- 1Department of Neurosurgery, Affiliated Cixi Hospital, Wenzhou Medical University, Ningbo, China
- 2School of Pharmaceutical Science, Wenzhou Medical University, Wenzhou, China
- 3Beijing Hui-Long-Guan Hospital, Peking University, Beijing, China
- 4School of Mental Health, Wenzhou Medical University, Wenzhou, China
- 5Cixi Biomedical Research Institute, Wenzhou Medical University, Ningbo, China
- 6Psychosomatic Medicine Research Division, Inner Mongolia Medical University, Hohhot, China
- 7Zhejiang Provincial Clinical Research Center for Mental Disorders, the Affiliated Wenzhou Kangning Hospital, Wenzhou Medical University, Wenzhou, China
Introduction: Patients with alcohol use disorder (AUD) often experience repeated withdrawal. Impulsivity is the most relevant factor influencing successful withdrawal. Brain-derived neurotrophic factor (BNDF) and fibroblast growth factor 21 (FGF21) are associated with impulsivity. Previous studies on the differential effects of BDNF or FGF21 on impulsivity have focused on single-gene effects and have inconsistent results. We aim to investigate the effects of BDNF rs6265 and FGF21 rs11665896, individually and together, on impulsivity during alcohol withdrawal in patients with AUD.
Methods: We recruited 482 adult Han Chinese males with AUD and assessed their impulsivity using the Barratt Impulsivity Scale. Genomic DNA was extracted and genotyped from peripheral blood samples. Statistical analysis was conducted on the data.
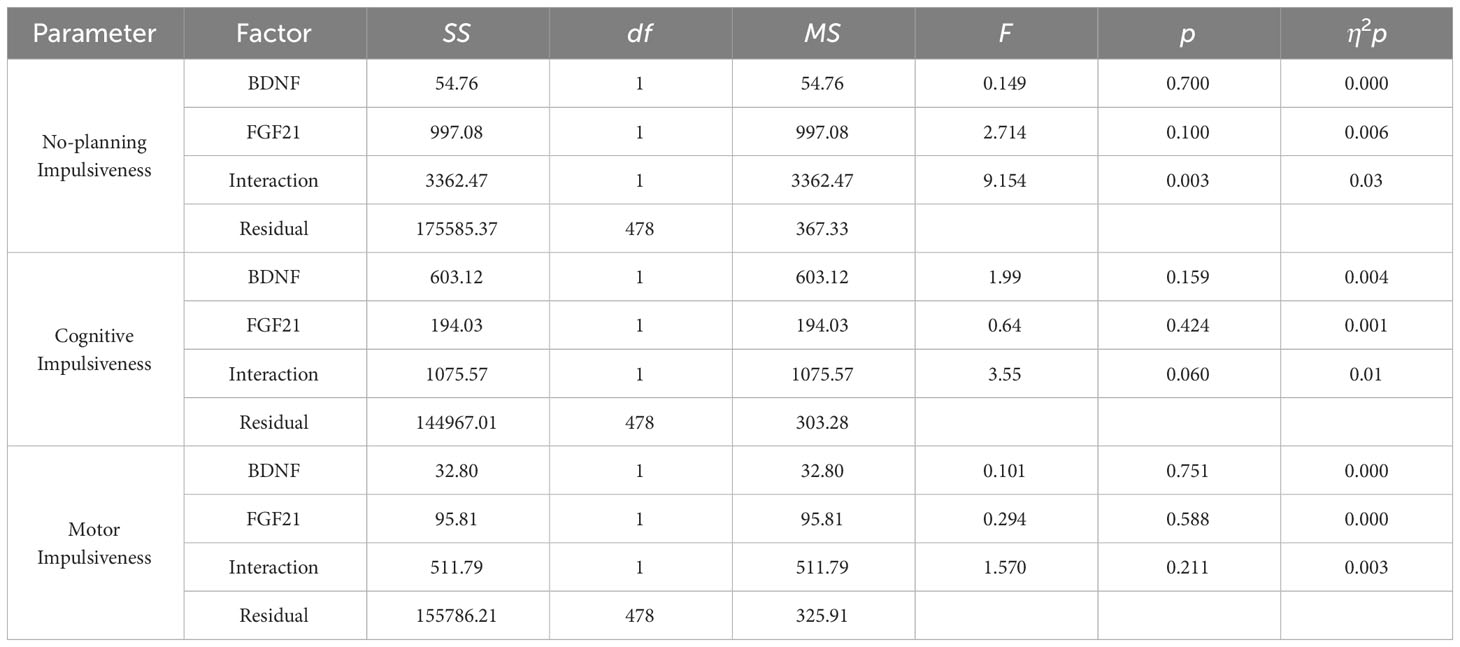
Results: The T-test and 2 × 2 analysis of variance were used to investigate the effects of the genes on impulsivity. There was a significant BDNF × FGF21 interaction on no-planning impulsiveness (F = 9.15, p = 0.003, η2p = 0.03). Simple main effects analyses and planned comparisons showed that BDNF rs6265 A allele × FGF21 rs11665896 T allele was associated with higher no-planning impulsiveness. Finally, hierarchical regression analyses revealed that only the interaction of BDNF and FGF21 accounted for a significant portion of the variance in no-planning impulsiveness.
Conclusion and significance: The combination of BDNF rs6265 A allele and FGF21 rs11665896 T allele may increase impulsivity and discourage alcohol withdrawal. Our study provides a possible genetic explanation for the effects of associated impulsivity in patients with AUD from the perspective of gene-gene interactions.
1 Introduction
Alcohol is the most widely used addictive substance in the world, and China’s alcohol market has become one of the world’s largest (1). People with harmful drinking patterns often suffer from alcohol use disorder (AUD) that impairs health and functioning (2). AUD is a global health problem ranking seventh among the leading causes of death (3). When long-term drinkers suddenly stop drinking, AUD can cause alcohol withdrawal syndrome, which is an understudied subtype of AUD (4).
Alcohol withdrawal syndrome can lead to impulsivity, which is a constituent of human behavior in which individuals assume appropriate risks and pursue novel opportunities (5, 6). Impulsivity is associated with many at-risk behaviors, including suicidality, substance abuse, and criminal actions (7, 8). It is essential to identify subjects with high impulsivity tendencies, which can help alcohol-dependent patients successfully quit drinking quickly and reduce the risk of harmful behaviors. Not all individuals experience impulsivity in the context of alcohol withdrawal (9). Studies showed that impulsive behavior is associated with genetics linking individual differences to specific allelic variants arising from single-nucleotide polymorphisms (10, 11). Several genes are associated with impulsivity and aggression in alcohol-dependent individuals, including brain-derived neurotrophic factor (BDNF) (12), FGF21 (13), tryptophan hydroxylase type 2 (14), 5-HT receptor 2A (15), and catechol-O-methyl transferase (16).
BDNF is the most prevalent growth factor in the central nervous system. BDNF participates in developing the central nervous system and neuronal plasticity (17). The most common BDNF single-nucleotide polymorphism (SNP) in humans is at codon 66 (rs 6265), which can lead to a val-to-met (V66M) protein variant (18). Studies showed that BDNF is associated with impulsivity, and can be a biological marker for impulsive behavior (19). Findings regarding the BDNF rs6265 Met allele and impulsivity have been inconsistent. Bergman and Su et al. found that the BDNF rs6265 Met allele was associated with higher impulsivity in children with attention-deficit hyperactivity disorder and methamphetamine abusers (20, 21). In contrast, Boscutti et al. found that the BDNF rs6265 Met/Met genotype was associated with reduced impulsivity levels (22).
Fibroblast growth factor 21 (FGF21) is a member of the FGF19 superfamily (23). It mediates its biological effects by binding to a co-receptor, β-klotho (24). FGF21 can cross the blood-brain barrier (25) and is significantly associated with alcohol craving (26). FGF21 can modulate the functions of hypothalamic-pituitary-adrenal axis, which is associated with suicide and impulsive aggression (27, 28). A study found that a decrease in FGF21 level associated with serotonin and dopamine in the cerebrospinal fluid leads to higher impulsivity (29); the transcription level of FGF21 was influenced by miRNA. FGF21 rs11665896 is located at the 3′UTR region, where target sites for miRNAs are located, and the change of G for T resulting from this SNP could affect miRNA binding, reducing FGF21 transcription (30).
Studies found that BDNF and FGF21 are associated with alcohol dependence and impulsivity (31, 32). In addition, the link between BDNF SNP and AUD has been reported in some critical literature. An epidemiological study of 377 Japanese male alcoholics reported that, for the G196A genotype, people with the A allele develop alcohol abuse earlier than those who do not (33). Regarding the effect of Val66Met polymorphism of the BDNF gene on AUD, epidemiological studies have shown that BDNF gene Val66Met polymorphism increases vulnerability to alcohol dependence (34). However, another animal study suggests that BDNF Val66Met polymorphism dose not play a significant role in the genetic predisposition to alcohol dependence or violent tendencies (35). The association of BDNF and FGF21 genes’ variations and interactions with AUD-related impulsivity needs further investigation. Therefore, this study investigated the effects of two-gene variants, BDNF rs6265 and FGF21 rs11665896, individually and in combination, on impulsivity in AUD patients during alcohol withdrawal.
2 Materials and methods
2.1 Participants
We recruited 482 participants from several hospitals in northern China. All participants are Han Chinese men hospitalized for alcohol use disorders. The inclusion criteria were diagnosis of alcohol dependence by at least two trained psychiatrists according to the DSM-IV and sufficient literacy skills. The exclusion criteria were 1) a history of substance abuse or dependence other than nicotine, 2) participants or their first-degree relatives with a history of severe psychiatric or neurological disorders, and 3) cardiovascular, liver, or kidney disease.
All AUD patients voluntarily or passively came to the hospital on the day of drinking or the day after their last drinking session due to mental and behavioral disorders caused by alcohol consumption. Then, they experienced three weeks of abstinence in the hospital. After that, the participants were asked to complete questionnaires and provide a blood sample for DNA extraction. All patients provided written informed consent and were told the blood sample would be subjected to a gene assay. The institutional review board of the Inner Mongolian Medical University approved the study. All procedures performed in this study involving human participants followed the Helsinki Declaration.
2.2 Impulsivity
Impulsivity was assessed using the Barratt Impulsiveness Scale (BIS-11) Chinese Version. The BIS contains 30 items rated on a five-point Likert scale ranging from “no” to “always.” The BIS evaluates impulsivity in three domains: no-planning, motor, and cognitive impulsiveness (36). A higher score on the BIS indicates a higher level of impulsivity. The BIS has high internal consistency with a Cronbach’s α of 0.80 (37).
2.3 Genotyping
Genomic DNA was extracted from each participant 5 mL of peripheral blood using standard techniques. The BDNF rs6265 and FGF21 rs11665896 SNPs were genotyped using 5’ nuclease fluorescent TaqManTM primers (Applied Biosystems, Foster City, CA). Reactions were performed according to the manufacturer’s protocol. All laboratory procedures were carried out in a manner blind to case-control status. The conditions of polymerase chain reaction were as follows: 50°C for 2 min, 95°C for 10 min, followed by 50 cycles of 95°C for 15 s and 60°C for 1 min. BDNF rs6265 was genotyped with primers: (forward) 5’ GGACTCTGGAGAGGTGAAT-3’ and (reverse) 5’ CTCATCAGCTCTTCTATC -3’. FGF21 rs11665896 was genotyped with primers: (forward) 5’ TGTGTGGTGTCTGAGGGAAG-3’ and (reverse) 5’ GAAGTCAAGAGATGGAGAGCA-3’. Ten percent of the DNA samples were duplicated randomly and tested, and no-fault genotyping was found.
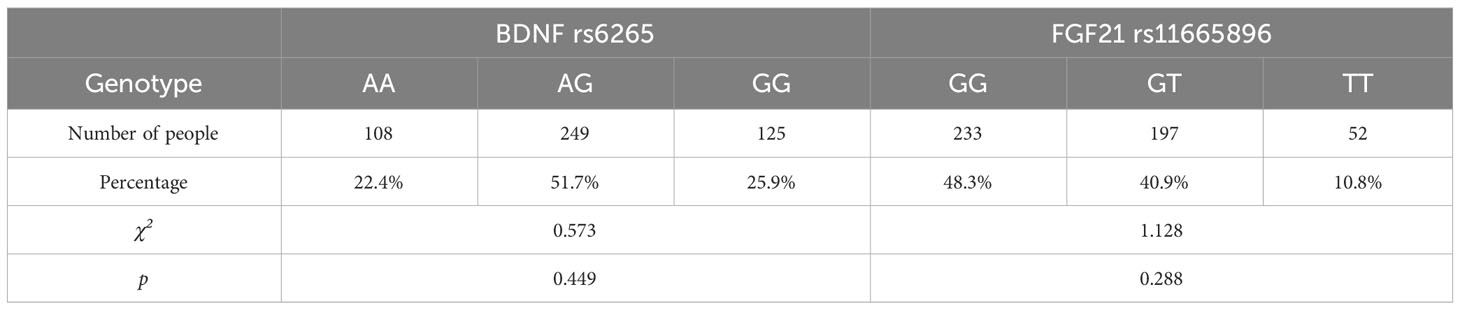
For the BDNF gene, there were 125 GG, 108 AA and 249 AG carriers. We grouped AA with AG to form an “A allele” (n = 355), with the remaining carriers categorized as “GG homozygote” (n = 125) genotype. For the FGF21 gene, there were 233 GG, 52 TT and 197 GT carriers. We grouped TT with GT to form a “T allele” (n = 249) with the remaining carriers categorized as “GG homozygote” (n = 233) genotype.
2.4 Statistical analyses
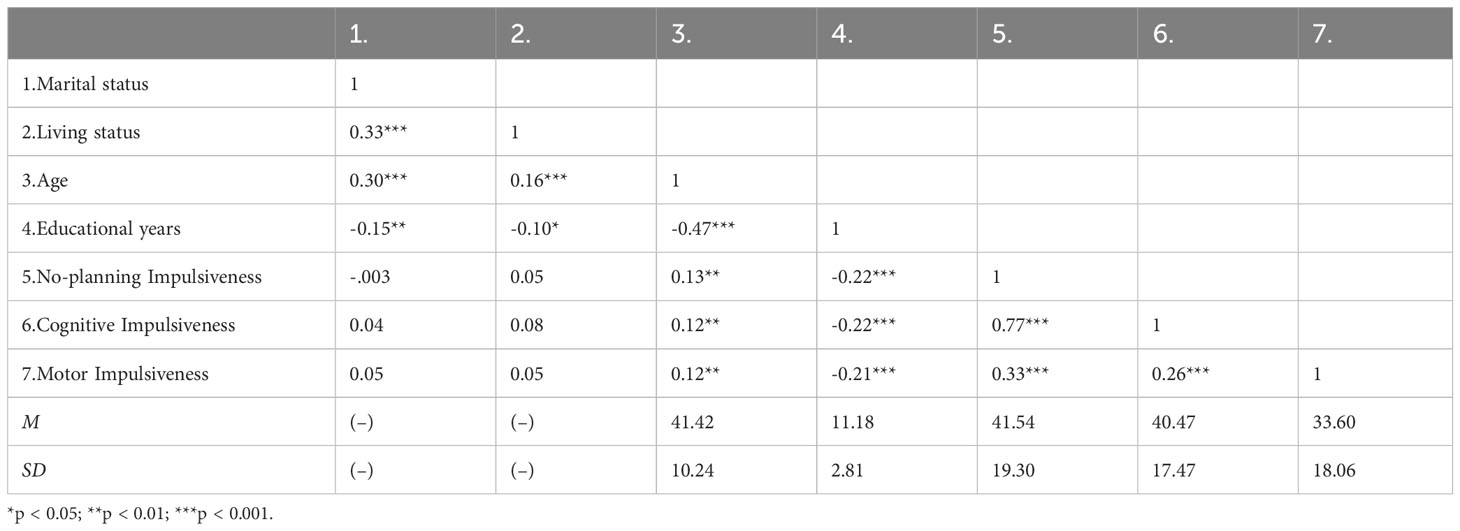
Pearson correlations were examined between marital status, living status, age, educational years, no-planning, cognitive, and motor impulsiveness. Then, the Hardy-Weinberg equilibrium for genotype distributions of BDNF rs6265 and FGF21 rs11665896 was tested using the c2 test for goodness of fit. To investigate the effects of genes on impulsivity, we conducted independent t-tests for each genotype and a 2 × 2 analysis of variance to examine BDNF rs6265 × FGF21 rs11665896 interaction on individual dimensions of impulsivity. Significant interactions were explored using simple main effects analyses and planned comparisons. Finally, we conducted hierarchical regression analyses to determine the specific and interactional effects of BDNF rs6265 × FGF21 rs11665896 on no-planning impulsiveness.
3 Results
3.1 Descriptive statistics
We included 482 participants. The mean age was 41.42 ± 10.24 years. The average number of education years was 11.18 ± 2.81 years. Most participants were married (71.6%) and living with family (78.4%). The impulsivity scores were no-planning impulsiveness (41.54 ± 19.30), cognitive impulsiveness (40.47 ± 17.47), and motor impulsiveness (33.60 ± 18.06). Correlation analyses revealed that general demographic, age and educational years were significantly correlated with impulsivity (Table 1). Age was significantly positively associated with impulsivity (|r|s ≥ 0.12, ps < 0.01), and educational years were significantly negatively associated with impulsivity (|r|s ≥ 0.21, ps < 0.001). Marital status and living status showed no significant correlations with impulsivity.
3.2 Effect of BDNF rs6265 and FGF21 rs11665896 genotypes on impulsivity
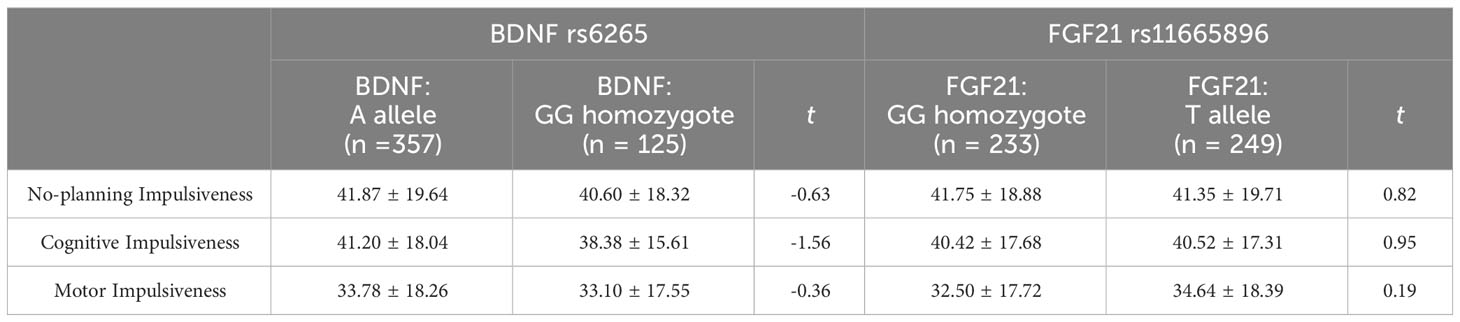
Before performing a single-gene effect test, Hardy-Weinberg equilibrium was established for each gene (Table 2). BDNF rs6265 and FGF21 rs11665896 were in Hardy-Weinberg equilibrium (χ2 = 0.573, p = 0.449; χ2 = 1.128, p = 0.288). The results of single-gene effect showed that there were no significant differences of impulsivity between BDNF rs6265 A allele carriers and GG homozygote carriers (ts < 1.56, p > 0.10), and there were no significant differences of impulsivity between FGF21 rs11665896 T allele carriers and GG homozygote carriers (ts < 0.95, p > 0.19) (Table 3). There were no significant single-gene effects of BDNF rs6265 or FGF21 rs11665896 on AUD-related impulsivity.
3.3 Effect of the interaction of BDNF rs6265 and FGF21 rs11665896 genotypes on impulsivity
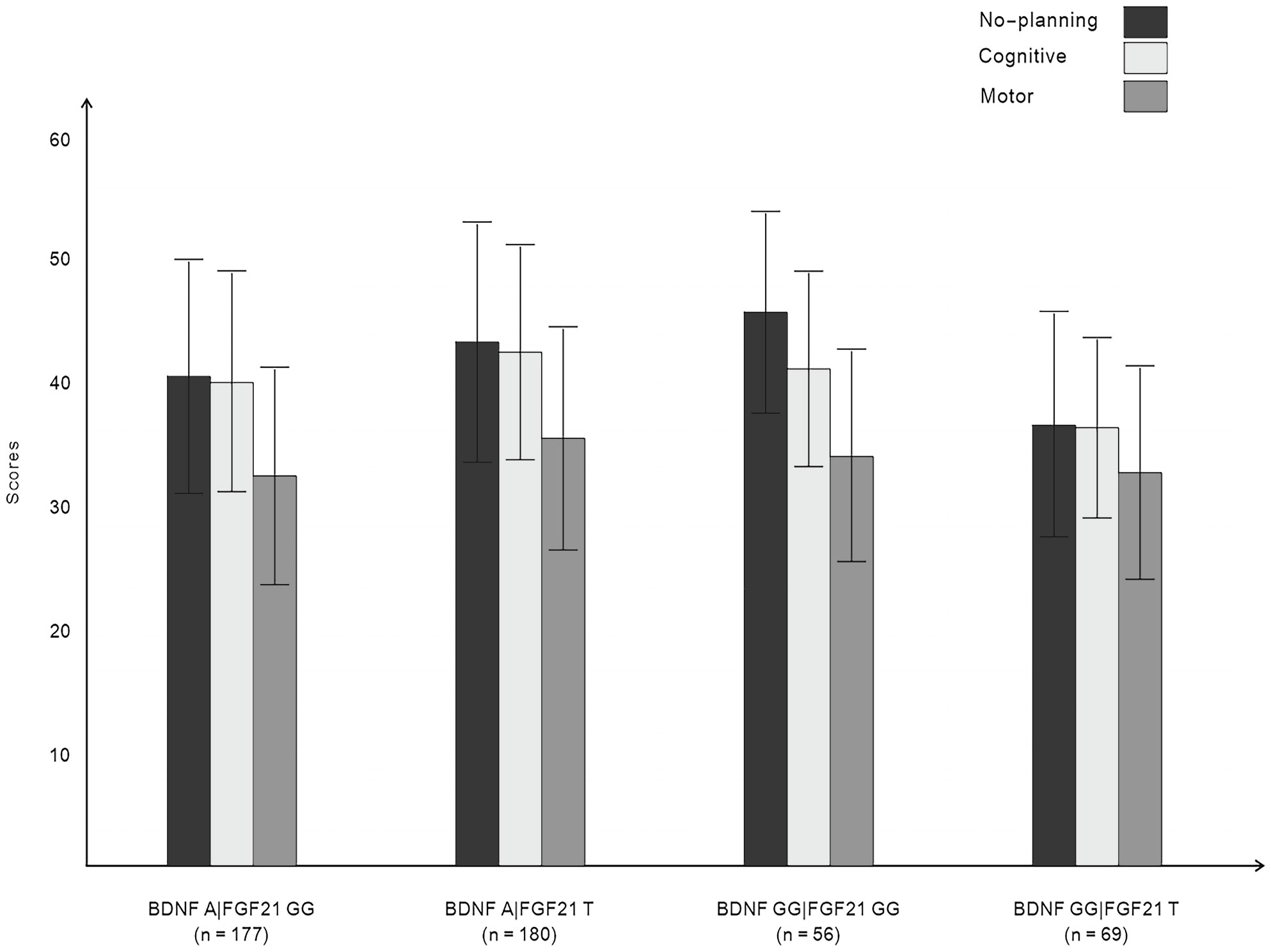
Figure 1 displays the impulsivity scores for the four allelic groups. BDNF rs6265 A allele and FGF21 rs11665896 T allele carriers showed higher levels of impulsivity. BDNF rs6265 GG homozygote and FGF21 rs11665896 T allele carriers showed lower levels of impulsivity. We subjected these impulsivity scores to analysis of variance with two between-subjects factors of BDNF rs6265 (A allele/GG homozygote) and FGF21 rs11665896 (T allele/GG homozygote) (Table 4). There was a significant BDNF × FGF21 interaction on no-planning impulsiveness (F = 9.15, p = 0.003, η2p = 0.03). However, this interaction effect was absent on cognitive impulsiveness (F = 3.55, p = 0.060, η2p = 0.01) and motor impulsiveness (F = 1.57, p = 0.21, η2p = 0.003).
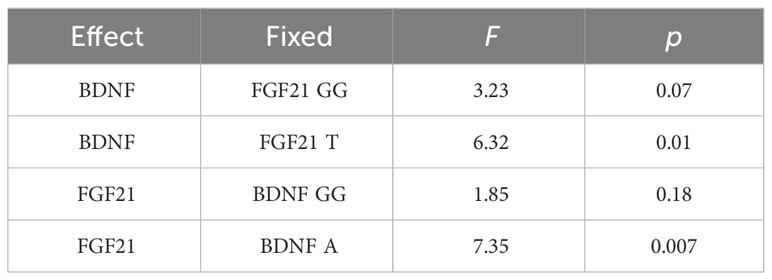
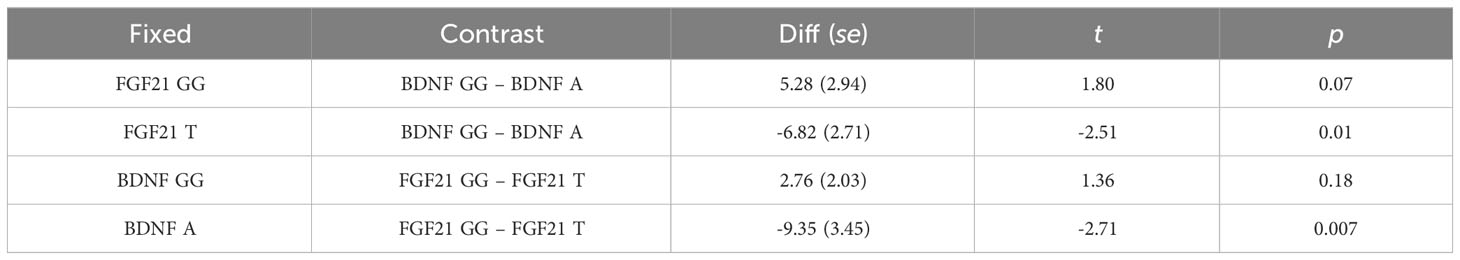
Simple main effects analysis (Table 5) and planned comparisons (Table 6) were conducted to explore this significant interaction. There was a significant BDNF effect in FGF21 T allele carriers (F = 6.32, p = 0.01) but not in FGF21 GG homozygote carriers (F = 3.32, p = 0.07). There was a significant FGF21 effect in BDNF A allele carriers (F = 7.35, p = 0.007) but not in BDNF GG homozygote carriers (F = 1.85, p = 0.18). The planned comparisons showed higher no-planning impulsiveness for the BDNF rs6265 A allele than for BDNF rs6265 GG homozygote carriers (t = -2.51, p = 0.01) in the FGF21 T allele group and higher no-planning impulsiveness for FGF21 rs11665896 T allele relative to FGF21 rs11665896 GG homozygote carriers (t = -2.71, p = 0.007) in the BDNF A allele group. This significant difference was also absent in the FGF21 GG homozygote group (t = 1.80, p = 0.07) and the BDNF GG homozygote group (t = 1.36, p = 0.18).
Finally, multivariate regression analysis was performed for the primary and interaction effects of BDNF and FGF21 on impulsiveness. BDNF rs6265 and FGF21 rs11665896 were entered as predictors of impulsiveness. Only the interaction of BDNF and FGF21 accounted for a significant portion of the variance in no-planning impulsiveness (β = -0.14, t = 2.195, p = 0.029). This significant interaction was absent for cognitive impulsiveness and motor impulsiveness.
4 Discussion
We investigated the effects of BDNF rs6265 and FGF21 rs11665896 on impulsivity with alcohol dependence during withdrawal. Previous studies showed that BDNF played a significant role in the processes of alcohol withdrawal syndrome (31), and FGF21 is related to AUD (32). The present study found no significant single-gene effect of BDNF rs6265 or FGF21 rs11665896 on AUD-related impulsivity. However, in the interaction analysis with two genes, we found that the interaction of BDNF rs6265 × FGF21 rs11665896 had a significant effect on no-planning impulsiveness in participants with AUD. When the BDNF rs6265 A allele was combined with the FGF21 rs11665896 T allele, no-planning impulsiveness was higher in patients with AUD.
BDNF rs6265 A allele and FGF21 rs11665896 T allele caused the production of mature protein (BNNF and FGF21) to decrease (30, 38). In a sample of individuals who had attempted suicide (39) and in attention-deficit hyperactivity disorder patients (40), low blood BDNF concentrations were associated with high levels of impulsivity. Low FGF21 levels in the cerebrospinal fluid led to impulsivity (29). Studies have shown that the higher the impulsivity, the higher the risk of being drink. Impulsivity can motivate alcohol consumption in a variety of ways. Stamates et al. found that individuals with high levels of impulsivity appear to have more significant negative internal and external motives for drinking (41). Moreover, high trait impulsivity, as measured with the BIS-11, may result in/from heavy drinking patterns in young social drinkers (42). In addition, impulsivity is related to a higher risk of relapse in alcohol use disorders (43, 44). FGF21 and BDNF have many similar biological effects. They have neuroprotective effects such as improving cognitive function and neurodegeneration (45–48). BDNF and FGF21 levels change after drinking alcohol (49, 50), suggesting there may be an interaction between BDNF and FGF21. Studies found that muscle-derived mediators such as FGF21 and cathepsin-B (CTSB) can pass through the blood-brain barrier and enhance neuroprotective markers including irisin and BDNF (51). Kang et al. found that FGF21 activated the AMPKα/SIRT1 signaling pathway, directly or indirectly inhibiting NF-κB expression and enhancing BDNF expression (45). Mice with the livers of BDNF mutants fed a high-fat diet contained abnormal levels of peroxisome proliferator-activated receptor and FGF21 transcripts (52).
In the present study, the BDNF rs6265 A allele or FGF21 rs11665896 T allele corresponded to lower BDNF and FGF21 protein levels. Decreases in FGF21 or BDNF may lead to a decrease in the other protein. The combination of the BDNF rs6265 A allele and the FGF21 rs11665896 T allele may correspond to the lowest BDNF and FGF21 levels in vivo.
Long-term heavy drinking can lead to inflammation and apoptosis (53, 54). Pro-BDNF and BDNF may produce opposite biological functions by signaling through p75NTR and TrkB, respectively (55). Anastasia et al. found that Met66proBDNF binds more effectively to the p75NTR/sortilin complex receptor than Val66proBDNF (56). Pro-BDNF-p75NTR activates the JNK pathway to upregulate p53 expression and initiate apoptosis (57). BDNF depletion is associated with neuroinflammation and neuronal apoptosis in Alzheimer’s disease (58). FGF21 relieved numerous inflammation-related metabolic disorders, including metabolic syndrome and cardiovascular diseases (59). Lu et al. found that FGF21 reduced senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the SIRT1-mTOR signaling pathway (60). These findings suggest that the effects of BDNF and FGF21 on impulsivity may be mediated by inflammation and apoptosis.
The mechanisms underlying higher impulsivity may also be associated with neurotransmitter changes, including serotonin and dopamine (61, 62). BDNF regulates mood, cognition, and response to stress and interacts with serotonergic, glutamatergic, cholinergic and dopaminergic neurotransmission (63, 64). Xu et al. found that the effect of CSF FGF21 on impulsivity may be related to the regulating effects of FGF21 on serotonin and dopamine in CSF (29). These findings suggest that BDNF and FGF21 may affect impulsivity by regulating neurotransmitters such as serotonin and dopamine.
The present study has some limitations. First, the measure of impulsivity used self-report scales, and data were collected using self-rating scales; therefore, reporting bias is unavoidable. Second, our study was cross-sectional and should be extended to longitudinal studies. Third, this study only included male patients. Nevertheless, some studies have found sex differences in drinking behavior, neural systems, and AUD treatment (65, 66). Schulte et al. found that while girls and boys may be facing similar vulnerabilities to problems with alcohol, boys begin to carry more risk as they move toward young adulthood (67). Agabio et al. found that women were older than men at the age of first drink, regular drinking, and onset of AUD, and progressed faster than men from regular use to AUD onset (68). More importantly, the sex difference occurs in BDNF SNP reported by alcohol-related studies. Female hBDNFVal/Val mice exhibited a greater propensity toward stable ethanol self-administration than male mice of the same genotype in the operant paradigm (6). Male Met68BDNF mice exhibited a preference for alcohol over social interaction and were insensitive to the acute anxiolytic action of alcohol, which was driven by malfunction of BDNF in the ventral hippocampus of male mice (69). Therefore, further research in female patients with AUD is warranted in the future. Fourth, our study examined only one genotype of BDNF or FGF21 and should be investigated using gene set analysis. Finally, specific mechanisms should be explored using relevant molecular biomolecular methods. The strengths of this study include our more profound understanding of the impact of AUD-related impulsivity at the two-gene level.
5 Conclusion
In summary, our findings suggest that combinations of genotypes of BDNF and FGF21 have different effects on impulsivity during withdrawal in alcohol-dependent patients. The BDNF rs6265 A allele × FGF21 rs11665896 T allele is associated with higher no-planning impulsiveness. The combination of BDNF rs6265 A allele and FGF21 rs11665896 T allele maybe a risk to increase impulsivity, which is not conducive to quitting in alcohol-dependent patients. There is a lack of research on AUD based on genetic variations. Further study on genetic targets may improve understanding of AUD and provide treatment methods for AUD patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.
Ethics statement
The studies involving humans were approved by the institutional review board of the Inner Mongolian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SY: Data curation, Investigation, Validation, Writing – original draft. FW: Formal analysis, Resources, Writing – original draft. LS: Formal analysis, Software, Writing – original draft. XQL: Investigation, Writing – original draft. SL: Investigation, Writing – original draft. YC: Investigation, Writing – original draft. LLC: Investigation, Writing – original draft. ZP: Investigation, Writing – original draft. YK: Methodology, Project administration, Supervision, Writing – original draft. Y-HC: Methodology, Project administration, Supervision, Writing – original draft. WW: Methodology, Project administration, Supervision, Writing – original draft. LC: Methodology, Project administration, Supervision, Writing – original draft. XKL: Conceptualization, Data curation, Supervision, Writing – review & editing. CT: Conceptualization, Data curation, Supervision, Writing – review & editing. YL: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Xinjiang Uyghur Autonomous Region (2018D01C228, FW); the Beijing Natural Science Foundation (7152074, FW); and the Opening Project of Zhejiang Provincial Top Key Discipline of Pharmaceutical Sciences (YL).
Acknowledgments
We thank Guanghui Shen for his statistic help and all the teachers and students for their involvement in this study. We also thank all the participants in our study for their time and cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hu A, Jiang H, Dowling R, Guo L, Zhao X, Hao W, et al. The transition of alcohol control in China 1990-2019: impacts and recommendations. Int J Drug Policy. (2022) 105:103698. doi: 10.1016/j.drugpo.2022.103698
2. Patel AK, Balasanova AA. Treatment of alcohol use disorder. Jama. (2021) 325:596. doi: 10.1001/jama.2020.2012
3. Collaborators GBDA. Alcohol use and burden for 195 countries and territories, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet. (2018) 392:1015–35. doi: 10.1016/S0140-6736(18)31310-2
4. Huang MC, Schwandt ML, Chester JA, Kirchhoff AM, Kao CF, Liang T, et al. Fkbp5 moderates alcohol withdrawal severity: human genetic association and functional validation in knockout mice. Neuropsychopharmacol. (2014) 39:2029–38. doi: 10.1038/npp.2014.55
5. Smith PH, Homish GG, Leonard KE, Collins RL. Marijuana withdrawal and aggression among a representative sample of U.S. Marijuana users. Drug Alcohol Depend. (2013) 132:63–8. doi: 10.1016/j.drugalcdep.2013.01.002
6. Hogan NL, Jaehne EJ, Bak S, Djouma E, van den Buuse M. Brain-derived neurotrophic factor val66met induces female-specific changes in impulsive behaviour and alcohol self-administration in mice. Behav Brain Res. (2021) 401:113090. doi: 10.1016/j.bbr.2020.113090
7. Pettinati HM, O’Brien CP, Dundon WD. Current status of co-occurring mood and substance use disorders: A new therapeutic target. Am J Psychiatry. (2013) 170:23–30. doi: 10.1176/appi.ajp.2012.12010112
8. Richard-Lepouriel H, Kung AL, Hasler R, Bellivier F, Prada P, Gard S, et al. Impulsivity and its association with childhood trauma experiences across bipolar disorder, attention deficit hyperactivity disorder and borderline personality disorder. J Affect Disord. (2019) 244:33–41. doi: 10.1016/j.jad.2018.07.060
9. Schuckit MA, Danko GP, Smith TL, Hesselbrock V, Kramer J, Bucholz K. A 5-year prospective evaluation of dsm-iv alcohol dependence with and without a physiological component. Alcohol Clin Exp Res. (2003) 27:818–25. doi: 10.1097/01.Alc.0000067980.18461.33
10. Bevilacqua L, Goldman D. Genetics of impulsive behaviour. Philos Trans R Soc Lond B Biol Sci. (2013) 368:20120380. doi: 10.1098/rstb.2012.0380
11. Rutter M. Gene-environment interdependence. Dev Sci. (2007) 10:12–8. doi: 10.1111/j.1467-7687.2007.00557.x
12. Nedic G, Perkovic MN, Sviglin KN, Muck-Seler D, Borovecki F, Pivac N. Brain-derived neurotrophic factor val66met polymorphism and alcohol-related phenotypes. Prog Neuropsychopharmacol Biol Psychiatry. (2013) 40:193–8. doi: 10.1016/j.pnpbp.2012.09.005
13. Xu J, Wu F, Wang F, Yang F, Liu M, Lou M, et al. The interaction of single nucleotide polymorphisms on fibroblast growth factor 19 superfamily genes is associated with alcohol dependence-related aggression. Front Genet. (2021) 12:695835. doi: 10.3389/fgene.2021.695835
14. Zhou Z, Roy A, Lipsky R, Kuchipudi K, Zhu G, Taubman J, et al. Haplotype-based linkage of tryptophan hydroxylase 2 to suicide attempt, major depression, and cerebrospinal fluid 5-hydroxyindoleacetic acid in 4 populations. Arch Gen Psychiatry. (2005) 62:1109–18. doi: 10.1001/archpsyc.62.10.1109
15. Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, et al. Impaired repression at a 5-hydroxytryptamine 1a receptor gene polymorphism associated with major depression and suicide. J Neurosci. (2003) 23:8788–99. doi: 10.1523/jneurosci.23-25-08788.2003
16. Kia-Keating BM, Glatt SJ, Tsuang MT. Meta-analyses suggest association between comt, but not htr1b, alleles, and suicidal behavior. Am J Med Genet B Neuropsychiatr Genet. (2007) 144b:1048–53. doi: 10.1002/ajmg.b.30551
17. Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. (2012) 64:238–58. doi: 10.1124/pr.111.005108
18. Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The bdnf val66met polymorphism affects activity-dependent secretion of bdnf and human memory and hippocampal function. Cell. (2003) 112:257–69. doi: 10.1016/s0092-8674(03)00035-7
19. Li S, Lu C, Kang L, Li Q, Chen H, Zhang H, et al. Study on correlations of bdnf, pi3k, akt and creb levels with depressive emotion and impulsive behaviors in drug-naïve patients with first-episode Schizophrenia. BMC Psychiatry. (2023) 23:225. doi: 10.1186/s12888-023-04718-8
20. Bergman O, Westberg L, Lichtenstein P, Eriksson E, Larsson H. Study on the possible association of brain-derived neurotrophic factor polymorphism with the developmental course of symptoms of attention deficit and hyperactivity. Int J Neuropsychopharmacol. (2011) 14:1367–76. doi: 10.1017/s1461145711000502
21. Su H, Tao J, Zhang J, Xie Y, Sun Y, Li L, et al. An association between bdnf val66met polymorphism and impulsivity in methamphetamine abusers. Neurosci Lett. (2014) 582:16–20. doi: 10.1016/j.neulet.2014.08.030
22. Boscutti A, Pigoni A, Delvecchio G, Lazzaretti M, Mandolini GM, Girardi P, et al. The Influence of 5-HTTLPR, BDNF Rs6265 and COMT Rs4680 polymorphisms on impulsivity in bipolar disorder: the role of gender. Genes (Basel). (2022) 13:482. doi: 10.3390/genes13030482
23. Dolegowska K, Marchelek-Mysliwiec M, Nowosiad-Magda M, Slawinski M, Dolegowska B. Fgf19 subfamily members: fgf19 and fgf21. J Physiol Biochem. (2019) 75:229–40. doi: 10.1007/s13105-019-00675-7
24. Yie J, Wang W, Deng L, Tam LT, Stevens J, Chen MM, et al. Understanding the physical interactions in the fgf21/fgfr/B-klotho complex: structural requirements and implications in fgf21 signaling. Chem Biol Drug Des. (2012) 79:398–410. doi: 10.1111/j.1747-0285.2012.01325.x
25. Hsuchou H, Pan W, Kastin AJ. The fasting polypeptide fgf21 can enter brain from blood. Peptides. (2007) 28:2382–6. doi: 10.1016/j.peptides.2007.10.007
26. Epperlein S, Gebhardt C, Rohde K, Chakaroun R, Patt M, Schamarek I, et al. The effect of fgf21 and its genetic variants on food and drug cravings, adipokines and metabolic traits. Biomedicines. (2021) 9:345. doi: 10.3390/biomedicines9040345
27. Belvederi Murri M, Prestia D, Mondelli V, Pariante C, Patti S, Olivieri B, et al. The hpa axis in bipolar disorder: systematic review and meta-analysis. Psychoneuroendocrinol. (2016) 63:327–42. doi: 10.1016/j.psyneuen.2015.10.014
28. Stanley B, Michel CA, Galfalvy HC, Keilp JG, Rizk MM, Richardson-Vejlgaard R, et al. Suicidal subtypes, stress responsivity and impulsive aggression. Psychiatry Res. (2019) 280:112486. doi: 10.1016/j.psychres.2019.112486
29. Xu J, Wu F, Li Y, Wang F, Lin W, Qian S, et al. Fibroblast growth factor 21 associating with serotonin and dopamine in the cerebrospinal fluid predicts impulsivity in healthy subjects. BMC Neurosci. (2021) 22:68. doi: 10.1186/s12868-021-00676-7
30. Ruiz-Padilla AJ, Morales-Hernandez G, Ruiz-Noa Y, Alonso-Castro AJ, Lazo-de-la-Vega-Monroy ML, Preciado-Puga MDC, et al. Association of the 3’utr polymorphism (Rs11665896) in the fgf21 gene with metabolic status and nutrient intake in children with obesity. J Pediatr Endocrinol Metab. (2019) 32:921–8. doi: 10.1515/jpem-2018-0546
31. Peregud DI, Korolkov AI, Baronets VY, Lobacheva AS, Arkus ML, Igumnov SA, et al. [Contents of bdnf, mir-30a-5p and mir-122 during alcohol withdrawal syndrome]. BioMed Khim. (2022) 68:218–27. doi: 10.18097/pbmc20226803218
32. Ho MF, Zhang C, Moon I, Wei L, Coombes B, Biernacka J, et al. Genome-wide association study for circulating fgf21 in patients with alcohol use disorder: molecular links between the snhg16 locus and catecholamine metabolism. Mol Metab. (2022) 63:101534. doi: 10.1016/j.molmet.2022.101534
33. Matsushita S, Kimura M, Miyakawa T, Yoshino A, Murayama M, Masaki T, et al. Association study of brain-derived neurotrophic factor gene polymorphism and alcoholism. Alcohol Clin Exp Res. (2004) 28:1609–12. doi: 10.1097/01.alc.0000145697.81741.d2
34. Benzerouk F, Gierski F, Gorwood P, Ramoz N, Stefaniak N, Hübsch B, et al. Brain-derived neurotrophic factor (Bdnf) val66met polymorphism and its implication in executive functions in adult offspring of alcohol-dependent probands. Alcohol. (2013) 47:271–4. doi: 10.1016/j.alcohol.2013.03.001
35. Tsai S-J, Liao D-L, Yu YWY, Chen T-J, Wu H-C, Lin C-H, et al. A study of the association of (Val66met) polymorphism in the brain-derived neurotrophic factor gene with alcohol dependence and extreme violence in Chinese males. Neurosci Lett. (2005) 381:340–3. doi: 10.1016/j.neulet.2005.02.043
36. Li X, Fei L, Xu D, Zhang Y, Yang S, Tong Y, et al. The reliability and validity of the Chinese revised version of the Barratt Impulsiveness Scale in community and university populations. Chin J Ment Health. (2011) 25:6. doi: 10.3969/j.issn.1000-6729.2011.08.013
37. Yao S, Yang H, Zhu X, Auerbach RP, Abela JR, Pulleyblank RW, et al. An examination of the psychometric properties of the Chinese version of the barratt impulsiveness scale, 11th version in a sample of Chinese adolescents. Percept Mot Skills. (2007) 104:1169–82. doi: 10.2466/pms.104.4.1169-1182
38. Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, et al. Variant brain-derived neurotrophic factor (Bdnf) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type bdnf in neurosecretory cells and cortical neurons. J Neurosci. (2004) 24:4401–11. doi: 10.1523/jneurosci.0348-04.2004
39. Ambrus L, Sunnqvist C, Ekman R, Träskman-Bendz L, Westrin Å. Plasma brain-derived neurotrophic factor and psychopathology in attempted suicide. Neuropsychobiol. (2016) 73:241–8. doi: 10.1159/000446286
40. Cubero-Millán I, Ruiz-Ramos MJ, Molina-Carballo A, Martínez-Serrano S, Fernández-López L, MaChado-Casas I, et al. Bdnf concentrations and daily fluctuations differ among adhd children and respond differently to methylphenidate with no relationship with depressive symptomatology. Psychopharmacol (Berl). (2017) 234:267–79. doi: 10.1007/s00213-016-4460-1
41. Stamates AL, Schulz CT, Ehlke SJ, Thompson L, Lau-Barraco C, Kelley ML. Latent profiles of impulsivity facets and associations with drinking behaviors. Drug Alcohol Depend. (2021) 228:108979. doi: 10.1016/j.drugalcdep.2021.108979
42. Papachristou H, Nederkoorn C, Havermans R, van der Horst M, Jansen A. Can’t stop the craving: the effect of impulsivity on cue-elicited craving for alcohol in heavy and light social drinkers. Psychopharmacol (Berl). (2012) 219:511–8. doi: 10.1007/s00213-011-2240-5
43. Sliedrecht W, Roozen HG, Witkiewitz K, de Waart R, Dom G. The association between impulsivity and relapse in patients with alcohol use disorder: A literature review. Alcohol Alcohol. (2021) 56:637–50. doi: 10.1093/alcalc/agaa132
44. Reichl D, Enewoldsen N, Weisel KK, Fuhrmann L, Lang C, Saur S, et al. Association of impulsivity with quality of life and well-being after alcohol withdrawal treatment. J Clin Psychol. (2022) 78:1451–62. doi: 10.1002/jclp.23316
45. Kang K, Xu P, Wang M, Chunyu J, Sun X, Ren G, et al. Fgf21 attenuates neurodegeneration through modulating neuroinflammation and oxidant-stress. BioMed Pharmacother. (2020) 129:110439. doi: 10.1016/j.biopha.2020.110439
46. Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK. Gdnf, ngf and bdnf as therapeutic options for neurodegeneration. Pharmacol Ther. (2013) 138:155–75. doi: 10.1016/j.pharmthera.2013.01.004
47. Mandolini GM, Lazzaretti M, Pigoni A, Delvecchio G, Soares JC, Brambilla P. The impact of bdnf val66met polymorphism on cognition in Bipolar Disorder: A review: special section on “Translational and Neuroscience Studies in Affective Disorders” Section editor, Maria Nobile Md, Phd. This section of jad focuses on the relevance of translational and neuroscience studies in providing a better understanding of the neural basis of affective disorders. The main aim is to briefly summaries relevant research findings in clinical neuroscience with particular regards to specific innovative topics in mood and anxiety disorders. J Affect Disord. (2019) 243:552–8. doi: 10.1016/j.jad.2018.07.054
48. Zhen YF, Zhang J, Liu XY, Fang H, Tian LB, Zhou DH, et al. Low bdnf is associated with cognitive deficits in patients with type 2 diabetes. Psychopharmacol (Berl). (2013) 227:93–100. doi: 10.1007/s00213-012-2942-3
49. Logrip ML, Barak S, Warnault V, Ron D. Corticostriatal bdnf and alcohol addiction. Brain Res. (2015) 1628:60–7. doi: 10.1016/j.brainres.2015.03.025
50. Desai BN, Singhal G, Watanabe M, Stevanovic D, Lundasen T, Fisher FM, et al. Fibroblast growth factor 21 (Fgf21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol Metab. (2017) 6:1395–406. doi: 10.1016/j.molmet.2017.08.004
51. Lee H, Lim Y. The potential role of myokines/hepatokines in the progression of neuronal damage in streptozotocin and high-fat diet-induced type 2 diabetes mellitus mice. Biomedicines. (2022) 10:1521. doi: 10.3390/biomedicines10071521
52. Teillon S, Calderon GA, Rios M. Diminished diet-induced hyperglycemia and dyslipidemia and enhanced expression of pparalpha and fgf21 in mice with hepatic ablation of brain-derived neurotropic factor. J Endocrinol. (2010) 205:37–47. doi: 10.1677/joe-09-0405
53. Dukić M, Radonjić T, Jovanović I, Zdravković M, Todorović Z, Kraišnik N, et al. Alcohol, inflammation, and microbiota in alcoholic liver disease. Int J Mol Sci. (2023) 24:3735. doi: 10.3390/ijms24043735
54. Gao XM, Dong WH, Xia CL, Ma ZY, Wang Y, Sarra S, et al. Centranthera grandiflore alleviates alcohol-induced oxidative stress and cell apoptosis. Chin J Nat Med. (2022) 20:572–9. doi: 10.1016/s1875-5364(22)60181-x
55. Warnault V, Darcq E, Morisot N, Phamluong K, Wilbrecht L, Massa SM, et al. The bdnf valine 68 to methionine polymorphism increases compulsive alcohol drinking in mice that is reversed by tropomyosin receptor kinase B activation. Biol Psychiatry. (2016) 79:463–73. doi: 10.1016/j.biopsych.2015.06.007
56. Anastasia A, Deinhardt K, Chao MV, Will NE, Irmady K, Lee FS, et al. Val66met polymorphism of bdnf alters prodomain structure to induce neuronal growth cone retraction. Nat Commun. (2013) 4:2490. doi: 10.1038/ncomms3490
57. Chen J, Zhang T, Jiao S, Zhou X, Zhong J, Wang Y, et al. Probdnf accelerates brain amyloid-B Deposition and learning and memory impairment in appsweps1de9 transgenic mice. J Alzheimers Dis. (2017) 59:941–9. doi: 10.3233/jad-161191
58. Gao L, Zhang Y, Sterling K, Song W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl Neurodegener. (2022) 11:4. doi: 10.1186/s40035-022-00279-0
59. BonDurant LD, Potthoff MJ. Fibroblast growth factor 21: A versatile regulator of metabolic homeostasis. Annu Rev Nutr. (2018) 38:173–96. doi: 10.1146/annurev-nutr-071816-064800
60. Lu H, Jia C, Wu D, Jin H, Lin Z, Pan J, et al. Fibroblast growth factor 21 (Fgf21) alleviates senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the sirt1-mtor signaling pathway. Cell Death Dis. (2021) 12:865. doi: 10.1038/s41419-021-04157-x
61. Yip SW, Potenza MN. Application of research domain criteria to childhood and adolescent impulsive and addictive disorders: implications for treatment. Clin Psychol Rev. (2018) 64:41–56. doi: 10.1016/j.cpr.2016.11.003
62. Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJ, Gillan CM, et al. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr. (2014) 19:69–89. doi: 10.1017/s1092852913000801
63. Gratacòs M, González JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X. Brain-derived neurotrophic factor val66met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and Schizophrenia. Biol Psychiatry. (2007) 61:911–22. doi: 10.1016/j.biopsych.2006.08.025
64. Russo-Neustadt A. Brain-derived neurotrophic factor, behavior, and new directions for the treatment of mental disorders. Semin Clin Neuropsychiatry. (2003) 8:109–18. doi: 10.1053/scnp.2003.50014
65. Flores-Bonilla A, Richardson HN. Sex differences in the neurobiology of alcohol use disorder. Alcohol Res. (2020) 40:4. doi: 10.35946/arcr.v40.2.04
66. Blendberg JM, Àrnadóttir S, Tarp K, Bilberg R. Gender differences in alcohol treatment. Alcohol Alcohol. (2020) 29:agaa071. doi: 10.1093/alcalc/agaa071
67. Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev. (2009) 29:535–47. doi: 10.1016/j.cpr.2009.06.003
68. Agabio R, Pisanu C, Minerba L, Gessa GL, Franconi F. Gender differences among sardinians with alcohol use disorder. J Clin Med. (2021) 10:4688. doi: 10.3390/jcm10204688
69. Moffat JJ, Sakhai SA, Hoisington ZW, Ehinger Y, Ron D. The bdnf val68met polymorphism causes a sex specific alcohol preference over social interaction and also acute tolerance to the anxiolytic effects of alcohol, a phenotype driven by malfunction of bdnf in the ventral hippocampus of male mice. Psychopharmacol (Berl). (2023) 240:303–17. doi: 10.1007/s00213-022-06305-3
Keywords: alcohol use disorder, alcohol withdrawal, impulsivity, BDNF, FGF21
Citation: Yang S, Wang F, Sun L, Liu X, Li S, Chen Y, Chen L, Pan Z, Kang Y, Chen Y-H, Wang W, Chen L, Li X, Tang C and Liu Y (2024) The effects of BDNF rs6265 and FGF21 rs11665896 polymorphisms on alcohol use disorder-related impulsivity in Han Chinese adults. Front. Psychiatry 15:1339558. doi: 10.3389/fpsyt.2024.1339558
Received: 16 November 2023; Accepted: 26 March 2024;
Published: 24 April 2024.
Edited by:
Javier Navarro-Zaragoza, University of Murcia, SpainCopyright © 2024 Yang, Wang, Sun, Liu, Li, Chen, Chen, Pan, Kang, Chen, Wang, Chen, Li, Tang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanlong Liu, YmVuamFtaW5seWxAd211LmVkdS5jbg==; Chonghui Tang, dGFuZ2VnZ0AxNjMuY29t; Xiaokun Li, eGlhb2t1bmxpQHdtdS5lZHUuY24=
†These authors share first authorship
 Shizhuo Yang
Shizhuo Yang Fan Wang
Fan Wang Lanrong Sun4†
Lanrong Sun4† Siyuan Li
Siyuan Li Yingjie Chen
Yingjie Chen Yimin Kang
Yimin Kang Yu-Hsin Chen
Yu-Hsin Chen Wei Wang
Wei Wang Li Chen
Li Chen Yanlong Liu
Yanlong Liu