
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Public Health , 25 October 2024
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1378701
 Kacy D. Nowak1
Kacy D. Nowak1 Morgan A. Lane2
Morgan A. Lane2 Armand Mbanya3
Armand Mbanya3 Jasmine R. Carter1
Jasmine R. Carter1 Brianna A. Binion3,4
Brianna A. Binion3,4 Daniel O. Espinoza2
Daniel O. Espinoza2 Matthew H. Collins2
Matthew H. Collins2 Christopher D. Heaney5,6,7
Christopher D. Heaney5,6,7 Nora Pisanic5
Nora Pisanic5 Kate Kruczynski5
Kate Kruczynski5 Kristoffer Spicer5
Kristoffer Spicer5 Magdielis Gregory Rivera5
Magdielis Gregory Rivera5 Felicia Glover8
Felicia Glover8 Tolulope Ojo-Akosile1
Tolulope Ojo-Akosile1 Robert F. Breiman1,2
Robert F. Breiman1,2 Evan J. Anderson2,8,9†
Evan J. Anderson2,8,9† Felipe Lobelo1,3
Felipe Lobelo1,3 Jessica K. Fairley1,2*
Jessica K. Fairley1,2*Background: A wide range of household secondary infection rates has been reported, and the role of children in population transmission dynamics for SARS-CoV-2 remains ill-defined. We sought to better understand household infection early in the pandemic.
Methodology: A cross-sectional study of 17 households in the Atlanta metropolitan area with at least one child and one case of COVID-19 in the prior 1–4 months were recruited between December 2020 and April 2021. Self-collected saliva samples were tested on a multiplexed platform to detect IgG antibodies that bind to SARS-CoV-2 antigens. Secondary infection rates (SIR) were calculated and compared.
Results: We report results on 17 families, including 66 individuals. We found an average SIR of 0.58; children and adults were similarly infected (62% children vs. 75% adults) (p = 0.2). Two out of 17 households had a pediatric index per our definition. Number of pediatric infections per household (p = 0.18), isolation (p = 0.34), and mask wearing (p = 0.80) did not differ significantly among households with an SIR above the mean vs. those with SIR below the mean. Households with higher SIR also had a higher number of symptomatic cases (p < 0.001).
Discussion: We demonstrated high household SIRs at the early stages of the pandemic in late 2020 to early 2021 with similar impact on children and adults. The ease of collecting saliva and the detection of asymptomatic infections highlight the advantages of this strategy and potential for scale-up.
Elucidating the role children and adolescents play in the transmission of SARS-CoV-2 as well as the impact of household transmission on them has been a challenge due to under testing, school closures at the start of the pandemic, and a high estimated proportion of asymptomatic pediatric infections (16–50%) (1). However, as the pandemic continued, pediatric cases rose due to evolving viral genetics and relaxation of non-pharmacological interventions. The American Academy of Pediatrics (AAP) reported that as of January 2022, almost 8.5 million (17.4% of total cases) pediatric cases of SARS-CoV-2 infection were diagnosed in the United States (U.S) (2). Hospitalization and severe COVID-19 infection rates among children rose as the Delta variant surged, and increased further with the emergence of the Omicron variant. Although pediatric SARS-CoV-2 hospitalization rates have been lower compared to older age groups, pediatric hospitalization rates went up significantly with the Omicron wave due to the sheer number of infections (3).
SARS-CoV-2 household transmission studies have ranged in methodology and outcomes. At the beginning of the pandemic, studies showed relatively low secondary infection rates (6–51%), but these were predominantly in Asia where index cases were often isolated from the rest of the household (4). As Europe and North America began to study household transmission, SIR were found to be consistently higher than those initial estimates, with an SIR up to 70% found in minority households in North Carolina (5–13). At the same time, while the likelihood of transmission from pediatric case was debated and found to have mixed results, several studies concluded that children were not primary drivers of transmission and that schools were safe to reopen (14–16). However, SIR of pediatric vs. adult index cases was found to be similar in situations where the index case could be identified (10–12, 17). And while a more recent meta-analysis published in 2022 analyzing 95 articles (48 studies included in the meta-analysis) demonstrated a pooled household SIR from pediatric index cases lower than that of adult index cases [0.20 (95% CI 0.15–0.26) vs. 0.64 (95% CI 0.64–0.85)], this difference did not hold up with more recent SARS-CoV-2 viral variants (18).
However, the majority of household transmission studies involved either retrospective identification of symptomatic cases or time-intensive longitudinal studies during the acute symptomatic infection phase (5, 19, 20). Both approaches likely missed a substantial proportion of infections and therefore biased secondary infection rates (SIR) estimates. Use of serology can improve asymptomatic case detection with select studies demonstrating SIR in children ranging from 7.6 to 48% when serology was used (7, 9, 21).
In Georgia, USA, data on household transmission, especially in households with children, have been limited. Previous studies have provided some insight but often lacked comprehensive testing strategies, such as serological testing, to accurately determine secondary infection rates (SIR). For instance, a survey of households with pediatric index cases reported an average SIR of 45.7%, which is likely an underestimate due to the reliance on symptomatic testing alone (22). Give these gaps, our cross-sectional study aimed to investigate secondary infection rates SARS-CoV-2 infection during the early stages of the pandemic in households with children using a saliva-based IgG test and compare SIRs according to individual and household-level characteristics. We hypothesized that children in households would be infected with SARS-CoV-2 at similar rates as the adult members and that index cases would not be solely confined to adults. Our findings have the potential to inform targeted public health strategies and interventions, especially in managing and preventing the spread of COVID-19 within households. By understanding that children can be infected at similar rates as adults and recognizing that index cases are not confined to adults, we can better design measures to protect all household members and curb the spread of the virus more effectively. This study contributes valuable data to the ongoing efforts to understand and combat COVID-19, particularly in family and household settings where the risk of transmission is high.
For this cross-sectional study, households were recruited from December 2020 to April 2021 and were identified through adult individuals who tested positive for SARS-CoV-2 infection at outpatient screening clinics at Emory Healthcare and through study advertisements on social media and university listservs. Households were eligible to participate if at least one member was under the age of 18 years and if one or more individuals tested positive for SARS-CoV-2 within 4–16 weeks prior to the study visit (dates of infection were late September 2020 through February 2021). This study was performed in a 1-4-month window to limit the likelihood that a subsequent SARS-CoV-2 infection, in the interim. All household members needed to agree to participate for the household to be eligible. Reported information about the index case such as timing of SARS-CoV-2 testing was collected retrospectively. At the time of this study, repeated infection was very rare. However, we did exclude any household members who reported a previous known infection.
Individuals then self-collected saliva samples using an Oracol™ device by rubbing a sponge in the gingival space on both sides of the participant’s mouth for 1 minute. Saliva samples were processed and then tested on a Luminex platform for IgG antibodies that bind to the SARS-CoV-2 nucleocapsid (N), receptor binding domain (RBD), and spike (S) proteins as previously described (23). A sample was defined as SARS-CoV-2 IgG positive if reactive to the GenScript N antigen and either the RBD or S Spike proteins as previously determined during validation of the assay (23). Total IgG was measured in samples testing negative for SARS-CoV-2 IgG to exclude false negatives due to insufficient sample quality. Samples without meeting a validated threshold of total IgG were classified as indeterminate. Since this study was conducted earlier in the pandemic, repeated infections were less common, and most individuals had not been infected more than once yet due to remote schooling, social distancing, and masking efforts. Therefore, detected antibodies were unlikely due to previous infections occurring before the study time period, and were thus considered as an infection occurring during the household transmission period. Individual and household surveys were completed electronically in RedCap by individuals after the study visit (24) and included individual demographics, symptoms, pre-existing medical conditions, exposures, and a household survey describing infection timelines and isolation procedures in the household.
A case was defined as a household member with a reported positive molecular test for SARS-CoV-2 result, a reported positive SARS-CoV-2 antigen test, or a positive SARS-CoV-2 salivary antibody test. A household index case was defined as the case who received the first confirmed SARS-CoV-2 test result or had the earliest symptom onset date among the household cluster. A household contact was defined as anyone living in the same residence as the index case at the time of infection. The outcome variable assessed was the secondary infection rate (SIR) which was defined as the proportion of secondary cases in a household infected from the index case over the total number of household contacts. The SIR was then dichotomized in order to compare households with an SIR below the study sample mean and households above the mean in order to identify factors association with a higher SIR.
Statistical analysis was performed using a t-test or Fisher’s Exact test as appropriate to explore differences between household SIR and household characteristics during the time of infection. Independent variables included whether the index case was an adult (≥18 years old) or child, number of individuals in the household, whether the index case was symptomatic, and whether bedrooms and/or bathrooms were shared in the household at the time of infection. While this was an exploratory pilot study, to assess the adequacy of the sample size, we estimated that SIR in households with adult index cases would be 4 times more likely to have a high SIR compared to households with pediatric index cases. If adult index cases were twice as common as pediatric, and assuming a power of 0.80, and 95% significance level, a sample size of 24 households would be ideal. All statistical analyses were performed using Rstudio 1.3.1073.
Seventeen households were enrolled with a total of 66 individuals, 34 (52%) of which were under 18 years old. The median age was 15.5 (IQR = 32.3), with 30 (46%) males and 36 (55%) females. Fifty-five of the 66 individuals (83%) reported testing at the time of acute infection, of which 35 (64%) were positive for SARS-CoV-2 molecular or antigen testing.
From the 66 saliva samples tested, 42 (64%) were positive for SARS-CoV-2 IgG antibody, 20 (30%) negative, and 4 (6%) were indeterminate due to low total IgG in the saliva sample. These 4 indeterminates were children who were either negative for SARS-CoV-2 PCR testing or did not get tested, therefore, we excluded them from the analysis given the uncertainty of their infection status. Six individuals with a negative SARS-CoV-2 PCR test were positive for SARS-CoV-2 IgG as defined in the methods. Among cases who reported a positive SARS-CoV-2 molecular or antigen test, four individuals (11.4%) received negative antibody test results. Furthermore, we were able to use antibody test results to assign infection status to nine individuals who did not receive SARS-CoV-2 testing at the time of household infection (five had positive antibodies and four had negative antibodies). Including self-reported SARS-CoV-2 test results (antigen or PCR) and salivary antibody test results, 46 individuals (70%) were defined as having had SARS-CoV-2 infection. The average household positivity of SARS-CoV-2 infections for the 17 families was 0.71 (SD = 0.3), meaning on average, 71% of household members were infected during the household episode. The average SIR was 0.58 (SD = 0.4) and the median SIR was 0.67 (range 0–1.0). Twenty-one children were infected (62% of children) versus 25 adults (78% of adults) (p = 0.19). All positive SARS-CoV2 cases known at the time of infection had either a COVID test or onset symptoms within 8 days of the household index case.
Among those who were infected, the average symptomatic case frequency in each household was 0.66 (SD = 0.4). Six children were asymptomatic (29% of infected children) and four adults were asymptomatic (16% of infected adults). The median age of the 49 household contacts was 10 (IQR = 29) years and for the 17 index cases was 40 (IQR = 13.0) years with nine being female. Fifteen (88%) index cases were symptomatic and four (24%) reported a comorbid chronic medical condition.
Children under 18 years made up 52% of the study sample but only two (12%) of the index cases. The ages of the child index cases were three and 15. The household SIR for the child index cases were 1.0 and 0.43 (average = 0.71). Both child index cases attended either in-person daycare or school (one of which had a known school exposure). There were more symptomatic cases in the high SIR group vs. low (Table 1), although not controlled for household size (on average 3 vs. 1, p < 0.001). There was no significant difference in isolation and masking behaviors between the low and high SIR groups (Table 1). No participants were fully vaccinated at the time of the household infection, although three adults were within 1 week after the first dose of an mRNA vaccine when the household infection occurred.
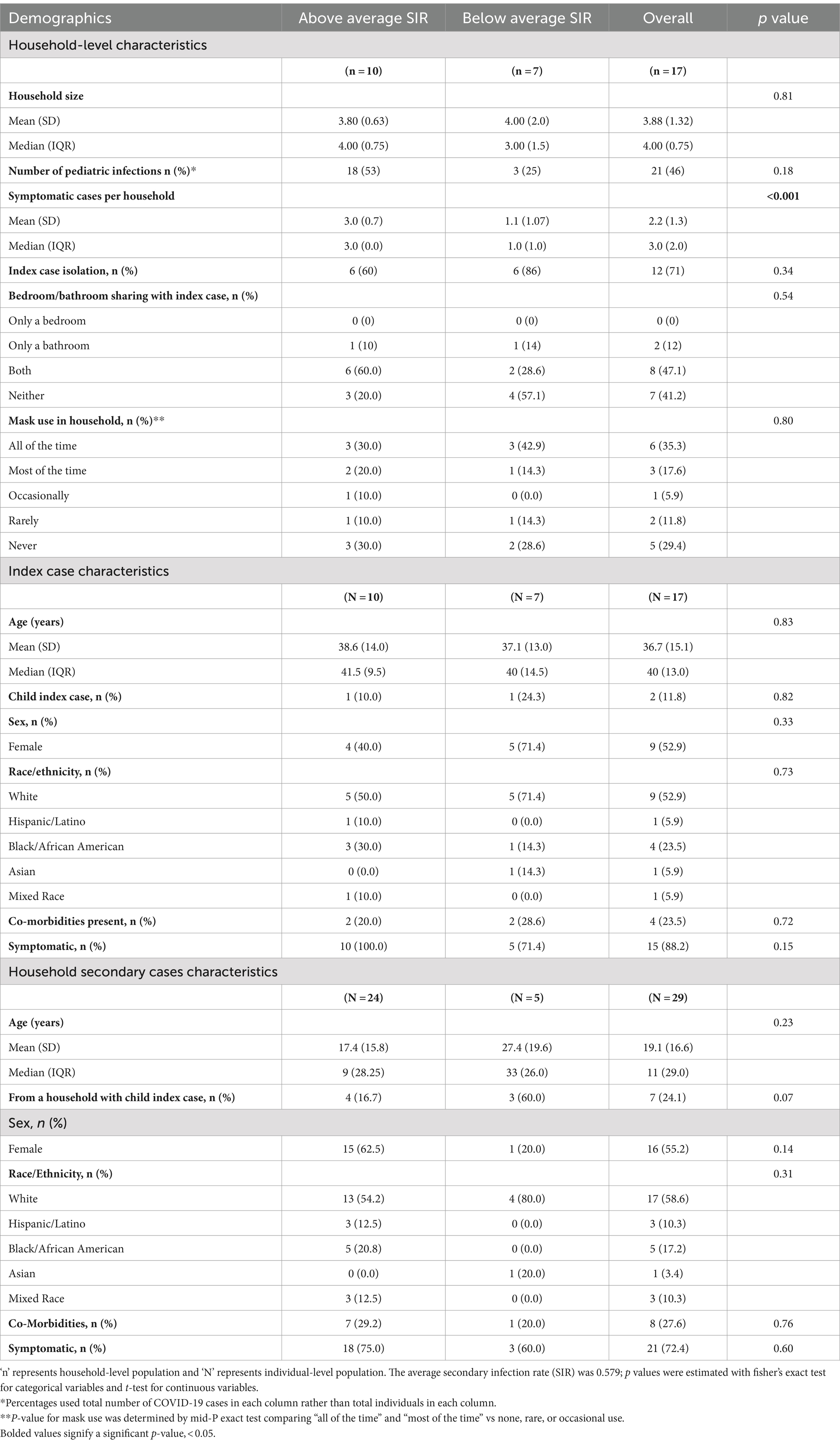
Table 1. Individual and household demographics stratified by average secondary infection rate (SIR).
We found an overall high secondary household infection rate of 58%, with 62% of children and 78% of adults infected with SARS-CoV-2. While small in scope, observing children who are both becoming infected and transmitting SARS-CoV-2 (in the case of likely index cases) to multiple household members is notable and aligns with higher estimates of SIR in the literature (9, 14). As observed with other respiratory viruses, our data also highlight the potential risk for children initiating transmission clusters among their close relatives, including those that may be a higher risk for adverse COVID-19 outcomes due to age and other medical comorbidities. Since repeated infections were not common at the time of the study, we assume that most of the “asymptomatic antibody positive” infections happened during the household infection. Not only did early studies have lower SIR (6–51%) than what we found, they also concluded that pediatric index cases were associated with lower household transmission (9, 14, 16, 25, 26). These studies that identified lower fewer pediatric than adult index cases were likely impacted by school closures and limited by methodologic factors such as under testing of asymptomatic cases. Additionally, many studies did not enroll the entire household which may have resulted in underestimation of SIR (5, 22, 27). While we had many more households with adult index cases, the SIR did not differ by adult vs. pediatric index case nor were children less likely to be infected compared to adults.
Our SIR was more in line with European and North American studies early in the pandemic where SIR were found to be to be consistently higher than those initial estimates, like a study that found an SIR of 70% found in in North Carolina (5–13).
Interestingly, household-level isolation measures did not differ between high or low SIR, but these data were limited by an overall small number of households. This was not consistent with other studies, many of which showed statistically significant associations between NPI usage (isolation, mask usage) and lower secondary transmission in households (9, 28–30). It is possible that in some of our cases, household transmission had already occurred before infection status was known, and therefore, before household mitigation activities could have prevented spread. A larger sample size may be needed to differentiate the potential effect of mitigation on the likelihood of household transmission. As no participants were fully vaccinated during the time of the household infection, vaccination did not impact household transmission. Furthermore, since the IgG positivity was dependent on the N antigen (not a target of the available vaccines in the U.S) it did not matter if individuals were vaccinated between the household infection and the study visit. While this analysis was pre-Delta and pre-Omicron, we would expect even higher secondary infection rates with Delta and Omicron than we found in this study, albeit possibly mitigated in part due to more widespread vaccination.
Our study had 10 asymptomatic cases, two of which were definite index cases. Both index cases were the sole case in their household which allowed for a confident assignment of these index cases. Other studies have also found asymptomatic cases when antibody tests are used (31). Households in our study with higher SIR also had more symptomatic cases (3 vs. 1, p = 0.001), This could suggest the presence of more transmissible virus in the household even if the index case being symptomatic did not differ between high and low SIR (p = 0.15). Prior studies have been mixed to whether symptomatic index cases were associated with higher SIR (26, 32). A larger sample size may have better defined both of these factors. Other index case characteristics aside from age and symptom status, like race and co-morbidity also did not differ among households with high and low SIR.
Lastly, the findings of this study demonstrate the challenge of precisely defining the role children play in the transmission of SARS-CoV-2, because if children are asymptomatic and either do not get tested or test negative but are truly a case, many pediatric cases go unreported and onward transmission unrecognized. We recognize that household transmission dynamics became more complicated as the pandemic progressed beyond 2021 with repeat infections, vaccinations, and more transmissible variants. That being said, this project benefited from the lack of these complicating factors and is informative in situations and locales with low vaccination rates, low prior infections, and potential future variants with high degrees of immune evasion.
Overall, this study demonstrated a high household SIRs in late 2020 / early 2021 in Atlanta, GA, where proportions of children and adults acquiring infection in the household were similar. In addition, pediatric index cases resulted in multiple subsequent household infections. Recruiting entire households and collecting saliva swabs for antibody tests on all members (infants and up) was convenient and feasible as it did not require invasive phlebotomy or a skilled research nurse. The ease of sample collection was a major outcome of the study and demonstrates the scalability and acceptability of this epidemiologic study design. The multiplex assay also has the advantage of differentiating between natural and vaccinated individuals, which is important in studying transmission in the setting of vaccination.
We also overcame methodologic limitations of previous studies, which were unable to account for asymptomatic infections given challenges of drawing blood in children, by employing a multiplexed saliva-based antibody assay that highly correlates with serum antibodies (15). In addition, the sensitivity and specificity of the assay (98 and 99%, respectively, with the N antigens used in this study) as well as validation tests showing minimal concern about cross-reactivity with common human coronaviruses, also support the use of the assay (23). This scalable, minimally invasive tool can complement household transmission studies and provide a more acceptable alternative to venipuncture in young children, with the ultimate goal to better inform public health policies especially those involving children, school, and vulnerable populations. While some of the benefits of an antibody tool are lost with repeat infections in the same individual, refinement of the antigen–antibody combinations, like the use of IgM or antigens that differ across variants, could maintain utility of this multiplexed approach. Determining the frequency of asymptomatic infections, especially as variants emerge, is important as there are still populations, such as older adults and immunocompromised, who can have poor outcomes from SARS-CoV-2 infection.
Our study had several limitations. Primarily, the results are descriptive, the study design is cross-sectional, and we acknowledge the lack of inferential statistical analyses to identify the relationships between dependent and independent variables. This limitation is partly due to the constraints of our sample size, which restricts the extent of statistical analysis we can perform. Despite these limitations, we have conducted univariate comparisons to the best of our ability given the sample. This study focused on describing the early days of the pandemic in households with COVID-19 in Georgia, USA, during Fall 2020 to Winter 2021. Ethical approvals restricted us from collecting data from screened individuals who did not agree to participate, hindering our understanding of potential selection biases and affecting the generalizability of our findings. The different recruitment methods may have introduced biases that are difficult to quantify and account for in our analysis. Lastly, our sample size being shy of the calculated ideal of 24 households, misclassification of the index case, and the possibility that a family member had been previously infected at an earlier point in the pandemic may have impacted our results. An index case could have also been asymptomatic and not discernible in our study since antibody tests cannot determine temporality.
The SARS-CoV-2 pandemic is not over and other viral pandemic are predicted in the near future. Having demonstrated the feasibility of studying household SARS-CoV-2 infections with this non-invasive assay, it can also be used as a model for studying the next highly contagious virus like SARS-CoV-2. To improve the robustness and accuracy of our results, future studies should aim to maximize the sample size and incorporate more comprehensive inferential statistical methods. We also recommend longitudinal studies for documenting causality.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Emory University Institutional Review Board, Kaiser Permanente Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participant or the participants’ legal guardians/next of kin.
KN: Conceptualization, Writing – original draft, Writing – review & editing, Formal analysis. ML: Project administration, Writing – review & editing, Data curation, Investigation. AM: Data curation, Investigation, Methodology, Writing – review & editing. JC: Data curation, Writing – review & editing. BB: Data curation, Investigation, Writing – review & editing. DE: Data curation, Investigation, Writing – review & editing. MC: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – review & editing. CH: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – review & editing. NP: Data curation, Investigation, Methodology, Writing – review & editing. KK: Data curation, Validation, Writing – review & editing. KS: Data curation, Writing – review & editing. MR: Data curation, Writing – review & editing. FG: Data curation, Writing – review & editing. TO-A: Data curation, Writing – review & editing. RB: Conceptualization, Investigation, Supervision, Writing – review & editing. EA: Conceptualization, Investigation, Supervision, Writing – review & editing. FL: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing. JF: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the SOM I3 /WHSC Synergy/Kaiser Permanente Georgia COVID-19 Collaboration; Johns Hopkins COVID-19 Research and Response Program award (CDH); FIA Foundation (CDH); NIAID grant R21AI139784 (CDH, MHC, and NP) and UL1 TR000424.
We would like to thank Kathy Stephens and Laila Hussaini and others at the Emory Children’s Center for their support of this study. In addition, we appreciate the assistance of Jennifer Breiman at Emory for her assistance with the study. We would also like to express our gratitude to all the families that dedicated their time to the study.
EJA has consulted for Pfizer, Sanofi Pasteur, Janssen, and Medscape, and his institution receives funds to conduct clinical research unrelated to this manuscript from MedImmune, Regeneron, PaxVax, Pfizer, GSK, Merck, Sanofi-Pasteur, Janssen, and Micron. He also serves on a safety monitoring board for Kentucky BioProcessing, Inc. and Sanofi Pasteur. His institution has also received funding from NIH to conduct clinical trials of Moderna and Janssen COVID-19 vaccines.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Almadhi, MA, Abdulrahman, A, Sharaf, SA, AlSaad, D, Stevenson, NJ, Atkin, SL, et al. The high prevalence of asymptomatic SARS-CoV-2 infection reveals the silent spread of COVID-19. Int J Infect Dis. (2021) 105:656–61. doi: 10.1016/j.ijid.2021.02.100
2. Pediatrics AAP. Children and COVID-19: state-level data report 2022. Report No. American Academy of Pediatrics and Children’s Hospital Association. (2022).
3. CfDCaP. C-N. Laboratory-confirmed COVID-19 associated hospitalizations. (2022) Available at: https://gis.cdc.gov/grasp/COVIDNet/COVID19_3.html.
4. Thompson, HA, Mousa, A, Dighe, A, Fu, H, Arnedo-Pena, A, Barrett, P, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) setting-specific transmission rates: a systematic review and Meta-analysis. Clin Infect Dis. (2021) 73:e754–64. doi: 10.1093/cid/ciab100
5. Wang, Z, Ma, W, Zheng, X, Wu, G, and Zhang, R. Household transmission of SARS-CoV-2. J Infect. (2020) 81:179–82. doi: 10.1016/j.jinf.2020.03.040
6. Jiang, XL, Zhang, XL, Zhao, XN, Li, CB, Lei, J, Kou, ZQ, et al. Transmission potential of asymptomatic and Paucisymptomatic severe acute respiratory syndrome coronavirus 2 infections: a 3-family cluster study in China. J Infect Dis. (2020) 221:1948–52. doi: 10.1093/infdis/jiaa206
7. Kuwelker, K, Zhou, F, Blomberg, B, Lartey, S, Brokstad, KA, Trieu, MC, et al. Attack rates amongst household members of outpatients with confirmed COVID-19 in Bergen, Norway: a case-ascertained study. Lancet Reg Health Eur. (2021) 3:100014. doi: 10.1016/j.lanepe.2020.100014
8. Reukers, DFM, van Boven, M, Meijer, A, Rots, N, Reusken, C, Roof, I, et al. High infection secondary attack rates of severe acute respiratory syndrome coronavirus 2 in Dutch households revealed by dense sampling. Clin Infect Dis. (2022) 74:52–8. doi: 10.1093/cid/ciab237
9. Galow, L, Haag, L, Kahre, E, Blankenburg, J, Dalpke, AH, Luck, C, et al. Lower household transmission rates of SARS-CoV-2 from children compared to adults. J Infect. (2021) 83:e34–6. doi: 10.1016/j.jinf.2021.04.022
10. Lewis, NM, Chu, VT, Ye, D, Conners, EE, Gharpure, R, Laws, RL, et al. Household transmission of severe acute respiratory syndrome Coronavirus-2 in the United States. Clin Infect Dis. (2021) 73:1805–e1813. doi: 10.1093/cid/ciaa1166
11. Laws, RL, Chancey, RJ, Rabold, EM, Chu, VT, Lewis, NM, Fajans, M, et al. Symptoms and transmission of SARS-CoV-2 among children — Utah and Wisconsin, march–may 2020. Pediatrics. (2021) 147:e2020027268. doi: 10.1542/peds.2020-027268
12. Burke, RM, Calderwood, L, Killerby, ME, Ashworth, CE, Berns, AL, Brennan, S, et al. Patterns of virus exposure and presumed household transmission among persons with coronavirus disease, United States, January-April 2020. Emerg Infect Dis. (2021) 27:2323–32. doi: 10.3201/eid2709.204577
13. Cerami, C, Popkin-Hall, ZR, Rapp, T, Tompkins, K, Zhang, H, Muller, MS, et al. Household transmission of severe acute respiratory syndrome coronavirus 2 in the United States: living density, viral load, and disproportionate impact on communities of color. Clin Infect Dis. (2022) 74:1776–85. doi: 10.1093/cid/ciab701
14. Shapiro Ben David, S, Rahamim-Cohen, D, Tasher, D, Geva, A, Azuri, J, and Ash, N. COVID-19 in children and the effect of schools reopening on potential transmission to household members. Acta Paediatr. (2021) 110:2567–73. doi: 10.1111/apa.15962
15. Ludvigsson, JF. Children are unlikely to be the main drivers of the COVID-19 pandemic - a systematic review. Acta Paediatr. (2020) 109:1525–30. doi: 10.1111/apa.15371
16. Kim, J, Choe, YJ, Lee, J, Park, YJ, Park, O, Han, MS, et al. Role of children in household transmission of COVID-19. Arch Dis Child. (2021) 106:709–11. doi: 10.1136/archdischild-2020-319910
17. Spielberger, BD, Goerne, T, Geweniger, A, Henneke, P, and Elling, R. Intra-household and close-contact SARS-CoV-2 transmission among children - a systematic review. Front Pediatr. (2021) 9:613292. doi: 10.3389/fped.2021.613292
18. Chen, F, Tian, Y, Zhang, L, and Shi, Y. The role of children in household transmission of COVID-19: a systematic review and Meta-analysis. Int J Infect Dis. (2022) 122:266–75. doi: 10.1016/j.ijid.2022.05.016
19. Shah, K, Saxena, D, and Mavalankar, D. Secondary attack rate of COVID-19 in household contacts: a systematic review. QJM. (2020) 113:841–50. doi: 10.1093/qjmed/hcaa232
20. Jing, QL, Liu, MJ, Zhang, ZB, Fang, LQ, Yuan, J, Zhang, AR, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. (2020) 20:1141–50. doi: 10.1016/S1473-3099(20)30471-0
21. Bi, Q, Lessler, J, Eckerle, I, Lauer, SA, Kaiser, L, Vuilleumier, N, et al. Insights into household transmission of SARS-CoV-2 from a population-based serological survey. Nat Commun. (2021) 12:3643. doi: 10.1038/s41467-021-23733-5
22. Teherani, MF, Kao, CM, Camacho-Gonzalez, A, Banskota, S, Shane, AL, Linam, WM, et al. Burden of illness in households with severe acute respiratory syndrome coronavirus 2-infected children. J Pediatric Infect Dis Soc. (2020) 9:613–6. doi: 10.1093/jpids/piaa097
23. Heaney, CD, Pisanic, N, Randad, PR, Kruczynski, K, Howard, T, Zhu, X, et al. Comparative performance of multiplex salivary and commercially available serologic assays to detect SARS-CoV-2 IgG and neutralization titers. J Clin Virol. (2021) 145:104997. doi: 10.1016/j.jcv.2021.104997
24. Harris, PA, Taylor, R, Thielke, R, Payne, J, Gonzalez, N, and Conde, JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
25. Li, W, Zhang, B, Lu, J, Liu, S, Chang, Z, Peng, C, et al. Characteristics of household transmission of COVID-19. Clin Infect Dis. (2020) 71:1943–6. doi: 10.1093/cid/ciaa450
26. Verberk, JDM, de Hoog, MLA, Westerhof, I, van Goethem, S, Lammens, C, Ieven, G, et al. Transmission of SARS-CoV-2 within households: a remote prospective cohort study in European countries. Eur J Epidemiol. (2022) 37:549–61. doi: 10.1007/s10654-022-00870-9
27. Pitman-Hunt, C, Leja, J, Jiwani, ZM, Rondot, D, Ang, J, and Kannikeswaran, N. Severe acute respiratory syndrome-Coronavirus-2 transmission in an Urban Community: the role of children and household contacts. J Pediatric Infect Dis Soc. (2021) 10:919–21. doi: 10.1093/jpids/piaa158
28. Senol, Y, and Avci, K. Identification of risk factors that increase household transmission of COVID-19 in Afyonkarahisar, Turkey. J Infect Dev Ctries. (2022) 16:927–36. doi: 10.3855/jidc.16145
29. Liu, PY, Gragnani, CM, Timmerman, J, Newhouse, CN, Soto, G, Lopez, L, et al. Pediatric household transmission of severe acute respiratory Coronavirus-2 infection-Los Angeles County, December 2020 to February 2021. Pediatr Infect Dis J. (2021) 40:e379–81. doi: 10.1097/INF.0000000000003251
30. Donnelly, MAP, Chuey, MR, Soto, R, Schwartz, NG, Chu, VT, Konkle, SL, et al. Household transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) alpha variant-United States, 2021. Clin Infect Dis. (2022) 75:e122–32. doi: 10.1093/cid/ciac125
31. Soriano-Arandes, A, Gatell, A, Serrano, P, Biosca, M, Campillo, F, Capdevila, R, et al. Household severe acute respiratory syndrome coronavirus 2 transmission and children: a network prospective study. Clin Infect Dis. (2021) 73:e1261–9. doi: 10.1093/cid/ciab228
32. Sumner, KM, Karron, RA, Stockwell, MS, Dawood, FS, Stanford, JB, Mellis, A, et al. Impact of age and symptom development on SARS-CoV-2 transmission in households with children-Maryland, New York, and Utah, august 2020-October 2021. Open Forum Infect Dis. (2022) 9:ofac390. doi: 10.1093/ofid/ofac390
Keywords: severe acute respiratory syndrome coronavirus 2, COVID-19, pediatric COVID-19, household and family, communicable disease transmission, SARS-CoV-2
Citation: Nowak KD, Lane MA, Mbanya A, Carter JR, Binion BA, Espinoza DO, Collins MH, Heaney CD, Pisanic N, Kruczynski K, Spicer K, Rivera MG, Glover F, Ojo-Akosile T, Breiman RF, Anderson EJ, Lobelo F and Fairley JK (2024) High SARS-CoV-2 secondary infection rates in households with children in Georgia, United States, Fall 2020—Winter 2021. Front. Public Health. 12:1378701. doi: 10.3389/fpubh.2024.1378701
Received: 30 January 2024; Accepted: 08 October 2024;
Published: 25 October 2024.
Edited by:
Emanuela Del Giudice, Sapienza University of Rome, ItalyReviewed by:
Pragya Sharma, Maulana Azad Medical College, IndiaCopyright © 2024 Nowak, Lane, Mbanya, Carter, Binion, Espinoza, Collins, Heaney, Pisanic, Kruczynski, Spicer, Rivera, Glover, Ojo-Akosile, Breiman, Anderson, Lobelo and Fairley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica K. Fairley, amVzc2ljYS5mYWlybGV5QGVtb3J5LmVkdQ==
†Present address: Evan J. Anderson, Moderna, Inc, Cambridge, MA, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.