- 1The First Affiliated Hospital, College of Clinical Medicine of Henan University of Science and Technology, Luoyang, Henan, China
- 2Department of Gastroenterology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
- 3Department of Gastroenterology, Huanghe Sanmenxia Hospital, Sanmenxia, Henan, China
- 4Department of Cardiovascular Medicine, Huanghe Sanmenxia Hospital, Sanmenxia, Henan, China
- 5Department of General Medicine, Dongpu Branch of The First Affiliated Hospital, Jinan University, Guangzhou, China
Objectives: Increasing concern about air pollution’s impact on public health underscores the need to understand its effects on non-neoplastic digestive system diseases (NNDSD). This study explores the link between air pollution and NNDSD in China.
Methods: We conducted a national cross-sectional study using 2015 data from the China Health and Retirement Longitudinal Study (CHARLS), involving 13,046 Chinese adults aged 45 and above from 28 provinces. Satellite-based spatiotemporal models estimated participants’ exposure to ambient particulate matter (3-year average). An analysis of logistic regression models was conducted to estimate the association between air pollutants [particulate matter with a diameter ≤ 2.5 μm (PM2.5) or ≤10 μm (PM10), sulfur dioxide (SO2), nitrogen dioxide (NO2), ozone (O3), and carbon monoxide (CO)] and NNDSD. Interaction analyses were conducted to examine potential modifiers of these associations.
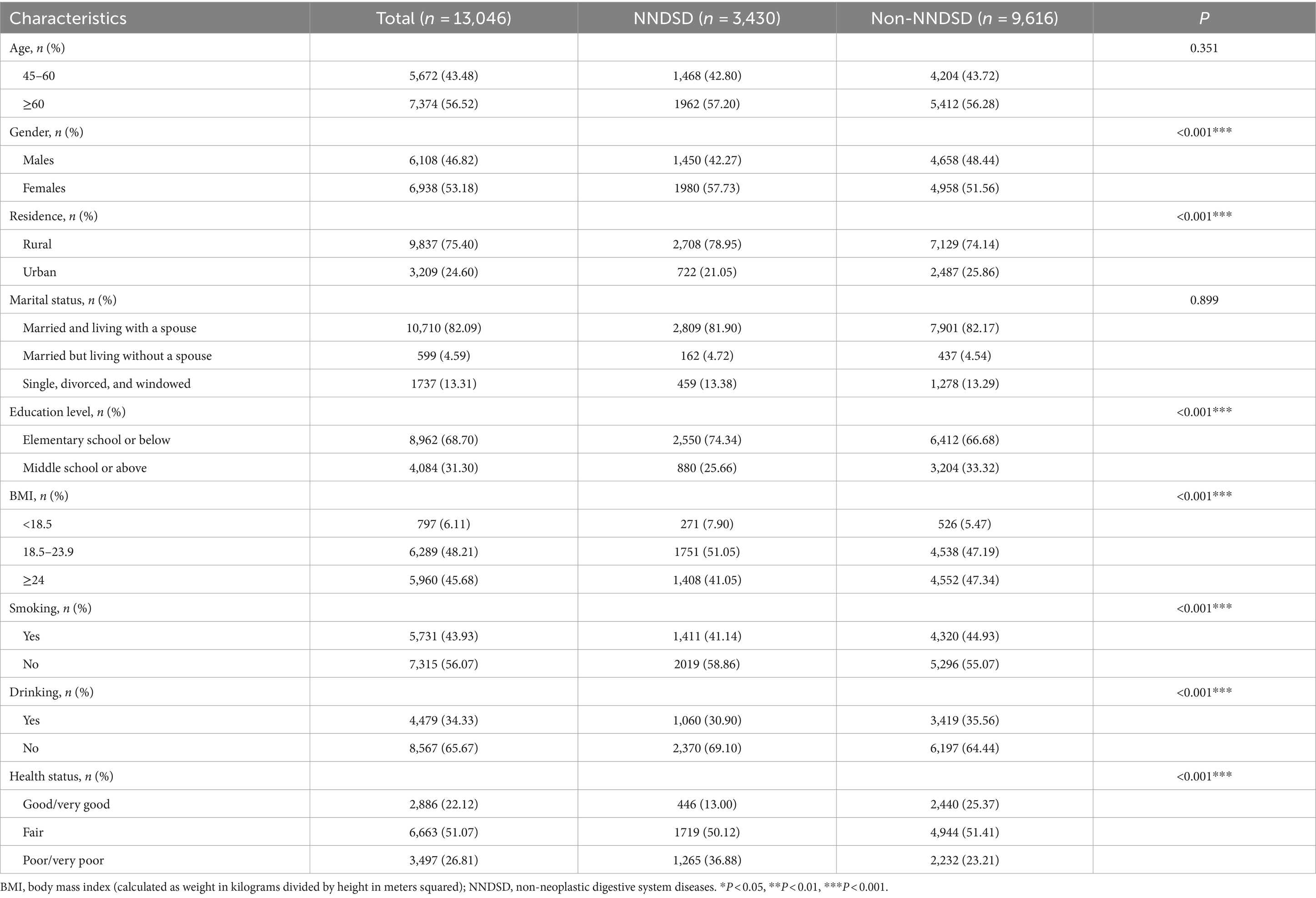
Results: The prevalence of NNDSD among participants was 26.29%. After adjusted for multivariate factors, we observed a 6% [odd ratio (OR) = 1.06, 95% confidence interval (CI): 0.94, 1.19], 23% (OR = 1.23, 95% CI: 1.09, 1.38), 26% (OR = 1.26, 95% CI: 1.12, 1.41), 30% (OR = 1.30, 95% CI: 1.16, 1.46), 13% (OR = 1.13, 95% CI: 1.01, 1.27) and 27% (OR = 1.27, 95% CI: 1.13, 1.43) increase in NNDSD risk with an interquartile range increase in PM2.5 (23.36 μg/m3), PM10 (50.33 μg/m3), SO2 (17.27 μg/m3), NO2 (14.75 μg/m3), O3 (10.80 μg/m3), and CO (0.42 mg/m3), respectively. Interaction analyses showed that PM2.5, SO2, and O3 had stronger effects on NNDSD risk among older adults, highly educated individuals, smokers, and married people, respectively.
Conclusion: This study demonstrates that long-term exposure to PM2.5, PM10, SO2, NO2, O3, and CO is positively associated with NNDSD risk in Chinese adults aged 45 and above. Implementing intervention strategies to enhance air quality is essential for reducing the burden of NNDSD.
1 Background
Non-neoplastic digestive system diseases (NNDSD) are non-cancerous digestive disorders, encompassing conditions such as gastroenteritis, gastroesophageal reflux disease (GERD), inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and liver diseases, among others (1, 2). There were 100,317 deaths related to non-malignant gastrointestinal diseases in the United States in 2019 (3). The incidence of digestive disorders has been increasing in China and other countries with Western societies (4). Research is increasingly revealing a pathogenic continuum between certain NNDSD and the emergence of malignant tumors of the gastrointestinal tract (5, 6). Millions worldwide suffer from these diseases, which also impose significant economic burdens, including high medical costs and lost work hours (7). Consequently, NNDSD represents a significant aspect of public health, and identifying risk factors for NNDSD is crucial for preventing and controlling NNDSD.
Industrialization, urbanization, and urban population growth have made air pollution one of the most critical environmental factors (8). Exposure to chronic air pollution [particulate matter with a diameter ≤ 2.5 μm (PM2.5) or ≤10 μm (PM10), sulfur dioxide (SO2), nitrogen dioxide (NO2), ozone (O3), and carbon monoxide (CO)] has been associated with respiratory diseases, cardiometabolic diseases, and cancer (9–12). Air pollution has been linked to digestive disorders such as enteritis, appendicitis, IBD, liver disease, and peptic ulcers. An association has been established between air pollution exposure and a higher incidence of NNDSD in urban populations (13–15). Our methodology extends previous research by incorporating a broader spectrum of air pollutants and a larger sample size. Prior studies primarily focused on PM2.5 and its impacts; our study included comprehensive pollutants such as PM10, SO2, NO2, O3, and CO, allowing for a more detailed analysis of air quality impacts on NNDSD. The effects of air pollution on digestive diseases have also been studied from a mechanistic perspective. Environmental exposure directly impacts epithelial cells, increases intestinal permeability, induces oxidative stress, triggers systemic inflammation, and alters gut microbiota (16–18). Despite their prevalence, the link between environmental factors, especially air pollution, and NNDSD remains largely unexplored. Particularly evident is this gap in China and other developing countries, where air pollution levels are high and large-scale population-based studies are lacking.
We conducted a national cross-sectional study to investigate the relationship between six major air pollutants (PM2.5, PM10, SO2, NO2, O3, and CO) and NNDSD to develop feasible strategies for preventing and controlling the disease.
2 Methods
2.1 Study population
The China Health and Retirement Longitudinal Study (CHARLS) selected participants from a national cohort of residents aged 45 and older. To gather representative data for middle-aged and older Chinese people, the study used GIS software and multistage probability sampling across 28 provinces. In accordance with the Declaration of Helsinki, Peking University’s Ethics Review Board approved the CHARLS protocol (IRB00001052-11015). Informed consent was obtained from all participants.
CHARLS started in 2011 and has been conducted every 2–3 years since then. Evaluations of Chinese air pollution levels have predominantly been conducted since 2013. Based on the CHARLS dataset from 2015, we conducted a cross-sectional study of 21,095 middle-aged and older adult who underwent medical examinations. We excluded data with missing key information: age (n = 364), body mass index (n = 3,348), smoking status (n = 11), alcohol consumption (n = 9), health status (n = 307), marital status (n = 2), residence (n = 86), education level (n = 2,945), and participants under 45 years old (n = 977). These exclusions were necessary to maintain the integrity and the analytical robustness of our study findings. The study included 13,046 middle-aged and older adult individuals from 28 provinces in China (Supplementary Figure S1).
2.2 Definition of NNDSD
A physician-diagnosed NNDSD was identified as one of the outcomes of interest in the 2015 questionnaire. During the follow-up questionnaire, the self-report question (‘Have you been diagnosed by a physician with a gastric or other non-neoplastic disease of the digestive system?’) was used to determine if the patient had been diagnosed with NNDSD. A subject who answered ‘yes’ to this question was defined as having NNDSD.
2.3 Air pollution exposure assessment
Between 2013 and 2015, a geocoding system was employed to identify the residential addresses of study subjects. These geocoded addresses were then used by Artificial Intelligence (AI) to calculate ground-level concentrations of air pollutants such as PM2.5, PM10, SO2, NO2, O3 and CO for each individual. The data were derived from the ChinaHighAirPollutants (CHAP) dataset and were spatially resolved to 0.1° (approximately 10 km). To estimate ambient PM2.5, PM10, SO2, NO2, CO, and O3, surface measurements, remote sensing products, atmospheric reanalysis and model simulations were used. With surface measurements, a 10-fold cross-validation was performed to determine R2 and the root mean square error (RMSE) for all daily mean estimates of all air pollutants (Supplementary Table S1). Annual air pollution exposures were calculated based on each participant’s county-level home address. For the main effects analyses, we calculated each participant’s long-term air pollution exposure as the 3-year average of PM2.5, PM10, SO2, NO2, O3, and CO levels before the 2015 CHARLS survey. For sensitivity analyses, we used the two-year average air pollution concentrations.
2.4 Covariates
Covariate data from CHARLS 2015 were used to prepare this study. As a demographic covariate, age ranges (45–60 years, ≥60 years), gender (male, female), residence (rural or urban), educational level (primary school and below, secondary school or above), marital status (married/cohabiting, unmarried, separated/divorced/widowed). The variables of health behavior included Body Mass Index (BMI) (underweight, normal weight, overweight/obese), smoking status (no or yes), alcohol consumption status (no or yes), and health status (good/very good, fair, poor/very poor).
2.5 Statistical analyses
We used descriptive statistics to compare the basic characteristics of participants, presenting categorical variables as numbers (percentages). Differences between non-NNDSD and NNDSD participants were assessed using the Chi-squared test for independence. The strength and direction of the association between air pollutants were quantified using Pearson’s correlation coefficients, which measure linear correlations between variables (Supplementary Table S2).
Logistic regression was used to examine the impact of 3-year average air pollutant concentrations on NNDSD. We first developed a crude model without adjustments. The base model adjusted for five covariates (gender, age, residence, education level, marital status), and the main model was fully adjusted for additional covariates (smoking, drinking, BMI, health status). NNDSD was expressed as odds ratios (OR) for each increment of the interquartile range (IQR) in air pollutants with corresponding 95% confidence interval (CI). Subgroup analyses and interaction tests were conducted within the logistic regression framework to explore potential modifications of the air pollution effects on NNDSD by various factors, including sex, age, place of residence, education level, marital status, smoking, alcohol consumption, BMI, and health status. Interaction terms were added to the models to statistically test for these effects.
To verify the robustness of our results, we performed sensitivity analyses. First, we reran the analyses using the average air pollution concentrations for the 2 years before 2015. Second, we excluded participants who self-reported poor or very poor health. We also conducted propensity score matching (PSM) using a nearest neighbor ratio of 1:1 without replacement and a caliper width of 0.05. We conducted the analyses using SPSS Statistics 26, R software (version 4.2.2), and Python3.11. R was used for spatial data analysis, statistical modeling, and generating forest plots, with packages like ggplot2, plyr, maptools, forestplot, and matchit. Python was used for air pollution mapping, employing libraries such as geopandas, matplotlib.pyplot, osgeo, and gdal. Statistical significance was determined by a two-tailed p-value of less than 0.05.
3 Results
3.1 Characteristics of study participants
In total, 13,046 participants were enrolled in this study, and the flowchart of participant enrollment can be seen in Supplementary Figure S1. Based on 13,046 participants from 28 provinces in China, Figure 1 illustrates their distribution. A summary of the basic characteristics of the study participants is provided in Table 1. The incidence rate of NNDSD was 26.29%. About 56.52% of the NNDSD cases were over 60 years old, and 53.18% of these were female. The majority of participants with NNDSD were married or cohabiting. Additionally, 26.81% of NNDSD cases were in poor health. There were 75.40% of participants who lived in rural areas and 68.70% who had only a primary school education or less. Aside from age and marital status, there was a significant difference between non-NNDSD and NNDSD patients with respect to sociodemographic and health behavioral characteristics.
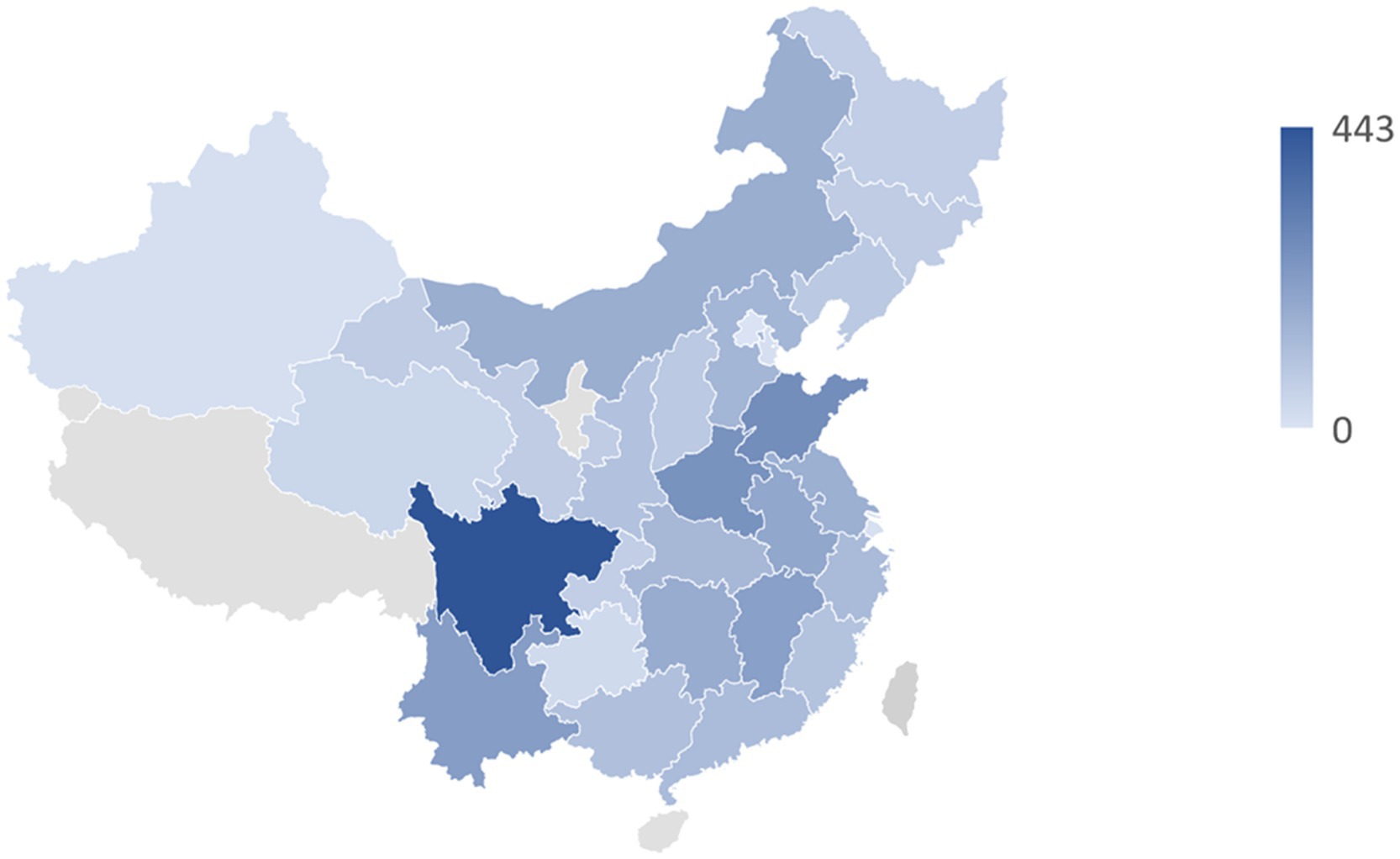
Figure 1. The geographical distribution of 13,046 middle-aged and older participants in 28 provinces of China.
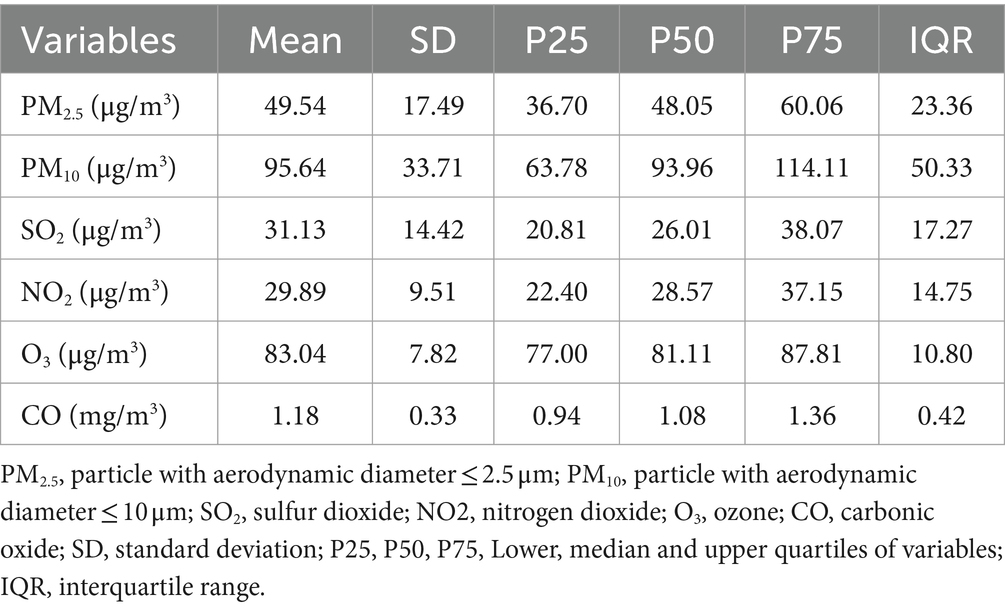
A three-year average ambient concentration of PM2.5, PM10, SO2, NO2, O3, and CO for the six air pollutants is shown in Table 2 and Figure 2 at 49.54 ± 17.49 μg/m3 for PM2.5, 95.64 ± 33.71 μg/m3 for PM10, 31.13 ± 14.42 μg/m3 for SO2, 29.89 ± 9.51 μg/m3 for NO2, 83.04 ± 7.82 μg/m3 for O3 and 1.18 ± 0.33 mg/m3 for CO. Additionally, Pearson correlation analysis revealed strong correlations among several pollutants. Specifically, PM2.5 and PM10 were the most strongly correlated (r = 0.89), indicative of their common sources or similar atmospheric behaviors. Notably strong correlations were also observed between NO2 and PM2.5 (r = 0.86), as well as between CO and SO2 (r = 0.82). The range of correlation coefficients between the major pollutants (PM2.5, PM10, SO2, NO2, O3, and CO) varied from 0.70 to 0.89. O3 showed a distinct pattern with lower correlation coefficients compared to other pollutants, as shown in Supplementary Table S2.
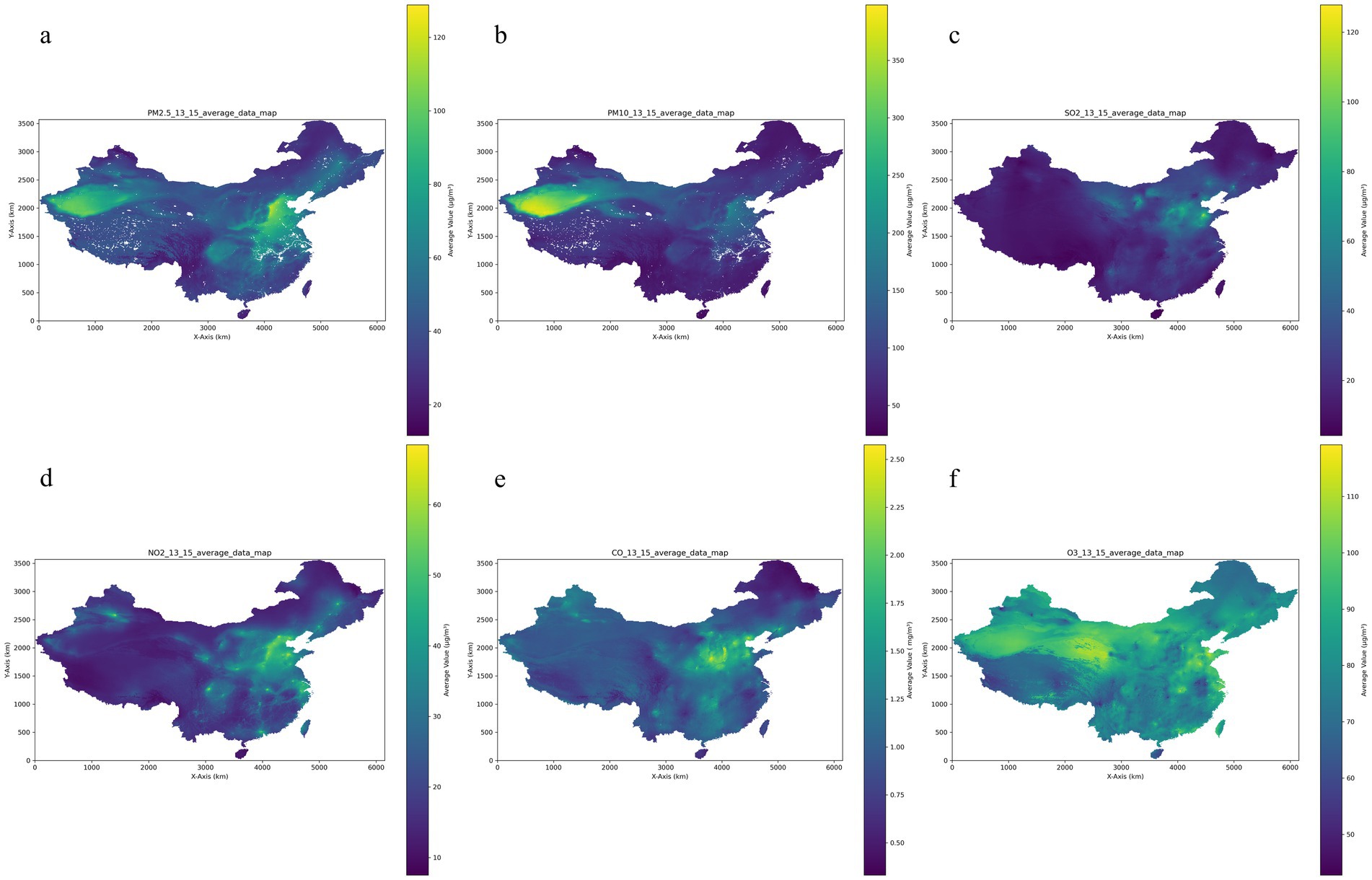
Figure 2. Air pollution distribution over 13–15 years of follow-up. (a) PM2.5: particle with aerodynamic diameter ≤ 2.5 μm; (b) PM10: particle with aerodynamic diameter ≤ 10 μm; (c) SO2: sulfur dioxide; (d) NO2: nitrogen dioxide; (e) CO: carbon monoxide; (f) O3: ozone.
3.2 Associations between air pollutants and NNDSD
The association between each air pollutant and NNDSD is shown in Figure 3 and Supplementary Table S3. Higher levels of exposure to air pollutants (PM2.5, PM10, SO2, NO2, O3, and CO) are associated with an increased risk of NNDSD. According to the crude model, for each additional exposure IQR of PM2.5, PM10, SO2, NO2, O3 and CO, the OR for NNDSD was 1.19 (95%CI: 1.06, 1.33), 1.36 (95%CI:1.22, 1.52), 1.44 (95%CI: 1.28, 1.61), 1.48 (95%CI: 1.32, 1.66), 1.30 (95%CI: 1.16, 1.46), and 1.36 (95%CI: 1.21, 1.53). Significant associations in the base model persisted after adjusting for demographic covariates. Taking into account all covariates, including age, sex, place of residence, education, marital status, body mass index, smoking, drinking, and health status, we observed a 6% (fully adjusted OR = 1.06, 95%CI: 0.94, 1.19), 23% (fully adjusted OR = 1.23, 95%CI: 1.09, 1.38), 26% (fully adjusted OR = 1.26, 95%CI: 1.12, 1.41), 30% (fully adjusted OR = 1.30, 95%CI: 1.16, 1.46), 13% (fully adjusted OR = 1.13, 95%CI: 1.01, 1.27) and 27% (fully adjusted OR = 1.27, 95%CI: 1.13, 1.43) increase in NNDSD risk with a IQR increase in PM2.5, PM10, SO2, NO2, O3, and CO exposure in the main model, respectively. PM2.5’s p-value in the main model is 0.34(>0.05), and the rest <0.05.
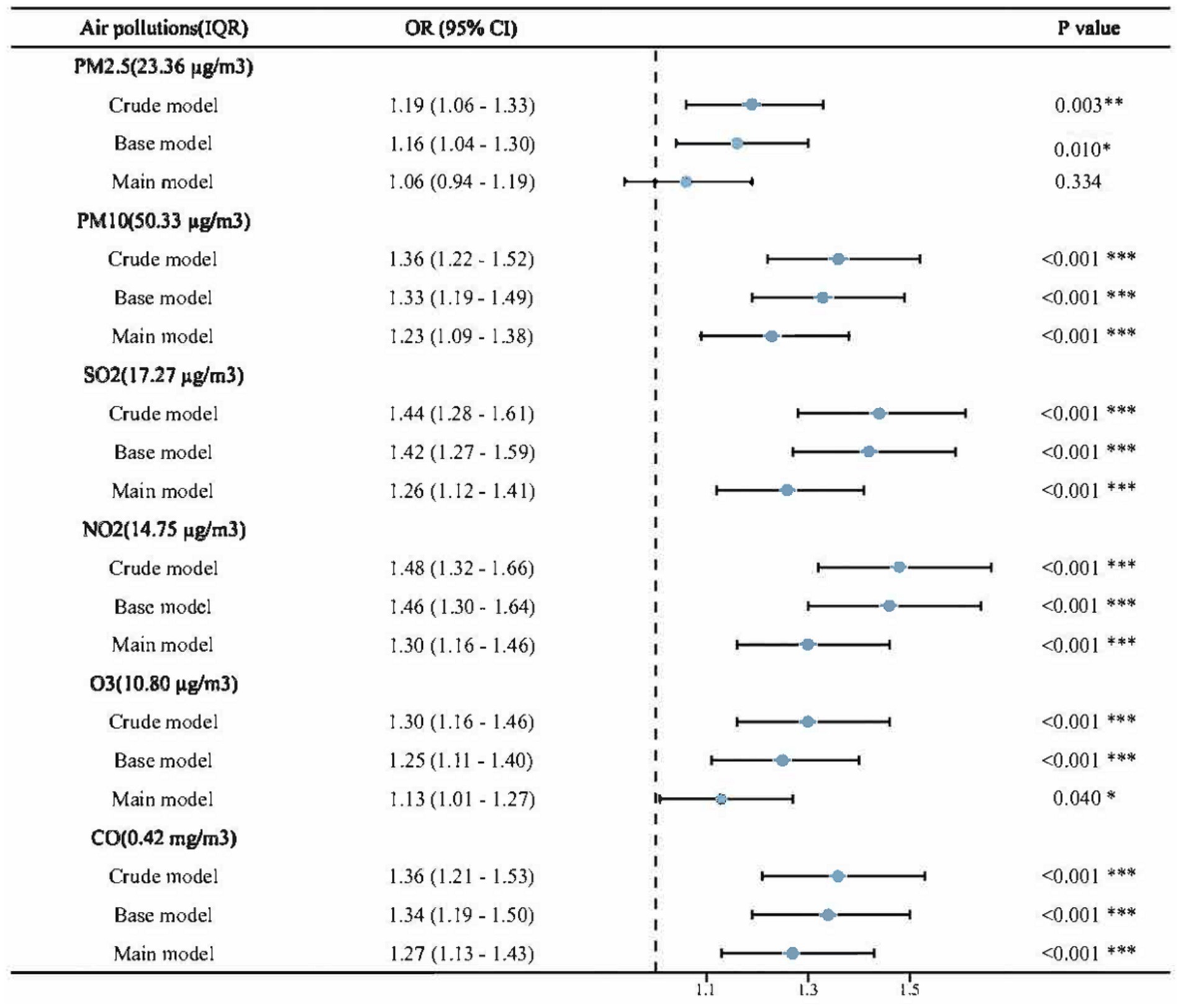
Figure 3. Associations between air pollution and NNDSD, per IQR increment in air pollutants. Crude model: no adjustment; Base model: Adjusted for age, gender, education level, marital status and residence; Main model: Base model + smoking, drinking, body mass index and health status. PM2.5, particle with aerodynamic diameter ≤ 2.5 μm; PM10, particle with aerodynamic diameter ≤ 10 μm; SO2, sulfur dioxide; NO2, nitrogen dioxide; CO, carbonic oxide; O3, ozone; NNDSD, non-neoplastic digestive system diseases; IQR, interquartile range; OR, odds ratios; CI, confidence interval. *p < 0.05, **p < 0.01, ***p < 0.001.
3.3 Subgroup and interaction analyses of air pollutants and NNDSD
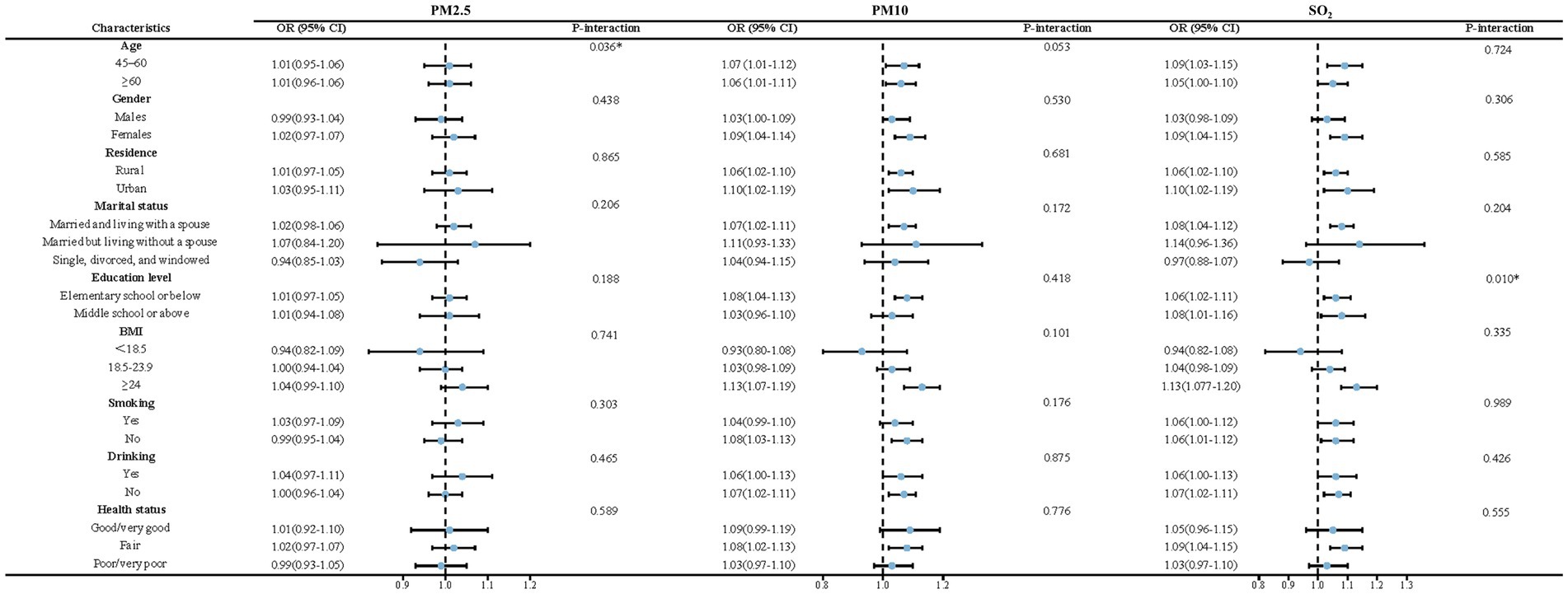
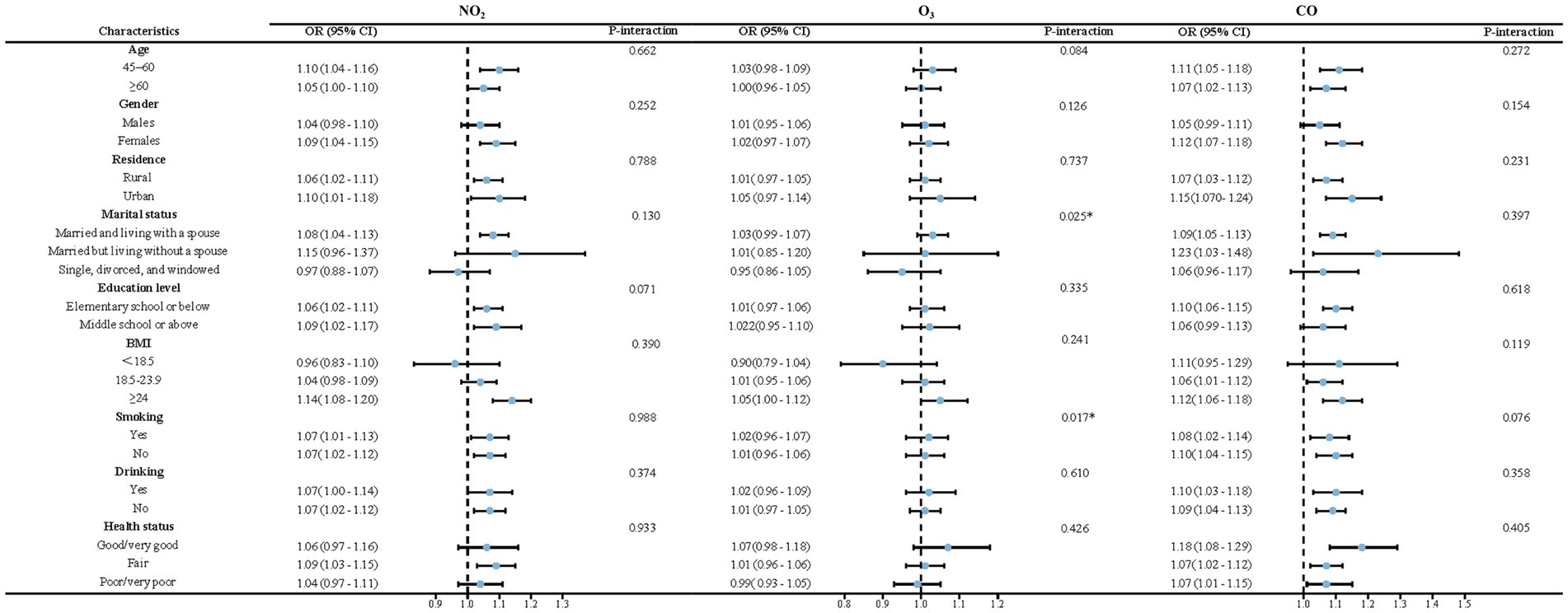
The results of the subgroup and interaction analyses are presented in Figures 4, 5. According to our findings, the following factors may modify the association between air pollution and NNDSD: PM2.5 significantly increased NNDSD risk in participants over 60 years of age (OR = 1.01, 95%CI: 0.96, 1.06), compared to participants aged 45–60 years (OR = 1.01, 95%CI: 0.95, 1.06) (P interaction = 0.04). In participants with higher education (OR = 1.08, 95%CI: 1.01, 1.16) as compared to those with lower education (OR = 1.06, 95%CI: 1.02, 1.11), SO2 had a greater effect on NNDSD in participants with higher education (P interaction = 0.01). In the group of smokers (OR = 1.02, 95%CI: 0.96, 1.07) (P interaction = 0.02) and of married or cohabiting couples (OR = 1.03, 95%CI: 0.99, 1.07) (P interaction = 0.03), ambient O3 had a greater effect on the rise in NNDSD.
3.4 Sensitivity analyses
The sensitivity analyses for the association between air pollution and NNDSD consistently demonstrated statistical significance in line with the main results. When the mean exposure concentration of air pollutants was reduced from 3 to 2 years, we observed that all the above significant associations remained strong (Supplementary Table S4). Additionally, we conducted sensitivity analyses that excluded poor/very poor health, which showed effect sizes and statistical significance similar to the main results, except for the association between exposure to O3 and NNDSD (Supplementary Table S5). In order to eliminate other potential confounders, sensitivity analyses were conducted when a 1:1 PSM was performed. Lastly, 3,430 participants with NNDSD were paired with the same number of participants non-NNDSD, and there were no significant differences in baseline characteristics between the two groups (Supplementary Table S7). Overall, these sensitivity analyses indicated that the main analysis results were robust (Supplementary Table S6).
4 Discussion
This study was one of the few national epidemiologic studies in China that investigated the relationship between continuous exposure to air pollutants and NNDSD. The purpose of this study was to investigate the effects of PM2.5, PM10, SO2, NO2, O3, and CO on NNDSD among middle-aged and older adult Chinese residents. Our findings revealed significant associations between several common air pollutants and the risk of NNDSD, with varying strengths across different pollutants. Nearly the entire global population is exposed to airborne pollution, recognized as a significant environmental health risk (19). In terms of policymaking aimed at protecting the public health from air pollution, our findings supported the hypothesis that air pollution exposure adversely affects NNDSD.
PM2.5 and PM10 are particulate air pollutants. Our study revealed that PM2.5 and PM10 demonstrate the strongest correlation in Pearson correlation analysis. Current research established a significant association between PM10 and NNDSD, persisting even after adjustment for confounding factors. Conversely, PM2.5 showed significant associations only in crude and base models, failing to maintain statistical significance in the main model. There appeared to be a difference in impact between air pollution particles of different sizes on digestive system health, with PM10 having a more pronounced effect in our study population than PM2.5.
This observation was corroborated by prior research. A comprehensive cross-sectional analysis involving 90,086 participants in southwest China demonstrated a positive association between prolonged exposure to environmental particulate matter and metabolically associated fatty liver disease. Additionally, animal studies have identified a steatohepatitis-like phenotype and liver fibrosis associated with such exposures, reinforcing the broader implications of particulate pollutants on liver health (20, 21). According to a cross-sectional study conducted in the United States, hospitalized patients with higher ambient PM2.5 exposure were more likely to develop non-alcoholic fatty liver disease (22). An extensive nationwide time-series study examined the adverse effects of short-term PM2.5 exposure on various digestive disorders, including intestinal infections, esophageal disease, gastritis, appendicitis, liver disease, gastrointestinal bleeding, and non-infectious gastroenteritis (23). On cooler days, there has been a significant increase in peptic ulcer hospitalizations in Taipei with rising levels of PM10 (24).
Research indicated that the impact of particulate air pollutants on the digestive system was primarily mediated through the ingestion of contaminants in food and water, as well as the direct effects of gaseous pollutants on the gastrointestinal tract during swallowing (25, 26). The deposition of air pollution particles in the lungs and the resultant epithelial cell responses, including the production of free radicals and inflammation, lead to the disruption of the alveolar barrier. These factors can subsequently enter the bloodstream, directly affecting intestinal epithelial cells and increasing intestinal permeability (27–30). Additionally, exposure to PM2.5 was associated with elevated levels of cluster of differentiation and systemic inflammation (31, 32). Animal studies demonstrate that PM10 exposure in mice leads to elevated pro-inflammatory cytokine levels in the small and large intestines, potentially stimulating alveolar macrophages to produce inflammatory factors present in the bloodstream during air pollution episodes (33, 34). Furthermore, studies have found that particulate air pollutants significantly altered the gut microbiota composition in both animals and humans, potentially increasing susceptibility to mucosal inflammation (17, 28, 30, 35).
We found a close association between SO2 levels and an increased risk of NNDSD. In subgroup and interaction analysis, we observed that the impact of environmental air pollutant SO2 on NNDSD was more pronounced among subjects with higher education. Residents near the Madin Dam in the State of Mexico showed a significant correlation between chronic exposure to SO₂ and liver function impairment. Specifically, individuals exposed to high levels of SO₂ exhibit elevated levels of lipid peroxidation products and oxidative stress markers in their blood, which are biomarkers indicating hepatocellular damage and dysfunction (36). In a study involving more than 2.7 million adults in Northwest China, each 10 μg/m3 increase in SO₂ concentration was associated with a 2.7-fold increase in the incidence risk of metabolically associated fatty liver disease (37). The increase in SO₂ levels have exacerbated inflammation and oxidative stress in the liver, both key drivers in the development of metabolically associated fatty liver disease. Research on pregnant women found that an increase in environmental SO₂ levels during the second trimester was associated with an increased risk of intrahepatic cholestasis of pregnancy (38). Long-term exposure to SO₂ may have increased pro-inflammatory cytokines such as IL-6 and IL-1β, and decreased hepatic bile transport proteins, leading to the accumulation of bile acids in the liver (39, 40).
A significant association between NO2 levels and NNDSD was observed. In our main model, an increase in NO2 exposure by one IQR was associated with a 30% increase in risk. An analysis of 8,566 older adult cases recorded between 2005 and 2010 by Tian et al. (41) found that a short-term increase in ambient NO2 levels may have increased the risk of peptic ulcer bleeding and related hospitalizations in Hong Kong’s older adult population. In a large-scale cohort study that investigated the associations between air pollutants and the risk of 12 gastrointestinal diseases, positive correlations were observed between NO2 and NOx with the risk of peptic ulcers and chronic gastritis (42). According to other studies, long-term exposures to NO2 were associated with ulcerative colitis but not Crohn’s disease (43). Research involving 329,048 adults in Taiwan and Hong Kong between 2001 and 2018 demonstrated that rising concentrations of NO₂ were linked to an increased risk of Non-Alcoholic Fatty Liver Disease (NAFLD) and its associated advanced fibrosis (44). Empirical evidence on the relationship between NO2 and digestive system diseases remained limited. As a component of traffic-related air pollution, NO2 was also found to be associated with elevated levels of cytokeratin-18, which may be linked to liver damage (45). NO2 swallowed through belching may trigger a nitrification reaction in the stomach, leading to redox interactions that disrupt the intestinal lining and promote gastrointestinal diseases (46).
Our study found that exposure to O3 is significantly associated with NNDSD, especially among smokers and those who are married or cohabiting. One research has shown that for every one standard deviation increase in O3 concentration, NAFLD decreases by 12%, and the incidence of advanced fibrosis decreases by 11%. This suggested that higher levels of O3 might have a protective effect against these conditions (44). Conversely, a study from Northwest China indicated that elevated O3 levels were linked to a slight increase in the risk of Metabolically Associated Fatty Liver Disease (37). Additionally, research from Korea reported a positive correlation between short-term exposure to O3 and elevated levels of gamma-glutamyl transferase (47). An analysis of the 7-day cumulative average of terrestrial O3 conducted by a Canadian researcher suggests that higher levels of environmental O3 exposure may increase the risk of perforated appendicitis (48). Kaplan’s findings indicated that higher 5-day average ozone levels increased appendicitis rates, especially in summer, with a greater impact on younger individuals than older adults. The specific mechanisms by which O3 influenced appendicitis incidence remained unclear, but inhaling or ingesting air pollutants likely triggered inflammatory responses in humans (49). Himuro (50) demonstrated that intrarectal administration of ozone gas causes transient epithelial cell damage, which is specifically caused by damage to DNA replication and cell cycle pathways. Exposure to O3 stimulated the production of tumor necrosis factors IL-6 and IL-8 and promotes systemic inflammation in humans, which may be a key factor in the pathogenesis of these diseases (51, 52).
In our study, exposure to CO was associated with an increased risk of NNDSD. There is limited literature on the impact of CO on digestive system disorders. Chinese researchers found that CO levels were significantly elevated on days of outpatient visits for enterocolitis (53). A study in Yichang, China, demonstrated that for every 1 mg/m3 increase in CO levels, there is a 19.04% (95% CI: 8.39, 29.68%) rise in daily outpatient visits for gastrointestinal diseases. From 2002 through 2007, Seo et al. (54) found that CO may increase the risk of GERD based on medical utilization data from the National Health Insurance Institute of Korea. Short-term exposure to CO air pollution was associated with decreased microvascular endothelial function (55). Microvascular dysfunction may have contributed to poor mucosal healing, refractory inflammatory ulcers, and intestinal injury in IBD patients, resulting in diminished vasodilatory capacity and inadequate tissue perfusion (56). CO’s high affinity for myoglobin may cause weakness in the lower esophageal sphincter, potentially leading to myasthenia gravis (57).
Our findings underscored the broader public health implications, suggesting that reducing air pollution levels could alleviate the burden of digestive system diseases among adults, particularly in areas with high pollution. Although many epidemiological studies link sustained air pollutant exposure to NNDSD risk, the underlying biological mechanisms remain unclear (58, 59). A biological and epidemiological study will be necessary to elucidate the mechanisms by which air pollution affects cognitive function in the future.
5 Limitations
There are several advantages to our study. Firstly, it is a nationwide study conducted in China, it offers a high level of generalizability. Secondly, this is the first study to investigate the relationship between air pollution exposure and NNDSD. Furthermore, we considered more air pollutants than previous studies, including the rarely considered NO2. However, we also have some limitations to our study. While we used satellite-based spatio-temporal modeling to estimate air pollution levels, exposure assessments were based on community locations, which may not fully represent individual exposures. Additionally, many individuals were excluded due to missing data on the variables, which may have led to a selection bias. Lastly, the findings may be affected by questionnaire and recall bias among older subjects. A lack of detailed types of NNDSD in this study also prevented a detailed study of life environmental factors on specific subtypes of NNDSD.
6 Conclusion
Our study suggests that chronic exposure to ambient air pollution may increase the prevalence of NNDSD among adults aged 45 and older in China. Particulates and gaseous pollutants like PM2.5, PM10, SO2, NO2, O3, and CO are significantly associated with NNDSD. This underscores the need for targeted air quality policies, which could reduce the NNDSD burden and prevent many cases.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found: https://charls.pku.edu.cn.
Ethics statement
In accordance with the Declaration of Helsinki, Peking University’s Ethics Review Board approved the CHARLS protocol (IRB00001052-11015). Informed consent was obtained from all participants.
Author contributions
YK: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. SD: Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization, Writing – original draft. WD: Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. WY: Conceptualization, Data curation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – review & editing. YY: Conceptualization, Data curation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – review & editing. LQ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Guangdong Provincial Medical Science and Technology Research Fund Project (Grant No. B2024202) and the High-Level Talent Research Project of the Affiliated Hospital of Guangdong Medical University (Grant No. GCC20230037).
Acknowledgments
Gratitude is extended to all the staff members involved in the creation of the China Health and Retirement Longitudinal Study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1372156/full#supplementary-material
References
1. Dawra, S, Behl, P, Srivastava, S, Manrai, M, Chandra, A, Kumar, A, et al. Non-neoplastic disorders in an aging gut: concise review. Egypt J Intern Med. (2023) 35:7. doi: 10.1186/s43162-023-00189-1
2. Okun, SD, and Lewin, DN. Non-neoplastic pancreatic lesions that may mimic malignancy. Semin Diagn Pathol. (2016) 33:31–42. doi: 10.1053/j.semdp.2015.09.005
3. Peery, AF, Crockett, SD, Murphy, CC, Jensen, ET, Kim, HP, Egberg, MD, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2021. Gastroenterology. (2022) 162:621–44. doi: 10.1053/j.gastro.2021.10.017
4. Peng, J, Xu, H, and Tang, X. Global inequalities in the burden of digestive diseases from 1990 to 2019: Findings from the global burden of disease study 2019. Gastroenterology. (2024) 166:223–224.e1. doi: 10.9734/bpi/dhrni/v3/1533
5. Schmocker, RK, and Lidor, AO. Management of non-neoplastic Gastric Lesions. Surg Clin North Am. (2017) 97:387–403. doi: 10.1016/j.suc.2016.11.011
6. Uemura, N, Okamoto, S, Yamamoto, S, Matsumura, N, Yamaguchi, S, Yamakido, M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. (2001) 345:784–9. doi: 10.1056/NEJMoa001999
7. Wang, R, Li, Z, Liu, S, and Zhang, D. Global, regional, and national burden of 10 digestive diseases in 204 countries and territories from 1990 to 2019. Front Public Health. (2023) 11:1061453. doi: 10.3389/fpubh.2023.1061453
8. Yin, P, Brauer, M, Cohen, AJ, Wang, H, Li, J, Burnett, RT, et al. The effect of air pollution on deaths, disease burden, and life expectancy across China and its provinces, 1990-2017: an analysis for the global burden of disease study 2017. Lancet Planet Health. (2020) 4:e386–98. doi: 10.1016/S2542-5196(20)30161-3
9. GBD. Diabetes and air pollution collaborators. Estimates, trends, and drivers of the global burden of type 2 diabetes attributable to PM2·5 air pollution, 1990-2019: An analysis of data from the Global Burden of Disease Study 2019. Lancet Planet Health. (2022) 6:e586–e600. doi: 10.1016/S2542-5196(22)00122-X
10. Bowe, B, Artimovich, E, Xie, Y, Yan, Y, Cai, M, and Al-Aly, Z. The global and national burden of chronic kidney disease attributable to ambient fine particulate matter air pollution: a modelling study. BMJ Glob Health. (2020) 5:e002063. doi: 10.1136/bmjgh-2019-002063
11. Safiri, S, Carson-Chahhoud, K, Noori, M, Nejadghaderi, SA, Sullman, MJM, Ahmadian Heris, J, et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: results from the global burden of disease study 2019. BMJ. (2022) 378:e069679. doi: 10.1136/bmj-2021-069679
12. Turner, MC, Krewski, D, Diver, WR, Pope, CA 3rd, Burnett, RT, Jerrett, M, et al. Ambient air pollution and Cancer mortality in the Cancer prevention study II. Environ Health Perspect. (2017) 125:087013. doi: 10.1289/EHP1249
13. Ji, Y, Su, X, Zhang, F, Huang, Z, Zhang, X, Chen, Y, et al. Impacts of short-term air pollution exposure on appendicitis admissions: evidence from one of the most polluted cities in mainland China. Front Public Health. (2023) 11:1144310. doi: 10.3389/fpubh.2023.1144310
14. Dorofeyev, A, Dorofeyeva, A, Borysov, A, Tolstanova, G, and Borisova, T. Gastrointestinal health: changes of intestinal mucosa and microbiota in patients with ulcerative colitis and irritable bowel syndrome from PM(2.5)-polluted regions of Ukraine. Environ Sci Pollut Res Int. (2023) 30:7312–24. doi: 10.1007/s11356-022-22710-9
15. Guo, B, Huang, S, Li, S, Han, X, Lin, H, Li, Y, et al. Long-term exposure to ambient PM2.5 and its constituents is associated with MAFLD. JHEP Report. (2023) 5:100912. doi: 10.1016/j.jhepr.2023.100912
16. Feng, J, Cavallero, S, Hsiai, T, and Li, R. Impact of air pollution on intestinal redox lipidome and microbiome. Free Radic Biol Med. (2020) 151:99–110. doi: 10.1016/j.freeradbiomed.2019.12.044
17. Mutlu, EA, Engen, PA, Soberanes, S, Urich, D, Forsyth, CB, Nigdelioglu, R, et al. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part Fibre Toxicol. (2011) 8:19. doi: 10.1186/1743-8977-8-19
18. Bosch, AJT, Rohm, TV, AlAsfoor, S, Low, AJY, Baumann, Z, Parayil, N, et al. Diesel exhaust particle (DEP)-induced glucose intolerance is driven by an intestinal innate immune response and NLRP3 activation in mice. Part Fibre Toxicol. (2023) 20:25. doi: 10.1186/s12989-023-00536-8
19. Fuller, R, Landrigan, PJ, Balakrishnan, K, Bathan, G, Bose-O'Reilly, S, Brauer, M, et al. Pollution and health: a progress update. Lancet Planet Health. (2022) 6:e535–47. doi: 10.1016/S2542-5196(22)00090-0
20. Guo, B, Guo, Y, Nima, Q, Feng, Y, Wang, Z, Lu, R, et al. Exposure to air pollution is associated with an increased risk of metabolic dysfunction-associated fatty liver disease. J Hepatol. (2022) 76:518–25. doi: 10.1016/j.jhep.2021.10.016
21. Zheng, Z, Zhang, X, Wang, J, Dandekar, A, Kim, H, Qiu, Y, et al. Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models. J Hepatol. (2015) 63:1397–404. doi: 10.1016/j.jhep.2015.07.020
22. VoPham, T, Kim, NJ, Berry, K, Mendoza, JA, Kaufman, JD, and Ioannou, GN. PM(2.5) air pollution exposure and nonalcoholic fatty liver disease in the Nationwide inpatient sample. Environ Res. (2022) 213:113611. doi: 10.1016/j.envres.2022.113611
23. Gu, J, Shi, Y, Zhu, Y, Chen, N, Wang, H, Zhang, Z, et al. Ambient air pollution and cause-specific risk of hospital admission in China: a nationwide time-series study. PLoS Med. (2020) 17:e1003188. doi: 10.1371/journal.pmed.1003188
24. Tsai, SS, and Chiu, HF. Yang CY: ambient air pollution and hospital admissions for peptic ulcers in Taipei: a time-stratified case-crossover study. Int J Environ Res Public Health. (2019) 16:1916. doi: 10.3390/ijerph16111916
25. Beamish, LA, Osornio-Vargas, AR, and Wine, E. Air pollution: An environmental factor contributing to intestinal disease. J Crohns Colitis. (2011) 5:279–86. doi: 10.1016/j.crohns.2011.02.017
26. Möller, W, Häussinger, K, Winkler-Heil, R, Stahlhofen, W, Meyer, T, Hofmann, W, et al. Mucociliary and long-term particle clearance in the airways of healthy nonsmoker subjects. J Appl Physiol. (2004) 97:2200–6. doi: 10.1152/japplphysiol.00970.2003
27. Frey, HC, Adams, PJ, Adgate, JL, Allen, GA, Balmes, J, Boyle, K, et al. The need for a tighter particulate-matter air-quality standard. N Engl J Med. (2020) 383:680–3. doi: 10.1056/NEJMsb2011009
28. Mutlu, EA, Comba, IY, Cho, T, Engen, PA, Yazıcı, C, Soberanes, S, et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ Poll. (1987) 240:817–30. doi: 10.1016/j.envpol.2018.04.130
29. Maeda, T, Miyazono, Y, Ito, K, Hamada, K, Sekine, S, and Horie, T. Oxidative stress and enhanced paracellular permeability in the small intestine of methotrexate-treated rats. Cancer Chemother Pharmacol. (2010) 65:1117–23. doi: 10.1007/s00280-009-1119-1
30. Salim, SY, Kaplan, GG, and Madsen, KL. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut Microbes. (2014) 5:215–9. doi: 10.4161/gmic.27251
31. Pope, CA 3rd, Bhatnagar, A, McCracken, JP, Abplanalp, W, Conklin, DJ, and O'Toole, T. Exposure to fine particulate air pollution is associated with endothelial injury and systemic inflammation. Circ Res. (2016) 119:1204–14. doi: 10.1161/CIRCRESAHA.116.309279
32. Zhang, Y, Wang, S, Zhu, J, Li, C, Zhang, T, Liu, H, et al. Effect of atmospheric PM2.5 on expression levels of NF-κB genes and inflammatory cytokines regulated by NF-κB in human macrophage. Inflammation. (2018) 41:784–94. doi: 10.1007/s10753-018-0732-8
33. Salim, SY, Jovel, J, Wine, E, Kaplan, GG, Vincent, R, Thiesen, A, et al. Exposure to ingested airborne pollutant particulate matter increases mucosal exposure to bacteria and induces early onset of inflammation in neonatal IL-10-deficient mice. Inflamm Bowel Dis. (2014) 20:1129–38. doi: 10.1097/MIB.0000000000000066
34. van Eeden, SF, Tan, WC, Suwa, T, Mukae, H, Terashima, T, Fujii, T, et al. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med. (2001) 164:826–30. doi: 10.1164/ajrccm.164.5.2010160
35. Ran, Z, An, Y, Zhou, J, Yang, J, Zhang, Y, Yang, J, et al. Subchronic exposure to concentrated ambient PM2.5 perturbs gut and lung microbiota as well as metabolic profiles in mice. Environ Pollut. (1987) 272:115987. doi: 10.1016/j.envpol.2020.115987
36. Ruiz-Lara, K, García-Medina, S, Galar-Martínez, M, Parra-Ortega, I, Morales-Balcázar, I, Hernández-Rosas, NA, et al. The evaluation of liver dysfunction and oxidative stress due to urban environmental pollution in Mexican population related to Madin dam, state of Mexico: a pilot study. Environ Sci Pollut Res Int. (2023) 30:6950–64. doi: 10.1007/s11356-022-22724-3
37. Ji, W, Cheng, Y, Tang, S, Gu, K, Liao, H, Li, L, et al. Exposure to ambient air pollution and metabolic dysfunction-associated fatty liver disease: findings from over 2.7 million adults in northwestern China. Ecotoxicol Environ Saf. (2024) 272:116109. doi: 10.1016/j.ecoenv.2024.116109
38. Li, C, Yu, JL, Xu, JJ, He, YC, Qin, KZ, Chen, L, et al. Interactive effects of ambient air pollution and sunshine duration on the risk of intrahepatic cholestasis of pregnancy. Environ Res. (2022) 215:114345. doi: 10.1016/j.envres.2022.114345
39. Kim, JW, Park, S, Lim, CW, Lee, K, and Kim, B. The role of air pollutants in initiating liver disease. Toxicol Res. (2014) 30:65–70. doi: 10.5487/TR.2014.30.2.065
40. Geier, A, Dietrich, CG, Voigt, S, Kim, SK, Gerloff, T, Kullak-Ublick, GA, et al. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology. (2003) 38:345–54. doi: 10.1053/jhep.2003.50317
41. Tian, L, Qiu, H, Sun, S, Tsang, H, Chan, KP, and Leung, WK. Association between emergency admission for peptic ulcer bleeding and air pollution: a case-crossover analysis in Hong Kong's elderly population. Lancet Planet Health. (2017) 1:e74–81. doi: 10.1016/S2542-5196(17)30021-9
42. Li, J, He, C, Ying, J, Hua, B, Yang, Y, Chen, W, et al. Air pollutants, genetic susceptibility, and the risk of incident gastrointestinal diseases: a large prospective cohort study. Environ Res. (2024) 247:118182. doi: 10.1016/j.envres.2024.118182
43. Li, FR, Wu, KY, Fan, WD, Chen, GC, Tian, H, and Wu, XB. Long-term exposure to air pollution and risk of incident inflammatory bowel disease among middle and old aged adults. Ecotoxicol Environ Saf. (2022) 242:113835. doi: 10.1016/j.ecoenv.2022.113835
44. Bo, Y, Lin, C, Guo, C, Wong, M, Huang, B, Lau, A, et al. Chronic exposure to ambient air pollution and the risk of non-alcoholic fatty liver disease: a cross-sectional study in Taiwan and Hong Kong. Ecotoxicol Environ Saf. (2024) 275:116245. doi: 10.1016/j.ecoenv.2024.116245
45. Hsieh, S, Leaderer, BP, Feldstein, AE, Santoro, N, McKay, LA, Caprio, S, et al. Traffic-related air pollution associations with cytokeratin-18, a marker of hepatocellular apoptosis, in an overweight and obese paediatric population. Pediatr Obes. (2018) 13:342–7. doi: 10.1111/ijpo.12228
46. Ma, L, Hu, L, Feng, X, and Wang, S. Nitrate and nitrite in health and disease. Aging Dis. (2018) 9:938–45. doi: 10.14336/AD.2017.1207
47. Kim, KN, Lee, H, Kim, JH, Jung, K, Lim, YH, and Hong, YC. Physical activity- and alcohol-dependent association between air pollution exposure and elevated liver enzyme levels: An elderly panel study. J Prev Med Public Health. (2015) 48:151–69. doi: 10.3961/jpmph.15.014
48. Kaplan, GG, Tanyingoh, D, Dixon, E, Johnson, M, Wheeler, AJ, Myers, RP, et al. Ambient ozone concentrations and the risk of perforated and non-perforated appendicitis: a multicity case-crossover study. Environ Health Perspect. (2013) 121:939–43. doi: 10.1289/ehp.1206085
49. Kaplan, GG, Dixon, E, Panaccione, R, Fong, A, Chen, L, Szyszkowicz, M, et al. Effect of ambient air pollution on the incidence of appendicitis. CMAJ. (2009) 181:591–7. doi: 10.1503/cmaj.082068
50. Himuro, H. The effect of ozone on colonic epithelial cells. Kurume Med J. (2018) 64:75–81. doi: 10.2739/kurumemedj.MS644002
51. Thompson, AM, Zanobetti, A, Silverman, F, Schwartz, J, Coull, B, Urch, B, et al. Baseline repeated measures from controlled human exposure studies: associations between ambient air pollution exposure and the systemic inflammatory biomarkers IL-6 and fibrinogen. Environ Health Perspect. (2010) 118:120–4. doi: 10.1289/ehp.0900550
52. Bocci, V, Valacchi, G, Corradeschi, F, and Fanetti, G. Studies on the biological effects of ozone: 8. Effects on the total antioxidant status and on interleukin-8 production. Mediat Inflamm. (1998) 7:313–7. doi: 10.1080/09629359890820
53. Xu, C, Kan, HD, Fan, YN, Chen, RJ, Liu, JH, Li, YF, et al. Acute effects of air pollution on enteritis admissions in Xi'an, China. J Toxicol Environ Health A. (2016) 79:1183–9. doi: 10.1080/15287394.2016.1227006
54. Seo, HS, Hong, J, and Jung, J. Relationship of meteorological factors and air pollutants with medical care utilization for gastroesophageal reflux disease in urban area. World J Gastroenterol. (2020) 26:6074–86. doi: 10.3748/wjg.v26.i39.6074
55. Zhang, X, Staimer, N, Tjoa, T, Gillen, DL, Schauer, JJ, Shafer, MM, et al. Associations between microvascular function and short-term exposure to traffic-related air pollution and particulate matter oxidative potential. Environ Health. (2016) 15:81. doi: 10.1186/s12940-016-0157-5
56. Britzen-Laurent, N, Weidinger, C, and Stürzl, M. Contribution of blood vessel activation, remodeling and barrier function to inflammatory bowel diseases. Int J Mol Sci. (2023) 24:5517. doi: 10.3390/ijms24065517
57. Ny, L, Alm, P, Ekström, P, Larsson, B, Grundemar, L, and Andersson, KE. Localization and activity of haem oxygenase and functional effects of carbon monoxide in the feline lower oesophageal sphincter. Br J Pharmacol. (1996) 118:392–9. doi: 10.1111/j.1476-5381.1996.tb15415.x
58. Zhou, M, Wang, H, Zeng, X, Yin, P, Zhu, J, Chen, W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2019) 394:1145–58. doi: 10.1016/S0140-6736(19)30427-1
Keywords: digestive disease, air pollution, older adult, CHARLS, Chinese
Citation: Kou Y, Du S, Du W, Ye W, Yang Y and Qin L (2024) Exposure to air pollution and non-neoplastic digestive system diseases: findings from the China health and retirement longitudinal study. Front. Public Health. 12:1372156. doi: 10.3389/fpubh.2024.1372156
Edited by:
Alessandra Pulliero, University of Genoa, ItalyReviewed by:
Worradorn Phairuang, Chiang Mai University, ThailandLeila Moftakhar, Abadan University of Medical Sciences, Iran
Copyright © 2024 Kou, Du, Du, Ye, Yang and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Qin, enpxcTc3N0AxMjYuY29t; Yuping Yang, eWFuZ3l1cGluZ2NobkAxNjMuY29t
†These authors have contributed equally to this work
 Yanqi Kou
Yanqi Kou Shenshen Du
Shenshen Du Weiwei Du4†
Weiwei Du4† Yuping Yang
Yuping Yang