- 1Department of Epidemiology, Wolaita Sodo University, Sodo, Ethiopia
- 2Institute of Health, Jimma University, Oromia, Ethiopia
Background: Tuberculosis (TB) is a key community health problem in numerous settings, predominantly in sub-Saharan Africa (SSA). TB is the second most lethal infectious disease worldwide. Around 1.6 million people died from TB in 2021. TB prevention and control strategies are difficult to implement in prison, especially in sub-Saharan Africa, owing to overcrowding and poor ventilation. Thus, this systematic review and meta-analysis aimed to synthesize the estimated pooled prevalence of tuberculosis among prisoners in sub-Saharan Africa.
Materials and methods: Electronic biomedical databases such as Google Scholar, Web of Science, PubMed/Medline, EMBASE, and Science Direct were used to systematically explore candidate studies published until December 2022. Data extraction was performed using a Microsoft Excel spreadsheet. The estimated pooled prevalence of tuberculosis was determined using a fixed-effects model. Cochrane Q-test and I2 statistics were used to check heterogeneity statistically across different studies. Begg’s rank and Egger’s tests were performed to assess evidence of possible publication bias.
Results: A total of 40 articles involving 59,300 prisoners were included in this systematic review and meta-analysis. The pooled prevalence of tuberculosis was 4.02% (95% CI: 2.68–5.36). We found the highest prevalence using Gene X pert as a diagnostic method, which was 4.97 (95% CI: 2.22–7.73). There is no evidence of publication bias.
Conclusion: The outcome of this review revealed a high prevalence of tuberculosis among prisoners in sub-Saharan Africa. To reach the “End Tuberculosis strategy” by 2030, early identification of cases through screening on entry and periodical active case finding is important. Moreover, prevention and prompt treatment after diagnosis must be implemented to limit transmission to the general population.
Systematic review registration: https://www.crd.york.ac.uk/prospero/#searchadvanced, identifier (CRD42023428933).
Introduction
Mycobacterium tuberculosis complex (MTBC) causes tuberculosis. When a person coughs, sneezes, talks, or sings, droplet nuclei are produced, which spread from person to person through the air (1, 2). Coughing for more than 2 weeks, fever, weight loss, and sputum production can occur in conjunction with hemoptysis, loss of appetite, night sweats, and fatigue, which are expressive clinical signs in patients positive for pulmonary tuberculosis (3).
In 2021, approximately 1.6 million people died and 10.6 million people contracted tuberculosis (TB) worldwide. Low-and middle-income countries accounted for 80% of cases and deaths, with 23% of new cases in the World Health Organization (WHO) Africa region. TB is the second biggest killer among infectious diseases and the 13th leading cause of death worldwide (1).
Globally, there were approximately 11 million people imprisoned in 2018. An increment of approximately 24% was observed between 2000 and 2018 globally. The imprisoned population in Africa has increased by 29% in recent years, and the tuberculosis burden in this region is the highest compared to other WHO regions (1). The prison system is a potential area for transmitting communicable diseases such as tuberculosis due to overcrowding, poor ventilation, inadequate lighting, illicit drug use, difficulty accessing health services, lack of or precarious basic sanitation housing infrastructure, and malnutrition (1, 3–5).
In developing countries, TB is more common in prisons than in the general population, and prisons in SSA are riskier due to the high number of incarcerated people per cell block, ventilation systems, nutrition-related issues, and high prevalence of human immunodeficiency virus (HIV) (6–9). TB prevalence among prisoners from 24 SSA countries ranges from 0.4 to 16.3% (10).
Prison staff are at risk of contracting tuberculosis due to their interaction with their inmates, which leads to the spread of the disease to their families and communities. This suggests that tuberculosis in prison is a concern for society as a whole, not just for prisoners (7). Therefore, compared to other regions in the world, SSA is one of the regions with a high burden of TB; therefore, this systematic review and meta-analysis helps to update the prevalence of tuberculosis among prisoners, inform policymakers, and improve approaches to prisoners.
Materials and methods
Reporting
The results were reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) statement (11). The article screening was based on the PRISMA 2009 statement, and the selection process has been shown using a PRISMA-P flow diagram. This review is registered in PROSPERO with registration number CRD42023428933.
Search methods and strategies
To identify potentially relevant articles, we performed exhaustive searches of electronic databases with no date limits on Google Scholar, Web of Science, PubMed/MEDLINE, Science Direct, PubMed/MEDLINE, and EMBASE. All searches were limited to articles written in English and human studies. We conducted a manual search for additional relevant studies using references from retrieved articles and related systematic reviews to identify original articles that may have been overlooked. The following keywords were used to generate search strings or terms: prevalence, magnitude, Tuberculosis, Pulmonary Tuberculosis, Mycobacterium infections, Prisoners, and sub-Saharan Africa. Advanced search databases were built with the above-mentioned terms in mind, using “Medical Subject Headings (MeSH) [((((“Prevalence”) OR “Burden” OR “Magnitude”) AND “tuberculosis” AND “prisoners”)) AND sub-Saharan Africa].
Inclusion and exclusion criteria
All studies on the prevalence of tuberculosis among prisoners in sub-Saharan Africa were included. Furthermore, this systematic review and meta-analysis included all cross-sectional studies on prisoners published in English and conducted in sub-Saharan Africa. Review papers, case series, case reports, abstracts, and qualitative studies were also barred from consideration.
Outcome measurement
One major finding that emerged from this systematic review and meta-analysis is the estimation of the pooled prevalence of tuberculosis among prisoners in sub-Saharan Africa. A tuberculosis-positive patient has a Mycobacterium tuberculosis complex found in a clinical specimen, whether by smear, culture, or WHO-recommended rapid diagnosis (such as Xpert MTB/RIF).
Data extraction and quality assessment
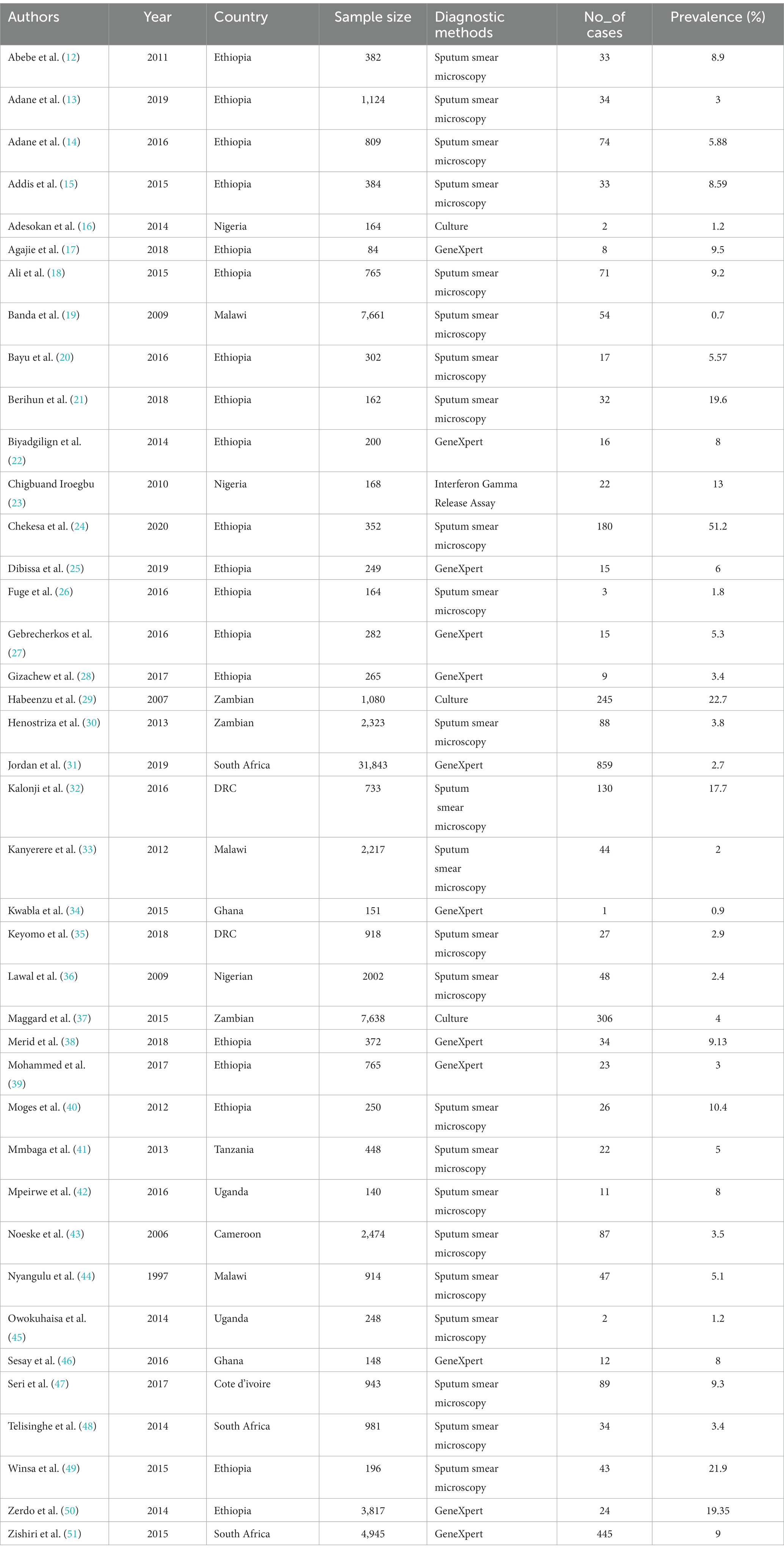
Endnote citation manager software version X9 for Windows was used to import retrieved studies from the databases, and manual removal was performed for duplicated articles. All articles were screened by three independent reviewers for predefined inclusion and exclusion criteria (abstract and title), followed by a full-text review. If disagreements regarding the inclusion of studies could not be resolved, a fourth investigator was invited to reach an agreement. Excel spreadsheet software was used to extract the data from the included studies. The spreadsheet included the first author’s name, publication year, study design, country, sample size, diagnostic methods, and number of cases (Table 1).
Statistical analysis
The analysis was carried out using the statistical software STATA Version 14.1 (StataCorp, College Station, Texas, United States), and heterogeneity was checked across studies by computing the I2 statistical test. If the I2 values were 0, 25, 50, and 75%, we assumed no, low, medium, and high heterogeneity across studies. A meta-analysis using a fixed-effects model with 95% confidence intervals (CI) was performed to analyze the pooled prevalence of tuberculosis among prisoners (I2,16.3% p = 0.188). A visual inspection of the funnel plot was performed to check for evidence of publication bias, followed by Begg’s rank and Egger’s tests, with a value of p of less than 0.05 used as a cut-off point. Leave-one-out sensitivity analysis was also performed to assess the impact of a small study. The analysis was carried out step-by-step, excluding the study, to assess the effect of each study on the pooled prevalence of tuberculosis. A forest plot was used to estimate pooled prevalence.
Results
Selection of studies
A search of the biomedical electronic databases yielded 352 published and unpublished studies. Although 244 duplicate articles were identified and removed, 108 were included in the screening. After 64 studies were removed based on title and abstract screening, 44 studies remained. Finally, 40 studies that met the eligibility criteria were included in the final analysis to estimate the pooled prevalence of tuberculosis among sub-Saharan African prisoners. The full selection process is illustrated in Figure 1.
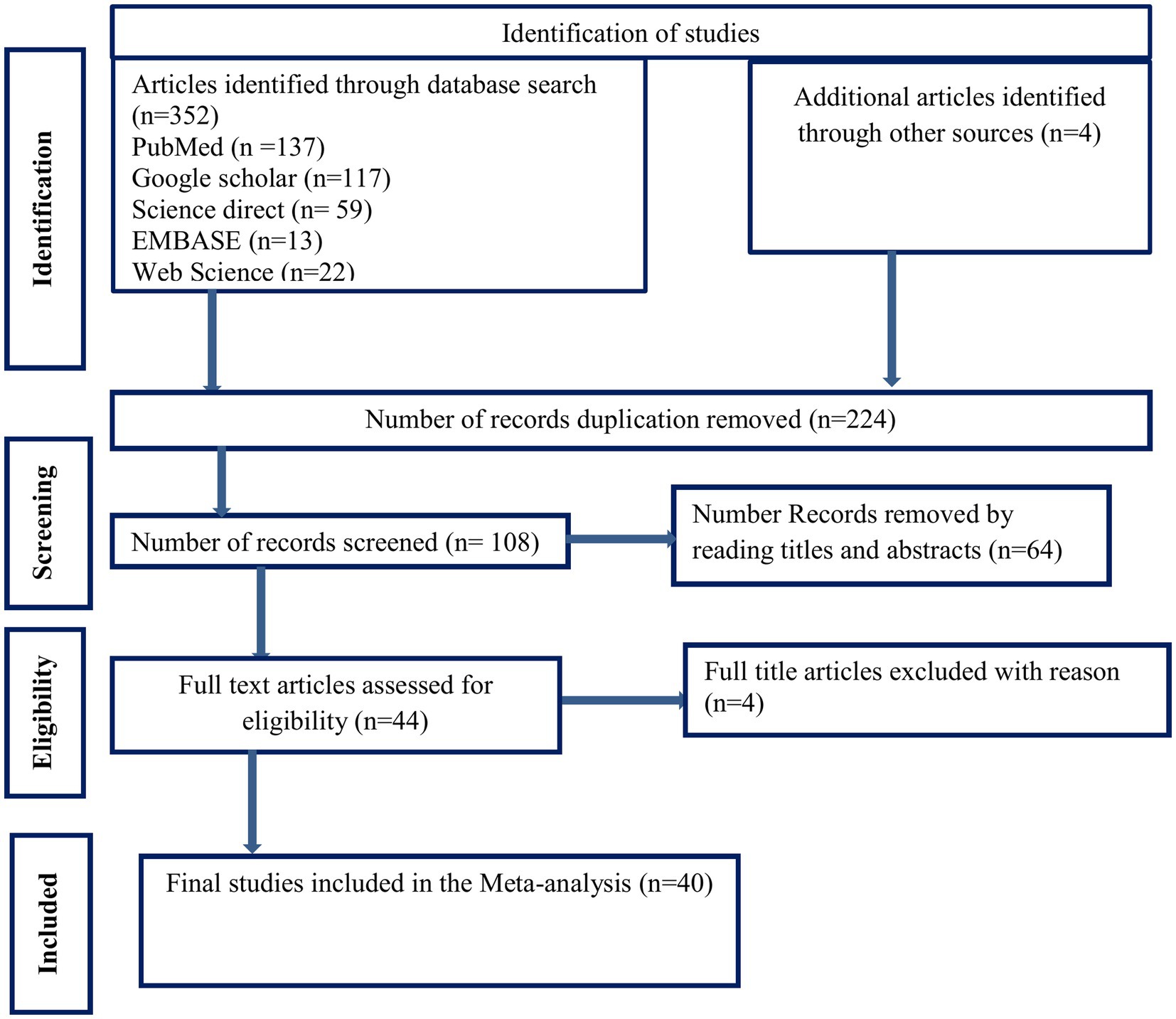
Figure 1. PRISMA flow diagram of articles screened and the selection process on tuberculosis among prisons in sub-Saharan, 2023.
Included studies characteristics
Among the 40 included studies, there were 19 studies were from Ethiopia; 3 from South Africa; 3 from Nigeria, Malawi, and Zambia; 2 from the Democratic Republic of Congo (DRC), Uganda, and Ghana; and 1 from Cameroon, Tanzania, and Cote d’Ivoire. The included study sample size ranged from 84 (2) to 31,843 (3), with 80,608 prisoners. Observational and interventional studies published between 1997 and 2020 were included. All 40 articles had a cross-sectional design. All included studies were facility-based (Table 1 illustrates the included studies’ baseline characteristics).
The pooled prevalence of tuberculosis among prisoners in sub-Saharan Africa
The pooled prevalence of tuberculosis among sub-Saharan African prisoners was 4.02% (95% CI: 2.68–5.36). The forest plot shows that statistical heterogeneity was low (I2 = 16.3%; p 0.188). As a result, we used a fixed effects model to estimate the pooled prevalence of tuberculosis (Figure 2).
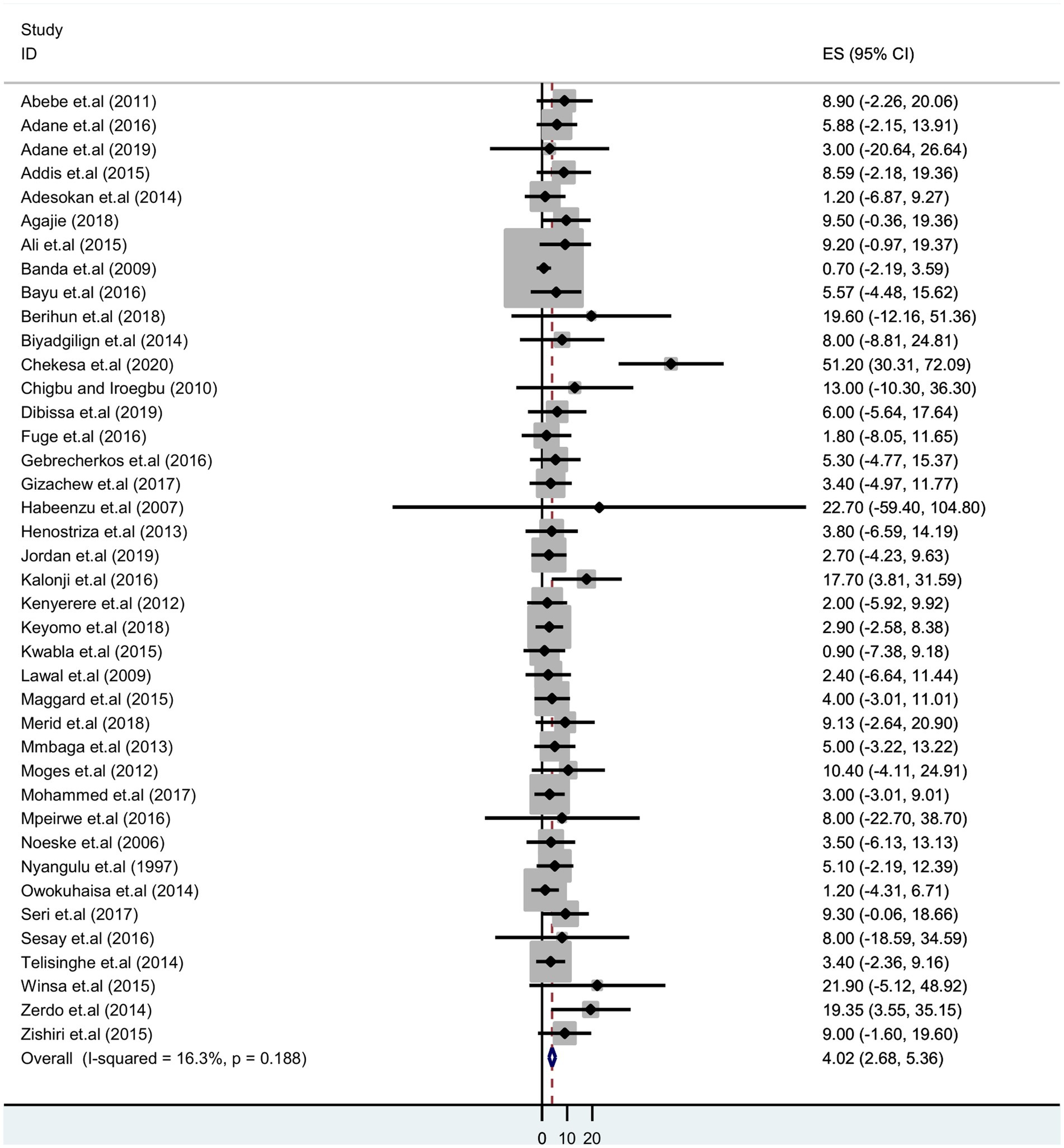
Figure 2. Forest plot showing pooled prevalence estimate of tuberculosis infection among prisoners in sub-Saharan Africa, 2023.
Sub-group analysis
A sub-group analysis based on diagnostic methods and country setting was performed to identify potential sources of heterogeneity. It shows the highest detection of tuberculosis was by Gene Xpert, which was 4.97% (95% CI: 2.22–7.73); sputum smear microscopy was 3.53% (95% CI: 1.92–5.13) and culture was 2.88% (95% CI: 2.40–8.16) (Figure 3). Thus, we observed country variation in the prevalence of tuberculosis in this study. The prevalence of TB was found to range between 7.10 (95% CI: 4.58–9.62) in Ethiopia and 1.37 (95% CI:-1.17–3.91) in Malawi (Figure 4).
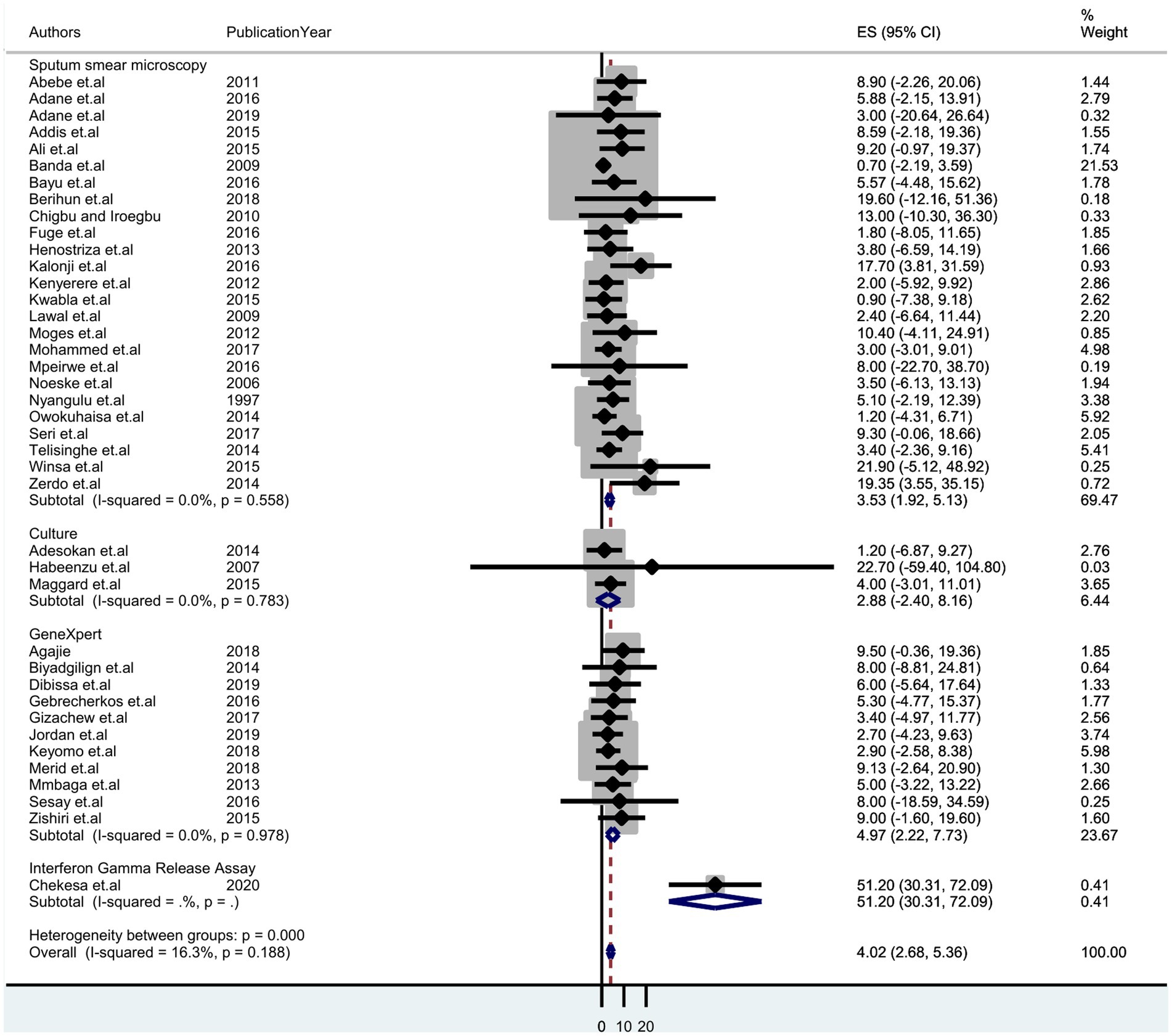
Figure 3. Forest plot displaying subgroup analysis on the pooled prevalence of tuberculosis by diagnostic method among prisoners in sub-Saharan Africa, 2023.
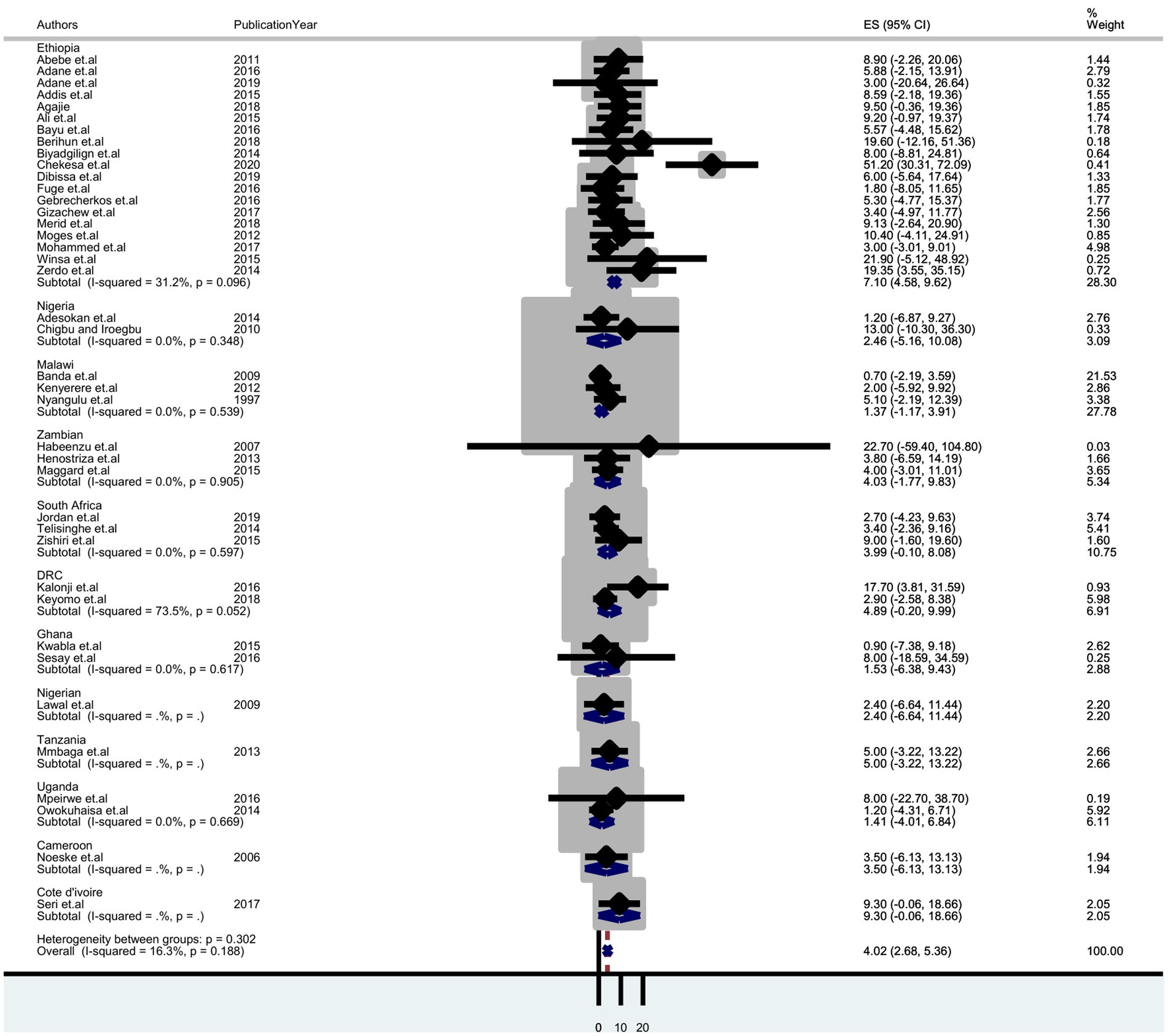
Figure 4. Forest plot displaying subgroup analysis on the pooled prevalence of tuberculosis by country among prisoners in sub-Saharan Africa, 2023.
Meta-regression
Meta-regression was used to identify factors associated with the pooled prevalence of tuberculosis among prisoners while keeping continuous variables in mind. For the meta-regression, publication year and sample size were considered. Meta-regression analysis revealed no statistically significant relationship between the pooled prevalence of tuberculosis among prisoners and publication year or sample size (Table 2).
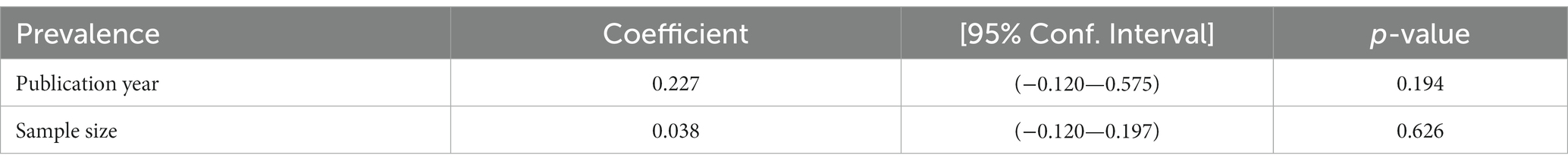
Table 2. Meta-regression to identify the source of heterogeneity for the pooled prevalence of tuberculosis among prisoners in sub-Saharan Africa, 2023.
Publication bias
To assess possible publication bias, a visually inspected funnel plot was used, which was statistically supported by Egger’s and Begg’s rank regression tests. The symmetrical distribution of the included studies in a large inverted funnel demonstrated the absence of a publication bias. With p-values of (p = 0.26) and (p = 0.15), respectively, the Egger and Begg rank tests revealed no publication bias among the included articles to estimate the pooled prevalence of tuberculosis among prisoners in sub-Saharan Africa (Figure 5).
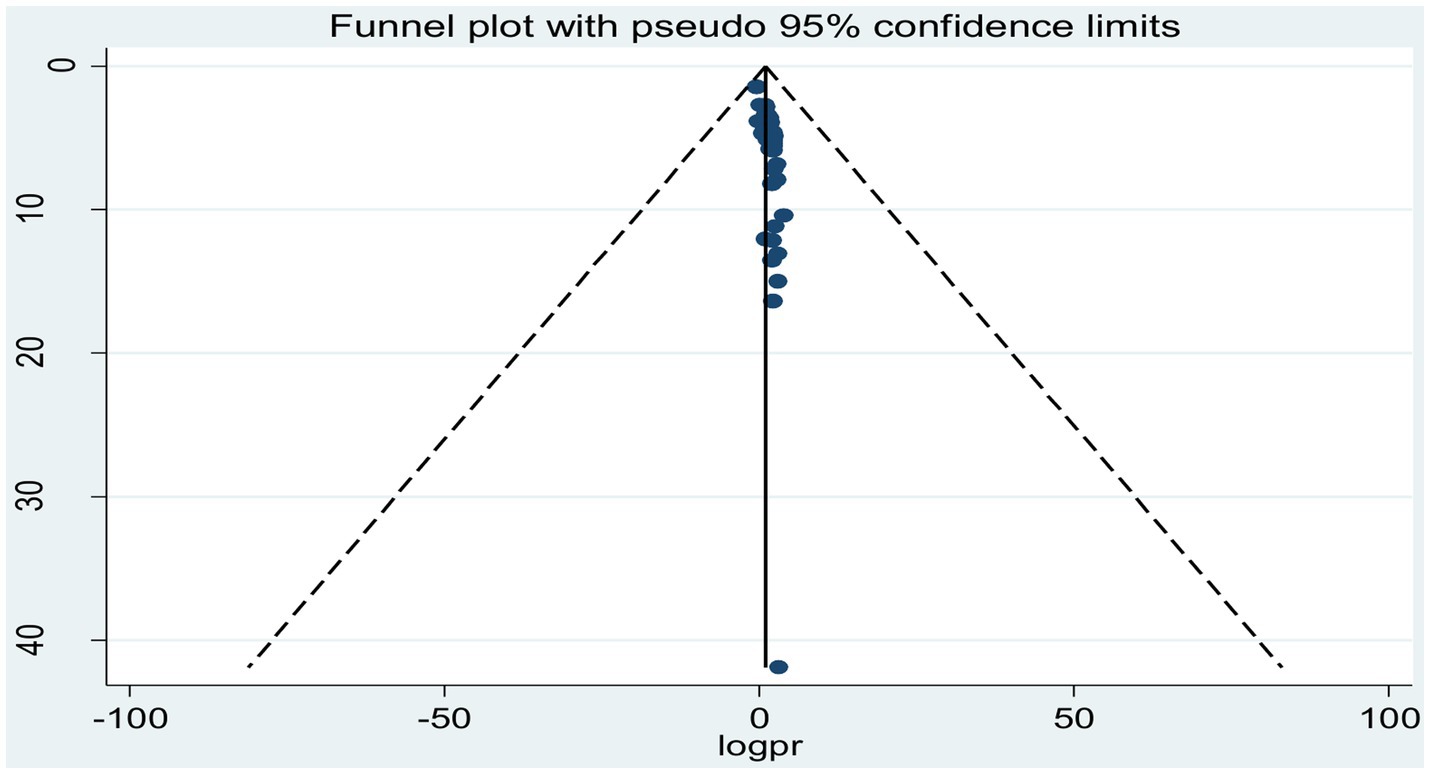
Figure 5. Funnel plot showing publication bias of studies reporting the pooled prevalence of tuberculosis among prisoners in sub-Saharan Africa, 2023.
Sensitivity analysis
By excluding each study one at a time, a leave-out-one sensitivity analysis was used to determine the effect of a single study on the pooled prevalence of tuberculosis among prisoners in sub-Saharan Africa. According to the findings, no single study had a significant impact on the pooled estimate of tuberculosis among prisoners in sub-Saharan Africa (Table 3).
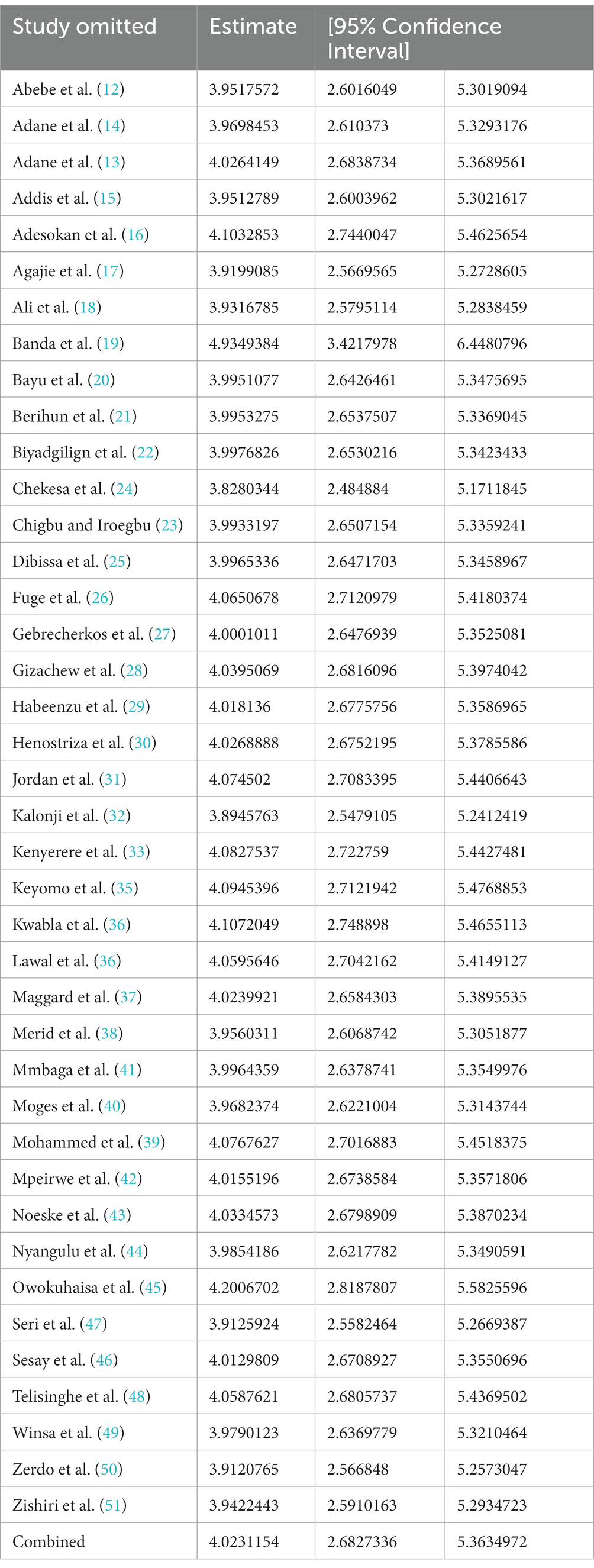
Table 3. Sensitivity analysis for the pooled prevalence of tuberculosis among prisoners in sub-Saharan Africa, 2023.
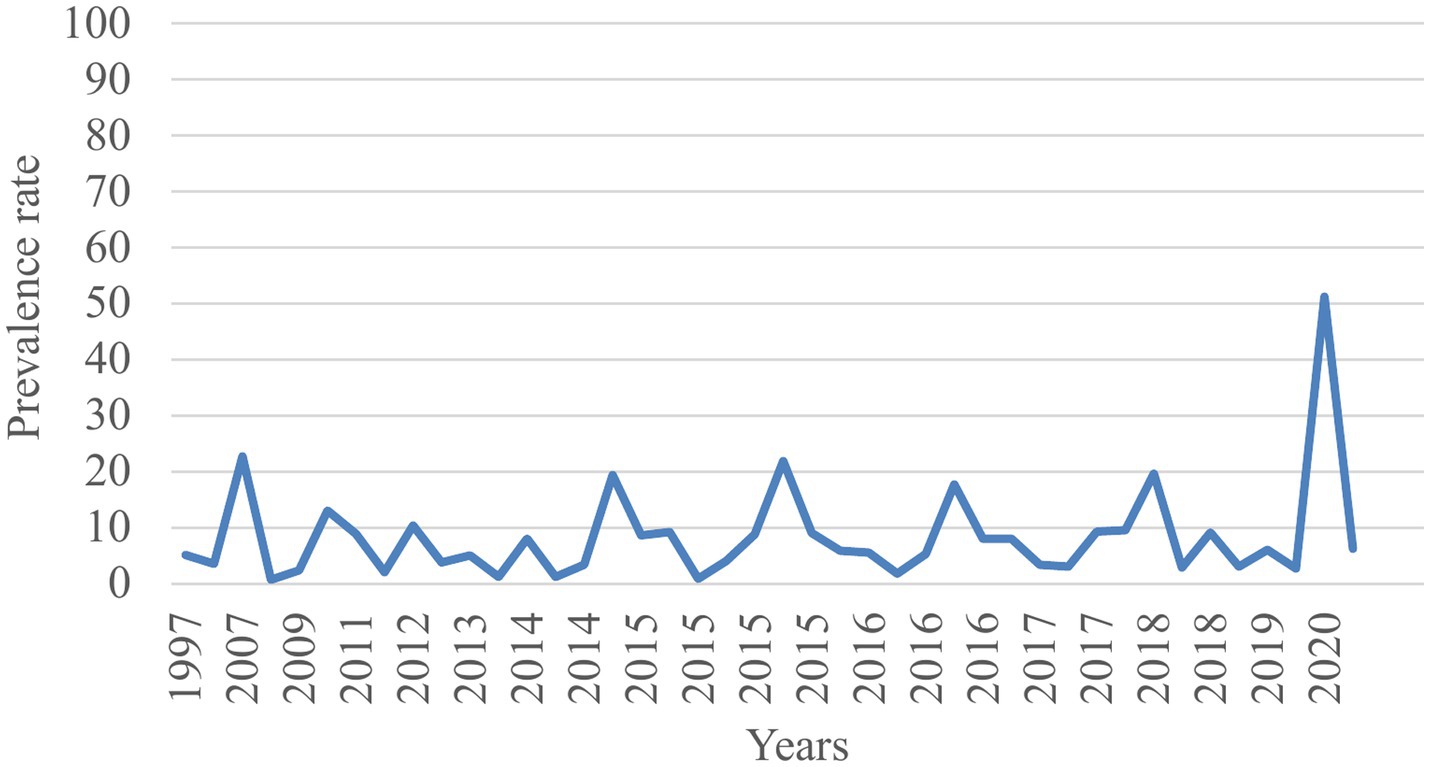
Trends of TB prevalence
The trend analysis indicated that despite efforts to eradicate TB, the disease burden among prisoners in sub-Saharan Africa continued to rise from 1997 to 2020 (Figure 6).
Discussion
There is evidence that the number of people developing tuberculosis is increasing in many low-and middle-income countries, and between 2019 and 2021, the number of deaths from tuberculosis also increased (1). In prisons, infectious diseases such as tuberculosis may spread more easily because segregation criteria are based on criminal characteristics rather than on public health concerns (52). As a result, the goal of this systematic review and meta-analysis was to report the most recent estimated pooled prevalence of tuberculosis among prisoners in sub-Saharan Africa.
The prevalence of tuberculosis among household contacts was 3.29% (95% CI: 2.35–4.23) (52). A recent systematic review has documented a 3- to 1,000-fold increase in the prevalence of TB in prisons compared to the general population (22). In SSA, it is estimated to be 6–30 times higher than that in the general population (53). According to the results of the current systematic review and meta-analysis, the pooled prevalence of tuberculosis among prisoners in sub-Saharan Africa was 4.02% (95% CI: 2.68–5.36). This finding is consistent with findings from Tajikistan 4.5% (54), South Africa 2.7% (51), and Ethiopia (4.0%) (55). This could be attributed to the similarity in tuberculosis diagnostic methods used in the incarcerated population.
However, the pooled prevalence of tuberculosis in this systematic review and meta-analysis was lower than that in an Ethiopian systematic review and meta-analysis (8.33%) (2). Furthermore, it was lower than that in studies conducted in Brazil 27.8% (56), Malaysia 7.7% (57), Nepal 10% (55), Iran 7.9% (58), South Africa (8.8%) (11), and Zambia (6.4%) (26). The lower prevalence found in this study could be attributed to differences in geographical location and the number of rooms with prisoners with poor ventilation.
The pooled prevalence of tuberculosis among prisoners in the current meta-analysis was higher than that in studies conducted in Brazil (1.89%) (59), Thailand (2.1%) (60), and Peru (2.5%) (61). The higher prevalence of tuberculosis in our study might be due to overcrowding and the difference in the incarcerated years of inmates.
Sub-group analysis of the pooled prevalence of tuberculosis among prisoners in sub-Saharan Africa showed no statistically significant difference (p = 0.188). Using diagnostic methods, tuberculosis was detected by Gene Xpert (4.97%), sputum smear microscopy (3.53%), and culture (2.88%). Xpert MTB/RIF’s suitability and feasibility as an MTB diagnostic method are attributed to its suitability and feasibility as a quick, reliable, controllable, simple, and cost-effective test (62). Gene Xpert uses DNA PCR technology to detect MTB and rifampicin resistance mutations simultaneously (63).
The sub-group analysis of this review also showed that the prevalence of tuberculosis among prisoners was higher in Ethiopia (7.10%) compared to other countries in sub-Saharan Africa. The variation in the prevalence of pulmonary TB within countries in prisons could be due to differences in diagnostic techniques, screening methods, overcrowding, and sociocultural and socioeconomic factors among the study participants.
An ongoing intervention for Tuberculosis (TB) in sub-Saharan Africa is the implementation of active case-finding and treatment programs within prisons. This involves screening all inmates for TB, providing treatment for those who test positive, and implementing infection control measures to prevent the spread of the disease within the prison environment. Additionally, TB preventive therapy is provided to high-risk inmates, such as those with HIV or other underlying health conditions, which helps to reduce the overall burden of TB within the prison population (64).
Strengths and limitations of the study
The strength of this review is that it follows the recommended PRISMA guidelines. We also rigorously searched the literature in different databases and identified eligible studies. Moreover, in the present review, the heterogeneity among studies was low. While interpreting the results of this systematic review and meta-analysis, we considered the limitations of this review. We were forced to compare our findings with those of primary studies in some parts of the discussion because of a lack of adequate systematic reviews and meta-analyzes. The other limitation of this review is that we only considered articles written in the English language, which may result in the exclusion of other articles. Last but not least, we found studies conducted in 13 SSA countries, which may not represent prisoners throughout the whole region.
Conclusion
The pooled prevalence of tuberculosis among prisoners in sub-Saharan Africa was prominently high based on this systematic review and meta-analysis. Therefore, to reach the end of the global TB epidemic, improvement in the prison setting is important. Screening on entry to the prison, periodical TB symptom screening, TB prevention training and information dissemination among the health staff in the prison and the inmates, and immediate treatment of diseased prisoners are important these measure to be put in place. Finally, this will help with the early identification and diagnosis of tuberculosis, which will reduce multidrug-resistant tuberculosis occurrence.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
The study was conceptualized and developed by YS and TM, who also conducted data analysis and interpretation and wrote the first draft. GA and YS built the search strategy, extracted the data, and assessed the quality of the studies included. The writing was reviewed and edited by YS and TM. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank the primary study authors who contributed to this systematic review and meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CI, Confidence Interval; HIV/AIDS, Human immune Virus/Acquired immune deficiency syndrome; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyzes; PTB, Pulmonary Tuberculosis; SSA, sub-Sahara Africa; WHO, World Health Organization.
References
2. Melese, A, and Demelash, H. The prevalence of tuberculosis among prisoners in Ethiopia: a systematic review and meta-analysis of published studies. Arch Public Health. (2017) 75:1–9. doi: 10.1186/s13690-017-0204-x
3. Harries, AD, Maher, D, Graham, S, Gilks, C, and Nunn, P. TB/HIV: a clinical manual. Geneva: World Health Organization (2004).
4. Bone, A, Aerts, A, Grzemska, M, Kimerling, M, Kluge, H, Levy, M, et al. Tuberculosis control in prisons: A manual for programme managers. Geneva: World Health Organization (2000).
5. Walmsley, R (2018). World prison population list. World prison brief. Institute for Crime and Justice Policy Research, London: Birkbeck. Available at: https://wwwprisonstudies.org
6. Dara, M, Acosta, CD, Melchers, NVV, Al-Darraji, HA, Chorgoliani, D, Reyes, H, et al. Tuberculosis control in prisons: current situation and research gaps. Int J Infect Dis. (2015) 32:111–7. doi: 10.1016/j.ijid.2014.12.029
7. Dara, M. Croix-rouge Cidl, santé Omdl. Guidelines for the control of tuberculosis in prisons. Washington, DC: USAID (2009).
8. Lambert, LA, Armstrong, LR, Lobato, MN, Ho, C, France, AM, and Haddad, MB. Tuberculosis in jails and prisons: United States, 2002−2013. Am J Public Health. (2016) 106:2231–7. doi: 10.2105/AJPH.2016.303423
9. Reyes, H, and Coninx, R. Pitfalls of tuberculosis programmes in prisons. BMJ. (1997) 315:1447–50. doi: 10.1136/bmj.315.7120.1447
10. Moyo, N, Tay, EL, and Denholm, J. ‘Know your epidemic’: are prisons a potential barrier to TB elimination in an Australian context? Trop Med Infect Dis. (2018) 3:93. doi: 10.3390/tropicalmed3030093
11. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. PRISMA group* t. preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
12. Abebe, D, Bjune, G, Ameni, G, Biffa, D, and Abebe, F. Prevalence of pulmonary tuberculosis and associated risk factors in eastern Ethiopian prisons. Int J Tuberc Lung Dis. (2011) 15:668–73. doi: 10.5588/ijtld.10.0363
13. Adane, K, Spigt, M, Winkens, B, and Dinant, G-J. Tuberculosis case detection by trained inmate peer educators in a resource-limited prison setting in Ethiopia: a cluster-randomised trial. Lancet Glob Health. (2019) 7:e482–91. doi: 10.1016/S2214-109X(18)30477-7
14. Adane, K, Spigt, M, Ferede, S, Asmelash, T, Abebe, M, and Dinant, G-J. Half of pulmonary tuberculosis cases were left undiagnosed in prisons of the Tigray region of Ethiopia: implications for tuberculosis control. PLoS One. (2016) 11:e0149453. doi: 10.1371/journal.pone.0149453
15. Addis, Z, Adem, E, Alemu, A, Birhan, W, Mathewos, B, Tachebele, B, et al. Prevalence of smear positive pulmonary tuberculosis in Gondar prisoners, north West Ethiopia. Asian Pac J Trop Med. (2015) 8:127–31. doi: 10.1016/S1995-7645(14)60302-3
16. Adesokan, H, Cadmus, E, Adeyemi, W, Lawal, O, Ogunlade, C, Osman, E, et al. Prevalence of previously undetected tuberculosis and underlying risk factors for transmission in a prison setting in Ibadan, South-Western Nigeria. Afr J Med Med Sci. (2014) 43:45–50.
17. Agajie, M, Disassa, H, Birhanu, M, and Amentie, M. Prevalence of pulmonary tuberculosis and associated factors in prisons of Benishangul Gumuz region, Western Ethiopia. Prevalence. (2018) 6. doi: 10.26821/IJSRC.6.9.2018.6808
18. Ali, S, Haileamlak, A, Wieser, A, Pritsch, M, Heinrich, N, Loscher, T, et al. Prevalence of pulmonary tuberculosis among prison inmates in Ethiopia, a cross-sectional study. PLoS One. (2015) 10:e0144040. doi: 10.1371/journal.pone.0144040
19. Banda, H, Gausi, F, Harries, A, and Salaniponi, F. Prevalence of smear-positive pulmonary tuberculosis among prisoners in Malawi: a national survey. Int J Tuberc Lung Dis. (2009) 13:1557–9.
21. Berihun, YA, Nguse, TM, and Gebretekle, GB. Prevalence of tuberculosis and treatment outcomes of patients with tuberculosis among inmates in Debrebirhan prison, north Shoa Ethiopia. Ethiop J Health Sci. (2018) 28:347–54. doi: 10.4314/ejhs.v28i3.13
22. Biadglegne, F, Rodloff, AC, and Sack, U. A first insight into high prevalence of undiagnosed smear-negative pulmonary tuberculosis in northern Ethiopian prisons: implications for greater investment and quality control. PLoS One. (2014) 9:e106869. doi: 10.1371/journal.pone.0106869
23. Chigbu, LN, and Iroegbu, CU. Incidence and spread of Mycobacterium tuberculosis-associated infection among aba Federal prison inmates in Nigeria. J Health Popul Nutr. (2010) 28:327–32.
24. Chekesa, B, Gumi, B, Chanyalew, M, Zewude, A, and Ameni, G. Prevalence of latent tuberculosis infection and associated risk factors in prison in east Wollega zone of western Ethiopia. PLoS One. (2020) 15:e0233314. doi: 10.1371/journal.pone.0233314
25. Dibissa, KE, Waktole, ZD, and Tolessa, BE. Prevalence of pulmonary tuberculosis and associated factors among prisoners in Western Oromia, Ethiopia: A cross-sectional study. bio Rxiv. (2019):869727. doi: 10.1101/869727
26. Fuge, TG, and Ayanto, SY. Prevalence of smear positive pulmonary tuberculosis and associated risk factors among prisoners in Hadiya zone prison, Southern Ethiopia. BMC Res Notes. (2016) 9:201. doi: 10.1186/s13104-016-2005-7
27. Gebrecherkos, T, Gelaw, B, and Tessema, B. Smear positive pulmonary tuberculosis and HIV co-infection in prison settings of North Gondar zone, Northwest Ethiopia. BMC Public Health. (2016) 16:1091. doi: 10.1186/s12889-016-3761-y
28. Gizachew Beza, M, Hunegnaw, E, and Tiruneh, M. Prevalence and associated factors of tuberculosis in prisons settings of east Gojjam zone, Northwest Ethiopia. Int J Bacteriol. (2017) 2017:1–7. doi: 10.1155/2017/3826980
29. Habeenzu, C, Mitarai, S, Lubasi, D, Mudenda, V, Kantenga, T, Mwansa, J, et al. Tuberculosis and multidrug resistance in Zambian prisons, 2000–2001. Int J Tuberc Lung Dis. (2007) 11:1216–20.
30. Henostroza, G, Topp, SM, Hatwiinda, S, Maggard, KR, Phiri, W, Harris, JB, et al. The high burden of tuberculosis (TB) and human immunodeficiency virus (HIV) in a large Zambian prison: a public health alert. PLoS One. (2013) 8:e67338. doi: 10.1371/journal.pone.0067338
31. Jordan, A, Podewils, L, Castro, K, Zishiri, V, and Charalambous, S. Prevalence and risk factors of tuberculosis disease in south African correctional facilities in 2015. Int J Tuberc Lung Dis. (2019) 23:1198–204. doi: 10.5588/ijtld.18.0782
32. Kalonji, GMDCG, Okenge Ngongo, L, Kazumba Nsaka, D, Kabengele, T, Tshimungu Kandolo, F, Ilunga-Ilunga, F, et al. Prevalence of tuberculosis and associated risk factors in the central prison of Mbuji-Mayi, Democratic Republic of Congo. Trop Med Health. (2016) 44:30. doi: 10.1186/s41182-016-0030-9
33. Kanyerere, H, Banda, R, Gausi, F, Salaniponi, F, Harries, A, Mpunga, J, et al. Surveillance of tuberculosis in Malawian prisons. Public Health Action. (2012) 2:10–4. doi: 10.5588/pha.11.0022
34. Kwabla, M, Ameme, D, and Nortey, P. Pulmonary tuberculosis and its risk factors among inmates of a Ghanaian prison. Int J Trop Dis Health. (2015) 9:1–10. doi: 10.9734/IJTDH/2015/17246
35. Kayomo, MK, Hasker, E, Aloni, M, Nkuku, L, Kazadi, M, Kabengele, T, et al. Outbreak of tuberculosis and multidrug-resistant tuberculosis, Mbuji-Mayi central prison, Democratic Republic of the Congo. Emerg Infect Dis. United states of America: Emerging Infectious Diseases (2018) 24:2029–35. doi: 10.3201/eid2411.180769
36. Lawal, M, Omili, M, Bello, T, Onuha, L, and Haruna, A. Tuberculosis in a Nigerian medium security prison. Benin J Postgrad Med. (2009) 11:1–8. doi: 10.4314/bjpm.v11i1.48840
37. Maggard, KR, Hatwiinda, S, Harris, JB, Phiri, W, Krüüner, A, Kaunda, K, et al. Screening for tuberculosis and testing for human immunodeficiency virus in Zambian prisons. Bull World Health Organ. (2015) 93:93–101. doi: 10.2471/BLT.14.135285
38. Merid, Y, Woldeamanuel, Y, Abebe, M, Datiko, D, Hailu, T, Habtamu, G, et al. High utility of active tuberculosis case finding in an Ethiopian prison. Int J Tuberc Lung Dis. (2018) 22:524–9. doi: 10.5588/ijtld.17.0635
39. Mohammed, SA. M. tuberculosis among jail inmates of Ethiopian prisons: Risk factors, molecular epidemiology and drug resistance: Lmu. Munich: LMU Munich (2017).
40. Moges, B, Amare, B, Asfaw, F, Tesfaye, W, Tiruneh, M, Belyhun, Y, et al. Prevalence of smear positive pulmonary tuberculosis among prisoners in North Gondar zone prison, Northwest Ethiopia. BMC Infect Dis. (2012) 12:1–7. doi: 10.1186/1471-2334-12-352
41. Mmbaga, VM. Prevalence and factors associated with pulmonary tuberculosis among prisoners in Dar es salaam, Tanzania, 2012. Dar es Salaam: Muhimbili University of Health and Allied Sciences (2013).
42. Mpeirwe, M, Rugera, S, and Boum, Y II. Diagnosis of tuberculosis in a high TB-HIV environment using microscopy and culture: the example of Kakiika prison-Kyamugorani, Mbarara, Uganda. J. Sci. Technol. (Ghana). (2016) 36:29–32. doi: 10.4314/just.v36i1.5
43. Noeske, J, Kuaban, C, Amougou, G, Piubello, A, and Pouillot, R. Pulmonary tuberculosis in the central prison of Douala. East Afr Med J. (2006) 83:25–30. doi: 10.4314/eamj.v83i1.9357
44. Nyangulu, D, Harries, A, Kang'Ombe, C, Yadidi, A, Chokani, K, Cullinan, T, et al. Tuberculosis in a prison population in Malawi. Lancet. (1997) 350:1284–7. doi: 10.1016/S0140-6736(97)05023-X
45. Owokuhaisa, J, Thokerunga, E, and Bazira, J. Prevalence of pulmonary tuberculosis among prison inmates at Mbarara central prison, South Western Uganda. Adv Res. (2014) 2:618–25. doi: 10.9734/AIR/2014/10676
46. Sesay, F. Prevalence of pulmonary tuberculosis and human Immuno-deficiency virus among inmates in Nsawam medium security prison in Ghana. Accra: University of Ghana (2016).
47. Séri, B, Koffi, A, Danel, C, Ouassa, T, Blehoué, M-A, Ouattara, E, et al. Prevalence of pulmonary tuberculosis among prison inmates: a cross-sectional survey at the correctional and detention Facility of Abidjan, Côte d'Ivoire. PLoS One. (2017) 12:e0181995. doi: 10.1371/journal.pone.0181995
48. Telisinghe, L, Fielding, KL, Malden, JL, Hanifa, Y, Churchyard, GJ, Grant, AD, et al. High tuberculosis prevalence in a south African prison: the need for routine tuberculosis screening. PLoS One. (2014) 9:e87262. doi: 10.1371/journal.pone.0087262
49. Winsa, BB, and Mohammed, AE. Investigation on pulmonary tuberculosis among Bedele Woreda prisoners, Southwest Ethiopia. Int J Biomed Sci Eng. (2015) 3:69–73. doi: 10.11648/j.ijbse.20150306.11
50. Zerdo, Z, Medhin, G, Worku, A, and Ameni, G. Prevalence of pulmonary tuberculosis and associated risk factors in prisons of Gamo Goffa zone, South Ethiopia: a cross-sectional study. Am J Health Res. (2014) 2:291–7. doi: 10.11648/j.ajhr.20140205.21
51. Zishiri, V, Charalambous, S, Shah, MR, Chihota, V, Page-Shipp, L, Churchyard, GJ, et al. Implementing a large-scale systematic tuberculosis screening program in correctional facilities in South Africa. Open forum infectious diseases. Oxford: Oxford University Press (2015).
52. Seid, G, Alemu, A, Dagne, B, Sinshaw, W, and Gumi, B. Tuberculosis in household contacts of tuberculosis patients in sub-Saharan African countries: a systematic review and meta-analysis. J Clin Tuberc Other Mycobact Dis. (2022) 29:100337. doi: 10.1016/j.jctube.2022.100337
54. Winetsky, DE, Almukhamedov, O, Pulatov, D, Vezhnina, N, Dooronbekova, A, and Zhussupov, B. Prevalence, risk factors and social context of active pulmonary tuberculosis among prison inmates in Tajikistan. PLoS One. (2014) 9:e86046. doi: 10.1371/journal.pone.0086046
55. Shrestha, G, Yadav, DK, Gautam, R, Mulmi, R, Baral, D, and Pokharel, PK. Pulmonary tuberculosis among male inmates in the largest prison of eastern Nepal. Tuberc Res Treat. (2019) 2019:1–7. doi: 10.1155/2019/3176167
56. Valença, MS, Scaini, JLR, Abileira, F, Gonçalves, CV, Von Groll, A, and Silva, PEA. Prevalence of tuberculosis in prisons: risk factors and molecular epidemiology. Int J Tuberc Lung Dis. (2015) 19:1182–7. doi: 10.5588/ijtld.15.0126
57. Al-Darraji, HAA, Altice, FL, and Kamarulzaman, A. Undiagnosed pulmonary tuberculosis among prisoners in Malaysia: an overlooked risk for tuberculosis in the community. Tropical Med Int Health. (2016) 21:1049–58. doi: 10.1111/tmi.12726
58. Alavi, SM, Bakhtiarinia, P, Eghtesad, M, Albaji, A, and Salmanzadeh, S. A comparative study on the prevalence and risk factors of tuberculosis among the prisoners in Khuzestan, south-West Iran. Jundishapur. J Microbiol. (2014) 7:e18872. doi: 10.5812/jjm.18872
59. Pelissari, D, Kuhleis, D, Bartholomay, P, Barreira, D, Oliveira, C, de Jesus, R, et al. Prevalence and screening of active tuberculosis in a prison in the south of Brazil. Int J Tuberc Lung Dis. (2018) 22:1166–71. doi: 10.5588/ijtld.17.0526
60. Morasert, T, Worapas, W, Kaewmahit, R, and Uphala, W. Prevalence and risk factors associated with tuberculosis disease in Suratthani central prison, Thailand. Int J Tuberc Lung Dis. (2018) 22:1203–9. doi: 10.5588/ijtld.17.0654
61. Salazar-De La Cuba, AL, Ardiles-Paredes, DF, Araujo-Castillo, RV, and Maguiña, JL. High prevalence of self-reported tuberculosis and associated factors in a nation-wide census among prison inmates in Peru. Tropical Med Int Health. (2019) 24:328–38. doi: 10.1111/tmi.13199
62. Metcalf, T, Soria, J, Montano, SM, Ticona, E, Evans, CA, Huaroto, L, et al. Evaluation of the GeneXpert MTB/RIF in patients with presumptive tuberculous meningitis. PLoS One. (2018) 13:e0198695. doi: 10.1371/journal.pone.0198695
63. Elbrolosy, AM, El Helbawy, RH, Mansour, OM, and Latif, RA. Diagnostic utility of GeneXpert MTB/RIF assay versus conventional methods for diagnosis of pulmonary and extra-pulmonary tuberculosis. BMC Microbiol. (2021) 21:1–10. doi: 10.1186/s12866-021-02210-5
Keywords: tuberculosis, prison, meta-analysis, sub-Saharan Africa, systematic review
Citation: Sisay Asgedom Y, Ambaw Kassie G and Melaku Kebede T (2023) Prevalence of tuberculosis among prisoners in sub-Saharan Africa: a systematic review and meta-analysis. Front. Public Health. 11:1235180. doi: 10.3389/fpubh.2023.1235180
Edited by:
Belaineh Girma Belaineh, International Training and Education Center for Health (I-TECH), United StatesReviewed by:
Raymond Salanga Dankoli, World Health Organisation, UkraineAndargachew Kumsa Erena, Ministry of Health, Ethiopia
Copyright © 2023 Sisay Asgedom, Ambaw Kassie and Melaku Kebede. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yordanos Sisay Asgedom, yordusisay@gmail.com
 Yordanos Sisay Asgedom
Yordanos Sisay Asgedom