
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 23 November 2023
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1244357
This article is part of the Research TopicWorld Antimicrobial Awareness WeekView all 25 articles
 Abiola Senok1,2*†
Abiola Senok1,2*† Jens Thomsen3,4†
Jens Thomsen3,4† Najiba M. Abdulrazzaq5,6
Najiba M. Abdulrazzaq5,6 The UAE AMR Surveillance Consortium§
The UAE AMR Surveillance Consortium§ Godfred Antony Menezes7‡
Godfred Antony Menezes7‡ Carole Ayoub Moubareck8‡
Carole Ayoub Moubareck8‡ Dean Everett4,9,10‡
Dean Everett4,9,10‡Introduction: Although pneumococcal conjugate vaccines (PCV) have been effective in reducing the burden of Streptococcus pneumoniae infections, there is a paucity of data on the relationship with antimicrobial resistance (AMR) trends in the Arabian Gulf region. This study was carried out to assess S. pneumoniae resistance trends in the United Arab Emirates (UAE) where PCV-13 vaccination was introduced in 2011.
Methods: Retrospective analysis of S. pneumoniae demographic and microbiological data collected as part of the national AMR surveillance program from 2010 to 2021 was carried out. A survey of reporting sites and hand searching of annual reports of local health authorities was carried out to identify data on S. pneumoniae serotypes as this is not included in the AMR surveillance database.
Results: From 2010 to 2021, 11,242 non-duplicate S. pneumoniae isolates were reported, increasing from 324 in 2010 to 1,115 in 2021. Factoring in annual increment in the number of surveillance sites, the number of isolates per site showed an upward trajectory from 2015 to 2018 and declined in 2020 with the onset of the pandemic. The majority of isolates (n/N = 5,751/11,242; 51.2%) were from respiratory tract specimens with 44.5% (n/N = 2,557/5,751) being nasal colonizers. Up to 11.9% (n/N = 1,337/11,242) were invasive pneumococcal disease (IPD) isolates obtained from sterile site specimens including blood (n = 1,262), cerebrospinal (n = 52), pleural (n = 19) and joint (n = 4) fluid; and were predominantly from pediatric patients. The downward trend for amoxicillin and for penicillin G at the non-meningitis and meningitis as well as oral penicillin breakpoints was statistically significant. In contrast, increasing trends of resistance were seen for levofloxacin, moxifloxacin, trimethoprim/sulfamethoxazole and erythromycin. IPD and non-IPD isolates showed similar demographic and AMR trends. None of the surveillance sites carried out S. pneumoniae serotyping and handsearching of annual reports did not yield this information.
Conclusion: The increasing trend of pneumococcal disease and AMR with emergence of isolates with MDR phenotype despite is of concern. In the absence of S. pneumoniae serotyping the role of non-vaccine serotypes in driving this pattern remains unknown. There is an urgent need for serotype, genomic and AMR surveillance of S. pneumoniae isolates in the UAE.
Streptococcus pneumoniae is a major cause of disease in children and adults with associated high burden of morbidity and mortality. In 2015, the global mortality rate attributed to pneumococcal infection was 45 deaths (29-56) per 100,000 among children aged 1–59 months (1). The spectrum of clinical manifestations ranges from non-invasive disease such as otitis media, to invasive pneumococcal disease (IPD) such as meningitis and bacteremia. In a recent report on the global analysis of lower respiratory tract infections, pneumococcal pneumonia caused more deaths than all other etiologies combined accounting for 1,189,937 deaths in 2016 (2). Although there are >100 serotypes of S. pneumoniae (3, 4), only a limited number are responsible for most IPD. The introduction of vaccines played a role in reducing the burden of morbidity and mortality associated with common vaccine preventable infectious diseases (5, 6). Indeed, this has been demonstrable with pneumococcal conjugate vaccines (PCV), wherein the initial introduction of the seven-valent pneumococcal conjugate vaccine (PCV7) and the subsequent 13-valent vaccine (PCV13) have been effective in reducing the burden of pneumococcal disease in children and adults (7).
Antimicrobial resistance (AMR) is a major global health threat with ~700,000 attributable deaths annually and a projected increase to 10 million by 2050 (8). It has been suggested that by reducing the numbers of the target microbe (both antibiotic susceptible and resistant strains) in circulation, vaccination programs could be a promising additional weapon in the fight against AMR (6, 9). Furthermore, with fewer occurrences of clinical infections following vaccination, a drop in antibiotic utilization is expected which could reduce selection pressure and emergence of resistance strains. For S. pneumoniae, pneumococcal carriage has been described as a critical source of horizontal spread in the community and the effect of vaccination on reduction of nasopharyngeal colonization could also impact antibiotic resistance (10, 11). However, the occurrence of serotype replacement by non-vaccine serotypes and the association with antibiotic resistant pneumococcal strains could negate the expected reduction in AMR trends (7, 9, 10, 12). This highlights the need for surveillance of AMR in S. pneumoniae particularly the tracking of emergent trends after the onset of a PCV vaccination program.
The UAE is highly cosmopolitan with dynamic population movement of large numbers of expatriate residents and tourists from across the world. Hence the emergence and dissemination of AMR pathogens is a concern particularly with reports of novel and variant strains in circulation (13, 14). In the 2011–2013 Survey of Antibiotic Resistance (SOAR) in the Gulf States, susceptibility of S. pneumoniae to most of the antibiotics tested was found to be consistently lower in UAE compared to other countries in the region (15). An earlier report by Senok et al. (16) had identified a high level of penicillin resistance, elevated macrolide and fluoroquinolone resistance and the occurrence of multidrug resistance phenotypes among S. pneumoniae isolated between 2004 and 2006 from patients with community acquired respiratory tract infections in the UAE. In addition, despite the significant risk factors for pneumococcal disease in the Arabian Gulf region, and the calls for heightened pneumococcal surveillance, there remains a paucity of published literature on the burden of pneumococcal infections (17). Indeed, in the UAE, the only two published studies on pneumococcal burden are based on single center data obtained prior to the 2007 introduction of PCV-7 (18, 19). These studies showed a higher incidence rate of pneumococcal disease relative to developed countries (19) with S. pneumoniae as causative agent of 9% of community acquired pneumonia (18). To address this gap in the literature, this report describes S. pneumoniae epidemiology and antibiotic resistance trends in the UAE over a twelve-year period.
This study is a retrospective data analysis for the twelve-year period 2010–2021. This timeframe includes one year prior to the 2011 introduction of PCV-13 in the UAE. AMR trends in S. pneumoniae were assessed by analysis of routine patient care national level AMR surveillance data.
The national AMR surveillance data is collected from a network of participating healthcare facilities and diagnostic laboratories across the country. These include primary, secondary and tertiary care facilities across governmental and private healthcare sectors. All data are collected from routine patient care, cleaned, and analyzed using a unified platform1 as described by Thomsen et al. (20). Training on data collection is provided to ensure quality assurance, standardization and accuracy. The fully anonymized data includes demographic data (age, gender, nationality, hospital site/location etc.), clinical and microbiological data such as specimen source and antibiogram. Pediatric age group was defined as newborn up to 18 years and those aged 19 years and above were categorized as adults. As clinical diagnosis is not routinely included in the dataset, the isolation of S. pneumoniae from blood, cerebrospinal fluid and other normally sterile body sites (e.g., joint, pleural and pericardial fluid) were used as indicators of IPD in line with the Centers for Disease Control and Prevention IPD case definition (https://ndc.services.cdc.gov/case-definitions/invasive-pneumococcal-disease-2017/).
The participating centers used at least one commercial, automated system for bacterial identification and antimicrobial susceptibility testing. These automated systems include VITEK® (BioMérieux SA, Craponne, France), BD Phoenix™ (Becton Dickinson, New Jersey, USA) and MicroScan WalkAway (Beckman Coulter, Brea, CA, USA) and were used in conformity with manufacturer guidelines. Only one laboratory relied solely on manual system for bacterial identification using API® (Analytical Profile Index. BioMérieux SA, Craponne, France). Two laboratories used manual antimicrobial testing methods (disc diffusion/Kirby Bauer). For the reporting of antimicrobial resistance, CLSI breakpoints were routinely applied by reporting sites and at the central level to determine susceptibility profiles of isolates (21).
Having an understanding of the S. pneumoniae serotype in circulation is of tremendous importance. However, the national AMR surveillance dataset does not include the crucial information on S. pneumoniae serotypes. To determine if S. pneumoniae serotyping was being carried out and if so to obtain data on the serotypes that have identified, we used two approaches to source for this data. Firstly, participating sites and laboratories in the national AMR surveillance program were requested via email questionnaire to indicate if S. pneumoniae serotyping is currently being undertaken or had previously been carried out in the last ten years and if so, they were requested to provide the data if available. Secondly, handsearching of publicly available annual reports (for the years 2010–2020) of the health authorities namely Department of Health, Abu Dhabi (DOH), Dubai Health Authority (DHA), and the Ministry of Health and Prevention (MoHAP) for reported data on S. pneumoniae serotypes and IPD was carried out. The DOH Quarterly Communicable Disease Bulletins (https://www.doh.gov.ae/en/resources/publication) and Open Data Dashboard (https://www.doh.gov.ae/en/resources/opendata), the DHA statistical report (https://www.dha.gov.ae/en/open-data) and MOHAP Opendata dashboard (https://mohap.gov.ae/en/open-data/mohap-open-data) were searched.
Data analysis was routinely carried out using the WHONET 2023 software. For additional statistical analysis other software packages used were IBM SPSS Statistics, version 28.0 (IBM SPSS Software), and EpiInfoTM for Windows v7.2.4.2022, Centers for Disease Control and Prevention. Statistical significance of temporal trends for antimicrobial resistance was calculated if data from at least five consecutive years was available. Statistical significance of trends is expressed as a p-value, calculated by a Chi-square for trend test (extended Mantel-Haenszel). A p-value of <0.05 was considered statistically significant. A 95% confidence interval is determined for the percentage of resistance and susceptibility based on the Wilson Score Interval with or without continuity correction method for calculating confidence intervals for a sample proportion (normal approximation to a binomial distribution) (22).
The number of reporting sites increased from 22 in 2010 to 317 in 2021 (Figure 1). These comprised of primary, secondary and tertiary care facilities across the public and private health sectors. From 2014 to 2021, the participating centers were distributed across all the seven emirates in the country in contrast to the period 2010–2012 when data was only obtained from Abu Dhabi emirate and from five emirates in 2013.
From 2010 to 2021, 11,242 non-duplicate isolates (representative of patients associated with S. pneumoniae) were reported, increasing from 324 in 2010 to 1,115 in 2021 (Figure 2). When normalized for the increased number of reporting sites per annum, after an initial decline between 2011 and 2014, the number of isolates per site increased between 2015 and 2018 with a plateau in 2019 (Figure 2). A sharp decline in reported S. pneumoniae isolates was observed in 2020 during the COVID-19 pandemic (Figure 2). The demographic distribution of the patients from whom isolates were obtained revealed a male preponderance with majority of patients being in the pediatric age group (Table 1). S. pneumoniae were predominantly isolated from respiratory tract specimens, which include nasopharyngeal swabs, sputum, bronchoalveolar lavage, tracheal aspirate and pleural fluid (n/N = 5,751/11,242; 51.2%). Isolates from nasopharyngeal swab specimens (indicative of colonization) represented 22.7% (n/N = 2,557/11,242) of all reported isolates and 44.5% (n/N = 2,557/5,751) of respiratory tract isolates. Figure 3 shows the distribution of specimen types where S. pneumoniae were isolated from. The isolation of S. pneumoniae in specimens from sterile sites which included blood, cerebrospinal, pleural and joint fluid was used as a marker of IPD. Up to 11.9% (n/N = 1,337/11,242) of all the S. pneumoniae isolates were from these sterile sites. Comparison of patients' demographics for IPD and non-IPD isolates revealed male preponderance in both groups with higher occurrence of hospitalization among IPD patients (51.1%), as compared to non-IPD patients (25.3%) (Table 2). There were more adult patients with IPD in contrast to the higher proportion of pediatric patients in the non-IPD group (Table 2). Patients with IPD were associated with a higher mortality rate (2.2%) as compared to patients with non-IPD (0.7%) (p < 0.001) (Table 2). Inpatients with IPD were associated with longer duration of hospitalization (median: 7 days), as compared to inpatients with non-IPD (median: 5 days). Changes in trend for IPD and non-IPD over time was only observed for nationality, with a decline in the percentage of Emirati nationals with IPD from 50.9% in 2010 to 33.3% in 2021, as well as those with non-IPD from 65.9% in 2010 to 40.4% in 2021.
Figure 4 shows the antimicrobial resistance trend for the beta lactam class of antibiotics. A statistically significant downward trend was observed for penicillin G at the non-meningitis and meningitis as well as oral penicillin breakpoints, and a similar trend was observed for amoxicillin (Figure 4). Despite the downward resistance trend, the proportion of S. pneumoniae isolates resistant to penicillin G at the meningitis breakpoint was over 45% which was much higher compared to other beta lactam antibiotics. For cefuroxime, although data was only available for 2017–2021, an upward trend in resistance was observed (Figure 4). For levofloxacin and moxifloxacin, although the proportion of resistant isolates was low across the study period (under 10%), there was an increasing trend which was statistically significant (Figure 5). Fluctuations were observed in the resistance trends for erythromycin, clindamycin, tetracycline and trimethoprim/sulfamethoxazole. However, the overall trends showed an upward trajectory which was statistically significant for trimethoprim/sulfamethoxazole (Figure 5). The proportion of multidrug resistant (MDR) isolates (resistance to 3 or more classes of antibiotics) increased from 16.4% (in 2013) to 42.2% in 2021 and this upward trend was statistically significant (p < 0.001; Figure 6). A comparison of IPD and non-IPD isolates did not reveal any differences between these two groups in the upward trend of MDR phenotype which was sustained at over 30% from 2018 to 2021 (Figure 6).
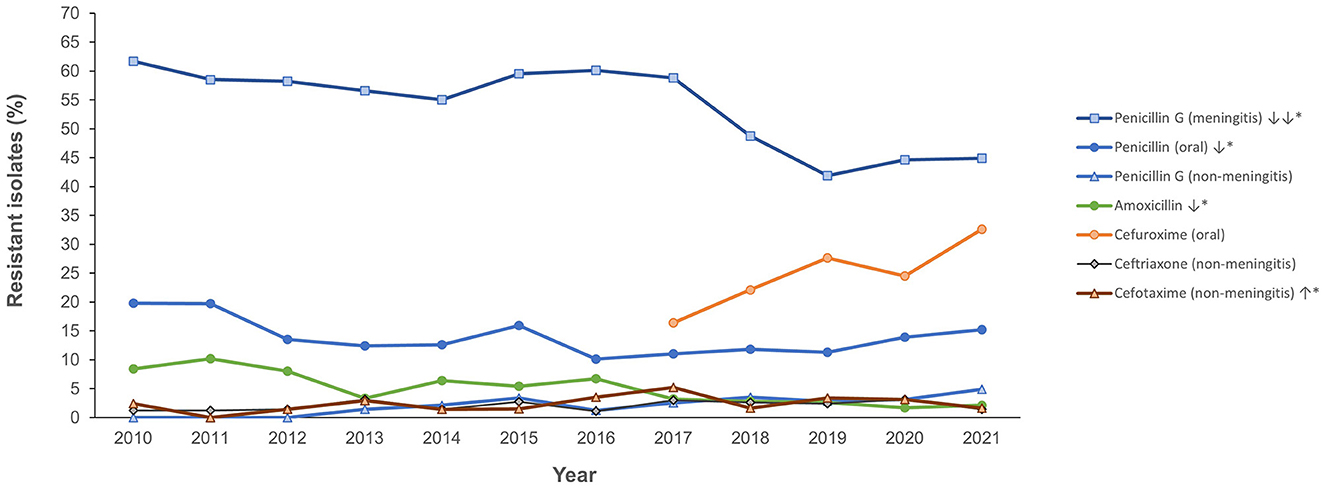
Figure 4. Resistance trend for beta lactam antibiotics (2010–2021). *Trend is statistically significant (p < 0.05).
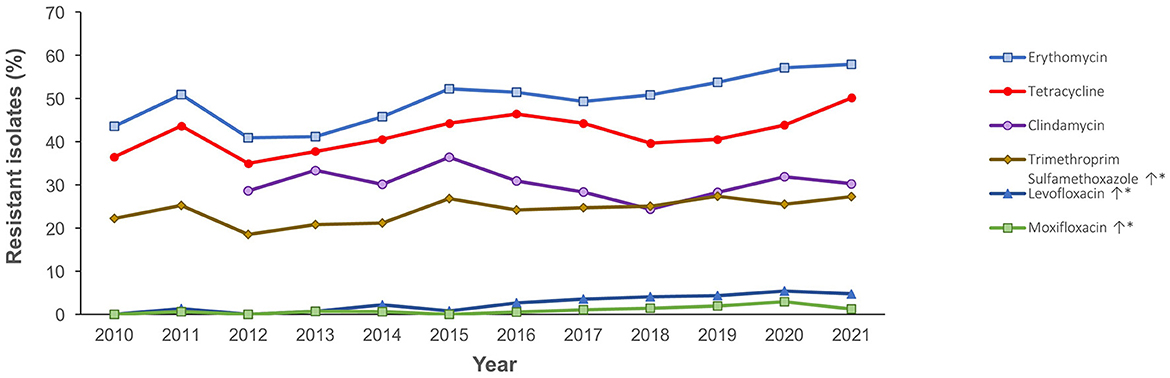
Figure 5. Resistance trend for other antibiotics (2010–2021). *Trend is statistically significant (p < 0.05).
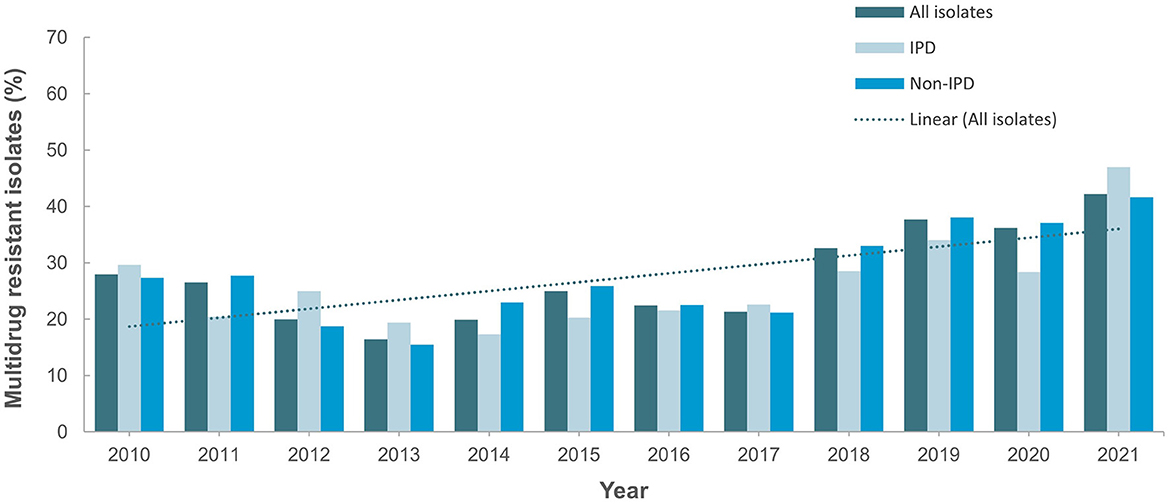
Figure 6. Trend for percentage multidrug-resistant isolates (2010–2021). Trend is statistically significant (p < 0.05).
None of the surveillance sites are currently conducting serotyping for S. pneumoniae and have not undertaken any serotyping in the preceding years (2010–2021). Hence no S. pneumoniae serotyping data was available for analysis. Handsearching of publicly available annual reports of health authorities for the period 2010–2020 did not yield data for S. pneumoniae serotype and burden of IPD.
National surveillance programs are crucial for monitoring trends in pneumococcal disease and AMR patterns over time. We present the analysis of S. pneumoniae surveillance and AMR trends in the UAE over a period of 12 years (2010–2021). Our findings provide the first comprehensive epidemiological profile of pneumococcal disease in the UAE and the changing AMR trends which are of significance for clinical management. We demonstrate increased identification of S. pneumoniae in alignment with the increasing number of reporting sites over the data collection period. When normalized for number of study sites a sustained increasing trend of isolates per site observed from 2015 to 2019 which is consistent with global reports of rising pneumococcal disease burden (1, 23–25). The sharp decline observed in reported isolates in 2020 is suggestive of reduction in S. pneumoniae transmission during the first year of the COVID-19 pandemic. This could be attributable to the intense COVID-19 non-pharmacological interventions such as social distancing, masking and hand hygiene practices as well as the rapid change in healthcare-seeking behavior such as increased use of telemedicine and decreased hospital visits during the pandemic (26, 27). However, decreased testing and reporting during this period might have also resulted in the underestimation burden of pneumococcal disease.
Our findings show a male preponderance which may be a reflection of the demographic distribution of the UAE population but is also in keeping with reported literature from other studies (28, 29). The reasons for the male preponderance in pneumococcal disease are not fully understood hence further research is needed to elucidate the underlying factors as well as identify the potential implications for prevention and treatment strategies. The fact that most of our patients were in the pediatric age group is particularly relevant because nasopharyngeal carriage of S. pneumoniae is more prevalent in children compared to adults (30, 31). Indeed, nasopharyngeal isolates accounted for 22.7% of all isolates irrespective of specimen source and specifically for respiratory tract specimens, 44.4% of isolates were nasopharyngeal colonizers. Children are more likely to be colonized with S. pneumoniae due to their immature immune systems and increased exposure to respiratory pathogens and pneumococcal colonization contributes to the risk of IPD in children (24, 32). These findings underscore the need for targeted preventive measures to protect this vulnerable population (32). Vaccination against S. pneumoniae is particularly important in the pediatric population, as it has been associated with reduction in pneumococcal nasopharyngeal carriage leading to a reduced risk of IPD (33, 34).
Understanding the dynamics of pneumococcal disease and antimicrobial resistance is essential for developing robust intervention and treatment strategies. High levels of occurrence of nasopharyngeal carriage of S. pneumoniae can lead to the selection and spread of antibiotic resistant strains. Elevated levels of resistance to penicillin, macrolides and fluoroquinolone as well as the occurrence of multidrug resistance phenotype were reported in S. pneumoniae isolates in the UAE in a study carried out prior to the advent of the PCV vaccination program (16). In that report, of the 100 isolates identified between 2004 and 2006 only 57% were penicillin susceptible (16). Our findings which show high occurrence of penicillin resistant S. pneumoniae suggests that these isolates are now endemic in our setting. In contrast to the downward trend observed for beta-lactam antibiotics, resistance to quinolones, and macrolides showed an upward trend which is in keeping with findings from other countries in the region (35, 36). In Kuwait, the downward trend in penicillin resistance, has been shown to be associated with the introduction of the PCV and circulating serotypes (32, 35). The finding of similar trends of resistance for the two macrolide antibiotics aligns with previous report from the UAE which demonstrated high occurrence of level of cross-resistance between erythromycin and clindamycin (16). The ramifications of the increasing occurrence of S. pneumoniae MDR strains are immense including limitations on the effectiveness of antibiotics for the treatment of pneumococcal infections, which will further increase healthcare costs and the burden of disease. These findings underscore the need for continued surveillance and implementation of effective antibiotic stewardship programs to combat this growing AMR threat.
IPD and non-IPD differ in terms of their pathogenesis and clinical presentation. IPD is a severe and potentially life-threatening infection associated with invasion of sterile body sites while non-IPD is typically less severe and limited to non-sterile sites (37). The findings from this study represent the first insight into IPD burden in the UAE encapsulating the period post-commencement of the pneumococcal vaccination program. Expectedly, higher hospitalization with longer duration was observed for IPD patients. However, the high occurrence of MDR phenotype in both IPD and non-IPD isolates is a cause for concern as it suggests that emergence of resistant strains of S. pneumoniae is ongoing in our setting.
Our findings reveal the absence of data on S. pneumoniae serotypes circulating in our setting which is a limitation of the existing surveillance dataset. The emergence of non-vaccine serotypes and their increasing antimicrobial resistance is a reminder of the importance of ongoing surveillance to monitor changes in pneumococcal serotypes and their AMR patterns. Such data can inform the development of new and improved pneumococcal vaccines that provide coverage against a wider range of clinically relevant serotypes. We advocate for the urgent initiation of a national S. pneumoniae serotyping and genomic surveillance program as data from such initiative will be useful in guiding policy decisions for the introduction of new pneumococcal vaccines and vaccination schedules. In addition, such surveillance data will be useful for mapping genomic changes associated with serotype switching from vaccine pressure, as well as provide early warnings for emergence of resistance and the spread of global clones. The occurrence of a high percentage of missing data and information about specific population risk groups are limitations observed in this national AMR surveillance dataset. This highlights the need for continued provision of training to personnel at participating sites as well as expansion of the clinical parameters included in the dataset.
Our analysis of the national S. pneumoniae AMR surveillance data provides insights into the evolving patterns of pneumococcal disease and antimicrobial resistance in the UAE. The findings highlight the need for the introduction of a S. pneumoniae serotype surveillance program to guide the pneumococcal vaccination program, as well as continued AMR monitoring and targeted intervention measures to address the growing threat of antibiotic resistance.
The datasets presented in this article are not readily available because the National AMR Surveillance database managed by the UAE Ministry of Health and Prevention (MOHAP) contains confidential health information. Requests to access the datasets should be directed to https://mohap.gov.ae/.
Ethical approval for this study was provided by the Ministry of Health and Prevention Research Ethics Committee (MOHAP/DXB-REC/D.D.D/No.131/2021; MOHAP/DXB-REC/J.J.J./No. 86/2023), Dubai Scientific Research Ethics Committee (DSREC-GL17-2023), and Abu Dhabi Health Research and Technology Ethics Committee (DOH/ZHCD/2023/1316). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Conceptualization, data interpretation, and manuscript review and editing: AS, JT, NA, GM, CA, and DE. Data collection: AS, JT, NA, GM, CA, DE, and The UAE AMR surveillance consortium. Formal analysis and manuscript preparation: AS and JT. All authors have read and agreed to the published version of the manuscript.
The study received funding from Pfizer Global Medical Grants (#69983713). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The authors wish to thank the UAE Ministry of Health and Prevention (MOHAP), regional health and public health authorities (DHA, DoH/ADPHC), AMR Focal points at participating sites, and all healthcare professionals and other experts involved in national AMR surveillance in the UAE for their continuous collaboration and support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^WHONET. The laboratory database software. https://whonet.org/.
1. Wahl B, O'Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, et al. Burden of streptococcus pneumoniae and haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. (2018) 6:e744–57. doi: 10.1016/S2214-109X(18)30247-X
2. Collaborators GBDLRI. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. (2018) 18:1191–210. doi: 10.1016/S1473-3099(18)30310-4
3. Ganaie F, Saad JS, McGee L, van Tonder AJ, Bentley SD, Lo SW, et al. A new pneumococcal capsule type, 10D, is the 100th serotype and has a large cps fragment from an oral streptococcus. MBio. (2020) 11. doi: 10.1128/mBio.00937-20
4. Ganaie FA, Saad JS, Lo SW, McGee L, van Tonder AJ, Hawkins PA, et al. Novel pneumococcal capsule type 33E results from the inactivation of glycosyltransferase WciE in vaccine type 33F. J Biol Chem. (2023) 299:105085. doi: 10.1016/j.jbc.2023.105085
5. Rappuoli R, Pizza M, Del Giudice G, De Gregorio E. Vaccines, new opportunities for a new society. Proc Natl Acad Sci U S A. (2014) 111:12288–93. doi: 10.1073/pnas.1402981111
6. Pletz MW, Maus U, Krug N, Welte T, Lode H. Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Agents. (2008) 32:199–206. doi: 10.1016/j.ijantimicag.2008.01.021
7. Feldman C, Anderson R. Recent advances in the epidemiology and prevention of streptococcus pneumoniae infections. F1000Res. (2020) 9:F1000. doi: 10.12688/f1000research.22341.1
8. Review on Antimicrobial Resistance Tackling a crisis for the health and wealth of nations. (2014). London: Review on Antimicrobial Resistance. Available online at: https://amr-review.org/ (accessed October 31, 2023).
9. Lo SW, Gladstone RA, van Tonder AJ, Lees JA, du Plessis M, Benisty R, et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: an international whole-genome sequencing study. Lancet Infect Dis. (2019) 19:759–69. doi: 10.1016/S1473-3099(19)30297-X
10. Swarthout TD, Fronterre C, Lourenço J, Obolski U, Gori A, Bar-Zeev N, et al. High residual carriage of vaccine-serotype streptococcus pneumoniae after introduction of pneumococcal conjugate vaccine in Malawi. Nat Commun. (2020) 11:2222. doi: 10.1038/s41467-020-15786-9
11. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. (2004) 4:144–54. doi: 10.1016/S1473-3099(04)00938-7
12. Chaguza C, Cornick JE, Andam CP, Gladstone RA, Alaerts M, Musicha P, et al. Population genetic structure, antibiotic resistance, capsule switching and evolution of invasive pneumococci before conjugate vaccination in Malawi. Vaccine. (2017) 35:4594–602. doi: 10.1016/j.vaccine.2017.07.009
13. Senok A, Nassar R, Celiloglu H, Nabi A, Alfaresi M, Weber S, et al. Genotyping of methicillin resistant staphylococcus aureus from the United Arab Emirates. Sci Rep. (2020) 10:18551. doi: 10.1038/s41598-020-75565-w
14. Sonnevend A, Ghazawi AA, Hashmey R, Jamal W, Rotimi VO, Shibl AM, et al. Characterization of carbapenem-resistant enterobacteriaceae with high rate of autochthonous transmission in the Arabian peninsula. PLoS ONE. (2015) 10:e0131372. doi: 10.1371/journal.pone.0131372
15. Jamsheer A, Rafay AM, Daoud Z, Morrissey I, Torumkuney D. Results from the survey of antibiotic resistance (SOAR) 2011-13 in the Gulf states. J Antimicrob Chemother. (2016) 71:i45–61. doi: 10.1093/jac/dkw064
16. Senok A, Al-Zarouni M, Al-Najjar J, Nublusi A, Panigrahi D. Antimicrobial resistance among streptococcus pneumoniae and haemophilus influenzae isolates in the United Arab Emirates: 2004-2006. J Infect Dev Ctries. (2007) 1:296–302. doi: 10.3855/jidc.367
17. Feldman C, Abdulkarim E, Alattar F, Al Lawati F, Al Khatib H, Al Maslamani M, et al. Pneumococcal disease in the Arabian Gulf: recognizing the challenge and moving toward a solution. J Infect Public Health. (2013) 6:401–9. doi: 10.1016/j.jiph.2013.06.004
18. Al-Muhairi S, Zoubeidi T, Ellis M, Nicholls MG, Safa W, Joseph J. Demographics and microbiological profile of pneumonia in United Arab Emirates. Monaldi Arch Chest Dis. (2006) 65:13–8. doi: 10.4081/monaldi.2006.580
19. Howidi M, Muhsin H, Rajah J. The burden of pneumococcal disease in children less than 5 years of age in Abu Dhabi, United Arab Emirates. Ann Saudi Med. (2011) 31:356–9. doi: 10.4103/0256-4947.83214
20. Thomsen JT, Abdulrazzaq H, Alrand HA, and The UAE AMR Surveillance Consortium. Surveillance of Antimicrobial Resistance in the United Emirates: the early implementation phase. Front. Public Health. (2023) 11:1247627. doi: 10.3389/fpubh.2023.124762
21. Institute CaLS. Performance Standards for Antimicrobial Susceptibility Testing, 24th Informational Supplement (M100-S24). Wayne, PA: CLSI 2014.
22. Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat. (1998) 52:119–26. doi: 10.1080/00031305.1998.10480550
23. Jimbo-Sotomayor R, Armijos-Acurio L, Proaño-Espinosa J, Segarra-Galarza KandSánchez-Choez X. Morbidity and mortality due to pneumococcal disease in children in ecuador from 2005 to 2015. J Glob Infect Dis. (2020) 12:124–8. doi: 10.4103/jgid.jgid_125_19
24. O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. (2009) 374:893–902. doi: 10.1016/S0140-6736(09)61204-6
25. Valenzuela MT, O'Loughlin R, De La Hoz F, Gomez E, Constenla D, Sinha A, et al. The burden of pneumococcal disease among Latin American and Caribbean children: review of the evidence. Rev Panam Salud Publica. (2009) 25:270–9. doi: 10.1590/S1020-49892009000300011
26. Kaur R, Schulz S, Fuji N, Pichichero M. COVID-19 pandemic impact on respiratory infectious diseases in primary care practice in children. Front Pediatr. (2021) 9:722483. doi: 10.3389/fped.2021.722483
27. Li Y, Guo Y, Duan Y. Changes in streptococcus pneumoniae infection in children before and after the COVID-19 pandemic in Zhengzhou, China. J Infect. (2022) 85:e80–1. doi: 10.1016/j.jinf.2022.05.040
28. Gutiérrez F, Masiá M, Mirete C, Soldán B, Rodríguez JC, Padilla S, et al. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J Infect. (2006) 53:166–74. doi: 10.1016/j.jinf.2005.11.006
29. Wagenvoort GH, Sanders EA, Vlaminckx BJ, de Melker HE, van der Ende A, Knol MJ. Sex differences in invasive pneumococcal disease and the impact of pneumococcal conjugate vaccination in the Netherlands, 2004 to 2015. Euro Surveill. (2017) 22:30481. doi: 10.2807/1560-7917.ES.2017.22.10.30481
30. Marchisio P, Esposito S, Schito GC, Marchese A, Cavagna R, Principi N. Nasopharyngeal carriage of streptococcus pneumoniae in healthy children: implications for the use of heptavalent pneumococcal conjugate vaccine. Emerg Infect Dis. (2002) 8:479–84. doi: 10.3201/eid0805.010235
31. Narwortey DK, Owusu-Ofori A, Slotved H-C, Donkor ES, Ansah PO, Welaga P, et al. Nasopharyngeal carriage of streptococcus pneumoniae among healthy children in Kassena-Nankana districts of Northern Ghana. BMC Infect Dis. (2021) 21:661. doi: 10.1186/s12879-021-06302-5
32. Mokaddas E, Albert MJ. Serotype distribution and penicillin-non-susceptibility of Streptococcus pneumoniae causing invasive diseases in Kuwait: a 10-year study of impact of pneumococcal conjugate vaccines. Expert Rev Vaccines. (2016) 15:1337–45. doi: 10.1080/14760584.2016.1198698
33. Mokaddas E, Albert MJ. Impact of pneumococcal conjugate vaccines on burden of invasive pneumococcal disease and serotype distribution of streptococcus pneumoniae isolates: an overview from Kuwait. Vaccine. (2012) 30:G37–40. doi: 10.1016/j.vaccine.2012.10.061
34. Løvlie A, Vestrheim DF, Aaberge IS, Steens A. Changes in pneumococcal carriage prevalence and factors associated with carriage in Norwegian children, four years after introduction of PCV13. BMC Infect Dis. (2020) 20:29. doi: 10.1186/s12879-019-4754-0
35. Johny M, Babelly M, Al-Obaid I, Al-Benwan K, Udo EE. Antimicrobial resistance in clinical isolates of Streptococcus pneumoniae in a tertiary hospital in Kuwait, 1997-2007: implications for empiric therapy. J Infect Public Health. (2010) 3:60–6. doi: 10.1016/j.jiph.2010.02.003
36. Torumkuney D, Mokaddas E, Jiman-Fatani A, Ageel A, Daoud Z, Bouferraa Y, et al. Results from the Survey Of Antibiotic Resistance (SOAR) 2015-17 in the Middle East (Kuwait, Lebanon and Saudi Arabia): data based on CLSI, EUCAST (dose-specific) and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints. J Antimicrob Chemother. (2020) 75:i60–75. doi: 10.1093/jac/dkaa084
37. Berical AC, Harris D, Dela Cruz CS, Possick JD. Pneumococcal vaccination strategies. an update and perspective. Ann Am Thorac Soc. (2016) 13:933–44. doi: 10.1513/AnnalsATS.201511-778FR
Keywords: Streptococcus pneumoniae, pneumococcal conjugate vaccine, serotyping, invasive pneumococcal disease, antimicrobial resistance
Citation: Senok A, Thomsen J, Abdulrazzaq NM, The UAE AMR Surveillance Consortium, Menezes GA, Ayoub Moubareck C and Everett D (2023) Antimicrobial resistance in Streptococcus pneumoniae: a retrospective analysis of emerging trends in the United Arab Emirates from 2010 to 2021. Front. Public Health 11:1244357. doi: 10.3389/fpubh.2023.1244357
Received: 22 June 2023; Accepted: 24 October 2023;
Published: 23 November 2023.
Edited by:
Dalal Hammoudi Halat, Qatar University, QatarReviewed by:
Feroze A. Ganaie, University of Alabama at Birmingham, United StatesCopyright © 2023 Senok, Thomsen, Abdulrazzaq, The UAE AMR Surveillance Consortium, Menezes, Ayoub Moubareck and Everett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abiola Senok, YWJpb2xhLnNlbm9rQG1icnUuYWMuYWU=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
§The UAE AMR Surveillance Consortium group members are listed in the Appendix
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.