
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health , 20 July 2023
Sec. Aging and Public Health
Volume 11 - 2023 | https://doi.org/10.3389/fpubh.2023.1169083
This article is part of the Research Topic Population Aging and Older Health in an Era of Digitalization: Empirical Findings and Implications View all 11 articles
Background: Frailty is a dynamic and complex geriatric condition characterized by multi-domain declines in physiological, gait and cognitive function. This study examined whether digital health technology can facilitate frailty identification and improve the efficiency of diagnosis by optimizing analytical and machine learning approaches using select factors from comprehensive geriatric assessment and gait characteristics.
Methods: As part of an ongoing study on observational study of Aging, we prospectively recruited 214 individuals living independently in the community of Southern China. Clinical information and fragility were assessed using comprehensive geriatric assessment (CGA). Digital tool box consisted of wearable sensor-enabled 6-min walk test (6MWT) and five machine learning algorithms allowing feature selections and frailty classifications.
Results: It was found that a model combining CGA and gait parameters was successful in predicting frailty. The combination of these features in a machine learning model performed better than using either CGA or gait parameters alone, with an area under the curve of 0.93. The performance of the machine learning models improved by 4.3–11.4% after further feature selection using a smaller subset of 16 variables. SHapley Additive exPlanation (SHAP) dependence plot analysis revealed that the most important features for predicting frailty were large-step walking speed, average step size, age, total step walking distance, and Mini Mental State Examination score.
Conclusion: This study provides evidence that digital health technology can be used for predicting frailty and identifying the key gait parameters in targeted health assessments.
As the population ages, frailty is becoming a major challenge for public healthcare. Frailty is a condition that affects many older people and is characterized by a decline in physical function, decreased resilience to stressors, and a higher risk of negative health outcomes such as falls, hospitalization, and death (1). Patients with frailty commonly show multifaceted clinical symptoms, phenotypic heterogeneity, and fluctuating manifestations that challenge the comprehensive appraisal of the condition (2, 3). Undiagnosed frailty is common in older people, since it typically has no explicit connection to a defined medical issue; therefore, frailty frequently remains untreated until later stages. Early detection of the risk of frailty is essential especially in the early stages, as such identification would facilitate the implementation of treatments to slow down declines and reduce adverse outcomes.
Currently, the prediction of frailty is performed using questionnaires and tests of physical activity, muscle strength or gait. The most widely used assessment is the fried performance (FP) test (4), which includes measures of weight loss, exhaustion, low physical activity, weakness, and slow walking speed. However, the FP test only evaluates the physical aspects of frailty, while frailty is acknowledged to be a multifaceted state that includes not only physical dimensions, but also social, cognitive, and psychological dimensions (5, 6). Gait-related mobility is a key physical ability that has been linked to frailty and is often used as a predictor of future health outcomes (7, 8).
During the aging process, the loss of function is intrinsic to all the physiological systems, including the Central and peripheral nervous systems, musculo-skeletal system, and cardiopulmonary system. The most dramatic and significant changes is the decline in limb muscle, which can lead to changes in gait parameters such as walking speed, cadence, and stride-length (9, 10). Gait speed, which is a quantifiable index of ambulatory ability and a major predictor of future health outcomes (11), is commonly used as an outcome in the research of frailty.
Advances in technologies associated with wearable devices has enabled the collection of more precise parameters regarding other spatial and temporal gait variables in addition to the commonly observed gait speed. These technologies are valid and practical, and they provide a promising, cost-effective digital method in standardizing the data collection and analysis of gait function with improved efficiency and accessibility both in the clinical setting and within seniors’ living communities (12–14).
With improved multidisciplinary diagnostic approaches, some researchers have predicted frailty status based on CGA, but this approach has several limitations. For example, questions within the CGA frequently require advanced knowledge and thus might not accurately reflect the mental status of subjects. Concurrently, with the evolution of computer science and artificial intelligence, many researchers would like to predict frailty using ML approaches (Table 1) (15–23). Previous studies in this regard have commonly utilized easy-to-access epidemiological datasets or electronic health records to construct ML algorithms (24); the nature of these databases mean that these algorithms are potentially limited to the use of a single dimension. Furthermore, little research has analyzed Asian populations, which have very different socio-economic profiles as compared with Western populations (16).
Therefore, the objectives of this study were to (A) obtain the relevant gait parameters using wearable sensor and to analyze their association with deterioration of physical function and evolution into frailty, and to (B) develop an ML frailty risk prediction model suited for an Asian population, using a combination of both wearable sensor-based gait analysis and CGA.
In total, we consecutively recruited 214 community-dwelling volunteers from the course of the anti-aging study, a prospective cohort study conducted to investigates the association of frailty with health. All subjects were recruited through advertisements in two ways. First, recruitment was conducted at four communities centers in Guangzhou (the capital of the Guangdong Province in the southeastern region of China). Second, with the assistance of the District Health Center and GPs clinics, recruitment invitations was handed out via online announcement to older individuals on Wechat platforms. Eligibility criteria for participants were: aged 60–95 years, having adequate auditory and visual acuity, and the ability to ambulate with or without any walking aids or assistance of others. Exclusion criteria included orthopedic or neurological complications or other relevant medical conditions that might restrict walking speed and natural movement. All subjects were required to complete CGA including but not limited to a standardized questionnaire that collected demographic information, medical and medication records, as well as multidimensional clinical assessment, including anthropometric evaluation, emotional evaluation, and neuropsychological evaluation.
The parameters of CGA included the patients’ demographic data, including age, gender, education level, marital status, employment position and measurement data. The clinical measurement data involved multimorbidity (defined by the coexistence of >2 chronic conditions), polypharmacy (defined as currently using >5 drugs), depression disorder (25) (defined by scores ≥10 on the 9-item Patient Health Questionnaire, PHQ-9), anxiety disorder (26) (defined by scores ≥10 on the 7-item Generalized Anxiety Disorder, GAD-7), cognitive function (27, 28) (assessed by the Mini-Mental State Examination, MMSE and the Chinese versions of the Montreal Cognitive Assessment, MoCA-BC) and neuropsychiatric function (29, 30) (assessed by the Mild Behavioral Impairment Checklist, [MBI-C]). A detailed description of how these features were defined is also provided in Table 2.
To obtain an objective and quantitative assessment of gait parameters, all participants were instructed to complete a 6-min walk test (6MWT) using a wearable sensor (Ambulosono Sensor System) (13, 31). The sensor was connected to the iOS Gait Reminder App, which can issue auditory instructions while continuously recording step size via an iOS gyroscope and accelerometers, after corrections for limb length, angular excursion, signal filtering, and drift. Participant were instructed to walk independently if possible, and were permitted to use walking aid (e.g., walker or cane) if needed.
The outcome measures used to categorize no-frailty and frailty was assessed utilizing the five components specified in the FP test (4), including: self-reported unintentional weight loss of 10 pounds or more within the last year; self-reported exhaustion; slowness stratified by gender and height; weakness via grip strength test using a hand dynamometer; and low physical activity based on the short version Minnesota Leisure Time Activity questionnaire (32). FP scores range from 0 to 5 points, with higher scores indicating more severe frailty. Based on the results, individuals with scores of 0 through 2 were categorized into the no-frailty group, and those with scores of 3 or more were included in the frailty group. This scale has exhibited high validity and has become a gold standard for classifying frailty in older adults (33).
In this study, five widely accepted and extensively used supervised ML models were applied: random forests (RF); decision trees (DT); naïve Bayes; neural network (NN) and stochastic gradient descent (SGD).
DT is a powerful ML algorithm capable of performing classification tasks. Advantages of DT include simplicity, interpretability, ability to model nonlinear data and ability to handle outliers during training (34), but weaknesses of the simple decision tree are instability and a risk of over fitting. RF, a popular ensemble classification method, combines multiple learning algorithms to achieve better performance. RF models generally outperform those generated by DT in terms of accuracy. Naive Bayes is a supervised probabilistic machine learning algorithm for probabilistic classification that relies on Bayes’ theorem with an assumption of strong independence between the input features (35). Neural network is a mathematical computing model that imitates the construction of biological neural networks and that is commonly used in classification tasks with various applications (36).We also attempted to apply SGD (37), an algorithm for optimization problems arising in high-dimensional inference tasks.
An overview of the ML approach used in this study is shown in Figure 1. After imputation of missing values by multiple interpolation, the values of input features were standardized to ensure that each feature had the same influence on the cost function in designing the ML models. In this study, the data were randomly divided, with 70% used for training and the remaining 30% used as test data. Within the 70% training set, the data was split into ten random folds for cross validation to guard against overfitting. The rigorous use of holdout method with random resampling and stratified k-fold cross-validation ensured the validity and generalizability of the findings, and helped to mitigate potential biases in the analysis. We used average values as they are robust to outlying predictions.
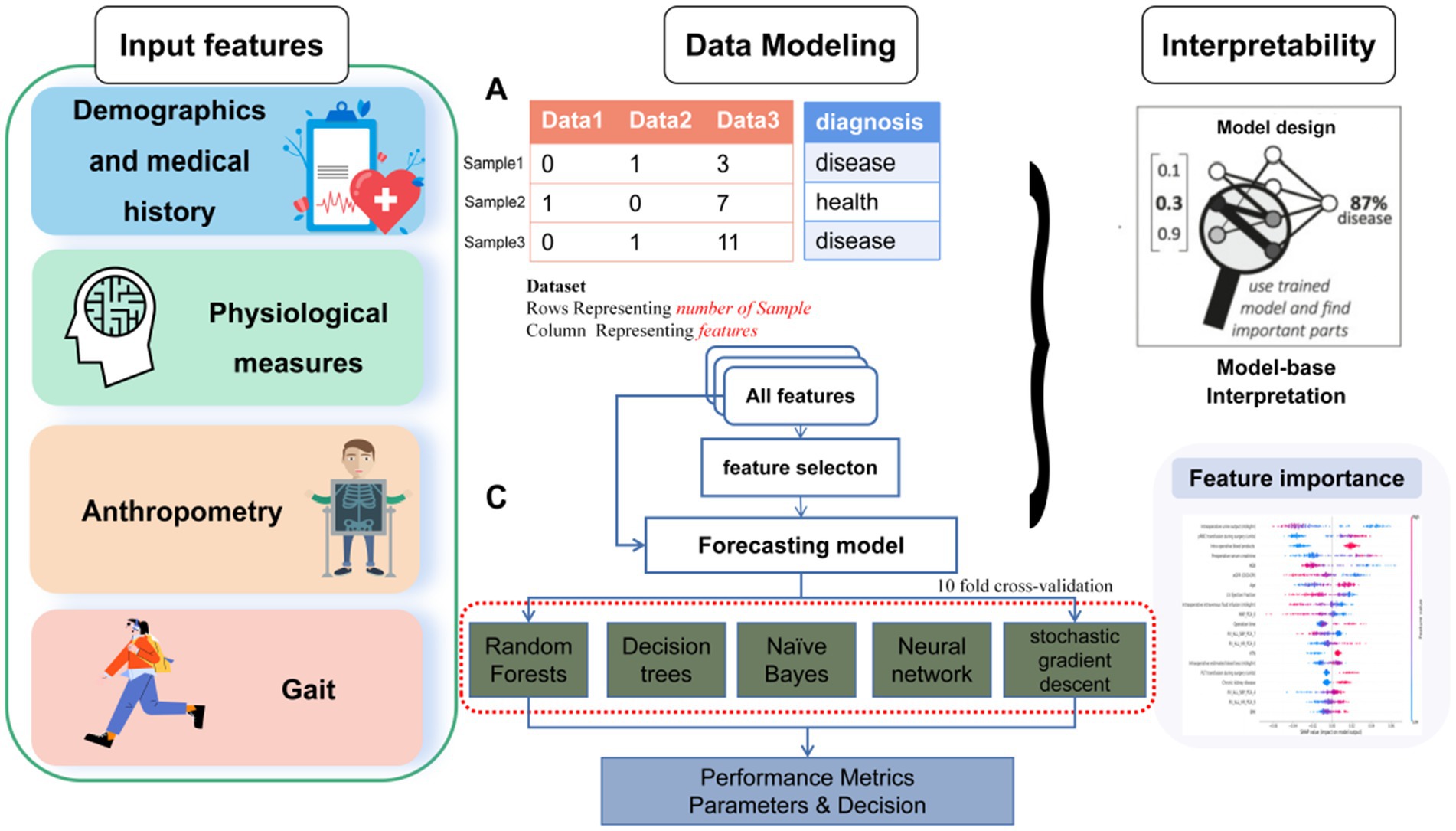
Figure 1. Overall data acquisition, feature extraction, feature selection, data classification analysis and machine learning flow for the classification of frailty.
When not all features have significant class discrimination information, using feature selection methods can help to remove the irrelevant and redundant features. This method can reduce the computational time and improve classification performance. To determine the lowest number of demographic features, clinical features and sensor-derived gait sequence features required to best identify frailty, optimal feature selection using either the independent samples t-test or the chi-square test was applied, depending on each feature’s types. Sixteen of the features showed a significant difference between the no-frailty and frailty groups, and thus they were used as independent variables for optimal feature selection.
The performance of the ML models was evaluated using AUC score, sensitivity, specificity, precision, F1-score and accuracy. We aimed to find the simplest model that achieves the highest accuracy. As the original training data were imbalanced (13.1% frailty), such data can result in biased estimates of training performance; hence, with the best performing model chosen by average AUC score, which can be understood as the probability that a randomly chosen no-frailty patient will have a score lower than a randomly chosen frailty patient. SHapley Additive exPlanation (SHAP) values were used to provide consistent and locally accurate attribution values for each feature within each prediction model (38).
All statistical analyses and calculations were performed using R software and Python (version 3.9.7; Python Software Foundation). The categorical variables were expressed as total numbers and percentages, with the chi-square test used for comparison between groups. Normally continuous variables were expressed as x̅ ± s, and the independent samples t-test for was used for comparisons between groups; non-normally continuous variables were expressed as median and IQR, with a Mann–Whitney U test used for comparisons between groups. The inspection level α = 0.05, and we considered any difference statistically significant if p < 0.05.
Table 3 compares the detailed demographic and clinical characteristics of each group. A total of 214 subjects were included in the study, among which 28 (13.1%) subjects were classified into the frailty group. Compared with the no-frailty group, subjects in the frailty group were of older age (74.3 ± 8.4 vs. 68.1 ± 6.1 years) and had lower BMI (22.2 ± 2.7 vs. 23.5 ± 2.9). Moreover, the frailty group had a higher proportion of comorbid conditions (74.4% vs. 31.7%), polypharmacy (35.7% vs. 8.6%) and depression (32.1% vs. 14.0%) with significant difference. The results also showed that the scores on the Mini Mental State Examination (MMSE) and the Montreal Cognitive Assessment from subjects in the frailty group were significantly lower than those of subjects in the no-frailty group (p < 0.01). There were no significant differences in gender or education between two groups (Table 3).
Table 4 shows gait parameters in relation to the presence of frailty. In comparison to subjects in the no-frailty group, those in the frailty group had slower gait speed (57.3 ± 17.6 vs. 70.1 ± 15.2 m/min), lower step walking distance (338.6 ± 96.7 vs. 420.5 ± 91.3 m), lower total cadence (113.5 ± 11.7 vs. 120.4 ± 15.2 step/min), shorter step size (0.508 ± 0.143 vs. 0.593 ± 0.107 m), and higher average step time (0.533 ± 0.059 vs. 0.502 ± 0.051 s).
We performed feature selection analyses among the various feature categories to investigate and identify crucial feature signatures for our models. The input data used were the gait features based on the outputs from wearable sensor and the demographic and clinical features derived from CGA. As illustrated in Table 5, the ML models achieved up to 63.5% accuracy, 88.2% specificity, 56.5% sensitivity, 98.4% precision and an F1-score of 65.0% using demographic and clinical features. When using the gait features, the ML models achieved up to 65.6% accuracy, 83.8% specificity, 65.3% sensitivity, 97.8% precision and F1-score of 65.6%. In comparison, the ML models that employed all features achieved the highest performance, with up to 71.1% accuracy, 89.5% specificity, 61.3% sensitivity, 97.0% precision, and an F1-score of 68.0%.
The receiver operating characteristic (ROC) curves of each ML model using different features are shown in Figure 2. All ML models achieved significant improvements in discrimination by using selected features as compared to the indiscriminate use of all features. In particular, RF exhibited an AUC gain of 4.3% when using selected features as compared to using all features, a gain of 6.2% when using selected features as compared to all demographic and clinical features, and a gain of 11.4% when using selected features as compared to using all gait sequence features. Since the RF model outperformed other ML models according to accuracy and AUC, it was selected for all downstream analyses.
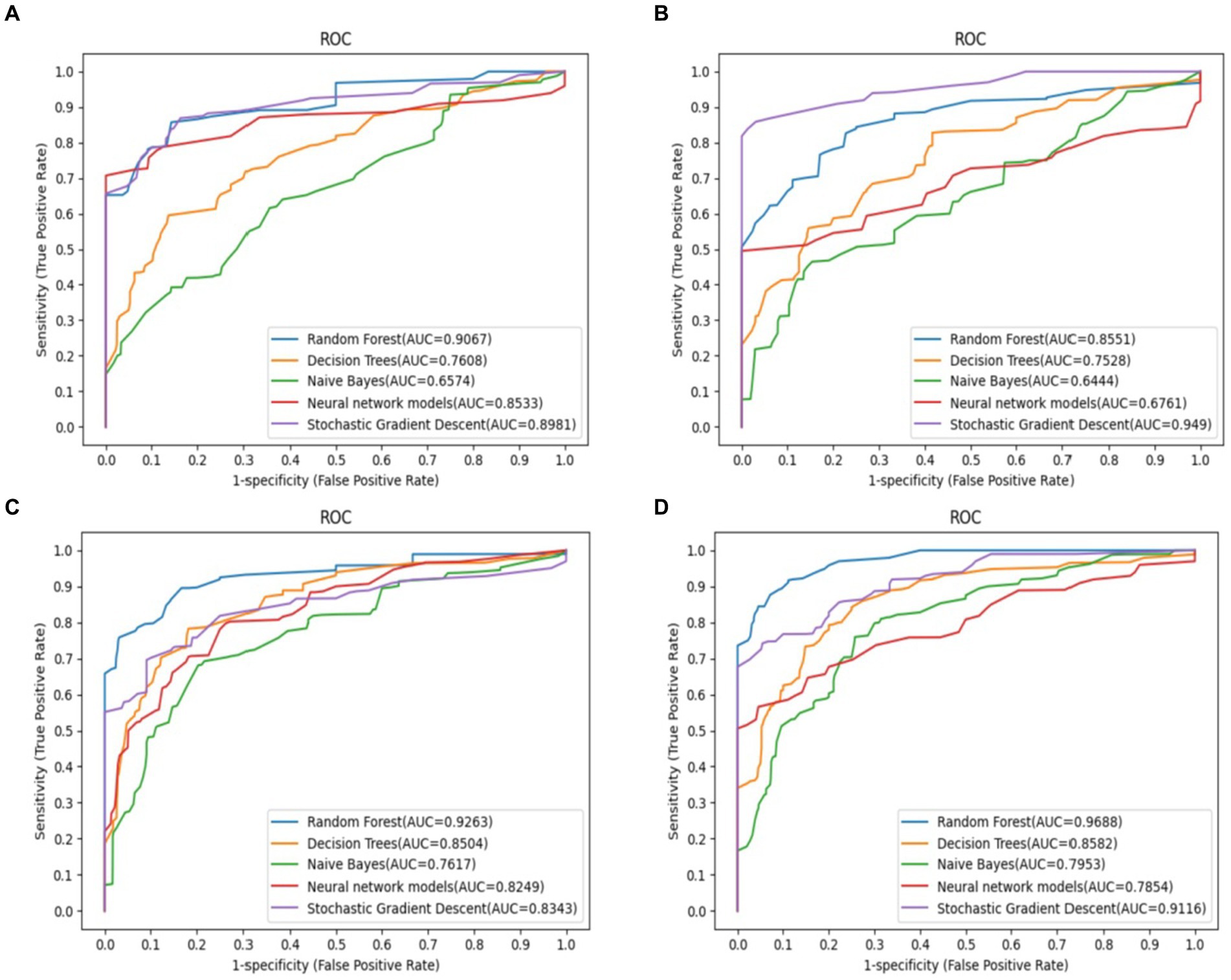
Figure 2. Predictive performance of different feature selection in predict the outcomes of frailty model. The RF model demonstrated the most favorable performance. (A) Demographic and clinical prediction (AUC = 0.907), (B) gait sequence prediction (AUC = 0.855), (C) all feature prediction (AUC = 0.926), (D) selected feature prediction (AUC = 0.969).
Figure 3 shows the SHAP summary plot of RF using selected features, with the features contributing to the model in descending order of average absolute SHAP values. This plot depicts the relationships of values to SHAP values in the training dataset. According to the prediction model, the higher the SHAP value of a feature, the more likely frailty becomes. As observed in the plot, the five most important predictors in the prediction model were large step walking speed, average step size, age, total step walking distance, and MMSE score.
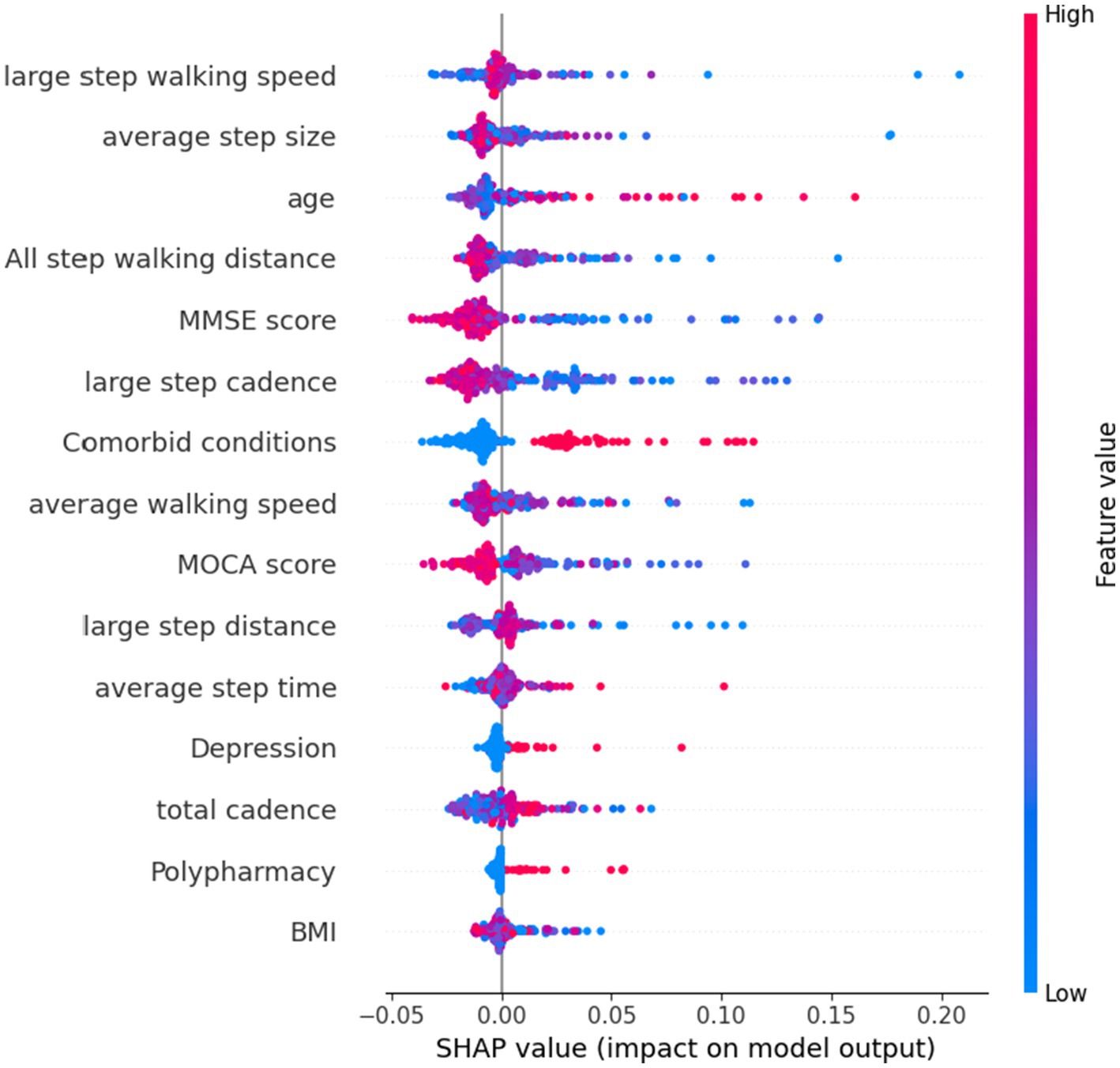
Figure 3. SHAP plot of top features influencing our model’s prediction of frailty using all features.
Frailty is a common condition among older adults that is characterized by a decline in physical and cognitive function and an increased risk of adverse health outcomes. Identifying frailty on the early stage can help healthcare professionals implement hierarchical strategies to prevent or delay its onset and manage potential conditions. In this study, we adopted a set of analytical and machine-learning approaches to analyze the relationship between frailty and a combination of CGA and wearable sensor-derived gait parameters in community-dwelling older adults.
To achieve this, we used statistical methodologies to extract a subset of uncorrelated components associated with frailty predictors, which were then used to independently identify and visualize multiple dimensions associated with frailty. Our results indicate that machine learning methods are effective in predicting frailty and that using a combination of both CGA characteristics and wearable sensor data improved the performance of our model compared to using either features separately. This multifaceted combination of features provides a comprehensive perspective on frailty, allowing the model to capture the intricate relationship between various factors.
Our final model, which was processed using a random forest machine learning method, achieved an impressive area under the curve (AUC) score of 0.926, indicating a high level of accuracy compared with previous estimates, ranging from 0.58 up to 0.92 (15–23). In contrast to other traditional predictive formulas, such as the electronic medical records-based model, administrative records-based model, and sensor-based model, the classification results of our model outperformed other traditional models. This enhanced performance may be attributed to our study design and to the synergistic approach that combined CGA and wearable sensor data, accompanied by rigorous feature selection and optimization processes.
Furthermore, we also calculated the accuracy of each model when processing different complements of patient characteristics, in addition to overall model performance. We found that using a narrower set of carefully selected features achieved significant improvements in discrimination relative to models that included all available features. This reduced the dimensionality of our dataset and allowed our model to focus on the most relevant features for predicting frailty. In previous study, Williamson and colleagues (20) reported that frailty was predicted using a large number of features of electronic medical record database without feature selection as effectively, which can lead to overfitting and reduced generalization of the model.
This limitation of the number of features needed would be helpful in clinical settings due to difficulties in collecting consistent data from older patients, especially with functional data or data indicating cognitive and emotional status. Notably, the top five features found to be important in predicting frailty were step walking speed, average step size, age, total step walking distance, and MMSE score. These results are consistent with previous research that has identified gait and physical activity parameters measured by wearable sensors as being associated with physical frailty and has found that certain gait parameters, such as percentage time standing, percentage time walking, walking cadence, and longest walking bout, are effective digital biomarkers for identifying frailty (21, 39). Gait stability, as determined by double-limb support time, step time and stride time, and long short-term memory have also been found to produce the highest discriminative power in identifying frailty using the RF model (40). Accordingly, our findings suggest that healthcare professionals treating older patients should focus on these five features, as they may indicate the risk of future frailty.
In particular, the strength of the gait parameters in our analysis, accounting for 50% of the predictive power, demonstrates the importance of these motor characteristics as a measure of frailty. The 6MWT is a valid, reliable and sensitive measure of functional performance capacity that has been found to be useful in evaluating frailty in older adults (41). Using digital methods to perform the 6MWT, such as wearable sensors or GPS devices, an economical and convenient way to provide diagnostic and clinical information, can significantly improve the accuracy and reliability of the test results compared to manual methods (42, 43).
CGA has also been used to identify risks of adverse events such as cognitive impairment, mortality, functional decline, surgical complications, and chemotherapy toxicity among frailty patients (44, 45). This is the first study ever done to show the benefits of utilizing a combination of CGA and wearable sensor data in predicting frailty. Our research adds to the existing knowledge by revealing the efficacy of employing an integrated approach, using both CGA and wearable sensor data in predicting frailty and a limited number of judiciously selected features in a machine learning model. It also highlights the importance of gait-related measures for frailty prediction. Previous studies have used various artificial intelligence (AI) models to analyze frailty and predict frailty risk in older adults using different types of data, including clinical records (15–18, 24, 46), physical function data (47, 48), and wearable sensor data (21, 22, 49). Additionally, the clinical implications of our method were summarized as follows:
A. The integration of both CGA and wearable sensors objectively measure gait parameters offers healthcare professionals comprehensive understand of frailty risk, enabling early intervention and better management of pre-clinical condition to prevent adverse health outcomes.
B. In terms of feature selection, our study identified the five most important indexs in the prediction model as large step walking speed, average step size, age, total step walking distance, and MMSE score. Clinicians can prioritize these factors when assessing frailty risk in older patients, allowing for a more effective and targeted approach that streamlines data collection and reduces the burden of frailty on patients and healthcare systems.
C. In terms of gait assessment, we depicted wearable sensors for auto digital gait data analysis such as the 6MWT that can provide more accurate and reliable results classification of frailty individuals compared to traditional manual methods, improving the overall quality of frailty evaluations.
D. In terms of the clinical setting, the application of ML techniques to analyze and predict frailty risk has proven beneficial in identifying at-risk individuals. These methods have shown promising results in identifying relevant features and interactions, particularly when numerous variables are involved, allowing for timely interventions and more personalized care plans.
Considering the implications of a future digital health approach, we aimed to capture pertinent gait components based on 6MWT that can be utilized to remotely predict the risk of frailty using wearable sensors. With this approach, patients would able to be long-term digital monitored without having to undergo an in-person clinic visit to assess their physical frailty. However, this study is subject to several limitations that need to be addressed. Since, the 6MWT is a long-distance walking test that places higher demands on the abilities, functional status, and reserves of older adults. Our recruitment threshold of walking behavior, a minimum test time of 6 min, was achievable for the vast majority of this population with or without any walking aids. However, the physical state of all senior persons cannot be accurately represented by our community-sourced volunteer recruiting. While the physical condition of older adult in the community is generally acceptable, the degree of frailty may exhibit more pronounced manifestation within older institutions, such as nursing homes.
Throughout the research process, some patients were unable to complete the full test due to their limited capacity for sustained long-distance walking. For safety reasons, these individuals had to be excluded from the study. While we would handle missing data appropriately, this may impact the experimental results and potentially weaken the test’s effectiveness. Consequently, the restricted source of the samples supply diminishes the representativeness, universality, and generalizability of our study findings, thereby reducing their therapeutic usefulness. Although there are some intrinsic limitations to the test, the 6MWT can be potentially performed in the vast majority of geriatric population, thus key gait parameters can be served as an important tool for early frailty screening, diagnostic assessment, and early prevention in older adults (50).
Future study is warranted to validate and generalize these findings to other populations and settings, as well as to explore the potential of integrating additional data sources and advanced machine learning algorithms to further enhance the ability of ML to identify the risk of frailty. Ultimately, the insights gained from this study have the potential to significantly impact clinical practice, leading to improved identification and management of frailty in older adults, enhancing their quality of life and overall health outcomes.
Overall, our findings suggest that combining CGA and wearable sensor-derived gait parameters can improve the accuracy of frailty prediction models. The use of digital measures of gait, such as the 6MWT, plays a crucial role in enhancing the model’s predictive power and should be considered by healthcare professionals when evaluating frailty risk in older patients. Due to the rapid rate at which wearable sensor-based data is being collected, high-performance data processing is becoming increasingly important. Further research is needed to determine the generalizability of these findings to other populations and settings.
The datasets presented in this article are not readily available because the datasets used and/or analyzed during the current study could be obtained from the corresponding author with reasonable requests. Requests to access the datasets should be directed to FX, eHVmdXBpbmcxOTgzQGd6dWNtLmVkdS5jbg==.
The studies involving human participants were reviewed and approved by Ethics Review Board of Guangdong Provincial Hospital of Chinese Medicine Ethics Committee (reference: B2017-168-01). The patients/participants provided their written informed consent to participate in this study.
SF, ZY, and FX conceived and designed the study. SF, RP, and QX recruited the participants, collected the data for the manuscript and provided substantial feedback. SF, JY, ZP, and BH analyzed and interpreted the data. SF, BH, and FX wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
The study was supported by the National Natural Science Foundation of China, No. 81974560; Guangzhou Science and Technology Plan Project, No. 2023A03J0736; Yan Dexin Academic Lessons learned Studio, The Second Hospital of Chinese Medicine [2014] No. 89; Scientific and technological research project of Guangdong Provincial Hospital of Chinese Medicine, No. YN2019ZWB03; Guangdong Provincial Key Laboratory of Diagnosis and Treatment of Major Neurological Diseases, No. 2020B1212060017; Guangdong Provincial Clinical Research Center for Neurological Diseases, No. 2020B1111170002; Southern China International Joint Research Center for Early Intervention and Functional Rehabilitation of Neurological Diseases, No. 2015B050501003 and 2020A0505020004; Guangdong Provincial Engineering Center for Major Neurological Disease Treatment, Guangdong Provincial Translational Medicine Innovation Platform for Diagnosis and Treatment of Major Neurological Disease, Guangzhou Clinical Research and Translational Center for Major Neurological Diseases, No. 201604020010 and Guangdong Basic and Applied Basic Research Foundation, No. 2021B1515120062. Funders played no role in this study (the design, data collection, execution, analysis, interpretation of data and writing the manuscript).
We appreciate the cooperation of the staff of the Guangdong Provincial Hospital of Chinese Medicine.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dent, E , Martin, FC , Bergman, H , Woo, J , Romero-Ortuno, R , and Walston, JD . Management of frailty: opportunities, challenges, and future directions. Lancet. (2019) 394:1376–86. doi: 10.1016/S0140-6736(19)31785-4
2. Looman, WM , Fabbricotti, IN , Blom, JW , Jansen, APD , Lutomski, JE , Metzelthin, SF, et al. The frail older person does not exist: development of frailty profiles with latent class analysis. BMC Geriatr. (2018) 18:84. doi: 10.1186/s12877-018-0776-5
3. Stolz, E , Mayerl, H , and Freidl, W . Fluctuations in frailty among older adults. Age Ageing. (2019) 48:547–52. doi: 10.1093/ageing/afz040
4. Fried, LP , Tangen, CM , Walston, J , Newman, AB , Hirsch, C , Gottdiener, J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–57. doi: 10.1093/gerona/56.3.M146
5. Solfrizzi, V , Scafato, E , Lozupone, M , Seripa, D , Schilardi, A , Custodero, C, et al. Biopsychosocial frailty and the risk of incident dementia: the Italian longitudinal study on aging. Alzheimers Dement. (2019) 15:1019–28. doi: 10.1016/j.jalz.2019.04.013
6. Henry, JD , Coundouris, SP , Mead, J , Thompson, B , Hubbard, RE , and Grainger, SA . Social frailty in late adulthood: social cognitive and psychological wellbeing correlates. J Gerontol B Psychol Sci Soc Sci. (2022) 78:87–96. doi: 10.1093/geronb/gbac157
7. Zhou, H , Park, C , Shahbazi, M , York, MK , Kunik, ME , Naik, AD, et al. Digital biomarkers of cognitive frailty: the value of detailed gait assessment beyond gait speed. Gerontology. (2022) 68:224–33. doi: 10.1159/000515939
8. Chu, WM , Kristiani, E , Wang, YC , Lin, YR , Lin, SY , Chan, WC, et al. A model for predicting fall risks of hospitalized elderly in Taiwan – a machine learning approach based on both electronic health records and comprehensive geriatric assessment. Front Med. (2022) 9:937216. doi: 10.3389/fmed.2022.937216
9. Pirker, W , and Katzenschlager, R . Gait disorders in adults and the elderly: a clinical guide. Wien Klin Wochenschr. (2017) 129:81–95. doi: 10.1007/s00508-016-1096-4
10. Millan-Domingo, F , Tarazona-Santabalbina, FJ , Carretero, A , Olaso-Gonzalez, G , Viña, J , and Gomez-Cabrera, MC . Real-life outcomes of a multicomponent exercise intervention in community-dwelling frail older adults and its association with nutritional-related factors. Nutrients. (2022) 14:5147. doi: 10.3390/nu14235147
11. Fritz, S , and Lusardi, M . White paper: walking speed: the sixth vital sign. J Geriatr Phys Ther. (2001) 32:2–5. doi: 10.1519/00139143-200932020-00002
12. Hu, B . Application of wearable technology in clinical walking and dual task testing. J Transl Int Med. (2019) 7:87–9. doi: 10.2478/jtim-2019-0019
13. Chomiak, T , Watts, A , Burt, J , Camicioli, R , Tan, SN , McKeown, MJ, et al. Differentiating cognitive or motor dimensions associated with the perception of fall-related self-efficacy in Parkinson's disease. NPJ Parkinsons Dis. (2018) 4:26. doi: 10.1038/s41531-018-0059-z
14. Hu, B , and Chomiak, T . Wearable technological platform for multidomain diagnostic and exercise interventions in Parkinson's disease. Int Rev Neurobiol. (2019) 147:75–93. doi: 10.1016/bs.irn.2019.08.004
15. Ambagtsheer, RC , Shafiabady, N , Dent, E , Seiboth, C , and Beilby, J . The application of artificial intelligence (AI) techniques to identify frailty within a residential aged care administrative data set. Int J Med Inform. (2020) 136:104094. doi: 10.1016/j.ijmedinf.2020.104094
16. Aponte-Hao, S , Wong, ST , Thandi, M , Ronksley, P , McBrien, K , Lee, J, et al. Machine learning for identification of frailty in Canadian primary care practices. Int J Popul Data Sci. (2021) 6:1650. doi: 10.23889/ijpds.v6i1.1650
17. le Pogam, MA , Seematter-Bagnoud, L , Niemi, T , Assouline, D , Gross, N , Trächsel, B, et al. Development and validation of a knowledge-based score to predict Fried's frailty phenotype across multiple settings using one-year hospital discharge data: The electronic frailty score. EClinicalMedicine. (2022) 44:101260. doi: 10.1016/j.eclinm.2021.101260
18. Tarekegn, A , Ricceri, F , Costa, G , Ferracin, E , and Giacobini, M . Predictive modeling for frailty conditions in elderly people: machine learning approaches. JMIR Med Inform. (2020) 8:e16678. doi: 10.2196/16678
19. Koo, D , Lee, AR , Lee, E , and Kim, IK . Development of a frailty detection model using machine learning with the Korean frailty and aging cohort study data. Healthc Inform Res. (2022) 28:231–9. doi: 10.4258/hir.2022.28.3.231
20. Tyler Williamson, P , Aponte-Hao, S , Mele, B , Lethebe, BC , Leduc, C , Thandi, M, et al. Developing and validating a primary care EMR-based frailty definition using machine learning. Int J Popul Data Sci. (2020) 5:1344. doi: 10.23889/ijpds.v5i1.1344
21. Park, C , Mishra, R , Golledge, J , and Najafi, B . Digital biomarkers of physical frailty and frailty phenotypes using sensor-based physical activity and machine learning. Sensors (Basel). (2021) 21:5289. doi: 10.3390/s21165289
22. Kraus, M , Saller, MM , Baumbach, SF , Neuerburg, C , Stumpf, UC , Böcker, W, et al. Prediction of physical frailty in orthogeriatric patients using sensor insole-based gait analysis and machine learning algorithms: cross-sectional study. JMIR Med Inform. (2022) 10:e32724. doi: 10.2196/32724
23. Minici, D , Cola, G , Giordano, A , Antoci, S , Girardi, E , Bari, MD, et al. Towards automated assessment of frailty status using a wrist-worn device. IEEE J Biomed Health Inform. (2022) 26:1013–22. doi: 10.1109/JBHI.2021.3100979
24. Wu, Y , Jia, M , Xiang, C , and Fang, Y . Latent trajectories of frailty and risk prediction models among geriatric community dwellers: an interpretable machine learning perspective. BMC Geriatr. (2022) 22:900. doi: 10.1186/s12877-022-03576-5
25. Levis, B , Benedetti, A , and Thombs, BD . Accuracy of patient health questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. (2019) 365:l1476. doi: 10.1136/bmj.l1476
26. Löwe, B , Decker, O , Müller, S , Brähler, E , Schellberg, D , Herzog, W, et al. Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Med Care. (2008) 46:266–74. doi: 10.1097/MLR.0b013e318160d093
27. Chen, KL , Xu, Y , Chu, AQ , Ding, D , Liang, XN , Nasreddine, ZS, et al. Validation of the Chinese version of montreal cognitive assessment basic for screening mild cognitive impairment. J Am Geriatr Soc. (2016) 64:e285–90. doi: 10.1111/jgs.14530
28. Zhuang, L , Yang, Y , and Gao, J . Cognitive assessment tools for mild cognitive impairment screening. J Neurol. (2021) 268:1615–22. doi: 10.1007/s00415-019-09506-7
29. Ismail, Z , Agüera-Ortiz, L , Brodaty, H , Cieslak, A , Cummings, J , Fischer, CE, et al. The Mild Behavioral Impairment Checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis. (2017) 56:929–38. doi: 10.3233/JAD-160979
30. Hu, S , Patten, SB , Fick, G , Smith, EE , and Ismail, Z . Validation of the mild behavioral impairment checklist (MBI-C) in a clinic-based sample. Alzheimers Dement. (2019) 15:P365. doi: 10.1016/j.jalz.2019.06.872
31. Chomiak, T , Sidhu, AS , Watts, A , Su, L , Graham, B , Wu, J, et al. Development and Validation of Ambulosono: A Wearable Sensor for Bio-Feedback Rehabilitation Training. Sensors (Basel). (2019) 19:686. doi: 10.3390/s19030686
32. Taylor, HL , Jacobs, DR Jr, Schucker, B , Knudsen, J , Leon, AS , and Debacker, G . A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. (1978) 31:741–55. doi: 10.1016/0021-9681(78)90058-9
33. Schwenk, M , Mohler, J , Wendel, C , D''Huyvetter, K , Fain, M , Taylor-Piliae, R, et al. Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: baseline results of the Arizona frailty cohort study. Gerontology. (2015) 61:258–67. doi: 10.1159/000369095
34. Smith, A , Anand, H , Milosavljevic, S , Rentschler, KM , Pocivavsek, A , and Valafar, H . Application of machine learning to sleep stage classification. Proce Int Conf Comput Sci Comput Intell. (2021) 2021:349–54. doi: 10.1109/csci54926.2021.00130
35. Muhammad, LJ , Islam, MM , Usman, SS , and Ayon, SI . Predictive data mining models for novel coronavirus (COVID-19) infected patients' recovery. SN Comput Sci. (2020) 1:206. doi: 10.1007/s42979-020-00216-w
36. Kriegeskorte, N , and Golan, T . Neural network models and deep learning. Curr Biol. (2019) 29:R231–6. doi: 10.1016/j.cub.2019.02.034
37. Nguyen, B , Morell, C , and De Baets, B . Scalable large-margin distance metric learning using stochastic gradient descent. IEEE Trans Cybern. (2020) 50:1072–83. doi: 10.1109/TCYB.2018.2881417
38. Lundberg, SM , Erion, G , Chen, H , DeGrave, A , Prutkin, JM , Nair, B, et al. From local explanations to global understanding with explainable AI for trees. Nat Mach Intell. (2020) 2:56–67. doi: 10.1038/s42256-019-0138-9
39. Park, C , Mishra, R , Sharafkhaneh, A , Bryant, MS , Nguyen, C , Torres, I, et al. Digital biomarker representing frailty phenotypes: the use of machine learning and sensor-based sit-to-stand test. Sensors (Basel). (2021) 21:3258. doi: 10.3390/s21093258
40. Jung, D , Kim, J , Kim, M , Won, CW , and Mun, KR . Frailty assessment using temporal gait characteristics and a long short-term memory network. IEEE J Biomed Health Inform. (2021) 25:3649–58. doi: 10.1109/JBHI.2021.3067931
41. Bortone, I , Sardone, R , Lampignano, L , Castellana, F , Zupo, R , Lozupone, M, et al. How gait influences frailty models and health-related outcomes in clinical-based and population-based studies: a systematic review. J Cachexia Sarcopenia Muscle. (2021) 12:274–97. doi: 10.1002/jcsm.12667
42. Rubin, DS , Ranjeva, SL , Urbanek, JK , Karas, M , Madariaga, MLL , and Huisingh-Scheetz, M . Smartphone-based gait cadence to identify older adults with decreased functional capacity. Digit Biomark. (2022) 6:61–70. doi: 10.1159/000525344
43. Mueller, A , Hoefling, HA , Muaremi, A , Praestgaard, J , Walsh, LC , Bunte, O, et al. Continuous digital monitoring of walking speed in frail elderly patients: noninterventional validation study and longitudinal clinical trial. JMIR Mhealth Uhealth. (2019) 7:e15191. doi: 10.2196/15191
44. Lee, H , Lee, E , and Jang, IY . Frailty and comprehensive geriatric assessment. J Korean Med Sci. (2020) 35:e16. doi: 10.3346/jkms.2020.35.e16
45. Ma, L , Zhang, L , Zhang, Y , Li, Y , Tang, Z , and Chan, P . Cognitive frailty in China: results from China comprehensive geriatric assessment study. Front Med. (2017) 4:174. doi: 10.3389/fmed.2017.00174
46. Sajeev, S , Champion, S , Maeder, A , and Gordon, S . Machine learning models for identifying pre-frailty in community dwelling older adults. BMC Geriatr. (2022) 22:794. doi: 10.1186/s12877-022-03475-9
47. Eskandari, M , Parvaneh, S , Ehsani, H , Fain, M , and Toosizadeh, N . Frailty identification using heart rate dynamics: a deep learning approach. IEEE J Biomed Health Inform. (2022) 26:3409–17. doi: 10.1109/JBHI.2022.3152538
48. Akbari, G , Nikkhoo, M , Wang, L , Chen, CPC , Han, DS , Lin, YH, et al. Frailty level classification of the community elderly using Microsoft Kinect-based skeleton pose: a machine learning approach. Sensors (Basel). (2021) 21:4017. doi: 10.3390/s21124017
49. Abbas, M, Somme, D, and Jeannes, RB, (Eds.) Machine Learning-Based Physical Activity Tracking with a View to Frailty Analysis. 2020 42nd Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) in conjunction with the 43rd Annual Conference of the Canadian Medical and Biological Engineering Society; (2020).
Keywords: digital health technology, wearable sensor, machine learning, prediction model, frailty, gait
Citation: Fan S, Ye J, Xu Q, Peng R, Hu B, Pei Z, Yang Z and Xu F (2023) Digital health technology combining wearable gait sensors and machine learning improve the accuracy in prediction of frailty. Front. Public Health. 11:1169083. doi: 10.3389/fpubh.2023.1169083
Received: 18 February 2023; Accepted: 30 June 2023;
Published: 20 July 2023.
Edited by:
Bijan Najafi, Baylor College of Medicine, United StatesReviewed by:
Juan Luis Sánchez González, University of Salamanca, SpainCopyright © 2023 Fan, Ye, Xu, Peng, Hu, Pei, Yang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhong Pei, cGVpemhvbmdAbWFpbC5zeXN1LmVkdS5jbg==; Zhimin Yang, eWFuZ3lvQHZpcC50b20uY29t; Fuping Xu, eHVmdXBpbmcxOTgzQGd6dWNtLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.