, even though <50% of the information comes from the null instrument (25); (4) weighted mode is consistent when the largest subset of instruments which identify the same causal effect are valid instruments, even if the majority of others are invalid (39). The assessment of causality between exposure and outcome was quantified as odds ratios (OR) and their respective 95% confidence intervals (CI). Statistical significance was indicated by a p < 0.05.
In addition, we used Cochran’s Q-test to detect heterogeneity among the selected SNPs, and if heterogeneity existed (p < 0.05) (40). To identify potential pleiotropy, we tested for MR-Egger-intercept horizontal pleiotropy, with a p-value for the intercept >0.05 indicating that no horizontal pleiotropy existed (41). Meanwhile, we removed individual SNPs one by one using the leave-one-out method and calculated the meta-effect estimates and confidence intervals for the remaining SNPs (42). It was used to test the effect of individual SNPs on causal inference.
In this study, R software (version 4.0.2) was used for all data analysis as well as for drawing statistical plots, mainly R packages (TwoSampleMR, Pacman, Matrix, and Mendelian randomization) were used for data analysis (43). These packages are free for free on the R Software website.2
3 Results
3.1 MR results
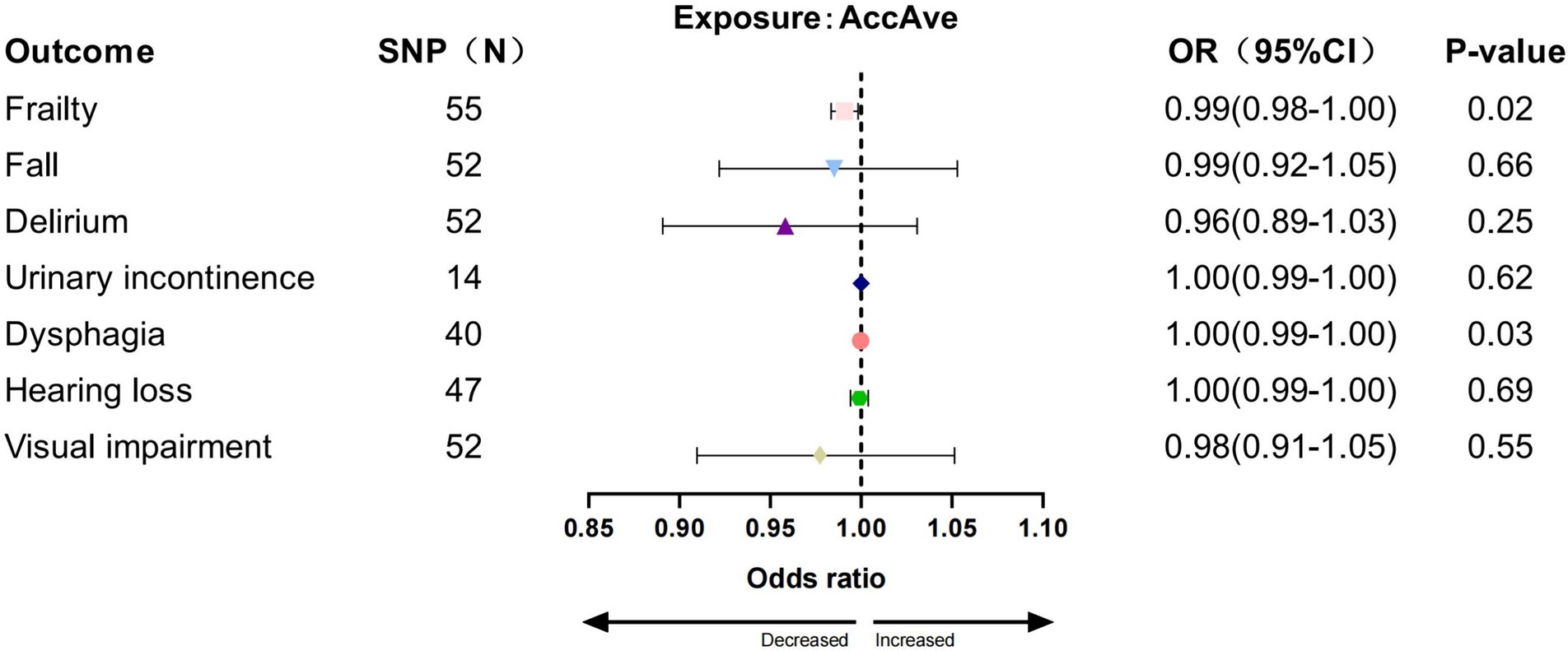
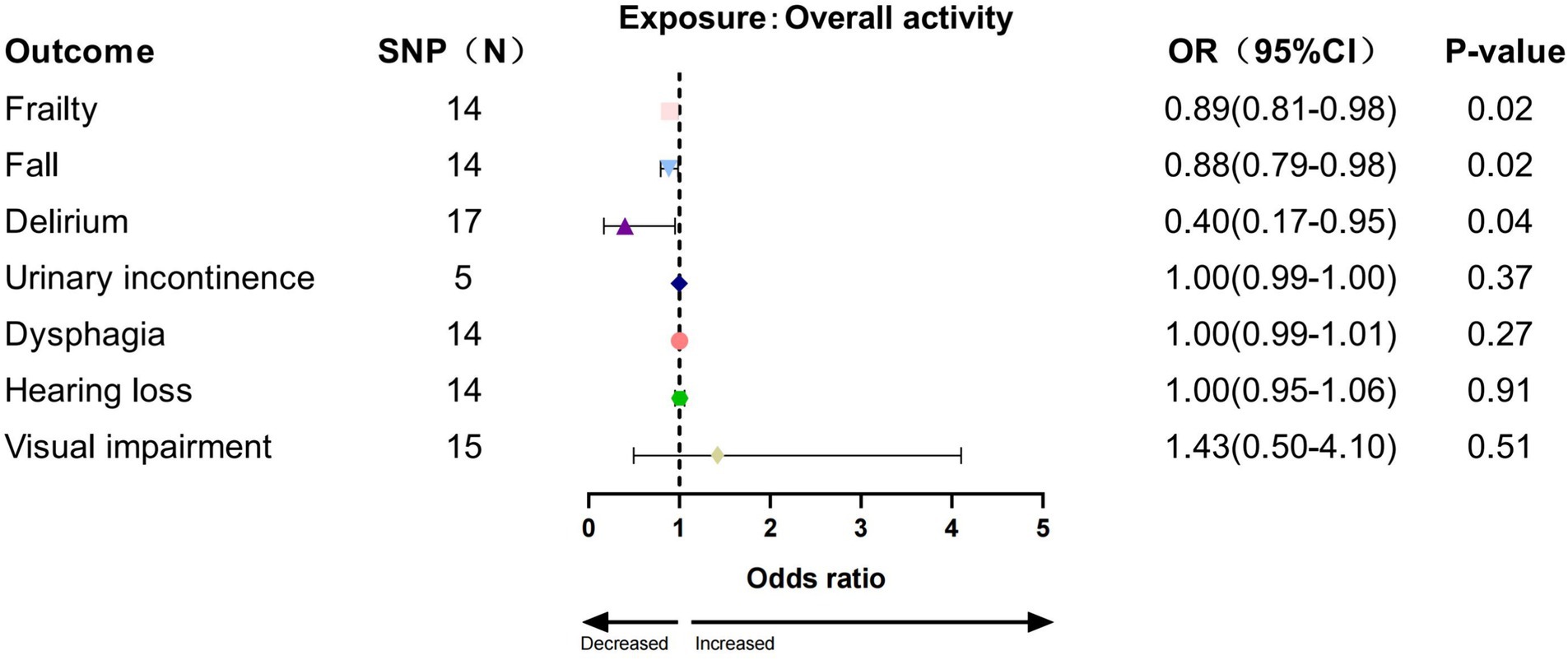
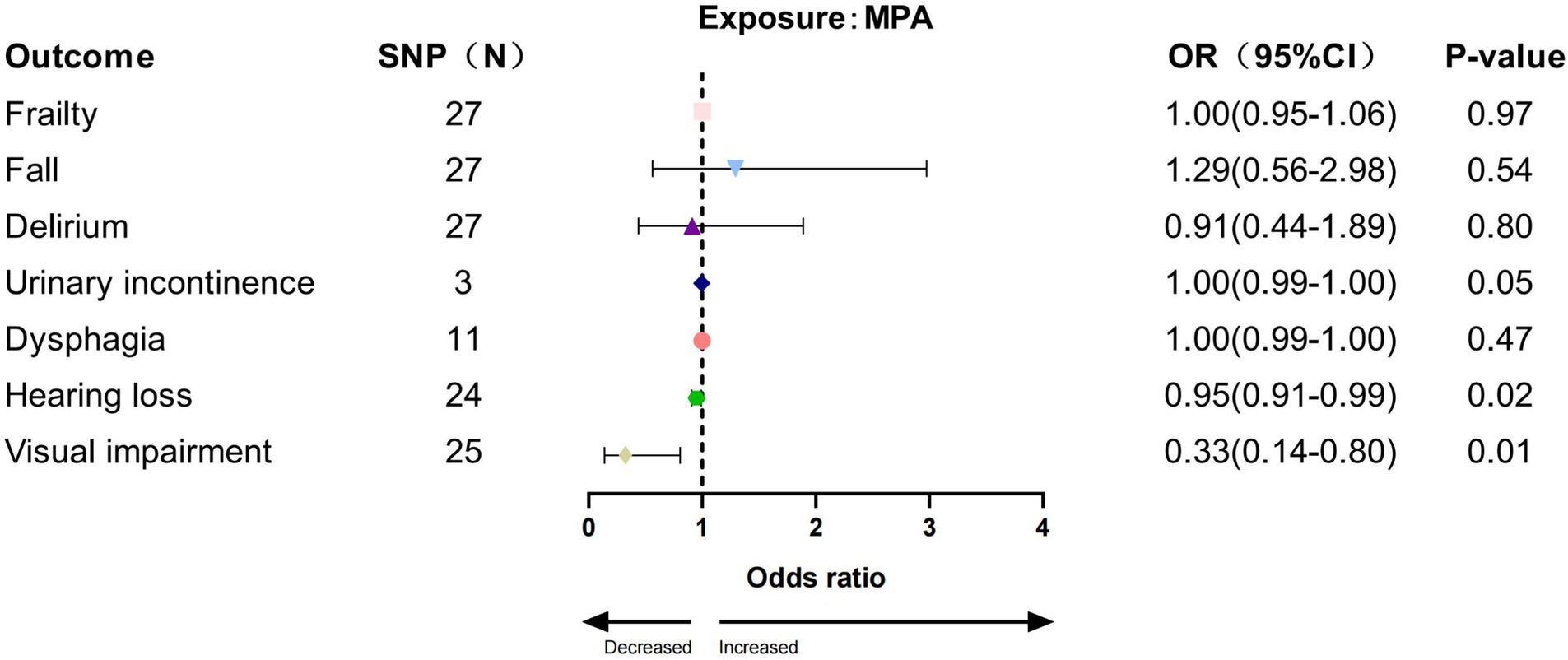
In the IVW analysis, we found a significant causal relationship between AccAve and a low risk of two GSs (frailty, OR = 0.99, 95% CI:0.98–0.99, p = 0.01; dysphagia, OR = 0.99, 95% CI: 0.99–0.99, p = 0.03; Figure 2). The weighted median, weighted mode, and MR-Egger analyses yielded similar patterns of effects (Supplementary Table S7). Similarly, there was a causal relationship between genetically predicted accelerometer-based “overall activity” PA and a low risk of two GSs (frailty, OR = 0.89, 95% CI: 0.81–0.98, p = 0.01; delirium, OR = 0.40, 95% CI: 0.16–0.95, p = 0.03; Figure 3) according to the IVW method. As shown in Figure 4, there was a significant causal relationship between accelerometer-based MPA and a low risk of three GSs (urinary incontinence, OR = 0.99, 95% CI: 0.99–1.00, p = 0.04; hearing loss, OR = 0.94, 95% CI: 0.90–0.99, p = 0.02; visual impairment, OR = 0.33, 95% CI: 0.13–0.80, p = 0.01).
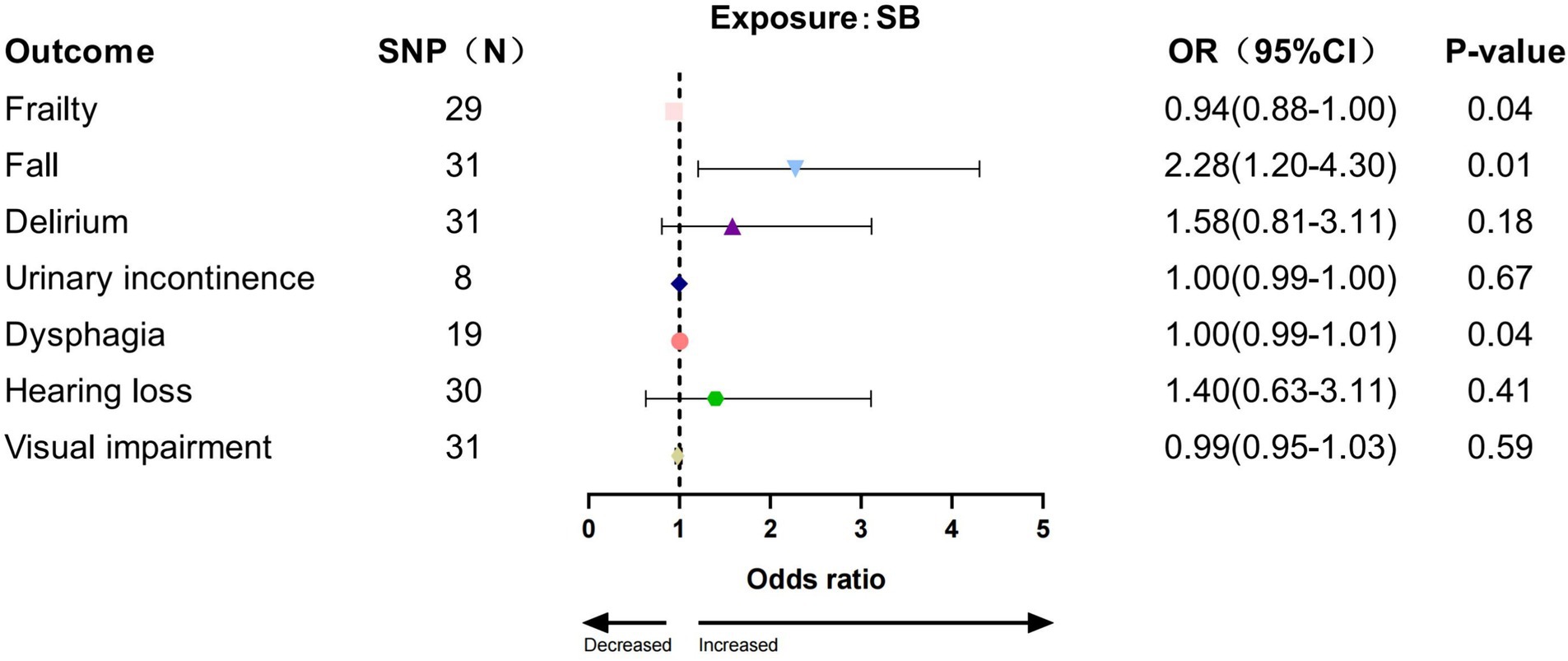
Among the tested SB phenotypes, IVW analysis indicated that accelerometer assessed SB increased the risk for three GSs (frailty: p = 0.039, OR = 0.939, 95% CI: 0.885–0.997; fall: p = 0.011, OR = 2.28, 95% CI: 1.20–4.30; dysphagia: p = 0.044, OR = 1.004, 95% CI: 1.000–1.007, Figure 5). The results from other MR methods showed a consistent direction (Supplementary Table S7).
3.2 Sensitivity analysis
To assess the robustness of the above results, a series of sensitivity analyses, including Cochran’s Q-test, MR Egger intercept test, and “leave-one-out,” were conducted (Supplementary Table S8). All p-values of the MR-Egger intercept tests were > 0.05, indicating that no horizontal pleiotropy existed. Meanwhile, heterogeneity was observed in the Cochran’s Q-test between AccAve and Frailty (Q = 54, p < 0.05). Although heterogeneity was observed in specific findings, it did not render the MR estimations inaccurate as the random-effect IVW method used in this work has the potential to mitigate the overall variability.
In the “leave-one-out” approach (Supplementary Figures S1–S10), each line represents a single nucleotide polymorphism (SNP). Black dots represent the meta-effect estimates obtained when eliminating that particular SNP, while horizontal lines reflect the appropriate confidence intervals. The red line indicates the position of the zero effect. As depicted in the diagram, the total effect estimate remains mostly unchanged when eliminating each SNP. Additionally, all error lines are positioned either to the right or left of zero, indicating a higher level of reliability in the data.
4 Discussion
To the best of our knowledge, this is the first MR study to investigate the genetic association of PA, SB, and seven prevalent GSs (frailty, falls, delirium, urinary incontinence, dysphagia, hearing loss, and visual impairment). According to the data shown in the results, genetically predicted accelerometer-based PA (AccAve, overall activity, MPA) was associated with a lower risk of 6 out of 7 GSs (frailty, dysphagia, delirium, urinary incontinence, hearing loss, and visual impairment), and accelerometer-based SB was associated with an increased risk of GSs (frailty, falls, dysphagia). Sensitivity analyses also indicated that results were robust in general. These findings provided a better understanding that PA is, in general, superior to SB in the older adult and had clinical implications for patients and caregivers.
In a longitudinal cohort study involving 1,735 European community-dwelling older adults, regular PA was associated with preserving or enhancing frailty (11). In another prospective, single-center cohort study with 132 participants aged 60 and older, PA was linked to a decreased incidence of delirium, particularly among women (18). Furthermore, a cross-sectional questionnaire-based study investigated the association between dysphagia risk and daily PA, as well as leisure-time exercise, in 3,070 community-dwelling Japanese older adults. The findings revealed that a higher level of leisure-time PA was associated with a reduced risk of dysphagia (12). However, research examining the relationship between PA and the mentioned three GS in older adults has yielded conflicting results. For instance, in a randomized controlled trial with 1,635 participants aged 70–89, the findings indicated that an MPA program did not correlate with a decreased risk of frailty (44). The present study aligns with this perspective. Additionally, a previous study identified a potential causal relationship between MPA and a reduced risk of urinary incontinence (45), hearing loss (46) and visual impairment in older adults (47). Our study, in supporting the aforementioned findings, further substantiates the potential causal relationship for them. Unfortunately, our study did not identify a potential causal relationship between accelerometer-based PA and fall risk. We have access to an explanation for this. In the MR study, SNPs associated with PA were used as instrumental variables to explore whether there is any link between exposure factors and outcome variables (48). This methodology enabled us to clearly and definitively determine the distinct influence of specific genetic variations on these outcomes, without any interference from other intricate elements (49). However, falls are not just attributed to individual genetic variations but also encompass intricate interplays between balance, muscle strength, environmental risk factors, and substance use (50, 51). Therefore, future research should look at the involvement of multiple SNPs and other complex factors in falls to provide a more complete approach to fall prevention in older persons.
There are various potential mechanisms can be used to illustrate the inverse association between PA and GSs. First, PA could induce several neuromuscular adaptations to slow down the aging-related decline in muscle function, which is suspected to be closely linked to the development of GSs (52, 53). Recent studies have shown that PA leads to the increase in peak firing frequencies of motoneurons and drives motor neuron activation, resulting in enhanced performance and function of the motor unit, which is the foundation for maintaining muscle strength in older adults (54, 55). Second, PA provides a healthy anti-inflammatory environment, largely by releasing muscle-derived myokines that accelerate myocardial regeneration and prevent age-related loss (56). Additionally, PA increases the availability of several growth factors to achieve delayed cognitive decline in the older adult and thus plays a protective role in GSs (57). According to reports, memory loss and cognitive impairment could be risk factors for GSs (58). Animal and human studies have shown that PA enhances brain health, and thus cognitive health, by increasing the availability of several growth factors in the neurotrophin family including BDNF, IGF-1, and VEGF (59, 60).
Another significant discovery in this MR study was the causal association between SB and a higher risk of three GSs (frailty, falls, dysphagia), which aligns with several investigations. A cross-sectional study in rural China revealed that older persons who spent 8 or more hours per day being sedentary were more susceptible to frailty than those who spent <4 h per day being sedentary (61). The adjusted analysis in another observational study with 411 participants demonstrated that there were independent associations between SB and frailty (62). Therefore, the older adult needed to enhance their awareness of reducing SB to control the GSs. In addition to frailty, previous observational studies have demonstrated that SB is associated with an increased risk of falls for older adults living in the community (63, 64). Findings from a meta-analysis provided further evidence in favor of this perspective (65). This can be explained by the fact that SB causes muscle weakness (66), reduced bone mass (67), and sarcopenia in older adults, consequently heightening the risk of frailty and falls (68). Furthermore, this study presents new evidence suggesting a possible causative association between SB and dysphagia in older persons. Specifically, we found that SB was related to an increased risk of dysphagia. One possible explanation is that SB leads to progressive weakening of muscles throughout the body, including the laryngeal and neck muscles associated with swallowing (69). This may affect the coordination and efficiency of swallowing in older adults (70).
There are certain strengths of our study. Compared with previous observational studies, we employed an MR study design to assess the causal associations between genetically predicted PA, SB, and GSs (18, 71). This design could minimize the potential biases due to confounding and reverse causality in the observational studies. Another advantage of this study is that the measures of exposures were obtained using accelerometers, which helps to eliminate potential recall and reaction bias (72). Additionally, the population bias was avoided as the populations under study were all individuals of European ancestry.
However, several limitations in our study need to be addressed. First, the sample selection might introduce bias. Our data predominantly come from Europe, which may not fully represent the global population or specific populations. This geographic and demographic limitation could affect the generalizability of our findings. Second, the representativeness of the sample should be considered. Although we employed rigorous random sampling techniques, the inherent variability in genetic backgrounds and environmental exposures across different populations might influence the results. Future studies with more diverse and larger samples are warranted to confirm our findings. Third, accelerometers also possess some constraints. Measuring posture and other types of PA and SB such as inactive, light exercise, and non-ambulatory activity is challenging (73). Therefore, our study focused primarily on overall PA and SB metrics in the sample. Additional research is required to investigate the correlation between various forms of PA, SB, and GSs. Lastly, although we drew on the largest available GWAS, some identified few genome-wide significant SNPs, which could result in relatively weak genetic instruments. To address this, we incorporated thresholds established in previous MR studies, employed additional SNPs as instruments, and performed sensitivity analyses (19, 74, 75). This approach aimed to mitigate the potential influence of alternative thresholds on the outcomes, thus enhancing the reliability of our findings.
5 Conclusion
The present study used a genetic approach and revealed that PA (AccAve, overall activity, MPA) was potentially causally associated with a lower risk of some of the six GSs (frailty, dysphagia, delirium, urinary incontinence, hearing loss, and visual impairment), whereas the accelerometer-based SB was potentially causally associated with a greater risk of three GSs (frailty, falls, and dysphagia). Overall, this study supports the hypothesis that strengthening PA and reducing SB are effective strategies for reducing GSs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
JC: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YL: Writing – original draft, Supervision. JY: Writing – original draft, Supervision. XZ: Writing – original draft, Validation, Supervision. YP: Writing – original draft, Methodology, Project administration.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the collaborators of this study for their time and effort in our research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1406303/full#supplementary-material
Footnotes
References
1. Inouye, SK, Studenski, S, Tinetti, ME, and Kuchel, GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. (2007) 55:780–91. doi: 10.1111/j.1532-5415.2007.01156.x
2. Akbarzadeh, MA, and Hosseini, MS. Is COVID-19 really a geriatric syndrome? Ageing Res Rev. (2022) 79:101657. doi: 10.1016/j.arr.2022.101657
3. Wanigatunga, AA, Cai, Y, Urbanek, JK, Mitchell, CM, Roth, DL, Miller, ER, et al. Objectively measured patterns of daily physical activity and phenotypic frailty. J Gerontol A Biol Sci Med Sci. (2022) 77:1882–9. doi: 10.1093/gerona/glab278
4. Ahmed, N, Mandel, R, and Fain, MJ. Frailty: an emerging geriatric syndrome. Am J Med. (2007) 120:748–53. doi: 10.1016/j.amjmed.2006.10.018
5. Sanford, AM, Morley, JE, Berg-Weger, M, Lundy, J, Little, MO, Leonard, K, et al. High prevalence of geriatric syndromes in older adults. PLoS One. (2020) 15:e0233857. doi: 10.1371/journal.pone.0233857
6. Bishop, AJ, Martin, P, and Poon, L. Happiness and congruence in older adulthood: a structural model of life satisfaction. Aging Ment Health. (2006) 10:445–53. doi: 10.1080/13607860600638388
7. Leslie, DL, Marcantonio, ER, Zhang, Y, Leo-Summers, L, and Inouye, SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. (2008) 168:27–32. doi: 10.1001/archinternmed.2007.4
8. Fong, TG, and Inouye, SK. The inter-relationship between delirium and dementia: the importance of delirium prevention. Nat Rev Neurol. (2022) 18:579–96. doi: 10.1038/s41582-022-00698-7
9. Bernabei, R, Landi, F, Calvani, R, Cesari, M, Del Signore, S, Anker, SD, et al. Multicomponent intervention to prevent mobility disability in frail older adults: randomised controlled trial (SPRINTT project). BMJ. (2022) 377:e068788. doi: 10.1136/bmj-2021-068788
10. Leslie, DL, Zhang, Y, Bogardus, ST, Holford, TR, Leo-Summers, LS, and Inouye, SK. Consequences of preventing delirium in hospitalized older adults on nursing home costs. J Am Geriatr Soc. (2005) 53:405–9. doi: 10.1111/j.1532-5415.2005.53156.x
11. Zhang, X, Tan, SS, Franse, CB, Bilajac, L, Alhambra-Borrás, T, Garcés-Ferrer, J, et al. Longitudinal association between physical activity and frailty among community-dwelling older adults. J Am Geriatr Soc. (2020) 68:1484–93. doi: 10.1111/jgs.16391
12. Maehara, T, Nishimura, R, Yoshitake, A, Tsukamoto, M, Kadomatsu, Y, Kubo, Y, et al. Association of daily physical activity and leisure-time exercise with dysphagia risk in community-dwelling older adults: a cross-sectional study. Sci Rep. (2023) 13:10893. doi: 10.1038/s41598-023-37605-z
13. Ramsey, KA, Rojer, AGM, D'Andrea, L, Otten, RHJ, Heymans, MW, Trappenburg, MC, et al. The association of objectively measured physical activity and sedentary behavior with skeletal muscle strength and muscle power in older adults: a systematic review and meta-analysis. Ageing Res Rev. (2021) 67:101266. doi: 10.1016/j.arr.2021.101266
14. Onambele-Pearson, G, Wullems, J, Doody, C, Ryan, D, Morse, C, and Degens, H. Influence of habitual physical behavior-sleeping, Sedentarism, physical activity-on bone health in community-dwelling older people. Front Physiol. (2019) 10:408. doi: 10.3389/fphys.2019.00408
15. Hannan, M, Collins, EG, Phillips, SA, Quinn, L, Steffen, A, and Bronas, UG. The influence of sedentary behavior on the relationship between cognitive function and vascular function in older adults with and without chronic kidney disease. Nephrol Nurs J Am Nephrol Nurses Assoc. (2021) 48:553–61. doi: 10.37526/1526-744X.2021.48.6.553
16. da Silva Coqueiro, R, de Queiroz, BM, Oliveira, DS, das Merces, MC, Oliveira Carneiro, JA, Pereira, R, et al. Cross-sectional relationships between sedentary behavior and frailty in older adults. J Sports Med Phys Fitness. (2017) 57:825–30. doi: 10.23736/S0022-4707.16.06289-7
17. Ozaki, E, Matsui, D, Kuriyama, N, Tomida, S, Nukaya, Y, and Koyama, T. Association between sedentary time and falls among middle-aged women in Japan. Healthcare. (2022) 10:2354. doi: 10.3390/healthcare10122354
18. Lee, SS, Lo, Y, and Verghese, J. Physical activity and risk of postoperative delirium. J Am Geriatr Soc. (2019) 67:2260–6. doi: 10.1111/jgs.16083
19. Choi, KW, Chen, CY, Stein, MB, Klimentidis, YC, Wang, MJ, Koenen, KC, et al. Assessment of bidirectional relationships between physical activity and depression among adults: a 2-sample Mendelian randomization study. JAMA Psychiatry. (2019) 76:399–408. doi: 10.1001/jamapsychiatry.2018.4175
20. Strath, SJ, Kaminsky, LA, Ainsworth, BE, Ekelund, U, Freedson, PS, Gary, RA, et al. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the American Heart Association. Circulation. (2013) 128:2259–79. doi: 10.1161/01.cir.0000435708.67487.da
21. Davey Smith, G, and Hemani, G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. (2014) 23:R89–98. doi: 10.1093/hmg/ddu328
22. Gupta, V, Walia, GK, and Sachdeva, MP. 'Mendelian randomization': an approach for exploring causal relations in epidemiology. Public Health. (2017) 145:113–9. doi: 10.1016/j.puhe.2016.12.033
23. Bowden, J, Davey Smith, G, and Burgess, S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
24. Burgess, S, Butterworth, A, and Thompson, SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
25. Bowden, J, Davey Smith, G, Haycock, PC, and Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
26. Gala, H, and Tomlinson, I. The use of Mendelian randomisation to identify causal cancer risk factors: promise and limitations. J Pathol. (2020) 250:541–54. doi: 10.1002/path.5421
27. Marth, G, Yeh, R, Minton, M, Donaldson, R, Li, Q, Duan, S, et al. Single-nucleotide polymorphisms in the public domain: how useful are they? Nat Genet. (2001) 27:371–2. doi: 10.1038/86864
28. Skrivankova, VW, Richmond, RC, Woolf, BAR, Davies, NM, Swanson, SA, Vander Weele, TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. (2021) 375:n2233. doi: 10.1136/bmj.n2233
29. Goodyear, MD, Krleza-Jeric, K, and Lemmens, T. The declaration of Helsinki. BMJ. (2007) 335:624–5. doi: 10.1136/bmj.39339.610000.BE
30. Klimentidis, YC, Raichlen, DA, Bea, J, Garcia, DO, Wineinger, NE, Mandarino, LJ, et al. Genome-wide association study of habitual physical activity in over 377,000 UK biobank participants identifies multiple variants including CADM2 and APOE. Int J Obes. (2018) 42:1161–76. doi: 10.1038/s41366-018-0120-3
31. Guo, W, Key, TJ, and Reeves, GK. Accelerometer compared with questionnaire measures of physical activity in relation to body size and composition: a large cross-sectional analysis of UK biobank. BMJ Open. (2019) 9:e024206. doi: 10.1136/bmjopen-2018-024206
32. Doherty, A, Smith-Byrne, K, Ferreira, T, Holmes, MV, Holmes, C, Pulit, SL, et al. GWAS identifies 14 loci for device-measured physical activity and sleep duration. Nat Commun. (2018) 9:5257. doi: 10.1038/s41467-018-07743-4
33. Thiyagalingam, S, Kulinski, AE, Thorsteinsdottir, B, Shindelar, KL, and Takahashi, PY. Dysphagia in older adults. Mayo Clin Proc. (2021) 96:488–97. doi: 10.1016/j.mayocp.2020.08.001
34. Mueller, YK, Monod, S, Locatelli, I, Büla, C, Cornuz, J, and Senn, N. Performance of a brief geriatric evaluation compared to a comprehensive geriatric assessment for detection of geriatric syndromes in family medicine: a prospective diagnostic study. BMC Geriatr. (2018) 18:72. doi: 10.1186/s12877-018-0761-z
35. Byrska-Bishop, M, Evani, US, Zhao, X, Basile, AO, Abel, HJ, Regier, AA, et al. High-coverage whole-genome sequencing of the expanded 1000 genomes project cohort including 602 trios. Cell. (2022) 185:3426–40. doi: 10.1016/j.cell.2022.08.004
36. Pierce, BL, Ahsan, H, and Vanderweele, TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
37. Hemani, G, Zheng, J, Elsworth, B, Wade, KH, Haberland, V, Baird, D, et al. The MR-base platform supports systematic causal inference across the human phenome. eLife. (2018) 7:34408. doi: 10.7554/eLife.34408
38. Bowden, J, Del Greco, MF, Minelli, C, Davey Smith, G, Sheehan, N, and Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. (2017) 36:1783–802. doi: 10.1002/sim.7221
39. Hartwig, FP, Davey Smith, G, and Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
40. Greco, MF, Minelli, C, Sheehan, NA, and Thompson, JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. (2015) 34:2926–40. doi: 10.1002/sim.6522
41. Burgess, S, and Thompson, SG. Interpreting findings from Mendelian randomization using the MR-egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
42. Nolte, IM . Metasubtract: an R-package to analytically produce leave-one-out meta-analysis GWAS summary statistics. Bioinformatics. (2020) 36:4521–2. doi: 10.1093/bioinformatics/btaa570
43. Rasooly, D, and Peloso, GM. Two-sample multivariable Mendelian randomization analysis using R. Curr Protocols. (2021) 1:e335. doi: 10.1002/cpz1.335
44. Trombetti, A, Hars, M, Hsu, FC, Reid, KF, Church, TS, Gill, TM, et al. Effect of physical activity on frailty: secondary analysis of a randomized controlled trial. Ann Intern Med. (2018) 168:309–16. doi: 10.7326/M16-2011
45. Lee, AH, and Hirayama, F. Physical activity and urinary incontinence in older adults: a community-based study. Curr Aging Sci. (2012) 5:35–40. doi: 10.2174/1874609811205010035
46. Kuo, PL, Di, J, Ferrucci, L, and Lin, FR. Analysis of hearing loss and physical activity among US adults aged 60-69 years. JAMA Netw Open. (2021) 4:e215484. doi: 10.1001/jamanetworkopen.2021.5484
47. Gleeson, M, Sherrington, C, and Keay, L. Exercise and physical training improve physical function in older adults with visual impairments but their effect on falls is unclear: a systematic review. J Physiother. (2014) 60:130–5. doi: 10.1016/j.jphys.2014.06.010
48. Didelez, V, and Sheehan, N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. (2007) 16:309–30. doi: 10.1177/0962280206077743
49. Freidin, MB, Stalteri, MA, Wells, PM, Lachance, G, Baleanu, AF, Bowyer, RCE, et al. An association between chronic widespread pain and the gut microbiome. Rheumatology. (2021) 60:3727–37. doi: 10.1093/rheumatology/keaa847
50. Elliott, S, Painter, J, and Hudson, S. Living alone and fall risk factors in community-dwelling middle age and older adults. J Community Health. (2009) 34:301–10. doi: 10.1007/s10900-009-9152-x
51. Shapiro, A, and Melzer, I. Balance perturbation system to improve balance compensatory responses during walking in old persons. J Neuroeng Rehabil. (2010) 7:32. doi: 10.1186/1743-0003-7-32
52. Taylor, D . Physical activity is medicine for older adults. Postgrad Med J. (2014) 90:26–32. doi: 10.1136/postgradmedj-2012-131366
53. Taylor, JA, Greenhaff, PL, Bartlett, DB, Jackson, TA, Duggal, NA, and Lord, JM. Multisystem physiological perspective of human frailty and its modulation by physical activity. Physiol Rev. (2023) 103:1137–91. doi: 10.1152/physrev.00037.2021
54. Nùñez-Lisboa, M, Valero-Breton, M, and Dewolf, AH. Unraveling age-related impairment of the neuromuscular system: exploring biomechanical and neurophysiological perspectives. Front Physiol. (2023) 14:1194889. doi: 10.3389/fphys.2023.1194889
55. Hunter, SK, Pereira, HM, and Keenan, KG. The aging neuromuscular system and motor performance. J Appl Physiol. (2016) 121:982–95. doi: 10.1152/japplphysiol.00475.2016
56. Vazquez-Guajardo, M, Rivas, D, and Duque, G. Exercise as a therapeutic tool in age-related frailty and cardiovascular disease: challenges and strategies. Can J Cardiol. (2024). doi: 10.1016/j.cjca.2024.01.005
57. Dao, E, Hsiung, GR, and Liu-Ambrose, T. The role of exercise in mitigating subcortical ischemic vascular cognitive impairment. J Neurochem. (2018) 144:582–94. doi: 10.1111/jnc.14153
58. Hopkins, RO, Suchyta, MR, Farrer, TJ, and Needham, D. Improving post-intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. (2012) 186:1220–8. doi: 10.1164/rccm.201206-1022CP
59. Choi, JW, Jo, SW, Kim, DE, Paik, IY, and Balakrishnan, R. Aerobic exercise attenuates LPS-induced cognitive dysfunction by reducing oxidative stress, glial activation, and neuroinflammation. Redox Biol. (2024) 71:103101. doi: 10.1016/j.redox.2024.103101
60. Farrukh, S, Habib, S, Rafaqat, A, Sarfraz, A, Sarfraz, Z, and Tariq, H. Association of exercise, brain-derived neurotrophic factor, and cognition among older women: a systematic review and meta-analysis. Arch Gerontol Geriatr. (2023) 114:105068. doi: 10.1016/j.archger.2023.105068
61. Zhou, Y, Yuan, Y, Wang, X, Qi, K, Zhang, S, Zhang, Y, et al. Sedentary behavior and physical frailty among rural older adults in China: the moderating effect of social isolation. J Am Med Dir Assoc. (2023) 25:500–5. doi: 10.1016/j.jamda.2023.08.020
62. Santos, ISD, Silva, CFR, Ohara, DG, Matos, AP, Pinto, A, and Pegorari, MS. Association between frailty syndrome and sedentary behavior among community-dwelling older adults in the Amazon region: a cross-sectional study. Med J. (2021) 139:226–33. doi: 10.1590/1516-3180.2020.0546.r1.14122020
63. Copeland, JL, Ashe, MC, Biddle, SJ, Brown, WJ, Buman, MP, Chastin, S, et al. Sedentary time in older adults: a critical review of measurement, associations with health, and interventions. Br J Sports Med. (2017) 51:1539. doi: 10.1136/bjsports-2016-097210
64. Gallibois, M, Handrigan, G, Caissie, L, Cooling, K, Hébert, J, Jarrett, P, et al. The effect of a standing intervention on falls in long term care: a secondary analysis of a randomized controlled trial. Can Geriatrics J. (2023) 26:247–52. doi: 10.5770/cgj.26.656
65. Jiang, Y, Wang, M, Liu, S, Ya, X, Duan, G, and Wang, Z. The association between sedentary behavior and falls in older adults: a systematic review and meta-analysis. Front Public Health. (2022) 10:1019551. doi: 10.3389/fpubh.2022.1019551
66. Yang, CW, Li, CI, Li, TC, Liu, CS, Lin, CH, Lin, WY, et al. The joint association of insulin sensitivity and physical activity on the skeletal muscle mass and performance in community-dwelling older adults. Exp Gerontol. (2017) 95:34–8. doi: 10.1016/j.exger.2017.05.006
67. Zusman, EZ, Dawes, M, Fleig, L, McAllister, MM, Cook, WL, Guy, P, et al. Older Adults' sedentary behavior and physical activity after hip fracture: results from an outpatient rehabilitation randomized controlled trial. J Geriatr Phys Ther. (2001) 42:E32–8. doi: 10.1519/JPT.0000000000000193
68. da Silva, VD, Tribess, S, Meneguci, J, Sasaki, JE, Garcia-Meneguci, CA, Carneiro, JAO, et al. Association between frailty and the combination of physical activity level and sedentary behavior in older adults. BMC Public Health. (2019) 19:709. doi: 10.1186/s12889-019-7062-0
69. Pinto, AJ, Bergouignan, A, Dempsey, PC, Roschel, H, Owen, N, Gualano, B, et al. Physiology of sedentary behavior. Physiol Rev. (2023) 103:2561–622. doi: 10.1152/physrev.00022.2022
70. Panebianco, M, Marchese-Ragona, R, Masiero, S, and Restivo, DA. Dysphagia in neurological diseases: a literature review. Neurol Sci Off J Italian Neurol Soc Clin Neurophysiol. (2020) 41:3067–73. doi: 10.1007/s10072-020-04495-2
71. Huang, TY, Chou, MY, Liang, CK, Lin, YT, Chen, RY, and Wu, PF. Physical activity plays a crucial role in multidomain intervention for frailty prevention. Aging Clin Exp Res. (2023) 35:1283–92. doi: 10.1007/s40520-023-02412-z
72. Dowd, KP, Szeklicki, R, Minetto, MA, Murphy, MH, Polito, A, Ghigo, E, et al. A systematic literature review of reviews on techniques for physical activity measurement in adults: a DEDIPAC study. The Int J Behav Nutr Phys Activity. (2018) 15:15. doi: 10.1186/s12966-017-0636-2
73. Hu, S, Xing, H, Wang, X, Zhang, N, and Xu, Q. Causal relationships between Total physical activity and ankylosing spondylitis: a Mendelian randomization study. Front Immunol. (2022) 13:887326. doi: 10.3389/fimmu.2022.887326
74. Chen, X, Hong, X, Gao, W, Luo, S, Cai, J, Liu, G, et al. Causal relationship between physical activity, leisure sedentary behaviors and COVID-19 risk: a Mendelian randomization study. J Transl Med. (2022) 20:216. doi: 10.1186/s12967-022-03407-6
Keywords: accelerometer-based, physical activity, sedentary behavior, geriatric syndromes, mendelian randomization
Citation: Chen J, Lu Y, Yao J, Zhang X and Pan Y (2024) The relationship between accelerometer-based physical activity, sedentary behavior, and seven common geriatric syndromes: a two-sample Mendelian randomization study. Front. Public Health. 12:1406303. doi: 10.3389/fpubh.2024.1406303
Edited by:
Yun Gao, Sichuan University, ChinaReviewed by:
Dongdong Qin, Yunnan University of Chinese Medicine, ChinaTeresa Dianne Hawkes, Applied Research Solutions, United States
Copyright © 2024 Chen, Lu, Yao, Zhang and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Pan, cGFueWFuZ0BzZHUuZWR1LmNu
 Jiping Chen
Jiping Chen Yanyu Lu
Yanyu Lu JiaWei Yao2
JiaWei Yao2 Xianliang Zhang
Xianliang Zhang Yang Pan
Yang Pan