
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 27 March 2024
Sec. Environmental Health and Exposome
Volume 12 - 2024 | https://doi.org/10.3389/fpubh.2024.1373044
 Yu-Jie Du1,2†
Yu-Jie Du1,2† Zhang-Wei Lu1,2†
Zhang-Wei Lu1,2† Kai-Di Li1,2
Kai-Di Li1,2 Yi-Yu Wang1,2
Yi-Yu Wang1,2 Hong Wu1,2
Hong Wu1,2 Rong-Gui Huang1,2
Rong-Gui Huang1,2 Xue Jin1,2
Xue Jin1,2 Yi-Yuan Wang1,2
Yi-Yuan Wang1,2 Jing Wang1,2
Jing Wang1,2 An-Yi Geng1,2
An-Yi Geng1,2 Bao-Zhu Li1,2,3*
Bao-Zhu Li1,2,3*Objectives: To investigate the causal relationships between pneumoconiosis and rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and gout.
Methods: The random-effects inverse variance weighted (IVW) approach was utilized to explore the causal effects of the instrumental variables (IVs). Sensitivity analyses using the MR-Egger and weighted median (WM) methods were did to investigate horizontal pleiotropy. A leave-one-out analysis was used to avoid the bias resulting from single-nucleotide polymorphisms (SNPs).
Results: There was no causal association between pneumoconiosis and SLE, RA or gout in the European population [OR = 1.01, 95% CI: 0.94–1.10, p = 0.74; OR = 1.00, 95% CI: 0.999–1.000, p = 0.50; OR = 1.00, 95% CI: 1.000–1.001, p = 0.55]. Causal relationships were also not found in pneumoconiosis due to asbestos and other mineral fibers and SLE, RA and gout [OR = 1.01, 95% CI: 0.96–1.07, p = 0.66; OR = 1.00, 95% CI: 1.00–1.00, p = 0.68; OR = 1.00, 95% CI: 1.00–1.00, p = 0.20].
Conclusion: Our study suggests that pneumoconiosis may have no causal relationship with the three inflammatory immune diseases.
The inflammatory immune response is a vital immune defense mechanism. Tissues go through a normal process involving self-defense and damage repair when they are damaged, or infected by toxins or bacteria, or caused by other factors (1). An excessive inflammatory immune response will cause body damage, leading to inflammatory immune diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), gout, and so on. It has been a significant public health issue that has had a negative impact on both long-term social and economic development as well as human health. This class of disorders is characterized by systemic inflammatory immunological dysfunction, but the precise cause and mechanism are yet unknown. It has been revealed that occupational exposure to particulate matter and environmental variables are significant contributors to the pathogenesis of this disease.
Occupational populations might develop inflammatory immunological disorders. People exposed to silica, for instance, have a relative risk of RA that is more than three times higher (2). Of those occupational populations with RA, SLE, and other disorders, 14.1% are extensively exposed to dust, chemicals, and other inorganic and organic pollutants. Immune disorders were substantially correlated with overall occupational environmental exposure [OR (95%CI) = 1.29 (1.11–1.49)] (3). Exposure to inorganic dust, such as silica and asbestos, is the most likely cause of pneumoconiosis and further contributes to a relatively high incidence rate ratio of immune system diseases (1.99) (3). And numerous epidemiological studies have demonstrated that pneumoconiosis has a strong correlation with the development of gout, RA, SLE, and other inflammatory immunological disorders. A nationwide cohort study had indicated that miners exposed to silica have an increased possibility of developing SLE and RA (4). In Japan, reports regarding thirty cases of pneumoconiosis with RA, SLE, and other illnesses have been presented (5). Patients with RA who are exposed to inorganic dust combined with several distinct pulmonary nodules, mainly peripheral to the lung, is called Caplan’s syndrome (6). At the time of diagnosis, a large number of individuals with Caplan’s syndrome had at least mild pneumoconiosis (7). According to a case–control study conducted in Sweden, women who are exposed to dust have an increased probability of developing gout and may also be at risk for other inflammatory disorders (8). This might be relevant to the mechanism of hyperuricemia, the process of sodium urate crystallization, or the inflammatory reaction to the crystals after prolonged exposure to dust. Pneumoconiosis patients have been reported to have a higher level of polyclonal gamma-globulin, particularly IgG, and a high positivity rate for autoantibodies such anti-nuclear antibodies (9). Pneumoconiosis is associated with increased autoantibodies, immune complexes, and overproduction of immunoglobulins, including rheumatoid factors (10, 11). Additionally, research on animals has demonstrated that crystalline silica (cSiO2) increased the risk of immunological disorders (12). Following a week-long exposure to cSiO2, the mice had highly elevated TNF-α levels, acquired autoantigens associated with lupus, and decreased levels in blood IgG, a hallmark of systemic inflammation. And it triggered autoantibodies against the autoantigens—collagen II, fibronectin, etc.—that are linked to RA. Besides, acute exposure to cSiO2 in lupus-prone mice conduced to lung inflammation, the generation of pro-inflammatory cytokines, and the activation of B- and T-cells, all of which hasten the onset of autoimmune disorders (13).
Nevertheless, Studies have constraints in their ability to detect and investigate isolated exposures and are unable to confirm the pathophysiology or causation of pneumoconiosis and inflammatory immune disorders, despite indications of a link between the two diseases. Two-sample Mendelian randomization (MR) method, as an effective method using genetic variation as instrumental variables (IVs), has been used to explore the causal associations between inflammatory immune diseases and other diseases, such as healthy lifestyle, atopic dermatitis, and hormonal factors (14–16). Genetic variation could minimize confounding, give stronger causal reasoning, and overcome some of the limitations of classic observational research since it is unaffected by other variables or the external environment. Therefore, we explored into the causative connections between pneumoconiosis and gout, SLE, and RA using the MR analysis.
A two-sample MR study was conducted to find causal relationships. The two-sample MR package in R (version 4.2.3) was used. Since our analysis was based on aggregated summary-level data, ethical approval was unneeded. Figure 1 illustrates our study’s assumptions and design. The MR analysis had its basis on three primary assumptions: (1) IVs are supposed to be strongly linked with exposures; (2) they should be independent from confounding factors associated with the relationship between the exposures and outcomes; (3) relevance only through exposures to influence the results (17). The flowchart of our study design is shown in Figure 2.
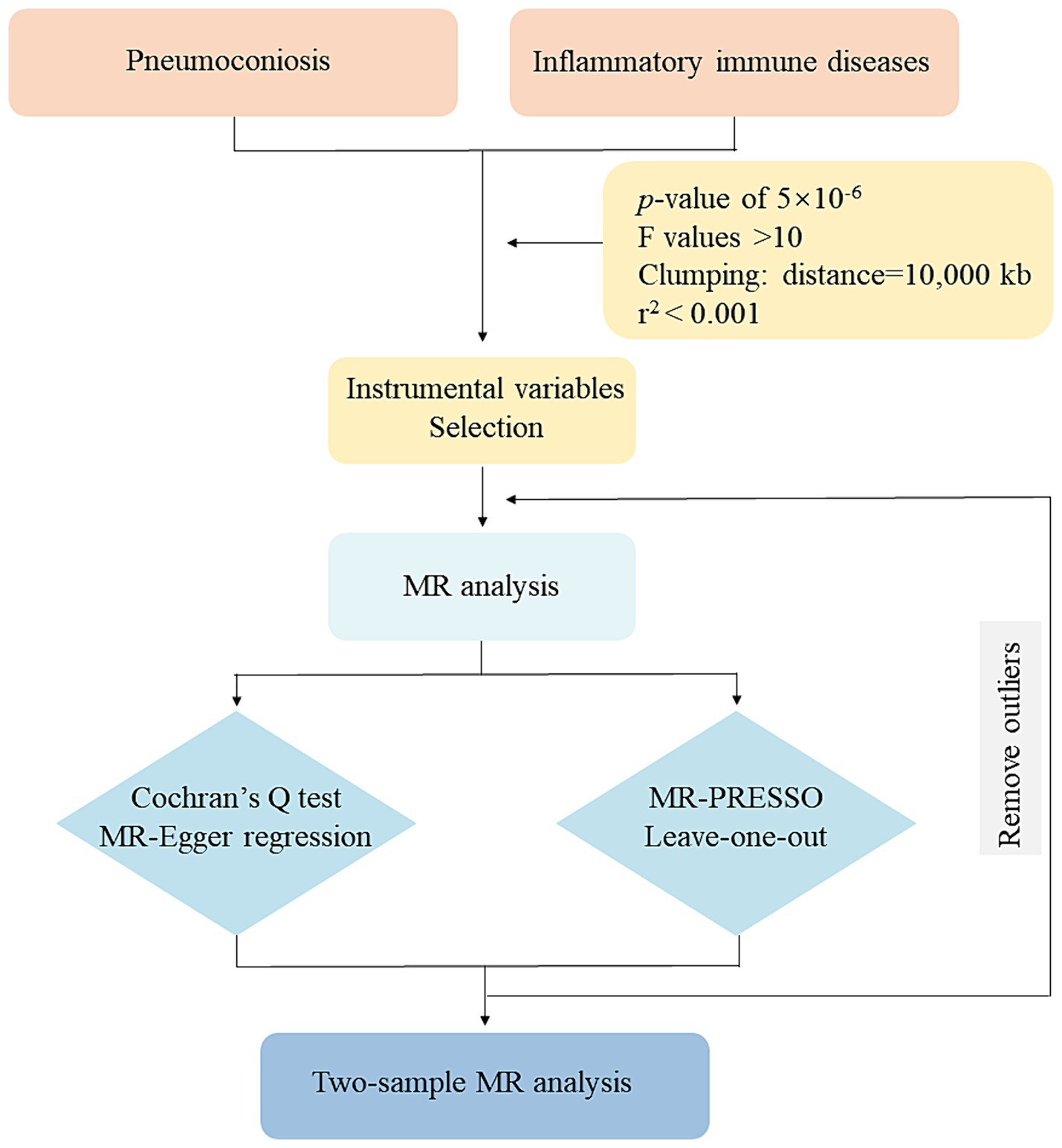
Figure 2. Flowchart of this MR study. MR: Mendelian randomization; MR-PRESSO: MR-pleiotropy residual sum and outlier.
Regarding IVs, we chose SNPs that showed a substantial exposure association (p < 5 × 10−6). And the F and r2 values were calculated to ensure each SNP’s validity. Linkage disequilibrium (LD) was eliminated in our study (r2 < 0.001, 10,000 kb). Palindromic SNPs with intermediate allele frequencies and SNPs with F-values <10 were removed (18). A comprehensive summary of the SNPs that were substantially linked to our exposures was presented in Supplementary Tables S1–S3.
All GWAS data was obtained from the IEU.1 Two exposures fall into one occupational disease and the GWAS data was from the FinnGen consortium2 and the European Bioinformatics Institute GWAS Catalogue.3 The exposures we selected including pneumoconiosis (ID: ebi-a-GCST90018900) and pneumoconiosis due to asbestos and other mineral fibers (ID: finn-b-J10_ASBESTPNEUMOC).
Three outcomes’ GWAS data were all from the European Bioinformatics Institute GWAS Catalogue. The inflammatory immune diseases considered to be analyzed were as follows: SLE (ID: ebi-a-GCST90018917), RA (ID: ebi-a-GCST90038685), and gout (ID: ebi-a-GCST90038687). Since all of the samples were European in origin, there were no racial differences. Table 1 presents the exposure and outcome summary information.
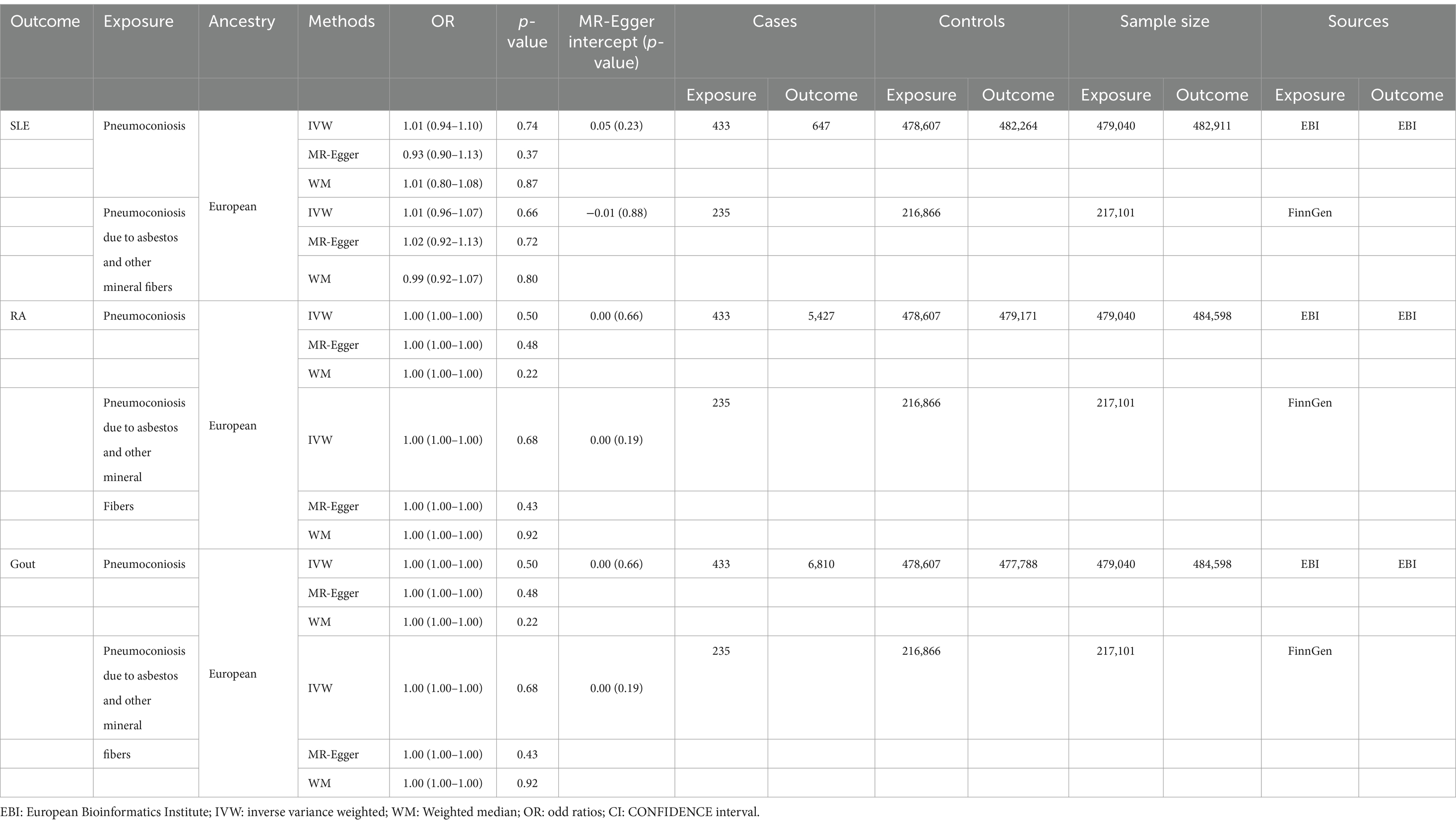
Table 1. Descriptive details and Mendelian randomization estimates of pneumoconiosis and SLE, RA and gout.
The MR-Egger method, weighted median (WM), inverse variance weighted (IVW) method, simple mode, and weighted mode were the methods employed. And the main analytical approach used to evaluate the causal links was the IVW method. Besides, the chosen SNPs had no connection to smoking or alcohol consumption, which may be confounding factors for inflammatory immune diseases.
For validation of the accuracy of the results we obtained, sensitivity analyses involving the Cochran’s Q test, MR-PRESSO, MR-Egger regression, and leave-one-out analysis were performed. Cochran’s Q test was utilized to assess SNPS heterogeneity (p > 0.05 indicates no heterogeneity) (19). Genetic diversity that influences outcomes through alternative pathways is known as pleiotropy (20). With MR-PRESSO, pleiotropy at the gene level was examined. It adjusts for the influence of genetic variants on causal estimates and identifies variants with pleiotropic effects. The presence of pleiotropy was also assessed by applying MR-Egger regression. An intercept that is statistically significant is able to identify pleiotropy. It computes the linear regression of the genetic variations on the outcome. To assess the overall influence of certain genetic variations, a leave-one-out analysis was used. The removal of one genetic variant at a time can be done with this method. Furthermore, by recalculating the causal estimate, the effect of each unique genetic variation may be determined (21).
No causal association was discovered in results of pneumoconiosis, pneumoconiosis due to asbestos and other mineral fibers on SLE, listed in Table 1, using the IVW method (OR: 1.01, p = 0.74; OR: 1.01, p = 0.66). This correlation was also not detected using the WM method and MR-Egger approach (Supplementary Figures S1A,B). We consider that our results are credible because there was no significant heterogeneity (p = 0.50; p = 0.57) and no horizontal pleiotropy (p = 0.23; p = 0.88) (Supplementary Figures S2A,B). The forest plot is showed in Figure 3. The stability of the MR estimates was further validated through a leave-out test (Supplementary Figures S3A,B).
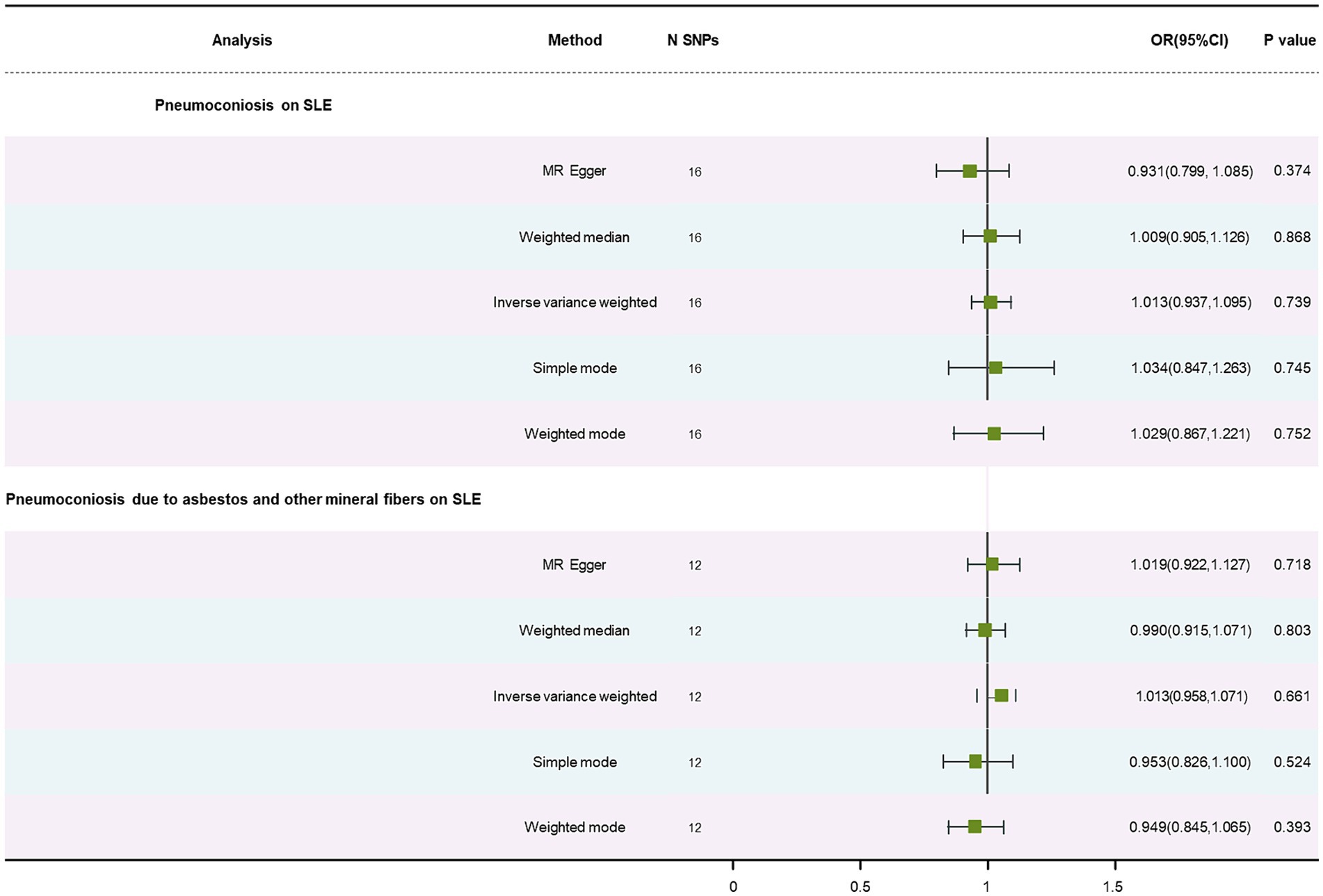
Figure 3. Forest plot of SNPs associated with pneumoconiosis and pneumoconiosis due to asbestos and other mineral fibers and their risk on SLE.
Using the IVW approach, no statistically significant association was found in results on RA, presented in Table 1 (OR: 1.00, p = 0.50; OR: 1.00, p = 0.68). The WM and the MR-Egger method similarly failed to find this relationship (Supplementary Figures S1C,D). There was no significant heterogeneity (p = 0.38; p = 0.18) and no horizontal pleiotropy (p = 0.66; p = 0.19) (Supplementary Figures S2C,D). The forest plot is depicted in Figure 4. A leave-out test was used to confirm the stability of the MR estimations (Supplementary Figures S3C,D).
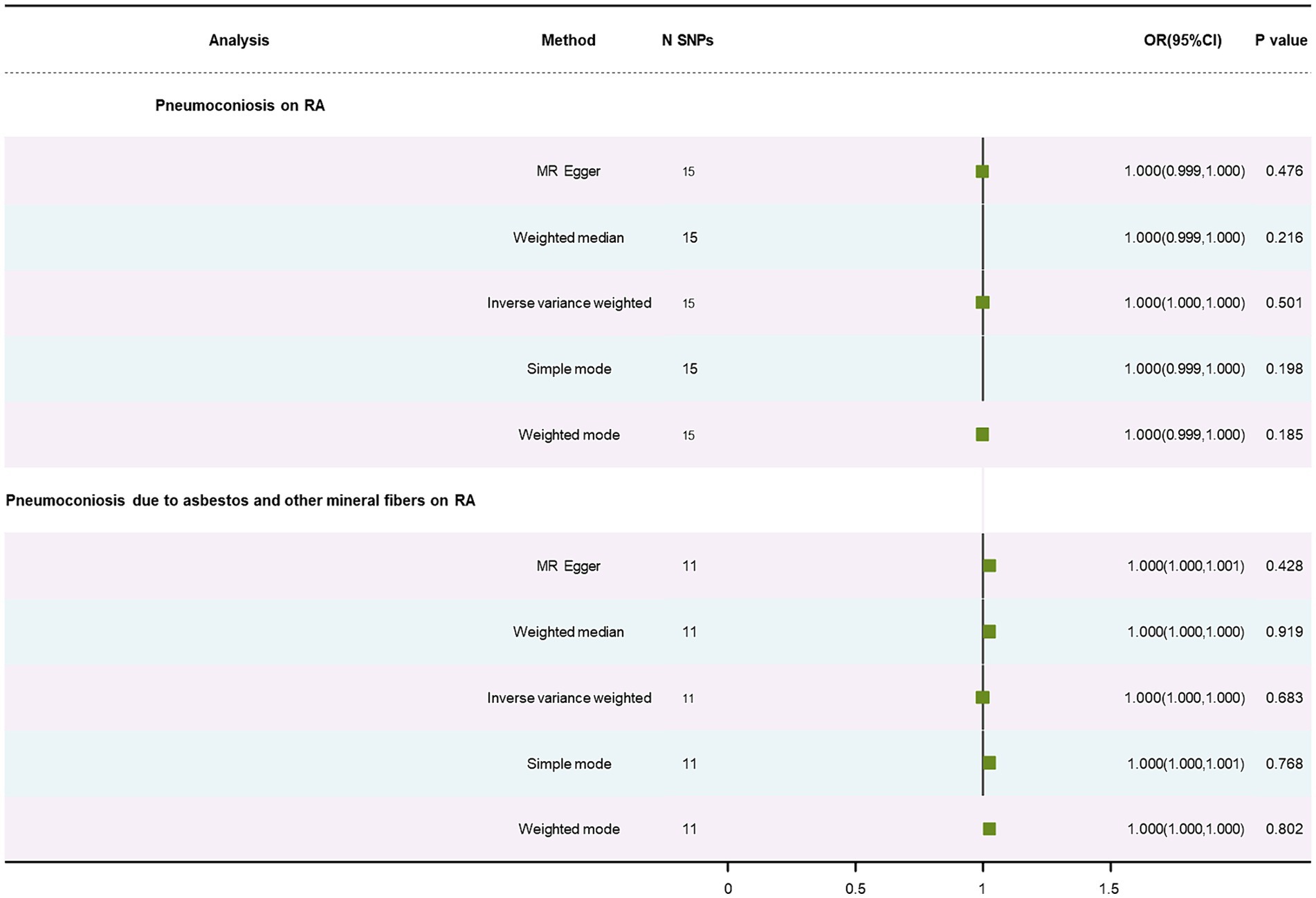
Figure 4. Forest plot of SNPs associated with pneumoconiosis and pneumoconiosis due to asbestos and their risk on RA.
No causal relationship was found in results on gout, displayed in Table 1, using the IVW method (OR: 1.00, p = 0.55; OR: 1.00, p = 0.20). This correlation was also failed to find using the WM method and MR-Egger approach (Supplementary Figures S1E,F). Pneumoconiosis had heterogeneous outcomes for gout, while pneumoconiosis due to asbestos and other mineral fibers did not (p = 0.01; p = 0.47). Our analysis indicated no horizontal pleiotropy (p = 0.19; p = 0.77) (Supplementary Figures S2E,F). The forest plot is showed in Figure 5. A leave-out test was applied to assess the stability of the MR estimates (Supplementary Figures S3E,F).
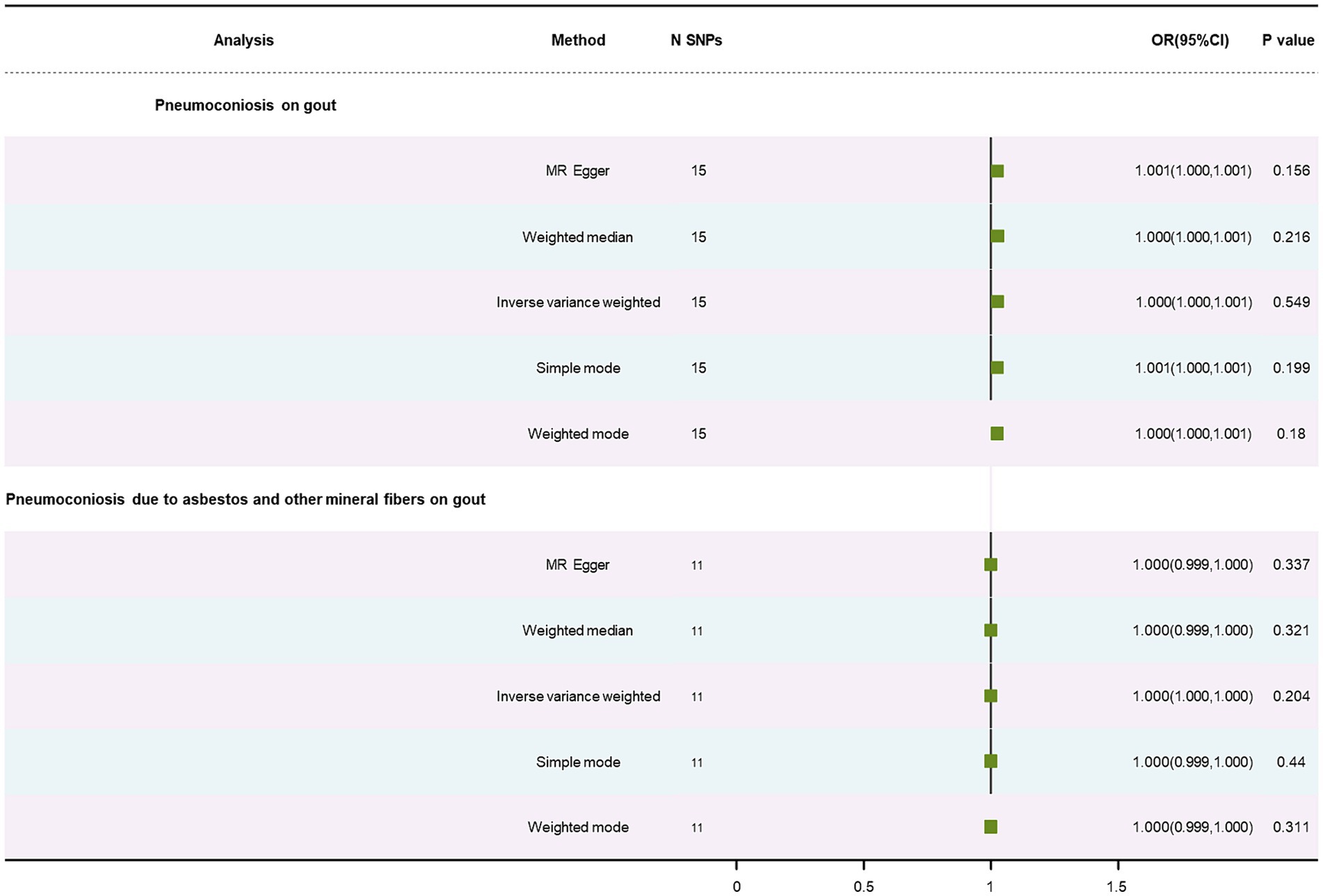
Figure 5. Forest plot of SNPs associated with pneumoconiosis and pneumoconiosis due to asbestos and their risk on gout.
To our knowledge, this is the first study to investigate the causal connection between pneumoconiosis and inflammatory immune diseases. Our results indicates that pneumoconiosis and SLE, RA, and gout are not causally related. Nevertheless, further research is needed to validate and clarify the mechanism.
Although the etiology and pathogenesis of inflammatory immune diseases are still unclear, they might be related to environmental factors. In some investigations, the development of RA, SLE, and other inflammatory immunological disorders is linked to occupational exposure to external airborne agents (EAA) (22). Workers in different manufacturing and construction sectors, as well as miners and quarry workers, might be frequently exposed to EAA, including cSiO2, asbestos, and steam (23). Pneumoconiosis is triggered directly by exposure to cSiO2 (a major factor), asbestos, etc. These elements have been linked to an elevated incidence and severity of inflammatory immunological disorders (2, 24). These environmental pollutants can be used as a molecular pattern that causes damage once they enter the human body, producing the autoinflammatory response that is typified by increased production of reactive oxygen species, increased macrophage flux, NF-κB activation, tumor necrosis factor, IL-1β, and IL-6, among other pro-inflammatory cytokines (25). These triggers cause B cells and dendritic cells to get stimulated, which in turn produces a lot of antibodies and autoreactive T lymphocytes. This destroys immunological tolerance and causes inflammatory immune diseases (26, 27).
New Zealand mixed mice exposed to silicate showed reduced blood IgG, a biomarker of systemic inflammation, and lifted growth of TNF-α, IL-6, B-cell activating factor, and IgM. Further still, cSiO2 also causes an autoantibody reaction to autoantigens linked to RA and SLE (28, 29). Pneumoconiosis patients’ serum cytokine analysis also led to some discoveries. Serum IL-6 appeared to be included in the same factor as ANCA levels and antinuclear antibody titers. Apart from boosting responder T cells and lowering Treg cells, IL-6 also contributes a great deal to T helper cell polarization towards Th17 cells, preventing cSiO2-induced lung inflammation via an IL-1B-dependent pathway (30, 31). The predominant factor influencing the level of inflammation is the fraction of quartz, a part of the respirable dust responsible for coal dust (32). However, a number of studies consistently demonstrate that the development of pneumoconiosis is not influenced by the quartz concentration of mixed dust. For instance, pneumoconiosis is less common in German coal mines where quartz concentrations are greater (33). Conversely, a coal mine with a lower proportion of quartz was shown to have a higher prevalence of pneumoconiosis (34). In French miners, high quartz concentrations have not been linked to pulmonary fibrosis (35). A review goes into great length on animal studies, in vitro evaluations, and epidemiological evidence supporting the hypothesis that iron concentration in coal, not quartz, raises the risk of pneumoconiosis in coal miners (36). It also mentioned that a potential biomarker might be the changed ferritin levels that coal workers experience after taking in coal dust. Moreover, clay samples significantly inhibited quartz-induced lung inflammation (37). Thus, the entire burden of pneumoconiosis may be entirely attributed to occupational exposure to gases, smoke, and particulate matter through risk factors (38). Rather than inflammation, the degree of occupational exposure concentration and exposure duration plays an essential part in the development and course of pneumoconiosis (39). Information on blood markers of systemic inflammation is not available in the database. Lung inflammation due to quartz content in inhaled dust may not cause a high lung burden or develop pneumoconiosis (40). Therefore, our study suggests that there is no causal relationship between pneumoconiosis and the three inflammatory immune diseases.
There are several limitations in previous observational studies. Firstly, the number of cases of SLE, RA, and gout caused by pneumoconiosis may have been underestimated and misclassified because the patients included were mainly diagnosed as outpatients. Secondly, many studies lacked case information and were therefore unable to control for relevant confounders, including family history and lifestyles. Thirdly, the history of dust exposure was incomplete, including information regarding the usage of personal protective equipment, which is the optimum approach for reducing cSiO2 exposure and preventing pneumoconiosis in workers.
There are also some limitations in our study to consider. First, we relaxed the selection criterion (p < 5 × 10−6, r2 = 0.001, kb = 10,000) as there were not numerous SNPS that fit the inclusion requirements. This might result in some false positives. Besides, the study focused on European populations, and it is unknown if other populations show the same link. Finally, we did not obtain GWAS data for pneumoconiosis caused by other exposures, and etiological categorization could not be analyzed. In conclusion, we did not find a causal relationship between pneumoconiosis and the three inflammatory immune diseases.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Y-JD: Writing – original draft. Z-WL: Writing – original draft. K-DL: Writing – original draft. Y-YuW: Writing – original draft. HW: Writing – original draft. R-GH: Writing – original draft. XJ: Writing – original draft. Y-YuaW: Writing – original draft. JW: Writing – original draft. A-YG: Writing – original draft. B-ZL: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (81803310); the Research Fund of Anhui Institute of translational Medicine (2021zhyx-C21); Center for Big Data and Population Health of IHM (JKS2022023); Clinical Medical Research Transformation Project in Anhui Province (202304295107020026); the Peak Discipline of Public Health and Preventive Medicine, Anhui Medical University.
We appreciate all the volunteers who participated in this study. We are grateful to the Open GWAS for providing GWAS summary statistics.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1373044/full#supplementary-material
1. Pollard, KM. Silica, silicosis, and autoimmunity. Front Immunol. (2016) 7:97. doi: 10.3389/fimmu.2016.00097
2. Stolt, P, Källberg, H, Lundberg, I, Sjögren, B, Klareskog, L, Alfredsson, L, et al. Silica exposure is associated with increased risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis. (2005) 64:582–6. doi: 10.1136/ard.2004.022053
3. Lee, S, Ma, X, and Lee, W. Association between exposure to external airborne agents and autoimmune disease. Ecotoxicol Environ Saf. (2023) 263:115334. doi: 10.1016/j.ecoenv.2023.115334
4. Li, X, Sundquist, J, Sundquist, K, and Zöller, B. Occupational risk factors for systemic lupus erythematosus: a nationwide study based on hospitalizations in Sweden. J Rheumatol. (2012) 39:743–51. doi: 10.3899/jrheum.110789
5. Matsuoka, Y, Tomita, M, Yoshino, I, and Hosoda, Y. Relationship between autoimmune diseases and pneumoconiosis. Sangyo Igaku. (1992) 34:421–31. doi: 10.1539/joh1959.34.421
6. Schreiber, J, Koschel, D, Kekow, J, Waldburg, N, Goette, A, and Merget, R. Rheumatoid pneumoconiosis (Caplan’s syndrome). Eur J Intern Med. (2010) 21:168–72. doi: 10.1016/j.ejim.2010.02.004
7. Honma, K, and Vallyathan, V. Rheumatoid pneumoconiosis: a comparative study of autopsy cases between Japan and North America. Ann Occup Hyg. (2002) 46:265–7. doi: 10.1093/annhyg/46
8. Sigurdardottir, V, Jacobsson, L, Schiöler, L, Svärd, A, Dehlin, M, and Toren, K. Occupational exposure to inorganic dust and risk of gout: a population-based study. RMD Open. (2020) 6:e001178. doi: 10.1136/rmdopen-2020-001178
9. Bas, S, Genevay, S, Meyer, O, and Gabay, C. Anti-cyclic citrullinated peptide antibodies, IgM and IgA rheumatoid factors in the diagnosis and prognosis of rheumatoid arthritis. Rheumatology. (2003) 42:677–80. doi: 10.1093/rheumatology/keg184
10. Lakos, G, Soos, L, Fekete, A, Szabo, Z, Zeher, M, Horvath, IF, et al. Anti-cyclic citrullinated peptide antibody isotypes in rheumatoid arthritis: association with disease duration, rheumatoid factor production and the presence of shared epitope. Clin Exp Rheumatol. (2008) 26:253–60.
12. Chauhan, PS, Wagner, JG, Benninghoff, AD, Lewandowski, RP, Favor, OK, Wierenga, KA, et al. Rapid induction of pulmonary inflammation, autoimmune gene expression, and ectopic lymphoid Neogenesis following acute silica exposure in lupus-prone mice. Front Immunol. (2021) 12:635138. doi: 10.3389/fimmu.2021.635138
13. Gottschalk, TA, Tsantikos, E, and Hibbs, ML. Pathogenic inflammation and its therapeutic targeting in systemic lupus erythematosus. Front Immunol. (2015) 6:550. doi: 10.3389/fimmu.2015.00550
14. Xia, Z, Liu, J, and Zu, Y. Systemic lupus erythematosus and atopic dermatitis: a two sample Mendelian randomization study. Clin Rheumatol. (2024) 43:1311–1317. doi: 10.1007/s10067-024-06900-z
15. Zhang, J, Fang, XY, Leng, R, Chen, HF, Qian, TT, Cai, YY, et al. Metabolic signature of healthy lifestyle and risk of rheumatoid arthritis: observational and Mendelian randomization study. Am J Clin Nutr. (2023) 118:183–93. doi: 10.1016/j.ajcnut.2023.04.034
16. Jiang, LQ, Zhang, RD, Musonye, HA, Zhao, HY, He, YS, Zhao, CN, et al. Hormonal and reproductive factors in relation to the risk of rheumatoid arthritis in women: a prospective cohort study with 223 526 participants. RMD Open. (2024) 10:e003338. doi: 10.1136/rmdopen-2023-003338
17. Hemani, G, Zheng, J, Elsworth, B, Wade, KH, Haberland, V, Baird, D, et al. The MR-base platform supports systematic causal inference across the human phenome. eLife. (2018) 7:e34408. doi: 10.7554/eLife.34408
18. Palmer, TM, Lawlor, DA, Harbord, RM, Sheehan, NA, Tobias, JH, Timpson, NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. (2012) 21:223–42. doi: 10.1177/0962280210394459
19. Bowden, J, Del Greco, MF, Minelli, C, Davey Smith, G, Sheehan, N, and Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. (2017) 36:1783–802. doi: 10.1002/sim.7221
20. Verbanck, M, Chen, CY, Neale, B, and Do, R. Publisher correction: detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:1196. doi: 10.1038/s41588-018-0164-2
21. Hemani, G, Tilling, K, and Davey, SG. Correction: orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. (2017) 13:e1007149. doi: 10.1371/journal.pgen.1007149
22. Celen, H, Dens, AC, Ronsmans, S, Michiels, S, and De Langhe, E. Airborne pollutants as potential triggers of systemic autoimmune rheumatic diseases: a narrative review. Acta Clin Belg. (2022) 77:874–82. doi: 10.1080/17843286.2021.1992582
23. Torén, K, Blanc, PD, Naidoo, R, Murgia, N, Stockfelt, L, and Schiöler, L. Cumulative occupational exposure to inorganic dust and fumes and invasive pneumococcal disease with pneumonia. Int Arch Occup Environ Health. (2022) 95:1797–804. doi: 10.1007/s00420-022-01848-6
24. Lee, S, Hayashi, H, Mastuzaki, H, Kumagai-Takei, N, and Otsuki, T. Silicosis and autoimmunity. Curr Opin Allergy Clin Immunol. (2017) 17:78–84. doi: 10.1097/ACI.0000000000000350
25. Barragán-Martínez, C, Speck-Hernández, CA, Montoya-Ortiz, G, Mantilla, RD, Anaya, JM, and Rojas-Villarraga, A. Organic solvents as risk factor for autoimmune diseases: a systematic review and meta-analysis. PLoS One. (2012) 7:e51506. doi: 10.1371/journal.pone.0051506
26. Mayeux, JM, Escalante, GM, Christy, JM, Pawar, RD, Kono, DH, and Pollard, KM. Silicosis and silica-induced autoimmunity in the diversity outbred mouse. Front Immunol. (2018) 9:874. doi: 10.3389/fimmu.2018.00874
27. Källberg, H, Ding, B, Padyukov, L, Bengtsson, C, Rönnelid, J, Klareskog, L, et al. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. (2011) 70:508–11. doi: 10.1136/ard.2009.120899
28. Brown, JM, Archer, AJ, Pfau, JC, and Holian, A. Silica accelerated systemic autoimmune disease in lupus-prone New Zealand mixed mice. Clin Exp Immunol. (2003) 131:415–21. doi: 10.1046/j.1365-2249.2003.02094
29. Bates, MA, Brandenberger, C, Langohr, I, Kumagai, K, Harkema, JR, Holian, A, et al. Silica triggers inflammation and ectopic lymphoid neogenesis in the lungs in parallel with accelerated onset of systemic autoimmunity and glomerulonephritis in the lupus-prone NZBWF1 mouse. PLoS One. (2015) 10:e0125481. doi: 10.1371/journal.pone.0125481
30. Lo Re, S, Dumoutier, L, Couillin, I, van Vyve, C, Yakoub, Y, Uwambayinema, F, et al. IL-17A-producing gammadelta T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis. J Immunol. (2010) 184:6367–77. doi: 10.4049/jimmunol.0900459
31. Song, L, Weng, D, Dai, W, Tang, W, Chen, S, Li, C, et al. Th17 can regulate silica-induced lung inflammation through an IL-1b-dependent mechanism. J Cell Mol Med. (2014) 18:1773–84. doi: 10.1111/jcmm.12341
32. Walton, WH, Dodgson, J, Hadden, GG, and Jacobsen, M. The effect of quartz and other non-coal dusts in coal workers’ pneumoconiosis. ln: WH Walton, ed, Inhaled particles IV, old Woking, United Kingdom: Unwin Brothers. (1977) p. 669–689.
33. Lebouffant, L, Daniel, H, and Martin, JC. Quartz as a Causative Agent in Pneumoconiotic Lesions in Coal Miners. In: Industrial health and medicine series, No. 19. Luxembourg: Commission of the European communities-ESC (1977). 1–60.
34. Hurley, JF, Burns, J, Copland, L, Dodgson, J, and Jacobsen, M. Coal workers’ with simple pneumoconiosis with exposure to dust at 10 British coalmines. Br J Ind Med. (1982) 39:120–7. doi: 10.1136/oem.39.2.120
35. Rivers, D, Wise, ME, King, EJ, and Nagelschmidt, G. Dust content, radiology and pathology in simple pneumoconiosis of coal workers’. Br J Occup Med. (1960) 17:87–108. doi: 10.1016/B978-1-4832-1329-3.50041-X
36. McCunney, RJ, Morfeld, P, and Payne, S. What component of coal causes coal workers’ pneumoconiosis? J Occup Environ Med. (2009) 51:462–71. doi: 10.1097/JOM.0b013e3181a01ada
37. Stone, V, Jones, R, Rollo, K, Duffin, R, Donaldson, K, and Brown, DM. Effect of coal mine dust and clay extracts on the biological activity of the quartz surface. Toxicol Lett. (2004) 149:255–9. doi: 10.1016/j.toxlet.2003.12.036
38. GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390:1345–422. doi: 10.1016/S0140-6736(17)32366-8
39. Schlünssen, V, Mandrioli, D, Pega, F, Momen, NC, Ádám, B, Chen, W, et al. The prevalences and levels of occupational exposure to dusts and/or fibers (silica, asbestos and coal): a systematic review and meta-analysis from the WHO/ILO joint estimates of the work-related burden of disease and injury. Environ Int. (2023) 178:107980. doi: 10.1016/j.envint.2023.107980
Keywords: inflammatory immune diseases, causal relationship, rheumatoid arthritis, systemic lupus erythematosus, gout
Citation: Du Y-J, Lu Z-W, Li K-D, Wang Y-Y, Wu H, Huang R-G, Jin X, Wang Y-Y, Wang J, Geng A-Y and Li B-Z (2024) No causal association between pneumoconiosis and three inflammatory immune diseases: a Mendelian randomization study. Front. Public Health. 12:1373044. doi: 10.3389/fpubh.2024.1373044
Received: 19 January 2024; Accepted: 14 March 2024;
Published: 27 March 2024.
Edited by:
Mohiuddin Md. Taimur Khan, Washington State University Tri-Cities, United StatesReviewed by:
Ayse Malatyali, University of Central Florida, United StatesCopyright © 2024 Du, Lu, Li, Wang, Wu, Huang, Jin, Wang, Wang, Geng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bao-Zhu Li, bGJ6ODg3MzBAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.