- 1Department of Psychiatry, Chonnam National University Medical School, Gwangju, South Korea
- 2Department of Cardiology, Chonnam National University Medical School, Gwangju, South Korea
Background: Considering the association of inflammation with suicide and acute coronary syndrome (ACS), we investigated the individual and interactive effects of serum tumor necrosis factor-alpha (sTNFα) levels and two polymorphisms (−850 C/T and −308 G/A) on suicidal ideation (SI) after ACS.
Methods: The SI status using items on the Montgomery–Åsberg Depression Rating Scale (MADRS), related covariates including sociodemographic and clinical characteristics, sTNFα levels, and tumor necrosis factor-alpha (TNF-α) polymorphisms were evaluated in 969 patients within 2 weeks after ACS. Of the patients, 711 were evaluated 1 year later for SI. Multivariate logistic regression models were used to calculate individual and interactive associations after adjusting for the covariates.
Results: Higher (vs. lower) sTNFα levels and the −850 C/T or T/T (vs. C/C) polymorphism were significantly associated with SI 2 weeks after ACS, while only higher sTNFα levels were significantly associated with SI after 1 year. Significant interactive effects were detected between sTNFα (higher) levels and the −850 C/T (C/C or C/T) polymorphism on SI 2 weeks after ACS and between the two (−850 CC or CT and −308 G/A or AA) polymorphisms on SI 1 year after ACS.
Conclusions: The sTNFα level and two polymorphisms (−850C/T and −308 G/A), separately or in combination, could be time-specific biomarkers for SI in ACS. Focused interventions for ACS patients at risk of SI might reduce the suicidal burden in patients with ACS.
Introduction
Suicide is a global health issue, and much effort has been dedicated to identify patients at risk of suicide and devise preventive strategies. However, no markers have been validated to predict SI, where suicide is a complex, multifactorial phenomenon involving interactions between various environmental stressors (e.g., childhood adversities and physical illness) and individual factors including hopelessness, temperament, and biological mechanisms [e.g., sensory processing, serotonergic system function, hypothalamic–pituitary–adrenergic (HPA) axis activity, inflammatory status, and genetic vulnerabilities] (1–4). Life-threatening physical illnesses, including acute coronary syndrome (ACS), are risk factors for suicide because of their psychological (e.g., hopelessness and psychiatric comorbidities) and biological (e.g., inflammation and a heightened stress response due to HPA axis activity) effects (5, 6). Previous epidemiological and clinical studies demonstrated an increased risk of suicide in ACS patients (6, 7). Suicide/suicidal behavior (SB) includes suicidal ideation (SI), suicidal attempts (SA), and death due to suicide. SI is associated with poor long-term cardiac outcomes in ACS patients (8). Therefore, it is important to investigate the pathophysiology and biological markers of suicide in ACS patients.
Previous studies reported that abnormal inflammatory responses, including changes in cytokines, contribute to SB, which includes SI and SA (9, 10). Additionally, inflammation is a well-established pathomechanism underlying ACS (11). Cytokines are key modulators of inflammation and promising biomarkers of SB in ACS patients (12). Tumor necrosis factor-alpha (TNF-α) is a proinflammatory cytokine produced by T cells, glial cells, and neurons. Tumor necrosis factor-alpha activates the serotonergic system of the brain by stimulating serotonin transport and decreasing extracellular serotonin (13). Accordingly, many studies have reported that an increase in the TNF-α level and expression is associated with SI, SA, and death (14–17). However, other studies have reported no such association (18, 19) or a negative association has been reported (20). For the studies of TNF-α polymorphism, TNF-α-308 G/A is associated with SA (21) and male suicide (22). However, others reported no such associations (23, 24).
Despite accumulating evidence for a high risk of suicidality in ACS patients (6, 7), and the associations between suicide and cytokine abnormalities (9, 10), no study has investigated the association between cytokine imbalance (including TNF-α) and suicidality in ACS patients. The TNF-α level is affected by TNF-α gene polymorphisms; −850T and −308A alleles result in higher TNF-α levels compared to −850C and −308G alleles (25, 26). Interventions focused on suicide prevention are needed for patients at high risk of SI within 2 weeks, and 1 year after, ACS onset, based on their TNF-α status (level and polymorphism). We hypothesized that increased inflammation within 2 weeks of ACS onset, evidenced by a higher TNF-α serum level or polymorphisms that increase TNF-α expression, may be associated with SI in ACS patients. Additionally, individual and interactive effects of TNF-α serum levels and polymorphisms on SI in ACS patients may vary according to the time elapsed since ACS. Therefore, we investigated the individual effects of the serum TNF-α level and polymorphisms (−850C/T and −308G/A), as well as their interactive effect, on SI within 2 weeks, and 1 year after, ACS onset.
Materials and Methods
Study Overview
This study was a secondary analysis from an observational prospective study of Korean DEPression in ACS (K-DEPACS), which embraced part of a randomized controlled trial [Escitalopram for DEPression in ACS (EsDEPACS) study; ClinicalTrial.gov identifier: NCT00419471] (27). We recruited 1,152 consecutive ACS patients hospitalized at the Department of Cardiology, Chonnam National University Hospital, South Korea. Of these patients, 969 who met the inclusion criteria and consented to both participation and phlebotomy constituted the baseline sample (Figure 1). The K-DEPACS study included patients males and females aged 18–85 years, diagnosed with ACS [ST-segment elevation myocardial infarction (MI) was diagnosed on the basis of continuous chest pain ≥30 min, a new ST-segment elevation ≥2 mm on ≥2 contiguous electrocardiographic leads, and creatine kinase-MB (CK-MB) values ≥3 × normal limit; non-ST-segment elevation MI was diagnosed on the basis of chest pain and an increase in cardiac biochemical marker, without new ST-segment elevation; and unstable angina was diagnosed on the basis of chest pain within the last 72 h with or without ST-T segment changes or an increase in cardiac biochemical markers] and able to complete the questionnaires, understand the objectives of the study, and provide written, informed consent. Patients with any of following were excluded from K-DEPACS: ACS during hospitalization for another indication, ACS within 3 months after a coronary artery bypass graft surgery, uncontrolled hypertension [systolic blood pressure (BP) >180 mmHg or diastolic BP >100 mmHg], resting heart rate <40/min, severe medical disorders that are life threatening or may interfere with ACS recovery, or clinically significant laboratory abnormalities. Additional inclusion criteria for EsDEPACS included a Beck Depression Inventory (28) score >10 and a major or minor depressive disorder diagnosed on the basis of the DSM-IV. Additional exclusion criteria for EsDEPACS were concurrent use of class I antiarrhythmic medications, reserpine, guanethidine, clonidine, methyldopa, lithium, anticonvulsants, antipsychotics, or antidepressants; previously diagnosed neuropsychiatric disorders such as dementia, Parkinson's disease, brain tumor, psychosis, bipolar disorder, alcoholism, or other substance dependence; pregnancy; and inclusion in ongoing trials of another drug.
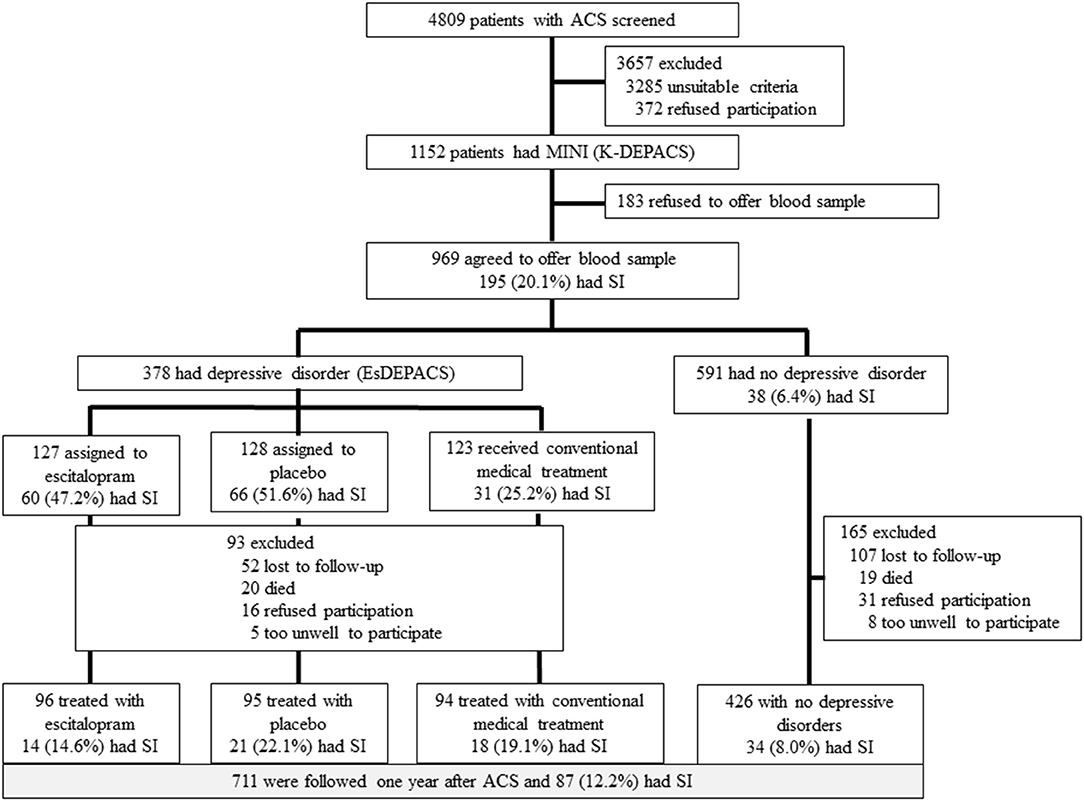
Figure 1. Flow diagram of the recruitment procedure. ACS, acute coronary syndrome; MINI, Mini-International Neuropsychiatric Interview; SI, suicidal ideation; K-DEPACS, Korean DEPression in Acute Coronary Syndrome study; EsDEPACS, Escitalopram for DEPression in Acute Coronary Syndrome study.
Baseline assessments of SI and sociodemographic characteristics, cardiovascular risk factors, and current cardiac status (as described in section “Evaluation of SI and Covariates” in section “Materials and Methods”) were made within 2 weeks (mean = 6.3 ± 2.4 days) after ACS; 711 patients were evaluated at 1 year after ACS onset. The Chonnam National University Hospital Institutional Review Board approved K-DEPACS and EsDEPACS, and all subjects provided written informed consent.
Evaluation of SI and Covariates
SI was measured within 2 weeks of the onset of ACS and after 1 year, using the suicide-related items of the Montgomery–Åsberg Depression Rating Scale (MADRS) (29). The MADRS consists of 10 items (total score range: 0–60). For the assessment of suicide-related items, participants were asked whether they thought life was worth living and whether they had ever planned a suicide attempt. Scores on the items ranged from 0 (life satisfaction) to 6 (explicit plans for suicide). The SI was defined by a score of 2 (fleeting suicidal thoughts) or more, as described previously (30). Although the suicide-related item of MADRS used in this study is a component of the depression rating scale, it was used in previous clinical trials and was found to be sensitive to changes in SI (30).
Data on the covariates strongly associated with SI in ACS patients (31) were evaluated within 2 weeks after ACS: (i) sociodemographic and depression characteristics including the age, sex, years of education, living status (living alone or not), type of residence (owned or rented), current occupation (employed or not), personal and family histories of depression, and depression diagnosis and treatment allocation status (escitalopram, placebo, treatment as usual); (ii) cardiovascular risk factors, including personal and family history of ACS, diagnosed hypertension and diabetes mellitus, hypercholesterolemia (fasting serum total cholesterol level >200 mg/dl or ongoing treatment), obesity (body mass index >25 kg/m2), and reported current smoking status and statin use; and (iii) the current cardiac status including ACS severity according to the Killip classification (32), left ventricular ejection fraction (echocardiography), heart rate (electrocardiography), and serum cardiac biomarkers (troponin I and CK-MB).
Depression was diagnosed on the basis of the DSM-IV criteria using the structured Mini International Neuropsychiatric Interview (33), within 2 weeks of ACS onset and after 1 year. The Killip classification is a simple clinical tool (classes I–IV) to stratify ACS patients according to the risk of poor clinical outcomes (34). Cardiologists who participated in our study and were blind to the depression status of participants determined the Killip classification.
Serum TNF-α and TNF-α Polymorphism
Venous blood from participants in a fasting state was collected and used for assays in present analyses. Serum tumor necrosis factor-alpha (sTNFα) levels were evaluated using a solid-phase sandwich enzyme-linked immunosorbent assay kit (Invitrogen, Camarillo, CA, USA), and the TNF-α polymorphisms were analyzed using polymerase chain reaction (PCR) and PCR-based restriction fragment length polymorphism assays. Polymorphism selection and allele detection methods are described in Supplementary Table 1. Serum tumor necrosis factor-alpha levels were categorized into lower and higher groups using the median values. The TNF-α −850C/T and −308G/A polymorphisms were classified into two groups, “C/C” and (“C/T” or “T/T”) and “G/G” and (“G/A” or “A/A”), considering their infrequency.
Statistical Analyses
Baseline characteristics were compared between patients with and without SI within 2 weeks of ACS onset, and after 1 year, using Student's t-tests or χ2-tests. Since the factors that affect SI during the acute and chronic phases might differ (35, 36), SI within 2 weeks of ACS onset and after 1 year were considered as separate dependent variables. Significant factors of SI within 2 weeks and 1 year after ACS onset (p < 0.05) were included as separate covariates in the multivariate analyses.
To evaluate the associations between sTNFα levels and polymorphisms, sTNFα levels were compared between patients with and without SI using Student's t-tests. A multivariate logistic regression model adjusted for relevant covariates was used to test the individual effects of sTNFα levels and polymorphisms on SI. To test for interactive effects between sTNFα levels and polymorphisms, participants were stratified based on their polymorphisms status (−850C/T and −308G/A). Then, the associations between sTNFα level and SI status were calculated using the same multivariate logistic regression models after adjusting for relevant covariates. To test for the interactive effects between two polymorphisms, participants were stratified by the TNF-α −850 C/T polymorphism status, and then the associations between TNF-α −308 G/A and SI status were analyzed using the same multivariate logistic regression models after adjusting for relevant covariates. The SPSS 21.0 software (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
Baseline Characteristics and Recruitment
All patients (N = 4,809) admitted with a recent ACS were approached for study participation. Among these patients, 1,152 were eligible for inclusion in the study and agreed to participate. Of these, 969 participants who agreed to blood/genetic testing were included in the present analyses. No significant differences were observed in baseline characteristics between those who did and those who did not undergo blood/genetic test. Of the 969 patients, 711 (73%) were followed for 1 year. The 258 patients lost to follow-up were older and had a higher Killip lass (p-value < 0.05). SI was identified in 195 (20.1%) and 87 (12.2%) patients within 2 weeks of ACS onset and after 1 year, respectively. The baseline characteristics associated with SI within 2 weeks of ACS onset and after 1 year were considered covariates (Supplementary Table 2).
Individual Effects of sTNFα Level and the TNF-α Polymorphisms on SI Status
Serum tumor necrosis factor-alpha levels were significantly higher in ACS patients with a higher frequency of the −850T and −308A alleles (all P-values < 0.05, Supplementary Figure 1). Table 1 summarizes the individual effects of the sTNFα level and the polymorphisms on SI. SI within 2 weeks after ACS was associated with a higher sTNFα level at baseline and a higher frequency of TNF-α −850 C/T or T/T alleles after adjusting for covariates. The significance of the association with the TNF-α –308 G/A allele was lost after adjustment. SI at 1 year after ACS was associated with a higher baseline sTNFα level, while no association was found with any of the TNF-α polymorphisms. All polymorphisms were in Hardy–Weinberg equilibrium (all P > 0.05).
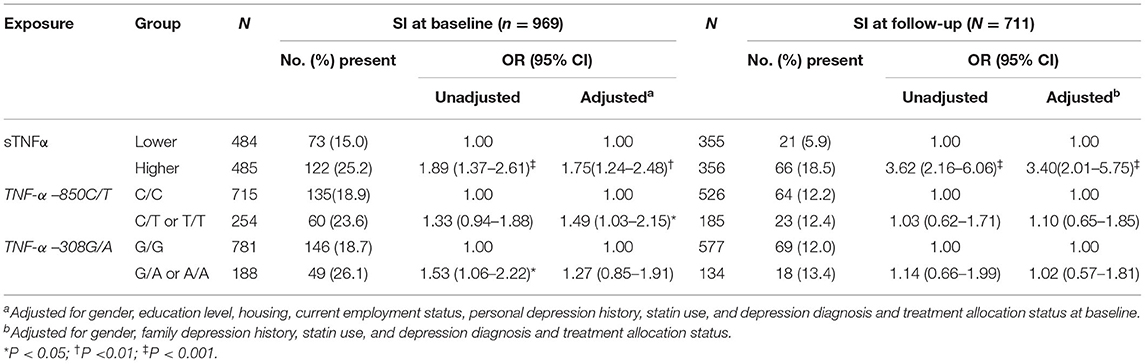
Table 1. Individual associations of serum tumor necrosis factor-alpha (sTNFα) level and two TNF-α polymorphisms with SI.
Interactive Effects of sTNFα Level and TNF-α Polymorphisms According to SI Status
The sTNFα levels (continuous variables) were compared by SI status for each TNF-α polymorphism (Table 2). SI within 2 weeks after ACS was significantly associated with a higher sTNFα level only in ACS patients with the −850 C/T or T/T polymorphism, while SI at 1 year after ACS was correlated with a higher sTNFα level only in ACS patients with the –308 G/G genotype.
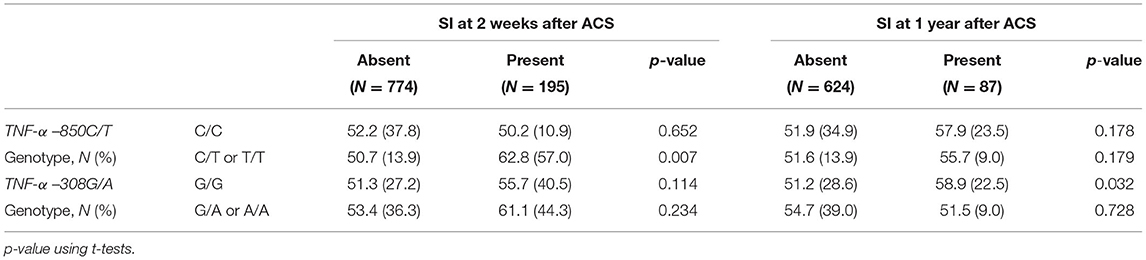
Table 2. Tumor necrosis factor-α (TNF-α) mean (SD) pg/ml serum concentrations by TNF-α two polymorphisms and depressive disorder status at 2 weeks and at 1 year after acute coronary syndrome (ACS).
The interactive effect between the sTNFα level (binary variable) and the TNF-α polymorphisms are described in Figure 2. SI at baseline was significantly associated with a higher sTNFα level in the presence of the −850 C/T or T/T genotype after adjusting for covariates. SI at 1 year after ACS was significantly associated with a higher sTNFα level in the presence of the –308 G/G genotype after adjustment, but no significant interaction was found. Instead, significant interactions were found between the two (−850 CC or CT and –308 G/A or AA) polymorphisms on SI at 1 year after ACS.
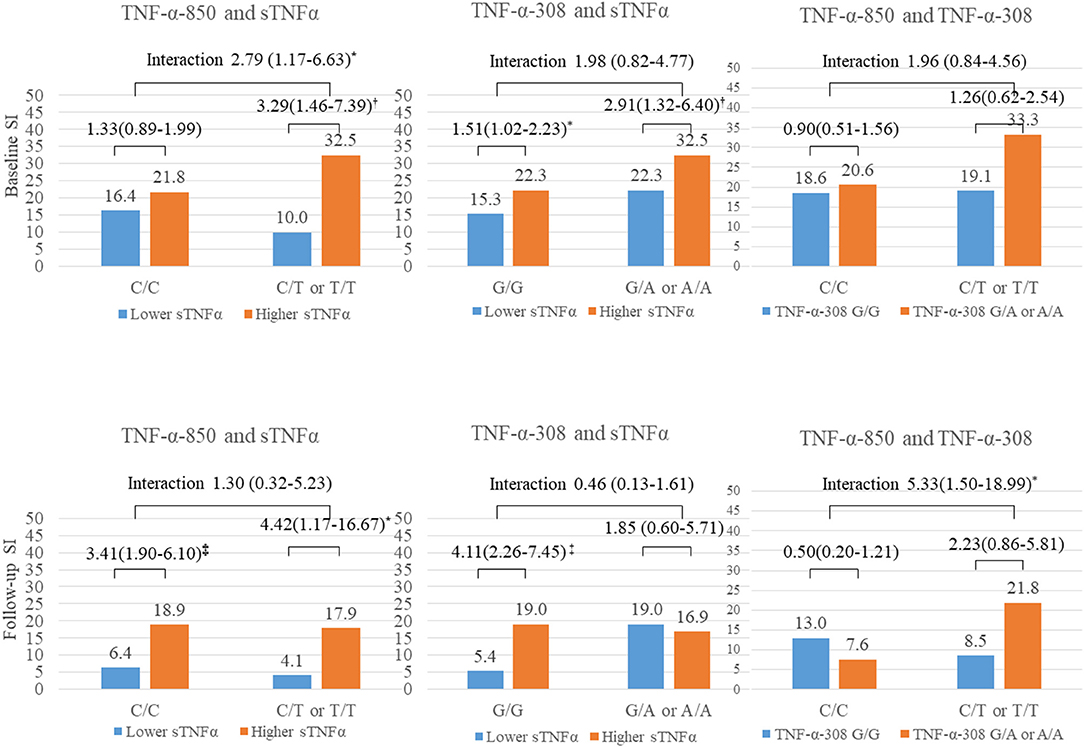
Figure 2. Interactive effect of tumor necrosis factor-alpha (sTNFα) level and two polymorphisms on suicidal ideation (SI) 2 weeks and 1 year after acute coronary syndrome (ACS). TNF-α, tumor necrosis factor-alpha; sTNFα, serum TNF-α level; SI, suicidal ideation. *P < 0.05; P < 0.01; P < 0.001.
Discussion
The main findings of this 1-year longitudinal study of ACS patients were that SI during the acute phase of ACS was significantly associated with higher baseline sTNFα levels and the −850 C/T or T/T polymorphism, both individually and interactively. SI during the chronic phase of ACS was associated with higher sTNFα levels individually and significantly interacted with the two polymorphisms. SI is an early and mild form of SB. However, a study including participants from several countries reported that 60% of patients transition from SI to a SA during the first year after SI onset (37). Therefore, it is essential to detect SI in the early phase after onset of severe physical illness to prevent SA.
In this study, higher sTNFα levels within 2 weeks after ACS were associated with SI, both within 2 weeks and after 1 year. This suggests that an increase in TNF-α immediately after ACS plays a role in the pathophysiology of SI in ACS patients. The association between TNF-α and suicide has not been confirmed in the psychiatric or general population. Higher TNF-α levels are associated with SA [plasma, (14)], suicidal death [brain, (15, 16)], and SI in depressed patients [serum, (17)]. However, TNF-α levels are not associated with SI, SA, or death [CSF and plasma, (19); plasma, (18)], whereas decreased TNF-α levels have been associated with SI in depressed adolescents [plasma, (20)]. These inconsistent findings may be due to differences in study design, including suicide-related outcomes, study participants, and the bio-samples measured. Moreover, no longitudinal study has been performed on the association between TNF-α level and later SI, except the antidepressant response on changes in SI (17, 38). The heightened inflammatory status of the sTNFα level following ACS (11) may have contributed to susceptibility to SI in response to a stressful event such as ACS through an interaction with the serotonergic system and neuroplasticity (39, 40).
It was interesting that the interactive effect of the TNF-α polymorphisms and sTNFα level on SI differed by time elapsed after ACS was diagnosed. The TNF-α −850 C/T polymorphism was associated with SI within 2 weeks after ACS individually and interactively with a higher sTNFα level. The significant interaction means that the association between a higher sTNFα level and SI within 2 weeks after ACS was prominent in the presence of the risk allele (−850 T). This inherited vulnerability, particularly the TNF-α −850 C/T or T/T polymorphism, which is associated with a higher sTNFα level (25), may potentiate the deleterious effect of an increased sTNFα level in response to ACS (11, 41). This proinflammatory state contributed to patients who faced the life-threatening event of ACS being susceptible to SI even in the acute phase of ACS. Although there is a lack of previous studies on the association between the TNF-α −850 C/T polymorphism and suicide ranging from SI to SA, our findings provide evidence for inflammatory dysregulation underlying SB and provide insight for future studies investigating the association between TNF-α and suicide to consider the interaction between the sTNFα level and these polymorphisms.
No interactions were detected between the sTNFα level and individual polymorphisms on SI at 1 year after ACS, but a significant interaction was identified between the two polymorphisms and SI. During the chronic phase of ACS when adaptation to the changes after ACS has occurred, the interactive effect of the two TNF-α genetic polymorphisms rather than a single inherited polymorphism may have contributed to ACS patients experiencing SI. Although no previous studies have considered the interaction between the two TNF-α polymorphisms and underlying mechanisms, future research with larger samples is needed to ascertain these time-specific and synergistic genetic associations underlying SI in ACS patients.
This study had several strengths. It was the first longitudinal study to investigate the associations between TNF-α measured within 2 weeks and SI within 2 weeks, and at 1 year after, ACS onset. Additionally, this was the first study to investigate the individual and interactive effects of sTNFα levels and two polymorphisms (−850 C/T and −308 G/A) on SI. Moreover, many clinical and psychosocial covariates were assessed using validated instruments for psychiatric and cardiovascular evaluations. All consecutive eligible patients with recent ACS were recruited and followed up after 1 year, which reduced the error due to discordant examination intervals and increased sample homogeneity.
There were several limitations to this study. First, SI was measured using suicide-related items on a depression scale, rather than by a dedicated psychometric instrument (such as the Columbia Classification Algorithm of Suicide Assessment) (42). Although the suicide-related items of the MADRS are sensitive to changes in SI (30), future studies using scales (e.g., Columbia Classification Algorithm of Suicide Assessment) that are more specific and sensitive to SI assessment are needed to confirm our findings. Second, our findings on SI may not apply to SB in general in patients with ACS. Nevertheless, SI alone imposes a considerable healthcare burden (43) and can lead to severe SB (44). Third, the frequent co-occurrence of SI and depression needs further evaluation. In this study, 38 (80.5%) of 195 ACS patients had SI within 2 weeks of ACS onset, while 77 (88.5%) of 87 ACS patients with SI had depression at 1 year after ACS. SI is significantly associated with depression. In fact, it is one of the diagnostic criteria for depression (45). Previous studies have reported unique, shared, and interactive pathophysiological mechanisms underlying depression and suicide (46, 47). Inflammatory dysregulation has also been suggested as an underlying mechanism of depression, particularly in ACS patients during the acute phase of illness (48). In this study, the relationship between SI and TNFα levels/polymorphisms remained significant despite adjusting for depression status/treatment as a confounding factor. However, the association of SI with depression should be considered when interpreting the results of our study. Fourth, sTNFα levels were measured only once (at baseline); therefore, longitudinal associations between changes in sTNFα levels and SI could not be evaluated. Also, this study included a relatively small sample. However, polymorphism studies require large sample sizes. Moreover, we did not evaluate mental disorder history, including anxiety disorders, which may affect SI and should be considered as a potential covariate. Additionally, healthy controls were not included in this study, which precluded comparison of sTNFα levels between ACS patients and healthy controls. Future studies including healthy controls will be needed. Finally, attrition should be considered when interpreting the results. Of the participants, 84% agreed to undergo blood/genetic testing, and 73% of these patients were followed up. There was no difference between patients who did and did not undergo blood/genetic testing, but patients lost to follow-up were more likely to be older and have worse cardiac function.
In conclusion, a significant individual and interactive effect of sTNFα levels and the −850 C/T polymorphism on SI within 2 weeks after ACS onset was identified. A significant individual effect of the sTNFα level and interactive effects of the two TNF-α polymorphisms on SI at 1 year after ACS onset were observed. Our findings suggest that sTNFα levels and polymorphisms (−850 C/T and −308 G/A), individually or in combination, could be used as time-specific biomarkers of SI in ACS patients. In other words, measuring the sTNFα levels within 2 weeks after ACS and/or 850 C/T polymorphism can identify ACS patients at high risk of SI during the acute phase of the illness. Measuring the sTNFα levels or both polymorphisms (−850 C/T and –308 G/A) within 2 weeks can identify ACS patients at high risk for SI in the chronic phase of the illness who require preventive management. Detection of SI in ACS patients is crucial because SI is associated with poor long-term cardiac outcomes in ACS patients (8). Evaluation of sTNFα levels and polymorphisms in ACS patients may help clinicians to identify vulnerable patients who may benefit from focused, collaborative, and preventive strategies for SI. Our result will provide a basis for future research on the role of TNF-α in SI in ACS patients. Long-term clinical outcome studies are required on the effects of early identification of SI and preventive management in ACS patients. Further studies are warranted to evaluate the reproducibility of our results and cost-effectiveness of testing for sTNFα levels and polymorphisms.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the inquries to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by The Chonnam National University Hospital Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
H-JK and J-MK conducted the data analysis and drafted the article. J-WK, J-YL, S-WK, and I-SS helped to analyze the data and to draft the article. YH, YA, and M-HJ helped to recruit the participants and perform cardiac assessment and management. All authors contributed the article and approved the submitted version.
Funding
The study was funded by a grant of the National Research Foundation of Korea Grant (NRF-2020M3E5D9080733 and NRF-2020R1A2C2003472) to J-MK.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.739823/full#supplementary-material
References
1. Mann JJ, Rizk MM. A brain-centric model of suicidal behavior. Am J Psychiatry. (2020), 177:902–16. doi: 10.1176/appi.ajp.2020.20081224
2. Pompili M, Rihmer Z, Akiskal H, Amore M, Gonda X, Innamorati M, et al. Tempraments mediate suicidal risk and psychopathology among patients with bipolar disorders. Compr Psychiatry. (2012) 53:280–5. doi: 10.1016/j.comppsych.2011.04.004
3. Serafini G, Gonda X, Pompili M, Rihmer Z, Amore M, Engel-Yeger B. The relationship between sensory processing patterns, alexithymia, traumatic childhood experiences and quality of life among patients with unipolar and bipolar disorders. Child Abuse Negl. (2016) 62:39–50. doi: 10.1016/j.chiabu.2016.09.013
4. Slavich GM, Auerbach RP. Stress and its sequelae: depression, suicide, inflammation, and physical illness. In: Butcher JN, Hooley JM, editors. APA Handbook of Psychopathology: Vol. 1. Psychopathology: Understanding, Assessing, and Treating Adult Mental Disorders. Washington, DC: American Psychological Association (2018). p. 375–402. doi: 10.1037/0000064-016
5. Costanza A, Amerio A, Aguglia A, Escelsior A, Serafini G, Berardelli I, et al. When sick brain and hopelessness meet: some aspects of suicidality in the Neurological patient. CNS Neurol Disord Drug Targets. (2020), 19:257–63. doi: 10.2174/1871527319666200611130804
6. Larsen KK, Agerbo E, Christensen B, Søndergaard J, Vestergaard M. Myocardial infarction and risk of suicide: a population-based case-control study. Circulation. (2010) 122:2388–93. doi: 10.1161/CIRCULATIONAHA.110.956136
7. Nascimento ER, Maia AC, Soares-Filho G, Nardi AE, Cardoso A. Predictors of suicidal ideation in coronary artery disease. Compr Psychiatry. (2015) 57:16–20. doi: 10.1016/j.comppsych.2014.10.017
8. Kim JM, Stewart R, Lee HJ, Kang HJ, Bae KY, Kim SW, et al. Impact of suicidal ideation on long-term cardiac outcomes in patients with acute coronary syndrome: sex-specific differences. Psychother Psychosom. (2018) 87:311–2. doi: 10.1159/000489788
9. Serafini G, Parisi VM, Aguglia A, Amerio A, Sampogna G, Fiorillo A, et al. A Specific Inflammatory profile underlying suicide risk? Systematic review of the main literature findings. Int J Environ Res Public Health. (2020) 17:2393. doi: 10.3090/ijeroh17072393
10. Ducasse D, Olié E, Guillaume S, Artéro S, Courtet P. A meta-analysis of cytokines in suicidal behavior. Brain Behav Immun. (2015) 46:203–11. doi: 10.1016/j.bbi.2015.02.004
11. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. (2005) 111:3481–8. doi: 10.1161/CIRCULATIONAHA.105.537878
12. Felger JC, Capuron L. Special Issue: The intersection of inflammation and metabolism in neuropsychiatric disorders. Brain Behav Immun. (2021) 93:331–4. doi: 10.1016/j.bbi.2020.12.025
13. Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. (2006) 31:2121–31. doi: 10.1038/sj.npp.1301029
14. Janelidze S, Mattei D, Westrin A, Träskman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain Behav Immun. (2011) 25:335–9. doi: 10.1016/j.bbi.2010.10.010
15. Pandey GN, Rizavi HS, Zhang H, Bhaumik R, Ren X. Abnormal protein and mRNA expression of inflammatory cytokines in the prefrontal cortex of depressed individuals who died by suicide. J Psychiatry Neurosci. (2018) 43:376–85. doi: 10.1503/jpn.170192
16. Wang Q, Roy B, Turecki G, Shelton RC, Dwivedi Y. Role of complex epi-genetic switching in tumor necrosis factor-alpha upregulation in the prefrontal cortex of suicide subjects. Am J Psychiatry. (2018) 175:262–74. doi: 10.1176/appi.jp.2017.16070759
17. Choi KW, Jang EH, Kim AY, Kim H, Park MJ, Byun SW, et al. Predictive inflammatory biomarkers for change in suicidal ideation in major depressive disorders and panic disorder: a 12-week follow-up study. J Psychiatr Res. (2021) 133:73–81. doi: 10.1016/j.jpsychires.2020.12.011
18. Li Z, Qi D, Chen J, Zhang C, Yi Z, Yuan C, et al. Venlafaxine inhibits the upregulation of plasma tumor necrosis factor-alpha (TNF-α) in the Chinese patients with major depressive disorder: a prospective longitudinal study. Psychoneuroendocrinology. (2013) 38:107–14. doi: 10.1016/j.psyneuen.2012.05.005
19. Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. (2009) 66:287–92. doi: 10.1016/j.biopsych.2009.01.030
20. Gabbay V, Klein RG, Guttman LE, Babb JS, Alonso CM, Nishawala M, et al. 2009. A preliminary study of cytokines in suicidal and nonsuicidal adolescents with major depression. J Child Adolesc Psychopharmacol. (2009) 19:423–30. doi: 10.1089/cap.2008.0140
21. Kim Y-K, Hong J-P, Hwang J-A, Lee H-J, Yoon H-K, Lee B-H, et al. TNF-alpha −308G>A polymorphism is associated with suicide attempts in major depressive disorder. J Affect Disord. (2013) 150:668–72. doi: 10.1016/j.jad.2013.03.019
22. Omrani MD, Bushehri B, Bagheri M, Salari-Lak S, Alipour A, Anoshae MR, et al. Role of IL-10-1082, IFN-γ+874, and TNF-α-308 genes polymorphism in suicidal behavior. Arch Suicide Res. (2009) 13:330–9. doi: 10.1080/13811110903266418
23. Sáiz PA, García-Portilla P, Paredes B, Arango C, Morales B, Alvarez V, et al. Association study of interleukin-1 gene complex and tumor necrosis factor alpha gene with suicide attempts. Psychiatr Genet. (2008) 18:147–50. doi: 10.1097/YPG.0b013e3282fb002a
24. Lang X, Trihn TH, Wu HE, Tong Y, Xiu M, Zhang XY. Association between TNF-alpha polymorphism and the age of first suicide attempt in chronic patients with schizophrenia. Aging. (2020) 12:1433–45. doi: 10.18631/aging.102692
25. McCusker SM, Curran MD, Dynan KB, McCullagh CD, Urguhart DD, Middleton D, et al. Association between polymorphism in regulatory region of gene encoding tumour necrosis factor alpha and risk of Alzheimer's disease and vascular dementia: a case–control study. Lancet. (2001) 357:436–9. doi: 10.1016/s0140-6736(00)04008-3
26. Wilson AG, Symons JA, McDowell TL, Mcdevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA. (1997) 94:3195–9. doi: 10.1073/pnas.94.7.3195
27. Kim JM, Bae KY, Kang HJ, Kim SW, Shin IS, Hong YJ. Design and methodology for the Korean observational and escitalopram treatment studies of depression in acute coronary syndrome: K-DEPACS and EsDEPACS. Psychiatry Investig. (2014) 11:89–94. doi: 10.4306/pi.2014.11.1.89
28. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. (1961) 4:561–71. doi: 10.1001/archpsyc.1961.01710120031004
29. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. (1979) 134:382–9. doi: 10.1192/bjp.134.4.382
30. Ballard ED, Luckenbaugh DA, Richards EM, Salls TL, Brutsché NE, Ameli R, et al. Assessing measures of suicidal ideation in clinical trials with a rapid-acting antidepressant. J Psychiatr Res. (2015) 68:68–73. doi: 10.1016/j.jpsychires.2015.06.003
31. Kim JM, Kang HJ, Bae KY, Kim SW, Shin IS, Hong YJ, et al. Determinants and escitalopram treatment effect on suicidal ideation in patients with acute coronary syndrome: findings from the K-DEPACS and EsDEPACS studies. Int J Caridol. (2016) 219:225–30. doi: 10.1016/j.ijcard.2016.06.048
32. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The mini-international neuropsychiatric interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. (1998) 59(Suppl):22–33.
33. Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. (1967) 20;457–64. doi: 10.1016/0002-9149(67)90023-9
34. El-Menyar A, Zubaid M, AlMahmeed W, Sulaiman K, AlNabti A, Singh R, et al. Killip classification in patients with acute coronary syndrome: insight from a multicentre registry. Am J Emerg Med. (2012) 30:97–103. doi: 10.1016/j.ajem.2010.10.011
35. Kang HJ, Bae KY, Kim SW, Shin IS, Hong YJ, Ahn Y. BDNF methylation and suicidal ideation in patients with acute coronary syndrome. Psychiatry Ingestig. (2018) 15:1094–97. doi: 10.30773/pi.2018.09.20
36. Kim JM, Kim SW, Kang HJ, Bae KY, Shin IS, Kim JT, et al. Serotonergic genes and suicidal ideation 2 weeks and 1 year after stroke in Korea. Am J Geriatr Psychiatry. (2014), 22:980–8. doi: 10.1016/j.jagp.2013.06.001
37. Nock MK, Borges G, Bromet EJ, Alonso J, Angermeyer M, et al. Cross-national prevalence and risk factors for suicidal ideation, plans, and attempts. Br J Psychiatry. (2008), 192:98–105. doi: 10.1192/bjp.bp.107.040113
38. Martinez JM, Garakani A, Yehuda R, Gorman JM. Proinflammatory and resiliency proteins in the CSF of patients with major depression. Depress Anx. (2012) 29:32–8. doi: 10.1002/da.20876
39. Yamada K, Iida R, Miyamoto Y, Saito K, Sekikawa K, Seishima M, et al. Neurobehavioral alterations in mice with a targeted deletion of the tumor necrosis factor-alpha gene: implications for emotional behavior. J Neuroimmunol. (2000) 111:131–8. doi: 10.1016/s0165-5728(00)00375-1
40. Neumann H, Schweigreiter R, Yamashita T, Rosenkranz K, Wekerle H, Barde YA. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. J Neurosci. (2002) 22:854–62. doi: 10.1523/JNEUROSCI.22-03-00854.2002
41. Gouweleeuw L, Wajant H, Maier O, Eisel ULM, Blankesteijn WM, Schoemaker RG. 2021. Effects of selective TNFR1 inhibition or TNFR2 stimulation, compared to non-selective TNF inhibition on (neuro)inflammation and behavior after myocardial infarction in male mice. Brain Behav Immun. (2021) 93:156–71. doi: 10.1016/j.bbi.2021.01.001
42. Posner K, Oquendo MA, Gould M, Stanley B, Davies M. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA's pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. (2007) 164:1035–43. doi: 10.1176/ajp.2007.164.7.1035
43. van Spijker BA, van Straten A, Kerkhof AJ, Hoeymans N, Smit F. Disability weights for suicidal thoughts and non-fatal suicidal attempts. J Affect Disord. (2011) 134:341–7. doi: 10.1016/j.jad.2011.05.020
44. Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia–suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. (2011) 168:1266–77. doi: 10.1176/appi.qpj.2011.10111704
45. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington VA: American Psychiatric Association (2013).
46. Mann JJ, Currier DM. Stress, genetic and epigenetic effect on the neurobiology of suicidal behaviour and depression. Eur Psychiatry. (2010), 25:268–71. doi: 10.1016/j.eurpsy.2010.01.009
47. Kalin NH. Insights into suicide and depression. Am J Psychiatry. (2020), 177:877–80. doi: 10.1176/appi.ajp.2020.20081207
Keywords: acute coronary syndrome, depression, tumor necrosis factor-α, gene association study, interaction
Citation: Kang H-J, Kim J-W, Lee J-Y, Kim S-W, Shin I-S, Hong YJ, Ahn Y, Jeong M-H and Kim J-M (2021) Time-Specific Associations of Tumor Necrosis Factor-α Levels and Polymorphisms (−850 C/T or −308 G/A) With Suicidal Ideation in Acute Coronary Syndrome Patients. Front. Psychiatry 12:739823. doi: 10.3389/fpsyt.2021.739823
Received: 12 July 2021; Accepted: 17 August 2021;
Published: 23 September 2021.
Edited by:
Domenico De Berardis, Azienda Usl Teramo, ItalyReviewed by:
Daria Smirnova, Samara State Medical University, RussiaMohsen Khosravi, Zahedan University of Medical Sciences, Iran
Gianluca Serafini, San Martino Hospital (IRCCS), Italy
Alessandra Costanza, Université de Genève, Switzerland
Zohreh Halvaiepour, Isfahan University of Medical Sciences, Iran
Copyright © 2021 Kang, Kim, Lee, Kim, Shin, Hong, Ahn, Jeong and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jae-Min Kim, am1raW0mI3gwMDA0MDtjaG9ubmFtLmFjLmty
 Hee-Ju Kang1
Hee-Ju Kang1 Ju-Yeon Lee
Ju-Yeon Lee Sung-Wan Kim
Sung-Wan Kim