- 1The Brain Cognition and Brain Disease Institute, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China
- 2Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions, Shenzhen, China
- 3Department of Psychiatry, National Clinical Research Center for Mental Disorders, The Second Xiangya Hospital of Central South University, Changsha, Hunan, China
- 4Department of Medical Genetics, Center for Medical Genetics, School of Basic Medical Sciences, Peking University Health Science Center, Beijing, China
- 5Neuroscience Research Institute, Peking University, Beijing, China
- 6Key Laboratory for Neuroscience, Ministry of Education of China and National Health Commission of China, Beijing, China
- 7Autism Research Center, Peking University Health Science Center, Beijing, China
- 8State Key Laboratory of Primate Biomedical Research, Institute of Primate Translational Medicine, Kunming University of Science and Technology, Kunming, China
Serine/threonine protein kinases are involved in axon formation and neuronal polarization and have recently been implicated in autism spectrum disorder (ASD) and neurodevelopmental disorders (NDD). Here, we focus on BRSK2, which encodes brain-specific serine/threonine protein kinase 2. Although previous studies have reported 19 unrelated patients with BRSK2 pathogenic variation, only 15 of 19 patients have detailed clinical data. Therefore, more case reports are needed to enrich the phenotype associated with BRSK2 mutations. In this study, we report a novel de novo frameshift variant (c.442del, p.L148Cfs*39) identified by exome sequencing in a 16 year-old Chinese boy with ASD. The proband presented with attention-deficit, auditory hallucinations, limb tremor, and abnormal brain electrical activity mapping. This study expands the phenotypic spectrum of BRSK2-related cases and reveals the highly variable severity of disorders associated with BRSK2.
Introduction
Children with autism spectrum disorder (ASD, also known as autism) share some symptoms, such as differences in social communication, and stereotyped, repetitive, or restricted behaviors or interests, based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) (1). The male-to-female ratio of patients with ASD is 4.2 (2). In addition, the prevalence of autism continues to increase, with serious implications for affected families and society.
Autism has a strong and diverse genetic background. Recent large cohort studies revealed that BRSK2 has strong statistical support and is a genome-wide significant risk gene for ASD (3). Brain selective kinase 2 (BRSK2) is a serine/threonine protein kinase that belongs to the AMPK-related protein kinase family, which also includes BRSK1 and 11 other kinases (4). BRSK2 was found to be selectively expressed in the mouse brain, and exhibited the highest expression in the brain among various human organs (5). Recent studies using KO mice suggested that BRSK1 and BRSK2 are essential for the development of polarity of forebrain neurons, which realizes distinct properties of axons and dendrites (6). In addition, BRSK2 mutations have been reported to be associated with ASD and neurodevelopmental disorders (NDD) (3). However, detailed clinical courses have not been described in many cases of BRSK2 mutations.
In this study, clinical exome sequencing was performed on an ASD patient and his family members, and the results revealed the presence of c.442del, p.L148Cfs*39, which is a de novo BRSK2 pathogenic mutation, in this proband. Notably, the patient exhibited acousma and abnormal EEG. This study expands the phenotypic spectrum associated with BRSK2 mutations.
Materials and methods
Psychological assessment
The Autism Diagnostic Observation Scale-Second Edition (ADOS) was used in autism clinical judgment. This scale is one of the most frequently used research tools, which has a standardized structure (7). Scale for assessment of negative symptoms (SANS) has 24 items which was used to measure the severity of negative symptoms in schizophrenia. Its score ranges between 0-120. Scale for assessment of positive symptoms (SAPS) has 35 items and its score ranges between 0 and 165 (8). The Wechsler Intelligence Scale for Children-Fifth Edition (WISC-V) is a valuable IQ test tool for assessing cognitive abilities in children between the ages of 6 and 16 years old (9).
EEG recording and data analysis
The EEG recording were obtained with 16-electrode Stellate Harmonie EEG systems (Natus Medical Incorporated). The EEG signals were preprocessed using a 0.1–100 Hz band-pass filter and the data was analyzed using Harmonie software (Stellate HARMONIR 7.0).
Sample preparation and DNA extraction
Peripheral blood of the proband and his family members was sampled by using EDTA tubes at The Second Xiangya Hospital of Central South University. DNA extraction was achieved by utilizing a DNA Blood Midi/Mini kit (Qiagen, Germany), and DNA concentrations were measured by utilizing a DNA Assay Kit (Qubit®, Life Technologies, USA).
Exome sequencing and variant prioritization
Exome sequencing of genomic DNA samples of the patient and his family members was executed using the Nonaseq 6000 platform (Illumina, USA). An exome library was developed by utilizing xGen Exome Research Panel V1.0 (Integrated DNA Technologies, USA). The raw paired-end reads were aligned to hg38/GRCh38, which serves as a reference genome, with BWA Enrichment. The Genome Analysis Tool Kit (GATK) was used to call variants. ANNOVAR was employed for annotation of Variant Call Format (VCF) acquired previously. The Human Genome Mutation Database (HGMD) and 1,000 Genomes Project were applied to characterize the detected variants. All variants were categorized based on mutation, location, and frequency. The threshold of low frequency filter was minor allele frequency (MAF) < 0.05. The synonymous SNVs and unannotated variants were discarded, and only SNVs observed in splice sites or exons were further investigated. Missense variants were predicted by utilizing the bioinformatics mutation prediction software programs (SIFT). The variations were categorized into groups of benign, likely benign, uncertain significance, likely pathogenic, and pathogenic by using American College of Medical Genetics and Genomics (ACMG). The AlphaFold tool was used to model and visualize the mutant and wild-type protein structures.
Sanger sequencing
The BRSK2 mutation was confirmed by Sanger sequencing of exon5, as well as its flanking intron regions (NM_001256627.2) of the proband and his family members. DNA amplification was achieved by utilizing PCR with gene-specific primers, and purification of the PCR products was achieved by utilizing a PCR Purification Kit (Qiagen, Germany). Additionally, Sanger sequencing using the ABI 3730xl DNA Analyzer (Applied Biosystems, USA) was executed on the purified PCR products to confirm BRSK2 mutation, and the results were investigated by utilizing SnapGene V.4.1.9 (SnapGene, USA).
Results
Clinical description
The patient was a 16-year-old boy who is the first child of a healthy, non-consanguineous couple. There was no family history of neurodevelopmental disorders. He was born at 39 weeks gestation with the following auxological parameters: length 50 cm and weight 3,900 g. The mother reported that the proband began crawling at 8 months, walking independently at the age of 1 year, saying single words at the age of 2 years, and saying simple sentences at 30 months. Meanwhile, eye contact with the patient exhibited no problems, and sphincter control was obtained at 24 months. At the age of 5 years, the proband was still unable to perform fine motor tasks, such as tying shoelaces, but interaction with peers was normal. The patient entered school at the age of 6, but exhibited severe difficulties in learning Chinese and inattention. The mother reported that the boy had auditory hallucinations and giggled involuntarily at the age of 11. The boy told his mother that he heard a male teacher talking to him and telling him to do things. This patient was first diagnosed with mental retardation in the Children's hospital in Tianjin at the age of 11. After 2 years of functional training and rehabilitation exercises, there was no significant improvement. However, the patient was found to have limb tremors at the age of 13. Although the proband did not have any dietary changes, he had a sleep disorder. He was too agitated to fall asleep for 2 days every month. The Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V) was used to test the patient's level of intellectual functions. His full-scale intelligence quotient (FSIQ) score was 38, which was below 70, and he was considered to have moderate intellectual disability. The Autism Diagnostic Observation Schedule (ADOS) score and the autism cut-off score of the proband were 18 and 10, respectively. The scale for the Assessment of Positive Symptoms (SAPS) and the scale for the Assessment of Negative Symptoms (SANS) were also used to assess the patient's schizophrenia symptoms. The scores of SAPS and SANS were 10 and 40, respectively, both below the cut-off score of 50. Based on these assessment results, the boy was second diagnosed with autistic spectrum disorder at Xiangya Hospital at the age of 13. The MRI results of his brain were normal, while the EEG results showed abnormal changes in brain electrical activity mapping (BEAM). The power of the theta band increased in the patient compared to typically developing (TD) boy of the same age (see Figure 1). Although the BEAM of this patient was altered, his mother reported that he never had a seizure.
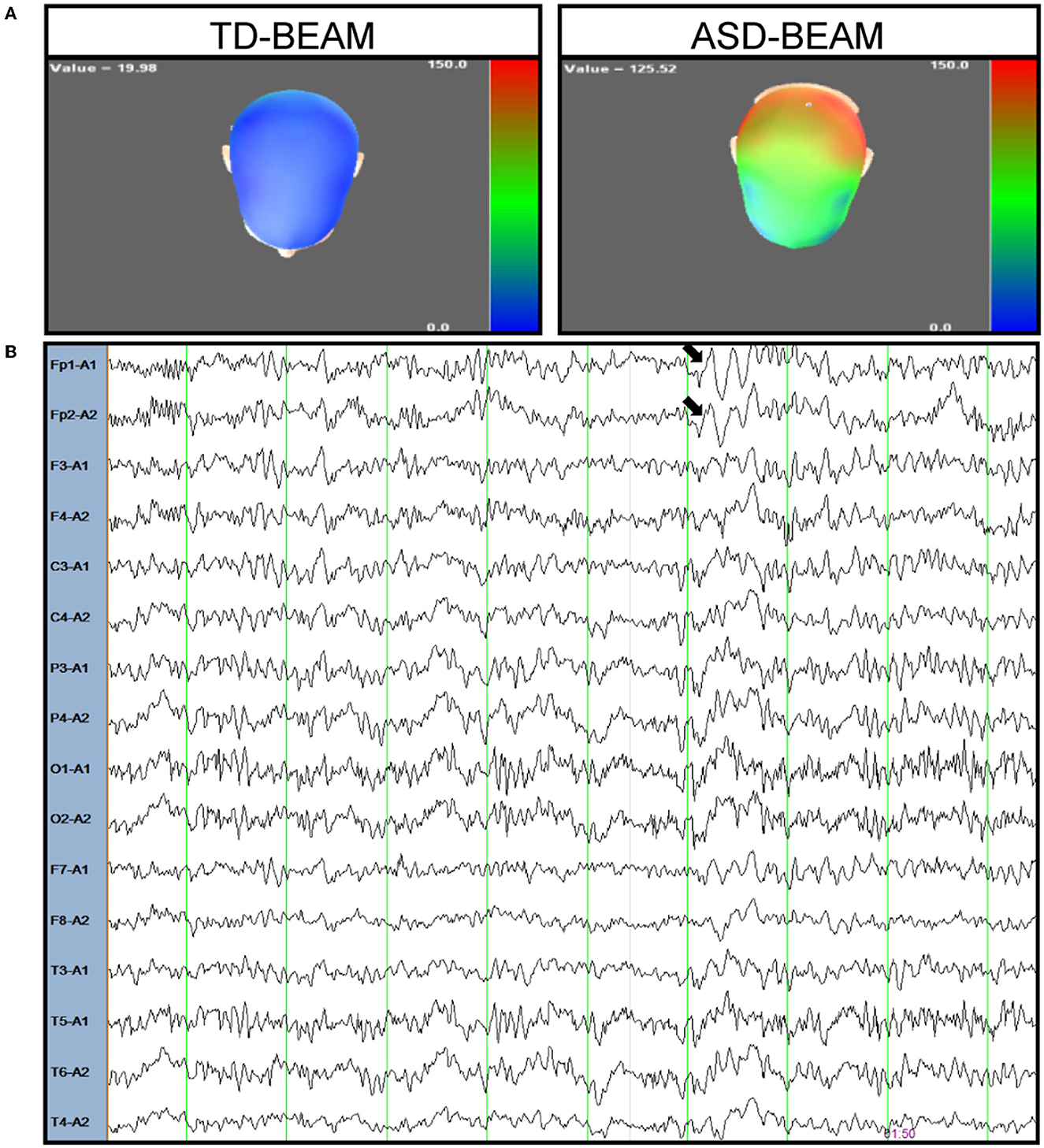
Figure 1. Electroencephalogram of the patient. (A) The power of theta band was observed increased in the patient (right) compared to a typical developing boy (left), colors depicted represent power (μV2) within theta bands across regions. Blue areas correspond to the lower power for theta band and the red areas correspond to higher power. (B) A representative EEG trace recorded from the patient during resting condition. The arrow marks the increase in the theta band in frontal brain region.
Through literature review, we identified 19 cases with BRSK2 pathogenic mutations. Fifteen of the 19 cases reported partial clinical data. All cases with partial clinincal data were diagnosed with autism and presented with speech delay (100%, 15/15). Three of the 15 cases were female, and their age ranged from 3 to 19 years at first report. Twelve of the 15 cases presented with motor delay (80%, 12/15). In addition, some were reported to have sleep disorders (20%, 3/15), feeding problems (13.34%, 2/15), epilepsy (13.34%, 2/15), and schizophrenia (6.67%, 1/15). Details are presented in Supplementary Table S1.
Genetic analysis
Exome sequencing was performed on the proband and his family members. The mean depth of coverage was 20X. The mapping rates of all samples exceeded 98%. The analysis revealed the presence of c.442del, p.L148Cfs*39, which is a de novo frameshift variant in exon 5 of BRSK2 gene on chromosome 11 (Figure 2A). Sanger sequencing demonstrated that the heterozygous variant was present in the proband, but not in his parents or his sister (Figure 2B). This C-deletion mutation in BRSK2 leads to a premature translation termination codon and a 187 amino acid truncated protein (Figure 2C). This de novo frameshift deletion was identified for the first time in this study and is not present in the SPARK or SFARI gene databases (Figure 2D) (one sequence deletion, two non-sense variants, six frameshift variants, six missense variants, and six splice-site variants).
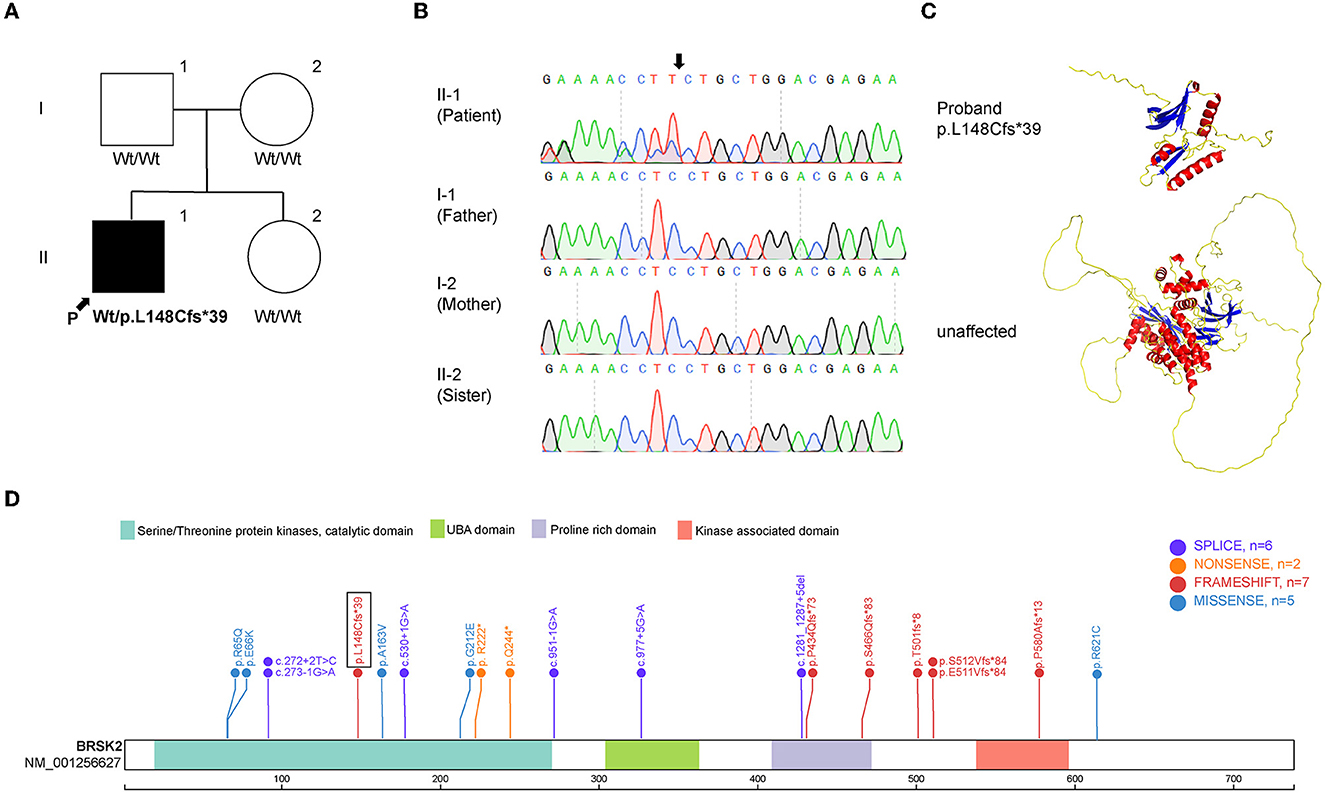
Figure 2. Mutation analysis of BRSK2. (A) Pedigree of the family described in this report. (B) Sequences around the BRSK2 mutation in the patient and his family. The patient carries a C deletion, which leads to a loss function variant, and his family carrier normal sequence. (C) The computational modeling of wild type and mutant BRSK2 in human. (D) Genomic structure of BRSK2 mutations reported previously are shown and the mutation found in this study is shown in a box.
Discussion
This study reports a novel pathogenic BRSK2 variant in an ASD patient. The proband presented with speech delays, attention-deficit, and acousma. Exome sequencing demonstrated the presence of c.442del, p.L148Cfs*39, which is a de novo frameshift variant predicted to be deleterious. Previous publications have reported 19 non-sense, splice alteration, frameshift, and deleterious missense variations in BRSK2. These mutations were likely responsible for the phenotypes of these patients with or without ASD (3, 10–16). We compared the reported clinical phenotypes caused by the mutations located in the same catalytic domain. There were four missense mutations, three splice alterations, two non-sense mutations and one single-base deletion in the same domain. Three of the four missense mutations were all G to A variations, but they caused different symptoms, including intermittent horizontal nystagmus, sleep disorder and undescended testis. One splice alteration caused mild gait ataxia and tremor in a female patient. This symptom is similar to the limb tremor of the patient in our study. Although facial features have been reported in some probands, no consistent set of features was observed in our case. Previous publications indicated the heterogeneity among different mutation sites. Hence, more detailed case reports are necessary to expand the phenotypic spectrum associated with BRSK2 mutations. In this study, abnormal brain electrical activity mapping and acousma were reported for the first time in an ASD patient with BRSK2 mutation. Although the patient's theta band power was altered, he never had seizures. In previous studies, Pablo Billeke and his group assessed the electroencephalographic activity of ASD and TD subjects during a working memory task. They found that impaired theta modulation correlated with autistic symptoms (17), which is consistent with our findings. It has been suggested that the alteration of the theta band may be related to the physiopathology of ASD.
BRSK2, also known as SAD-A, is located at 11p15.5 and encodes 736 amino acids. The protein comprises multiple domains, including a proline-rich domain (aa 424-468), a kinase-associated domain (KA1; aa 530-653), a protein kinase (aa 19-270), and a ubiquitin-associated domain (UBA; aa 297-339) (18). BRSK2 is highly conserved in evolution and exclusively expressed in the vertebrate brain (14). In fact, BRSK2 is involved in axonogenesis and cortical neuron polarization (4, 6, 19, 20). Previous studies have reported that BRSK2 interacts with NDD-associated genes such as autism and developmental delay (DD) and/or intellectual disability (ID). BRSK2 can phosphorylate TSC2 and suppress mTORC1 activity (21, 22). As a component of the TSC signaling pathway, TSC2 regulates cell size and growth. The TSC signaling pathway is associated with autophagy during early axonal growth. Also, BRSK2 interacts with PTEN, which is associated with various developmental disorders (e.g., autism). PTEN knockout mice exhibit neuronal structure malformation and autistic features caused by aberrant TSC-mTORC1 signaling (23, 24). A previous publication reported that single mutant BRSK1 or BRSK2 mice were healthy and fertile, but BRSK1 and BRSK2 double knockout mice showed perinatal lethality with a severe defects in axon differentiation and died within 2 h after birth (4). Conversely, another study demonstrated that on a C57BL/6N background, BRSK2 is essential for cortical development. The BRKS2 knockout mice died within a few days after birth (10). Meanwhile, BRSK2-mutant zebrafish exhibited ASD-like features (e.g., developmental delay, social impairment) (4, 14).
BRSK1, also known as SAD-B, is the homolog of BRSK2. BRSK1 acts as a multifunctional regulator, by phosphorylating its downstream proteins it is involved in many biological processes. BRSK1 can phosphorylateγ-tubulin to regulate centrosome duplication, and phosphorylate CAST to control active zone vesicle recycling for synaptic depression (25, 26). Furthermore, BRSK1 knockout mice showed impaired contextual fear learning. BRSK1 plays a critical role in controlling vesicle release properties and regulating hippocampal function in the mature brain (27). However, when we queried the SFARI Gene database, which tracks the ever-expanding genetic risk factors of autism, surprisingly, we found that BRSK1 has not yet been included among the autism risk genes. Although the evidence suggests that BRSK1 and BRSK2 play a key roles in cortical and neurodevelopmental processes, the functional compensation between BRSK1 and BRSK2 has not been sufficiently studied. Hence, more work is needed to investigate the function of BRSK1 and BRSK2.
In conclusion, we report a pathogenic de novo BRSK2 mutation in an ASD patient, and our findings expand the phenotypic spectrum associated with BRSK2 mutations.
Data availability statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
LY and TW developed the writing plan and drafted the manuscript. XL and YS performed the autism clinical evaluation. YH, ML, HW, and QL performed the experiments. ZL, LY, and QL developed the figures. LY reviewed and approved the final manuscript. All authors have read and agreed to published version of the manuscript.
Funding
This work was supported by research grants from the National Natural Science Foundation of China (NSFC, No. 32000728 and NSFC-Guangdong Joint Fund-U20A6005), Shenzhen Science and Technology Program (JCYJ20220818101608018), and supported, in part, by the Fundamental Research Funds for the Central Universities starting fund (BMU2022RCZX038) and grant from the NSFC (No. 82201314) to TW.
Acknowledgments
We thank Xiu Sun and Dr. Xujun Wu for performing the EEG recording. We also thank Dr. Robert K. Naumann for improving the language of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JL declared a shared parent affiliation with the author TW to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1205204/full#supplementary-material
References
1. DaWalt LS, Taylor JL, Movaghar A, Hong J, Kim B, Brilliant M, et al. Health profiles of adults with autism spectrum disorder: differences between women and men. Autism Res. (2021) 14:1896–904. doi: 10.1002/aur.2563
2. Zeidan J, Fombonne E, Scorah J, Ibrahim A, Durkin MS, Saxena S, et al. Global prevalence of autism: a systematic review update. Autism Res. (2022) 15:778–90. doi: 10.1002/aur.2696
3. Zhou X, Feliciano P, Shu C, Wang T, Astrovskaya I, Hall JB, et al. Integrating de novo and inherited variants in 42,607 autism cases identifies mutations in new moderate-risk genes. Nat Genet. (2022) 54:1305–19. doi: 10.1038/s41588-022-01148-2
4. Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. (2005) 307:929–32. doi: 10.1126/science.1107403
5. Nakanishi M, Higashi Y, Sanes JR, Shimada S, Ugawa S, Pan YA, et al. Isozyme-specific role of SAD-A in neuronal migration during development of cerebral cortex. Cerebral Cortex. (2019) 29:3738–51. doi: 10.1093/cercor/bhy253
6. Dhumale P, Menon S, Chiang J, Puschel AW. The loss of the kinases SadA and SadB results in early neuronal apoptosis and a reduced number of progenitors. PLoS ONE. (2018) 13:e0196698. doi: 10.1371/journal.pone.0196698
7. Frigaux A, Evrard R, Lighezzolo-Alnot J. ADI-R and ADOS and the differential diagnosis of autism spectrum disorders: Interests, limits and openings. Encephale. (2019) 45:441–8. doi: 10.1016/j.encep.2019.07.002
8. Samiei M, Hedayati K, Mirabzadeh Ardekani A, Dolatshahi B, Daneshmand R, Samadi R. Obsessive-compulsive disorder in hospitalized patients with schizophrenia. Basic Clin Neurosci. (2016) 7:323–30. doi: 10.15412/J.BCN.03070405
9. Na SD, Burns TG. Wechsler intelligence scale for children-V: test review. Appl Neuropsychol Child. (2016) 5:156–60. doi: 10.1080/21622965.2015.1015337
10. Hiatt SM, Thompson ML, Prokop JW, Lawlor JMJ, Gray DE, Bebin EM, et al. Deleterious variation in BRSK2 associates with a neurodevelopmental disorder. Am J Hum Genet. (2019) 104:701–8. doi: 10.1016/j.ajhg.2019.02.002
11. Iossifov I, O'Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. (2014) 515:216–21. doi: 10.1038/nature13908
12. Feliciano P, Zhou X, Astrovskaya I, Turner TN, Wang T, Brueggeman L, et al. Exome sequencing of 457 autism families recruited online provides evidence for autism risk genes. NPJ Genom Med. (2019) 4:19. doi: 10.1038/s41525-019-0093-8
13. De Rubeis R, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. (2014) 515:209–15. doi: 10.1038/nature13772
14. Deng J, Wang Y, Hu M, Lin J, Li Q, Liu C, et al. Deleterious variation in BR serine/threonine kinase 2 classified a subtype of autism. Front Mol Neurosci. (2022) 15:904935. doi: 10.3389/fnmol.2022.904935
15. Woodbury-Smith M, Lamoureux S, Begum G, Nassir N, Akter H, O'Rielly DD, et al. Mutational landscape of autism spectrum disorder brain tissue. Genes. (2022) 13. doi: 10.3390/genes13020207
16. Mahjani B, De Rubeis SC, Gustavsson M, Mulhern M, Xu X, et al. Prevalence and phenotypic impact of rare potentially damaging variants in autism spectrum disorder. Mol Autism. (2021) 12:65. doi: 10.1186/s13229-021-00465-3
17. Larrain-Valenzuela J, Zamorano F, Soto-Icaza P, Carrasco X, Herrera C, Daiber F, et al. Theta and alpha oscillation impairments in autistic spectrum disorder reflect working memory deficit. Sci Rep. (2017) 7:14328. doi: 10.1038/s41598-017-14744-8
18. Guo Z, Tang W, Yuan J, Chen X, Wan B, Gu X, et al. BRSK2 is activated by cyclic AMP-dependent protein kinase A through phosphorylation at Thr260. Biochem Biophys Res Commun. (2006) 347:867–71. doi: 10.1016/j.bbrc.2006.06.178
19. Lilley BN, Krishnaswamy A, Wang Z, Kishi M, Frank E, Sanes JR, et al. Kinases control the maturation of nerve terminals in the mammalian peripheral and central nervous systems. Proc Natl Acad Sci USA. (2014) 111:1138–43. doi: 10.1073/pnas.1321990111
20. Fogarty S, Hardie DG. C-terminal phosphorylation of LKB1 is not required for regulation of AMP-activated protein kinase, BRSK1, BRSK2, or cell cycle arrest. J Biol Chem. (2009) 284:77–84. doi: 10.1074/jbc.M806152200
21. Tamir TY, Bowman BM, Agajanian MJ, Goldfarb D, Schrank TP, Stohrer T, et al. Gain-of-function genetic screen of the kinome reveals BRSK2 as an inhibitor of the NRF2 transcription factor. J Cell Sci. (2020) 133. doi: 10.1242/jcs.241356
22. Saiyin H, Na N, Han X, Fang Y, Wu Y, et al. BRSK2 induced by nutrient deprivation promotes Akt activity in pancreatic cancer via downregulation of mTOR activity. Oncotarget. (2017) 8:44669–81. doi: 10.18632/oncotarget.17965
23. Bright NJ, Carling D, Thornton C. Investigating the regulation of brain-specific kinases 1 and 2 by phosphorylation. J Biol Chem. (2008) 283:14946–54. doi: 10.1074/jbc.M710381200
24. Nie J, Han X, Shi Y. SAD-A and AMPK kinases: the “yin and yang” regulators of mTORC1 signaling in pancreatic beta cells. Cell Cycle. (2013) 12:3366–9. doi: 10.4161/cc.26496
25. Alvarado-Kristensson M, Rodriguez MJ, Silio V, Valpuesta JM, Carrera AC, SADB. phosphorylation of gamma-tubulin regulates centrosome duplication. Nat Cell Biol. (2009) 11:1081–92. doi: 10.1038/ncb1921
26. Mochida S, Hida Y, Tanifuji S, Hagiwara A, Hamada S, Abe M, et al. SAD-B Phosphorylation of CAST controls active zone vesicle recycling for synaptic depression. Cell Rep. (2016) 16:2901–13. doi: 10.1016/j.celrep.2016.08.020
Keywords: BRSK2, autism, de novo mutation, exome sequence, clinical phenotype
Citation: Hu Y, Li M, Shen Y, Wang T, Liu Q, Lu Z, Wang H, Luo X and Yang L (2023) Case report: A novel frameshift mutation in BRSK2 causes autism in a 16-year old Chinese boy. Front. Psychiatry 14:1205204. doi: 10.3389/fpsyt.2023.1205204
Received: 13 April 2023; Accepted: 26 June 2023;
Published: 21 August 2023.
Edited by:
Fengyu Zhang, Global Clinical and Translational Research Institute, United StatesReviewed by:
Aislinn Joanmarie Williams, The University of Iowa, United StatesJun Li, Peking University Sixth Hospital, Peking University, China
Copyright © 2023 Hu, Li, Shen, Wang, Liu, Lu, Wang, Luo and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixin Yang, lx.yang@siat.ac.cn; Xuerong Luo, luoxuerong@csu.edu.cn
†These authors have contributed equally to this work
 Yu Hu
Yu Hu