- 1Li Ka Shing Knowledge Institute, St. Michael’s Hospital, Toronto, ON, Canada
- 2Institute for Clinical Evaluative Sciences, Toronto, ON, Canada
- 3Department of Family and Community Medicine, University of Toronto, Toronto, ON, Canada
- 4Department of Family and Community Medicine, St. Michael’s Hospital, Toronto, ON, Canada
- 5Children’s Hospital of Eastern Ontario Research Institute, Ottawa, ON, Canada
- 6Department of Psychiatry, University of Ottawa, Ottawa, ON, Canada
- 7School of Epidemiology and Public Health, University of Ottawa, Ottawa, ON, Canada
- 8Azrieli Adult Neurodevelopmental Centre, Centre for Addiction and Mental Health, Toronto, ON, Canada
- 9Department of Psychiatry, University of Toronto, Toronto, ON, Canada
- 10Autism Research Centre, Bloorview Research Institute, Holland Bloorview Kids Rehabilitation Hospital, Toronto, ON, Canada
- 11Department of Pediatrics, University of Toronto, Toronto ON, Canada
- 12Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON, Canada
- 13Li Ka Shing Centre for Healthcare Analytics Research and Training, Unity Health Toronto, Toronto, ON, Canada
- 14Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 15Institute of Health Policy, Management, and Evaluation, University of Toronto, Toronto, ON, Canada
- 16Department of Medicine, University of Toronto, Toronto, ON, Canada
Background: In 2011, the Canadian Alliance for Monitoring Effectiveness and Safety of Antipsychotics in Children (CAMESA) published guidelines for the metabolic monitoring of antipsychotic-treated children and youth. Population-based studies examining adherence to these guidelines are needed to ensure the safe use of antipsychotics in children and youth.
Methods: We conducted a population-based study of all Ontario residents aged 0 to 24 who were newly dispensed an antipsychotic between April 1, 2018, and March 31, 2019. We estimated prevalence ratios (PRs) and 95% confidence intervals (CI) associating sociodemographic characteristics with the receipt of baseline and follow-up (3- and 6-month) laboratory testing using log-Poisson regression models.
Results: Overall, 6,505 of 27,718 (23.5%) children and youth newly dispensed an antipsychotic received at least one guideline-recommended baseline test. Monitoring was more prevalent among individuals aged 10 to 14 years (PR 1.20; 95% CI 1.04 to 1.38), 15 to 19 years (PR 1.60; 95% CI 1.41 to 1.82), and 20 to 24 years (PR 1.71; 95% CI 1.50 to 1.94) compared to children under the age of 10. Baseline monitoring was associated with mental health-related hospitalizations or emergency department visits in the year preceding therapy (PR 1.76; 95% CI 1.65 to 1.87), a prior diagnosis of schizophrenia (PR 1.20; 95% CI 1.14 to 1.26) or diabetes (PR 1.35; 95% CI 1.19 to 1.54), benzodiazepine use (PR 1.13; 95% CI 1.04 to 1.24), and receipt of a prescription from a child and adolescent psychiatrist or developmental pediatrician versus a family physician (PR 1.41; 95% CI 1.34 to 1.48). Conversely, monitoring was less frequent in individuals co-prescribed stimulants (PR 0.83; 95% CI 0.75 to 0.91). The prevalence of any 3- and 6-month follow-up monitoring among children and youth receiving continuous antipsychotic therapy at these time points was 13.0% (1,179 of 9,080) and 11.4% (597 of 5,261), respectively. Correlates of follow-up testing were similar to those of baseline monitoring.
Conclusion: Most children initiating antipsychotic therapy do not receive guideline-recommended metabolic laboratory monitoring. Further research is needed to understand reasons for poor guideline adherence and the role of clinician training and collaborative service models in promoting best monitoring practices.
Introduction
Children and youth receiving treatment with antipsychotic drugs are at risk of various metabolic disorders, including hyperglycemia, dyslipidemia, and hyperprolactinemia (1, 2). In addition to their association with new-onset type 2 diabetes and sudden death, adverse metabolic effects and metabolic syndrome during childhood also predict increased adult cardiovascular risk (3–8). Therefore, early detection and management of adverse metabolic effects are required for children and youth treated with antipsychotics.
Several practice guidelines have been published for the metabolic monitoring of antipsychotic-treated children and youth (9–14). Generally, the guidelines are in agreement with the need for baseline and follow-up monitoring, with some variation in recommended follow-up intervals at which testing should be performed and the specific tests recommended. In 2011, the Canadian Alliance for Monitoring Effectiveness and Safety of Antipsychotics in Children (CAMESA) published recommendations for monitoring antipsychotic adverse effects in children and youth (15). Generally, these guidelines recommend monitoring of specified anthropomorphic and metabolic parameters, including laboratory assessments of glucose, lipids, serum transaminases, and prolactin at baseline and following 3- and 6-months of treatment, with some recommendations varying according to the specific antipsychotic drug used (15). However, despite the increased use of these medications over time, several studies have found low adherence to CAMESA’s laboratory monitoring recommendations. Specifically, a retrospective chart review of 294 antipsychotic-treated children and adolescents found that baseline and follow-up laboratory monitoring occurred in less than 20% of patients (16). Low rates of adherence to CAMESA-recommended laboratory monitoring were also observed in a retrospective study of 345 antipsychotic-treated children and youth referred to a psychopharmacology clinic at a children’s specialty hospital (11). This study additionally found no appreciable change in monitoring following the publication of CAMESA guidelines, with 35 and 39% of patients receiving any laboratory monitoring in the periods preceding and following publication (17). Another chart audit of 180 children and youth found that baseline and follow-up laboratory monitoring occurred in less than 50% of patients 5 years following the publication of CAMESA guidelines (18). In the only Canadian population-based study, the prevalence of baseline and follow-up (3 months to 1 year) metabolic monitoring in 6916 Alberta children and youth was 17 and 35%, respectively (19). Furthermore, most children (58%) did not undergo guideline-recommended testing during follow-up, and laboratory monitoring was less prevalent in treatment naïve children and youth (19).
Yet, despite these findings, additional research examining adherence to recommended laboratory monitoring in antipsychotic-treated children and youth is needed, for several reasons. First, most Canadian research is based on chart audits of small numbers of children and youth (16–18). Moreover, the lone Canadian population-based study was conducted just 3 years following the publication of the CAMESA guidelines (19). Population-based studies evaluating guideline adherence over a longer period of follow are needed to understand whether best monitoring practices have been adopted into clinical practice. Second, few population-based studies have explored whether disparities exist in the receipt of guideline-recommended laboratory monitoring. This is important because such disparities can result in a higher frequency of adverse health outcomes in specific sub-populations of patients. In one United States study using Medicaid claims data, the strongest determinants of glucose testing were the presence of multiple mental health co-morbidities and being hospitalized or seen in an emergency department, while having two or more medical office visits and serious mental illness were associated with lipid testing (20). Similar findings have been observed in more recent studies, with serious mental illness and greater contact with the health care system being determinants of metabolic monitoring (16, 21, 22). The setting in which care is being delivered has also been examined as a determinant of metabolic monitoring, with one study finding low adherence to monitoring guidelines across child psychiatry, pediatric and family practice clinics (23). However, this study explored relatively few sociodemographic and clinical correlates of testing, limiting insight into which individuals are at greatest risk for guidelines not being followed. Specifically, it is unknown if guideline adherence varies according to socioeconomic status, prescriber type, the concomitant receipt of psychotropic medications that may increase the risk of adverse effects with antipsychotics (e.g., stimulants, benzodiazepines, antidepressants) or clinical conditions in which the use of antipsychotics is typically off-label, such as autism spectrum disorder and attention deficit/hyperactivity disorder (ADHD). Moreover, studies from the United States examining adherence to monitoring guidelines were limited to children and youth with specific forms of insurance, and therefore excluded large groups of individuals lacking insurance and who may be most risk of poor monitoring (20, 24, 25). Studies including all children and youth regardless of insurance status, are needed to better understand disparities in guideline-recommended monitoring and inform the development of strategies promoting adherence to best practices.
Between January 2018 and March 2019, all children and youth aged 24 and under in Ontario, Canada received prescription medication at no cost through a publicly-funded universal pharmacare program (26). Prescription drug data were therefore available for all children and youth during this period. Because all required laboratory testing is covered by the publicly-funded health care system and these data are also available for the entire population, we conducted a study exploring variation in the receipt of CAMESA-recommended laboratory monitoring among all antipsychotic-treated children and youth in Ontario. Our main objectives were to determine the prevalence of guideline-recommended laboratory testing at baseline and at 3 and 6 months following the commencement of treatment. We also sought to identify correlates of baseline and follow-up recommended monitoring.
Methods
Setting
We conducted a population-based cohort study of all Ontario residents aged 0 to 24 who were newly dispensed an antipsychotic between April 1, 2018, and March 31, 2019. We selected an upper age limit of 24 to align with the United Nations definition of youth as individuals between the ages of 15 and 24.1
Data sources
We used Ontario’s administrative health databases. These datasets were linked using unique encoded identifiers and analyzed at ICES (formerly known as the Institute for Clinical Evaluative Sciences). We identified claims for antipsychotics using the Ontario Drug Benefit database, which contains comprehensive records of all publicly-funded medications dispensed to Ontario residents. In addition, we identified prescriptions for stimulants and benzodiazepines using the Narcotics Monitoring System database, which contains comprehensive population records of all controlled-substances prescriptions dispensed from community pharmacies in Ontario, regardless of payer. We used the ICES Physician Database and Corporate Provider Database to determine prescriber specialty and the Registered Persons Database, a registry of all individuals eligible for the Ontario Health Insurance Plan (OHIP), to ascertain demographic characteristics for all children and youth dispensed antipsychotics over the study period. Specific demographic variables included age, sex, urban or rural residence, and neighborhood income quintile, derived by linking 2016 Census data to individual residential postal codes using the Postal Code Conversion File. To define patient comorbidity, we used the OHIP database to identify claims for physician services, and obtained diagnostic information from inpatient hospital admissions, emergency department visits and mental health-related hospitalizations using the Canadian Institute for Health Information’s Discharge Abstract Database, National Ambulatory Care Reporting System database and Ontario Mental Health Reporting System database, respectively. Specific mental health and neurodevelopmental conditions of interest included past diagnoses at any time prior to initiating an antipsychotic of schizophrenia or other psychotic disorders, ADHD, and autism spectrum disorder (see Supplementary Appendix for diagnostic codes). We also captured mental health hospitalizations and emergency department visits in the year preceding the initiation of antipsychotic therapy. We further categorized these encounters into diagnostic groups comprising substance use disorders, mood disorders, anxiety disorders, obsessive compulsive disorder, personality disorders and self-harm, based on the primary or main diagnosis (see Supplementary Appendix for codes). These definitions have been widely used for mental health system performance measurement in Canada (27–30). We identified individuals with pre-existing diabetes using the Ontario Diabetes Database, reasoning that these individuals may undergo more frequent laboratory monitoring for blood glucose.
To determine whether individuals prescribed antipsychotics received recommended laboratory monitoring, we used the OHIP database to identify claims for laboratory tests billed to the publicly funded health insurance plan and the Ontario Laboratory Information System (OLIS) database, an electronic repository containing information on provincial laboratory orders, patient demographics and provider information. OLIS includes data from hospital, commercial and public health laboratories, with 91% coverage of the province’s total annual laboratory testing as of 2016 (31). We excluded children and youth initiating antipsychotic treatment at the Hospital for Sick Children because this facility did not contribute data to OLIS during the study period. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
Study population and outcomes
We identified all individuals 0 to 24 years of age who were newly dispensed a prescription antipsychotic between April 1, 2018, and March 31, 2019, using the Ontario Drug Benefit database. Because the publicly-funded drug benefit program covers a maximum of 100 days’ supply of medication per prescription, we used a three-month look-back period to ascertain new use. We defined the prevalence of baseline laboratory monitoring as the number of individuals receiving at least one recommended laboratory test between 30 days before and 14 days after the first dispensing of a prescription antipsychotic divided by the total number of individuals newly dispensed an antipsychotic over the accrual period.
To examine the prevalence of follow-up testing 3 and 6 months after initiating antipsychotic therapy, we restricted our cohort to individuals newly dispensed a prescription antipsychotic between April 1, 2018, and September 30, 2018. We next defined a period of ongoing antipsychotic use as the dispensing of a prescription antipsychotic within 1.5 times the previous prescription days’ supply to allow a grace period between repeat prescriptions. This is a commonly used approach for preventing person-time on continuous therapy from being misclassified as unexposed in cases where individuals do not fill their prescription at precise intervals defined by the amount of medication dispensed or if the calculated or pharmacist-recorded days’ supply is too short (32).We censored individuals at the time of treatment discontinuation (no refills within 1.5 times the previous prescription days’ supply), turning 25 years old, or the end of follow-up (March 31, 2019), whichever occurred first. We did not censor individuals who switched between antipsychotics. We determined the prevalence of guideline-recommended testing at approximately 3 and 6 months after antipsychotic therapy initiation, defined as the number of individuals receiving at least one guideline-recommended laboratory test 60 to 120 days and 150 to 200 days following the first dispensing of an antipsychotic divided by the number of individuals still receiving ongoing treatment in those follow-up windows. The specific laboratory tests examined in all analyzes were glucose, serum transaminases, prolactin, total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, triglycerides, and other lipids.
Statistical analysis
We summarized baseline characteristics of children and youth initiating antipsychotics using counts and percentages for categorical variables and medians with interquartile ranges (IQR) for continuous variables. We determined the prevalence of baseline and follow-up testing for the overall sample and according to baseline demographic variables (i.e., sex, age-category, urban or rural residence, neighborhood income quintile), clinical and health system variables (i.e., comorbidity status, mental health-related hospital admissions or emergency department visits in the year preceding antipsychotic initiation, receipt of benzodiazepines, stimulants or antidepressants), and antipsychotic prescriber specialty (i.e., family physician, pediatrician, child and adolescent psychiatrist or developmental pediatrician, other). We ascertained individual comorbidity in the prior 2 years using the Johns Hopkins ACG® System Version 10 case-mix assignment software (33). We specifically used ACG® System Aggregated Diagnosis Groups (ADGs), which are clusters of diagnostic codes with similar severity and expected persistence. The count of ADGs ranges from 0 to 32, with higher values reflecting more comorbidity. We categorized comorbidity status as 0 to 5, 6 to 9 and ≥ 10 ADGs.
We used multivariable log-Poisson regression models with robust variance estimators to quantify the associations between demographic, clinical and health systems variables with the receipt of baseline and follow-up laboratory monitoring (34, 35). We expressed results as prevalence ratios (PRs) and 95% confidence intervals (CI).
Results
We identified 27,718 children and youth who were newly dispensed an antipsychotic medication between April 1, 2018, and March 31, 2019. Most (n = 27,222; 98.2%) received a second-generation antipsychotic. The median duration of continuous use was 7.6 weeks (interquartile range 4.3 to 18.1 weeks). A slight majority (n = 14,186; 51.2%) were female, and the median age was 19 years (IQR 15 to 21), with 9.4% being younger than 10 (n = 2,591). About one-third of the cohort (n = 9,382; 33.9%) had a mental health-related hospitalization or emergency department visit in the year before receiving an antipsychotic (Table 1). In addition, 3,827 (13.8%), 7,498 (27.1%) and 2,887 (10.4%) individuals had healthcare encounters for schizophrenia, ADHD and autism spectrum disorder, respectively, at any time before receiving an antipsychotic. Overall, 7,363 (27.6%) underwent laboratory testing at least once during the baseline, 3- and 6-month follow-up periods, with 64 (0.23%) individuals being monitored at all three time points.
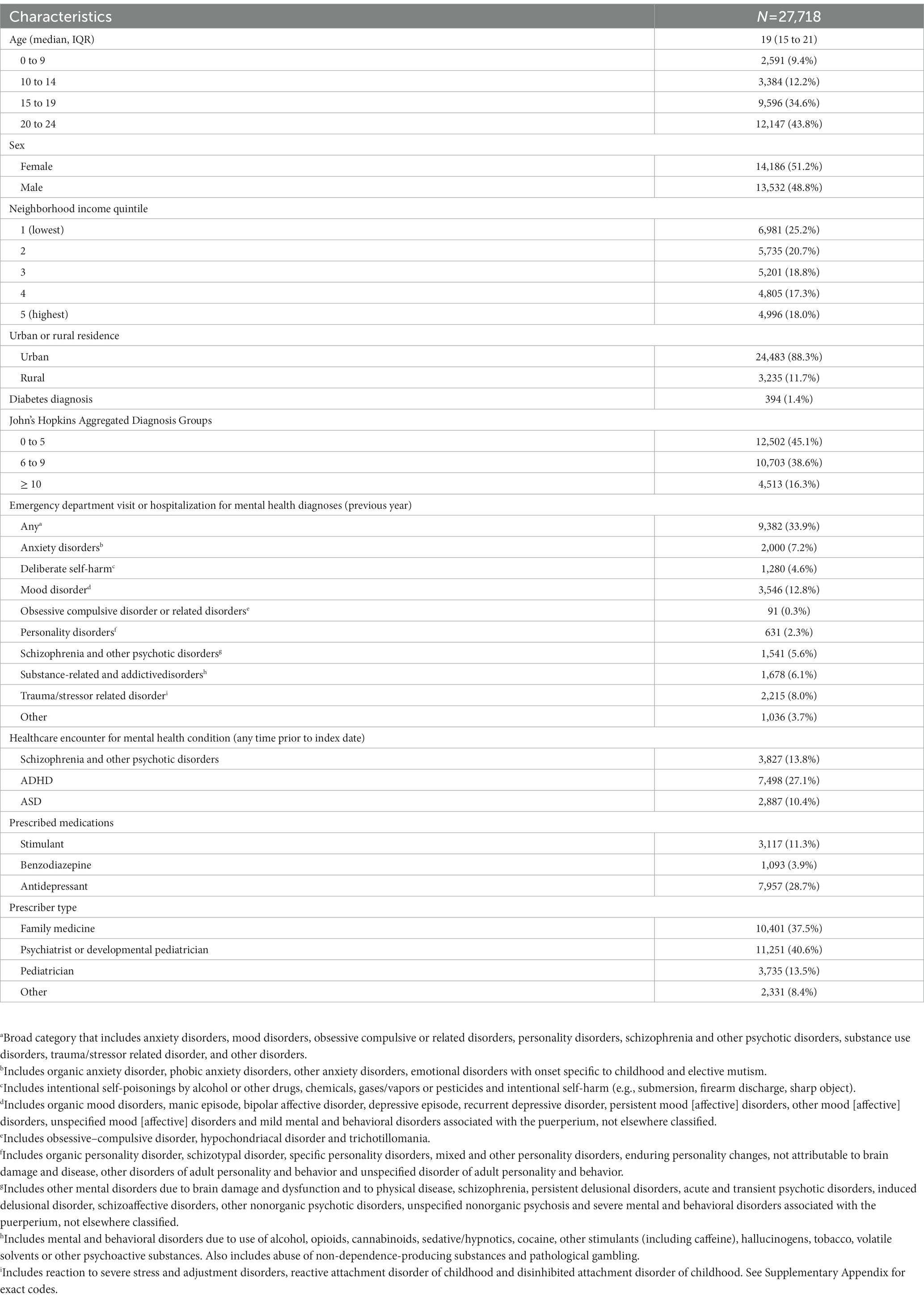
Table 1. Characteristics of children and youth newly dispensed an antipsychotic between April 1, 2018, and March 31, 2019.
Baseline monitoring
Overall, 6,505 (23.5%) antipsychotic-treated children and youth received at least one guideline-recommended baseline test. Monitoring was more prevalent in children treated with first-generation antipsychotics (211/496; 42.5%) than second-generation antipsychotics (6,294/27,222; 23.1%). Blood glucose and serum transaminase testing were most common, received by 5,870 (21.2%) and 4,706 (17.0%) of children and youth, with lipid (n = 2,180; 7.9%) and prolactin (n = 720; 2.6%) testing undertaken less frequently. Following multivariable adjustment, baseline monitoring was more prevalent among those aged 10 to 14 years (PR 1.20; 95% CI 1.04 to 1.38), 15 to 19 years (PR 1.60; 95% CI 1.41 to 1.82) and 20 to 24 years (PR 1.71; 95% CI 1.50 to 1.94) relative to individuals younger than 10 (Table 2). The frequency of baseline monitoring was slightly greater in the highest-income relative to the lowest-income neighborhoods (25.1% vs. 23.4%; PR 1.09; 95% CI 1.02 to 1.16). In addition, the prevalence of monitoring was higher in children and youth with any mental health-related hospitalizations or emergency department visits in the year prior to initiating therapy (37.4% vs. 16.3%; PR 1.76; 95% CI 1.65 to 1.87), those with a past diagnosis of schizophrenia or psychotic disorder (37.6% vs. 21.2%; PR 1.20; 95% CI 1.14 to 1.26), individuals with pre-existing diabetes (36.3% vs. 23.3%; PR 1.35; 95% CI 1.19 to 1.54) and individuals who were co-prescribed benzodiazepines (32.0% vs. 23.1%; PR 1.13; 95% CI 1.04 to 1.24). Baseline monitoring was also more frequent among children and youth whose treatment was initiated by a child and adolescent psychiatrist or developmental pediatrician relative to a family physician (31.4% vs. 18.2%; PR 1.41, 95% CI 1.34 to 1.48). Baseline monitoring was also more prevalent among those with 6 to 9 ADGs (PR 1.41; 95% CI 1.34 to 1.48) relative to those with 5 or fewer ADGs. Conversely, monitoring was less common in individuals with a diagnosis of ADHD (19.4% vs. 25.0%; PR 0.86; 95% CI 0.81 to 0.90) and autism spectrum disorder (16.3% vs. 24.3%; PR 0.89, 95% CI 0.82 to 0.97), as well as those co-prescribed stimulants (13.7% vs. 24.7%; PR 0.83; 95% CI 0.75 to 0.91) or antidepressants (22.8% vs. 23.7%; PR 0.89; 95% CI 0.85 to 0.93). Although baseline monitoring was more frequent among females relative to males (24.6% vs. 22.3%), females were less likely to undergo baseline monitoring following multivariable analysis (PR 0.95; 95% CI 0.91 to 0.99) (Table 2).
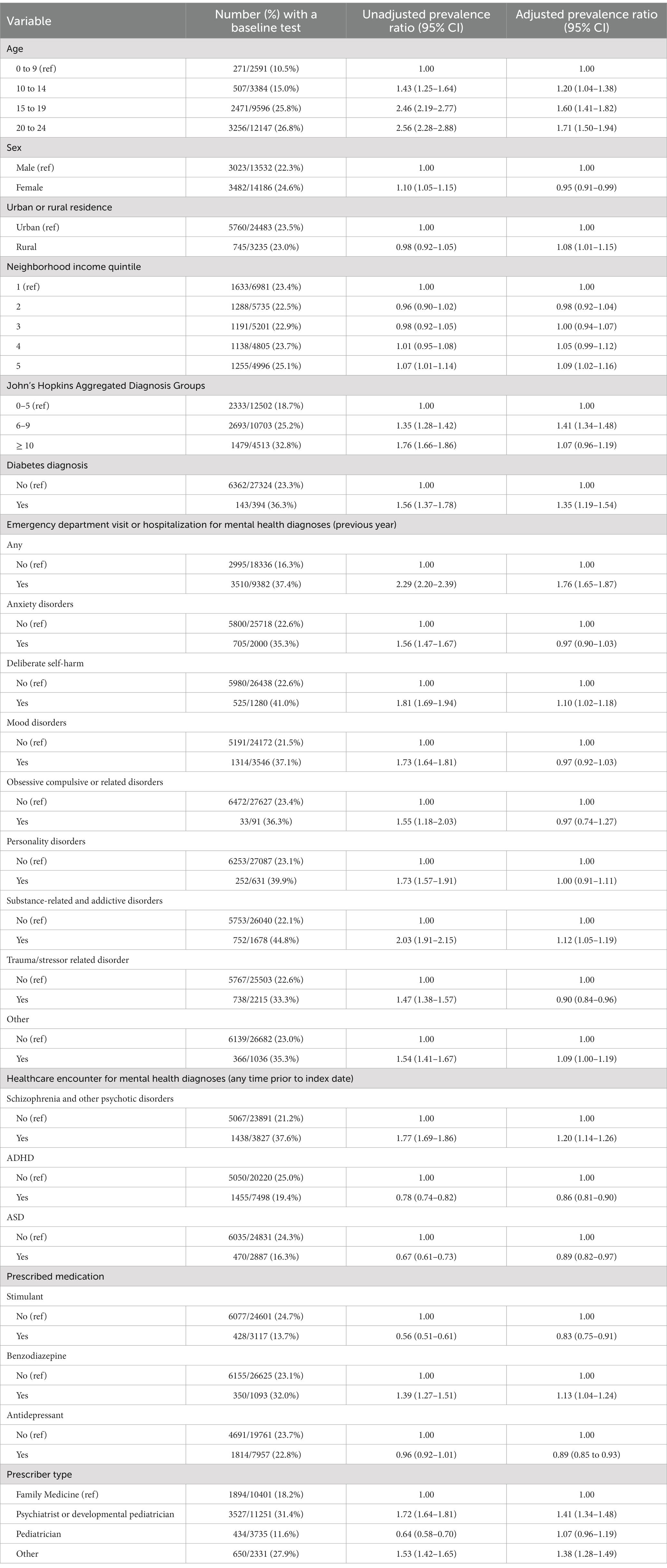
Table 2. Prevalence ratios for receipt of any baseline monitoring test among children and youth newly dispensed an antipsychotic between April 1, 2018 and March 31, 2019.
Follow-up monitoring
The prevalence of any follow-up laboratory testing at 3 months and 6 months among children and youth receiving continuous antipsychotic therapy at these time points was 13.0% (1,179 of 9,080) and 11.4% (597 of 5,261), respectively. Follow-up monitoring was more frequent in individuals receiving first generation antipsychotics, with 24.8% (28 of 113) and 21.5% (14 of 65) children and youth receiving continuous treatment at 3- and 6-months receiving any follow-up monitoring. Respective figures for children and youth receiving second generation antipsychotics were 12.8% (1,151 of 8,967) and 11.2% (583 of 5,196). Similar to baseline testing, the receipt of any 3- or 6-month follow-up testing was higher among older children and youth, those with prior diagnoses of schizophrenia or diabetes, those with a higher ADG comorbidity score, individuals co-prescribed benzodiazepines, and those with prescriptions written by a child and adolescent psychiatrist or developmental pediatrician (Table 3). Females were more likely than males to undergo 3-month (16.1% vs. 10.1%; PR 1.26, 95% CI 1.11 to 1.42) and 6-month follow-up testing (14.1% vs. 9.1%; PR 1.27, 95% CI 1.07 to 1.51).
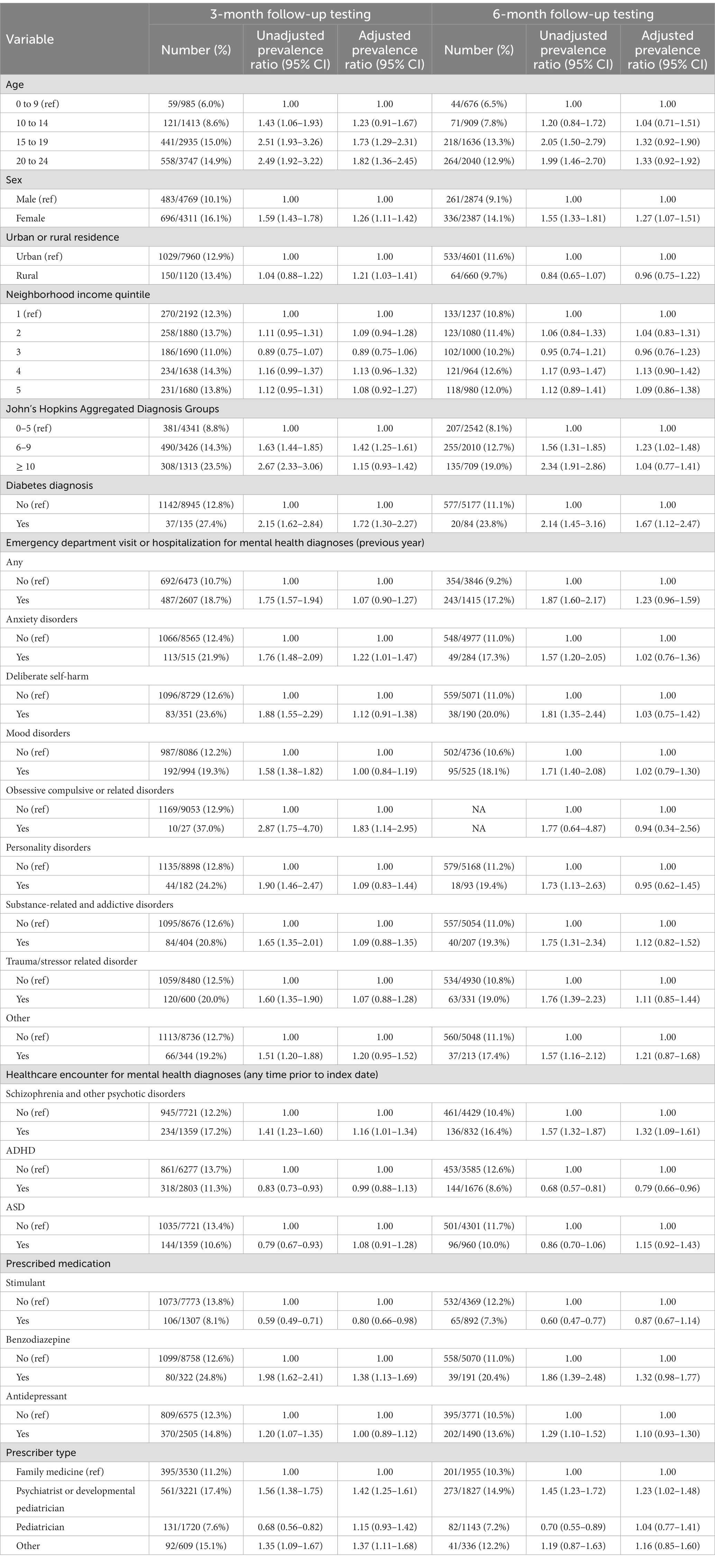
Table 3. Prevalence ratios for receipt of any 3- and 6-month laboratory monitoring among children and youth newly dispensed an antipsychotic between April 1, 2018, and March 31, 2019.
Discussion
In our population-based study of children and youth initiating antipsychotic therapy, we found that fewer than one in four children and youth initiating antipsychotic therapy received any baseline laboratory monitoring. Follow-up monitoring was also infrequent, with only 13.0 and 11.4% of individuals receiving 3- and 6-month testing, respectively. Baseline and follow-up monitoring were more common among older recipients, those co-prescribed benzodiazepines, those with a past diagnosis of diabetes or schizophrenia and other psychotic disorders, individuals with a mental-health related hospitalization or emergency department visit in the year prior to commencing therapy, and those prescribed antipsychotics by a child and adolescent psychiatrist or developmental pediatrician. However, monitoring was infrequent even in these patients. Overall, our findings suggest that best laboratory monitoring practices for children and youth receiving antipsychotics are occurring infrequently 7 years following the publication of the CAMESA guidelines.
Our study has important implications for clinical practice. The potential for iatrogenic harm with antipsychotics in children and youth is well documented, with metabolic abnormalities such as dyslipidemia, hyperglycemia and obesity occurring early during treatment and imparting a 2- to 3-fold increase in risk for type 2 diabetes relative to children and youth who are not treated with these drugs (1–4). This risk increases with higher cumulative doses, longer treatment duration, and adjunctive antidepressant use, and remains elevated after discontinuation (3, 4, 36). Moreover, adverse metabolic effects developing in childhood may increase the risk of early and long-term cardiovascular disease (6–8). In light of the potential for serious adverse effects and the low prevalence of monitoring in all children and youth, tailored interventions are needed to promote best monitoring practices when antipsychotics are prescribed. This assertion is supported by past studies from Canada and the United States finding small changes in monitoring before and after the publication of clinical guidelines (17, 37, 38). Furthermore, changes in monitoring practices observed in studies of quality improvement educational initiatives targeting prescribers have been modest, with a systematic review of six studies finding median postintervention glucose, lipid, and waist circumference screening rates of 39, 37 and 16%, respectively (39). These findings suggest that the publication of guidelines and interventions targeting clinician knowledge and practice enhancements are insufficient for improving metabolic screening rates in antipsychotic-treated children and youth.
Our data do not allow us to conclusively determine whether low guideline adherence reflects a lack of awareness among clinicians and families for the need for regular monitoring or challenges obtaining blood work at regular intervals among families overburdened by multiple appointments and competing needs for children and youth with mental health and neurodevelopmental conditions (40–43).
Patient comfort and education regarding the need for regular blood work may represent possible individual-level barriers to adopting metabolic monitoring recommendations in routine clinical practice. This is consistent with our findings that past diagnoses of diabetes and schizophrenia and prior mental health-related hospitalizations and emergency department visits were associated with a higher prevalence of baseline and follow-up monitoring, as these individuals may be more comfortable with or aware of the need for routine monitoring involving routine blood work. Our finding that laboratory monitoring was associated with increasing age may also reflect greater comfort with regular blood work in adolescents and youth, as past research has found higher levels of distress with venipuncture among younger children relative to adolescents (44). Similarly, anxiety related to medical procedures among children with neurodevelopmental disorders such as ADHD and autism spectrum disorder may explain the lower monitoring prevalence in these children and youth (45, 46). Toolkits developed to support obtaining blood work from children and youth with neurodevelopmental disorders may help facilitate routine metabolic monitoring when antipsychotics are prescribed for children and youth with neurodevelopmental disorders (47). Similarly, patient-centered programs offering flexible laboratory appointment times, blood work conducted in the patient’s home rather than an office setting, and additional support to promote patient comfort may help overcome anxiety and fear associated with specimen collection, although research is required to ascertain their effectiveness in promoting regular monitoring (46, 48). The higher prevalence of baseline and follow-up monitoring among individuals receiving benzodiazepines may reflect the short-term use of these drugs for procedural anxiolysis. Although this practice has been found to be generally well tolerated, studies of prophylactic benzodiazepines in children have generally focused on operative and diagnostic procedures, and the efficacy and safety for reducing anxiety related to blood work is unknown (49). Our finding of a slightly higher prevalence of baseline monitoring in children and youth living in the highest relative to the lowest income neighborhoods suggests that structural interventions addressing barriers such as the costs of attending separate medical and laboratory appointments and time away from work may be needed to support low-income families in obtaining recommended monitoring.
Provider-level barriers may also contribute to low rates of recommended monitoring, with non-specialist physicians being reluctant to undertake metabolic monitoring without access to expert guidance on the management of abnormalities. This is consistent with our finding of a higher screening prevalence among children and youth seen by child and adolescent psychiatrists and developmental pediatricians compared to primary care providers and past research showing a general awareness of and agreement with the need for metabolic monitoring among psychiatrists (50–52). A similar finding has been observed in a United States study, in that the odds of receiving metabolic monitoring were 42% higher in children and youth receiving shared care including a mental health provider relative to treatment from a primary care provider alone (53). Shared care models, including telemedical health programs connecting primary care providers to pediatric mental health specialists, represent a promising mechanism for optimizing antipsychotic prescribing and monitoring in children and youth (54, 55). Training clinicians to provide specialist-level care to children and youth with mental health and neurodevelopmental conditions is another approach for promoting best practices in antipsychotic laboratory monitoring of children and youth. This approach has been implemented in Ontario through Project Extension of Community Health Outcomes (ECHO) (56, 57). Project ECHO Ontario uses a continuing professional education model to train health care providers throughout Ontario to provide specialist-level care to children and youth with mental health and neurodevelopmental conditions. However, whether such initiatives can improve adherence to metabolic monitoring in antipsychotic-treated children and youth remains unknown, with further evaluation needed. In addition, further research is needed to investigate the role of health system- and organizational-level characteristics (e.g., presence of laboratory facilities onsite) and interventions addressing such barriers on adherence to monitoring guidelines.
Our study has some limitations. First, we could not ascertain the prevalence of monitoring for other guideline-recommended parameters, such as blood pressure, body mass index and waist circumference. Past research suggests that adherence to these monitoring parameters is higher than that for those requiring routine blood work (51, 52). It is therefore possible that clinicians may be undertaking metabolic monitoring only in those children demonstrating changes in anthropomorphic measures. However, even if this were the case, the prevalence of metabolic monitoring remains inadequate given that most antipsychotic-treated children and youth experience weight gain or obesity within 12 weeks of initiating treatment (2). Second, we could not determine the indication for antipsychotic use. Prior Canadian research indicates that these drugs are generally used off-label for children and youth with ADHD, conduct disorders and mood disorders (19, 58). Third, diagnostic categories of mental health and neurodevelopmental conditions were derived using administrative algorithms published in earlier studies and reports, and were not validated for use in this study (27, 59). Fourth, we could not account for the dose of antipsychotic in our analyzes. Fifth, we could not ascertain whether glucose and lipid testing were performed under fasting conditions. Finally, our study was conducted in a single Canadian province in the year when universal funding of medications for all children and youth was introduced, potentially limiting the generalizability of our findings. However, our study includes all children and youth newly initiating antipsychotic drugs in a setting of publicly funded healthcare and pharmacare. Our findings suggest that barriers unrelated to health insurance status continue to undermine the uptake of guideline-recommended laboratory monitoring in antipsychotic-treated children and youth and may be transferable to similar settings where prescription drugs and routine healthcare are provided at no cost to children and youth.
In conclusion, most children and youth initiating antipsychotic therapy do not undergo guideline-recommended baseline and follow-up metabolic monitoring. Further research clarifying reasons for poor guideline uptake is needed to inform the development and evaluation of interventions that optimize laboratory metabolic monitoring in children and youth treated with antipsychotics. In addition, research examining the role of alternative healthcare delivery models, such as telehealth and programs training primary care physicians to provide specialist care, prophylactic procedural anxiolysis and toolkits to assist with blood draws is warranted to determine whether such interventions can improve rates of metabolic monitoring.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The datasets generated and/or analyzed during the current study are not publicly available due to data sharing agreements which prohibit ICES from making the data set publicly available. The data set from this study is held securely in coded form at ICES, and access may be granted to those who meet pre-specified criteria for confidential access, available at https://www.ices.on.ca/DAS,understanding that the programs may rely upon coding templates or macros that are unique to ICES. Requests to access these datasets should be directed to https://www.ices.on.ca/DAS.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the legislation and the institutional requirements.
Author contributions
TA, TW, TG, MP, MT, KP, WG, MM, YL, and DJ conceptualized and designed the study, and were involved in the interpretation of the data. TW conducted the analyzes. TA drafted the initial manuscript. All authors critically reviewed the manuscript for intellectual content, revised the manuscript, and approved the final manuscript as submitted and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This study was funded by the Canadian Institutes of Health Research (funding reference number 166149).
Acknowledgments
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). Parts of this material are based on data and/or information compiled and provided by the Canadian Institute of Health Information (CIHI) and the Ontario Ministry of Health. The analyzes, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from ©Canada Post Corporation and Statistics Canada. We thank IQVIA Solutions Canada Inc. for use of their Drug Information File. Tara Gomes holds a Canada Research Chair in Drug Policy Research and Evaluation.
Conflict of interest
MP has received consulting fees for unrelated work from Addis & Associates/Roche and the Government of Nova Scotia. MT has received consulting fees for unrelated work from Green Shield Canada and the Canadian Agency for Drugs and Technologies in Health. TG has received funding from the Ontario MOH and the Canadian Agency for Drugs and Technologies in Health for unrelated work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1172559/full#supplementary-material
Footnotes
References
1. Cohen, D, Bonnot, O, Bodeau, N, Consoli, A, and Laurent, C. Adverse effects of second-generation antipsychotics in children and adolescents: a Bayesian meta-analysis. J Clin Psychopharmacol. (2012) 32:309–16. doi: 10.1097/JCP.0b013e3182549259
2. Correll, CU, Manu, P, Olshanskiy, V, Napolitano, B, Kane, JM, and Malhotra, AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. (2009) 302:1765–73. doi: 10.1001/jama.2009.1549
3. Bobo, WV, Cooper, WO, Stein, CM, Olfson, M, Graham, D, Daugherty, J, et al. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiat. (2013) 70:1067–75. doi: 10.1001/jamapsychiatry.2013.2053
4. Rubin, DM, Kreider, AR, Matone, M, Huang, YS, Feudtner, C, Ross, ME, et al. Risk for incident diabetes mellitus following initiation of second-generation antipsychotics among Medicaid-enrolled youths. JAMA Pediatr. (2015) 169:e150285. doi: 10.1001/jamapediatrics.2015.0285
5. Ray, WA, Stein, CM, Murray, KT, Fuchs, DC, Patrick, SW, Daugherty, J, et al. Association of Antipsychotic Treatment with Risk of unexpected death among children and youths. JAMA Psychiat. (2019) 76:162–71. doi: 10.1001/jamapsychiatry.2018.3421
6. Koskinen, J, Magnussen, CG, Sinaiko, A, Woo, J, Urbina, E, Jacobs, DR Jr, et al. Childhood age and associations between childhood metabolic syndrome and adult risk for metabolic syndrome, type 2 diabetes mellitus and carotid intima media thickness: the international childhood cardiovascular cohort consortium. J Am Heart Assoc. (2017) 6:e005632. doi: 10.1161/JAHA.117.005632
7. Magnussen, CG, Koskinen, J, Chen, W, Thomson, R, Schmidt, MD, Srinivasan, SR, et al. Pediatric metabolic syndrome predicts adulthood metabolic syndrome, subclinical atherosclerosis, and type 2 diabetes mellitus but is no better than body mass index alone: the Bogalusa heart study and the cardiovascular risk in young Finns study. Circulation. (2010) 122:1604–11. doi: 10.1161/CIRCULATIONAHA.110.940809
8. Turer, CB, Brady, TM, and de Ferranti, SD. Obesity, hypertension, and dyslipidemia in childhood are key modifiable antecedents of adult cardiovascular disease: a call to action. Circulation. (2018) 137:1256–9. doi: 10.1161/CIRCULATIONAHA.118.032531
9. American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of atypical antipsychotic medications in children and adolescents. (2023). Available at: https://www.aacap.org/App_Themes/AACAP/docs/practice_parameters/Atypical_antipsychotic_Medications_Web.pdf. (Accessed April 17, 2023).
10. McClellan, J, Kowatch, R, and Findling, RL, Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. (2007) 46:107–25. doi: 10.1097/01.chi.0000242240.69678.c4
11. McClellan, J, and Stock, S. American Academy of Child and Adolescent Psychiatry (AACAP) committee on quality issues (CQI). Practice parameter for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. (2013) 52:976–90. doi: 10.1016/j.jaac.2013.02.008
12. Gleason, MM, Egger, HL, Emslie, GJ, Greenhill, LL, Kowatch, RA, Lieberman, AF, et al. Psychopharmacological treatment for very young children: contexts and guidelines. J Am Acad Child Adolesc Psychiatry. (2007) 46:1532–72. doi: 10.1097/chi.0b013e3181570d9e
13. Scotto Rosato, N, Correll, CU, Pappadopulos, E, Chait, A, Crystal, S, and Jensen, PS. Treatment of maladaptive aggressive in youth steering committee. Treatment of maladaptive aggression in youth: CERT guidelines II. Treatments and ongoing management. Pediatrics. (2012) 129:e1577–86. doi: 10.1542/peds.2010-1361
14. Steiner, H, and Remsing, L, Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. (2007) 46:126–41. doi: 10.1097/01.chi.0000246060.62706.af
15. Pringsheim, T, Panagiotopoulos, C, Davidson, J, and Ho, J. Canadian Alliance for monitoring effectiveness and safety of antipsychotics in children (CAMESA) guideline group. Evidence-based recommendations for monitoring safety of second-generation antipsychotics in children and youth. Paediatr Child Health. (2011) 16:581–9. doi: 10.1093/pch/16.9.581
16. Coughlin, M, Goldie, CL, Tranmer, J, Khalid-Khan, S, and Tregunno, D. Patient, treatment, and health care utilization variables associated with adherence to metabolic monitoring practices in children and adolescents taking second-generation antipsychotics. Can J Psychiatr. (2018) 63:240–9. doi: 10.1177/0706743717751693
17. Kara, I, and Penner, M. Impact of antipsychotic guidelines on laboratory monitoring in children with neurodevelopmental disorders. J Child Adolesc Psychopharmacol. (2021) 31:79–83. doi: 10.1089/cap.2020.0096
18. Jazi, S, Ben-Amor, L, Abadie, P, Menard, ML, Choquette, R, Berthiaume, C, et al. Long-term metabolic monitoring of youths treated with second-generation antipsychotics 5 years after publication of the CAMESA guidelines are we making Progress? Surveillance Métabolique à long Terme des Jeunes Traités par Antipsychotiques de Deuxième Génération, Cinq ans Après la publication des Lignes Directrices Camesa: Faisons-nous des Progrès? Can J Psychiatr. (2021) 66:645–56. doi: 10.1177/0706743720974847
19. Chen, W, Cepoiu-Martin, M, Stang, A, Duncan, D, Symonds, C, Cooke, L, et al. Antipsychotic prescribing and safety monitoring practices in children and youth: a population-based study in Alberta, Canada. Clin Drug Investig. (2018) 38:449–55. doi: 10.1007/s40261-018-0626-4
20. Morrato, EH, Nicol, GE, Maahs, D, Druss, BG, Hartung, DM, Valuck, RJ, et al. Metabolic screening in children receiving antipsychotic drug treatment. Arch Pediatr Adolesc Med. (2010) 164:344–51. doi: 10.1001/archpediatrics.2010.48
21. Raebel, MA, Penfold, R, McMahon, AW, Reichman, M, Shetterly, S, Goodrich, G, et al. Adherence to guidelines for glucose assessment in starting second-generation antipsychotics. Pediatrics. (2014) 134:e1308–14. doi: 10.1542/peds.2014-0828
22. Hsueh, L, Iturralde, E, Slama, NE, Spalding, SR, and Sterling, SA. Cardiometabolic monitoring and sociodemographic and clinical characteristics of youths prescribed antipsychotic medications. Psychiatr Serv. (2023):appips20220151. doi: 10.1176/appi.ps.20220151
23. Wakefield, S, Aligeti, M, Rachamallu, V, Baronia, R, Aynampudi, R, Parmar, A, et al. Metabolic monitoring of child and adolescent patients on atypical antipsychotics by psychiatrists and primary care providers. Am J Ther. (2020) 27:e425–30. doi: 10.1097/MJT.0000000000000853
24. Haupt, DW, Rosenblatt, LC, Kim, E, Baker, RA, Whitehead, R, and Newcomer, JW. Prevalence and predictors of lipid and glucose monitoring in commercially insured patients treated with second-generation antipsychotic agents. Am J Psychiatry. (2009) 166:345–53. doi: 10.1176/appi.ajp.2008.08030383
25. Delate, T, Kauffman, YS, Botts, SR, Wong, C, and Gaughan, KM. Metabolic monitoring in commercially insured pediatric patients newly initiated to take a second-generation antipsychotic. JAMA Pediatr. (2014) 168:679–81. doi: 10.1001/jamapediatrics.2014.224
26. Ministry of Health and Long-Term Care. OHIP+: Children and youth Pharmacare key facts for prescribers. (2023). Available at: https://www.afhto.ca/wp-content/uploads/ohip_prescriber-002.pdf. (Accessed February 20, 2023).
27. The Mental Health and Addictions Scorecard and Evaluation Framework Research Team. The mental health of children and youth in Ontario: 2017 scorecard. Toronto, ON: Institute for Clinical Evaluative Sciences (2017).
28. Chiu, M, Guttmann, A, and Kurdyak, P. Mental health and addictions system performance in Ontario: an updated scorecard, 2009-2017. Healthc Q. (2020) 23:7–11. doi: 10.12927/hcq.2020.26340
29. Chiu, M, Gatov, E, Vigod, SN, Amartey, A, Saunders, NR, Yao, Z, et al. Temporal trends in mental health service utilization across outpatient and acute care sectors: a population-based study from 2006 to 2014. Can J Psychiatr. (2018) 63:94–102. doi: 10.1177/0706743717748926
30. Saunders, NR, Toulany, A, Deb, B, Strauss, R, Vigod, SN, Guttmann, A, et al. Acute mental health service use following onset of the COVID-19 pandemic in Ontario, Canada: a trend analysis. CMAJ Open. (2021) 9:E988–97. doi: 10.9778/cmajo.20210100
31. McArthur, E, Bota, SE, Sood, MM, Nesrallah, GE, Kim, SJ, Garg, AX, et al. Comparing five comorbidity indices to predict mortality in chronic kidney disease: a retrospective cohort study. Can J Kidney Health Dis. (2018) 5:205435811880541. doi: 10.1177/2054358118805418
32. Schneeweiss, S, and Avorn, J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. (2005) 58:323–37. doi: 10.1016/j.jclinepi.2004.10.012
33. The Johns Hopkins. ACG system. Baltimore: Johns Hopkins University. (2023). Available at: www.hopkinsacg.org. (Accessed January 11, 2023).
34. Zou, G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. (2004) 159:702–6. doi: 10.1093/aje/kwh090
35. Petersen, MR, and Deddens, JA. A comparison of two methods for estimating prevalence ratios. BMC Med Res Methodol. (2008) 8:9. doi: 10.1186/1471-2288-8-9
36. Pisano, S, Catone, G, Veltri, S, Lanzara, V, Pozzi, M, Clementi, E, et al. Update on the safety of second generation antipsychotics in youths: a call for collaboration among paediatricians and child psychiatrists. Ital J Pediatr. (2016) 42:51. doi: 10.1186/s13052-016-0259-2
37. Connolly, JG, Toomey, TJ, and Schneeweiss, MC. Metabolic monitoring for youths initiating use of second-generation antipsychotics, 2003-2011. Psychiatr Serv. (2015) 66:604–9. doi: 10.1176/appi.ps.201400222
38. Cotes, RO, Fernandes, NK, McLaren, JL, McHugo, GJ, Bartels, SJ, and Brunette, MF. Improving Cardiometabolic monitoring of children on antipsychotics. J Child Adolesc Psychopharmacol. (2017) 27:916–9. doi: 10.1089/cap.2017.0034
39. Melamed, OC, LaChance, LR, O'Neill, BG, Rodak, T, and Taylor, VH. Interventions to improve metabolic risk screening among children and adolescents on antipsychotic medication: a systematic review. J Child Adolesc Psychopharmacol. (2021) 31:63–72. doi: 10.1089/cap.2020.0115
40. Houtrow, AJ, and Okumura, MJ. Pediatric mental health problems and associated burden on families. Vulnerable Child Youth Stud. (2011) 6:222–33. doi: 10.1080/17450128.2011.580144
41. Busch, SH, and Barry, CL. Mental health disorders in childhood: assessing the burden on families. Health Aff (Millwood). (2007) 26:1088–95. doi: 10.1377/hlthaff.26.4.1088
42. Turan Gürhopur, FD, and Dalgıç, Aİ. Family burden among parents of children with intellectual disability. J Psychiatr Nurs. (2017) 8:9–16. doi: 10.14744/phd.2017.87609
43. Zhao, X, Page, TF, Altszuler, AR, Pelham, WE, Kipp, H, Gnagy, EM, et al. Family burden of raising a child with ADHD. J Abnorm Child Psychol. (2019) 47:1327–38. doi: 10.1007/s10802-019-00518-5
44. Humphrey, GB, Boon, CM, van Linden van den Heuvell, GF, and van de Wiel, HB. The occurrence of high levels of acute behavioral distress in children and adolescents undergoing routine venipunctures. Pediatrics. (1992) 90:87–91. doi: 10.1542/peds.90.1.87
45. Evans, DW, Canavera, K, Kleinpeter, FL, Maccubbin, E, and Taga, K. The fears, phobias and anxieties of children with autism spectrum disorders and down syndrome: comparisons with developmentally and chronologically age matched children. Child Psychiatry Hum Dev. (2005) 36:3–26. doi: 10.1007/s10578-004-3619-x
46. Davit, CJ, Hundley, RJ, Bacic, JD, and Hanson, EM. A pilot study to improve venipuncture compliance in children and adolescents with autism spectrum disorders. J Dev Behav Pediatr. (2011) 32:521–5. doi: 10.1097/DBP.0b013e3182245b09
47. Autism Speaks. ATN/AIR-P Parent's guide to blood draws. (2023). Available at: https://www.autismspeaks.org/tool-kit/atnair-p-parents-guide-blood-draws. (Accessed February 11, 2023).
48. Lifelabs. Serving patients with autism. (2023) Available at: https://www.lifelabs.com/patients/patient-centred-care/serving-patients-with-autism/. (Accessed February 11, 2023).
49. Kuang, H, Johnson, JA, Mulqueen, JM, and Bloch, MH. The efficacy of benzodiazepines as acute anxiolytics in children: a meta-analysis. Depress Anxiety. (2017) 34:888–96. doi: 10.1002/da.22643
50. Rodday, AM, Parsons, SK, Mankiw, C, Correll, CU, Robb, AS, Zima, BT, et al. Child and adolescent psychiatrists' reported monitoring behaviors for second-generation antipsychotics. J Child Adolesc Psychopharmacol. (2015) 25:351–61. doi: 10.1089/cap.2014.0156
51. McLaren, JL, Brunette, MF, McHugo, GJ, Drake, RE, and Daviss, WB. Monitoring of patients on second-generation antipsychotics: a National Survey of child psychiatrists. Psychiatr Serv. (2017) 68:958–61. doi: 10.1176/appi.ps.201500553
52. Walter, G, Delaroche, A, Soh, N, Hunt, G, Cleary, M, Malhi, G, et al. Side effects of second-generation antipsychotics: the experiences, views and monitoring practices of Australian child psychiatrists. Australas Psychiatry. (2008) 16:253–62. doi: 10.1080/10398560801958549
53. Shenkman, E, Thompson, L, Bussing, R, Forrest, CB, Woodard, J, Sun, Y, et al. Provider specialty and receipt of metabolic monitoring for children taking antipsychotics. Pediatrics. (2021) 147:e20200658. doi: 10.1542/peds.2020-0658
54. Thackeray, J, Crane, D, Fontanella, C, Sorter, M, Baum, R, and Applegate, M. A Medicaid quality improvement collaborative on psychotropic medication prescribing for children. Psychiatr Serv. (2018) 69:501–4. doi: 10.1176/appi.ps.201700547
55. Kelleher, KJ, Rubin, D, and Hoagwood, K. Policy and practice innovations to improve prescribing of psychoactive medications for children. Psychiatr Serv. (2020) 71:706–12. doi: 10.1176/appi.ps.201900417
56. Hostutler, CA, Valleru, J, Maciejewski, HM, Hess, A, Gleeson, SP, and Ramtekkar, UP. Improving Pediatrician's behavioral health competencies through the project ECHO Teleconsultation model. Clin Pediatr (Phila). (2020) 59:1049–57. doi: 10.1177/0009922820927018
57. Furlan, AD, Pajer, KA, Gardner, W, and MacLeod, B. Project ECHO: building capacity to manage complex conditions in rural, remote and underserved areas. Can J Rural Med. (2019) 24:115–20. doi: 10.4103/CJRM.CJRM_20_18
58. Pringsheim, T, Stewart, DG, Chan, P, Tehrani, A, and Patten, SB. The Pharmacoepidemiology of psychotropic medication use in Canadian children from 2012 to 2016. J Child Adolesc Psychopharmacol. (2019) 29:740–5. doi: 10.1089/cap.2019.0018
Keywords: antipsychotic agents/adverse effects, drug monitoring, adolescent, child, guidelines as topic
Citation: Antoniou T, Wang T, Pajer K, Gardner W, Lunsky Y, Penner M, Tadrous M, Mamdani M, Juurlink DN and Gomes T (2023) Adherence to antipsychotic laboratory monitoring guidelines in children and youth: a population-based study. Front. Psychiatry 14:1172559. doi: 10.3389/fpsyt.2023.1172559
Edited by:
Mirko Manchia, University of Cagliari, ItalyReviewed by:
Ginger E. Nicol, Washington University in St. Louis, United StatesAntonio Clavenna, Mario Negri Institute for Pharmacological Research (IRCCS), Italy
Copyright © 2023 Antoniou, Wang, Pajer, Gardner, Lunsky, Penner, Tadrous, Mamdani, Juurlink and Gomes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tony Antoniou, VG9ueS5hbnRvbmlvdUB1bml0eWhlYWx0aC50bw==
 Tony Antoniou
Tony Antoniou Tianru Wang2
Tianru Wang2 William Gardner
William Gardner Yona Lunsky
Yona Lunsky Melanie Penner
Melanie Penner Muhammad Mamdani
Muhammad Mamdani