
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 13 December 2023
Sec. Mood Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1290364
This article is part of the Research Topic Treatment Resistant Depression (TRD): epidemiology, clinic, burden and treatment View all 23 articles
Objective: This systematic review of randomized controlled studies (RCTs) and observational studies evaluated the efficacy and safety of stanford neuromodulation therapy (SNT) for patients with treatment-resistant depression (TRD).
Methods: A systematic search (up to 25 September, 2023) of RCTs and single-arm prospective studies was conducted.
Results: One RCT (n = 29) and three single-arm prospective studies (n = 34) met the study entry criteria. In the RCT, compared to sham, active SNT was significantly associated with higher rates of antidepressant response (71.4% versus 13.3%) and remission (57.1% versus 0%). Two out of the three single-arm prospective studies reported the percentage of antidepressant response after completing SNT, ranging from 83.3% (5/6) to 90.5% (19/21). In the three single-arm prospective studies, the antidepressant remission rates ranged from 66.7% (4/6) to 90.5% (19/21). No severe adverse events occurred in all the four studies.
Conclusion: This systematic review found SNT significantly improved depressive symptoms in patients with TRD within 5 days, without severe adverse events.
Major depressive disorder (MDD) is a leading cause of disability worldwide (1), and up to 55% of patients suffering from MDD fulfill the criteria of treatment-resistant depression (TRD) (2). Accumulating evidence has found that ketamine (3) and esketamine (4) had a rapid antidepressant, antisuicidal effects on TRD. Esketamine nasal spray has been approved as the first therapeutic agent for TRD (5). Furthermore, a real-world study found a significant reduction of depressive symptoms in patients suffering from TRD after receiving esketamine nasal spray (5). Apart from antidepressant medication, strategies such as vagus nerve stimulation (6), electroconvulsive therapy (7, 8), transcranial alternating current stimulation (9), and transcranial magnetic stimulation (TMS) [e.g., deep TMS (10), accelerated TMS (11), intermittent theta-burst stimulation (iTBS) (12), accelerated iTBS (13), bilateral TBS (14), and continuation TBS (15)], have been developed as a nonpharmacological alternative for the treatment of MDD.
iTBS has been approved in many countries in the treatment of TRD. However, efficiency has been less than desired and another treatment protocol (number and spacing of individual treatments) may provide a better outcome (16). Stanford neuromodulation therapy (SNT), a neuroscience-informed accelerated iTBS protocol, had been investigated as a solution to these limitations (17). For example, Cole et al. reported significant superiority of active SNT over sham stimulation in improving depressive symptoms in TRD (17). We conducted this systematic review of randomized controlled studies (RCTs) and single-arm prospective studies to examine the efficacy and safety of SNT for patients with TRD.
Following PICOS acronym, studies were selected and screened by three investigators (XJL, ZJQ and QML) for inclusion in this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (18). Participants: patients with TRD based on study-defined diagnostic criteria. For example, TRD was defined as failure to responding to at least two antidepressants from different classes at adequate dosages (19). Intervention vs. Comparison: active SNT plus antidepressants or antidepressants free versus sham SNT plus antidepressants or antidepressants free in RCTs; or SNT added to antidepressants or antidepressants free in single-arm prospective studies. Outcomes: Coprimary outcomes were study-defined response and remission. A secondary outcome was adverse events. Study: only published RCTs or single-arm prospective studies on the efficacy and safety of SNT, using resting-state functional connectivity Magnetic Resonance Imaging (fcMRI) to target high-dose iTBS (10 sessions of iTBS daily, 18,000 pulses/day, 5 consecutive days, and 90,000 total pulses), as an adjunctive treatment for TRD were considered. High-dose iTBS studies with different intervals between sessions, such as 50-min or 60-min, were approved. Studies on patients without TRD were excluded (20). Systematic reviews, retrospective studies, and case reports/series were not included.
We performed a systematic review of relevant literature from inception to 25 September, 2023, based on the Cochrane Library, PubMed, EMBASE and PsycINFO databases and reference lists from retrieved studies (16, 17, 21) to identify RCTs and single-arm prospective studies (single-group and before-after design) that examined the antidepressant effects of SNT for TRD. The following search terms were used: (“Stanford neuromodulation therapy” OR “Stanford accelerated intelligent neuromodulation therapy” OR SNT OR “High-dose spaced theta-burst stimulation”) AND (depress* OR dysphor* OR dysthymi* OR melanchol* OR antidepress* OR bipolar OR MDD). Study selection was performed independently by three investigators (XJL, ZJQ and QML).
Data extraction was performed independently by three investigators (XJL, ZJQ, and QML). If there were discrepancies, consensus was achieved between the investigators and then discussion was conducted with a senior investigator (WZ). Additionally, the first and/or corresponding authors were contacted as necessary to acquire any pertinent information that was missing.
For RCTs and single-arm prospective studies, the Cochrane risk of bias (22) and Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I) (23) were, respectively, used to assess the study quality independently by the three investigators (XJL, ZJQ, and QML).
As shown in Figure 1, 107 potentially relevant articles were identified, and finally one RCT (17) and three single-arm prospective studies (16, 21, 24) met the study entry criteria (Table 1). Four studies (n = 63) (16, 17, 21, 24) examined the efficacy and safety of adjunctive SNT for adult patients with TRD. The risk of bias of included studies is summarized in Tables 2, 3. Based on the Cochrane risk of bias tool, the double-blind RCT (17) was rated as low risk with regard to attrition bias and reporting bias (Table 2). In the RCT, compared to sham, active SNT was significantly associated with higher rates of antidepressant response (71.4% versus 13.3%) and remission (57.1% versus 0%) (17). Two out of the three single-arm prospective studies reported the rates of antidepressant response after completing SNT, ranging from 83.3% (5/6) (21) to 90.5% (19/21) (16). In the three single-arm prospective studies, the antidepressant remission rates ranged from 66.7% (4/6) (21), 83.3% (5/6) (24) to 90.5% (19/21) (16). Furthermore, Cole et al. found 70% of patients with TRD continued to fulfill response criteria at 1-month follow-up (16). Poydasheva et al. reported that 40% of patients with TRD met the criteria for both response and remission at the 3-month follow-up assessment (24). No severe adverse events occurred in the four studies (16, 17, 21).
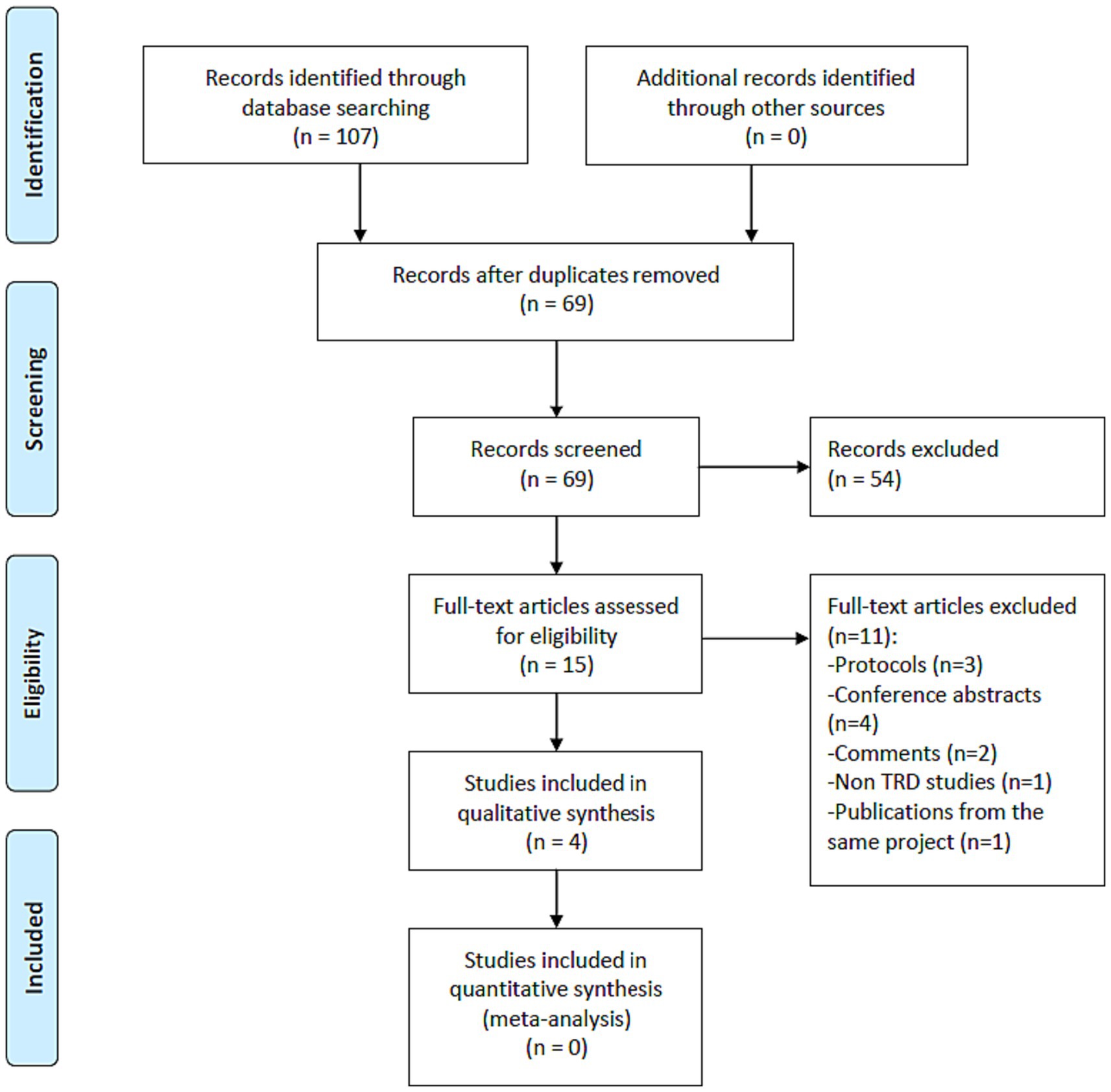
Figure 1. PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses; RCTs, randomized controlled trials; TRD, treatment-resistant depression.
This systematic review found SNT, using resting-state fcMRI to target high-dose iTBS, could significantly improve depressive symptoms in patients with TRD within 5 days, without severe adverse events. The rate of antidepressant remission (66.7–90.5%) reported in the included studies is higher than the corresponding figures for ketamine treatment (8.3%) (25), electroconvulsive therapy (48.0%) (26) and standard FDA-approved repetitive transcranial magnetic stimulation (rTMS) protocols (5.9%) (27). However, Lan et al. found that iTBS (one sessions/day) and high-frequency rTMS appeared to be equally effective in alleviating depressive symptoms for patients with TRD (10). A recent meta-analysis of RCTs (n = 239) found that the study-defined response was greater for active accelerated iTBS (≥2 sessions of iTBS daily) than sham stimulation (13).
The short duration protocol (5 days) of SNT is a non-invasive brain stimulation with proven efficacy in TRD which could be used in emergency or inpatient settings where rapid-acting treatments are needed. As previously described (16, 17, 21), this protocol for SNT consisted of 5 consecutive days (90,000 total pulses) with ten iTBS sessions per day (18,000 pulses/day and a 50-min intersession interval per session) delivered to the region of the left dorsolateral prefrontal cortex (DLPFC). This protocol was designated SNT, to distinguish it from other accelerated iTBS protocols which do not have a high overall pulse dose of stimulation (SNT versus standard iTBS protocols: 90,000 versus 18,000 pulses) and individualized targeting using fcMRI (28, 29). This systematic review of studies with iTBS at high doses involved different intersession intervals per session. Therefore, one single-arm prospective study with its protocol for SNT consisting of 5 consecutive days (18,000 pulses/day, 90,000 total pulses and a 60-min intersession interval per session) was also included (24). However, the individual contribution of each element in the improvement of TRD outcomes is unclear, and this should be further examined.
As a rapid therapeutic intervention for TRD, SNT seems to be comparable to glutamatergic modulators like esketamine (the S-enantiomer of ketamine) (30), exhibiting a greater affinity for the N-methyl-d-aspartate receptor compared to the R-enantiomer (31). The administration of esketamine via intravenous (32) or intranasal (31) routes has a rapid onset of antidepressant effects. For example, Daly et al. found that esketamine administered intranasally at doses of 28, 56, and 84 mg appeared to be effective in treating TRD (31). A retrospective study found that accelerated high-frequency rTMS (four times daily for five consecutive days over the left DLPFC) appears to be more effective than intranasal esketamine (33). However, there are currently no head-to-head comparison studies on TMS and esketamine in treating TRD.
This systematic review has several limitations. First, only one RCT (17) was detected and the total sample size of the included studies (n = 63) was relatively small. Second, of the included four studies, three (16, 17, 21) were conducted by the same team at a single site, limiting generalizability of these findings. Third, the systematic review was not registered as this is not compulsory in most academic journals. Fourth, long-term follow up period (e.g., longer than 3 months) was not adopted in included studies, although the persistence of the antidepressant effect remains an important issue for TMS treatments, with several studies emphasizing the urgency of developing maintenance protocols to prevent potential relapses (34). Despite these limitations, this systematic review preliminarily found that SNT protocol appeared to be effective and well tolerated by patients with TRD. SNT is distinct from standard once daily TMS. An advantage of standard once daily TMS (treatment time 40 min) is that it allows time for supportive care to be provided by staff. Accelerated treatment offers considerable alternative advantages which will call for reorganization and reorientation of treatment centers. Future research is warranted to confirm and expand the utilization of SNT as an adjunctive treatment for TRD.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
X-JL: Data curation, Writing – original draft. D-BC: Data curation, Writing – original draft. Q-ML: Data curation, Writing – original draft. Z-JQ: Writing – original draft. SP: Writing – review & editing. WZ: Writing – original draft. Y-TX: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Natural Science Foundation of China (82101609), China International Medical Exchange Foundation (Z-2018-35-2002), the Science and Technology Program of Guangzhou (2023A03J0839 and 2023A03J0436), Science and Technology Planning Project of Liwan District of Guangzhou (202201012), National Clinical Key specialty construction project [(2023) 33], The Natural Science Foundation Program of Guangdong (2023A1515011383), Guangzhou Municipal Key Discipline in Medicine (2021–2023), and Guangzhou High-level Clinical Key Specialty, Department of Emergency Medicine of National clinical key specialty and Guangzhou Research-oriented Hospital. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Friedrich, MJ . Depression is the leading cause of disability around the world. JAMA. (2017) 317:1517. doi: 10.1001/jama.2017.3826
2. Thomas, L, Kessler, D, Campbell, J, Morrison, J, Peters, TJ, Williams, C, et al. Prevalence of treatment-resistant depression in primary care: cross-sectional data. Br J Gen Pract. (2013) 63:e852–8. doi: 10.3399/bjgp13X675430
3. Marcantoni, WS, Akoumba, BS, Wassef, M, Mayrand, J, Lai, H, Richard-Devantoy, S, et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 - January 2019. J Affect Disord. (2020) 277:831–41. doi: 10.1016/j.jad.2020.09.007
4. McIntyre, RS, Carvalho, IP, Lui, LMW, Majeed, A, Masand, PS, Gill, H, et al. The effect of intravenous, intranasal, and oral ketamine in mood disorders: a meta-analysis. J Affect Disord. (2020) 276:576–84. doi: 10.1016/j.jad.2020.06.050
5. Martinotti, G, Vita, A, Fagiolini, A, Maina, G, Bertolino, A, Dell'Osso, B, et al. Real-world experience of esketamine use to manage treatment-resistant depression: a multicentric study on safety and effectiveness (REAL-ESK study). J Affect Disord. (2022) 319:646–54. doi: 10.1016/j.jad.2022.09.043
6. Zhang, X, Guo, YM, Ning, YP, Cao, LP, Rao, YH, Sun, JQ, et al. Adjunctive vagus nerve stimulation for treatment-resistant depression: a preliminary study. Int J Psychiatry Clin Pract. (2022) 26:337–42. doi: 10.1080/13651501.2021.2019789
7. İlhan Atagün, M, and Atay, CÖ. A systematic review of the literature regarding the relationship between oxidative stress and electroconvulsive therapy. Alpha Psychiatry. (2022) 23:47–56. doi: 10.5152/alphapsychiatry.2021.21584
8. Zheng, W, Li, XH, Zhu, XM, Cai, DB, Yang, XH, Ungvari, GS, et al. Adjunctive ketamine and electroconvulsive therapy for major depressive disorder: a meta-analysis of randomized controlled trials. J Affect Disord. (2019) 250:123–31. doi: 10.1016/j.jad.2019.02.044
9. Zheng, W, Cai, DB, Nie, S, Chen, JH, Huang, XB, Goerigk, S, et al. Adjunctive transcranial alternating current stimulation for patients with major depressive disorder: a systematic review and meta-analysis. Front Psych. (2023) 14:1154354. doi: 10.3389/fpsyt.2023.1154354
10. Lan, XJ, Yang, XH, Qin, ZJ, Cai, DB, Liu, QM, Mai, JX, et al. Efficacy and safety of intermittent theta burst stimulation versus high-frequency repetitive transcranial magnetic stimulation for patients with treatment-resistant depression: a systematic review. Front Psych. (2023) 14:1244289. doi: 10.3389/fpsyt.2023.1244289
11. Zheng, W, Zhang, XY, Xu, R, Huang, X, Zheng, YJ, Huang, XB, et al. Adjunctive accelerated repetitive transcranial magnetic stimulation for older patients with depression: a systematic review. Front Aging Neurosci. (2022) 14:1036676. doi: 10.3389/fnagi.2022.1036676
12. Blumberger, DM, Vila-Rodriguez, F, Thorpe, KE, Feffer, K, Noda, Y, Giacobbe, P, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet. (2018) 391:1683–92. doi: 10.1016/s0140-6736(18)30295-2
13. Cai, DB, Qin, ZJ, Lan, XJ, Liu, QM, Qin, XD, Wang, JJ, et al. Accelerated intermittent theta burst stimulation for major depressive disorder or bipolar depression: a systematic review and meta-analysis. Asian J Psychiatr. (2023) 85:103618. doi: 10.1016/j.ajp.2023.103618
14. Qin, ZJ, Huang, SQ, Lan, XJ, Shi, ZM, Huang, XB, Ungvari, GS, et al. Bilateral theta burst stimulation for patients with acute unipolar or bipolar depressive episodes: a systematic review of randomized controlled studies. J Affect Disord. (2023) 340:575–82. doi: 10.1016/j.jad.2023.08.065
15. Li, CT, Chen, MH, Juan, CH, Huang, HH, Chen, LF, Hsieh, JC, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. (2014) 137:2088–98. doi: 10.1093/brain/awu109
16. Cole, EJ, Stimpson, KH, Bentzley, BS, Gulser, M, Cherian, K, Tischler, C, et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry. (2020) 177:716–26. doi: 10.1176/appi.ajp.2019.19070720
17. Cole, EJ, Phillips, AL, Bentzley, BS, Stimpson, KH, Nejad, R, Barmak, F, et al. Stanford neuromodulation therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. (2022) 179:132–41. doi: 10.1176/appi.ajp.2021.20101429
18. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
19. Demyttenaere, K, and Van Duppen, Z. The impact of (the concept of) treatment-resistant depression: an opinion review. Int J Neuropsychopharmacol. (2019) 22:85–92. doi: 10.1093/ijnp/pyy052
20. Tang, NL, Chen, YH, Wang, WY, Sun, CZ, Wu, D, Sun, L, et al. A preliminary study of precise treatment for major depression patients with suicide ideation by individualized targeted robot assisted Stanford accelerated intelligent neuromodulation therapy (in Chinese). Chinese J. Psychiatry. (2022) 55:14–23. doi: 10.3760/cma.j.cn113661-20210527-00173
21. Williams, NR, Sudheimer, KD, Bentzley, BS, Pannu, J, Stimpson, KH, Duvio, D, et al. High-dose spaced theta-burst TMS as a rapid-acting antidepressant in highly refractory depression. Brain. (2018) 141:e18. doi: 10.1093/brain/awx379
22. Higgins, JP, Altman, DG, Gøtzsche, PC, Jüni, P, Moher, D, Oxman, AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
23. Sterne, JA, Hernán, MA, Reeves, BC, Savović, J, Berkman, ND, Viswanathan, M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
24. Poydasheva, AG, Bakulin, IS, Sinitsyn, DO, Zabirova, AH, Suponeva, NA, Maslenikov, NV, et al. Experience of stanford neuromodulation therapy in patients with treatment-resistant depression. Bull Russian State Med Univ. (2022) 4:31–7. doi: 10.24075/brsmu.2022.044
25. Zheng, W, Zhou, YL, Liu, WJ, Wang, CY, Zhan, YN, Li, HQ, et al. Investigation of medical effect of multiple ketamine infusions on patients with major depressive disorder. J Psychopharmacol. (2019) 33:494–501. doi: 10.1177/0269881119827811
26. Heijnen, WT, Birkenhäger, TK, Wierdsma, AI, and van den Broek, WW. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. (2010) 30:616–9. doi: 10.1097/JCP.0b013e3181ee0f5f
27. Taylor, SF, Bhati, MT, Dubin, MJ, Hawkins, JM, Lisanby, SH, Morales, O, et al. A naturalistic, multi-site study of repetitive transcranial magnetic stimulation therapy for depression. J Affect Disord. (2017) 208:284–90. doi: 10.1016/j.jad.2016.08.049
28. Desmyter, S, Duprat, R, Baeken, C, Van Autreve, S, Audenaert, K, and van Heeringen, K. Accelerated intermittent theta burst stimulation for suicide risk in therapy-resistant depressed patients: a randomized, sham-controlled trial. Front Hum Neurosci. (2016) 10:480. doi: 10.3389/fnhum.2016.00480
29. Duprat, R, Desmyter, S, Rudi de, R, van Heeringen, K, Van den Abbeele, D, Tandt, H, et al. Accelerated intermittent theta burst stimulation treatment in medication-resistant major depression: a fast road to remission? J Affect Disord. (2016) 200:6–14. doi: 10.1016/j.jad.2016.04.015
30. d'Andrea, G, Pettorruso, M, Lorenzo, GD, Mancusi, G, McIntyre, RS, and Martinotti, G. Rethinking ketamine and esketamine action: are they antidepressants with mood-stabilizing properties? Eur Neuropsychopharmacol. (2023) 70:49–55. doi: 10.1016/j.euroneuro.2023.02.010
31. Daly, EJ, Singh, JB, Fedgchin, M, Cooper, K, Lim, P, Shelton, RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. (2018) 75:139–48. doi: 10.1001/jamapsychiatry.2017.3739
32. Singh, JB, Fedgchin, M, Daly, E, Xi, L, Melman, C, De Bruecker, G, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. (2016) 80:424–31. doi: 10.1016/j.biopsych.2015.10.018
33. Pettorruso, M, d'Andrea, G, Di Carlo, F, De Risio, L, Zoratto, F, Miuli, A, et al. Comparing fast-acting interventions for treatment-resistant depression: an explorative study of accelerated HF-rTMS versus intranasal esketamine. Brain Stimul. (2023) 16:1041–3. doi: 10.1016/j.brs.2023.06.003
Keywords: stanford neuromodulation therapy, treatment-resistant depression, response, remission, systematic review
Citation: Lan X-J, Cai D-B, Liu Q-M, Qin Z-J, Pridmore S, Zheng W and Xiang Y-T (2023) Stanford neuromodulation therapy for treatment-resistant depression: a systematic review. Front. Psychiatry. 14:1290364. doi: 10.3389/fpsyt.2023.1290364
Received: 07 September 2023; Accepted: 07 November 2023;
Published: 13 December 2023.
Edited by:
Vassilis Martiadis, Department of Mental Health, ItalyReviewed by:
Bao-Liang Zhong, Wuhan Mental Health Center, ChinaCopyright © 2023 Lan, Cai, Liu, Qin, Pridmore, Zheng and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Tao Xiang, eHl1dGx5QGdtYWlsLmNvbQ==; Wei Zheng, emhlbmd3ZWkwNzAyQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.