
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 14 February 2025
Sec. Neuroimaging
Volume 16 - 2025 | https://doi.org/10.3389/fpsyt.2025.1458602
 Ying Liu1,2
Ying Liu1,2 Yi-Kuang Hsien1,2
Yi-Kuang Hsien1,2 Wenlong Su1,2
Wenlong Su1,2 Zhiqing Tang1,2
Zhiqing Tang1,2 Hui Li3
Hui Li3 Junzi Long1,2
Junzi Long1,2 Xingxing Liao1,2
Xingxing Liao1,2 Hao Zhang1,2,3*
Hao Zhang1,2,3*Background: Apathy is a prevalent psychiatric condition after stroke, affecting approximately 30% of stroke survivors. It is associated with slower recovery and an increased risk of depression. Understanding the pathophysiological mechanisms of post stroke apathy (PSA) is crucial for developing targeted rehabilitation strategies.
Methods: In this study, we recruited a total of 18 PSA patients, 18 post-stroke non-apathy (NPSA) patients, and 18 healthy controls (HCs). Apathy was measured using the Apathy Evaluation Scale (AES). Resting-state functional magnetic resonance imaging (rs-fMRI) was utilized to investigate spontaneous brain activity. We estimated the amplitude of low-frequency fluctuation (ALFF) across three different frequency bands (typical band: 0.01–0.08 Hz; slow-4: 0.027–0.073 Hz; slow-5: 0.01–0.027 Hz) and the fractional amplitude of low-frequency fluctuation (fALFF).
Results: Band-specific ALFF differences among the three groups were analyzed. Significant differences were found in the typical band within the left lingual gyrus, right fusiform gyrus, right superior temporal gyrus (STG), and left insula. In the slow-4 band, significant differences were observed in the left middle frontal gyrus (MFG) and right STG. In the slow-5 band, significant differences were identified in the left calcarine cortex and right insula. For fALFF values, significant differences were found in the left lingual gyrus and right thalamus. Moreover, positive correlations were observed between AES scores and the ALFF values in the right STG (r = 0.490, p = 0.002) in the typical band, left MFG (r = 0.478, p = 0.003) and right STG (r = 0.451, p = 0.006) in the slow-4 band, and fALFF values of the right thalamus (r = 0.614, p < 0.001).
Conclusion: This study is the first to investigate the neural correlates of PSA using voxel-level analysis and different ALFF banding methods. Our findings indicate that PSA involves cortical and subcortical areas, including the left MFG, right STG, and right thalamus. These results may help elucidate the neural mechanisms underlying PSA and could serve as potential neuroimaging indicators for early diagnosis and intervention.
Apathy is characterized by a measurable decline in goal-directed behaviors across the cognitive, emotional, and social dimensions of an individual’s life (1). It is a common neuropsychiatric symptom after stroke, which affects approximately 30% of stroke survivors (2, 3). Post-stroke apathy (PSA) is associated with functional disabilities, slower recovery, cognitive deficits and an increased risk of subsequent depression (4–6). However, the pathophysiological mechanisms that contribute to apathy in stroke patients are yet to be fully elucidated. Understanding these mechanisms could facilitate the development of more effective, targeted rehabilitation strategies for PSA.
Resting-state functional magnetic resonance(rs-fMRI), which assesses spontaneous fluctuations in blood-oxygen-level-dependent signals, serves as a robust non-invasive method for examining brain functional connectivity (7, 8). During rest, the brain is still active, resulting in slow fluctuations of blood flow that is seen as a marker of spontaneous brain activation (9). The amplitude and frequency of these fluctuations correlate with emotional regulation (10). The amplitude of low-frequency fluctuations (ALFF) quantifies the spontaneous activity amplitude of brain regions during resting state by calculating the average square root of the power spectral density within a specific low-frequency range (0.01-0.08Hz) (11). Further, the typical frequency bands can be divided into the following ranges: slow-5(0.01–0.027Hz), slow-4(0.027–0.073Hz), slow-3(0.073–0.198Hz), and slow-2 (0.198–0.25Hz) (9, 12). Studies indicate that various neural oscillation frequencies within the brain may exhibit sensitivity to activity across different brain regions. The slow-5 and slow-4 bands are primarily related to gray matter, whereas slow-3 and slow-2 bands are associated with white matter and can be affected by aliasing with respiratory and cardiac signals (9). The fractional amplitude of low-frequency fluctuations(fALFF) is a method that calculates the ratio of the power spectrum within the low-frequency range to the power spectrum across the entire frequency range (13). It also measures spontaneous neural activity, and is often preferred because it standardizes the power spectra and is robust against physiological noise (13). These rs-fMRI indices are widely utilized in the study of neurological disorders, such as Parkinson’s disease, Alzheimer’s disease, and stroke (14–16).
Neuroimaging studies have consistently linked the apathetic syndrome to disruptions in specific medial frontal cortex and subcortical structures, particularly the anterior cingulate cortex, medial orbitofrontal cortex, and ventral striatum (17, 18). These areas are integral parts of the brain’s reward system. Emerging physiological evidence indicates that reward processing is altered in apathetic patients, making them less sensitive to rewarding outcomes (19, 20). Previous studies have primarily associated apathy with abnormal activity in various brain regions, including the frontal lobe and the cingulate gyrus. For example, Parkinson’s patients with apathy show reduced ALFF in the right anterior cingulate gyrus in the slow-5 band and decreased fALFF in the right middle frontal gyrus in the typical band (21). Another study indicated decreased ALFF in left orbital middle frontal gyrus and bilateral superior frontal gyrus (22). Only one study has investigated the fALFF, finding significant differences in the left middle temporal regions, right anterior and middle cingulate regions, middle frontal region, and cuneus region in PSA patients (23). However, to date, no studies have explored changes in brain regions across different frequency bands in PSA patients. It is critical for elucidating the pathophysiological mechanisms underlying apathy after stroke.
In this study, we utilize ALFF and fALFF to investigate the changes in spontaneous brain activity during resting state in PSA patients. Based on prior study, we hypothesize that PSA patients will exhibit alterations in ALFF within the frontal lobes and subcortical region. Identifying abnormalities in spontaneous brain activity in PSA patients could elucidate potential therapeutic targets, thereby improving our understanding and management of PSA.
The inclusion criteria for this study were as follows: (1) a confirmed diagnosis of stroke with a disease duration of more than one month; (2) a diagnosis of apathy based on established consensus criteria, with an Apathy Evaluation Scale (AES) score greater than 38 (24, 25); (3) ages between 18 and 75 years; (4) were right-handed; (5) willing to participate to the study, and signed a written informed consent. The exclusion criteria were: (1) presence of severe liver or kidney diseases or a history of tumors; (2) contraindication for MRI; (3) refusal to participate in the study. This study adheres to the Helsinki Declaration and has been approved by the Medical Ethics Committee of the China Rehabilitation Research Center.
Apathy was assessed by the Apathy Evaluation Scale (AES), which includes 18 items evaluating the behavioral, cognitive, and emotional dimensions of apathy (26). The AES has a cutoff score of >38, with scores ranging from 18 to 72; higher scores indicate greater apathy. Depression was assessed by Hamilton Depression Rating Scale (HAMD). HAMD is the most commonly used depression scale because it has a high specificity to assess the severity of depression symptoms. The Cronbach’s α score of HAMD-24 is 0.88, and the κ-score is 0.92 (27). Cognitive function was assessed using the Mini-Mental State Examination (MMSE), a widely utilized tool for evaluating cognitive function globally (28, 29). The total cognition score ranges from 0 to 30 (30).
Neurological functional status was assessed using the National Institutes of Health Stroke Scale (NIHSS) and Barthel index. The NIHSS is a standardized tool used to quantify stroke severity (31). The total NIHSS score ranges from 0 to 42, with higher totals signifying greater stroke impact. The Barthel index was utilized to evaluate activities of daily living. The Barthel index includes ten self-care items including grooming, bathing, feeding, toilet use, dressing, walking, transferring from bed to chair, stair climbing, bowel control, and bladder control (32).
Participants underwent both T1 and rs-fMRI scanning. The data were collected on a Philips Ingenia 3T MRI scanner equipped with a 32-channel head coil. High resolution T1-weighted anatomical images were acquired (TR = 7.13ms, TE =3.22ms; FOV = 256 × 256 mm2; flip angle = 7°; 256 × 256 matrix; 192 slices; voxel size = 1 × 1 × 1 mm3). Rs-fMRI were acquired using a gradient echo planar imaging (EPI) sequence (TR = 2000ms, TE =30ms; FOV = 224 × 224 mm2; flip angle = 90°; 64 × 64 matrix; 32 slices; voxel size = 3.5 × 3.5 × 4.35 mm3). Participants were required to keep still and awoke during the entire scanning.
Data preprocessing was performed using the DPARSF (http://rfmri.org/DPARSF) toolkit based on the MATLAB 2021b (33). Preprocessing includes the following steps: (1) Conversion of DICOM format to NFITI format; (2) Removal of the first ten time points; (3) Time layer and head motion correction; (4) Each participant’s fMRI images were co-registered with their T1 images. Subsequently, the DARTEL method was employed to segment the T1 images into gray matter, white matter, and cerebrospinal fluid, followed by spatial transformation of the segmented gray matter probability maps. The same transformation parameters were then applied to the corresponding fMRI images; (5) Spatial smoothing was performed using a 8-mm full width at half maximum (FWHM) Gaussian kernel to reduce noise and enhance the reliability of the signal; (6) Detrend; (7) regression of head motion, brain white matter signal, cerebrospinal fluid signal, and global signal as covariates. In this study, the rs-fMRI data for subjects with head motion displacement >2.5mm or rotation >2.5° in any axis were discarded. Four participants were consequently excluded from the study due to head movement. A total of 18 PSA patients, 18 post stroke non-apathy (NPSA) patients and 18 health controls (HC) were finally included in the following analysis.
The typical bands (0.01-0.08Hz) (11), slow-5(0.01–0.027Hz) and slow-4(0.027–0.073Hz) were extracted. and the fALFF value was obtained by dividing the sum of ALFF values in this frequency band by the sum of amplitudes in the full frequency band. Normalization was performed by dividing each voxel’s ALFF or fALFF value within the specified frequency band by the brain-wide mean, yielding normalized metrics (mALFF and mfALFF) to quantify relative low-frequency fluctuations for statistical analysis.
The age, gender and years of education were compared using analysis of variance (ANOVA) in the PSA, NPSA, and HC group. The chi-square test was used to analyze the disease types and lesion sides. The duration of disease, as well as the AES, HAMD, MMSE, NIHSS, and Barthel Index scores, between the PSA and NPSA groups were analyzed using either the Mann-Whitney test or the t-test, depending on the distribution and nature of the data. The above data were analyzed by SPSS (version 25.0, Armonk, NY, USA). Statistical significance was set at p<0.05. Sex, age, and head motion were used as covariates in the ANOVA analysis to compare ALFF and fALFF values among the three groups. Post-hoc pairwise t-tests with Bonferroni correction were conducted to evaluate group differences in ALFF and fALFF values among the PSA, NPSA, and HC groups. Spearman correlation analysis was performed to assess the relationship between ALFF, fALFF values, and AES scores within the regions that showed significant differences between the PSA and NPSA groups.
In this study, 54 participants were selected for the present study, including 18 PSA patients, 18 NPSA patients and 18 HCs. A summary of the participants’ demographic and clinical data is presented in Table 1. There were no significant differences in gender (p =0.758), age (p = 0.779), education (p = 0.271), diagnosis (p = 0.735), lesion hemisphere (p =0.423) among three group. PSA group and NPSA group showed no significant difference in MMSE (p =0.158), HAMD (p =0.346), NHISS (p =0.447), BI (p =0.686). Compared with the NPSA group, the PSA patients exhibited significantly higher AES scores (p < 0.001).
In typical band (0.01–0.08 Hz), there are significant differences in left lingual, right fusiform, right superior temporal gyrus (STG), left insula among three group (voxel p<0.001, cluster p<0.05, GRF corrected, cluster size >30 voxels) (Table 2, Figure 1A). Post-hoc analyses showed increased ALFF values in the right fusiform and right STG in PSA group when compared with both NPSA and HC groups (p<0.05) (Figure 2A).
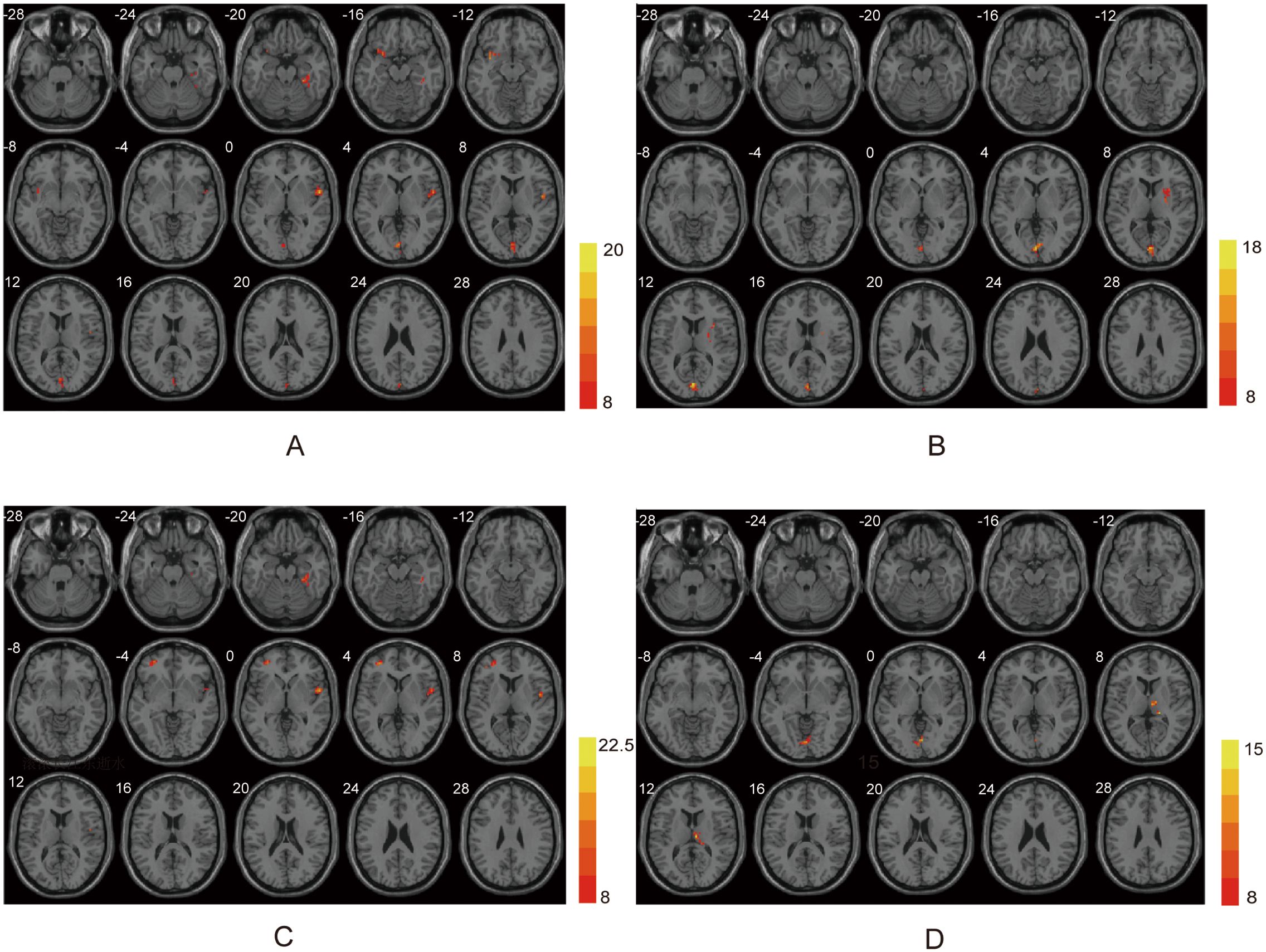
Figure 1. ANOVA differences in different frequency bands. (A) Brain regions showing typical band differences among PSA, NPSA and HC groups. (B) Brain regions showing slow-5 band differences among three groups. (C) Brain regions showing slow-4 band differences among three groups. (D) Brain regions showing fALFF differences among three groups.
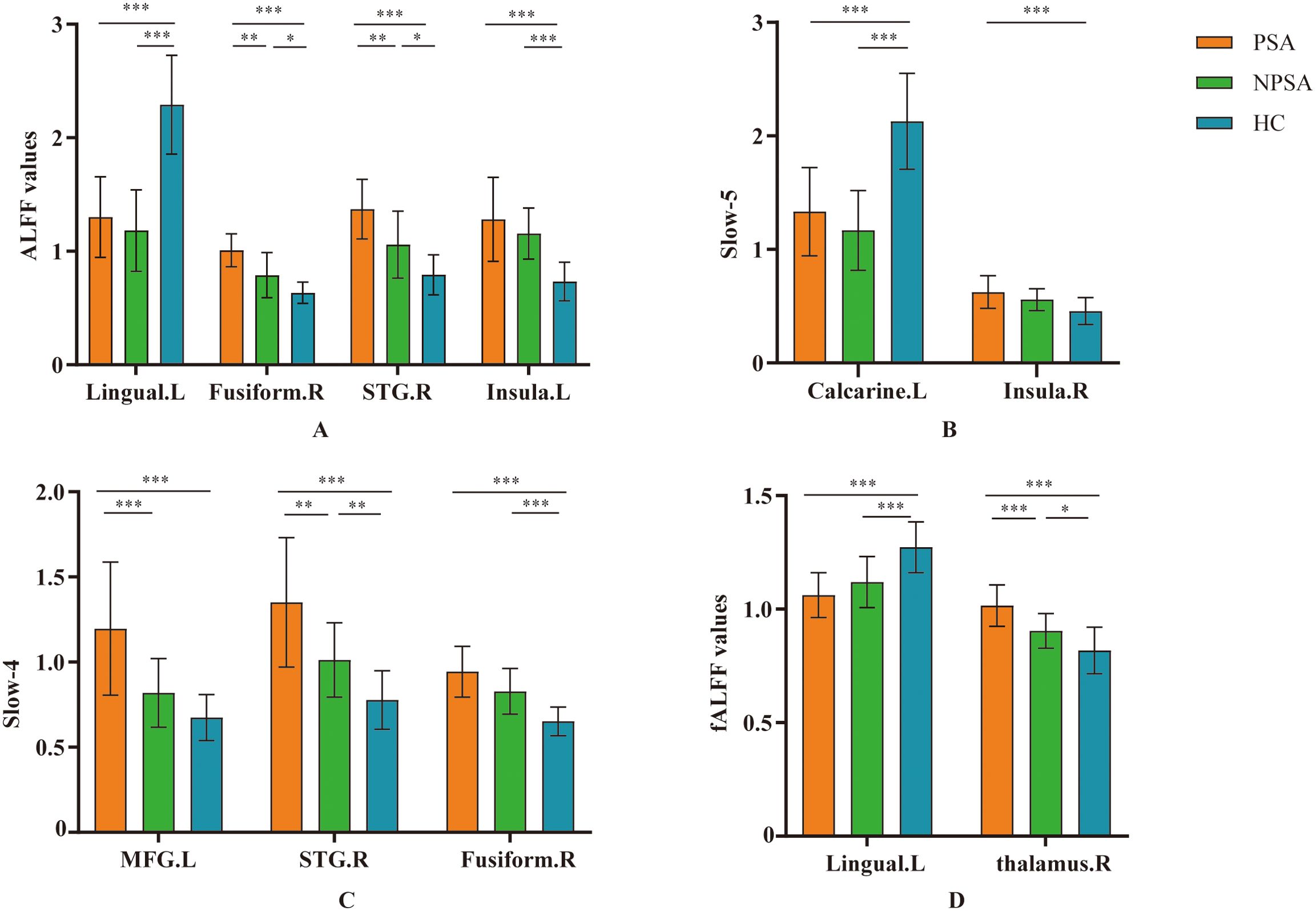
Figure 2. Post-hoc comparisons of ALFF between each pair of three groups. Between-group differences of ALFF in (A) typical band, (B) slow-5 band, (C) slow-4 band, and (D) fALFF. *p < 0.05, **p < 0.01, ***p < 0.001. L, left; R, right. MFG, middle frontal gyrus; STG, superior temporal gyrus.
In slow-5 band (0.01–0.027 Hz), there are significant differences in left calcarine and right insula among three group (voxel p<0.001, cluster p<0.05, GRF corrected, cluster size >30 voxels) (Table 2, Figure 1B). However, there are no significant difference in PSA group when compared with both NPSA and HC groups by post-hoc analyses (p<0.05) (Figure 2B).
In slow-4 band (0.027–0.073Hz), there are significant differences in left middle frontal gyrus (MFG), right STG, right fusiform among three group (voxel p<0.001, cluster p<0.05, GRF corrected, cluster size >30 voxels) (Table 2, Figure 1C). Post-hoc analyses exhibited increased left MFG and right STG in PSA group when compared with both NPSA and HC groups (p<0.05) (Figure 2C).
For fALFF values, there are significant differences in left lingual and right thalamus among three group (voxel p<0.001, cluster p<0.05, GRF corrected, cluster size >30 voxels) (Table 2, Figure 1D). Post-hoc analyses exhibited increased right thalamus in PSA group when compared with both NPSA and HC groups (p<0.05) (Figure 2D).
The ALFF values of right STG (r = 0.490, p = 0.002) in typical band are positively correlated with the AES scores in stroke patients. The ALFF values of left MFG (r = 0.478, p = 0.003) and right STG (r = 0.451, p = 0.006) in slow-4 band are positively correlated with the AES scores in stroke patients. The fALFF values of right thalamus is positively correlated with the AES scores in stroke patients (r = 0.614, p < 0.001) (Figure 3).
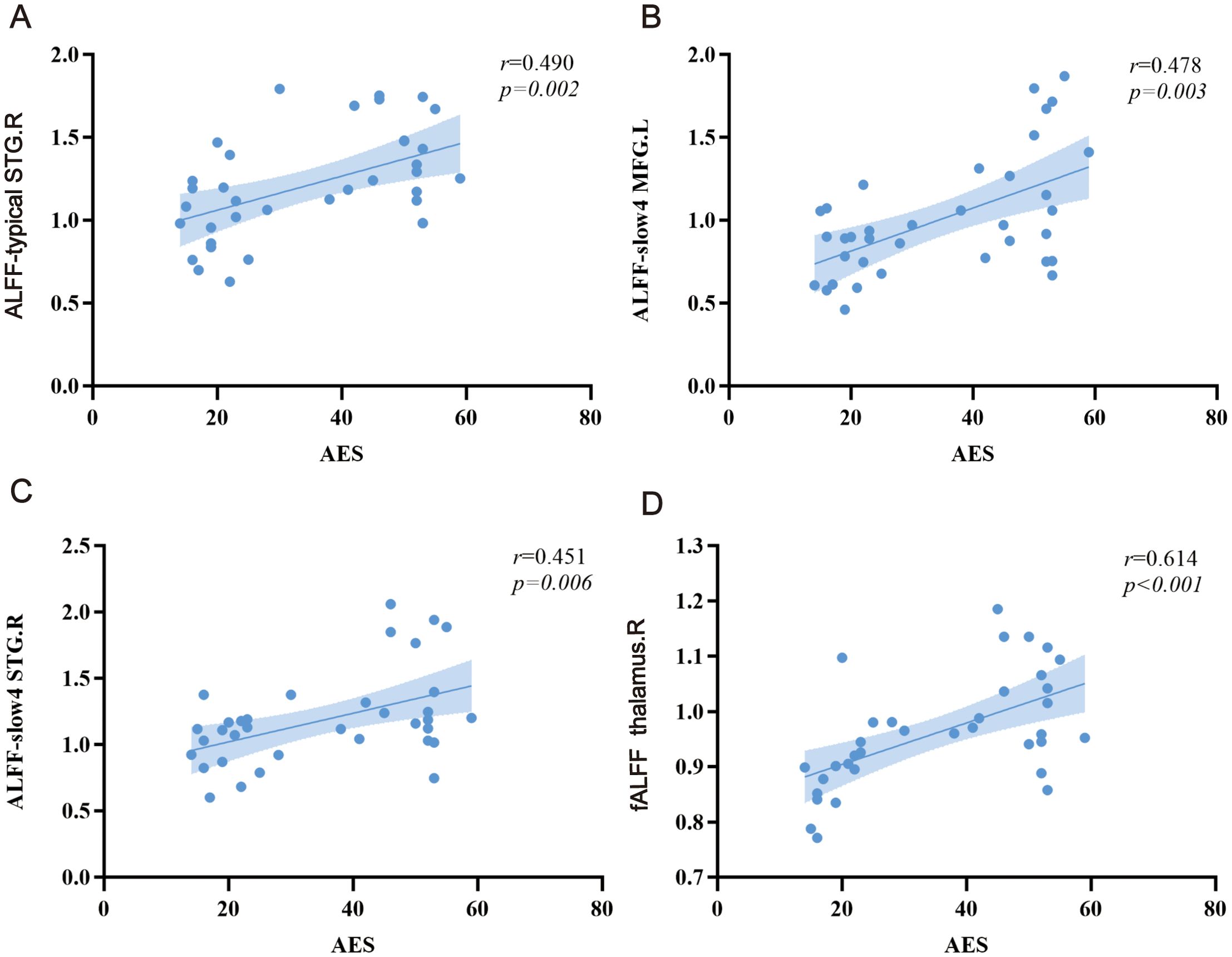
Figure 3. Relationship between ALFF alterations and apathy scores. (A) typical band, (B, C) slow-4, and (D) fALFF. L, left; R, right. MFG, middle frontal gyrus; STG, superior temporal gyrus.
This study utilizes different frequency bands of ALFF to investigate local functional abnormalities in PSA patients. Our findings reveal that the primary brain regions exhibiting abnormalities in PSA patients are concentrated in the frontal and temporal lobes, as well as the thalamus. Moreover, the ALFF values in the left MFG, right STG, and right thalamus are positively correlated with apathy scores.
The MFG, a core component of the dorsolateral prefrontal cortex (DLPFC), plays a critical role in regulating goal-directed behavior, motivation, and emotional responses, all of which are often impaired in apathy (34, 35). Dysfunction in the MFG has been strongly linked to apathy, a condition characterized by a quantitative reduction in voluntary, goal-directed behaviors. This is particularly evident in patients with focal prefrontal cortex lesions, where decreased reactivity to emotional and reward-related stimuli leads to deficits in decision-making. These deficits hinder the ability to accurately evaluate the consequences of actions within emotional and affective contexts, resulting in diminished motivation and impaired goal-directed behavior (36–38). Apathy is further characterized by a significant decrease in the motivation to set and pursue goals, independent of cognitive impairment, emotional distress, or depression (39). Structural imaging studies have consistently shown reduced gray matter volumes in individuals with apathy, particularly in the frontal and temporal lobes (40). In conditions such as cognitive impairment and Alzheimer’s disease, apathy has also been associated with increased white matter lesions and atrophy in frontal gray matter (41–43). Hypoactivity in frontal lobe regions, including the DLPFC, has been identified as a major contributor to apathy following stroke (44). In this study, we observed increased ALFF values in the left MFG, consistent with its critical role in integrating cognitive and motivational processes. This finding aligns with prior research linking DLPFC activity and connectivity with apathy severity. Reduced activity and connectivity within the DLPFC, including the MFG, has been associated with higher levels of apathy, particularly in tasks involving working memory, attentional processing, and motivational regulation. These findings underscore the centrality of the MFG in the neural mechanisms of apathy, highlighting its role in bridging cognitive and emotional processes to sustain goal-directed behavior (45).
The temporal lobe plays a critical role in emotion, memory, and social behavior, with STG serving as a key component of this region (46–48). Structural abnormalities in the temporal lobe, such as reduced inferior-temporal cortical thickness, have been identified as significant risk factors for apathy, particularly in Alzheimer’s disease and mild cognitive impairment (49, 50). Functional studies have further highlighted the STG’s essential role in the neural mechanisms of apathy. For instance, reduced functional connectivity between the right STG and regions such as the right supramarginal gyrus and the left precuneus has been significantly associated with self-reported apathy symptoms in older adults (51). These disrupted connections likely impair the integration of social and emotional information, contributing to decreased motivation and the manifestation of apathy. In stroke patients, functional abnormalities in the STG are further supported by changes in regional cerebral blood flow (rCBF). Okada et al. demonstrated that apathetic stroke patients exhibited significantly reduced rCBF in the right DLPFC and left frontotemporal regions, with apathy severity negatively correlated with rCBF in these areas (44, 51). This indicates that the STG not only plays a localized functional role but also influences motivational states through its broader network connections. Our findings further confirm the importance of the STG in PSA. PSA patients showed significantly increased ALFF values in the right STG across both the typical band and the slow-4 band. This increase in ALFF may reflect compensatory changes in local neural activity to counterbalance network dysfunction caused by disrupted functional connectivity. This result aligns with studies of first-episode, drug-naive major depressive disorder patients, who also exhibited significant increases in ALFF values in the right STG, suggesting that the STG may play a similar role in regulating emotion and motivation across different pathological conditions (52). Additionally, growing evidence suggests that post-stroke apathy arises not from damage to a single brain region but from the disconnection of a complex functional network (53). As a hub for integrating auditory, emotional, and social signals, the STG is critical for maintaining the functional coherence of these networks. Disruptions in STG activity and its connectivity patterns are likely to propagate through the network, leading to widespread impairments in emotional and motivational regulation.
In addition, beyond the changes in ALFF values in cortical regions, spontaneous activity in subcortical regions also changed in PSA patients. Our results showed that right fusiform and right thalamus exhibited increased ALFF values in PSA patient. A study indicated that patients with apathetic depression had lower functional connectivity between the nucleus accumbens and the thalamus (54). Apathy may be a prominent feature of thalamic strokes, particularly those involving the territory of the paramedian arteries, including the dorsomedian and intralaminar thalamic nuclei (55). The thalamus plays a crucial role in regulating emotional and behavioral processes due to its extensive connections, which are critical for emotional and motivational regulation. Research has shown that thalamic damage can trigger symptoms of apathy (55, 56). Our study further confirms the important role of the thalamus in apathy.
Finally, several brain regions, including the right STG, the left MFG, and the right thalamus, were found to be positively correlated with apathy score. These results suggest that PSA is closely related to functional changes in several brain regions, involving multiple aspects of emotion, motivation, and cognition (57). Alterations in right STG, left MFG, and right thalamic function may work together to cause a decrease in patients’ levels of emotion and motivation. In the future, ALFF changes in PSA patients at different stages of recovery could be tracked to understand the relationship between functional changes in brain regions and the evolution of apathy symptoms. Additionally, targeting these apathy-related brain regions with transcranial magnetic stimulation or other neuromodulation techniques could potentially improve symptoms. Combining various imaging techniques, such as electroencephalography, could further elucidate the specific mechanisms through which these brain regions regulate emotion and motivation.
There are a couple of limitations to our findings. First, the relatively small sample size of 54 participants may limit the reliability and statistical power of our findings. This limitation underscores the need for future studies with larger cohorts to validate and extend these results. Second, although our study focused on abnormal functional changes in specific brain regions using ALFF and fALFF at the voxel level, it did not explore the interactions within and between functional brain networks. Future studies could incorporate additional analytical approaches, such as functional connectivity analysis, to provide a more comprehensive understanding of the overall abnormalities in midbrain networks among PSA patients. This may offer deeper insights into the underlying pathological mechanisms of PSA. Third, we did not consider the different symptom dimensions of apathy. Further analysis is necessary to assess the relationship between different dimensions of apathy and brain function.
In conclusion, this is the first study to investigate the neural correlates of PSA based on voxel level using different ALFF banding methods. These findings reveal that PSA patients mainly involve cortical and subcortical areas including left MFG, right STG, and right thalamus. This may help to elucidate the neural mechanisms of PSA and may be a potential neuroimaging indicator for early diagnosis and intervention in PSA.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by China Rehabilitation Research Center (No.2020-050-1). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YL: Conceptualization, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. Y-KH: Conceptualization, Software, Visualization, Writing – original draft. WS: Methodology, Software, Writing – original draft. ZT: Data curation, Validation, Writing – review & editing. HL: Data curation, Validation, Writing – review & editing. JL: Formal analysis, Resources, Writing – review & editing. XL: Investigation, Resources, Writing – original draft. HZ: Project administration, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Robert P, Lanctôt KL, Agüera-Ortiz L, Aalten P, Bremond F, DeFrancesco M, et al. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur psychiatry: J Assoc Eur Psychiatrists. (2018) 54:71–6. doi: 10.1016/j.eurpsy.2018.07.008
2. Jorge RE, Starkstein SE, Robinson RG. Apathy following stroke. Can J Psychiatry Rev Can Psychiatr. (2010) 55:350–4. doi: 10.1177/070674371005500603
3. Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: A systematic review and meta-analysis. Cerebrovascular Dis (Basel Switzerland). (2013) 35:23–39. doi: 10.1159/000346076
4. Santa N, Sugimori H, Kusuda K, Yamashita Y, Ibayashi S, Iida M. Apathy and functional recovery following first-ever stroke. Int J Rehabil Res Internationale Z fur Rehabilitationsforschung Rev internationale recherches readaptation. (2008) 31:321–6. doi: 10.1097/MRR.0b013e3282fc0f0e
5. van Dalen JW, Moll van Charante EP, Nederkoorn PJ, van Gool WA, Richard E. Poststroke apathy. Stroke. (2013) 44:851–60. doi: 10.1161/strokeaha.112.674614
6. Withall A, Brodaty H, Altendorf A, Sachdev PS. A longitudinal study examining the independence of apathy and depression after stroke: the sydney stroke study. Int psychogeriatrics. (2011) 23:264–73. doi: 10.1017/s1041610209991116
7. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. (2007) 8:700–11. doi: 10.1038/nrn2201
8. van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fmri functional connectivity. Eur neuropsychopharmacology: J Eur Coll Neuropsychopharmacol. (2010) 20:519–34. doi: 10.1016/j.euroneuro.2010.03.008
9. Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. The oscillating brain: complex and reliable. Neuroimage. (2010) 49:1432–45. doi: 10.1016/j.neuroimage.2009.09.037
10. Gray JP, Müller VI, Eickhoff SB, Fox PT. Multimodal abnormalities of brain structure and function in major depressive disorder: A meta-analysis of neuroimaging studies. Am J Psychiatry. (2020) 177:422–34. doi: 10.1176/appi.ajp.2019.19050560
11. Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, et al. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional mri. Neuroimage. (2007) 36:144–52. doi: 10.1016/j.neuroimage.2007.01.054
12. Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Sci (New York NY). (2004) 304:1926–9. doi: 10.1126/science.1099745
13. Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, et al. An improved approach to detection of amplitude of low-frequency fluctuation (Alff) for resting-state fmri: fractional alff. J Neurosci Methods. (2008) 172:137–41. doi: 10.1016/j.jneumeth.2008.04.012
14. Guan X, Guo T, Zeng Q, Wang J, Zhou C, Liu C, et al. Oscillation-specific nodal alterations in early to middle stages parkinson's disease. Trans neurodegeneration. (2019) 8:36. doi: 10.1186/s40035-019-0177-5
15. Hu H, Wang L, Abdul S, Tang X, Feng Q, Mu Y, et al. Frequency-dependent alterations in functional connectivity in patients with alzheimer's disease spectrum disorders. Front Aging Neurosci. (2024) 16:1375836. doi: 10.3389/fnagi.2024.1375836
16. Li Q, Hu S, Mo Y, Chen H, Meng C, Zhan L, et al. Regional homogeneity alterations in multifrequency bands in patients with basal ganglia stroke: A resting-state functional magnetic resonance imaging study. Front Aging Neurosci. (2022) 14:938646. doi: 10.3389/fnagi.2022.938646
17. Le Heron C, Apps MAJ, Husain M. The anatomy of apathy: A neurocognitive framework for amotivated behaviour. Neuropsychologia. (2018) 118:54–67. doi: 10.1016/j.neuropsychologia.2017.07.003
18. Kos C, van Tol MJ, Marsman JB, Knegtering H, Aleman A. Neural correlates of apathy in patients with neurodegenerative disorders, acquired brain injury, and psychiatric disorders. Neurosci Biobehav Rev. (2016) 69:381–401. doi: 10.1016/j.neubiorev.2016.08.012
19. Martínez-Horta S, Riba J, de Bobadilla RF, Pagonabarraga J, Pascual-Sedano B, Antonijoan RM, et al. Apathy in parkinson's disease: neurophysiological evidence of impaired incentive processing. J Neurosci. (2014) 34:5918–26. doi: 10.1523/jneurosci.0251-14.2014
20. Muhammed K, Manohar S, Ben Yehuda M, Chong TT, Tofaris G, Lennox G, et al. Reward sensitivity deficits modulated by dopamine are associated with apathy in parkinson's disease. Brain. (2016) 139:2706–21. doi: 10.1093/brain/aww188
21. Xu H, Zhang M, Wang Z, Yang Y, Chang Y, Liu L. Abnormal brain activities in multiple frequency bands in parkinson's disease with apathy. Front Neurosci. (2022) 16:975189. doi: 10.3389/fnins.2022.975189
22. Shen YT, Li JY, Yuan YS, Wang XX, Wang M, Wang JW, et al. Disrupted amplitude of low-frequency fluctuations and causal connectivity in parkinson's disease with apathy. Neurosci Lett. (2018) 683:75–81. doi: 10.1016/j.neulet.2018.06.043
23. Jiang S, Zhang H, Fang Y, Yin D, Dong Y, Chao X, et al. Altered resting-state brain activity and functional connectivity in post-stroke apathy: an fmri study. Brain Sci. (2023) 13. doi: 10.3390/brainsci13050730
24. Robert P, Onyike CU, Leentjens AF, Dujardin K, Aalten P, Starkstein S, et al. Proposed diagnostic criteria for apathy in alzheimer's disease and other neuropsychiatric disorders. Eur psychiatry: J Assoc Eur Psychiatrists. (2009) 24:98–104. doi: 10.1016/j.eurpsy.2008.09.001
25. Mulin E, Leone E, Dujardin K, Delliaux M, Leentjens A, Nobili F, et al. Diagnostic criteria for apathy in clinical practice. Int J geriatric Psychiatry. (2011) 26:158–65. doi: 10.1002/gps.2508
26. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res. (1991) 38:143–62. doi: 10.1016/0165-1781(91)90040-v
27. Li J, Oakley LD, Brown RL, Li Y, Luo Y. Properties of the early symptom measurement of post-stroke depression: concurrent criterion validity and cutoff scores. J Nurs research: JNR. (2020) 28:e107. doi: 10.1097/jnr.0000000000000380
28. Hlávka JP, Yu JC, Lakdawalla DN. Crosswalk between the mini-mental state examination and the telephone interview for cognitive status (Tics-27/30/40). Alzheimer's dementia: J Alzheimer's Assoc. (2022) 18:2036–41. doi: 10.1002/alz.12569
29. Lawton M, Kasten M, May MT, Mollenhauer B, Schaumburg M, Liepelt-Scarfone I, et al. Validation of conversion between mini-mental state examination and montreal cognitive assessment. Mov Disord. (2016) 31:593–6. doi: 10.1002/mds.26498
30. Li J, Cacchione PZ, Hodgson N, Riegel B, Keenan BT, Scharf MT, et al. Afternoon napping and cognition in chinese older adults: findings from the China health and retirement longitudinal study baseline assessment. J Am Geriatrics Soc. (2017) 65:373–80. doi: 10.1111/jgs.14368
31. Goldstein LB, Samsa GP. Reliability of the national institutes of health stroke scale. Extension to non-neurologists in the context of a clinical trial. Stroke. (1997) 28:307–10. doi: 10.1161/01.str.28.2.307
32. Leung SO, Chan CC, Shah S. Development of a chinese version of the modified barthel index– validity and reliability. Clin Rehabil. (2007) 21:912–22. doi: 10.1177/0269215507077286
33. Yan CG, Wang XD, Zuo XN, Zang YF. Dpabi: data processing & Analysis for (Resting-state) brain imaging. Neuroinformatics. (2016) 14:339–51. doi: 10.1007/s12021-016-9299-4
34. Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. (2010) 35:192–216. doi: 10.1038/npp.2009.104
35. Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. (2006) 16:916–28. doi: 10.1093/cercor/bhj043
36. Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient evr. Neurology. (1985) 35:1731–41. doi: 10.1212/wnl.35.12.1731
37. Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. (2000) 10:295–307. doi: 10.1093/cercor/10.3.295
38. José RG, Samuel AS, Isabel MM. Neuropsychology of executive functions in patients with focal lesion in the prefrontal cortex: A systematic review. Brain Cognit. (2020) 146:105633. doi: 10.1016/j.bandc.2020.105633
39. Marin RS. Apathy: A neuropsychiatric syndrome. J neuropsychiatry Clin Neurosci. (1991) 3:243–54. doi: 10.1176/jnp.3.3.243
40. Grool AM, Geerlings MI, Sigurdsson S, Eiriksdottir G, Jonsson PV, Garcia ME, et al. Structural mri correlates of apathy symptoms in older persons without dementia: ages-reykjavik study. Neurology. (2014) 82:1628–35. doi: 10.1212/wnl.0000000000000378
41. Starkstein SE, Mizrahi R, Capizzano AA, Acion L, Brockman S, Power BD. Neuroimaging correlates of apathy and depression in alzheimer's disease. J neuropsychiatry Clin Neurosci. (2009) 21:259–65. doi: 10.1176/jnp.2009.21.3.259
42. Jonsson M, Edman A, Lind K, Rolstad S, Sjögren M, Wallin A. Apathy is a prominent neuropsychiatric feature of radiological white-matter changes in patients with dementia. Int J geriatric Psychiatry. (2010) 25:588–95. doi: 10.1002/gps.2379
43. Apostolova LG, Akopyan GG, Partiali N, Steiner CA, Dutton RA, Hayashi KM, et al. Structural correlates of apathy in alzheimer's disease. Dementia geriatric Cogn Disord. (2007) 24:91–7. doi: 10.1159/000103914
44. Okada K, Kobayashi S, Yamagata S, Takahashi K, Yamaguchi S. Poststroke apathy and regional cerebral blood flow. Stroke. (1997) 28:2437–41. doi: 10.1161/01.str.28.12.2437
45. Fazio L, Logroscino G, Taurisano P, Amico G, Quarto T, Antonucci LA, et al. Prefrontal activity and connectivity with the basal ganglia during performance of complex cognitive tasks is associated with apathy in healthy subjects. PloS One. (2016) 11:e0165301. doi: 10.1371/journal.pone.0165301
46. Olson IR, McCoy D, Klobusicky E, Ross LA. Social cognition and the anterior temporal lobes: A review and theoretical framework. Soc Cognit Affect Neurosci. (2013) 8:123–33. doi: 10.1093/scan/nss119
47. Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: A review of findings on social and emotional processing. Brain. (2007) 130:1718–31. doi: 10.1093/brain/awm052
48. Wong C, Gallate J. The function of the anterior temporal lobe: A review of the empirical evidence. Brain Res. (2012) 1449:94–116. doi: 10.1016/j.brainres.2012.02.017
49. Azocar I, Rapaport P, Burton A, Meisel G, Orgeta V. Risk factors for apathy in alzheimer's disease: A systematic review of longitudinal evidence. Ageing Res Rev. (2022) 79:101672. doi: 10.1016/j.arr.2022.101672
50. Guercio BJ, Donovan NJ, Ward A, Schultz A, Lorius N, Amariglio RE, et al. Apathy is associated with lower inferior temporal cortical thickness in mild cognitive impairment and normal elderly individuals. J neuropsychiatry Clin Neurosci. (2015) 27:e22–7. doi: 10.1176/appi.neuropsych.13060141
51. Jang JY, Han SD, Yew B, Blanken AE, Dutt S, Li Y, et al. Resting-state functional connectivity signatures of apathy in community-living older adults. Front Aging Neurosci. (2021) 13:691710. doi: 10.3389/fnagi.2021.691710
52. Yang C, Zhang A, Jia A, Ma JX, Sun N, Wang Y, et al. Identify abnormalities in resting-state brain function between first-episode, drug-naive major depressive disorder and remitted individuals: A 3-year retrospective study. Neuroreport. (2018) 29:907–16. doi: 10.1097/wnr.0000000000001054
53. Yang S, Hua P, Shang X, Cui Z, Zhong S, Gong G, et al. Deficiency of brain structural sub-network underlying post-ischaemic stroke apathy. Eur J Neurol. (2015) 22:341–7. doi: 10.1111/ene.12575
54. Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup JK, Lim KO, et al. Functional connectivity in apathy of late-life depression: A preliminary study. J Affect Disord. (2013) 149:398–405. doi: 10.1016/j.jad.2012.11.023
55. Carrera E, Bogousslavsky J. The thalamus and behavior: effects of anatomically distinct strokes. Neurology. (2006) 66:1817–23. doi: 10.1212/01.wnl.0000219679.95223.4c
56. Ioannidis AE, Kimiskidis VK, Loukopoulou E, Geroukis T, Vlaikidis N, Kosmidis MH. Apathy, cognitive dysfunction and impaired social cognition in a patient with bilateral thalamic infarction. Neurocase. (2013) 19:513–20. doi: 10.1080/13554794.2012.701645
Keywords: post stroke apathy, resting state fMRI, amplitude of low-frequency fluctuation, fractional amplitude of low-frequency fluctuation, superior temporal gyrus, middle frontal gyrus
Citation: Liu Y, Hsien Y-K, Su W, Tang Z, Li H, Long J, Liao X and Zhang H (2025) Frequency-dependent changes in the amplitude of low-frequency fluctuations in post stroke apathy: a resting-state fMRI study. Front. Psychiatry 16:1458602. doi: 10.3389/fpsyt.2025.1458602
Received: 13 August 2024; Accepted: 28 January 2025;
Published: 14 February 2025.
Edited by:
Yuka Kotozaki, Iwate Medical University, JapanReviewed by:
Kristina Meyer, Charité University Medicine Berlin, GermanyCopyright © 2025 Liu, Hsien, Su, Tang, Li, Long, Liao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Zhang, Y3JyY3poMjAyMEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.